Abstract
Tissue engineering and regenerative medicine (TE&RM) approaches to treating liver disease have the potential to provide temporary support with biohybrid-liver-assist devices or long-term therapy by replacing the diseased liver with functional constructs. A rate-limiting step for TE&RM strategies has been the loss of hepatocyte-specific functions after hepatocytes are isolated from their highly specialized in vivo microenvironment and placed in in vitro culture systems. The identification of a biologic substrate that can maintain a functional hepatocyte differentiation profile during in vitro culture would advance potential TE&RM therapeutic strategies. The present study compared two different biologic substrates for their ability to support human hepatocyte function in vitro: porcine-liver-derived extracellular matrix (PLECM) or MatrigelTM. Because Matrigel has been shown to be the most useful matrix for static, traditional hepatocyte culture, we directly compared PLECM with Matrigel in each experiment. Albumin secretion, hepatic transport activity, and ammonia metabolism were used to determine hepatocyte function. Hepatocytes cultured between two layers of PLECM or Matrigel showed equally high levels of albumin expression and secretion, ammonia metabolism, and hepatic transporter expression and function. We conclude that like Matrigel, PLECM represents a favorable substrate for in vitro culture of human hepatocytes.
Introduction
Liver disease is the 11th leading cause of death and results in over 25, 000 deaths per year in the United States.1 Allogeneic liver transplantation is the treatment of choice for patients with end-stage liver disease but is limited by both the high cost and severe shortage of donor organs.2 Both whole-organ xenotransplantation and hepatocyte transplantation provide alternative therapies but have had limited clinical success.3 Tissue engineering and regenerative medicine (TE&RM) approaches for the reconstruction of functional liver tissue are being widely investigated and typically involve the use of cells, scaffolds, and bioactive factors.3–6 The present study is predicated upon the hypothesis that the scaffold or substrate upon which hepatocytes are seeded is a critical determinant of cell phenotype and function.
In vitro culture of hepatocytes is associated with dedifferentiation; decreased hepatocyte-specific functions7 such as albumin secretion, hepatic transport activity, and ammonia metabolism; and loss of hepatocyte polarity. The identification of a substrate that can maintain a functional hepatocyte differentiation profile would represent an advancement toward development of a tissue-engineered liver substitute.
Individual extracellular matrix (ECM) components or combinations of desired ECM components have been used as substrates for hepatocyte culture systems for several decades.8–10 Although cell culture substrates composed of individual ECM proteins such as type-I collagen, laminin, and fibronectin have facilitated primary rat hepatocyte attachment, maintenance of selected liver-specific functions, and preserved hepatocyte polarity and morphology for short periods of culture, these systems are insufficient to prevent dedifferentiation of hepatocytes.11,12
Conventional in vitro culture models for hepatocytes include the use of a sandwich configuration in which hepatocytes are either placed between two layers of type-I collagen and Matrigel (MG) or cultured on type-I collagen overlaid with MG.9,13–16 Sandwich culture of primary hepatocytes maintains hepatocyte polarity and morphology better than hepatocytes cultured on a single layer of type-I collagen or MG.17–19 Further, hepatocyte-specific functions, such as albumin secretion, urea synthesis, and cytochrome P450 responsiveness and inducibility are enhanced when the sandwich culture model is used.9,12,13,15,20–23
We posed as a hypothesis that ECM derived from the liver itself might be a useful substrate for hepatocyte culture. Although matrix derived from human liver might be superior to that from other species for the maintenance of human hepatocyte function because of its availability, we first examined porcine-liver-derived ECM (PLECM). The present study investigated the ability of PLECM to support the following primary human hepatocyte (PHH)–specific functions in vitro: albumin secretion, hepatic transport activity, ammonia metabolism, and the mRNA levels of albumin, bile salt export pump (BSEP), and sodium taurocholate cotransporting polypeptide (NTCP).
Materials and Methods
Overview of experimental design
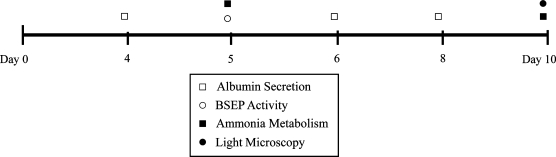
PHHs were harvested from six human donor livers of different age groups, sex, and medical histories. PHH viability was determined by trypan blue exclusion (Table 1). PHHs were cultured either (1) between two layers of MatrigelTM, which is referred to as an MG sandwich, or (2) on adsorbed PLECM overlaid with PLECM gel (PLECM sandwich). Albumin secretion, hepatic transport activity, and ammonia metabolism were evaluated at selected days during a 10-day culture period and were used as indicators of hepatocyte function (Fig. 1). The mRNA levels of albumin, BSEP, and NTCP were quantified.
Table 1.
