Abstract
Interactions with the extracellular matrix (ECM) play an important role in regulating cell function. Cells cultured in, or on, three-dimensional ECM recapitulate similar features to those found in vivo that are not present in traditional two-dimensional culture. In addition, both natural and synthetic materials containing ECM components have shown promise in a number of tissue engineering applications. Current materials available for cell culture and tissue engineering do not adequately reflect the diversity of ECM composition between tissues. In this paper, a method is presented for extracting solutions of proteins and glycoproteins from soft tissues and inducing assembly of these proteins into gels. The extracts contain ECM proteins specific to the tissue source with low levels of intracellular molecules. Gels formed from the tissue-derived extracts have nanostructure similar to ECM in vivo and can be used to culture cells as both a thin substrate coating and a thick gel. This technique could be used to assemble hydrogels with varying composition depending upon the tissue source, hydrogels for three-dimensional culture, as scaffolds for tissue engineering therapies, and to study cell–matrix interactions.
Introduction
Cell behavior is mediated by complex three-dimensional (3D) physical and biochemical interactions between cells and their extracellular microenvironment.1,2 Historically, two-dimensional (2D) culture systems have been used to gain insight into cell–extracellular matrix (ECM) interactions; however, cell responses to ECM function differently in 2D versus 3D systems.1,3–5 Cells cultured in, or on, ECM gels recapitulate many of their in vivo functions as they are exposed to physical and mechanical conditions that more closely approximate the in vivo environment.4
ECM-based biomaterials have played a prominent role in tissue engineering and regenerative medicine. Clinically successful tissue regeneration has been observed with collagen-based materials, small intestine submucosa, and decellularized tissues. Additional ECM materials that have been used for cell culture and preclinical tissue applications include tumor-derived Matrigel™ (BD Biosciences, Franklin Lakes, NJ) and synthetic ECM mimics. While some ECM-like properties can be engineered into synthetic materials, the properties of these materials are different from ECM in both their physical and biological complexity. Pure ECM components, including collagens, fibrin, and laminin-111, are widely used in 3D culture systems, but the diversity of ECM composition and structure is not present in these one-component models.
A commonly used 3D culture substrate is Matrigel, a matrix extract consisting primarily of specialized ECM proteins that make up basement membranes (BMs). BMs are functionalized layers of ECM that separate various cell types, including endothelial and epithelial cells, from the tissue stroma and play a significant role in regulating cell function. BMs are comprised of different isoforms of laminin, collagen, nidogen/entactin, and proteoglycans.6,7 Laminins are glycoproteins consisting of α, β, and γ chains assembled into a cross-shaped heterotrimer that play an important role in BM function. Laminin isoforms vary with each tissue and its developmental stage. The currently accepted laminin nomenclature defines heterotrimers by sequential Arabic numerals based on their chains (e.g., laminin α1, β1, and γ1 chains are denoted as laminin-111).8 Matrigel (also known as reconstituted BMs, laminin-rich ECM,5 and Cultrex)9 is a commercially available product consisting of some BM proteins at levels and ratios similar to those found in vivo.6,10 This solution of proteins is extracted from a spontaneously occurring mouse tumor, the Engelbreth-Holm-Swarm sarcoma,9,11 and assembled into a fibrous gel network at 37°C. These gels have been used in studies of cell migration, angiogenesis, tumor invasion, and tissue engineering.10,12 While sometimes referred to as laminin-rich ECM, the only laminin isoform present in Matrigel is laminin-111. This isoform plays an important role in early embryogenesis, but is not expressed in most mature organs. In adult tissues and organs, other laminin isoforms influence cell behavior. In terms of laminin composition, Matrigel does not approximate BM composition in most tissues.
Complex ECM structures from decellularized tissues have also been used extensively in tissue regeneration and reconstruction. These acellular biodegradable scaffolds promote tissue regeneration by simulating the biomechanical and physical architecture of the tissue. Small intestine submucosa is an ECM-rich material from porcine small intestine that is used for the regeneration of bladder tissue.13 Other xenogeneic ECMs have been under investigation as potential scaffolds for bone regeneration,14 skin reconstruction,15 bioengineered vessel grafts,16 and urologic tissue engineering.13 While these materials have been applied in a number of clinical situations, there are only a limited number of tissues that are available in a decellularized form.
The main objective of this study was to develop a method that allows for the isolation and reconstitution of protein and glycoprotein solutions from soft tissues into 3D fibrous networks for cell culture and tissue engineering applications. The composition of the extracted ECM varies with tissue source and contains minimal intracellular contaminants. The 3D nanostructure of the gels is similar to ECM in vivo, and the gels support integrin-mediated cell growth and endothelial cell (EC) differentiation. The technique described here could be used to design tissue-specific 3D models of cellular morphogenesis, scaffolds for tissue engineering therapies, or environments for guided stem cell differentiation. The gels could be used as systems analogous to Matrigel-based models, allowing investigation of tissue-specific ECM phenomenon. The method is described in sufficient detail to be applied in any lab for isolation and gelation of tissue-derived ECM-rich hydrogels.
Materials and Methods
Dermis and fat samples were harvested from the dorsal skin and subcutaneous fat, respectively, of male Long-Evans (pigmented) rats. Human glioblastoma multiforme (U87) and squamous carcinoma xenografts (SCC-61) were harvested from nude mice. Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Walkersville, MD).
Extraction of ECM proteins
Tissues were dissected, cut into small sections (1–2 mm in thickness), and suspended in dispase solution (Sigma, St. Louis, MO) at 2 mL/g of tissue and incubated for 15 min at 4°C. The sections were rubbed over a cell sieve to separate the intact cells from the remaining tissue. The tissues were homogenized in a high salt buffer solution (0.05 M Tris pH 7.4, 3.4 M sodium chloride, 4 μM of ethylenediaminetetraacetic acid, and 2 μM of N-ethylmaleimide) containing protease inhibitors (0.001 mg/mL pepstatin, 0.01 mg/mL aprotonin, 0.001 mg/mL leupeptin, 2 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride [Sigma]). The homogenized mixture was centrifuged three times at 7000 g for 15 min, and supernatant discarded. The pellet was incubated in 2 M urea buffer (0.15 M sodium chloride and 0.05 Tris pH 7.4) at 1 mL of buffer/g of tissue and stirred overnight at 4°C. The mixture was then centrifuged at 14,000 g for 20 min, and the supernatant stored, while the pellet was resuspended in the urea buffer. The pellet was homogenized and centrifuged again at 14,000 g for 20 min. The supernatants from both centrifugation steps were used in the following experiments.
