Abstract
Helminth infection is a worldwide health problem. In addition to directly causing disease, helminthic infection also affects the incidence and progression of other diseases by exerting immune modulatory effects. In animal models, infection with helminthic parasites can prevent autoimmune diseases and allergic inflammatory diseases, but worsens protective immunity to certain infectious pathogens. In this review, we summarize current findings regarding the effects of helminth infection on type 1 diabetes, tuberculosis, and asthma and discuss possible mechanisms through which helminthic parasites modulate host immunity. Investigating these mechanisms could lead to treatment strategies that specifically modulate the immune response as well as address fundamental questions in immunobiology.
Keywords: Parasites, Regulation, Inflammation, Immune disorders, Infectious diseases
Introduction
Hygiene hypothesis and helminthic infection
The “hygiene hypothesis” suggests that sanitizing the environment leads to an increased incidence of allergic disease in susceptible individuals [1, 2]. Recently, an extension of this original hypothesis has suggested that improved hygiene may also increase susceptibility to certain autoimmune disorders [3, 4]. A corollary to this hypothesis is that exposure to certain parasites and microbes in early years of life might prevent such diseases [4]. This may result from environmental pathogens that stimulate the development and maintenance of well-regulated immune responses. Among these pathogens, helminthic parasites are particularly potent at inducing immune modulation, and a modification of the hygiene hypothesis suggests a particularly important role for these eukaryotic pathogens [5].
Helminth infections affect 1.5 billion people in the world [6]. The characteristic immune response includes: eosinophilia, mucosal mast cell hyperplasia, elevated IgE secretion, increased production of Th2 cytokines, and expansion of regulatory T-cells (Tregs). Clinical manifestations include malnutrition, anemia, impaired growth, retarded cognitive development, mucohemorrhagic diarrhea, and severe morbidity [7–10]. In some cases, invading parasites can also modulate the immune system, a characteristic that may have evolved to evade host immune surveillance and impair development of effective host responses. It should be noted that this effect of the parasite on the host immune response might also influence the host response to other infectious and non-infectious agents [11]. Intriguingly, geographic regions with a high burden of helminth infection have a lower incidence of many inflammatory disorders such as asthma, rheumatoid arthritis (RA), type 1 diabetes (T1D), multiple sclerosis (MS), and inflammatory bowel diseases (IBD) [4]. Alternatively, helminth infection increases the severity of certain infectious diseases such as Mycobacterium tuberculosis [12] and reduces the potency of Bacillus Calmette-Guérin (BCG) vaccination [13]. Thus, parasites may have both beneficial and harmful effects on the immune response. In this review, we focus on the immunomodulatory effect of helminth parasites on the development of an autoimmune disease, T1D; an infectious disease, tuberculosis (TB); and an allergic response, asthma. Other recently published articles have reviewed parasite infection in inflammatory bowel disease [14, 15], rheumatoid arthritis [16], and multiple sclerosis [17, 18]. Taken together, studies of the effects of helminths on these different inflammatory disorders suggest that the Th2-type response to helminths can trigger both Th2 cytokines and T regulatory cell responses that together can modulate both Th1-type inflammation and allergy-associated Th2-type inflammation. In this review, we emphasize the helminth-induced immune regulatory mechanism(s) shown to be effective in controlling specific inflammatory conditions; it is of course likely that future studies may identify additional mechanisms or combinations thereof. We believe this information is relevant in understanding how helminths influence other diseases and may provide a rationale for the use of helminth-based therapies for inflammatory disorders and for the development of more effective vaccines against microbes in helminth-infected populations.
Helminth infection and T1D
T1D is an organ-specific autoimmune disease in which the pancreatic β cells are specifically destroyed by pathogenic IFN-γ-producing Th1 T cells [19]. It affects approximately 0.2% to 0.3% of children in the USA and ten times that rate in first-degree relatives of patients with T1D. Its incidence has significantly increased in Westernized societies in the past three decades [4]. Although T1D is a multigenetic disorder [20], it is also influenced by environmental factors [21, 22].
Recently, the influence of helminthic infection on the onset and development of T1D has drawn increasing attention. First, there is an inverse association between T1D and infectious diseases such as filariasis, and soil-transmitted helminths [4]. Second, in mice, parasites and associated products significantly decreased the rate of spontaneous T1D and inhibited beta-cell/islet infiltration [23–25]. These findings raise the possibility that helminth infection may act as a protective factor against human T1D.
Th2 response
How helminthic infection suppresses T1D remains unclear. Destructive insulitis in non-obese diabetic (NOD) mice is associated with increased numbers of interferon-γ (IFN-γ)-producing cells and can be inhibited by IL-4 [26]. Furthermore, expression of IL-4 in the pancreas inhibited cyclophosphamide-induced diabetes [27] and exogenous administration of IL-4 or IL-4 plus IL-10 in vivo decreased islet infiltration and sustained normal blood glucose levels [28, 29]. The possible mechanisms by which IL-4 prevents T1D are summarized in Fig. 1. Helminths are excellent agents to induce highly polarized Th2-type responses [30]. Indeed, infection of NOD mice with helminths shifted a Th1-type response to a Th2-type response and blocked the development of T1D [24, 25]. However, whether exogenous IL-4 or helminth infection-induced IL-4 mediates the control of T1D directly or through stimulation of other immune regulatory pathways is unclear.
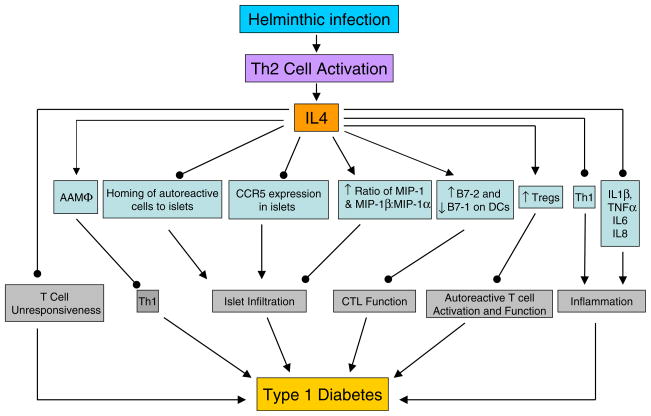
Fig. 1.
IL-4 may use the following pathways to inhibit T1D: (1) IL-4 can overcome the T cell unresponsiveness, thought to trigger T1D in NOD mice [130], by restoring NOD thymic and peripheral T cell proliferation [131]; (2) IL-4 suppresses the migration of auto-reactive cells to inflammatory sites [132]; (3) IL-4 treatment attenuates CCR5 mRNA expression in islets and increases MIP-1β production with an elevated ratio of MIP-1β and MCP-1: MIP-1α in the pancreas, which correlates with increased diabetes resistance [133]; (4) IL-4 inhibits cytotoxic T lymphocyte (CTL) function by boosting B7.2 and attenuating B7.1 expression on DCs, and blocking B7.2 abrogates IL-4 mediated protection from T1D [134]; (5) IL-4 can elicit FoxP3-expressing CD4+CD25+ Tregs from CD4+CD25− precursors [135] and increase their suppressive function [136]; (6) IL-4 provides anti-inflammation function through suppressing the production of acute-phase cytokines such as IL-1β, TNF-α, IL-6, and IL-8 [137]; (7) IL-4 stimulates AAMΦs [138], which may downregulate Th1 pathogenic T cell responses; (8) IL-4 directly inhibits Th1 cells. All of these mechanisms may contribute to the protective function of IL-4 in diabetes
IL-10
Helminths may elicit regulatory cytokines such as IL-10 or transforming growth factor-beta (TGF-β). IL-10 is an anti-inflammatory cytokine and its administration has been shown to ameliorate T1D [26]. Immunization with Schistosoma mansoni antigens increased IL-10 production by T cells and impaired the adoptive transfer of diabetes with splenocytes from NOD mice [24]. Administration of recombinant human IL-10 [31] or IL-10 gene delivery [32] in NOD mice both significantly diminished insulitis and blocked diabetes onset. Blocking IL-10 function accelerated T1D and increased the ability of splenocytes from NOD mice to transfer T1D [33]. Mechanisms though which helminth-induced IL-10 may downregulate T1D are summarized in Fig. 2.