Donor Information for Human Hepatocyte Preparations
| Donor HH# | Age | Sex | Race | Drug history | Viability (%) |
|---|---|---|---|---|---|
| HH1400 | 21 | M | C | NK | 87 |
| HH1412 | 68 | M | C | Chemotherapy | 76 |
| HH1416 | 79 | F | ND | Chemotherapy | 83 |
| HH1417 | 53 | F | ND | None | 85 |
| HH1419 | 78 | M | ND | None | 79 |
| HH1423 | 51 | M | ND | None | 81 |
M, male; F, female; C, Caucasian; ND, data not provided; NK, no known chronic drug treatment.
FIG. 1.
Schematic of functional assays used to profile PHHs. Timeline represents days in culture. PHHs, primary human hepatocytes.
Reagents
Pepsin, sodium hydroxide, glycerol, dibasic sodium phosphate, monobasic sodium phosphate, dexamethasone, ethylenediaminetetraacetic acid (EDTA), and ethidium bromide were purchased from Sigma Chemical (St. Louis, MO). Matrigel was purchased from Becton Dickinson (Franklin Lakes, OR). Trypsin was purchased from Gibco (Carlsbad, CA). Type-I collagen was purchased from Becton Dickinson. Quant-iT PicoGreen Assay was obtained from Invitrogen–Molecular Probes (Eugene, OR). Triton-X 100 and sodium deoxycholic acid were purchased from Spectrum (Gardena, CA). Hepatocyte maintenance medium (HMM) was purchased from Lonza (Walkersville, MD). Penicillin, streptomycin, and bovine calf serum were purchased from Gibco. Trizol reagent was purchased from Invitrogen (Carlsbad, CA). Hank's balanced salt solution (HBSS) was purchased from Lonza. All quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR) reagents were purchased from Applied Biosystems (Foster City, CA). Cyclophilin, albumin, BSEP, and NTCP were synthesized by Applied Biosystems, and the assay identification numbers are cyclophilin, Hs03045993_gH; albumin, Hs00910222_m1; BSEP, Hs00184824_m1; and NTCP, Hs00161820_m1.
Isolation and preparation of PLECM
The methods used to isolate porcine liver ECM (PLECM) have been previously described.12,24,25 Livers were harvested from market weight pigs (∼110–130 kg) immediately after euthanasia. The tissues were rinsed with tap water and frozen until used. Each liver lobe was cut into 5-mm-thick slices with a rotating blade and subjected to three separate 30-min washes in deionized water with mechanical agitation on an orbital shaker. The sections were then gently massaged to aid in cell lysis and soaked in 0.02% trypsin/0.05% EDTA at 37°C for 1 h. The tissue was rinsed in deionized water, and the massaging was repeated followed by mechanical agitation of the liver sections in 3% Triton X-100 for 1 h. The rinsing and massaging process was repeated until all visible remnants of cellular material were removed. The tissue sections were then mechanically agitated in 4% sodium deoxycholic acid for 1 h followed by extensive rinsing in water. The remaining decellularized connective tissue matrix was referred to as PLECM.12
After processing, the PLECM was immersed in a solution of 0.1% peracetic acid followed by repeated rinses in water or phosphate-buffered saline (PBS) at pH 7.4.
Preparation of PLECM gels
The method used to prepare a gel form of harvested ECM has been previously described.26 The protocol was modified to produce a gel form of PLECM.
First, a powdered form of PLECM was produced from sheets of PLECM. The hydrated, disinfected PLECM was lyophilized overnight. Lyophilized sheets of PLECM were cut into small pieces, then placed in a rotary knife mill (Wiley Mill) to create a particulate form of the PLECM. Particles less than 250 μm were collected by sieve through a #60 screen.
One gram of lyophilized PLECM powder and 100 mg of pepsin (2000–3000 U/mg) were mixed in 100 mL of 0.01 M HCl and stirred for ∼72 h at room temperature (25°C). The final viscous solution of digested PLECM or pregel solution had a pH of approximately 3.0–4.0.
Formation of PLECM gels was initiated by the addition of 0.1 N NaOH (1/10 of the volume of pregel solution) and 10 × PBS at pH 7.4 (1/9 of the volume of pregel solution) at 4°C. Inactivation of pepsin activity occurred when the pH was raised to 7.4. For gelation to occur, the solution was brought to the desired volume/concentration using cold (4°C) 1 × PBS at pH 7.4 and was then placed at 37°C. The prepared PLECM gel had a white color, smooth surface, and uniform consistency (Fig. 2).
FIG. 2.
Macroscopic view of decellularized porcine-liver-derived extracellular matrix (PLECM) tissue is shown in (A). After solubilization and gelation the material has a smooth topography (B, 10 × ) and uniform consistency. The surface of Matrigel is shown for comparison (C, 10 × ). Color images available online at www.liebertonline.com/ten.
Preparation of culture dishes
Plates were coated with PLECM (0.3 mg) and were dried overnight to six-well plates in a laminar flow hood. Plates were sealed in Parafilm and stored at 4°C until use. On the day of the experiment, MG (0.3 mg) was coated onto six-well plates at least 30 min before adding cells.