Gelation kinetics
A SPECTRAMAX absorbance microplate reader with SOFTMAX PRO (Molecular Devices, Menlo Park, CA) was used to measure absorbance (360 nm) as a measure of gel formation.17 Extracts were induced to form gels by the addition of acetic acid or incubation at 37°C. For the temperature-induced gelation all extract solutions were initially at 4°C. The solutions were placed into a 37°C plate reader incubator at time 0 of the kinetic reading.
DAPI staining and DNA assays for decellularization assessment
Tissues were stained with DAPI before and after decellularization and imaged under a fluorescence microscope. Hoechst dye (Molecular probes, Carlsbad, CA) was used to assess DNA content in the extracts. HUVECs lysed with 10% Triton-X and radio immunoprecipitation assay (RIPA) buffer were used as positive controls. Equal volumes of Hoechst dye (diluted 1:1000) were used for each sample, and solutions were incubated for 15 min at 37°C. The dye was detected using 360 nm excitation and 460 nm emission wavelengths. Additionally, PicoGreen DNA quantification kit (Molecular probes) was used to determine the DNA levels in dispase-treated versus untreated ECM extracts. The dye was detected using 480 nm excitation and 520 nm emission wavelengths.
Western blot analysis was performed for β-actin as an additional measure of intracellular contamination. Extracts were suspended in sodium dodecyl sulfate sample buffers and run on a 16% polyacrylamide gel in the presence of 5% β-mercaptoethanol. Proteins were transferred to a nitrocellulose membrane and incubated overnight with a rabbit monoclonal antibody against β-actin (Abcam, Cambridge, MA) diluted 1:5000. The membrane was incubated with a secondary antibody goat anti-rabbit horseradish peroxidase (Bio-Rad, Hercules, CA) diluted 1:5000. Immunoreactive proteins were detected using enhanced chemiluminescent western blotting substrate Super Signal (Pierce, Rockford, IL).
Composition analysis
Bicinchoninic acid (BCA) assays (Pierce) were used to determine total protein concentration. Sircol™ and Blyscan™ assays (Biocolor, Belfast, UK ) were used to determine the concentrations of collagens and sulfated glycosaminoglycans (sGAGs), respectively, in the extracts. The concentration of glycoproteins, including proteoglycans, in the extracts was determined using the glycoprotein isolation kit conA (Pierce). Total protein concentrations before and after glycoprotein isolation were analyzed using BCA protein assays.
Immunoblot analysis
Western blot analyses were performed to identify and characterize the presence of BM proteins in the extract solutions. Protein extracts were suspended in sample buffer with 5% β-mercaptoethanol and run on 10% and gradient 4–12% polyacrylamide gels. Primary and secondary antibodies were added and the proteins detected as described above. The following antibodies and dilutions were used: rabbit anti-collagen type IV (1:5000; Abcam), rabbit anti-laminin β3 (1:1000), rabbit anti-fibronectin (1:1000), rabbit anticollagen VII (1:1000), and rabbit anti-nidogen (1:500; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibody used was goat anti-rabbit (1:10,000; Bio-Rad).
ELISA analyses
Proteins in the extracts were further verified using indirect ELISAs. Fifty microliters of extracts diluted to a final concentration of 10 μg/mL was incubated at 4°C overnight in high absorbance 96-well plates (Nunc, Rochester, NY). The wells were washed and then blocked overnight with 5% milk in tris buffered saline (TBS). The wells were washed with TBS and incubated with the primary antibody for 2 h at room temperature. The primary antibodies used were the same as for western blot analysis and a rabbit anti-laminin α4 chain (a gift from Dr. Miner J. from Washington University in St. Louis). After incubation, the plate was washed and the antigen–antibody complex identified with an enzyme conjugated secondary antibody (goat anti-rat [Pierce] or goat anti-rabbit IgG (H + L) [Bio-Rad] depending on the primary antibody host). The plates were incubated for 45 min at room temperature. Tetramethylbenzidine (Sigma) solution was then added and incubated for 10 min before 2 N HCl was added to stop the reaction. Absorbance was measured at 450 nm.
Electron microscopy
Gels were prepared for scanning electron microscopy (SEM) by fixation in 4% glutaraldehyde prepared in a 0.1 M cacodylate buffer. The samples were rinsed twice and placed in 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h. The samples were then processed by dehydration through an ethanol series and critical point dried with CO2 (Polaron E 3000, Quorum Technologies Ltd, East Sussex, United Kingdom). The samples were sputter coated with gold/platinum (Polam, LOMO America Inc., Northbrook, IL) and imaged under a high-resolution scanning electron microscope (Hitachi, Pleasanton, CA). Gels were fixed in glutaraldehyde and analyzed using both SEM and transmission electron microscopy (TEM). Digital EM images were analyzed using Axiovision 4.3 software (Carl Zeiss MicroImaging, Thornwood, NY).
Cell culture
HUVECs and 3T3 fibroblasts (Cambrex, Walkersvile, MD) were seeded (5000 cells/well) on 96-well plates coated with 0, 0.1, and 1 mg/mL of protein extracts. The cells were suspended in 200 μL of growth medium (Lonza, Valais, Switzerland) and cultured for 7 days. The plates were processed every 2 days using MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay (Promega, Madison, WI). Hank's balanced salt solution was added to each well with 20 μL of the MTS solution and incubated for 1–3 h at 37°C. Absorbance was measured at 490 nm.
