Abstract
The emerging sciences of stem cell biology and cellular plasticity have led to the development of cell-based therapies for advanced human disease. Pre-clinical studies which defined the potential of bone marrow-derived mononuclear cells to repair damaged and dysfunctional myocardium led to the rapid advancement of these strategies to the clinic. Such rapid advancement has led to controversy regarding the appropriate conduct of such studies. In the United States, the National Heart, Lung, and Blood Institute established the Cardiovascular Cell Therapy Research Network (CCTRN) to facilitate the early translation of clinical trials of cell therapy for left ventricular dysfunction. The premise upon which the CCTRN was established was that multiple clinical trial sites would interact effectively with a Data Coordinating Center to perform early phase 1 and 2 clinical trials within a highly coordinated network structure. In order to develop this network, the unmet needs of the community needed to be defined, the clinical trials identified, and the structure to perform the studies needed to be established. This manuscript highlights the challenges in the development of the CCTRN and the approaches faced to define a network to perform clinical trials in human cell therapy of cardiovascular disease.
Keywords: Network, Cell Therapy, Biorepository, Management, Multicenter
Introduction
Early this decade, intriguing small-animal studies demonstrated that delivery of bone marrow-derived mononuclear cells enabled improved ventricular function following induced myocardial infarction [1–3]. These studies utilized intramyocardial delivery of bone marrow mononuclear cells in murine models of myocardial infarction. Cell delivery resulted in remarkable improvement in left ventricular function. These cells held the potential of enabling healing through transdifferentiation into cardiac cells and/or improvement in angiogenesis. The plausible mechanisms were either direct effects or perhaps via paracrine influences on local cells. These studies generated tremendous interest worldwide amongst basic and translational scientists, clinicians, and even patients.
The development of either novel approaches to treat left ventricular dysfunction in patients treated with standard of care or new adjunctive therapies in patients with poor prognoses was energizing, and the potential value of autologous therapies was self-evident. At the same time, several studies appeared in the literature that suggested that myocardial cells may be capable of replication and not be terminally differentiated as once suggested [4]. Demonstration of potency of resident myocardial cells provided general support to the concept that cells might provide new therapeutics for myocardial repair.
The emerging data from preclinical studies led to the rapid initiation of clinical trials of bone marrow mononuclear cell delivery in Europe. The clinical trials of Assmus [5] and Wollert [6] utilized clinically available methods of bone marrow harvest and isolation, and intracoronary delivery of cells using the stop flow technique. Global or regional assessments of left ventricular function were the prospectively declared primary response measures in subjects with acute myocardial infarction or chronic left ventricular dysfunction. Initial nonrandomized studies suggested that this approach might be performed safely and, in addition, provided a premonition of efficacy. These studies led to larger randomized clinical trials in Europe [7–11] in spite of animal data which challenged the concept of transdifferentiation of bone marrow-derived cells [12, 13]. The rapid development of cell-based therapies in Europe and the consideration of unmet needs of patients in the United States generated the attention of the National Heart, Lung, and Blood Institute (NHLBI) which explored appropriate venues of financial funding in this area [14]. In 2005, an NHLBI working group on the translation of cardiovascular cell-based therapies recommended the development of a Cardiovascular Cell Therapy Research Network (CCTRN).
Why a Network?
As part of the National Institutes of Health (NIH), the NHLBI has supported basic science research in stem and progenitor cell biology broadly and extensively. While multicenter clinical trials were either underway or already completed in Europe, several small phase 1 studies of cardiovascular cell therapy were progressing in the United States and elsewhere. It was decided that there was a need for support of large phase 1 or phase 2 clinical studies which might be beyond the usual NIH investment in basic science and small single center first-in-man studies which were typically funded through standard mechanisms. It was also believed that the science and data from early clinical studies did not support the development of larger phase 3 studies at that time.
The early application of novel clinical strategies is often focused on patients without other therapeutic options. By definition, the prevalence of the patients in any individual site may be limited [15]. In order to provide sufficient patients to complete the proposed phase 1 and 2 studies, it was thought important to perform the studies at multiple sites. Thus, taken together, these needs led to the application of the clinical trial network methodology to cell therapy of cardiovascular diseases.
