Abstract
Bacterial vaginosis (BV) is a commonly occurring vaginal infection that is associated with a variety of serious risks related to the reproductive health of women. Conventional antibiotic treatment for this condition is frequently ineffective because the antibiotics tend to inhibit healthy vaginal microflora along with the pathogens. Lactocin 160, a bacteriocin produced by healthy vaginal lactobacilli, is a promising alternative to antibiotics; this compound specifically inhibits the BV-associated vaginal pathogens such as Gardnerella vaginalis and Prevotella bivia without affecting the healthy microflora. This study investigates the molecular mechanism of action for lactocin 160 and reveals that this compound targets the cytoplasmic membrane of G. vaginalis, causing the efflux of ATP molecules and dissipation of the proton motive force.
Keywords: Probiotics, Lactocin 160, Bacterial vaginosis, Bacteriocin, Mode of action
Introduction
Bacterial vaginosis (BV) is characterized by replacement of healthy vaginal microflora, which predominantly consists of Lactobacillus spp., by a variety of potentially pathogenic species such as Gardnerella vaginalis, Prevotella, Bacteroides, Peptostreptococcus, and Mobiluncus spp. [12, 35]. Although BV is not a life-threatening condition, it has been linked to numerous complications related to the reproductive health of women. BV clearly elevates the risk of an infection following gynecological surgery, such as an abortion [19]. In pregnant women, BV may lead to intra-amniotic infections that can cause serious brain damage in the developing fetus [13, 29]. Women affected by BV during their pregnancy are also at risk of giving birth prematurely, resulting in a high rate of infant death [20, 31]. Finally, BV is recognized as a major risk factor for transmission and acquisition of certain STIs, including genital herpes and HIV infection [9, 10, 32]. In particular, the BV-associated pathogens G. vaginalis and P. bivia were shown to directly induce replication of the HIV virus in several cell lines [14, 15]. Furthermore, the study conducted by Cherpes et al. [10] suggests that genital tract shedding of HSV-2 is amplified by BV.
Conventional treatment of BV is by administration of the antibiotics clindamycin and metranidazole. The problem with these antibiotics is that they tend to inhibit native vaginal microflora along with the pathogens, thus contributing to a high (20%) rate of BV reoccurrence within one month of the therapy [36]. Many researchers now perceive and treat BV as an microecological imbalance rather than an infection [16]. Accordingly, the effective treatment for this condition should selectively target the pathogenic microorganisms, while allowing the healthy vaginal microflora to recover. The bacteriocins produced by vaginal lactobacilli have recently attracted the attention of the scientific community as a possible remedy against BV [2, 4, 33]. Bacteriocins are defined as ribosomally synthesized antimicrobial peptides which are generally active against microorganisms closely related to the producer strain [30]. Lactocin 160 is a 3.8-kDa antimicrobial peptide produced by a clinical strain of Lactobacillus rhamnosus isolated from a healthy human vagina. Preliminary characterization of this peptide suggests that it is a bacteriocin, and its sequencing is currently underway ([3, 21]; Aroutcheva et al. unpublished data). Lactocin 160 was shown to selectively inhibit clinical strains of G. vaginalis and P. bivia but not the healthy vaginal isolates [3], which makes it a promising alternative to antibiotics for prophylaxis and treatment of BV [3, 21]. Furthermore, both in vitro and in vivo models show that the topical application of lactocin 160 does not induce irritation of vaginal epithelia, indicating that it is safe for intravaginal applications [11].
The molecular mechanism of action of lactocin 160 has been investigated against Micrococcus luteus 10420, a model microorganism commonly used to study bacteriocins [21]. It was determined that lactocin 160 causes an efflux of ATP and dissipation of the transmembrane electric potential in M. luteus cells. In this study, we investigate the mechanism of action of lactocin 160 against G. vaginalis, one of the key pathogens involved in BV. Nisin was used as a model bacteriocin because the molecular mechanisms of action of this antimicrobial have been studied in great detail against a variety of bacterial species. Bacteriocins have been previously evaluated as a treatment for BV [4, 33]. However, to the best of our knowledge, this is the first report on the molecular mechanism of action of a bacteriocin against the BV-associated organism G. vaginalis. The apparent lack of research exploring the mode of action of antimicrobial peptides against this vaginal pathogen may be explained by the fact that G. vaginalis is an extremely fastidious organism, thus requiring every assay to be specifically tailored to its ideal growth or survival conditions.
Materials and Methods
Bacterial Strains and Growth Conditions
Frozen stock of G. vaginalis ATCC 14018 and L. rhamnosus 160 cultures were kept at −80°C in their appropriate growth medium supplemented with 15% glycerol. Brain heart infusion (BHI) broth (Difco, Sparks, MD) supplemented with 3% horse serum (JRH Biosciences, KS) was used for the proliferation of Gardnerella vaginalis, while MRS broth (Difco) was used to grow L. rhamnosus 160. The cells were grown under anaerobic conditions at 37°C without agitation. Both microorganisms were subcultured multiple times prior to being used in the experimental procedures.