EC network formation was investigated using a vasculogenesis assay. Gels were formed by placing 50 μL/well of ECM extract in a 96-well plate and incubating the solution for 1–4 h at 37°C. Subsequently, 2 × 104 HUVECs, a cell number commonly used for Matrigel morphogenesis assay,18,19 were seeded on the ECM gels. The cells were incubated at 37°C overnight and imaged 24 h later. In blocking assays, HUVECs were incubated for 15 min at 37°C in medium containing functional blocking antibodies directed against α6 (clone GoH3 at 50 μg/mL) and α3 (clone P1B5 diluted 1:20) integrins (Chemicon, Temecula, CA) before seeding on the gels. The number of sprouts formed and the length of the sprouts were measured using Axiovision program.
Invasion assay
Cell spheroids were produced by suspending NIH 3T3 fibroblasts (10,000 cells/well) in maintenance media (Dulbecco's modified Eagle's medium, 10% fetal bovine serum, and 1% penicillin–streptomycin) containing 0.25% (w/v) carboxymethylcellulose (Sigma-Aldrich, St. Louis, MO). Cells were seeded in nonadherent round-bottom 96-well plates (Griener, Frickenhausen, Germany) and incubated for 24 h at 37°C, 5% CO2. After the spheroids formed, they were removed from the 96-well plate and placed into the gels (six gels/group). Cell spheroids were placed into dermis-temperature gels and Matrigel (150 μL/well in a 96-well plate). Media was changed every other day, and gels were imaged daily for 7 days to monitor fibroblast invasion. The distance of invasion into the gels was measured using Axiovision program.
Statistical analysis
One-way ANOVA with Tukey Kramer post hoc tests (SigmaStat 3.5) were used for analysis between groups (each group contains at least n = 4), where p < 0.05 was considered statistically significant.
Results
Extraction and composition
Solutions were extracted from multiple tissues, including rat dermis, rat subcutaneous fat, and human glioblastoma (U87) and squamous cell carcinoma (SCC61) xenografts. The composition of the tissue extracts varied with the tissue source. Two tissue types, dermis and fat, were analyzed extensively using western blots and ELISAs for general ECM compounds and BM proteins known to be present within the respective tissues (Table 1). Although collagens, sGAGs, and proteoglycans were present in all extracts, the relative levels of these components varied. Collagen content in the fat and dermis extracts was statistically higher than Matrigel. The levels of sGAGs were significantly higher only in fat-derived extracts (Table 1). Glycoproteins, including proteoglycans, were slightly lower in the dermis- and fat-derived extracts when compared to Matrigel; however, no statistical difference was found (Table 1). Additionally, extracts contained significant levels of nidogen/entactin and collagen type IV (Fig. 1A, B), and ubiquitous BM proteins that contribute significantly to assembly of BMs into macromolecular complexes. Only dermal extracts contained the β3 chain of laminin (Fig. 1D), which is a subunit of laminin 332 (laminin-5) that plays an important role in epidermal–dermal junctions and skin homeostasis.20 Fat BMs contained significant levels of the α4 chain of laminin 411 (laminin-8) and fibronectin (Fig. 1C). Little is known about laminins in adipose tissue, but the presence of laminin 411 in fat BMs may be associated with the extensive vasculature in fat tissues.21 Fibronectin is known to be present in BMs of adipose tissues, particularly in areas rich in preadipocytes (Fig. 1C).22,23 Matrigel contained only laminin chains associated with laminin-111. These results indicate that the composition of the extracts varies with the tissue source and are distinct in composition from Matrigel (Table 1).
Table 1.
Summary of the Composition of Dermis and Fat Extracts and Matrigel
| Dermis | Fat | Matrigel | |
|---|---|---|---|
| % ECM/total protein concentration | |||
| sGAGs | 0.40 ± 0.08% | 2.80 ± 0.36%a | 0.29 ± 0.26% |
| Collagens | 59.02 ± 5.61%a | 59.27 ± 7.18%a | 38.90 ± 14.76% |
| Glycoproteins | 26.8 ± 0.4% | 25.4 ± 3.8% | 41.5 ± 2.3% |
| Groups | |||
| Conc. (mg/mL) | 10–17 | 1.8–5.2 | 10–12 |
| Collagen VII | + | − | − |
| Laminin α4 | + | + | − |
| Laminin β3 | + | − | − |
| Collagen IV | + | + | + |
| Nidogen | + | + | + |
| Fibronectin | + | + | − |
Indicates a statistically significant (p < 0.05) difference from Matrigel.
FIG. 1.
Tissue-derived extracts contained different levels of proteins, sGAGs, collagens, glycoproteins, and distinct BM components depending on the tissue source. Example western blots showing that nidogen (A) and collagen type IV (B) were present in dermis, fat, and Matrigel but at varying levels and molecular weights. Fibronectin was present in dermis and fat but not Matrigel (C). Laminin β3 was present in dermis but not any other tissue (D).
Decellularization verification
Tissues were initially subjected to urea extraction without decellularization. However, significant levels of intracellular components were found in the extracts. To address these issues, tissues were exposed briefly to treatment with dispase to minimize contamination by intracellular components before extraction. After dispase treatment, cells were separated from the bulk tissue using a sieve, and cell removal was checked under phase contrast microscopy. DAPI images of the tissue indicate some cell removal from the treated tissues (Fig. 2A, B). Numerous cell nuclei were seen in the tissue before dispase treatment, while limited nuclei were visible after treatment. Reduced contamination with intracellular proteins was further verified by western blots for β-actin (Fig. 2C) and quantitative DNA analysis (Fig. 2D, E). β-Actin, an intracellular protein, was present in the extracts from untreated tissues, but not detectable in extracts from tissues treated with dispase. DNA assay results reveal low levels of DNA present in the ECM-treated extracts (Fig. 2D, E) with statistically lower content than Matrigel (p < 0.001). PicoGreen DNA assay results indicate that intracellular contamination in the tissue-derived extracts is reduced by brief treatment with dispase (Fig. 2E, p < 0.05). Dispase treatment reduced DNA content from 462.2 ± 14.7 to 183.7 ± 10.2 ng/mL.
FIG. 2.