What is a Network?
The NHLBI has utilized the network approach to advance clinical trials in areas in which coordination of multiple centers is required to complete enrollment in sometimes complex clinical trials. A network is defined as a coordinated platform of professionals from multiple disciplines committed to enhancing the effectiveness and efficiency of multisite clinical investigation. It aims to improve the understanding and development of novel therapies and to better understand the outcomes of subjects enrolled. The network approach has been extensively utilized by the Lung branch for performing clinical investigations in asthma and other disease states since the early 1990s [16–18]. Concurrent with the establishment of the CCTRN, the NHLBI also established the Heart Failure Clinical Trial Network and the Cardiac Surgery Clinical Trial Network. Networks generally consist of multiple clinical sites with the shared goal of simultaneously performing integrated and complex clinical trials in a focused disease state. An important component of the network structure is the establishment of a Data Coordinating Center (DCC) which provides central administrative and regulatory management of the network on a daily basis. With this structure in mind, the request for application (RFA) for the CCTRN was published in 2006.
In the RFA, it was defined that the sites must contain senior scientific and clinical leadership that would not only conduct the clinical trials but also participate in writing and directing the proposed studies. Importantly, each site proposed potential clinical trials at the time of submission.
In the United States, all cell-based clinical trials are regulated by the Food and Drug Administration (FDA) through an investigational new drug (IND) application process. In general, clinical trials must be supported by preclinical safety, toxicity, and manufacturing/processing data. Therefore, at the time of application, it was important that sites have supportive data to propose a suitable large phase 1 or phase 2 clinical trial. That is, in general, sites supported their submissions by demonstrating their capacity to propose studies which could be supported through the IND process.
Current Network Structure
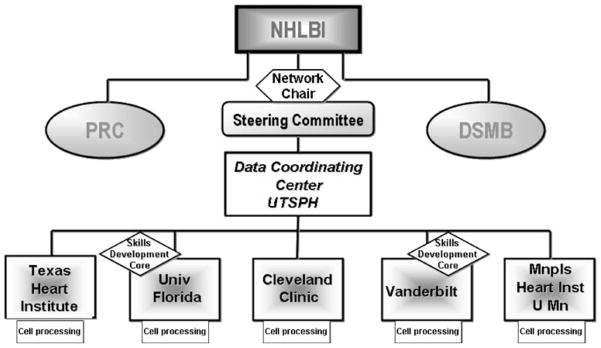
Five clinical sites were selected as clinical sites through a peer review process. These sites (and PIs) include University of Florida (Carl Pepine, M.D.), Cleveland Clinic Foundation (Steven Ellis, M.D.), Texas Heart Institute (James Willerson, M.D.), Minneapolis Heart Institute/University of Minnesota (Timothy Henry, M.D.), and Vanderbilt University (originally Douglas Vaughan, M.D. and currently David Zhao, M.D.). The Data Coordinating Center was awarded to the University of Texas-Houston School of Public Health (Lem Moyé, M.D., Ph.D). Robert D. Simari, M.D. (Mayo Clinic) was selected to chair the Steering Committee and Sonia Skarlatos Ph.D. was named the program officer from the NHLBI (Fig. 1).
Fig. 1.
Organizational structure of the CCTRN. PRC Protocol Review Committee, DSMB Data Safety and Monitoring Board
These sites each consist of an experienced multidisciplinary team with highly specialized skills in many areas. The skills required at each site include acquisition and purification of autologous bone marrow cells, characterization of the cellular product, assurance of aseptic techniques, storage and transport of final cell product(s), highly trained cardiovascular specialists to screen potential candidates and deliver the cell product(s); highly specialized techniques and equipment to assist in delivery and assess results (NOGA, c Magnetic resonance imaging (cMRI), etc.); and sufficient clinical research support staff to screen, recruit, enroll, and follow an adequate number of subjects to provide meaningful results.