Preparation and Use of the Antimicrobial Solutions
The partially purified preparation of lactocin 160 was obtained using the method previously described by Aroutcheva et al. [3], Dover et al. [11], and Li et al. [21]. The protocol was adapted for large-scale fermentation at the Cell Production and Recovery Facility (Waksman Institute, Rutgers University, NJ) and used to produce 50 l of L. rhamnosus 160 culture. All the purification procedures were followed as described by Aroutcheva et al. [3], leading to the production of 11.38 g of lyophilized, partially purified preparation which was subsequently used in the experimental procedures. The 10 AU ml−1 stock solution of lactocin 160 was prepared by dissolving 400 mg of the powder in 1 ml of distilled water. The solution was then filter-sterilized through a 0.2-μm syringe filter (NALGENE, Rochester, NY) and used within 4 h.
The 100 AU ml−1 stock solution of nisin was prepared by dissolving 10 mg of 2.5% commercial nisin preparation (Sigma-Aldrich) in 1 ml of nisin diluent (0.02 mol l−1 hydrochloric acid solution, pH 1.7). The solution was then filter-sterilized with a 0.2-μm filter (NALGENE) and used within 4 h.
The activity of the lactocin 160 preparation, as expressed in arbitrary units (AU), was determined using the 96-well plate method described by Naghmouchi et al. [28]. Briefly, the serial dilutions of lactocin 160 were prepared from the stock solution using sterile distilled water. Each well of the 96-well plate contained 100 μl of the lactocin 160 solution (various concentrations), 10 μl of the overnight G. vaginalis culture, and 90 μl of the bacterium’s growth medium. The plate was incubated anaerobically at 37°C for 72 h, and the lowest dilution of the lactocin 160 preparation that gave the full inhibition of the microbial growth was assigned 1 AU. The activity of the nisin preparation against G. vaginalis was assessed in a similar way.
Data presented in the Results and Discussion section illustrates the mode of action of lactocin 160 and nisin at 1 AU ml−1 so that a comparison between these two antimicrobials could be drawn. Higher concentrations of the antimicrobials were also evaluated with equivalent results (data not shown). The 4.44 mmol l−1 aqueous solution of lactic acid was used as a negative control for lactocin 160, as this is the lactic acid concentration previously reported for this partially purified antimicrobial preparation [11]. Lactic acid has some antimicrobial properties which had to be accounted for using the control [2]. Similarly, nisin diluent (20 mmol l−1 aqueous solution of hydrochloric acid) was used as a negative control for nisin.
The Membrane-Disruption (Ethidium Bromide) Assay
The cytoplasmic membrane integrity of the G. vaginalis cells was assessed using the method described by Benito et al. [7]. Briefly, the culture of G. vaginalis was grown anaerobically at 37°C to an OD600 of 0.6 and then aliquoted into microcentrifuge tubes. Ethidium bromide (Sigma-Aldrich, St. Louis, MO) was added to each tube at a final concentration of 100 μmol l−1. Immediately after the addition of ethidium bromide, cells were treated for 5 min with either 1 AU ml−1 lactocin 160 or the corresponding controls. Following the treatment, cells were washed twice with the equivalent volume of PBS so that any unbound ethidium bromide was eluted. Bound ethidium bromide was quantified via its fluorescence using a PerkinElmer LS-50B spectrofluorometer (PerkinElmer Life and Analytical Sciences, Inc., Boston, MA). The fluorescence measurements were taken at 22°C using quartz cuvettes (10 mm light path) with excitation and emission wavelengths of 493 and 610 nm, respectively, and with a slit width of 10 nm. Heat treatment (100°C for 10 min) was used as a positive control for membrane damage. Fluorescence of the experimental samples was normalized to the positive control with the assumption that those cells lysed by the heat experienced 100% membrane damage.
The ATP Assay
The effect of lactocin 160 on the cellular ATP content of G. vaginalis was elucidated using the method previously described by Li et al. [21]. Briefly, G. vaginalis was grown anaerobically at 37°C to an OD600 of 0.6. The cells were washed once with an equivalent volume of 50 mmol l−1 MES buffer (pH 6.5) and then re-suspended in 50 mmol l−1 MES at half the volume of the original culture. Subsequently, the cell suspension was energized with 0.2% glucose for 20 min, aliquoted into 1.5 ml microcentrifuge tubes and then treated for 5 min with either 1 AU ml−1 of lactocin 160 or the corresponding controls. To assess the total ATP levels of the treated cell suspension, 20 μl was mixed with 80 μl of DMSO (FisherBiotech, Fair Lawn, NJ) and then diluted with 4.9 ml of cold deionized water (4°C). DMSO lyses cells, hence inducing the release of intracellular ATP into the supernatant. For assessment of the extracellular ATP levels, 100 μl of the treated cell suspension was diluted with 4.9 ml of 50 mmol l−1 MES buffer (pH 6.5). ATP contents of the prepared solutions were determined using the commercially available ATP Bioluminescent Assay Kit (Sigma-Aldrich) following the manufacturer’s instructions. The total ATP levels of cell suspensions treated with the negative controls were very consistent; thus, their average was used to normalize all results and express them as percentage values.