Dispase treatment reduces contamination by intracellular proteins in the ECM extracts. Cells stained for DAPI are visible in tissues before decellularization (A) but are not apparent after dispase treatment (B). (C) Western blots were performed for β-actin as a measurement of cellular contamination. High levels of β-actin were seen in extracts from untreated tissues but not detected in extracts from dispase-treated tissues. (D) High levels of DNA were present in untreated but not in treated tissues. In fact, DNA levels in treated tissues were less than in Matrigel in both the Hoechst assay (D) and PicoGreen assay (E). *Statistically significant difference from negative control. #A statistically significant difference from Matrigel. Significance is defined as p < 0.001.
Gelation
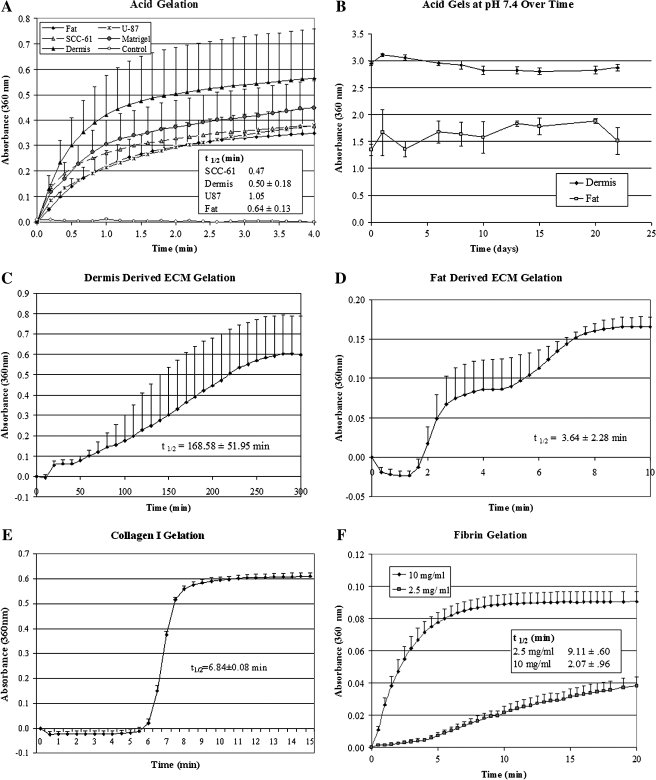
Previous attempts to extract ECM from tissues for cell culture studies have been limited due to the inability of the extracts to form gels. All extracts derived from this procedure rapidly assembled into 3D gels with a reduction in pH to 4.0 (Fig. 3A). This process was not reversed by equilibrating the gels at physiologic pH, and they maintained their structure under cell culture conditions (Fig. 3B). In addition to pH-induced gelation, extracts from adipose and dermis tissues assembled into gels with an increase in temperature to 37°C (Fig. 3C, D). In all cases, the process of temperature gelation was slower than pH-induced gelation. Although the ECM mechanism of assembly in vivo is not clear, it has been suggested that BM assembly in vivo is induced by a reduction in pH at cell surfaces.24,25 These extracts may provide a tool for studying this process. All tissue extracts could be assembled into gels by acid mechanisms of gelation and maintained this structure at pH 7.4 and 37°C.
FIG. 3.
ECM extract solutions gel with a reduction in pH or incubation at 37°C. Kinetic readings of normalized absorbance at 360 nm (a measure of gel formation) for different ECM extracts, including Matrigel, with a change in pH from 7.4 to 4.0 (A). After gelation, the acid gels were equilibrated at pH 7.4 and maintained their structure for a period of 22 days (B). The ECM extracts derived from dermis and fat can be induced to form a gel by increasing temperature to 37°C (C, D). Other ECM gelation kinetics graphs were produced including collagen I (2.0 mg/mL) (E) and fibrin (2.5 and 10 mg/mL concentration (F). The time at which absorbance is half its maximum is given as t1/2.
Nanostructure
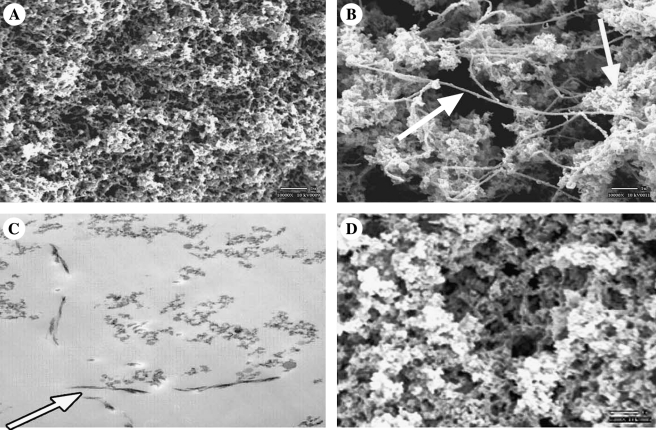
ECMs in vivo exist as a 3D network of fibers.26 The physical structure of the fibers plays an important role in their biological function. Cell behavior is influenced by both the pore and fiber diameters of these networks and their specific 3D orientation.27,28 The structure of gels formed from the protein extracts was examined using SEM and TEM. SEM images (Fig. 4A, B, D) revealed that the gels were made up of an interconnected array of fibers that is similar to the in vivo nanostructure of BMs. Pore and fiber diameters were comparable to literature values of BMs in vivo (Fig. 4 and Table 2) but varied with the mechanism of gelation. Acid-induced gels were more uniform in fiber and pore structure than temperature-induced gels (p < 0.001). Temperature-induced gels had larger mean pore and fiber diameters and showed the presence of two distinct fiber sizes (Fig. 4, arrows). Cross sections of the gels viewed with TEM (Fig. 4C) showed primarily flocculent fiber structure, the characteristic histological feature of TEM of BMs in tissue sections. However, occasional fibrils were observed in the TEM of the temperature-induced gels. Regardless of mechanism, fiber sizes were similar to literature values of Matrigel and fell within ranges for BMs in vivo (Table 2).
FIG. 4.