Network Management
To run a diverse network scattered across thousands of miles requires communication and coordination of effort (Fig. 2). The primary means of communication amongst the network are teleconferences. The Steering Committee (site PIs and Drs Moyé, Skarlatos and Simari) meet as a group biweekly. The entire CCTRN also meets biweekly on alternate weeks. These teleconferences provide a continuing and clear venue for communication and interaction amongst sites and leadership. Additionally, the cell-processing groups, study coordinators, and core labs meet regularly. The entire CCTRN meets face to face at least twice yearly at a different clinical site.
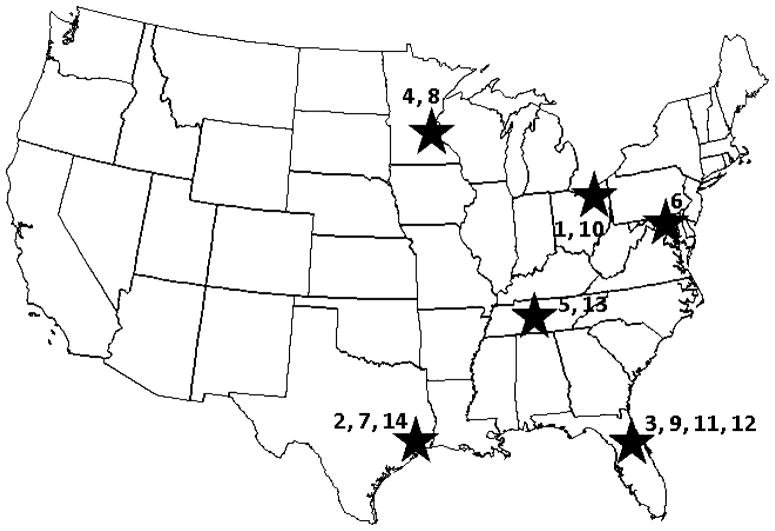
Fig. 2.
Map of CCTRN sites. Clinical Centers: 1 Cleveland Clinic, Cleveland, Ohio; 2 Texas Heart Institute; 3 University of Florida at Gainesville, Gainesville, Florida; 4 Minneapolis Heart Institute, Minneapolis, Minnesota; 5 Vanderbilt University Medical Center, Nashville, Tennessee; 6 Study Sponsor, NHLBI Bethesda, Maryland; 7 Data Coordinating Center, Houston, Texas; 8 Bio-Repository, Minneapolis, Minnesota; 9 Bio-Repository, Gainesville, Florida; 10 Echo Core Lab, Cleveland, Ohio; 11 MRI Core Lab, Gainesville, Florida; 12 MV02 Core Lab, Gainesville, Florida; 13 SPECT Core Lab, Nashville, Tennessee; 14 Cell Processing Quality Control Lab, Houston, Texas
The CCTRN has a website which allows for communication (www.cctrn.org) and a quarterly newsletter. In addition to information for investigators, this website has public access to provide for information regarding the clinical trials as well as information regarding stem cells in general. In addition, a Newsletter is produced discussing scientific and logistical issues.
Selection of Clinical Trials
At the initial meeting of the CCTRN members, each site presented the trials which they included in their response to the RFA. The Steering Committee (consisting of the five site PIs, the PI of the DCC, the NHLBI Program Officer, and the Steering Committee chair) selected the initial trials to pursue following presentation and deliberation. In addition to scientific and clinical criteria, the likelihood of obtaining an IND was highly relevant to the discussion.
At the core of any discussion of the development of new therapies is the identification of the patient population to be targeted. Disparate approaches may be taken. Targeting less sick subjects with less comorbidity allows for perhaps a less obscured view of adverse events and perhaps a clearer view of potential efficacy. Targeting a sicker population without other options or with poor prognoses avoids the exposure of risk to healthier populations. Following initial discussion (and subsequent regulatory review), the CCTRN identified populations with poor prognoses or lack of other options to study.
The CCTRN selected coronary artery disease patients with LV dysfunction (EF ≤45%) as the target population for its three initial trials. These trials explore effects of bone marrow mononuclear cells (BMMNCs) in the acute and chronic stages of the disease. Each of these trials uses SEPAX cell selection after the BM harvest and the primary outcome is assessed at 6 months.
