Abstract
Ginkgo biloba extract (GBE) facilitates blood flow, influences nitric oxide systems, and has a relaxant effect on smooth muscle tissue. These processes are important to the sexual response in women and, hence, it is feasible that GBE may have a therapeutic effect. The present study was the first to provide an empirical examination of the effects of both short- and long-term GBE administration on subjective and physiological (vaginal photoplethysmography) measures of sexual function in women with Sexual Arousal Disorder. A single dose of 300 mg GBE had a small but significant facilitatory effect on physiological, but not subjective, sexual arousal compared to placebo in 99 sexually dysfunctional women. The long-term effects of GBE on sexual function were assessed in 68 sexually dysfunctional women who were randomly assigned to 8 weeks treatment of either (1) GBE (300 mg/daily), (2) placebo, (3) sex therapy which focused on training women to attend to genital sensations, or (4) sex therapy plus GBE. When combined with sex therapy, but not alone, long-term GBE treatment significantly increased sexual desire and contentment beyond placebo. Sex therapy alone significantly enhanced orgasm function compared with placebo. Long-term GBE administration did not significantly enhance arousal responses beyond placebo. It was concluded that (1) neither short- or long-term administration of GBE alone substantially impacts sexual function in women, (2) a substantial placebo effect on sexual function exists in women with sexual concerns, and (3) teaching women to focus on genital sensations during sex enhances certain aspects of women’s sexual functioning.
Keywords: Ginkgo biloba, Female sexual dysfunction, Sex therapy, Vaginal photoplethysmography
Introduction
The Ginkgo biloba is an ancient tree that the Chinese have cultivated and held sacred for its health-promoting properties for over two millennia (Christen, Courtois, & Droy-Lefaix, 1995; DeFeudis, 1991). In recent decades, concentrated extracts from Ginkgo biloba leaves (GBE) have been sold in Western countries as herbal medicines for treating peripheral vascular disease and for enhancing cerebral blood flow (Smith, Maclennan, & Darlington, 1996). Indeed, a number of clinical trials have shown that GBE is effective in treating a wide range of problems associated with impaired circulation, including hearing problems, visual disturbances, edema, varicose veins, leg ulcers, stroke, and intermittent claudication (Cohen & Bartlik, 1998), as well as symptoms of cerebrovascular insufficiency, such as difficulties of concentration and memory, confusion, lack of energy, depressed mood, dizziness, and tinnitus (for review, see Kleijnen & Knipschild, 1992).
Although GBE has long been used to treat disorders involving restricted vascularity, only recently has its use in the treatment of sexual dysfunction been suggested. Ellison and DeLuca (1998) reported a case of a 37-year-old woman who was experiencing fluoxetine-induced sexual dysfunction (i.e., decreased sexual desire and arousal, decreased lubrication, delayed orgasm, vaginal anesthesia) that was relieved after 2 weeks of daily use of GBE (180–240 mg/day). In a 4-week, open trial in 33 women and 30 men, Cohen and Bartlik (1998) examined the effects of GBE (40 or 60 mg/twice daily) on antidepressant-induced sexual dysfunction. GBE was 84% effective in alleviating the antidepressant-induced sexual symptoms, with success rates being slightly higher in women than in men (91% and 76% for women and men, respectively). Detailed outcome measures or results were not provided in the report, but Cohen and Barlik did note that all phases of the sexual response cycle, including desire, arousal (erection and lubrication), orgasm, and resolution, were favorably enhanced.