Tissue-derived ECM extracts form gels with fibrous structure similar to in vivo matrix regardless of gelation mechanism. SEMs of dermis-derived extracts formed by pH-induced (A) and temperature-induced (B) gelation. (C) TEM image of temperature-induced, dermis-derived extract gel showing diffusively flocculent structure similar to that seen in BM structure in vivo. Arrows in (B) and (C) indicate presence of fibril structures distinct to matrix structures in temperature gels. (D) SEM of 3D U87-derived matrix assembled by pH gelation.
Table 2.
Summary of Mean Fiber and Pore Radii Calculated for Dermis- and U87-Derived ECM Extract Gels and Literature Values for Matrigel and BMs In Vivo
| Hydrogel | Fiber radius (nm) | Pore radius (nm) |
|---|---|---|
| U87-derived ECM-pH | 83 ± 25 | 475 ± 253 |
| Dermis-derived ECM-pH | 32 ± 15a | 160 ± 64a |
| Dermis-derived ECM-temperature | 55 ± 14 | 359 ± 143 |
| Matrigel | 35 ± 18 | 53 ± 3523 |
| In vivo (enamel epithelium) | 20–7551 | |
| In vivo (glomeruli) | 2–5 | 10–1339 |
| In vivo (aortic valve) | 16.2 ± 0.5 | 14.3 ± 223 |
| In vivo (bladder) | 26 ± 14 | 41 ± 24.524 |
| In vivo (dermal) | 25–4552 | |
| In vivo (corneal) | 46 ± 16 | 92 ± 3438 |
Indicates a statistically significant (p < 0.05) difference from ECM-temperature.
Cell culture
Progress in understanding cell–matrix interactions in proliferation, invasion, and angiogenesis has largely been driven by the ease of use of Matrigel, fibrin, and collagen in cell culture studies. Three-dimensional culture systems present a microenvironment more similar to that seen in vivo with distinct cell function and signaling mechanisms in comparison to conventional 2D culture.29 Fibroblasts and HUVECs were seeded on tissue culture flasks coated with a thin layer of dermis-derived extracts (0.1 mg/mL) or on 3D gels formed by incubating 1.0 mg/mL of extracts at 37°C. In 2D culture, there was a rapid increase in the number of cells with time, while in 3D culture, readings plateau after 3 days (Fig. 5A, B). These trends were observed for both cell types, agreeing with previous studies in which cell proliferation on soft 3D gels is lower than cells culture on stiff, 2D coated surfaces.30,31
FIG. 5.
Tissue-derived ECM gels reduce cellular proliferation and induce EC network formation. Regardless of cell type cellular proliferation is higher on 2D rather than 3D ECM-rich substrates. Cell numbers of HUVECs (A) and fibroblasts (B) increased rapidly when cells were cultured on 2D coating densities but plateau on 3D gels HUVECs seeded on dermis-derived extracts (C), fat-derived extracts (D), and Matrigel (E) assemble into an interconnected network with structure varying depending on the tissue source. Symbols * and # indicate statistically significant differences from the control and 2D culture, respectively. Significance is defined as p < 0.05.
EC network formation
The organization of ECs into an interconnected network has been observed in the culture of ECs on the surface of many natural gels.32 This phenomenon has been used as a method for evaluating pro- and antiangiogenic properties of soluble and matrix factors. Gels formed from all tissue extracts induced HUVEC assembly into network (Fig. 5C, D, E), but the structure of the network varied with the tissue source, possibly due to varying biochemical composition and mechanical properties of each matrix.27,33,34
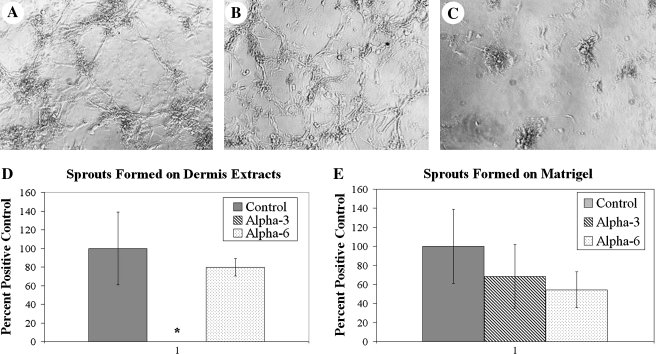
Cell morphogenesis in 3D culture is often used to investigate cell–matrix interactions influencing neovascularization,31 with blocking antibodies used as a first-pass screen of specific integrins contributing to the process.35,36 Integrin blocking assays were performed to investigate which integrins are involved in network assembly on tissue-derived gels. Antibodies to α3 and α6 integrins were added to ECs before seeding on dermis-derived gels and Matrigel. Inhibition of α6 integrin interactions blocked network formation on Matrigel but not on dermis extracts. Inhibition of α3 integrin showed the opposite effect (Fig. 6). When blocking α3 integrin, ECs did not form sprouts when compared to control (without integrin inhibition) and α6 integrin–inhibited wells. These results indicate that the integrins required for network formation vary with the tissue origin of the matrix.
FIG. 6.
Network formation on ECM is mediated by integrins in a tissue-dependent manner. After 24 h of incubation, network formation of HUVECs on dermis-derived extracts (A) without antibody inhibition, (B) in the presence of α6 integrin function blocking antibody (GoH3 50 μg/mL), and (C) in the presence of α3 integrin function blocking antibody (P1B5 diluted 1:20). (D) The percentage of sprouts formed on dermis-derived matrices shows that network formation is inhibited by blocking the α3 integrin but not α6. (E) Network formation on Matrigel is the opposite with inhibition by blocking the α6 integrin but not α3. *Statistically significant difference from the control. Significance is defined as p < 0.05.
