Summary
Brain regions beyond visual cortex are thought to be responsible for attention-related modulation of visual processing[1,2], but most evidence is indirect. Here we applied fMRI, including retinotopic mapping of visual areas, to patients with focal right-parietal lesions and left spatial neglect[3,4]. When attentional load at fixation was minimal, retinotopic areas in right visual cortex showed preserved responses to task-irrelevant checkerboards in the contralateral left hemifield, analogously to left visual cortex for right hemifield checkerboards, indicating a ‘symmetric’ pattern in either hemisphere with respect to contralateral stimulation under these conditions. But when attentional load at fixation was increased, a functional asymmetry emerged for visual cortex, with contralateral responses in right visual areas pathologically reduced (even eliminated for right V4/TEO), while left visual areas showed no such reduction in their contralateral response. These results reveal attention-dependent abnormalities in visual cortex after lesions in distant (parietal) regions. This may explain otherwise puzzling aspects of neglect[5,6], as confirmed here by additional behavioural testing.
Results and Discussion
Visuospatial neglect is a severe neurological disorder after right-hemisphere damage, often involving parietal cortex[3,4]. Neglect has multiple components[7], including losses of contralesional awareness that cannot be attributed to primary sensory or motor loss, but may involve pathological biases in attention[1,8]. Neurally, this might reflect disruption of influences from damaged regions (e.g. parietal cortex) upon activity in intact visual areas[1,2]. Recent functional neuroimaging in neglect patients showed some residual activation of intact visual cortex despite losses of awareness[9-11], plus anomalies in remaining fronto-parietal areas[12]. Evoked potential studies[13] indicate that unperceived left visual stimuli may produce reduced or suppressed P1/N1 components. But no study has directly tested whether neglect patients show abnormal attention-dependent activity in early, retinotopically-mapped visual cortex; nor how the functional response of their visual cortex may depend on attentional demand[14,15].
We used fMRI, including retinotopic mapping of V1-V3 plus V4/TEO, to examine how task-demand at central fixation may affect cortical responses to left visual field (LVF) or right (RVF) stimulation, after right parietal damage. We selected two patients with focal parietal lesions (Suppl. Fig. S1), but structurally preserved visual cortex and intact visual fields. They were scanned while performing tasks of minimal or increased attentional load at screen-center. Previous work in normals shows that increasing attentional load at fixation can reduce visual activations for task-irrelevant peripheral stimuli, ‘symmetrically’ for each hemifield[14]. Here the low-load task was minimal (‘no load’), simply requiring fixation on a central stream of coloured stimuli. The higher load required discrimination of rare colour targets in a similar central stream.
During either central task, checkerboards could appear in RVF, LVF, or bilaterally, or none (Fig. 1a), in a pseudorandom blocked order that was counterbalanced across the two central tasks (see Methods). We tested for any impact of attentional demand at central fixation on visual responses to peripheral task-irrelevant checkerboards, for retinotopically mapped regions corresponding to the checkerboard positions (Methods). We predicted that visual responses to checkerboards should be relatively normal during minimal load (consistent with intact visual fields), but might exhibit a pathologically ‘asymmetric’ pattern during increased task-demand at fixation, with activation reduced in right unlike left visual cortex.
Figure 1.