ΔpH Dissipation Assay
The effect of lactocin 160 on the transmembrane pH gradient of G. vaginalis cells (ΔpH) was determined using the method described by Molenaar et al. [24] with some modifications. To prepare the cells, an initial 20 ml culture was incubated anaerobically at 37°C until an OD600 of 0.6 was reached. The culture was centrifuged at 5000g, washed twice with 20 ml of 50 mmol l−1 potassium phosphate buffer (PPB, pH 6.0) and subsequently re-suspended in 200 μl of fresh PPB. The cell suspension was mixed with 20 μl of the pH-sensitive probe BCECF-AM (MP Biomedicals, Inc., Solon, OH), and incubated for 5 min at room temperature to allow the diffusion of the probe into the cytoplasm. The cells were then washed twice with 1 ml of 50 mmol l−1 PBS (pH 6.0) and resuspended in 200 μl of the equivalent buffer. Changes in the intracellular pH of the cells were monitored using a LS-50B spectrofluorometer (PerkinElmer). The fluorescent measurements were taken at 22°C in quartz cuvettes (10 mm light path) with excitation and emission wavelengths of 502 and 525 nm, respectively, and with slit widths of 5 nm for excitation and 15 nm for emission. Each cuvette containing 2 ml of 50 mmol l−1 PPB (pH 7.0) was spiked with 10 μl of the cells loaded with BCECF-AM. Sharp fluctuations in fluorescence intensity are generated when the sample compartment of the spectrofluorimeter is opened; therefore, it is always necessary to allow ca. 20 s for the signal to equilibrate. As the fluorescence signal equilibrated, the cells were energized with 2.2 mmol l−1 glucose. The resulting increase in the fluorescence of the probe indicates an elevation in the intracellular pH of the cells. Immediately after the signal equilibrated, cells were spiked with 5 μmol l−1 valinomycin (MP Biomedicals, Solon, OH) to convert the ΔΨ component of the proton motive force (PMF) into transmembrane pH gradient. Once again, the signal was allowed to equilibrate before cells were treated with either 1 AU ml−1 lactocin 160 or the corresponding controls. Finally, 2 μmol l−1 nigericin (MP Biomedicals) was used to completely dissipate any residual ΔpH.
ΔΨ Dissipation Assay
The effect of lactocin 160 on the transmembrane electrical potential of the cells (ΔΨ) was determined using a modified version of the method described by Sims et al. [34]. Briefly, 20 ml of G. vaginalis culture was incubated anaerobically at 37°C until an OD600 of 0.6 was reached. The cells were then centrifuged at 5000g, washed once with 20 ml of fresh growth medium and resuspended in 200 μl of fresh medium. The fluorescent probe 3,3′-dipropylthiadicarbocyanine iodide (DiSC3 (5)) was used to monitor changes in the ΔΨ of the cells. The fluorescence intensity of the probe was measured continuously in quartz cuvettes (10 mm light path) at 22°C using a LS-50B spectrofluorometer (PerkinElmer), with excitation and emission wavelengths of 643 and 666 nm, respectively, and a slit width of 10 nm. Each cuvette contained 2 ml of BHI broth supplemented with 3% horse serum; DiSC3 (5) probe was added to a final concentration of 5 μmol l−1. Next, 20 μl of the cell suspension was added to the system, resulting in a noticeable decrease in the fluorescence of the probe. As the signal stabilized, the cells were spiked with 5 μmol l−1 nigericin to convert ΔpH into ΔΨ; equilibration of the signal was followed by addition of either 1 AU ml−1 lactocin 160 or the negative controls. Finally, 2 μmol l−1 valinomycin was used to collapse any remaining ΔΨ.
Statistics
The ethidium bromide and the ATP assay were conducted at least twice, in duplicate. The results were analyzed using the Student’s t-test (P ≤ 0.01). Only one replicate was used for the ΔpH and ΔΨ dissipation assays, due to the time-sensitive nature of these assays; however, each of these experiments was repeated three times producing equivalent results.
Results and Discussion
The Cytoplasmic Membrane Damage Caused by Lactocin 160 in G. vaginalis Cells Could not be Detected Using the Ethidium Bromide Assay
The cell membrane is the primary site of action for most reported bacteriocins [17, 26]. In particular, bacteriocins such as enterocin P and lactococcin G form transient channels in the cytoplasmic membranes of their target cells [18, 25], while the activity of others, such as mersacidin and divergicin M35, may even lead to cell lysis [6, 8, 27].
The ethidium bromide assay is arguably the simplest way to assess the effect of an antimicrobial on the membrane integrity of a prokaryotic cell. Ethidium bromide is a fluorescent compound with high affinity for DNA molecules. It penetrates bacterial cells via damaged cytoplasmic membranes, and subsequently binds to the intracellular DNA. Alternatively, ethidium bromide may bind extracellular DNA molecules from lysed cells. The unbound, residual ethidium bromide is removed with the washing buffer [5, 7].