Isolation and culture of PHHs
Hepatocytes were isolated either from donor livers that were not used for transplantation or from areas of hepatic tissue not involved in tumor from tissue resections for cancer. All human subject protocols were reviewed and approved by the University of Pittsburgh Institutional Review Boards. No significant amounts of steatosis (fat) were observed in any of the livers. Viability of the hepatocytes at the time of plating was greater than 76%, as measured by trypan blue exclusion.
PHHs were isolated by a three-step collagenase perfusion of human liver27 and seeded in six-well plates precoated with either PLECM or MG at a density of 1.5 × 106 cells per well. Hepatocytes were seeded in HMM supplemented with 10−7 M dexamethasone, 10−7 M insulin, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% bovine calf serum. After 4 h of culture at 37°C, the medium was replaced with serum-free HMM with the supplements listed above. After 24 h of culture in serum-free HMM, unattached PHHs were removed by gentle agitation and the following overlays were added: MG substrata were overlaid with 0.3 mg/mL of MG, and PLECM substrata were overlaid with 0.3 mg/mL PLECM gel. The medium was changed every 24 h.
Light microscopy
PHHs were maintained in their respective culture conditions for 10 days. On day 10, PHHs were imaged at 100 × with a light microscope (Zeiss Axiovert 40CFL with Axiocam MRc5, Thornwood, NY).
Measurement of DNA content
Using a cell scraper, cells were harvested on ice in 150 μL cell harvest buffer containing dibasic sodium phosphate (11.5 g/L), monobasic sodium phosphate (2.6 g/L), EDTA (0.033 g/L), and 20% glycerol. The cell lysates were produced by sonication and stored at –20°C. Total DNA was quantified using the commercially available Quant-iT PicoGreen dsDNA Kit according to the manufacturer's instructions. For each group, a minimum of two samples were used to determine total DNA and each samples was measured in triplicate.
Isolation of total RNA
Total RNA from cell lysates was isolated using Trizol reagent as instructed by the manufacturer. RNA concentration was determined by spectrophotometer at 260 nm. The purity of RNA was determined by spectrophotometry using the 280 nm/260 nm ratio, and the integrity checked by agarose gel electrophoresis stained with ethidium bromide. RNA was stored at −80° until further use.
Quantitative real-time RT PCR
mRNA expression levels of albumin (from donors HH1400, HH1412, and HH1423), BSEP (from donors HH1416, HH1417, HH1419, and HH1423), and NTCP (from donors HH1416, HH1417, HH1419, and HH1423) were determined using TaqMan quantitative RT-PCR assays. From each group, 2 μg of total RNA was incubated with 5 U RNA qualified (RQ) DNase, 5 μL 10 × RQ buffer in a total volume of 10 μL at 37°C for 30 min. The samples were then incubated with 1 μL RQ DNase Stop Solution at 65°C for 10 min. The DNAse-treated RNA was then incubated with 1 μL of random hexamer primers (100 ng/L) at 70°C for 5 min for cDNA synthesis. cDNA generation continued with incubation at 37°C for 60 min with the following reagents: 1 μL deoxynucleotide triphosphate (dNTP) (10 mM of each), 5 μL MMLV 5 × , and 1 μL MMLV RT for a total volume of 12 μL.
TaqMan one-step RT-PCR assays were performed with 4 ng of each RNA sample in a final reaction volume of 72 μL prepared from TaqMan one-step RT-PCR Master Mix Reagents Kit. Assays were performed using an Applied Biosystems' ABI Prism 7900HT sequence detection system. An initial RT step occurred for 30 min at 48° and was subsequently followed by heating to 95° for 10 min followed by 40 cycles of 95° for 15 s and 60° for 1 min.
Measurement of albumin secretion
The conditioned medium was collected from donors HH1400, HH1412, and HH1423 on days 4, 6, 8, and 10, and stored at −20°C until analysis. The conditioned medium was not collected from all donor samples because of the limited availability of cells from selected donors. PHH secretion of albumin was measured using a commercially available kit (Bethyl Laboratories, Montgomery, TX). At least three samples were collected from each culture condition and each sample was measured in triplicate. All data were normalized to total DNA content.
Hepatic transport activity assay
Hepatic transport activity was measured for donors HH1416, HH1417, HH1419, and HH1423 as previously described.28 Briefly, on day 5, the medium was aspirated from PHHs and replaced with HBSS containing cations (calcium and magnesium) for 20 min at 37°C. After this incubation period, the HBSS (with cations) was aspirated and replaced with HBSS (with cations) containing 1 μM [3H]taurocholate. After incubation of PHHs with 1 μM [3H]taurocholate for 20 min at 37°C, the solution was aspirated and the PHHs were rinsed three times with ice-cold HBSS (with cations). PHHs were incubated with (1) HBSS containing cations or (2) HBSS without cations for 20 min. The medium was collected, and cells were harvested in 1 mL NaOH/sodium dodecyl sulfate solution using a cell scraper. Each sample (0.5 mL) was then counted using a liquid scintillation counter. The harvested cell solution was stored at −80°C until DNA quantification. At least three samples were collected from each culture condition and each sample was measured in triplicate. All data were normalized to total DNA content.