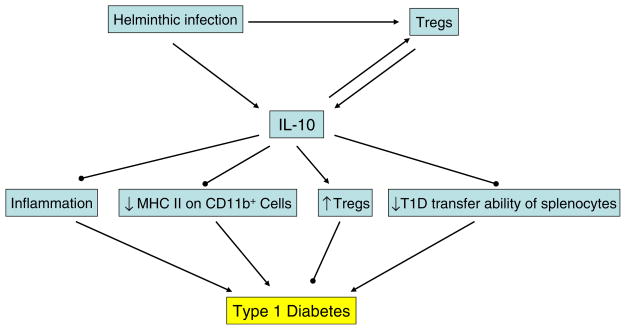
Fig. 2.
The function of IL-10 in preventing T1D: (1) IL-10 directly suppresses inflammatory responses; (2) IL-10 increases the percentage of CD4+ CD25+ regulatory T cells, dampens the MHC Class II expression on CD11b+ cells, and inhibits the ability of diabetogenic spleen cells to transfer T1D [139]
Tregs
CD4+CD25+ Tregs can be induced during helminth infection [34, 35]. A protective role of Tregs has been shown in T1D. In both humans and mice, decreased number or function of natural CD4+CD25+ T cells correlates with the tendency to develop or accelerate T1D [36, 37], although recent studies suggest that NOD mice do not have a decreased number of FoxP3+ Tregs [38]. Transfer of Tregs to NOD mice blocks diabetes development [39]. Moreover, cyclophosphamide accelerates T1D in NOD mice in part by reducing Tregs [39], suggesting that Tregs may contribute to the suppressive effect of helminths on the development of diabetes. As shown in Fig. 3, Tregs may use different pathways to prevent T1D.
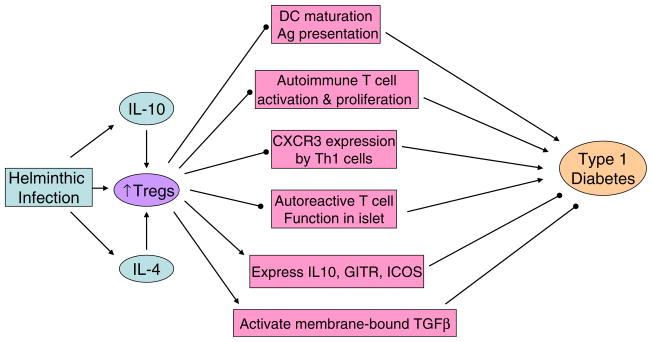
Fig. 3.
Treg cells prevent the onset of T1D through the following possible mechanisms: (1) Treg cells control T cell priming by inhibiting DC maturation and Ag presentation [140]. (2) Tregs suppress autoimmune T cell differentiation into T effector cells [141]. (3) Tregs inhibit chemokine receptor CXCR3 expression by Th1 cells that plays an important role in T cell pancreatic islet infiltration [141]. (4) Tregs restrain the auto reactive T cell function inside the islets [142]. (5) Tregs express high levels of IL-10, glucocorticoid induced tumor necrosis factor receptor family and Inducible T-cell COStimulator (ICOS) to suppress inflammation and tissue damage within the peripheral tissues [143, 144]. Blocking ICOS abrogates protective function of Tregs and accelerates the disease development [145]. (6) Tregs contribute to autoimmune tolerance through activating membrane-bound TGFβ [146]
Invariant natural killer T cells
Besides Tregs, invariant natural killer T cells (iNKT) cells have also been linked with T1D. In NOD mice, the number of iNKT cells was markedly reduced and increasing the number of iNKT cells in vivo prevented T1D [40]. In humans, iNKT cell clones isolated from patients with T1D failed to produce IL-4 upon T cell receptor stimulation [41]. Prevention of T1D by iNKT cells requires cell-contact or close-cell proximity [42] and depends on the activity of CD4+CD25+ Tregs [43] but not peripheral CD1d expression [44]. The requirement of IL-4 for iNKT cell-mediated protection against T1D is still controversial [42, 45].
iNKT cells may regulate the host response post parasite infection [46]. For example, they make IL-4 and IFNγ in vivo after S. mansoni infection [47]. Prevention of T1D in NOD mice by S. mansoni worm and egg antigens was associated with an increase of iNKT cells, suggesting that iNKT cell expansion and activation may contribute to helminth-induced protection from diabetes in NOD mice [24].
Alternatively activated macrophages
Alternatively activated macrophages (AAMΦs) can be induced by IL-4 during helminth infection [48]. AAMΦs express CD206 and IL-4Ralpha on the cell surface and metabolize arginine to polyamines or proline via the arginase pathway [48] in contrast to classically activated macrophages that metabolize arginine to nitric oxide via the inducible nitric oxide synthase pathway. In NOD mice, macrophages (MΦs) appear to contribute to the development of T1D [49] while depletion of MΦs inhibits the development of T1D [50]. It is likely that classically activated macrophages develop in NOD mice given the elevated Th1-type response. Helminth infection may instead trigger AAMΦ generation that suppresses the Th1-type response and associated classical activation of MΦ, thereby protecting rather than exacerbating insulitis and β-cell destruction. AAMΦs have been shown to suppress Th1-type responses during helminth infection [51] and to inhibit antigen-specific CD4+ activation through a TGF-β-dependent mechanism [52]. Recently, we found that following Heligmosomoides polygyrus (Hp) infection of NOD mice, AAMΦs are present in the pancreatic lymphoid nodes (Liu Q. et al., Infect Immun. 2009 Sep 14. [Epub ahead of print]), suggesting that they may play a role in the control of the Th1-type immune response, and thus contribute to the inhibition of T1D.
Helminthic infection and Mycobacterium tuberculosis
TB is a leading life-threatening infectious disease with 1.9 billion people infected and 1.9 million deaths each year [53]. Currently, mortality and morbidity remain alarmingly high for TB. Possible reasons include: the majority of people carry a latent infection and reactivation increases morbidity and mortality [54]; current vaccine candidates (BCG) mainly protect against TB meningitis in children, but not in latently infected individuals [55]; and co-infection with HIV significantly magnifies the risk of having active TB [56].
The finding that HIV co-infection significantly reduces immunity to MTB suggests that co-infection with other pathogens may also affect the protective immune response to MTB. Consistently, increasing evidence suggests helminths exert profound immunoregulatory effects: (1) There is a strong correlation between the intestinal helminth infection and the onset of active pulmonary TB [57] and enhancement in mycobacterial-specific immune responses occurs following anti-helminthic therapy [58]. (2) Helminthic infection reactivates latent TB infection [59]. (3) Helminthic infection weakens the efficacy of BCG vaccine [57]. In mice, infection with helminthic parasites such as S. mansoni prior to BCG vaccination markedly lowers the TB antigen-specific Th1-type response [12]. In humans, children born from worm-infected mothers show a compromised response to BCG vaccination [60]. Taken together, these studies suggest that helminth infection might worsen TB pathogenesis.