Cell invasion
Fibroblast spheroids were implanted into dermis-derived gels and Matrigel to examine 3D cell invasion (Fig. 7). The fibroblasts exhibited more rapid invasion into Matrigel than the dermis gels. By day 7, the fibroblasts had invaded 19.0 ± 4.0 × 105 μm2 of Matrigel and only 2.4 ± 0.7 × 105 μm2 of the dermis gels (p < 0.001). The level of invasion was much lower in the dermis gels but increased over time. At day 7, the levels were significantly higher than day 0 (p = 0.023), day 1 (p = 0.025), day 2 (p = 0.036), and day 4 (p = 0.047). The invasion was both slower in dermis and phenotypically distinct. Fibroblasts invaded Matrigel as interconnected sprouts, while they invaded the dermis gels as individual cells (arrows in Fig. 7).
FIG. 7.
Fibroblasts exhibited more rapid invasion into Matrigel than into dermis gels. Image of spheroid implanted in dermis-derived matrices (A) and Matrigel (B) at day 0. Fibroblasts invade Matrigel with continuous structures (D), while individual cells can be seen invading the dermis-derived gels (C, arrows) (scale bars in all images = 500 μm.) (E) Cells implanted in Matrigel showed an increase in invasion area at each time point, while cells placed in dermis-derived matrices showed an increase in area over day 0 at all time points and over day 1 at days 4, 6, and 7 (*statistical significance, p < 0.05).
Discussion
ECMs are networks of specialized proteins and proteoglycans that play a significant role in modulating cell function.9 The specific composition of the ECM varies with each tissue and due to disease.37 These differences in composition cannot be studied using materials currently available for cell culture. In this paper, we present a method for extracting solutions containing ECM components from soft tissues and inducing the extracts to assemble into gels for use in 3D cell culture and tissue engineering applications. The extracts contain a complex mixture of ECM proteins and proteoglycans, but the composition varies depending on the tissue source. The fibrous structure of the gels is similar in scale and architecture to BMs in vivo. The resultant matrices support tissue-specific, integrin-mediated cell growth and EC network formation.
The tissue extracts contained high levels of BM components. Studies with Matrigel and laminin-111 have contributed to the belief that BM assembly is a temperature-dependent process that occurs once cells secrete a minimum concentration threshold of BM proteins. Despite protein concentrations above these observed thresholds, extracts from only a subset of tissues assembled into gels after incubation at 37°C. The success of temperature gelation did not correlate with overall protein concentration. It is possible that temperature gelation depends on a threshold composition of laminin or other specific component. Interestingly, all extracts assembled into gels after a reduction in pH to ∼4.0. Assembly of laminin-111 triggered by a local acidic pH at the cell surface38 has been suggested as a mechanism to explain observations that in vivo protein levels in BMs may be lower than gelation thresholds observed in vitro.24 Our results potentially agree with this mechanism, as the resultant gels have structure similar to BMs in vivo.39 It is also possible that decreasing pH interferes with the solubility of the protein in urea.40 This technique provides a method for more detailed study into the mechanisms of BM assembly.
Regardless of gelation mechanism, the 3D structure of the gels were similar in structure and scale to in vivo matrices26,27,39,41,42 and could be used for cell culture. The acid gels maintained their structure after equilibration at pH 7.4. The structure and biological activity of the gels43 varies with mechanism of gelation. Temperature-induced gels had larger pore and fiber diameters than acid gels. Whether used for cell culture or tissue engineering, more detailed studies are needed to clarify the relationship between gelation mechanism and physical and biological properties of the resultant gels. The mechanism of gelation may alter gel degradation, the activity of proteins, or transport of molecules through the gels.
One of the most common materials used for 3D culture is Matrigel. While often treated as a model of general ECM behavior, Matrigel does not accurately represent all BMs. It consists primarily of laminin-111, an isoform not present in most human adult tissues.44 ECM extracts from dermis and adipose contained laminin chains other than those in laminin-111. Currently, it is not possible to fully characterize all laminin isoforms present in these complex tissue extracts due to limitations in antibody availability; however, it is clear that laminin environments distinct from any currently available can be assembled using this technique.
Cell morphogenesis in 3D culture is extensively applied as models of physiologic and pathologic processes.45,46 ECs proliferated on low concentrations of ECM extracts, but organized into interconnected networks on all ECM gels. The structure and integrins involved in network formation varied significantly with the extract source. The α6 integrin inhibited sprouts formation on Matrigel, while α3 integrin inhibited network formation on dermis-derived extracts. These results suggest that misleading conclusions may be drawn when using Matrigel as a model of general tissue behavior. Cell culture models of healthy and diseased tissue need to take into account the specific composition and structure of the ECM to understand cell–matrix variations with respect to the tissue site. This technique could be used to generate matrices for studying tissue-specific cell–ECM phenomenon.47–49
The technique proposed in this paper was applied to a number of different tissue sources, and we believe that it could be applied successfully to many other soft tissues. These soft tissue extracts could be used as scaffold materials for tissue engineering and regenerative medicine. This technique described here was derived from that used for isolating Matrigel. While in use for over 25 years,10,11 it is interesting that published techniques using similar approaches to other tissues are rare. It was insightful and fortuitous that Kleinman, Martin, and colleagues used Engelbreth-Holm-Swarm tumors as the source for Matrigel. Its high BM content10 allowed high concentrations of mostly BMs to be extracted that would assemble into gels at 37°C.10 A similar technique has been utilized to extract proteins from skeletal muscle (Myogel) and cartilage (Cartigel) tissues. Myogel formed a gel at 37°C; however, Cartigel had to be suspended in type I collagen gels for 3D cell culture.49,50 Supplementing the extracts with additional ECM components may confound experimental results using this model.49,51 The ECM extracts could be assembled into gels by a pH mechanism that would allow the use of these tissue-specific gels without additional components.
The high cellular content in many tissues hinders using urea solubility directly. A brief dispase treatment was used to remove some of the cells in the tissue before extraction. This technique is not as complicated and effective as clinically applied decellularization procedures and needs to be perfected or altered if ECM extracts will be used clinically.52 Because urea is not likely to lyse cells, contamination probably results from mechanical disruption of cells. The combination of initial cell removal followed by urea extraction greatly reduces contamination by cellular components. Dispase was selected because it is a mild metalloprotease, but it is possible that it results in some damage to the extracted proteins. Westerns reveal multiple protein isoforms for each BM component. This may be a reflection of the multiple isoforms possible for each protein or enzymatic breakdown. Further molecular studies are needed to better understand the effect of dispase on protein integrity. The technique can be used in the absence of dispase treatment, but care must be taken to consider effects from cellular components.