Ammonia metabolism assay
PHH metabolism of ammonia was measured on day 5 of culture for donors HH1400, HH1412, HH1419, and HH1423. The medium was aspirated and PHHs were incubated with 2.5 mM NH4Cl in HMM for two hours at 37°C. The conditioned medium was collected and the concentration of ammonia nitrogen was measured using a commercially available kit (Wako Pure Chemical Industries, Tokyo, Japan). At least three samples were collected from each culture condition and each sample was measured in triplicate. All data were normalized to total DNA content.
Statistical analysis
All values were calculated as mean ± standard deviation. All data were normalized to PHHs cultured on type-I collagen. A one-way analysis of variance with a post-hoc Tukey's multiple comparison procedure was performed. A p-value of <0.05 was considered statistically significant and all calculations were performed using SAS software v9.1 (Cary, NC).
Results
Effect of ECM on hepatocyte morphology
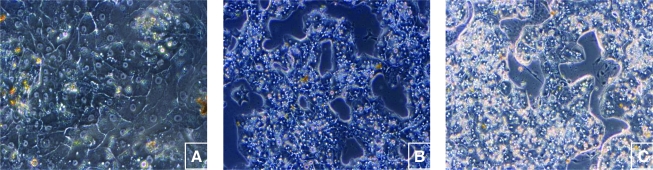
Our standard culture conditions utilize human hepatocytes on culture plates with absorbed type-I collagen (rat tail). We examined the morphology of the cells on PLECM and Matrigel and for these studies also compared the results to that observed on type-I collagen. As expected, hepatocytes cultured on type-I collagen without an overlay were flattened, and formed a confluent cell monolayer (Fig. 3A). In contrast, hepatocytes cultured in an MG (Fig. 3B) or PLECM (Fig. 3C) sandwich formed cord-like arrays in which the cells adhered to each other and pulled together and upward in three dimensions, which then exposed open areas of the culture surface between the hepatic cords (Fig. 3B, C).
FIG. 3.
PHHs maintained for 10 days under different ECM conditions (100 × ). PHHs were cultured (A) on type-I collagen; (B) between two layers of Matrigel; (C) or between two layers of PLECM. PHHs cultured in a Matrigel (MG) or PLECM sandwich formed chord-like arrays. Color images available online at www.liebertonline.com/ten.
DNA content
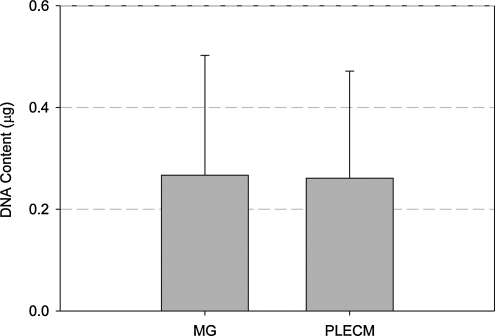
The relative attachment of hepatocytes to the different substrates was evaluated by determining the total amount of DNA recovered from each culture condition (Fig. 4). The total amount of DNA recovered from the cells plated in either an MG or a PLECM sandwich was not significantly different (Fig. 4). Thus, the cells showed similar attachment or plating efficiency on PLECM and Matrigel.
FIG. 4.
Mean DNA content of cell cultured from six livers at the time of final harvest (Day 10). Values represent mean ± standard deviation (SD).
Albumin mRNA expression
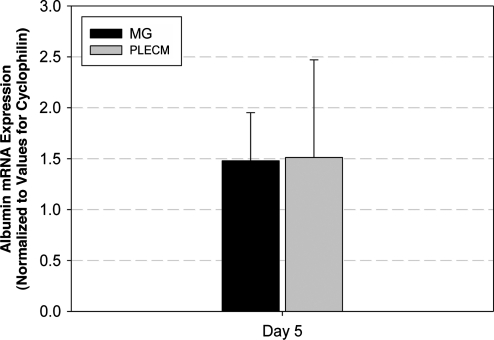
On day 5 of culture, mRNA levels of albumin were determined. Albumin mRNA amount from each culture condition was averaged and normalized to an internal control gene, cyclophilin. Albumin mRNA amounts of PHHs cultured in an MG or PLECM sandwich were identical (Fig. 5).
FIG. 5.
Average albumin mRNA expression of PHHs cultured in an MG or PLECM sandwich at day 5. Values represent mean ± SD of three livers.
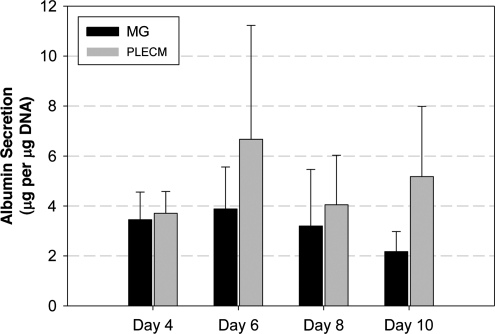
Albumin secretion
Albumin secretion by PHHs was measured on days 4, 6, 8, and 10 of culture. As shown in Figure 6, both MG and PLECM maintain albumin secretion equally well to the day 10 time point of culture, the scheduled end of the experiment.