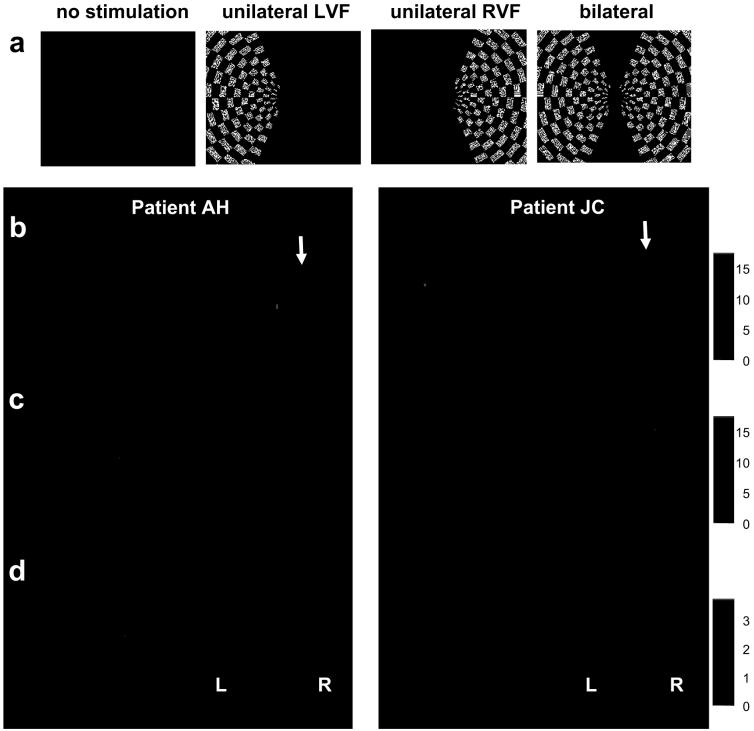
Paradigm and fMRI results. (a) A stream of successive small (0.5°) coloured stimuli appeared centrally at 1.25 Hz. These were either Os that patients had merely to fixate (‘no’ or minimal load); or Ts for which patients had to detect infrequent (7.5%) red targets (higher load). During either task, irrelevant peripheral checkerboards could be flashed in left, right, both, or neither hemifield, in pseudorandom order that was equivalent across the load tasks. (bc) Whole-brain SPM maps in patients AH and JC, showing activation under no-load in: (b) right occipito-temporal cortex for LVF>RVF checkerboards; or (c) activation of left occipito-temporal cortex for RVF>LVF checkerboards. A robust contralateral visual response is observed for each hemisphere under no-load, even on the right side where parietal damage exists (see arrows). (d) Whole-brain SPMs for each patient showing activation for no-load > higher-load central conditions. Higher activity is observed in right occipital cortex under no-load (i.e., increased attention demands at fixation reduce right occipital responses), without any such effect in left occipital cortex, nor anywhere beyond visual cortex.
Both patients showed similar fMRI results. We first examined effects of unilateral stimulation in LVF vs RVF (or vice-versa), under minimal task-load. Whole-brain SPMs revealed robust activation of contralateral occipital cortex, ‘symmetrically’ for the two hemispheres (Fig. 1bc), as normally expected. We next contrasted no- minus higher-load (initially across checkerboard conditions). In both patients, higher load reduced visual activation in right occipito-temporal areas, but with no such effect in left visual cortex (see Fig 1d). Increased demand at fixation thus introduced an ‘asymmetry’ into the previously ‘symmetric’ responses, diminishing activation of right visual cortex (responsive to LVF, Fig. 1b) but not left visual cortex (responsive to RVF, Fig. 1c).
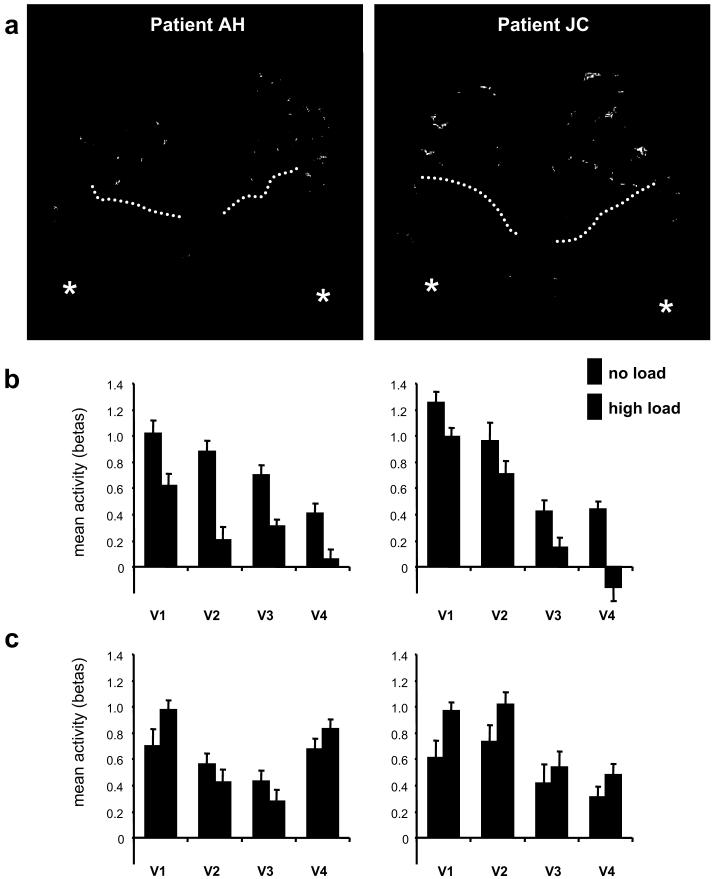
We separately mapped visual areas in each hemisphere for each patient, applying established retinotopic procedures (see Methods) to neglect patients for the first time. V1-V3 were readily identified along with V4/TEO in both hemispheres for both patients (Fig. 2a), indicating preserved basic retinotopy (see also Suppl. Fig. S2) despite parietal damage and neglect. Activity estimates were extracted from each retinotopic area, for the different conditions in the main load experiment (see Methods), and submitted to two complementary assessments.