Two trials (TIME and Late TIME) assess effects of cell delivery via stop flow approach (or placebo) following acute myocardial infarction (AMI) among patients with LVD persisting after successful PCI with stenting for a left anterior descending coronary artery-related occlusion. These studies address the hypothesis that delivery of BMMNCs improves global or regional LV function. In the TIME trial, subjects who present with an initial, large anterior MI and receive successful reperfusion are randomized using a two factor design to either cell (150×106) or placebo product delivery at either 3 or 7 days post-MI [19]. In the Late TIME trial, patients are randomized to receive either cells (150×106) or placebo at 14–21 days post-MI. For both trials, the primary measure of interest is LV function determined by cardiac MRI.
The third trial (FOCUS) addresses patients with chronic left ventricular dysfunction persisting >30 days after acute infarction who have no revascularization option. The FOCUS trial aims to deliver BMMNCs to patients, with either limiting HF symptoms (NYHA 2–3) or angina (CCS II–IV) and SPECT reversibility, via an intraventricular myocardial delivery catheter using electromechanical mapping guidance (NOGA). In this trial, patients are randomized 2:1 to either cell (100×106) or placebo product. The primary measure of interest includes a combination of change in MVO2, cardiac volumes, and ischemic burden as measured by SPECT.
Trial Approval Process
Once approved by the CCTRN, each trial was reviewed by a protocol review committee (PRC). The PRC is an independent panel of experts convened by the NHLBI and led by Victor Dzau M.D. (Duke). Once approved, the trials were reviewed by an NHLBI-sponsored Data Safety and Monitoring Board (DSMB) chaired by Keith March M.D. (University of Indiana).
Although the same cellular product is used in each study, the CCTRN requested two INDs from the FDA for the performance of these studies. As TIME and Late TIME share delivery systems and patient populations, they were approved under a single IND. FOCUS was approved as a separate IND. Both INDs are held by University of Texas Health Science Center-Houston with the DCC serving as the sponsor. Finally, each study was reviewed and approved by individual site Institutional Review Boards.
Cells and Cell Preparation
Central to any cell therapy strategy is definition and quality control of the final cellular product to be utilized. Based on the prior experience within the clinical sites (under site held INDs) and the growing worldwide experience, BMMNCs selected for endothelial markers were chosen for each of the three current studies. This choice also simplified isolation and purification issues. Due to the distance between sites, it was decided to isolate cells locally at each site with rigorous oversight from a central Quality Control Cell Processing Lab (QC-CPL; currently directed by Adrian Gee, M.D., Baylor Medical Center, Houston, USA). Local isolation by multiple stem cell processing labs, however, requires uniform methods of harvesting and purification. The CCTRN chose the Sepax System (Biosafe, Switzerland) for BMMNC isolation [20]. This system, which was approved for cord blood processing, is a closed system and allows for faster, more uniform BMMNC isolation. QC-CPL demonstrated that this system reduced variability between runs and increase the yield of CD34+ cells, which include stem and progenitor cells involved in repair of infarcted and ischemic tissue [21]. The Sepax system is used in all three studies.
Network investigators, in concert with the PRC, DSMB, and FDA decided to use a “placebo” (or “cell free control”) arm for each study. The decision to utilize a placebo is balanced between its clarifying influence on the attribution of differences in patient outcomes, on the one hand, and the potential for harm to subjects on the other. For Time and LATE TIME, it was decided to utilize a novel method of blinding for placebo delivery. BMMNCs prepared for delivery have a quite different appearance than albumen. For this reason, we developed an approach by where 100 μ of autologous blood is added to the 5% human serum albumen placebo to generate a similar appearance to the study cell product (Fig. 3).
Fig. 3.