FIG. 6.
Albumin daily secretion of PHHs cultured in an MG or PLECM sandwich at days 4, 6, 8, and 10. Values represent mean ± SD of three livers.
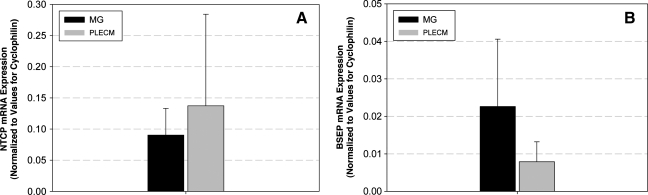
NTCP and BSEP mRNA expression
Because cell isolation disrupts cellular polarity and since hepatic transporter expression and function requires the reestablishment of that polarity, cells must be maintained in the sandwich culture for several days before analysis of transporter expression and function. Thus, on day 5 of culture, NTCP and BSEP mRNA levels were measured. There was variability in the expression of these two genes between cases, but as shown in Figure 7, there was no significant difference in PHHs cultured in either MG or PLECM with respect to NTCP (Fig. 7A) or BSEP (Fig. 7B) expression.
FIG. 7.
NTCP (A) and BSEP (B) mRNA expression levels of PHHs cultured in an MG or PLECM sandwich at day 5. Values represent mean ± SD of four livers. NTCP, sodium taurocholate cotransporting polypeptide; BSEP, bile salt export pump.
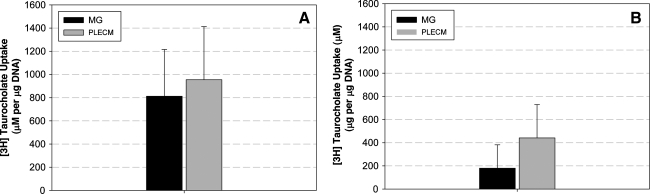
Hepatic transport activity
[3H]Taurocholate uptake and efflux are used as measurements of NTCP and BSEP hepatic transport activity, respectively. As shown in Figure 8, there was no difference between [3H]taurocholate uptake (Fig. 8A) or efflux (Fig. 8B) from PHHs cultured in either an MG or PLECM sandwich. Trends suggested that PHHs cultured with PLECM sandwich had the highest levels of [3H]taurocholate efflux, but there was no significant difference between the groups (p = 0.14).
FIG. 8.
Hepatic transport activity of PHHs. (A) [3H]Taurocholate uptake and (B) [3H]taurocholate efflux of PHHs. Values represent mean ± SD of four livers.
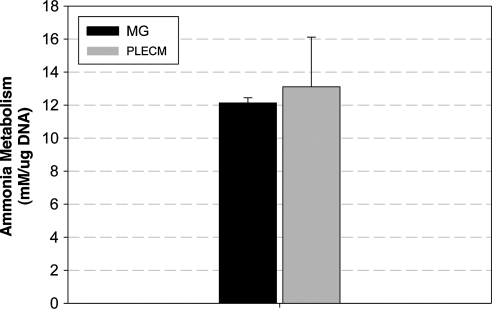
Ammonia metabolism
Ammonia metabolism is a function characteristic of mature, differentiated liver. Determination of ammonia metabolism was conducted on day 5 of culture. Results presented in Figure 9 indicate that there was no difference in the amount of ammonia metabolized by cells cultured in an MG or PLECM sandwich.
FIG. 9.
Ammonia metabolism of PHHs cultured for 5 days in an MG or PLECM sandwich. All data were normalized to PHHs cultured on type-I collagen. Values represent mean ± SD of four livers.
Discussion
When isolated and placed into culture, hepatocytes can lose differentiated gene expression and function quickly. In numerous studies, Matrigel has been shown to be the most effective culture substrate with regard to the maintenance of differentiated gene expression and function. In the present experiments, a gel form of ECM derived from porcine liver was shown to support human hepatocyte function in vitro at levels equal to Matrigel. Recent studies have suggested that tissue- and organ-specific ECM can promote site-appropriate differentiation of progenitor cells29,30 and maintain site-appropriate phenotype in in vitro culture systems.31 Since the ECM of each tissue and organ is produced by the resident cells and logically represents the ideal scaffold or substrate for these cells, it is intuitive that a substrate composed of liver-derived ECM would be favorable for hepatocytes.
It has recently been shown that biologic ECM scaffolds support tissue-specific cell populations. Hepatic sinusoidal endothelial cells maintained their differentiated phenotype the longest when cultured on ECM derived from the liver, as compared with sinusoidal endothelial cells cultured on ECM derived from small intestine or urinary bladder.31 These results suggest that ECM biologic scaffolds provide a set of unique, tissue-specific signals that is dependent upon the tissue from which an ECM is derived. The tissue-specificity of ECM scaffolds may explain why PHHs maintain their phenotype best when cultured on ECM derived from the liver compared with PHHs cultured with ECM derived from the Engelbreth–Holm–Swarm mouse sarcoma (MG).