Figure 2.
Retinotopically mapped results. (a) 3D reconstruction of occipital cortex and functionally defined retinotopic areas for each hemisphere, in each patient. Asterisks indicate foveal region at occipital pole, dotted lines indicate parieto-occipital sulcus. V1 in blue, V2 pink, V3 orange, V4/TEO red. See also Suppl. Figure S2 for flatmaps. (b) Extracted parameter estimates of activity (mean beta values from GLM analysis on each separate stimulation epoch, averaged across stimulus-responsive voxels within each area, with standard-error bars shown for the mean across epochs) showing responses of right retinotopic areas to contralateral stimulation in left visual field as a function of task-load at fixation (collapsed across LVF and bilateral checkerboards; see Suppl. Fig. S3 for separation of those), relative to the (subtracted) no-checkerboard baseline. Higher attentional load at fixation reduced contralateral responses for each right visual area, in both patients. It even eliminated the response to contralateral checkerboards for right V4/TEO. (c) Left visual areas did not show such reduction during higher load, leading to a significant difference between hemispheres for the attentional effect on responses to contralateral stimuli (i.e. a hemisphere x load x stimulation interaction; see main text).
We first extracted z-scores for peak voxels within each retinotopic area and hemisphere, for the contrast between no-load minus higher load in SPM (cf. the whole-brain maps in Fig. 1d). A striking asymmetry was apparent, with highly significant load effects for all retinotopically-defined ROIs within the right hemisphere for each patient (z-values ranging from 2.53 to 3.61, p-values from 0.006 to 0.0001; Suppl. Table S2), but no such effect in the left retinotopic ROIs (even when searching each region for the peak voxel in this contrast, z-values ranged from -0.15 to 1.16, with corresponding nonsignificant p-values from 0.117 to 0.619; Table S2).
The asymmetrical result was confirmed by a further analysis that directly compared hemispheres for visual areas. We ran a new GLM analysis of fMRI data in the main load experiment (see Methods), now modeling each appearance (‘epoch’) of each condition separately (the whole series was repeated 8 times per patient, 4 per run). We then extracted the parameter-estimates for each retinotopic ROI (averaged across its voxels) per epoch, and per condition. A randomized ANOVA was performed on these data with four factors of task load (high-low), hemisphere (right-left), checkerboard stimulation (contralateral-bilateral-none) and area (V1 to V4). For each patient, all main effects were significant (p<.001) except hemisphere (p>.11). The key interaction of load x hemisphere x visual stimulation was highly significant for both AH and JC (F2,336 = 8.88 and 5.29, respectively, both p<.005), due to higher central load leading to a reduction of the response to contralateral checkerboards for right but not left visual cortex (Fig. 2b and 2c). There was also a two-way interaction for load x stimuli (F2,336 = 8.35 and 3.82, p<.001), and for load x hemisphere (F1,336 = 96.9 and 74.1, p<.001). T-tests confirmed a significant reduction of responses to LVF stimuli (Fig. 2b) during high vs no load, in all right visual areas for patient AH (V1, t14=2.78, p=.015; V2, t14=5.47, p<.001; V3, t14=4.15, p=.001; V4/TEO, t14=6.55, p=.001) and in all areas except V2 (that showed only a trend in the same direction) for patient JC (V1, t14=2.51, p=.025; V2, t14=1.58, p=.13; V3, t14=3.68, p=.003; V4/TEO, t14=5.15, p=.001). There were no such significant reductions in left visual areas (Fig 2c). For completeness, we also ran a further ANOVA on the same epoch data, but now treating all experimental conditions (load x stimulation) as repeat factors and regions of interest (area x hemisphere) as nonrepeat. This revealed a similar pattern of significance (including the critical three-way interaction of load x hemisphere x stimulation, p<.001 for both patients).