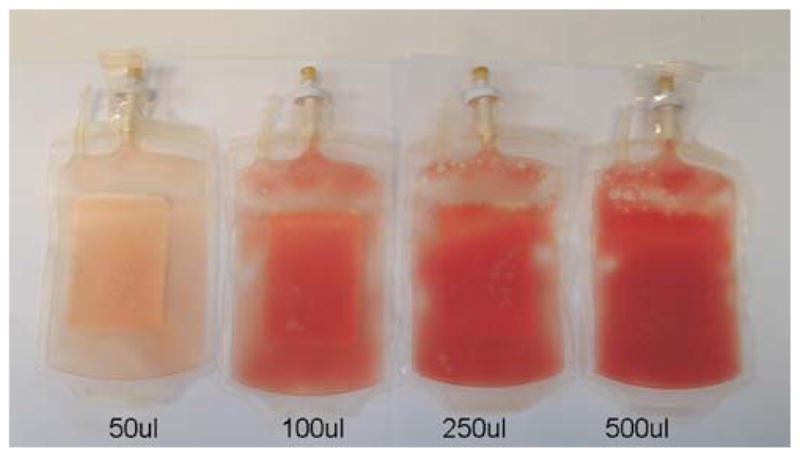
Placebo preparation. Autologous blood is added to 5% serum albumen to create a blinded preparation
Development of Biorepository
The purpose of the biorepository is to address an immediate unmet clinical need in cardiovascular research: to develop mechanistic understanding of cell-mediated repair. The biorepository is a joint effort between the University of Minnesota’s Center for Cardiovascular Repair and the University of Florida’s program in stem cell biology and regenerative medicine (UF). The specific aim of the biorepository within current CCTRN trials is to examine the relationship between cell therapy clinical outcomes and cell characteristics such as phenotype and function. As a core laboratory, the biorepository
provides storage of critical biomaterials from patients enrolled in CCTRN trials;
provides long-term integrity (up to 10 years) of these specimens;
provides phenotypic and functional analyses of bone marrow or blood samples freshly obtained from patients.
This is the first prospective analysis of both phenotype and function in the “active agent”-administered bone marrow in a cardiovascular cell therapy trial; and represents a further prospective analysis of changes in circulating cells and biomarkers in response to cell or placebo treatment.
The biorepository provides standardized analyses of bone marrow and peripheral blood samples for current and future ancillary studies within the CCTRN in accordance with prospective identification of cell characteristics. Because a sample of cells from each patient is stored, as new biomarkers, cytokines/chemokines, signaling molecules, and other potentially important elements become available, it will be possible to evaluate cell characteristics from all enrolled patients. Moreover, these bone marrow and peripheral blood characteristics after AMI can be assessed in context to study subject demographics (e.g., age, gender, and smoking) as well as clinical outcomes after injection (e.g., changes in wall motion and myocardial perfusion). This information and correlative studies should enrich the understanding of cell-mediated repair in multiple NHLBI-funded trials under the auspices of the network. The initial experience of these studies have been accepted for presentation at AHA 2009 Scientific Sessions in Orlando in November 2009.
Development of Satellites
Immediately upon the initiation of recruitment to research protocols, the network turned its attention to the development of satellites. Their goal is to develop these sites to bolster recruitment, diversify the trial population, and embed the network in the local research community in a fiscally conservative climate while ensuring all regulatory and safety requirements are met. Each satellite site will be linked to a primary clinical site and will recruit to studies in which cell delivery may be performed locally, and each satellite site will have its own independent research staff and investigators.
A completed application to add satellite sites is submitted to the Steering Committee (SC) by the petitioning center for evaluation in accordance with the terms of the CCTRN Satellite Site Agreement. Because of the prominence of cell processing in network protocols, access and utilization of designated clinical site cell processing resources is scrutinized. Satellite PIs and staff will be welcomed to participate (ex officio) in all SC activities. Regulatory compliance, including quality control review of regulatory documents and data submissions, site monitoring, fielding site’s questions, document control, and training and certification oversight will be performed by the central DCC with support and coordination of the clinical center. At this time, three satellites are actively screening and recruiting patients for the network.
Development of Cores
Response variables of interest for the protocols are image or procedure-based and require precision to minimize sample size and conserve resources. Core laboratories were established for these variables. The Echo Core Laboratory (Cleveland Clinic) and Cardiac MRI Core Laboratory (University of Florida) were established to provide service to all protocols. SPECT core laboratory (Vanderbilt University) and MV02 Core Laboratory (University of Florida) were established to provide specific service to the heart failure protocol. Each of these laboratories contributes data to a central database at the Coordinating Center, allowing their data to be integrated with the clinical data of the network.