Helminth-induced Th2 cytokines
Protection against MTB infection relies mainly on IFN-γ production by CD4+ Th1 cells. Helminthic infections are potent inducers of Th2 cells [61]. Compared to uninfected controls, TB patients have a markedly increased IL-4 [62] and Th2/Th1 (IL-4/IFN-γ) mRNA ratio [63]. Th2 cytokine predominance, especially IL-4, has been shown to correlate with the progression to active TB [64]. In MTB-infected animals, IL-4 deficiency extensively depressed the infection in lung and spleen, but rIL-4 administration enhanced bacterial burden to the level of wild-type mice [65]. Thus, IL-4 is a predictive indicator of disease progression in TB. Recently, it has been found that TB-infected patients had increased stability of endogenous IL-4 mRNA, but not IL-4 delta2 mRNA (a competitive antagonist of IL-4) [63]. The mechanisms through which IL-4 may impair control of M. tuberculosis involve multiple pathways (see Fig. 4).
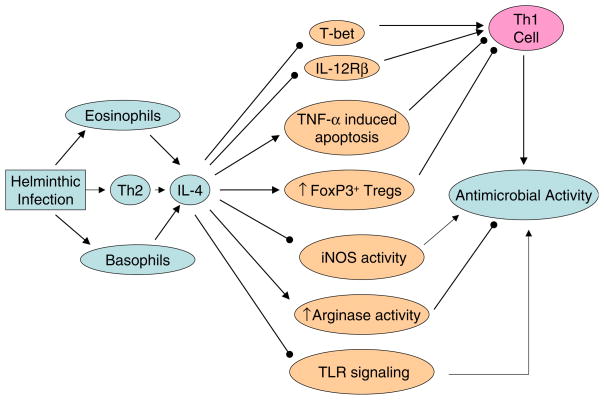
Fig. 4.
IL-4 promotes the pathogenesis and progression of TB. (1) IL-4 downregulates expression of the Th1-specific transcription factor T-bet [147] and also abrogates expression of IL-12Rβ to suppress the development of Th1 cells [148]. (2) IL-4 increases CD30 expression which causes TNFα-mediated apoptosis of infected T cells to weaken the anti-MTB immunity [149]. (3) IL-4 increases arginase activity [48] but suppresses iNOS activity [150]. (4) IL-4 promotes the generation of FoxP3+ regulatory T cells [135]. (5) IL-4 downregulates toll-like receptor-2 (TLR2) signaling [151]
Tregs
In addition to controlling pathogenic T cells and autoimmune inflammatory responses, Treg cells also play important roles during infectious disease. Treg cells can benefit pathogen survival by diminishing protective immunity [66]. Indeed, MTB infection increased the frequency of CD4+ Treg cells in patients with active disease compared to PPD+ healthy subjects. Moreover, IFN-γ production by CD4+ T-cells was markedly diminished in patients with active disease, while standard short-course chemotherapy significantly improved IFN-γ production and decreased Tregs frequency [67]. In another study, increased IL-10 and TGF-β mRNA have also been seen in TB patients who have increased Tregs [68]. As helminth infection induces Tregs in vivo [35], it may downregulate host immunity through Tregs [69].
Migration of DCs from the lung to DLNs
Inhaled MTB is phagocytosed by lung-resident alveolar MΦs, interstitial MΦs, and dendritic cells (DCs) following a pulmonary infection. However, only infected DCs migrate to the draining mediastinal LNs to initiate T cell priming [70] and cause MTB dissemination [71, 72]. In vivo depletion of DCs delayed MTB-specific T cell priming and resulted in exacerbated disease [73].
MTB infection induces high IL-12 release from DCs in a TLR9-dependent manner [74]. IL-12 is not only the prototypical Th1-inducing cytokine, but recently it has been shown that IL-12p40 homodimers have a role in positively influencing the activation and migration of MTB-infected DCs from the pulmonary site to the draining mediastinal LNs [75]. IL-10 can inhibit both the migratory [76] and IL-12 secretion capacity of DCs [77]. IL-10 is a major by-product of the helminth-induced Th2 response. We have found that intestinal helminths that migrate through the lung can suppress M. tuberculosis-induced Th1 effector cells (Liu Z. et al., unpublished observation), probably through inducing inhibitory effects on pulmonary DC function.
Th17 cells
The Th17 cell, a newly identified T effector lineage that expresses IL-17, can augment tissue inflammation in the lung and other organs [78]. Recent studies suggest that local secretion of IL-17 contributes to long-standing anti-microorganism responses throughout persistent bacterial infections [79]. Four weeks after Mycobacterium bovis BCG infection of IL-17 deficient mice, IFN-γ production by lung-infiltrating T cells is markedly reduced and granuloma formation is suppressed [80]. Also, Th17 cells can respond immediately to antigen and further mobilize Th1 cells to suppress pathogen survival in peripheral tissues during recall responses to MTB infection [81]. IL-17 may promote immunity to MTB by: (1) inducing initial neutrophil-driven inflammation [80]; (2) upregulating the expression of certain chemokines, which recruit IFN-γ-producing CD4+ T cells to the infection site [81]; (3) promoting IFN-γ production by CD3+ T cells [80]; or (4) favoring granuloma formation, which restricts bacterial spread [80]. IL-4 has also been shown to downregulate Th17 cell development in vitro [78] and in vivo (Liu Z., unpublished observation). It is thus possible that the increased incidence of TB following helminth infection may be partly due to in vivo inhibition of IL-17 production by helminth-induced IL-4.
Alternatively activated macrophages
Th1 effector cell function includes secretion of IFN-γ that activates MΦs to destroy intracellular pathogens including MTB. These “classically” activated MΦs upregulate inducible NO synthase (iNOS) and produce anti-mycobacterial reactive nitrogen intermediates that control both acute and chronic TB infection [82]. In contrast, helminth-induced AAMΦs upregulate arginase instead of iNOS [83]. Like the counter-regulation of Th1 and Th2 cells, reciprocal inhibition of iNOS and arginase also occurs as they compete for the common substrate-L-arginine [84]. Compared to IFN-γ activated bone marrow MΦs (BMMΦ), IL-4-primed BMMΦ showed delayed and moderately reduced responses to intracellular bacteria, which benefit from the intracellular persistence of MTB [85]. One explanation would be that IL-4 and IL-13 inhibit autophagy-induced elimination of intracellular Mycobacteria in MΦs in an Akt-dependent manner [86].
Arginase, a key enzyme characteristic of AAMΦs, converts L-arginine to proline, an important precursor of collagen. Arginase promotes collagen deposition and fibrosis of granulomas. Protective granulomas during MTB infection result in a localized inflammatory response and containment of MTB [87]. Although mycobacterial granulomas develop under the influence of IFNγ-producing Th1 cells, there is underlying low-level Th2-type activity that induces fibrosis in late-stage granulomas [88]. The fact that granuloma pathology can be shaped by the balance of iNOS and arginase [89] suggests that helminth infections can influence the evolution of tuberculous granulomas. Given that AAMΦs have wound healing effects [90], it is possible that AAMΦs induced by parasites benefit the recovery of MTB-infected tissue structure. AAMΦs induced by the filarial nematodes have an anti-proliferative effect on a range of different cell types, including antigen-specific T cells [91]. One mechanism may involve production of polyamines, a downstream metabolite of the arginase pathway, which have been shown to suppress Th1-type responses [92]. Whether helminth-induced AAMΦs can suppress MTB-specific T cell responses is an area warranting further investigation.