For both patients, the proportional size (see Methods) of load effects on right cortical responses to LVF checkerboards was maximal in V4/TEO (40-95%), larger (all p<.05) than for areas V1-V3 (15-35%). For right V4/TEO, the response to contralateral left checkerboards (compared with none) was actually abolished under increased attentional-load at fixation, with activation no longer differing significantly from the baseline condition with no peripheral stimuli (Fig. 2b).
Results for right retinotopic visual areas were similar for unilateral left and bilateral stimulation (i.e. for any condition driving contralesional LVF, see suppl. Fig. S3), but differed strikingly from the preserved response of left retinotopic areas to right checkerboards even with increased load (compare Figs. 2b-2c), again consistent with the whole-brain SPM results (Fig. 1d). Left visual areas typically showed either no significant impact of task demand, or if anything a tendency for increased response with higher load. Thus, right and left visual cortex behaved similarly and ‘symmetrically’ (with respect to contralateral stimulation) under minimal central demand; but a pathological visual ‘asymmetry’ emerged only under increased central demand. While there was some tendency for an asymmetry with central demand even in the baseline no-checkerboard condition, central load did not significantly affect right visual cortex in the absence of contralateral checkerboards, indicating that the most substantial impact of load on right visual cortex concerned its response to LVF stimulation, not just a ‘baseline shift’ [16].
Finally, we used a similar attention-load paradigm for a behavioural study in 6 other neglect patients, where visual objects were now presented in LVF or RVF instead of checkerboards. Object recognition was tested after short runs of either central task, showing symmetric hit-rates for LVF and RVF stimuli (35% vs 40%) after exposure under no load at fixation, but significantly worse recognition for LVF (11%) than RVF (28%) under higher load (see Supplemental Material). Again this asymmetry appeared only under central load, analogously to our fMRI results.
Our results reveal pathological functional changes in distant, structurally intact retinotopic visual cortex, for patients with neglect after right-parietal damage. These functional changes were attention-dependent. Patients showed a normal ‘symmetric’ pattern of visual activation in either hemisphere (for contralateral stimulation) under minimal load at fixation, but a pathological asymmetry during increased demand at fixation. This led to reduction (or for right V4/TEO, even elimination) of the right visual cortex response to contralateral peripheral stimuli, while left visual cortex showed no such reduction. This asymmetry under increased attentional load at fixation is unlike the symmetric effects of central load in normals[14], even for higher task demands.
A notable result in our patients was that right V4/TEO became functionally ‘blind’ to left checkerboards under higher attentional demand at fixation, a pattern never observed in healthy subjects[14], although normal attentional effects often increase across successive visual areas from V1 to V4/TEO[17-19]. The dramatic result for V4/TEO might conceivably relate to our colour task, though this aspect alone cannot explain the pathological asymmetry of load effects. Future studies could compare the impact of different types of load task, as well as different lesion sites in further patients. Our findings appear consistent with a major role for parietal cortex in attention[17,18], and with the view that impaired awareness for contralesional visual stimuli in neglect patients may involve disturbed influences from higher areas upon sensory pathways[1,2]. But while neglect is more frequent and severe after parietal damage, it can also arise after other lesions (e.g. frontal). Future work may determine whether such lesions can produce similar impacts on visual cortex, and if this involves concomitant changes in parietal activity[12].
Peripheral checkerboards were always task-irrelevant here, although salient and not unexpected. Any account in terms of possible division of attention between centre and periphery, or of limited resources in neglect patients, would still need to explain the critical asymmetry found under high central load only. The damaged regions in right parietal cortex may normally serve to enhance visual processing[2,17] whenever salient events occur in LVF while attention is otherwise engaged[20]. In the absence of such parietal influences on visual cortex, due to the lesion, retinotopic areas in visual cortex may then show abnormal functional responses, as demonstrated here.