To our knowledge, there has been only one placebo-controlled trial of GBE for antidepressant-induced sexual dysfunction (Kang, Lee, Kim, & Cho, 2002), and no studies examining the effects of GBE for sexual problems not induced by antidepressant medications. In the one placebo-controlled study published, 27 men and 10 women were randomly assigned to receive either GBE (120 mg/daily first 2 weeks; 160 mg/daily next 2 weeks; 240 mg/day remaining 4 weeks) or placebo for 2 months using a double-blind protocol. Sexual function was assessed by asking participants to rate a number of sexual items on a 5 point rating scale from 1 = severe impairments, to 5 = normal or the level of sexual function prior to antidepressant medication. The following four items were directly relevant to women’s sexual function: sexual desire, overall sexual function, orgasm frequency, and satisfaction with orgasm. Comparisons with baseline levels were made for each item at weeks 2, 4, and 8. Results indicated there were no statistically significant differences on any items between GBE and placebo except for the item of satisfaction with orgasm at week 8, which showed an improvement with placebo. Although a well-controlled study, the findings from this study do not allow conclusions to be drawn regarding the effectiveness of using GBE to treat women’s sexual dysfunction given the limited sample size, that the results were not reported separately by gender, and that sex related changes were not measured using a validated questionnaire.
There are many active agents found in GBE and it is unknown whether the therapeutic benefits of GBE are attributable to single active ingredients or the combined, or perhaps even synergistic, action of many ingredients. Ginkgo biloba leaves contain two major groups of substances: flavonoids (e.g., kaempferol, quercetin, isorhamnetin derivatives) and terpenes (e.g., ginkgolides, bilobalide) (van Beek, Scheeren, Rantio, Melger, & Lelyveld, 1991). Ginkgolides, which have not been found in any other living species, can be divided into several types (i.e., A, B, C) which differ only in the number and position of hydroxyl groups (Kleijnen & Knipschild, 1992). Although there may be differences in the composition of ginkgo preparations depending on the manufacturing process used, most preparations are standardized based on the amount of ginkgo–flavone glycosides (24%) and terpenoids (6%).
There are several viable means by which GBE might enhance women’s sexual function. One is via the vasoregulatory activity of GBE. Clinical and pharmacological studies have shown that GBE promotes increased blood flow both in the arteries and capillaries (Tyler, 1993). In a randomized double-blind study (Koltringer, Eber, Lind, Langsteger, & Wakonig, 1989) conducted on 60 patients, GBE (200 mg) given over four consecutive days led to increased skin perfusion and a decrease in blood viscosity and elasticity. Similarly, GBE significantly increased blood flow in nail-fold capillaries in a randomized single-blind crossover study, although conclusions from this study are limited given the small sample size (n = 10) (Jung, Morowietz, Kiesewetter, & Wenzel, 1990). Clinical reports also indicate GBE is effective in treating a variety of conditions responsive to improved circulation (Cohen & Bartlik, 1998). Genital vasocongestion is a marker of sexual arousal in women, and is the crucial process by which plasma transudation and subsequent lubrication of the epithelial surface of the vaginal wall occur. Given GBE’s ability to facilitate peripheral blood flow to various bodily regions (e.g., arms, legs, hands), it is reasonable to speculate that it might also be effective in facilitating blood flow to the genital region, thus enhancing sexual arousal mechanisms, particularly among women with sexual dysfunction who have subnormal capillary vascular function. Indeed, research indicates that sexual arousal problems are associated with vascular and clitoral insufficiency in some women (Park et al., 1997).
A second means by which GBE might facilitate sexual function is via the relaxation of the muscular cells of blood vessels. Several studies conducted on isolated rabbit aorta have shown that GBE induces a dose-dependent relaxing effect on vascular smooth muscle (e.g., Auguet & Clostre, 1983; Auguet, DeFeudis, & Clostre, 1982). More recently, Paick and Lee (1996) studied the effect of GBE on human and rabbit corpus cavernosal tissue. Both human and rabbit corpus cavernosal tissue, precontracted by norepinephrine, showed a potent relaxation response to subfractions of non-ginkolide non-flavonoid fraction, the component of ginkgo biloba extract believed to have the most potent relaxing effect on vascular smooth muscle.
In addition to the above mechanisms, Cohen and Bartlik (1998) speculated that GBE could enhance vascular flow to the genitals through inhibition of platelet-activating factor, in a similar manner to the mechanism by which it enhances cerebral perfusion, and by having a direct effect on prostaglandins, which are known to enhance sexual arousal in men. Recent research has also shown GBE to have nitric oxide-scavenging abilities (Marcocci, Maguire, Droy-Lefaix, & Packer, 1994), which point to its potential therapeutic value in treating conditions in which nitric oxide (NO) reactivity is important. In men, sexual stimulation leads to the production of NO and the subsequent release of guanylate cyclase. Guanylate cyclase converts guanosine triphosphate to cyclic guanosine monophosphate (cGMP) and cGMP produces relaxation of the smooth muscles of the penile arteries and corpus cavernosum, resulting in increased blood flow into the penis (Burnett, 1997). Evidence suggests that this may also occur in the clitoris.