Clinical Research
Training Cores
Over the past several years, considerable progress has been made relative to new knowledge in the field of cardiovascular (CV) regenerative medicine using cell therapy. In order to provide new investigators for this exciting field, specialized programs for the development of this future workforce are needed. To address this need, the CCTRN provided funding for two training cores: the University of Florida and Vanderbilt University (VN). The one at UF has been active for 2 years and its program is summarized below.
The goal of the CCTRN training core at UF is to empower young physician-scientists with knowledge and skills necessary for conducting pre-clinical research and early phase clinical trials in regenerative CV medicine though a structured training program (locally known as the UF CV Cell Therapy Scholars Program). Candidates for this training program include post-doctoral fellows and junior faculty (MD, MD/PhD, and PhD degrees) committed to a clinical research career in academic medicine. Candidates must be willing to commit at least 1 year full time to this training. Additionally, CV Cell Therapy Scholars who are fellows-in-training (CV medicine or CT surgery, vascular surgery, etc.) will be expected to continue research training, part time, when they return to complete their clinical fellowship training. The ultimate goal is that these scholars will each bring a novel idea related to CV cell therapy to an early phase clinical trial.
The goal of the CCTRN training core at VN is to provide a structured research apprenticeship with individualized mentored training with protected time for research and collaboration between basic and clinical scientists. Training core participants attend bi-weekly regenerative medicine work group meetings, grand rounds, cardiac cell therapy seminars and didactic courses. They also have formal career development through professional development plans, quarterly feedback meetings with mentor, and specific review of fulfillment of professional objectives. Participants in this program include clinical fellows, postdoctoral research fellows, residents, and PhD/MD or PhD students. Training core participants rotate through a variety of units depending on their research interests, these include: the stem cell transplantation unit, the cardiac catherization laboratory for intracoronary and intramyocardial delivery of cells, the cardiac MRI unit for optimal ascertainment of cardiac MRI, and basic science labs for stem cell research.
We believe that these training programs not only provide a high quality educational value but, in addition, directly address one of the most pertinent needs for the field of CV cell therapy: the lack of qualified personnel to support the growing portfolio of research (basic, translational, and clinical). The ultimate goal is that these scholars will each bring a novel idea related to CV cell therapy to an early phase clinical trial. Notably, targeted postgraduate programs have been successfully developed in related areas (i.e., gene therapy), which recognized that trained personnel are vital for a successful research mission.
Conclusion
Momentum for the development of cell therapy in cardiovascular disease accelerates with the development of guidelines [22] and delivery systems [23]. The Cardiovascular Cell Therapy Research Network was established by the NHLBI to develop, coordinate, and conduct multiple collaborative protocols testing the effects of stem cell therapy on cardiovascular disease. The network builds on contemporary findings of the cell therapy basic science community, translating this newly acquired information to the cardiac clinical setting in the phase I/II study paradigm. The network consists of five clinical research centers, a data coordinating center which provides trial management and data analysis, a cell processing quality control center, and six core laboratories. Together, these network components provide standardization of cell therapy preparation and endpoint measurements. By recruiting from multiple centers, the network accelerates the speed with which its studies can be completed, increases the generalizability of study findings, and amplifies the dissemination of its public health findings.