Such attention-dependent effects on neural visual responses may explain otherwise puzzling aspects of the neglect syndrome. In clinical behaviour and formal testing, neglect patients often seem to have fully functioning visual fields at one moment, yet appear blind in LVF the next[5,21]. Moreover, neglect severity can vary under different task conditions[6,22], as demonstrated here by our behavioural follow-up. Our fMRI results reveal that demand at current fixation can have critical consequences for functional responsivity of right versus left visual cortex in neglect patients. More generally, our study illustrates that combining fMRI with lesion approaches can reveal functional abnormalities in distant brain areas remote from the lesion[2,23], as shown here for attention-dependent abnormalities in visual cortex of neglect patients after parietal damage.
Experimental Procedures
Patients in neuroimaging study
Two patients with right-hemisphere stroke were selected due to focal lesions in right parietal cortex (Fig. S1), with left spatial neglect but intact visual fields, and preserved ability to maintain fixation during scanning. Neglect was diagnosed with standard tests at the time of fMRI investigation (Table S1).
Attentional task during scanning
The paradigm was similar to recent work in healthy subjects[14], using easier tasks as appropriate for neurological patients. Two successive experimental runs (∼12 min each) each comprised no-load and higher-load tasks, with their order counterbalanced across runs. Central Ts or Os appeared for 500 ms each (separated by 250 ms), with colour and T orientation pseudo-randomized, equiprobably across checkerboard conditions. An instruction display (10 sec) preceded each task block. Target onsets in the higher-load condition (red Ts) were unpredictable (7.5% of items, equiprobable across checkerboard conditions). Both patients showed accurate performance (AH 96% correct, JC 90% correct). During either task, large checkerboards (∼10×14°, sparing central 2° on either side) flickered (8 Hz) for epochs of 20 sec in LVF, RVF, both sides, or neither, in pseudorandom sequence (each appeared once in an otherwise random order, with a different random sequence of the 4 peripheral-stimulation conditions during each task block, but the actual order of the 4 peripheral stimulation conditions was identical overall for the two different load tasks). Each checkerboard condition (LVF, RVF, bilateral, or none) arose 4 times during each task in each run, thus 8 times in total. In other words, the full set of conditions was essentially repeated 8 times per patient. Patients were instructed to ignore the checkerboards. Three 20-sec empty periods (resting baseline) were included before and after each task. Continuous eye-tracking during fMRI confirmed correct central fixation across conditions (see Suppl. Material).
Retinotopic mapping
A standard visual mapping protocol was administered after the attentional tasks, comprising two separate runs as described elsewhere[14]; see also [24,25]. Stimulation by rotating checkerboard wedge (45° angle) was used to map polar angle; whereas an expanding annulus mapped eccentricity up to 14° from center-of-field (0.02 Hz period), sparing the central 2° on each side in both cases. Retinotopic stimulation (rotation or expansion) traversed the same parts of the visual field in which peripheral checkerboards were presented during the load task[14], so that we could assess attentional modulations specifically for stimulus-driven retinotopic regions (as defined by individual mapping) in the separate load experiment. Fixation was maintained on a coloured dot at screen-centre during mapping, as confirmed by online eye-tracking.
fMRI acquisition and analysis
The same scanning parameters were used in the attentional-load task and retinotopic mapping runs. Functional images were obtained using T2*-weighted transverse slices (TE = 40 ms; TR = 2.74 s; matrix size 64×64×36; voxel size: 3×3×3 mm3) with two series of 262 volumes for the attention-load experiment, and two series of 64 scans for retinotopic mapping. A high-resolution T1 anatomical volume image (matrix 256×176×256; voxel size: 1×1×1.5 mm3) was acquired in the same session. All time-series from each individual were realigned, time-corrected, and smoothed (4 mm FWHM) using SPM99 (http://fil.ion.ucl.ac.uk/spm/).