Immunohistochemical evaluation of the human clitoris revealed that NO is produced in this tissue (Burnett, 1997) and, with the exception that the clitoris does not contain a subalbugineal layer (which contributes to the rigidity of the penis), the clitoris may be considered the homologue of the penis (Toesca, Stolfi, & Cocchia, 1996). Arterial blood inflow is delivered via the clitoral cavernosal arteries and is regulated by helicine arteriolar smooth muscle tone (Park, Moreland, Goldstein, Atala, & Traish, 1998). NO has been implicated as a vasodilator in clitoral corpus cavernosum and vaginal muscularis smooth muscle relaxation (Azadoi et al., 1992; Burnett, 1997). Thus, impaired smooth muscle function may adversely impact the process of clitoral erection and vaginal engorgement and lubrication. Sildenafil (Viagra) acts on NO systems by prolonging the action of cGMP (thus inhibiting the metabolism of cGMP by cyclic nucleotide phosphodiesterase isozymes (PDE5) (Boolell et al., 1996) and has been highly effective in alleviating erectile dysfunction (e.g., Dinsmore et al., 1999; Goldstein et al., 1998; Marks, Duda, Dorey, Macairan, & Santos, 1999; Montorsi et al., 1999).
Evidence from placebo-controlled research is conflicting as to whether sildenafil may also be beneficial in treating sexual arousal dysfunction in women. Among pre-menopausal women with Sexual Arousal Disorder (SAD), Carusa, Intelisano, Lupo, and Agnello (2001) reported increased self-reported sexual arousal, orgasm, and enjoyment of sexual activity with sildenafil. Among post-menopausal women with SAD, Basson and Brotto (2003) reported decreased latency to orgasm, and increased subjective sexual arousal and perceptions of genital arousal with sildenafil, but only among women with low baseline levels of laboratory-induced genital arousal. In a large sample of 577 estrogenized and 204 estrogen-deficient women with SAD, Basson, McInnes, Smith, Hodgson, and Koppiker (2002) reported no significant impact of sildenafil on self-report measures of sexual arousal function.
In summary, GBE facilitates blood flow, influences NO systems, and has a relaxant effect on smooth muscle tissue. These processes are integral to sexual response in women. From a pharmacological perspective then, it is highly feasible that GBE may be effective in enhancing sexual function in women. GBE is extremely well tolerated (Maitra, Marcocci, Droy-Lefaix, & Packer, 1995), and animal studies suggest that GBE may exert anti-stress effects that are not explained by either classical antidepressant or anxiolytic activity (Porsolt, Martin, Lenegere, Fromage, & Drieu, 1990; Rapin, Lamproglou, Drieu, & DeFeudis, 1994).
The present study was designed to provide a comprehensive examination of the potential effectiveness of using GBE to treat sexual dysfunction in women with sexual arousal disorder with or without desire and/or orgasm concerns. The short term effects of GBE were examined using a double-blind protocol in which women participated in two counterbalanced sessions in which they received either 300 mg GBE or placebo 90 min prior to viewing a film series consisting of neutral and erotic scenarios. Sexual arousal was measured throughout the film presentation both subjectively and physiologically (vaginal photoplethysmograph). Based on evidence indicating GBE is effective in increasing vascular efficiency, we predicted levels of sexual arousal to the erotic videos would be significantly higher among all women during the sessions where they received GBE compared to placebo.