Lin et al. compared rat hepatocytes cultured on PLECM biologic scaffolds to well-characterized hepatocyte culture models (type-I collagen sandwich configuration or a single layer of type-I collagen).12 Hepatocytes survived up to 45 days on a sheet form of PLECM and several liver-specific functions such as albumin synthesis, urea production, and P-450 IA1 activity were markedly enhanced compared with the growth and metabolism of cells cultured on a single layer of type-I collagen.
Zeisberg et al. isolated liver-derived basement membrane matrix from human or bovine liver, and used the substrate for culture of human hepatocytes.19 Human hepatocytes adhered more efficiently to liver-derived basement membrane matrix and expressed lower levels of vimentin and cytokeratin-18, which are markers of hepatocyte dedifferentiation, compared with hepatocytes cultured on MG or type-I collagen. However, maintenance of liver-specific functions in vitro was not reported.19
Biologic scaffolds composed of ECM provide a more physiologically relevant culture substrate compared with reconstituted ECM proteins and have unique biochemical profiles that are dependent upon the tissue from which an ECM scaffold is derived. Minimally processed ECM scaffolds and gels, such as PLECM, provide a combination of ECM proteins derived from the liver that are present in physiologically relevant amounts (e.g., type-I, IV, VI, III, XI, and XIX collagen, heparin sulfate proteoglycan, ECM-bound growth factors, laminin, biglycan, tenascin, and fibronectin).24,32,33
The maintenance of hepatocyte-specific functions of PHHs cultured with PLECM or MG may be due to the composition of the respective ECM. MG contains the following ECM proteins: laminin, type-IV collagen, entactin, heparan sulfate proteoglycan, chondroitin sulfate proteoglycan, and ECM-bound growth factors.34–36 Laminin, the most abundant ECM protein in MG, is not a major constituent of adult liver ECM.37,38 Although MG lacks several of ECM proteins found in the liver, such as fibronectin and type-I collagen, it remains a useful substrate for hepatocyte culture. It is not surprising that an ECM derived from, and produced by, the cells of the liver would also be a useful substrate for hepatocytes culture. The structural and functional proteins of an ECM scaffold provide a physical substratum for the attachment and spatial organization of cells.39 Well-known biophysical properties such as three-dimensional (3D) surface topography play important roles in cell phenotype and behavior.40–44 The 3D surface topography of an ECM includes variation in pore diameter as well as variation in the distances between the pores,45 surface texture,43 hydrophobicity and/or hydrophilicity, and the presence or absence of a basement membrane.24 Obviously, the 3D surface topography of the gel form of PLECM differs greatly from that of the native liver ECM.
This is the first report of the use of these liver matrix preparations as substrate for hepatocyte culture. With time and experience we hope to improve on the preparation and utilization of liver matrix from porcine as well as human liver. In separate studies (in preparation), we have found that both human and pig liver matrix enhance the differentiation of placental stem cells toward the hepatic lineage. As the field of TE&RM moves toward the replacement of more complex tissues and 3D organs, it is likely that more specialized scaffolds, including those that are produced by human cells or tissues, may be needed to support multiple, functional cell phenotypes. The findings of the present study suggest that a liver-specific ECM substrate can help maintain appropriate hepatocyte phenotype and suggest that an ECM produced from human liver could similarly useful for TE&RM.
Disclosure Statement
No competing financial interests exist.