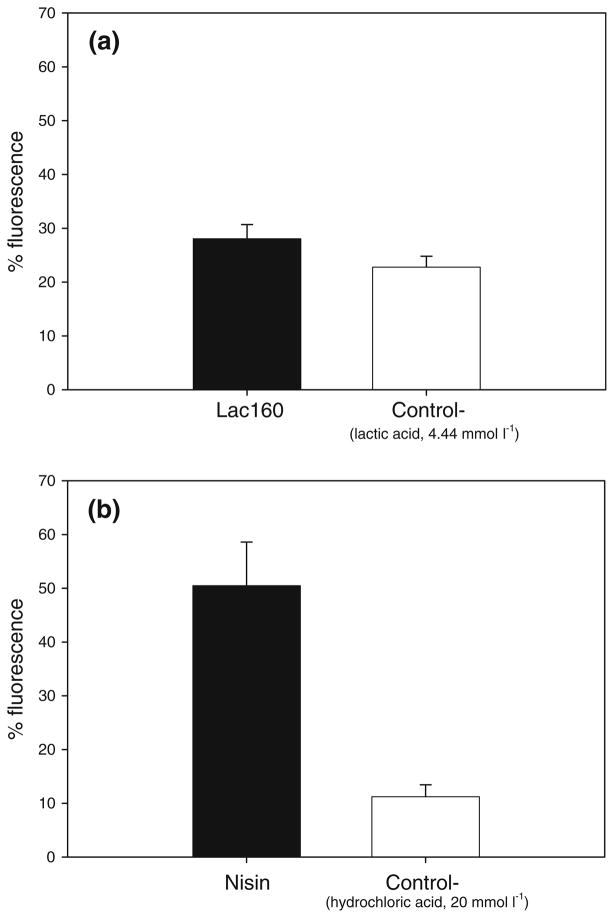
The results obtained from the ethidium bromide assay indicate that the bacteriocin nisin instigates severe membrane damage in G. vaginalis even at 1 AU ml−1 (Fig. 1b). In contrast, the membrane damage induced in G. vaginalis by lactocin 160 could not be detected using this assay (Fig. 1a).
Fig. 1.
Assessment of membrane integrity using the ethidium bromide assay. Lactocin 160 (Lac160) and the lactic acid solution (Control−) had an equivalent effect on the concentration of cell-bound ethidium bromide, indicating that the cytoplasmic membrane damage induced by lactocin 160 in G. vaginalis could not be determined through the ethidium bromide assay (a). Nisin, in contrast to lactocin 160, causes a detectable level of damage in the cytoplasmic membranes of G. vaginalis cells (b); the cells treated with nisin fluoresce with a significantly higher intensity than the cells treated with nisin diluent (Control−)
Lactocin 160 Induces Efflux of ATP in G. vaginalis
Bacteriocins frequently deplete the intracellular ATP pool of the cells they target. Efflux of ATP and/or of its precursor molecules (ADP, phosphates) may occur through cellular membranes perturbed by the activity of a bacteriocin [1, 17]. In aerobic cells, bacteriocins also prevent ATP synthesis through depletion of transmembrane gradients [26]. Finally, the cellular response repertoire to bacteriocins commonly involves expenditure of ATP.
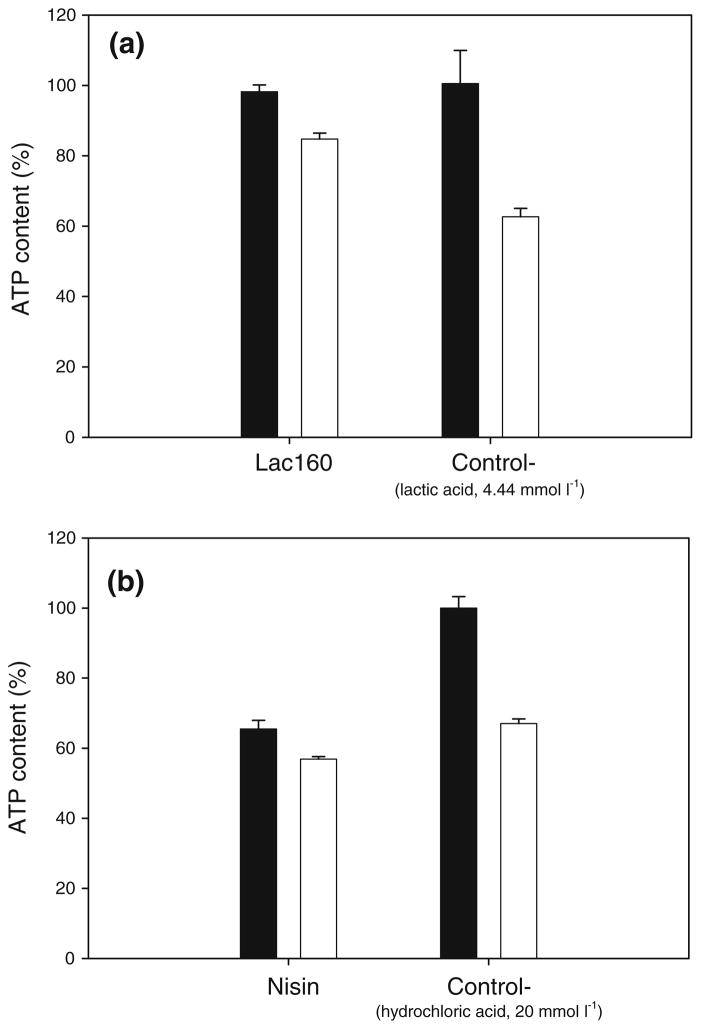
The activity of lactocin 160 induced a transmembrane efflux of ATP in G. vaginalis cells (Fig. 2a). Nisin, in contrast to lactocin 160, triggered the intracellular hydrolysis of ATP in G. vaginalis (Fig. 2b). It is important to note that the negative controls, including cells treated with PBS buffer (data not shown), had most of their ATP content externalized, indicating that G. vaginalis poorly tolerates the conditions of the assay.