The long-term effects of GBE on sexual function were assessed by randomly assigning participants to 8 weeks of either GBE, placebo, sex therapy, or sex therapy plus GBE, and comparing sexual outcome measures at 4 weeks (mid-treatment), and 8 weeks (post-treatment). Outcome measures included validated questionnaires, event logs, and laboratory measures of continuous subjective and physiological sexual arousal responses to erotic stimuli. Although speculative, we hypothesized that prolonged GBE treatment would exert a priming effect on vascular efficiency that would enhance vaginal engorgement to erotic stimuli. Thus, we predicted higher levels of physiological sexual arousal to erotic videos in the GBE and in the sex therapy plus GBE conditions compared with placebo or sex therapy alone. The sex therapy in this study focused primarily on training women to identify and attend to sexual sensations in the genitals. Based on this assumption, we predicted that subjective levels of sexual arousal to erotic videos would be highest in the sex therapy plus GBE condition, and a higher concordance between laboratory measures of subjective and physiological arousal would be seen in the sex therapy plus GBE condition compared with GBE, placebo, or sex therapy alone. Finally, we predicted that women in the sex therapy plus GBE condition would show the greatest level of improvement on validated measures of sexual arousal, orgasm and sexual satisfaction post-treatment compared with women in the GBE, placebo, or sex therapy conditions. To the extent that the subjective experience of sexual desire and arousal are closely related in women, we expected self-report measures of sexual desire to also be highest in the sex therapy plus GBE condition.
Method
Participants
Women (n = 99) aged 18–65 who were currently experiencing Sexual Arousal Disorder, with or without coexistent Hypoactive Sexual Desire Disorder and/or Orgasmic Disorder were recruited through radio public service announcements and advertisements in local newspapers. Of these 99 women, 36 reported sexual side effects secondary to anti-depressant medication (fluoxetine, sertraline, or paroxetine) use; 63 were not on antidepressant medication.
Participants were considered to have an antidepressant-induced sexual dysfunction if they reported an onset of Hypoactive Sexual Desire Disorder, Sexual Arousal Disorder, or Orgasmic Disorder no less than one week and no more than 3 months after beginning treatment with either fluoxetine, sertraline, or paroxetine; they had been receiving antidepressant treatment for a minimum of 10 weeks prior to beginning the study; and they described the sexual dysfunction as following the otherwise successful treatment with the antidepressant, and as being distinctly different from any sexual dysfunction they may have noticed prior to starting antidepressant treatment. In an effort to increase the homogeneity of input variables, potential participants receiving antidepressant medications other than fluoxetine, sertraline, or paroxetine medication were not included in the study (n = approximately 20).
There is wide variability in the mechanisms of action by which the antidepressants exert their effects, and also in the reported sexual side effects associated with antidepressant medications (e.g., Meston & Gorzalka, 1992). Side effects secondary to fluoxetine, sertraline, and paroxetine were chosen for examination in the present study because these drugs have a similar serotonergic mechanism of action in that they selectively inhibit the uptake of sertonin but, unlike some of the newer antidepressants (e.g., mirtazapine), they do not act on noradrenergic systems (Stimmel, Dopheide, & Stahl, 1997). In addition, fluoxetine, sertraline, and paroxetine have been associated with similar sexual side effects in women (e.g., Meston & Gorzalka, 1992; Rosen, Lane, & Menza, 1999).