References
- 1.Organ Procurement and Transplantation Network (OPTN) www.optn.org/data/ www.optn.org/data/
- 2.The Organ Procurement and Transplantation Network. 2008. www.optn.org/latestData/rptData.asp www.optn.org/latestData/rptData.asp
- 3.Allen J.W. Bhatia S.N. Engineering liver therapies for the future. Tissue Eng. 2002;8:725. doi: 10.1089/10763270260424097. [DOI] [PubMed] [Google Scholar]
- 4.Fiegel H.C. Kaufmann P.M. Bruns H. Kluth D. Horch R.E. Vacanti J.P. Kneser U. Hepatic tissue engineering: from transplantation to customized cell-based liver directed therapies from the laboratory. J Cell Mol Med. 2008;12:56. doi: 10.1111/j.1582-4934.2007.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borenstein J.T. Weinberg E.J. Orrick B.K. Sundback C. Kaazempur-Mofrad M.R. Vacanti J.P. Microfabrication of three-dimensional engineered scaffolds. Tissue Eng. 2007;13:1837. doi: 10.1089/ten.2006.0156. [DOI] [PubMed] [Google Scholar]
- 6.Ohashi K. Yokoyama T. Yamato M. Kuge H. Kanehiro H. Tsutsumi M. Amanuma T. Iwata H. Yang J. Okano T. Nakajima Y. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13:880. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 7.Schuetz E.G. Li D. Omiecinski C.J. Muller-Eberhard U. Kleinman H.K. Elswick B. Guzelian P.S. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol. 1988;134:309. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- 8.Michalopoulos G. Sattler G. Sattler C. Pitot H.C. Interaction of chemical carcinogens and drug-metabolizing enzymes in primary cultures of hepatic cells from the rat. Am J Pathol. 1976;85:755. [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton G.A. Jolley S.L. Gilbert D. Coon D.J. Barros S. LeCluyse E.L. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell-cell interactions. Cell Tissue Res. 2001;306:85. doi: 10.1007/s004410100429. [DOI] [PubMed] [Google Scholar]
- 10.Reid L.M. Gaitmaitan Z. Arias I. Ponce P. Rojkind M. Long-term cultures of normal rat hepatocytes on liver biomatrix. Ann NY Acad Sci. 1980;349:70. doi: 10.1111/j.1749-6632.1980.tb29516.x. [DOI] [PubMed] [Google Scholar]
- 11.Watt F.M. Cell culture models of differentiation. FASEB J. 1991;5:287. doi: 10.1096/fasebj.5.3.2001788. [DOI] [PubMed] [Google Scholar]
- 12.Lin P. Chan W.C. Badylak S.F. Bhatia S.N. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 13.Gross-Steinmeyer K. Stapleton P.L. Tracy J.H. Bammler T.K. Lehman T. Strom S.C. Eaton D.L. Influence of Matrigel-overlay on constitutive and inducible expression of nine genes encoding drug-metabolizing enzymes in primary human hepatocytes. Xenobiotica. 2005;35:419. doi: 10.1080/00498250500137427. [DOI] [PubMed] [Google Scholar]
- 14.LeCluyse E.L. Fix J.A. Audus K.L. Hochman J.H. Regeneration and maintenance of bile canalicular networks in collagen-sandwiched hepatocytes. Toxicol In Vitro. 2000;14:117. doi: 10.1016/s0887-2333(99)00096-x. [DOI] [PubMed] [Google Scholar]
- 15.Dunn J.C. Tompkins R.G. Yarmush M.L. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 16.Sidhu J.S. Farin F.M. Omiecinski C.J. Influence of extracellular matrix overlay on phenobarbital-mediated induction of CYP2B1, 2B2, and 3A1 genes in primary adult rat hepatocyte culture. Arch Biochem Biophys. 1993;301:103. doi: 10.1006/abbi.1993.1121. [DOI] [PubMed] [Google Scholar]
- 17.LeCluyse E.L. Audus K.L. Hochman J.H. Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol. 1994;266(6 Pt 1):C1764. doi: 10.1152/ajpcell.1994.266.6.C1764. [DOI] [PubMed] [Google Scholar]
- 18.Moghe P.V. Berthiaume F. Ezzell R.M. Toner M. Tompkins R.G. Yarmush M.L. Culture matrix configuration and composition in the maintenance of hepatocyte polarity and function. Biomaterials. 1996;17:373. doi: 10.1016/0142-9612(96)85576-1. [DOI] [PubMed] [Google Scholar]
- 19.Zeisberg M. Kramer K. Sindhi N. Sarkar P. Upton M. Kalluri R. De-differentiation of primary human hepatocytes depends on the composition of specialized liver basement membrane. Mol Cell Biochem. 2006;283:181. doi: 10.1007/s11010-006-2677-8. [DOI] [PubMed] [Google Scholar]
- 20.LeCluyse E. Madan A. Hamilton G. Carroll K. DeHann R. Parkinson A. Expression and regulation of cytochrome P450 enzymes in primary cultures of human hepatocytes. J Biochem Mol Toxicol. 2000;14:177. doi: 10.1002/(sici)1099-0461(2000)14:4<177::aid-jbt1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Silva J.M. Morin P.E. Day S.H. Kennedy B.P. Payette P. Rushmore T. Yergey J.A. Nicoll-Griffith D.A. Refinement of an in vitro cell model for cytochrome P450 induction. Drug Metab Dispos. 1998;26:490. [PubMed] [Google Scholar]
- 22.Kern A. Bader A. Pichlmayr R. Sewing K.F. Drug metabolism in hepatocyte sandwich cultures of rats and humans. Biochem Pharmacol. 1997;54:761. doi: 10.1016/s0006-2952(97)00204-9. [DOI] [PubMed] [Google Scholar]