Allergic asthma and helminth infection
Allergic asthma, a chronic reactive lung disease, is characterized by airway inflammation, enhanced sensitivity to external agents, and airflow obstruction. In many cases, asthma is caused by a Th2-type allergic response that induces lymphocyte-mediated airway inflammation [93].
Although allergic disorders have a genetic element [94], recent studies indicate that environmental factors also play an important role. People living in Western Europe have an increased incidence of asthma compared to genetically similar individuals who live in Eastern Europe [95]. Consistently, immigrants from developing countries in Western nations showed increased incidences of asthma even within the same country, whereas children raised in farms in suburban areas have lower incidences of asthma compared to those living in urban settings [96]. These findings suggest that environmental factors regulate the onset of asthma. Consistently, infection with S. mansoni reduces Th2-type immune responses to allergens and clinical manifestations of asthma in humans and in mice [97, 98].
As both allergic asthma and helminth infections trigger Th2-type responses, it is perhaps surprising that the immune response induced by helminths can control asthma. Apparently, helminths induce regulatory mechanisms associated with the Th2-type response that can control allergen-induced Th2-type immune responses. Although not yet well understood, a number of recent studies have indicated several pathways through which helminths may contribute to inhibition of allergic inflammatory responses [99–102].
IgE and IgG4
IgE plays an important role in the development of allergic asthma [103]. Allergen-specific IgE antibodies can bind to high-affinity receptors expressed on mast cells and basophils, which causes the release of inflammatory mediators that induce the allergic immune response and clinical symptoms [104]. Blocking IgE in patients slows asthma exacerbation and ameliorates clinical manifestations [104]. Since helminth infections induce potent polyclonal IgE production [30], it was predicted that the parasites would trigger allergic responses [105]. However, helminth-infected individuals are rarely allergic to the parasites and typically are less responsive to allergens [106]. Consistently, substantial negative correlations were found between total IgE levels and skin allergen tests in helminth-infected individuals [106]. This may be due to helminth-induced production of polyclonal IgE suppressing allergen-specific IgE production through competitive occupancy of IgE receptors on mast cells and basophils [107]. Indeed, treatment of helminth-infected children with anti-helminthic medication dramatically decreased total serum IgE levels but enhanced the sensitivity to skin-test [108]. However, in a murine asthma model, despite suppressed allergic responses, Heligmosomoides polygyrus infection significantly increased both total IgE and allergen-specific IgE, indicating that other mechanisms may also account for the protective effects [109].
Microfilaremic filarial infection usually induces high levels of IgG4 production in human beings [110]. Patients with chronic filarial infection rarely show allergic reactions to parasite antigens [111]. Further studies found that anti-filarial IgG4 antibody increased in these asymptomatic patients. Specific removal of IgG4 antibody from the sera reduced its ability to inhibit histamine, suggesting that IgG4 is responsible for mediating the asthma-blocking activity in asymptomatic patients [111]. Thus, high IgG4 production may also play a role in helminth-mediated protection from asthma.
Tregs
A number of studies suggest that Treg cells play an important role in controlling allergic responses. In humans, the persistence and exacerbation of asthma is correlated with reduced FoxP3 mRNA and frequency of Tregs in peripheral blood mononuclear cells [112], and the function of Treg cells may also be defective in allergic patients [113]. Reduction of allergic symptoms after allergen-specific immununotherapy correlates with IL-10-producing regulatory T cells [114]. In mice, Treg cells effectively suppress allergen-induced airway hyper reactivity and inflammation [115]. Helminth-induced Tregs may thus downregulate the host immune responses to allergens. Indeed, H. polygyrus infection increased CD4+CD25+ T cells in mesenteric LNs, and transfer of mesenteric LN cells from H. polygyrus-infected mice protected ovalbumin sensitized recipients from asthma [102]. Similarly, treatment with schistosome egg antigens increased the number and suppressive function of IL-10-producing Treg cells, decreased Th2 cytokines, and reduced antigen-induced airway inflammation in a murine asthma model [116]. These studies suggest that prevention of asthma by helminth infection may involve Treg cell-dependent pathways.
IL-10
Helminth infection can induce elevated IL-10 production [117]. IL-10 plays a role in downregulating inflammation-mediated allergic diseases. Diminished IL-10 secretion is observed in asthma patients [118]. Glucocorticoid treatment of asthma patients increased IL-10 production by alveolar MΦs [119]. The weal diameters of allergen-specific skin prick tests are negatively associated with IL-10 production [120]. These findings raise the possibility that IL-10 may contribute to helminth-mediated protection from asthma. Consistently, helminth infection reduces allergic airway inflammation and increases IL-10 production [109, 119]. IL-10 may prevent asthma through inhibition of mast cell degranulation [121], suppression of Th2 cytokine production [109, 122], induction of regulatory T cells [123], and/or direct inhibition of inflammation [124]. In contrast, some studies showed that helminth-induced suppression of asthma can occur through IL-10-independent pathways [101, 102].
Innate components
In addition to Treg cells, components of innate immunity induced by worm infection may also impede the development of asthma. Soluble molecules from S. mansoni eggs inhibit DC responsiveness to lipopolysaccharide (LPS) [125], while low-dose LPS promotes the development of Th2 allergic responses induced by inhaled ovalbumin [126]. In addition, a glycolipid from schistosomes modulates human DCs to induce IL-10-producing regulatory T cells [127]. Glycan-derived products from schistosome eggs also expand peritoneal MΦs that suppress T cell proliferation [128]. Similarly, schistosome worms enhance expression of programmed death ligand (PD-L1) on MΦs to induce T cell anergy [129]. Thus, helminth-mediated inhibition of asthma may also occur through modulation of DC or macrophage function.
Conclusion
Helminthic infections are widespread and represent a major global health problem. These pathogens elicit a strong, polarized Th2-type response that modulates regulatory innate and T cell populations. These different components of the response, Th2 cells, T regulatory cells, and innate regulatory populations, may, to some extent, be stimulated independently by helminth infection. The combined actions of these agents during the Th2-type response result in potent regulatory effects on Th1-type inflammation ranging from autoimmune diseases, such as T1D, to infectious diseases including TB. Furthermore, these immune responses evoked by helminths can control the pathologic Th2-type responses associated with asthma in experimental mouse models, suggesting that even responses mediated by Th2 cytokines are downregulated.
The actual helminth-induced immune regulatory mechanisms leading to control of both Th1- and Th2-type responses are not yet well understood. They may include Treg cells, AAMΦs, differentially activated DCs, IgE-producing B cells, IL-10 and/or TGFβ, Th2 cytokines, and differential expression of cell surface costimulatory molecules like PDL and Inducible T-cell COStimulator. As we continue to elucidate these immunoregulatory mechanisms induced by helminths and identify helminth structures that elicit them, we may gain important insights into developing new therapies for the treatment of inflammatory diseases.