Fig. 2.
The effect of lactocin160 and nisin on ATP content of G. vaginalis cells. Open bars represent the level of extracellular ATP, while closed bars represent the total ATP content (extracellular plus the intracellular ATP). Accumulation of extracellular ATP in the G. vaginalis culture treated with lactocin 160 (Lac 160) indicates that the antimicrobial induced the efflux of ATP across the cytoplasmic membrane of the cells (a). In contrast, nisin caused a significant decrease in the total ATP content of the culture, signifying that this bacteriocin triggered the intracellular hydrolysis of ATP in G. vaginalis (b). The corresponding negative controls for Lac 160 and nisin were the cells treated with the lactic acid solution and nisin diluent, respectively (both represented as Control−)
Lactocin 160 has putatively been described as a bacteriocin [3, 21], therefore, it is likely that, much like many other known bacteriocins, it exerts its effect by formation of transient pores in the cytoplasmic membrane of the target cell [17]. These transient channels may be ion specific, as they frequently differ in the size, charge, and in the duration of their existence [18, 25]. Hence, it is not surprising that lactocin 160 makes the target cytoplasmic membrane permeable to ATP but not to ethidium bromide, as described in the previous section. This further indicates that the antimicrobial activity (at 1 AU ml−1) is not due to cell lysis. The specificity of the channels formed by lactocin 160 can be evaluated further by elucidating the effect of this antimicrobial on various transmembrane potentials in G. vaginalis.
Lactocin 160 Completely Collapses both Components of the PMF in G. vaginalis Cells
A common consequence of the disruption of cytoplasmic membranes, caused by bacteriocins, is a collapse of the PMF, the electrochemical gradient essential for numerous cellular processes [23, 26]. Bacteriocins can dissipate both the electrical (ΔΨ) and chemical (ΔpH) components of the PMF [17], although some may specifically target only one of the gradients [18, 22].
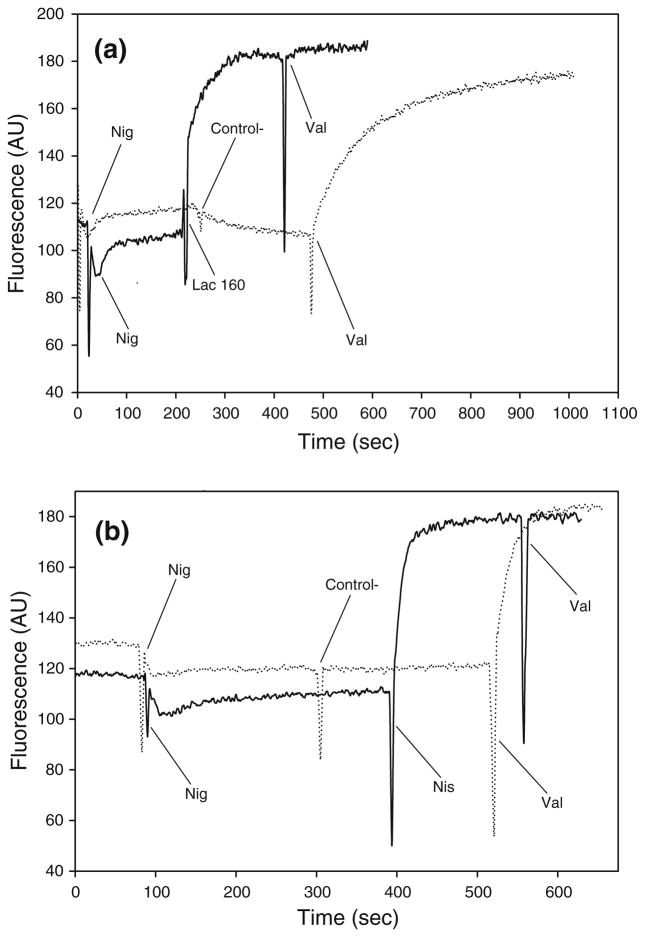
Lactocin 160, much like nisin, induced complete collapse of the transmembrane electric potential (ΔΨ) in G. vaginalis cells. The assay was conducted using the G. vaginalis growth medium; consequently there was no need to energize the cells through supplementation of glucose [34]. Following the addition of nigericin (K+/H+ exchanger), cells were treated with either lactocin 160 (Fig. 3a) or nisin (Fig. 3b), both of which resulted in a sharp increase in fluorescence, while the corresponding negative controls did not produce such an effect. Under the assay conditions, an increase in the fluorescence intensity of the probe is caused by depolarization of the cellular cytoplasmic membranes. The depletion of ΔΨ induced by either lactocin 160 or nisin was total, evident by the fact that final treatment with the ionophore valinomycin (used to dissipate any residual ΔΨ) did not lead to any additional increase in fluorescence intensity.