The broad application of 3D models using single ECM components, Matrigel, xenogeneic ECM, and synthetic matrices to study cell–matrix phenomenon suggests many areas where this technique could be applied. It could be used to further our understanding of cell–matrix interactions or the role of specific laminin isoforms in cancer and stem cell research.49,53,54 Applying this technique to the extraction of ECM from human tissues may help avoid concerns with currently used xenogeneic matrices for tissue engineering and regenerative medicine applications. The ability of these extracts to assemble into gels could be exploited to further increase our understanding of the fundamental mechanisms of BM/ECM assembly.
Acknowledgments
The authors would like to thank Dr. Hynda Kleinman for helpful comments, Linda Fox at Loyola University Medical Center for assistance with the electron microscopy, and Dr. Jeffrey H. Miner from Washington University in St. Louis for the generous gift of the antibody to the α4 chain of laminin. This work was supported by the Department of Veterans Affairs (EMB), the National Institutes of Health (RRW, ro1 ca 111423-01a1), the Bill and Melinda Gates Foundation (MLM), the National Science Foundation (EMB, 0052896 and 0731201), and the IIT Educational Research Initiative Fund (EMB).
Disclosure Statement
No competing financial interests exist.
References
- 1.Friedl P. Brocker E.B. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gumbiner B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen J.A. Swartz M.A. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;33:1469. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- 4.Schindler M. Nur E.K.A. Ahmed I. Kamal J. Liu H.Y. Amor N. Ponery A.S. Crockett D.P. Grafe T.H. Chung H.Y. Weik T. Jones E. Meiners S. Living in three dimensions: 3D nanostructured environments for cell culture and regenerative medicine. Cell Biochem Biophys. 2006;45:215. doi: 10.1385/CBB:45:2:215. [DOI] [PubMed] [Google Scholar]
- 5.Nelson C.M. Bissell M.J. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinman H.K. McGarvey M.L. Hassell J.R. Star V.L. Cannon F.B. Laurie G.W. Martin G.R. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 7.Martin G.R. Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- 8.Aumailley M. Bruckner-Tuderman L. Carter W.G. Deutzmann R. Edgar D. Ekblom P. Engel J. Engvall E. Hohenester E. Jones J.C. Kleinman H.K. Marinkovich M.P. Martin G.R. Mayer U. Meneguzzi G. Miner J.H. Miyazaki K. Patarroyo M. Paulsson M. Quaranta V. Sanes J.R. Sasaki T. Sekiguchi K. Sorokin L.M. Talts J.F. Tryggvason K. Uitto J. Virtanen I. von der Mark K. Wewer U.M. Yamada Y. Yurchenco P.D. A simplified laminin nomenclature. Matrix Biol. 2005;24:326. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Kleinman H.K. Philp D. Hoffman M.P. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14:526. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Kleinman H.K. Martin G.R. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Kleinman H.K. McGarvey M.L. Liotta L.A. Robey P.G. Tryggvason K. Martin G.R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 12.Kelly J.L. Findlay M.W. Knight K.R. Penington A. Thompson E.W. Messina A. Morrison W.A. Contact with existing adipose tissue is inductive for adipogenesis in matrigel. Tissue Eng. 2006;12:2041. doi: 10.1089/ten.2006.12.2041. [DOI] [PubMed] [Google Scholar]
- 13.Cheng E.Y. Kropp B.P. Urologic tissue engineering with small-intestinal submucosa: potential clinical applications. World J Urol. 2000;18:26. doi: 10.1007/PL00007071. [DOI] [PubMed] [Google Scholar]
- 14.Petrovic L. Schlegel A.K. Schultze-Mosgau S. Wiltfang J. Different substitute biomaterials as potential scaffolds in tissue engineering. Int J Oral Maxillofac Implants. 2006;21:225. [PubMed] [Google Scholar]
- 15.Bello Y.M. Falabella A.F. Eaglstein W.H. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2:305. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- 16.Walles T. Herden T. Haverich A. Mertsching H. Influence of scaffold thickness and scaffold composition on bioartificial graft survival. Biomaterials. 2003;24:1233. doi: 10.1016/s0142-9612(02)00490-8. [DOI] [PubMed] [Google Scholar]
- 17.Pollard T.D. The role of actin in the temperature-dependent gelation and contraction of extracts of Acanthamoeba. J Cell Biol. 1976;68:579. doi: 10.1083/jcb.68.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodwin A.M. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res. 2007;74:172. doi: 10.1016/j.mvr.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auerbach R. Akhtar N. Lewis R.L. Shinners B.L. Angiogenesis assays: problems and pitfalls. Cancer Metastasis Rev. 2000;19:167. doi: 10.1023/a:1026574416001. [DOI] [PubMed] [Google Scholar]
- 20.McGrath J.A. Pulkkinen L. Christiano A.M. Leigh I.M. Eady R.A. Uitto J. Altered laminin 5 expression due to mutations in the gene encoding the beta 3 chain (LAMB3) in generalized atrophic benign epidermolysis bullosa. J Invest Dermatol. 1995;104:467. doi: 10.1111/1523-1747.ep12605904. [DOI] [PubMed] [Google Scholar]
- 21.Hausman G.J. Richardson R.L. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 22.Haraida S. Nerlich A.G. Wiest I. Schleicher E. Lohrs U. Distribution of basement membrane components in normal adipose tissue and in benign and malignant tumors of lipomatous origin. Mod Pathol. 1996;9:137. [PubMed] [Google Scholar]
- 23.Pierleoni C. Verdenelli F. Castellucci M. Cinti S. Fibronectins and basal lamina molecules expression in human subcutaneous white adipose tissue. Eur J Histochem. 1998;42:183. [PubMed] [Google Scholar]