References
- 1.Liu AH. Hygiene theory and allergy and asthma prevention. Paediatr Perinat Epidemiol. 2007;21(Suppl 3):2–7. doi: 10.1111/j.1365-3016.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 2.Klement E, Lysy J, Hoshen M, Avitan M, Goldin E, Israeli E. Childhood hygiene is associated with the risk for inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2008;103(7):1775–1782. doi: 10.1111/j.1572-0241.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 3.Kamradt T, Goggel R, Erb KJ. Induction, exacerbation and inhibition of allergic and autoimmune diseases by infection. Trends Immunol. 2005;26(5):260–267. doi: 10.1016/j.it.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunol. 2006;28(10):515–523. doi: 10.1111/j.1365-3024.2006.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wickelgren I. Immunotherapy. Can worms tame the immune system? Science. 2004;305(5681):170–171. doi: 10.1126/science.305.5681.170. [DOI] [PubMed] [Google Scholar]
- 6.Kamal SM, El Sayed Khalifa K. Immune modulation by helminthic infections: worms and viral infections. Parasite Immunol. 2006;28(10):483–496. doi: 10.1111/j.1365-3024.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 7.Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Robinson BA, Bundy DA. Moderate to heavy infections of Trichuris trichiura affect cognitive function in Jamaican school children. Parasitology. 1992;104(Pt 3):539–547. doi: 10.1017/s0031182000063800. [DOI] [PubMed] [Google Scholar]
- 8.Hall A, Hewitt G, Tuffrey V, de Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4(Suppl 1):118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgiev VS. Parasitic infections. Treatment and developmental therapeutics. 1. Necatoriasis. Curr Pharm Des. 1999;5(7):545–554. [PubMed] [Google Scholar]
- 10.Dickson R, Awasthi S, Williamson P, Demellweek C, Garner P. Effects of treatment for intestinal helminth infection on growth and cognitive performance in children: systematic review of randomised trials. Bmj. 2000;320(7251):1697–1701. doi: 10.1136/bmj.320.7251.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sewell DL, Reinke EK, Hogan LH, Sandor M, Fabry Z. Immunoregulation of CNS autoimmunity by helminth and mycobacterial infections. Immunol Lett. 2002;82(1–2):101–110. doi: 10.1016/s0165-2478(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 12.Elias D, Akuffo H, Thors C, Pawlowski A, Britton S. Low dose chronic Schistosoma mansoni infection increases susceptibility to Mycobacterium bovis BCG infection in mice. Clin Exp Immunol. 2005;139(3):398–404. doi: 10.1111/j.1365-2249.2004.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias D, Akuffo H, Pawlowski A, Haile M, Schon T, Britton S. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine. 2005;23(11):1326–1334. doi: 10.1016/j.vaccine.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 14.Wang LJ, Cao Y, Shi HN. Helminth infections and intestinal inflammation. World J Gastroenterol. 2008;14(33):5125–5132. doi: 10.3748/wjg.14.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int J Parasitol. 2007;37(5):457–464. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Harnett W, Harnett MM. Filarial nematode secreted product ES-62 is an anti-inflammatory agent: therapeutic potential of small molecule derivatives and ES-62 peptide mimetics. Clin Exp Pharmacol Physiol. 2006;33(5–6):511–518. doi: 10.1111/j.1440-1681.2006.04400.x. [DOI] [PubMed] [Google Scholar]
- 17.Fleming J, Fabry Z. The hygiene hypothesis and multiple sclerosis. Ann Neurol. 2007;61(2):85–89. doi: 10.1002/ana.21092. [DOI] [PubMed] [Google Scholar]
- 18.Fleming JO, Cook TD. Multiple sclerosis and the hygiene hypothesis. Neurology. 2006;67(11):2085–2086. doi: 10.1212/01.wnl.0000247663.40297.2d. [DOI] [PubMed] [Google Scholar]
- 19.Shizuru JA, Taylor-Edwards C, Banks BA, Gregory AK, Fathman CG. Immunotherapy of the nonobese diabetic mouse: treatment with an antibody to T-helper lymphocytes. Science. 1988;240(4852):659–662. doi: 10.1126/science.2966437. [DOI] [PubMed] [Google Scholar]
- 20.She JX. Susceptibility to type I diabetes: HLA-DQ and DR revisited. Immunol Today. 1996;17(7):323–329. doi: 10.1016/0167-5699(96)10014-1. [DOI] [PubMed] [Google Scholar]
- 21.Oldstone MB. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science. 1988;239(4839):500–502. doi: 10.1126/science.3277269. [DOI] [PubMed] [Google Scholar]
- 22.Soltesz G, Patterson CC, Dahlquist G. Worldwide childhood type 1 diabetes incidence—what can we learn from epidemiology? Pediatr Diabetes. 2007;8(Suppl 6):6–14. doi: 10.1111/j.1399-5448.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 23.Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJ, et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21(4):169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 24.Zaccone P, Fehervari Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33(5):1439–1449. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 25.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75(1):397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raz I, Eldor R, Naparstek Y. Immune modulation for prevention of type 1 diabetes mellitus. Trends Biotechnol. 2005;23(3):128–134. doi: 10.1016/j.tibtech.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Mueller R, Bradley LM, Krahl T, Sarvetnick N. Mechanism underlying counterregulation of autoimmune diabetes by IL-4. Immunity. 1997;7(3):411–418. doi: 10.1016/s1074-7613(00)80362-3. [DOI] [PubMed] [Google Scholar]
- 28.Ko KS, Lee M, Koh JJ, Kim SW. Combined administration of plasmids encoding IL-4 and IL-10 prevents the development of autoimmune diabetes in nonobese diabetic mice. Mol Ther. 2001;4(4):313–316. doi: 10.1006/mthe.2001.0459. [DOI] [PubMed] [Google Scholar]
- 29.Lee M, Koh JJ, Han SO, Ko KS, Ki SW. Prevention of autoimmune insulitis by delivery of interleukin-4 plasmid using a soluble and biodegradable polymeric carrier. Pharm Res. 2002;19(3):246–249. doi: 10.1023/a:1014478515005. [DOI] [PubMed] [Google Scholar]
- 30.Gause WC, Urban JF, Jr, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24(5):269–277. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 31.Pennline KJ, Roque-Gaffney E, Monahan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol. 1994;71(2):169–175. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- 32.Goudy K, Song S, Wasserfall C, Zhang YC, Kapturczak M, Muir A, et al. Adeno-associated virus vector-mediated IL-10 gene delivery prevents type 1 diabetes in NOD mice. Proc Natl Acad Sci USA. 2001;98(24):13913–13918. doi: 10.1073/pnas.251532298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips JM, Parish NM, Drage M, Cooke A. Cutting edge: interactions through the IL-10 receptor regulate autoimmune diabetes. J Immunol. 2001;167(11):6087–6091. doi: 10.4049/jimmunol.167.11.6087. [DOI] [PubMed] [Google Scholar]
- 34.Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39(4):1098–1107. doi: 10.1002/eji.200838871. [DOI] [PubMed] [Google Scholar]
- 35.Finney CA, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection. Eur J Immunol. 2007;37(7):1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109(1):131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ott PA, Anderson MR, Tary-Lehmann M, Lehmann PV. CD4+CD25+ regulatory T cells control the progression from periinsulitis to destructive insulitis in murine autoimmune diabetes. Cell Immunol. 2005;235(1):1–11. doi: 10.1016/j.cellimm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Mellanby RJ, Thomas D, Phillips JM, Cooke A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology. 2007;121(1):15–28. doi: 10.1111/j.1365-2567.2007.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177(10):6603–6612. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- 40.Baxter AG, Kinder SJ, Hammond KJ, Scollay R, Godfrey DI. Association between alphabetaTCR+CD4−CD8− T-cell deficiency and IDDM in NOD/Lt mice. Diabetes. 1997;46(4):572–582. doi: 10.2337/diab.46.4.572. [DOI] [PubMed] [Google Scholar]