Fig. 3.
Lactocin 160, much like nisin, induces a complete collapse of transmembrane electrical potential (ΔΨ) in G. vaginalis. Two μmol l−1 nigericin (Nig) was initially used to convert ΔpH component of PMF into ΔΨ. A sharp increase in the fluorescence of DiSC3 (5), following the addition of lactocin 160 (Lac160, a) and nisin (Nis, b), signifies that these antimicrobials dissipate the transmembrane electric potential in G. vaginalis. In contrast, the corresponding negative controls, lactic acid solution, and nisin diluent, respectively (both designated as ‘Control−’), did not affect fluorescence of the probe. Two μmol l−1 valinomycin (Val) was ultimately used to completely collapse any residual ΔΨ
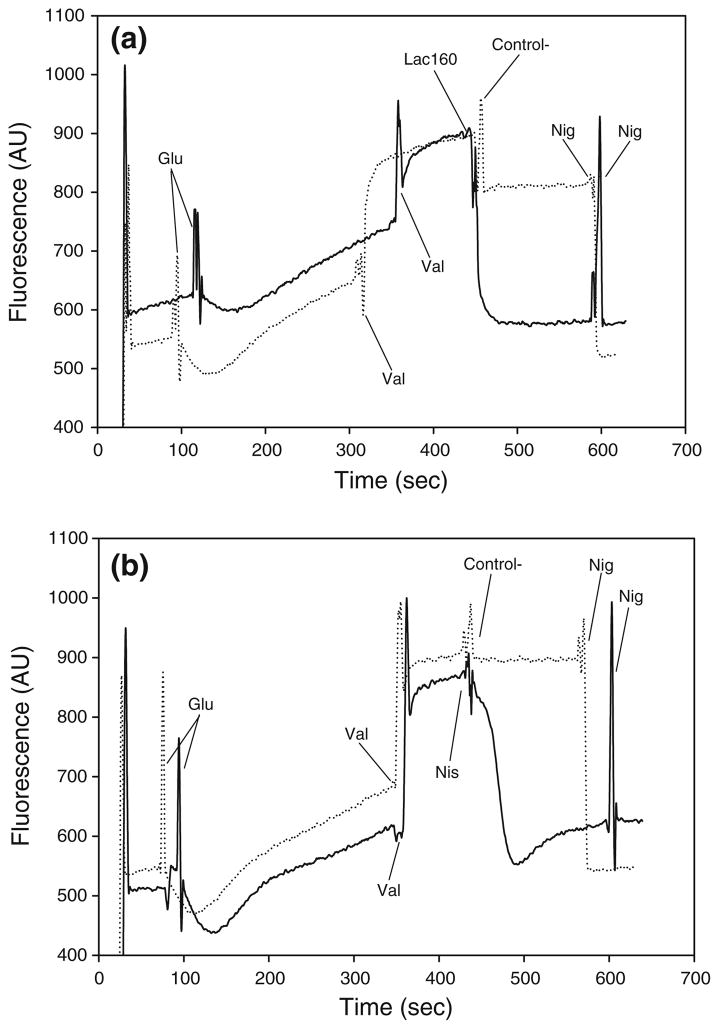
Lactocin 160, much like nisin, also induced the collapse of the ΔpH component of the PMF, as evidenced by a sharp decrease in the fluorescence of the energized, valinomycin-treated cells (Fig. 4a, b). A drop in the fluorescence intensity of the cells loaded with the pH-sensitive probe BCECF-AM, points to a decrease of their intracellular pH. Conditions of the experiment were selected so that the pH of the assay buffer would be lower than the intracellular pH (data not shown). Consequently, a bacteriocin-induced decrease in intracellular pH signifies the ability of the antimicrobial to dissipate the transmembrane pH gradient (ΔpH) of the target cell. As expected, the lactic acid solution used as a negative control for Lac160 slightly acidified cytoplasmic pH in G. vaginalis cells (Fig. 4a). This mild acidification of the cytoplasm, however, did not cause a complete collapse of ΔpH, since the subsequent addition of nigericin triggered a further decrease of the cellular cytoplasmic pH, ultimately leading to the complete dissipation of ΔpH. In contrast to their corresponding negative controls, both lactocin160 (Fig. 4a) and nisin (Fig. 4b) completely dissipated ΔpH in G. vaginalis; i.e., addition of the ionophore valinomycin did not cause a further fall in the fluorescence intensity of the probe. It is interesting to note that the drop in fluorescence caused by nisin is immediately followed by a minor rise before the signal stabilizes (Fig. 4a). This ‘secondary’ rise in fluorescence was not observed with either lactocin 160 or nigericin, even though the experiment was repeated numerous times. This observed effect may be related to the ability of nisin to trigger intracellular ATP hydrolysis in G. vaginalis cells, as illustrated in previous sections. Most importantly, this discrepancy between the cellular response to nisin and to lactocin 160 further confirms the hypothesis that although both antimicrobials target the cytoplasmic membrane of the pathogen, their precise mechanism of action may still differ.