- 24.Freire E. Coelho-Sampaio T. Self-assembly of laminin induced by acidic pH. J Biol Chem. 2000;275:817. doi: 10.1074/jbc.275.2.817. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y.S. Champliaud M.F. Burgeson R.E. Marinkovich M.P. Yurchenco P.D. Self-assembly of laminin isoforms. J Biol Chem. 1997;272:31525. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- 26.Brody S. Anilkumar T. Liliensiek S. Last J.A. Murphy C.J. Pandit A. Characterizing nanoscale topography of the aortic heart valve basement membrane for tissue engineering heart valve scaffold design. Tissue Eng. 2006;12:413. doi: 10.1089/ten.2006.12.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrams G.A. Murphy C.J. Wang Z.Y. Nealey P.F. Bjorling D.E. Ultrastructural basement membrane topography of the bladder epithelium. Urol Res. 2003;31:341. doi: 10.1007/s00240-003-0347-9. [DOI] [PubMed] [Google Scholar]
- 28.Kwon I.K. Kidoaki S. Matsuda T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: structural characteristics, mechanical properties and cell adhesion potential. Biomaterials. 2005;26:3929. doi: 10.1016/j.biomaterials.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Even-Ram S. Yamada K.M. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Gruber H.E. Hanley E.N., Jr. Human disc cells in monolayer vs 3D culture: cell shape, division and matrix formation. BMC Musculoskelet Disord. 2000;1:1. doi: 10.1186/1471-2474-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubota Y. Kleinman H.K. Martin G.R. Lawley T.J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francis M.E. Uriel S. Brey E.M. Endothelial cell-matrix interactions in neovascularization. Tissue Eng Part B Rev. 2008;14:19. doi: 10.1089/teb.2007.0115. [DOI] [PubMed] [Google Scholar]
- 33.Yurchenco P.D. Tsilibary E.C. Charonis A.S. Furthmayr H. Laminin polymerization in vitro. Evidence for a two-step assembly with domain specificity. J Biol Chem. 1985;260:7636. [PubMed] [Google Scholar]
- 34.Davis G.E. Senger D.R. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 35.Drake C.J. Hungerford J.E. Little C.D. Morphogenesis of the first blood vessels. Ann NY Acad Sci. 1998;857:155. doi: 10.1111/j.1749-6632.1998.tb10115.x. [DOI] [PubMed] [Google Scholar]
- 36.Nisato R.E. Tille J.C. Jonczyk A. Goodman S.L. Pepper M.S. alphav beta 3 and alphav beta 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis. 2003;6:105. doi: 10.1023/B:AGEN.0000011801.98187.f2. [DOI] [PubMed] [Google Scholar]
- 37.Streuli C.H. Bailey N. Bissell M.J. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens M.M. George J.H. Exploring and engineering the cell surface interface. Science. 2005;310:1135. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 39.Osawa T. Feng X.Y. Nozaka Y. Scanning electron microscopic observations of the basement membranes with dithiothreitol separation. Med Electron Microsc. 2003;36:132. doi: 10.1007/s00795-003-0214-3. [DOI] [PubMed] [Google Scholar]
- 40.Bennion B.J. Daggett V. The molecular basis for the chemical denaturation of proteins by urea. Proc Natl Acad Sci USA. 2003;100:5142. doi: 10.1073/pnas.0930122100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abrams G.A. Schaus S.S. Goodman S.L. Nealey P.F. Murphy C.J. Nanoscale topography of the corneal epithelial basement membrane and Descemet's membrane of the human. Cornea. 2000;19:57. doi: 10.1097/00003226-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Edwards A. Daniels B.S. Deen W.M. Hindered transport of macromolecules in isolated glomeruli. II. Convection and pressure effects in basement membrane. Biophys J. 1997;72:214. doi: 10.1016/S0006-3495(97)78660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uriel S. Huang J.J. Moya M.L. Francis M.E. Wang R. Chang S.Y. Cheng M.H. Brey E.M. The role of adipose protein derived hydrogels in adipogenesis. Biomaterials. 2008;29:3712. doi: 10.1016/j.biomaterials.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 44.Ekblom P. Lonai P. Talts J.F. Expression and biological role of laminin-1. Matrix Biol. 2003;22:35. doi: 10.1016/s0945-053x(03)00015-5. [DOI] [PubMed] [Google Scholar]
- 45.Lubarsky B. Krasnow M.A. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 46.Auerbach R. Lewis R. Shinners B. Kubai L. Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 47.Davis G.E. Camarillo C.W. Regulation of endothelial cell morphogenesis by integrins, mechanical forces, and matrix guidance pathways. Exp Cell Res. 1995;216:113. doi: 10.1006/excr.1995.1015. [DOI] [PubMed] [Google Scholar]
- 48.Stahl S. Weitzman S. Jones J.C. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J Cell Sci. 1997;110 (Pt 1):55. doi: 10.1242/jcs.110.1.55. [DOI] [PubMed] [Google Scholar]
- 49.Philp D. Chen S.S. Fitzgerald W. Orenstein J. Margolis L. Kleinman H.K. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23:288. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- 50.Abberton K.M. Bortolotto S.K. Woods A.A. Findlay M. Morrison W.A. Thompson E.W. Messina A. Myogel, a novel, basement membrane-rich, extracellular matrix derived from skeletal muscle, is highly adipogenic in vivo and in vitro. Cells Tissues Organs. 2008;188:347. doi: 10.1159/000121575. [DOI] [PubMed] [Google Scholar]
- 51.Sharma B. Elisseeff J.H. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 2004;32:148. doi: 10.1023/b:abme.0000007799.60142.78. [DOI] [PubMed] [Google Scholar]
- 52.Schaner P.J. Martin N.D. Tulenko T.N. Shapiro I.M. Tarola N.A. Leichter R.F. Carabasi R.A. Dimuzio P.J. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 53.Bissell M.J. Modelling molecular mechanisms of breast cancer and invasion: lessons from the normal gland. Biochem Soc Trans. 2007;35:18. doi: 10.1042/BST0350018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischbach C. Chen R. Matsumoto T. Schmelzle T. Brugge J.S. Polverini P.J. Mooney D.J. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]