- 41.Kent SC, Chen Y, Clemmings SM, Viglietta V, Kenyon NS, Ricordi C, et al. Loss of IL-4 secretion from human type 1a diabetic pancreatic draining lymph node NKT cells. J Immunol. 2005;175(7):4458–4464. doi: 10.4049/jimmunol.175.7.4458. [DOI] [PubMed] [Google Scholar]
- 42.Novak J, Beaudoin L, Griseri T, Lehuen A. Inhibition of T cell differentiation into effectors by NKT cells requires cell contacts. J Immunol. 2005;174(4):1954–1961. doi: 10.4049/jimmunol.174.4.1954. [DOI] [PubMed] [Google Scholar]
- 43.Ly D, Mi QS, Hussain S, Delovitch TL. Protection from type 1 diabetes by invariant NK T cells requires the activity of CD4+CD25+ regulatory T cells. J Immunol. 2006;177(6):3695–3704. doi: 10.4049/jimmunol.177.6.3695. [DOI] [PubMed] [Google Scholar]
- 44.Novak J, Beaudoin L, Park S, Griseri T, Teyton L, Bendelac A, et al. Prevention of type 1 diabetes by invariant NKT cells is independent of peripheral CD1d expression. J Immunol. 2007;178(3):1332–1340. doi: 10.4049/jimmunol.178.3.1332. [DOI] [PubMed] [Google Scholar]
- 45.Mi QS, Ly D, Zucker P, McGarry M, Delovitch TL. Interleukin-4 but not interleukin-10 protects against spontaneous and recurrent type 1 diabetes by activated CD1d-restricted invariant natural killer T-cells. Diabetes. 2004;53(5):1303–1310. doi: 10.2337/diabetes.53.5.1303. [DOI] [PubMed] [Google Scholar]
- 46.Trottein F, Mallevaey T, Faveeuw C, Capron M, Leite-de-Moraes M. Role of the natural killer T lymphocytes in Th2 responses during allergic asthma and helminth parasitic diseases. Chem Immunol Allergy. 2006;90:113–127. doi: 10.1159/000088884. [DOI] [PubMed] [Google Scholar]
- 47.Mallevaey T, Zanetta JP, Faveeuw C, Fontaine J, Maes E, Platt F, et al. Activation of invariant NKT cells by the helminth parasite Schistosoma mansoni. J Immunol. 2006;176(4):2476–2485. doi: 10.4049/jimmunol.176.4.2476. [DOI] [PubMed] [Google Scholar]
- 48.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19(4):448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee KU, Amano K, Yoon JW. Evidence for initial involvement of macrophage in development of insulitis in NOD mice. Diabetes. 1988;37(7):989–991. doi: 10.2337/diab.37.7.989. [DOI] [PubMed] [Google Scholar]
- 50.Jun HS, Santamaria P, Lim HW, Zhang ML, Yoon JW. Absolute requirement of macrophages for the development and activation of beta-cell cytotoxic CD8+ T-cells in T-cell receptor transgenic NOD mice. Diabetes. 1999;48(1):34–42. doi: 10.2337/diabetes.48.1.34. [DOI] [PubMed] [Google Scholar]
- 51.Brombacher F, Arendse B, Peterson R, Holscher A, Holscher C. Analyzing classical and alternative macrophage activation in macrophage/neutrophil-specific IL-4 receptor-alpha-deficient mice. Methods Mol Biol. 2009;531:225–252. doi: 10.1007/978-1-59745-396-7_15. [DOI] [PubMed] [Google Scholar]
- 52.Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J Immunol. 2006;176(11):6918–6927. doi: 10.4049/jimmunol.176.11.6918. [DOI] [PubMed] [Google Scholar]
- 53.Navin TR, McNabb SJ, Crawford JT. The continued threat of tuberculosis. Emerg Infect Dis. 2002;8(11):1187. doi: 10.3201/eid0811.020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stead WW. Pathogenesis of a first episode of chronic pulmonary tuberculosis in man: recrudescence of residuals of the primary infection or exogenous reinfection? Am Rev Respir Dis. 1967;95(5):729–745. doi: 10.1164/arrd.1967.95.5.729. [DOI] [PubMed] [Google Scholar]
- 55.Baumann S, Nasser Eddine A, Kaufmann SH. Progress in tuberculosis vaccine development. Curr Opin Immunol. 2006;18 (4):438–448. doi: 10.1016/j.coi.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 56.Haar CH, Cobelens FG, Kalisvaart NA, van der Have JJ, van Gerven PJ, van Soolingen D. Tuberculosis drug resistance and HIV infection, The Netherlands. Emerg Infect Dis. 2007;13(5):776–778. doi: 10.3201/eid1305.060334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elias D, Akuffo H, Britton S. Helminthes could influence the outcome of vaccines against TB in the tropics. Parasite Immunol. 2006;28(10):507–513. doi: 10.1111/j.1365-3024.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 58.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol. 2001;123(2):219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borkow G, Weisman Z, Leng Q, Stein M, Kalinkovich A, Wolday D, et al. Helminths, human immunodeficiency virus and tuberculosis. Scand J Infect Dis. 2001;33(8):568–571. doi: 10.1080/00365540110026656. [DOI] [PubMed] [Google Scholar]
- 60.Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW, et al. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162(11):6843–6848. [PubMed] [Google Scholar]
- 61.Liu Z, Liu Q, Pesce J, Anthony RM, Lamb E, Whitmire J, et al. Requirements for the development of IL-4-producing T cells during intestinal nematode infections: what it takes to make a Th2 cell in vivo. Immunol Rev. 2004;201:57–74. doi: 10.1111/j.0105-2896.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 62.Rook GA. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med. 2007;7(3):327–337. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 63.Dheda K, Chang JS, Huggett JF, Kim LU, Johnson MA, Zumla A, et al. The stability of mRNA encoding IL-4 is increased in pulmonary tuberculosis, while stability of mRNA encoding the antagonistic splice variant, IL-4delta2, is not. Tuberculosis (Edinb) 2007;87(3):237–241. doi: 10.1016/j.tube.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Ordway DJ, Costa L, Martins M, Silveira H, Amaral L, Arroz MJ, et al. Increased Interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J Infect Dis. 2004;190(4):756–766. doi: 10.1086/422532. [DOI] [PubMed] [Google Scholar]
- 65.Buccheri S, Reljic R, Caccamo N, Ivanyi J, Singh M, Salerno A, et al. IL-4 depletion enhances host resistance and passive IgA protection against tuberculosis infection in BALB/c mice. Eur J Immunol. 2007;37(3):729–737. doi: 10.1002/eji.200636764. [DOI] [PubMed] [Google Scholar]
- 66.Mason CM, Porretta E, Zhang P, Nelson S. CD4+ CD25+ transforming growth factor-beta-producing T cells are present in the lung in murine tuberculosis and may regulate the host inflammatory response. Clin Exp Immunol. 2007;148(3):537–545. doi: 10.1111/j.1365-2249.2007.03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ribeiro-Rodrigues R, Resende Co T, Rojas R, Toossi Z, Dietze R, Boom WH, et al. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144(1):25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173(7):803–810. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 69.Taylor MD, van der Werf N, Harris A, Graham AL, Bain O, Allen JE, et al. Early recruitment of natural CD4+ Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. Eur J Immunol. 2009;39(1):192–206. doi: 10.1002/eji.200838727. [DOI] [PubMed] [Google Scholar]
- 70.Bhatt K, Hickman SP, Salgame P. Cutting edge: a new approach to modeling early lung immunity in murine tuberculosis. J Immunol. 2004;172(5):2748–2751. doi: 10.4049/jimmunol.172.5.2748. [DOI] [PubMed] [Google Scholar]
- 71.Humphreys IR, Stewart GR, Turner DJ, Patel J, Karamanou D, Snelgrove RJ, et al. A role for dendritic cells in the dissemination of mycobacterial infection. Microbes Infect. 2006;8(5):1339–1346. doi: 10.1016/j.micinf.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 72.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179(4):2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 73.Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol. 2005;175(5):3268–3272. doi: 10.4049/jimmunol.175.5.3268. [DOI] [PubMed] [Google Scholar]