Contributor Information
Robert D. Simari, Email: simari.robert@mayo.edu, Mayo Clinic College of Medicine, 200 First Street SW, Rochester, MN 55905, USA
Lemuel A. Moyé, University of Texas, School of Public Health, Houston, USA
Sonia I. Skarlatos, National Heart Lung and Blood Institute, Bethesda, USA
Stephen G. Ellis, Cleveland Clinic Lerner College of Medicine, Cleveland, USA
David X. M. Zhao, Vanderbilt Heart and Vascular Institute, Nashville, USA
James T. Willerson, Texas Heart Institute, Houston, TX, USA
Timothy D. Henry, Minneapolis Heart Institute Foundation, Minneapolis, USA
Carl J. Pepine, University of Florida, Gainesville, USA
References
- 1.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson S, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 2.Kocher A, Schuster M, Szaboles M, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone marrow-derived angioblasts preventscardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nature Medicine. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 3.Jackson K, Majka S, Wang H, Pocius J, Hartley C, Majesky M, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. Journal of Clinical Investigation. 2001;107(11):1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrami A, Konrad UK, Kajstura J, Shao-Min S, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. New England Journal of Medicine. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 5.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOP-CARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 6.Wollert K, Meyer G, Lotz J, Ringes LS, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST randomized controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 7.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. New England Journal of Medicine. 2006;355(12):1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 8.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 9.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. New England Journal of Medicine. 2006;355(12):1199–1210. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 10.Assmus B, Honold J, Schächinger V, Britten M, Fischer-Rasokat U, Lehmann R, et al. Transcoronary transplantation of progenitor cells in acute myocardial infarction. New England Journal of Medicine. 2006;355(12):1222–1233. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Latif A, Bolli R, Tleyjeh I, Montori V, Perin E, Hornung C, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Archives of Internal Medicine. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 12.Murry C, Soonpaa M, Reinecke H, Nakajima H, Rubart M, Pasumarthi K, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 13.Balsam L, Wagers A, Christensen J, Kofidis T, Weissman I, Robbins R. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 14.Skarlatos S. New programs for gene- and cell-based therapies at NHLBI. Clinical Pharmacology and Therapeutics. 2007;82(3):334–336. doi: 10.1038/sj.clpt.6100289. [DOI] [PubMed] [Google Scholar]
- 15.Kiernan T, Boilson B, Sandhu G, Lennon R, Roger V, Barsness G, et al. Nonrevascularizable coronary artery disease following coronary artery bypass graft surgery: A population-based study in Olmsted County, Minnesota. Coronary Artery Disease. 2009;20(2):106–111. doi: 10.1097/MCA.0b013e3283239819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinchilli V, Drazen J, Fish J, Lemanske R, Jr, Lazarus S, Martin R. The clinical trials in the initial five-year award period of the Asthma Clinical Research Network. Controlled Clinical Trials. 2001;22:126S–134S. doi: 10.1016/s0197-2456(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 17.Kephart D, Chinchilli V, Hurd S, Cherniack R Asthma Clinical Research Network. The organization of the Asthma Clinical Research Network: a multicenter, multiprotocol clinical trials team. Controlled Clinical Trials. 2001;22:119S–125S. doi: 10.1016/s0197-2456(01)00161-1. [DOI] [PubMed] [Google Scholar]
- 18.Denlinger L, Sorkness C, Chinchilli V, Lemanske R. Guideline-defining asthma clinical trials of the NHLBI ACRN and CARE networks. Journal of Allergy and Clinical Immunology. 2007;119(1):3–13. doi: 10.1016/j.jaci.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traverse J, Henry T, Vaughan D, Ellis S, Pepine C, Willerson J, et al. Rationale and design for TIIME: a phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. American Heart Journal. 2009;158:356–363. doi: 10.1016/j.ahj.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aktas M, Radke T, Strauer B, Wernet P, Kogler G. Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cytotherapy. 2008;10(2):203–211. doi: 10.1080/14653240701851324. [DOI] [PubMed] [Google Scholar]
- 21.Cogle C, Wainman D, Jorgensen M, Guthrie S, Mames R, Scott E. Adult human hematopoietic cells provide functional hemangioblast activity. Blood. 2004;103(1):133–135. doi: 10.1182/blood-2003-06-2101. [DOI] [PubMed] [Google Scholar]
- 22.Nelson TJ, Behfar A, Terzic A. Stem cells: biologics for regeneration. Clinical Pharmacology and Therapeutics. 2008;84:620–623. doi: 10.1038/clpt.2008.146. [DOI] [PubMed] [Google Scholar]
- 23.Bartunek J, Sherman W, Vanderheyden M, Fernandez-Aviles F, Wijns W, Terzic A. Delivery of biologics in cardiovascular regenerative medicine. Clinical Pharmacology and Therapeutics. 2009;85:548–552. doi: 10.1038/clpt.2008.295. [DOI] [PubMed] [Google Scholar]