- 23.Jasmund I. Schwientek S. Acikgoz A. Langsch A. Machens H.G. Bader A. The influence of medium composition and matrix on long-term cultivation of primary porcine and human hepatocytes. Biomol Eng. 2007;24:59. doi: 10.1016/j.bioeng.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Brown B. Lindberg K. Reing J. Stolz D.B. Badylak S.F. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert T.W. Sellaro T.L. Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Freytes D.O. Martin J. Velankar S.S. Lee A.S. Badylak S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Strom S.C. Pisarov L.A. Dorko K. Thompson M.T. Schuetz J.D. Schuetz E.G. Use of human hepatocytes to study P450 gene induction. Methods Enzymol. 1996;272:388. doi: 10.1016/s0076-6879(96)72044-x. [DOI] [PubMed] [Google Scholar]
- 28.Kostrubsky V.E. Strom S.C. Hanson J. Urda E. Rose K. Burliegh J. Zocharski P. Cai H. Sinclair J.F. Sahi J. Evaluation of hepatotoxic potential of drugs by inhibition of bile-acid transport in cultured primary human hepatocytes and intact rats. Toxicol Sci. 2003;76:220. doi: 10.1093/toxsci/kfg217. [DOI] [PubMed] [Google Scholar]
- 29.Cepko C.L. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol. 1999;9:37. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- 30.Jadhav A.P. Mason H.A. Cepko C.L. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006;133:913. doi: 10.1242/dev.02245. [DOI] [PubMed] [Google Scholar]
- 31.Sellaro T.L. Ravindra A.K. Stolz D.B. Badylak S.F. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007;13:2301. doi: 10.1089/ten.2006.0437. [DOI] [PubMed] [Google Scholar]
- 32.Hodde J.P. Badylak S.F. Brightman A.O. Voytik-Harbin S.L. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 1996;2:209. doi: 10.1089/ten.1996.2.209. [DOI] [PubMed] [Google Scholar]
- 33.Voytik-Harbin S.L. Brightman A.O. Kraine M.R. Waisner B. Badylak S.F. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478. [PubMed] [Google Scholar]
- 34.Kleinman H.K. McGarvey M.L. Liotta L.A. Robey P.G. Tryggvason K. Martin G.R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 35.Futaki S. Hayashi Y. Yamashita M. Yagi K. Bono H. Hayashizaki Y. Okazaki Y. Sekiguchi K. Molecular basis of constitutive production of basement membrane components. Gene expression profiles of Engelbreth-Holm-Swarm tumor and F9 embryonal carcinoma cells. J Biol Chem. 2003;278:50691. doi: 10.1074/jbc.M304985200. [DOI] [PubMed] [Google Scholar]
- 36.Couchman J.R. Kapoor R. Sthanam M. Wu R.R. Perlecan and basement membrane-chondroitin sulfate proteoglycan (bamacan) are two basement membrane chondroitin/dermatan sulfate proteoglycans in the Engelbreth-Holm-Swarm tumor matrix. J Biol Chem. 1996;271:9595. doi: 10.1074/jbc.271.16.9595. [DOI] [PubMed] [Google Scholar]
- 37.Kato S. Otsu K. Ohtake K. Kimura Y. Yashiro T. Suzuki T. Akamatsu N. Concurrent changes in sinusoidal expression of laminin and affinity of hepatocytes to laminin during rat liver regeneration. Exp Cell Res. 1992;198:59. doi: 10.1016/0014-4827(92)90149-3. [DOI] [PubMed] [Google Scholar]
- 38.Reid L.M. Fiorino A.S. Sigal S.H. Brill S. Holst P.A. Extracellular matrix gradients in the space of Disse: relevance to liver biology. Hepatology. 1992;15:1198. doi: 10.1002/hep.1840150635. [DOI] [PubMed] [Google Scholar]
- 39.Hubbell J.A. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003;14:551. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Dow J.A. Clark P. Connolly P. Curtis A.S. Wilkinson C.D. Novel methods for the guidance and monitoring of single cells and simple networks in culture. J Cell Sci Suppl. 1987;8:55. doi: 10.1242/jcs.1987.supplement_8.4. [DOI] [PubMed] [Google Scholar]
- 41.Clark P. Connolly P. Curtis A.S. Dow J.A. Wilkinson C.D. Topographical control of cell behaviour: II. Multiple grooved substrata. Development. 1990;108:635. doi: 10.1242/dev.108.4.635. [DOI] [PubMed] [Google Scholar]
- 42.Clark P. Connolly P. Curtis A.S. Dow J.A. Wilkinson C.D. Topographical control of cell behaviour. I. Simple step cues. Development. 1987;99:439. doi: 10.1242/dev.99.3.439. [DOI] [PubMed] [Google Scholar]
- 43.den Braber E.T. de Ruijter J.E. Smits H.T. Ginsel L.A. von Recum A.F. Jansen J.A. Quantitative analysis of cell proliferation and orientation on substrata with uniform parallel surface micro-grooves. Biomaterials. 1996;17:1093. doi: 10.1016/0142-9612(96)85910-2. [DOI] [PubMed] [Google Scholar]
- 44.Chehroudi B. Soorany E. Black N. Weston L. Brunette D.M. Computer-assisted three-dimensional reconstruction of epithelial cells attached to percutaneous implants. J Biomed Mater Res. 1995;29:371. doi: 10.1002/jbm.820290312. [DOI] [PubMed] [Google Scholar]
- 45.Zeltinger J. Sherwood J.K. Graham D.A. Mueller R. Griffith L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7:557. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]