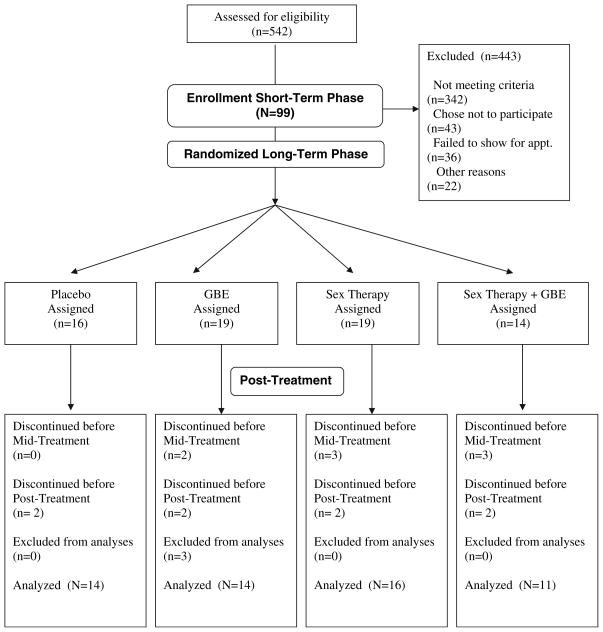
Table 1 describes participants’ enrollment. As illustrated in Table 1, a total of 443 women were excluded from the study. Of the women who did not meet criteria (n = 342), the majority was not in a relationship (n = 81) or did not meet diagnostic criteria for sexual dysfunction (n = 80), 45 women were on medications known to affect sexual dysfunction, and 40 had either undergone total hysterectomy or had a health condition that excluded them from the study (i.e., high blood pressure, diabetes, or thyroid disorder). Few women (n = 12) were breast feeding or pregnant and four reported a history of psychosis. Of the 43 women who chose not to participate, 13 reported feeling uncomfortable with the psychophysiological assessment procedures used in the study and 19 did not want to make the time commitment required for the treatment.
Table 1.
Participants flow-chart
 |
During the entry interview, inclusion and exclusion criteria were assessed. Participants were accepted in the study if they were between the ages of 18 and 65, were currently involved in a heterosexual relationship, and agreed that during the course of the study they would engage in at least two sexual encounters with their partners per week, would not use aspirin or other blood thinning pharmaceutical agents, and would use a medically accepted form of birth control. This latter criterion was required by the University of Texas Internal Review Board to protect participants against the potential unknown negative consequences of GBE on pregnancy. Participants were not included if they reported amenorrhea, were pregnant, lactating or less than 1 year post-partum, reported a bleeding disorder, had a history of major pelvic surgery, diabetes, neurological impairments, hypertension, or heart problems, reported either currently engaging in high levels of alcohol use or illicit drug use or reported being treated for drug, alcohol, or substance abuse within the previous 6 months. Participants were questioned as to whether they had ever received a psychiatric or psychological diagnosis in the past and, if so, whether they were currently experiencing any symptoms. Participants who were currently experiencing symptoms suggestive of an untreated Axis I psychiatric disorder were excluded from participation. Participants were also excluded if they were receiving any psychological intervention that specifically focused on sexuality issues, or if they posed a current, serious suicidal or homicidal risk. Participants currently taking anticoagulants, antihypertensives or beta blockers were excluded from the study. Table 2 shows the participants’ demographics.
Table 2.
Means and SDs for demographic characteristics for women in the short- and long-term study divided by conditions
| Acute sample (n = 99) | Chronic sample (n = 68) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 16) |
GBE (n = 19) |
Therapy (n = 19) |
GBE + Therapy (n = 14) |
|||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| Age (in years) | 25.30 | 7.81 | 24.92 | 4.14 | 25.82 | 6.81 | 27.82 | 10.26 | 25.40 | 5.36 |
| Length of relationship (in mos) | 2.60 | 1.53 | 2.75 | 0.97 | 2.55 | 1.44 | 2.09 | 1.58 | 2.70 | 1.16 |
| n | % | n | % | n | % | n | % | n | % | |
| Education | ||||||||||
| At least some college | 89 | 89.90 | 16 | 100.00 | 18 | 94.74 | 18 | 94.74 | 13 | 92.86 |
| Marital Status | ||||||||||
| Currently single | 48 | 48.49 | 9 | 56.25 | 12 | 63.16 | 10 | 52.63 | 6 | 42.86 |
| Married | 37 | 37.40 | 7 | 43.75 | 4 | 21.05 | 5 | 26.32 | 7 | 50.00 |
| Divorced | 14 | 14.14 | 0 | 0.00 | 3 | 15.79 | 4 | 21.05 | 1 | 7.14 |
| Ethnicity | ||||||||||
| Caucasian/White | 72 | 72.72 | 10 | 62.50 | 16 | 84.21 | 15 | 78.95 | 11 | 78.57 |
| African American | 3 | 3.03 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Native American | 1 | 1.01 | 1 | 6.25 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Hispanic/Latina | 13 | 13.13 | 1 | 6.25 | 2 | 10.53 | 3 | 15.79 | 2 | 14.29 |
| Asian American | 5 | 5.05 | 2 | 12.50 | 0 | 0.00 | 1 | 5.26 | 1 | 7.14 |
| Other | 1 | 1.01 | 1 | 6.25 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Mixed ethnicity | 4 | 4.04 | 1 | 6.25 | 1 | 5.26 | 0 | 0.00 | 0 | 0.00 |
| Sexual Orientation | ||||||||||
| Bisexual | 10 | 10.10 | 2 | 12.50 | 2 | 10.53 | 2 | 10.53 | 3 | 21.43 |