Fig. 4.
Lactocin 160, much like nisin, completely dissipated the transmembrane pH gradient (ΔpH) in G. vaginalis. Initially, the cells loaded with BCECF-AM were energized with 2.2 mmol l−1 glucose (Glu). Two μmol l−1 valinomycin (Val) was then used to convert the ΔΨ component of the PMF into ΔpH. Decrease in fluorescence intensity following the addition of lactocin 160 (Lac160, a), or nisin (Nis, b), indicate that these antimicrobials dissipate transmembrane pH gradient in G. vaginalis. Nisin diluent and lactic acid solutions, respectively, were used as the corresponding negative controls (Control−). Finally, 2 μmol l−1 nigericin (Nig) was used to completely collapse any residual ΔpH
Conclusion
This study has revealed that lactocin 160 dissipates both components of the proton motive force (ΔΨ and ΔpH), and induces the efflux of ATP in the vaginal pathogen, G. vaginalis. The activity of lactocin 160 against G. vaginalis is likely to be due to the formation of transient pores in the cytoplasmic membrane of the pathogen. Although the bacteriocin nisin may also dissipate ΔΨ and ΔpH by transient pore formation, the intrinsic characteristics of these channels are likely to differ from those formed by lactocin 160.
The safety and narrow spectrum of activity associated with lactocin 160 renders this antimicrobial agent as a promising candidate for the treatment and prophylaxis of bacterial vaginosis. A clear understanding of its mechanism of action, however, is vital for the intelligent design of effective formulations involving multiple hurdles. In general, cells have difficulty adapting to various unrelated stress factors; therefore, a combination of antimicrobials with different mechanisms of action is likely to have a synergistic effect against the target microorganism. By the same token, multiple hurdles make it less probable for resistant mutants to arise and be selected for.
Acknowledgments
This research was sponsored by NIH Grant “Natural antimicrobials against bacterial vaginosis” NCCAM NIH R21AT002897-01. The authors thank Katia Sutyak and Dr. Ruth Wirawan for the editorial work.
References
- 1.Abee T, Klaenhammer TR, Letellier L. Kinetic studies of the action of lactacin F, a bacteriocin produced by Lactobacillus johnsonii that forms poration complexes in the cytoplasmic membrane. Appl Environ Microbiol. 1994;60:1006–1013. doi: 10.1128/aem.60.3.1006-1013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol. 2001;185:375–379. doi: 10.1067/mob.2001.115867. [DOI] [PubMed] [Google Scholar]
- 3.Aroutcheva AA, Simoes JA, Faro S. Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infect Dis Obstet Gynecol. 2001;9:33–39. doi: 10.1155/S1064744901000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barberis IL, Pajaro MC, Godino SD, Aichino C. In vitro inhibition of the growth of Gardnerella vaginalis by bacteriocins produced by strains of Pseudomonas aeruginosa. Enferm Infecc Microbiol Clin. 1997;15:473–476. [PubMed] [Google Scholar]
- 5.Barker C, Park SF. Sensitization of Listeria monocytogenes to low pH, organic acids, and osmotic stress by ethanol. Appl Environ Microbiol. 2001;67:1594–1600. doi: 10.1128/AEM.67.4.1594-1600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer R, Dicks LM. Mode of action of lipid II-targeting lantibiotics. Int J Food Microbiol. 2005;101:201–216. doi: 10.1016/j.ijfoodmicro.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Benito A, Ventoura G, Casadei M, Robinson T, Mackey B. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl Environ Microbiol. 1999;65:1564–1569. doi: 10.1128/aem.65.4.1564-1569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brotz H, Bierbaum G, Markus A, Molitor E, Sahl HG. Mode of action of the lantibiotic mersacidin: inhibition of peptidoglycan biosynthesis via a novel mechanism? Antimicrob Agents Chemother. 1995;39:714–719. doi: 10.1128/AAC.39.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherpes TL, Meyn LA, Krohn MA, Hillier SL. Risk factors for infection with herpes simplex virus type 2: role of smoking, douching, uncircumcised males, and vaginal flora. Sex Transm Dis. 2003;30:405–410. doi: 10.1097/00007435-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis. 2005;40:1422–1428. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 11.Dover SE, Aroutcheva AA, Faro S, Chikindas ML. Safety study of an antimicrobial peptide lactocin 160, produced by the vaginal Lactobacillus rhamnosus. Infect Dis Obstet Gynecol. 2007;2007:78248. doi: 10.1155/2007/78248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falagas ME, Betsi GI, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect. 2007;13:657–664. doi: 10.1111/j.1469-0691.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 14.Hashemi FB, Ghassemi M, Roebuck KA, Spear GT. Activation of human immunodeficiency virus type 1 expression by Gardnerella vaginalis. J Infect Dis. 1999;179:924–930. doi: 10.1086/314674. [DOI] [PubMed] [Google Scholar]