- 74.Pompei L, Jang S, Zamlynny B, Ravikumar S, McBride A, Hickman SP, et al. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J Immunol. 2007;178(8):5192–5199. doi: 10.4049/jimmunol.178.8.5192. [DOI] [PubMed] [Google Scholar]
- 75.Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203(7):1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Demangel C, Bertolino P, Britton WJ. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur J Immunol. 2002;32(4):994–1002. doi: 10.1002/1521-4141(200204)32:4<994::AID-IMMU994>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 77.Hickman SP, Chan J, Salgame P. Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naive T cell polarization. J Immunol. 2002;168(9):4636–4642. doi: 10.4049/jimmunol.168.9.4636. [DOI] [PubMed] [Google Scholar]
- 78.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27(1):17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178(6):3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 81.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 82.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 84.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160(11):5347–5354. [PubMed] [Google Scholar]
- 85.Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K, Mollenkopf H, et al. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36(3):631–647. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- 86.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, et al. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27(3):505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 87.Aly S, Laskay T, Mages J, Malzan A, Lang R, Ehlers S. Interferon-gamma-dependent mechanisms of mycobacteria-induced pulmonary immunopathology: the role of angiostasis and CXCR3-targeted chemokines for granuloma necrosis. J Pathol. 2007;212(3):295–305. doi: 10.1002/path.2185. [DOI] [PubMed] [Google Scholar]
- 88.Wangoo A, Sparer T, Brown IN, Snewin VA, Janssen R, Thole J, et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous lung disease. J Immunol. 2001;166(5):3432–3439. doi: 10.4049/jimmunol.166.5.3432. [DOI] [PubMed] [Google Scholar]
- 89.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167(11):6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 90.Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, et al. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand J Immunol. 2001;53(4):386–392. doi: 10.1046/j.1365-3083.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- 91.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites—masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 92.Hasko G, Kuhel DG, Marton A, Nemeth ZH, Deitch EA, Szabo C. Spermine differentially regulates the production of interleukin-12 p40 and interleukin-10 and suppresses the release of the T helper 1 cytokine interferon-gamma. Shock. 2000;14(2):144–149. doi: 10.1097/00024382-200014020-00012. [DOI] [PubMed] [Google Scholar]
- 93.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104(8):1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoffjan S, Ober C. Present status on the genetic studies of asthma. Curr Opin Immunol. 2002;14(6):709–717. doi: 10.1016/s0952-7915(02)00393-x. [DOI] [PubMed] [Google Scholar]
- 95.Matricardi PM. Prevalence of atopy and asthma in eastern versus western Europe: why the difference? Ann Allergy Asthma Immunol. 2001;87(6 Suppl 3):24–27. doi: 10.1016/s1081-1206(10)62336-8. [DOI] [PubMed] [Google Scholar]
- 96.Yemaneberhan H, Bekele Z, Venn A, Lewis S, Parry E, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet. 1997;350(9071):85–90. doi: 10.1016/S0140-6736(97)01151-3. [DOI] [PubMed] [Google Scholar]
- 97.Mangan NE, van Rooijen N, McKenzie AN, Fallon PG. Helminth-modified pulmonary immune response protects mice from allergen-induced airway hyperresponsiveness. J Immunol. 2006;176(1):138–147. doi: 10.4049/jimmunol.176.1.138. [DOI] [PubMed] [Google Scholar]
- 98.Araujo MI, de Carvalho EM. Human schistosomiasis decreases immune responses to allergens and clinical manifestations of asthma. Chem Immunol Allergy. 2006;90:29–44. doi: 10.1159/000088879. [DOI] [PubMed] [Google Scholar]
- 99.Pacifico LG, Marinho FA, Fonseca CT, Barsante MM, Pinho V, Sales-Junior PA, et al. Schistosoma mansoni antigens modulate experimental allergic asthma in a murine model: a major role for CD4+ CD25+ Foxp3+ T cells independent of interleukin-10. Infect Immun. 2009;77(1):98–107. doi: 10.1128/IAI.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dittrich AM, Erbacher A, Specht S, Diesner F, Krokowski M, Avagyan A, et al. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J Immunol. 2008;180(3):1792–1799. doi: 10.4049/jimmunol.180.3.1792. [DOI] [PubMed] [Google Scholar]
- 101.Trujillo-Vargas CM, Werner-Klein M, Wohlleben G, Polte T, Hansen G, Ehlers S, et al. Helminth-derived products inhibit the development of allergic responses in mice. Am J Respir Crit Care Med. 2007;175(4):336–344. doi: 10.1164/rccm.200601-054OC. [DOI] [PubMed] [Google Scholar]
- 102.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202(9):1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320(5):271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 104.DuBuske LM. IgE, allergic diseases, and omalizumab. Curr Pharm Des. 2006;12(30):3929–3944. doi: 10.2174/138161206778559641. [DOI] [PubMed] [Google Scholar]
- 105.Knopf PM. Immunomodulation and allergy. Allergy Asthma Proc. 2000;21(4):215–220. doi: 10.2500/108854100778248836. [DOI] [PubMed] [Google Scholar]
- 106.Araujo MI, Lopes AA, Medeiros M, Cruz AA, Sousa-Atta L, Sole D, et al. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int Arch Allergy Immunol. 2000;123(2):145–148. doi: 10.1159/000024433. [DOI] [PubMed] [Google Scholar]
- 107.Lynch NR, Puccio FS, Di Prisco MC, Escudero JE, Nozzolino M, Hazel LA, et al. Association between allergic disease and reactivity to recombinant Der p 2 allergen of house dust mites in a tropical situation. J Allergy Clin Immunol. 1998;101(4 Pt 1):562–564. doi: 10.1016/s0091-6749(98)70367-7. [DOI] [PubMed] [Google Scholar]
- 108.Carvalho EM, Bastos LS, Araujo MI. Worms and allergy. Parasite Immunol. 2006;28(10):525–534. doi: 10.1111/j.1365-3024.2006.00894.x. [DOI] [PubMed] [Google Scholar]
- 109.Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006;177(3):1628–1635. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- 110.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3(9):733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 111.Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J Immunol. 1992;148 (9):2731–2737. [PubMed] [Google Scholar]
- 112.Xue K, Zhou Y, Xiong S, Xiong W, Tang T. Analysis of CD4+ CD25+ regulatory T cells and Foxp3 mRNA in the peripheral blood of patients with asthma. J Huazhong Univ Sci Technolog Med Sci. 2007;27(1):31–33. doi: 10.1007/s11596-007-0109-y. [DOI] [PubMed] [Google Scholar]
- 113.Xystrakis E, Boswell SE, Hawrylowicz CM. T regulatory cells and the control of allergic disease. Expert Opin Biol Ther. 2006;6(2):121–133. doi: 10.1517/14712598.6.2.121. [DOI] [PubMed] [Google Scholar]
- 114.Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4 +CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111(6):1255–1261. doi: 10.1067/mai.2003.1570. [DOI] [PubMed] [Google Scholar]