Whole-brain analysis was performed using the general linear model (GLM) as implemented in SPM[26]. For each patient, 8 experimental conditions (2 task-load x 4 peripheral stimulations) were modelled as boxcar waveforms convolved with a canonical hemodynamic response function for each scanning run (16 betas of interest per design matrix). Realignment parameters were entered as additional covariates to capture movement-related artifacts. Parameter estimates for each covariate were estimated for each voxel in each participant. Statistical parametric maps of the t-statistic (SPM[t]) were generated from linear contrasts between conditions, thresholded at p<0.001 uncorrected, with cluster-size > 20 voxels.
Retinotopic-mapping data were analyzed with standard procedures[24,25,27], as described elsewhere[14], using SPM[26] and MrGray/MrFlatMesh software[28]. Retinotopic stimulation was first modelled using a GLM with two regressors (sine and cosine functions with same frequency as stimulation wedges), plus movement parameters from image realignment. Phase maps were obtained for polar-angle and eccentricity activation (arctangent of sine/cosine ratio), using voxel-wise F-test at p<0.001. Colour-coded values were projected onto the flattened occipital cortical surface to identify boundaries between discrete areas [24,25,27], using MrGray and MrFlatMesh[29]; see Supplementary Figure S2A and S2B. Stimulus-responsive voxels were selected based on the combination (overlap) of activation to both rotating and expanding stimulation. We could reliably delineate ventral and dorsal portions of V1, V2, V3, plus ventral V4/TEO, with a similar number of voxels in both patients (total AH: 159 right, 168 left; JC: 138 right, 121 left) like healthy subjects [14,29].
Stimulus-responsive voxels in retinotopic areas were then projected back onto the original 3D brain volume (Fig. 2a), to extract activation values (betas) during the attentional-load experiment. These betas were obtained from a new GLM analysis of the main load experiment, in which each successive stimulation epoch (was now modelled separately (as an individual regressor), yielding 8 betas (4 epochs x 2 runs) for each of the four checkerboard conditions (bilateral, RVF, LVF or none) in each of the two attention (higher or no load) tasks (total 64 betas per patient). These betas were then averaged across voxels within each stimulus-responsive retinotopic region, to yield a robust unbiased measure. Data from V1-V3 were averaged across upper and lower fields[17,18], as these did not differ in the load experiment. Averaged beta values per area, hemisphere, condition, and epoch were submitted to ANOVA and t-tests with experimental conditions (load and stimulation) and region-of-interest (visual area and hemisphere) as randomized factors (but we also ran another ANOVA treating the experimental conditionsas repeat factors, which confirmed a similar outcome, see main text). Corresponding plots in Figs 2b and 2c had the no-checkerboard condition subtracted from them. In addition, to estimate the relative (proportional) size of load effects on different visual areas, we computed the mean difference between low minus high load conditions, normalized by response magnitude in the low-load condition (initially averaged across all conditions with contralateral stimuli, i.e., LVF and bilateral for right visual cortex, RVF and bilateral for left visual cortex; but see Fig. S3 for separation of unilateral and bilateral results). Finally, within each retinotopic area, we also extracted the peak z-score (see Table S2) obtained for the contrast of no-load minus higher load in the initial whole-brain SPM analysis (where only one beta value had been estimated for each of the experimental conditions per run).
Supplementary Material
Acknowledgements
Supported by grants from the Swiss National Science Foundation to PV (No 3200B0-114014) and from the Wellcome Trust and MRC to JD, who also holds a Royal Society-Leverhulme Trust Senior Research Fellowship. Our thanks to the patients and families for their time and cooperation, and to anonymous reviewers for helpful suggestions.