| Heterosexual | 89 | 89.90 | 14 | 87.50 | 17 | 89.47 | 17 | 89.47 | 11 | 78.57 |
Measures
Laboratory Measures of Sexual Arousal
Vaginal photoplethysmography (Sintchak & Geer, 1975) was used to measure vaginal pulse amplitude (VPA), an index of physiological sexual arousal in response to sexual stimuli. The VPA signal was sampled 80 times per second and the amplitude of each pulse wave was recorded in millivolts (mV) throughout the entire 3 min of a neutral video and 10 min of an erotic video. In accordance with previous studies of this nature (e.g., Meston & Heiman, 1998), obvious artifacts caused by movement or contractions of the pelvic muscles were easily detected and deleted following visual inspection of the data. Pulses affected by more subtle artifact were included in the analysis. Given that we computed an average of all pulses (approximately 900 pulses), we expected small errors due to artifacts to average out. Additionally, HLM analyses are expected to be only minimally affected by artifact. HLM is based on the computation of regression coefficients and regression lines are only minimally affected by the small outliers that plague VPA data.
Percentage of increase in VPA levels were calculated by computing the ratio between the average VPA during the entire section of the erotic video and the average VPA during the neutral video. All pulses were included in the analysis except for the obvious errors produced by movement artifact. When a continuous measure of VPA was needed for within participants analyses (i.e., the relationship between VPA and subjective sexual arousal), the amplitude of each pulse during the neutral and the erotic video was averaged across every 10 s intervals.
Subjective ratings of the participant’s sexual response throughout the exposure to the neutral and the erotic videos were measured with a device termed “arousometer.” The arousometer was a computer optical mouse (Intellimouse by Microsoft®) mounted on a wooden track divided into equally spaced intervals from “0” to “7.” At each numeric interval that the computer mouse passes, participants feel a slight resistance on the mouse indicating that they are changing from one numeric value to another. The resistance provided by the arousometer allowed participants to monitor the level of arousal that they were indicating without having to focus their attention away from the television screen. Subjective sexual arousal scores were first transformed into a 0–100 scale where 0 on the arousometer corresponded to a score of 0 and a 7 on the arousometer corresponded to a score of 100. Subsequently, the average of subjective sexual arousal was computed during the exposure to the neutral and erotic videos. Since all participants reported 0.00 subjective sexual arousal during the neutral video, the average of subjective sexual arousal during the erotic video corresponded to the score of subjective sexual arousal used for between-participants analyses. The average subjective sexual arousal for 10 s intervals throughout the neutral and the erotic videos was used for within-participants analyses (i.e., the analysis of the relationship between VPA and subjective sexual arousal).
Six sets of videos were counterbalanced across participants and used as stimuli for assessing laboratory measures of sexual arousal. Each video comprised 1 min of the word “relax,” 3 min of a travel documentary on Russian culture, and 10 min of an erotic video showing a heterosexual couple engaging in foreplay (2 ± 0.5 min), manual stimulation (3 ± 0.5 min), oral sex (2 ± 0.5 min), and vaginal penetration (3 ± 0.5 min). To examine whether the videos were comparable in their ability to enhance sexual arousal in women, a one-way ANOVA was computed using video number (1–6) watched during baseline as an independent variable, and VPA and continuous subjective sexual arousal during baseline as dependent variables. No significant differences were observed between the six videos for either percentage of VPA increase, F(5, 98) < 1, or average subjective sexual arousal during the erotic portion of the videos, F(5, 98) = 1.05. On average, women showed a maximum increase of 3.06 mV (SD = 5.24), mV in VPA (average of 30 s of maximum VPA during erotic video––average 30 s of maximum VPA during neutral video), and an increase in subjective sexual arousal between 22.63 and 34.29 units.