- 115.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202(11):1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang J, Zhao J, Yang Y, Zhang L, Yang X, Zhu X, et al. Schistosoma japonicum egg antigens stimulate CD4 CD25 T cells and modulate airway inflammation in a murine model of asthma. Immunology. 2007;120(1):8–18. doi: 10.1111/j.1365-2567.2006.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296(5567):490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 118.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97(6):1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 119.Magnan A, van Pee D, Bongrand P, Vervloet D. Alveolar macrophage interleukin (IL)-10 and IL-12 production in atopic asthma. Allergy. 1998;53(11):1092–1095. doi: 10.1111/j.1398-9995.1998.tb03821.x. [DOI] [PubMed] [Google Scholar]
- 120.Macaubas C, Sly PD, Burton P, Tiller K, Yabuhara A, Holt BJ, et al. Regulation of T-helper cell responses to inhalant allergen during early childhood. Clin Exp Allergy. 1999;29(9):1223–1231. doi: 10.1046/j.1365-2222.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 121.Royer B, Varadaradjalou S, Saas P, Guillosson JJ, Kantelip JP, Arock M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin Exp Allergy. 2001;31(5):694–704. doi: 10.1046/j.1365-2222.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 122.Araujo MI, Hoppe BS, Medeiros M, Jr, Carvalho EM. Schistosoma mansoni infection modulates the immune response against allergic and auto-immune diseases. Mem Inst Oswaldo Cruz. 2004;99(5 Suppl 1):27–32. doi: 10.1590/s0074-02762004000900005. [DOI] [PubMed] [Google Scholar]
- 123.Bellinghausen I, Konig B, Bottcher I, Knop J, Saloga J. Inhibition of human allergic T-helper type 2 immune responses by induced regulatory T cells requires the combination of interleukin-10-treated dendritic cells and transforming growth factor-beta for their induction. Clin Exp Allergy. 2006;36(12):1546–1555. doi: 10.1111/j.1365-2222.2006.02601.x. [DOI] [PubMed] [Google Scholar]
- 124.Tournoy KG, Kips JC, Pauwels RA. Endogenous interleukin-10 suppresses allergen-induced airway inflammation and nonspecific airway responsiveness. Clin Exp Allergy. 2000;30(6):775–783. doi: 10.1046/j.1365-2222.2000.00838.x. [DOI] [PubMed] [Google Scholar]
- 125.Kane CM, Cervi L, Sun J, McKee AS, Masek KS, Shapira S, et al. Helminth antigens modulate TLR-initiated dendritic cell activation. J Immunol. 2004;173(12):7454–7461. doi: 10.4049/jimmunol.173.12.7454. [DOI] [PubMed] [Google Scholar]
- 126.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196(12):1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277(50):48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 128.Atochina O, Daly-Engel T, Piskorska D, McGuire E, Harn DA. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1(+) macrophages that suppress naive CD4(+) T cell proliferation via an IFN-gamma and nitric oxide-dependent mechanism. J Immunol. 2001;167(8):4293–4302. doi: 10.4049/jimmunol.167.8.4293. [DOI] [PubMed] [Google Scholar]
- 129.Smith P, Walsh CM, Mangan NE, Fallon RE, Sayers JR, McKenzie AN, et al. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J Immunol. 2004;173(2):1240–1248. doi: 10.4049/jimmunol.173.2.1240. [DOI] [PubMed] [Google Scholar]
- 130.Zipris D, Lazarus AH, Crow AR, Hadzija M, Delovitch TL. Defective thymic T cell activation by concanavalin A and anti-CD3 in autoimmune nonobese diabetic mice. Evidence for thymic T cell anergy that correlates with the onset of insulitis. J Immunol. 1991;146(11):3763–3771. [PubMed] [Google Scholar]
- 131.Rapoport MJ, Jaramillo A, Zipris D, Lazarus AH, Serreze DV, Leiter EH, et al. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993;178(1):87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cameron MJ, Arreaza GA, Zucker P, Chensue SW, Strieter RM, Chakrabarti S, et al. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J Immunol. 1997;159(10):4686–4692. [PubMed] [Google Scholar]
- 133.Cameron MJ, Arreaza GA, Grattan M, Meagher C, Sharif S, Burdick MD, et al. Differential expression of CC chemokines and the CCR5 receptor in the pancreas is associated with progression to type I diabetes. J Immunol. 2000;165(2):1102–1110. doi: 10.4049/jimmunol.165.2.1102. [DOI] [PubMed] [Google Scholar]
- 134.King C, Mueller Hoenger R, Malo Cleary M, Murali-Krishna K, Ahmed R, King E, et al. Interleukin-4 acts at the locus of the antigen-presenting dendritic cell to counter-regulate cytotoxic CD8+ T-cell responses. Nat Med. 2001;7(2):206–214. doi: 10.1038/84659. [DOI] [PubMed] [Google Scholar]
- 135.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25-CD4+ precursors. J Immunol. 2005;175(9):6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 136.Maerten P, Shen C, Bullens DM, Van Assche G, Van Gool S, Geboes K, et al. Effects of interleukin 4 on CD25+CD4+ regulatory T cell function. J Autoimmun. 2005;25(2):112–120. doi: 10.1016/j.jaut.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 137.Morita Y, Yamamura M, Kawashima M, Aita T, Harada S, Okamoto H, et al. Differential in vitro effects of IL-4, IL-10, and IL-13 on proinflammatory cytokine production and fibroblast proliferation in rheumatoid synovium. Rheumatol Int. 2001;20(2):49–54. doi: 10.1007/s002960000074. [DOI] [PubMed] [Google Scholar]
- 138.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goudy KS, Burkhardt BR, Wasserfall C, Song S, Campbell-Thompson ML, Brusko T, et al. Systemic overexpression of IL-10 induces CD4+CD25+ cell populations in vivo and ameliorates type 1 diabetes in nonobese diabetic mice in a dose-dependent fashion. J Immunol. 2003;171(5):2270–2278. doi: 10.4049/jimmunol.171.5.2270. [DOI] [PubMed] [Google Scholar]
- 140.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172(8):4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 141.Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173(5):2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- 142.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202(10):1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–237. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 144.Bluestone JA. Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol. 2005;5(4):343–349. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
- 145.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4 +CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199(11):1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gregg RK, Jain R, Schoenleber SJ, Divekar R, Bell JJ, Lee HH, et al. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173(12):7308–7316. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- 147.Yin Z, Chen C, Szabo SJ, Glimcher LH, Ray A, Craft J. T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-gamma by gammadelta T cells. J Immunol. 2002;168(4):1566–1571. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- 148.Himmelrich H, Parra-Lopez C, Tacchini-Cottier F, Louis JA, Launois P. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor beta 2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J Immunol. 1998;161(11):6156–6163. [PubMed] [Google Scholar]
- 149.Seah GT, Rook GA. Il-4 influences apoptosis of mycobacterium-reactive lymphocytes in the presence of TNF-alpha. J Immunol. 2001;167(3):1230–1237. doi: 10.4049/jimmunol.167.3.1230. [DOI] [PubMed] [Google Scholar]
- 150.Coccia EM, Stellacci E, Marziali G, Weiss G, Battistini A. IFN-gamma and IL-4 differently regulate inducible NO synthase gene expression through IRF-1 modulation. Int Immunol. 2000;12(7):977–985. doi: 10.1093/intimm/12.7.977. [DOI] [PubMed] [Google Scholar]
- 151.Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, et al. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med. 2003;9(5):525–532. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]