- 15.Hashemi FB, Ghassemi M, Faro S, Aroutcheva A, Spear GT. Induction of human immunodeficiency virus type 1 expression by anaerobes associated with bacterial vaginosis. J Infect Dis. 2000;181:1574–1580. doi: 10.1086/315455. [DOI] [PubMed] [Google Scholar]
- 16.Hay P. Life in the littoral zone: lactobacilli losing the plot. Sex Transm Infect. 2005;81:100–102. doi: 10.1136/sti.2003.007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hechard Y, Sahl HG. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie. 2002;84:545–557. doi: 10.1016/s0300-9084(02)01417-7. [DOI] [PubMed] [Google Scholar]
- 18.Herranz C, Chen Y, Chung HJ, Cintas LM, Hernandez PE, Montville TJ, Chikindas ML. Enterocin P selectively dissipates the membrane potential of Enterococcus faecium T136. Appl Environ Microbiol. 2001;67:1689–1692. doi: 10.1128/AEM.67.4.1689-1692.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson PG, Bergstrom M, Forsum U, Jacobsson B, Strand A, Wolner-Hanssen P. Bacterial vaginosis. Transmission, role in genital tract infection and pregnancy outcome: an enigma. Acta Pathol Microbiol Immunol Scand. 2005;113:233–245. doi: 10.1111/j.1600-0463.2005.apm_01.x. [DOI] [PubMed] [Google Scholar]
- 20.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003;189:139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Aroutcheva AA, Faro S, Chikindas ML. Mode of action of lactocin 160, a bacteriocin from vaginal Lactobacillus rhamnosus. Infect Dis Obstet Gynecol. 2005;13:135–140. doi: 10.1080/10647440500148156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAuliffe O, Ryan MP, Ross RP, Hill C, Breeuwer P, Abee T. Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl Environ Microbiol. 1998;64:439–445. doi: 10.1128/aem.64.2.439-445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966;41:445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 24.Molenaar D, Abee T, Konings WN. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim Biophys Acta. 1991;1115:75–83. doi: 10.1016/0304-4165(91)90014-8. [DOI] [PubMed] [Google Scholar]
- 25.Moll G, Ubbink-Kok T, Hildeng-Hauge H, Nissen-Meyer J, Nes IF, Konings WN, Driessen AJ. Lactococcin G is a potassium ion-conducting, two-component bacteriocin. J Bacteriol. 1996;178:600–605. doi: 10.1128/jb.178.3.600-605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montville TJ, Bruno ME. Evidence that dissipation of proton motive force is a common mechanism of action for bacteriocins and other antimicrobial proteins. Int J Food Microbiol. 1994;24:53–74. doi: 10.1016/0168-1605(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 27.Naghmouchi K, Drider D, Fliss I. Action of divergicin M35, a class IIa bacteriocin, on liposomes and Listeria. J Appl Microbiol. 2007;102:1508–1517. doi: 10.1111/j.1365-2672.2006.03206.x. [DOI] [PubMed] [Google Scholar]
- 28.Naghmouchi K, Kheadr E, Lacroix C, Fliss I. Class I/Class IIa bacteriocin cross-resistance phenomenon in Listeria monocytogenes. Food Microbiol. 2007;24:718–727. doi: 10.1016/j.fm.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Newton ER, Piper J, Peairs W. Bacterial vaginosis and intraamniotic infection. Am J Obstet Gynecol. 1997;176:672–677. doi: 10.1016/s0002-9378(97)70568-4. [DOI] [PubMed] [Google Scholar]
- 30.Nissen-Meyer J, Nes IF. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch Microbiol. 1997;167:67–77. [PubMed] [Google Scholar]
- 31.Oakeshott P, Kerry S, Hay S, Hay P. Bacterial vaginosis and preterm birth: a prospective community-based cohort study. Br J Gen Pract. 2004;54:119–122. [PMC free article] [PubMed] [Google Scholar]
- 32.Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier SL, Rabe L, Gaydos CA, Quinn TC, Konde-Lule J. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 33.Simoes JA, Aroutcheva A, Heimler I, Shott S, Faro S. Bacteriocin susceptibility of Gardnerella vaginalis and its relationship to biotype, genotype, and metronidazole susceptibility. Am J Obstet Gynecol. 2001;185:1186–1190. doi: 10.1067/mob.2001.118144. [DOI] [PubMed] [Google Scholar]
- 34.Sims PJ, Waggoner AS, Wang CH, Hoffman JF. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974;13:3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- 35.St John E, Mares D, Spear GT. Bacterial vaginosis and host immunity. Current HIV/AIDS Rep. 2007;4:22–28. doi: 10.1007/s11904-007-0004-y. [DOI] [PubMed] [Google Scholar]
- 36.Weir E. Bacterial vaginosis: more questions than answers. Can Med Assoc J. 2004;171:448. doi: 10.1503/cmaj.1041174. [DOI] [PMC free article] [PubMed] [Google Scholar]