References
- 1.Driver J, Vuilleumier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. 2001;79:39–88. doi: 10.1016/s0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- 2.Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans R Soc Lond B Biol Sci. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karnath HO, Milner AD, Vallar G. The Cognitive and Neural Bases of Spatial Neglect. Oxford: Oxford University Press; 2002. [Google Scholar]
- 5.Walker R, Findlay JM, Young AW, Welch J. Disentangling neglect and hemianopia. Neuropsychologia. 1991;29:1019–1027. doi: 10.1016/0028-3932(91)90065-g. [DOI] [PubMed] [Google Scholar]
- 6.Rapcsak SZ, Verfaellie M, Fleet WS, Heilman KM. Selective attention in hemispatial neglect. Arch Neurol. 1989;46:178–182. doi: 10.1001/archneur.1989.00520380082018. [DOI] [PubMed] [Google Scholar]
- 7.Driver J, Vuilleumier P, Husain M. Spatial neglect and extinction. In: Gazzaniga M, editor. The new cognitive neurosciences. 3rd ed. Cambridge, MA: MIT Press; 2004. pp. 589–606. 589-606. [Google Scholar]
- 8.Rafal RD. Neglect. Current Opinion in Neurobiology. 1994;4:231–236. doi: 10.1016/0959-4388(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 9.Vuilleumier P, Sagiv N, Hazeltine E, Poldrack RA, Swick D, Rafal RD, Gabrieli JD. Neural fate of seen and unseen faces in visuospatial neglect: a combined event-related functional MRI and event-related potential study. Proc Natl Acad Sci U S A. 2001;98:3495–3500. doi: 10.1073/pnas.051436898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuilleumier P, Armony JL, Clarke K, Husain M, Driver J, Dolan RJ. Neural response to emotional faces with and without awareness: event-related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia. 2002;40:2156–2166. doi: 10.1016/s0028-3932(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 11.Rees G, Wojciulik E, Clarke K, Husain M, Frith C, Driver J. Unconscious activation of visual cortex in the damaged right hemisphere of a parietal patient with extinction. Brain. 2000;123(Pt 8):1624–1633. doi: 10.1093/brain/123.8.1624. [DOI] [PubMed] [Google Scholar]
- 12.Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- 13.Marzi CA, Girelli M, Miniussi C, Smania N, Maravita A. Electrophysiological correlates of conscious vision: evidence from unilateral extinction. J Cogn Neurosci. 2000;12:869–877. doi: 10.1162/089892900562471. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Attentional load and sensory competition in human vision: modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cereb Cortex. 2005;15:770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- 15.Pinsk MA, Doniger GM, Kastner S. Push-pull mechanism of selective attention in human extrastriate cortex. J Neurophysiol. 2004;92:622–629. doi: 10.1152/jn.00974.2003. [DOI] [PubMed] [Google Scholar]
- 16.Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 17.Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- 18.Kastner S, De Weerd P, Pinsk MA, Elizondo MI, Desimone R, Ungerleider LG. Modulation of sensory suppression: implications for receptive field sizes in the human visual cortex. J Neurophysiol. 2001;86:1398–1411. doi: 10.1152/jn.2001.86.3.1398. [DOI] [PubMed] [Google Scholar]
- 19.Mehta AD, Ulbert I, Schroeder CE. Intermodal selective attention in monkeys. II: physiological mechanisms of modulation. Cereb Cortex. 2000;10:359–370. doi: 10.1093/cercor/10.4.359. [DOI] [PubMed] [Google Scholar]
- 20.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 21.Russell C, Malhotra P, Husain M. Attention modulates the visual field in healthy observers and parietal patients. Neuroreport. 2004;15:2189–2193. doi: 10.1097/00001756-200410050-00009. [DOI] [PubMed] [Google Scholar]
- 22.Vuilleumier PO, Rafal RD. A systematic study of visual extinction. Between- and within-field deficits of attention in hemispatial neglect. Brain. 2000;123(Pt 6):1263–1279. doi: 10.1093/brain/123.6.1263. [DOI] [PubMed] [Google Scholar]
- 23.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 24.Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 25.Warnking J, Dojat M, Guerin-Dugue A, Delon-Martin C, Olympieff S, Richard N, Chehikian A, Segebarth C. fMRI retinotopic mapping--step by step. Neuroimage. 2002;17:1665–1683. doi: 10.1006/nimg.2002.1304. [DOI] [PubMed] [Google Scholar]
- 26.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- 27.Tootell RB, Reppas JB, Dale AM, Look RB, Sereno MI, Malach R, Brady TJ, Rosen BR. Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature. 1995;375:139–141. doi: 10.1038/375139a0. [DOI] [PubMed] [Google Scholar]
- 28.Wandell BA, Chial S, Backus BT. Visualization and measurement of the cortical surface. J Cogn Neurosci. 2000;12:739–752. doi: 10.1162/089892900562561. [DOI] [PubMed] [Google Scholar]
- 29.Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis. 2003;3:586–598. doi: 10.1167/3.10.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.