Sexual Function
A semi-structured clinical interview was conducted by an advanced student in clinical psychology with over 300 h of experience assessing sexual dysfunction in women. The interviewer first provided a definition of sexual desire, arousal, and orgasm based on the DSM-IV-TR criteria and then asked the participants to indicate whether in the previous 4 weeks they had experienced any difficulties related to each sexual dimension. The interviewer used a number of prompts to further investigate the nature and severity of the dysfunction. Participants were also questioned as to whether they were experiencing personal or relational distress as a consequence of their sexual difficulties.
Levels of sexual function and satisfaction were also assessed using two well-established validated questionnaires: the Female Sexual Function Index (FSFI; Rosen et al., 2000) and the Sexual Satisfaction Scale–Women (SSS-W; Meston & Trapnell, 2005). The FSFI is a 19-item questionnaire divided into six domains: desire (2 items), arousal (4 items), lubrication (4 items), orgasm (3 items), satisfaction (3 items), and pain (3 items). Response options differ by question but are based on a 5-point Likert scale rating system with some questions also including the response option of “no sexual activity.” Several studies on the psychometrics quality of the FSFI have reported adequate inter-item reliability with Cronbach’s α computed for the subscales ranging from .89 to .96; appropriate test–retest reliability (r = .79–.86) after 2 weeks, and good discriminant validity between women with and without Hypoactive Sexual Desire Disorder, Sexual Arousal Disorder, and Orgasmic Disorder (Meston, 2003; Rosen et al., 2000). Recently, a cut off score of 26.5 was shown to have adequate sensitivity and specificity for the distinction between women with and without female sexual dysfunction (Wiegel, Meston, & Rosen, 2005).
The SSS-W (Meston & Trapnell, 2005) is a 30-item questionnaire developed on the four domains of sexual satisfaction identified by the literature: compatibility, contentment, communication, and concern (personal, relational). Response options range from 1 = strongly agree, to 5 = strongly disagree. The psychometrics of this questionnaire indicate that it has good inter-item reliability (Cronbachs’ α ≥ .72), acceptable test–retest reliability after a lapse of 1 month (r = .62–.79), and the construct validity of the instrument was confirmed with a principal component analysis. The full scale score differentiated between women with (M = 88.8) and without (M = 123.4) sexual dysfunction.
In agreement with the guidelines on clinical trials for sexual treatments provided by the Food and Drug Administration (FDA Guidance document, 2000), an event log was created for the assessment of treatment-induced changes in the sexual function of the participants. Participants were provided with event logs and were asked to complete the event after each sexual activity that did or did not include a partner. The log was composed of seven items that addressed satisfaction with the sexual arousal response (physical and mental sexual arousal), satisfaction with orgasm, levels of desire before the sexual activity, and general experience of sexual pleasure during the activity. Each item was scored on a 6-point Likert scale that ranged from “none” or “not satisfied” to “very much” or “extremely satisfied.” An analysis of the psychometrics properties of these event logs has shown that self-report questionnaires such as the FSFI are more sensitive at identifying the clinical changes induced by treatment than are event logs (Rellini & Meston, 2006). The results from the events logs were included in this study as a supplement to the descriptions provided by the FSFI and the SSS-W on the changes experienced by the participants in the study.
Conditions
Short-term GBE and Long-term GBE
GBE
The GBE used for this study was a standardardized extract of GBE leaves, termed GBE 761, manufactured by Schwabe GMBH in Germany, and distributed in U.S. under Nature’s Way Brand. This product contains concentrated (50:1) ginkgo biloba leaf extract standardized to 24% ginkgo flavone glycosides and 6% terpen lactose. It is the most widely used form of GBE in clinical trials. Each pill contained 300 mg of the GBE 761 formula. This dosage was selected based on studies that have indicated GBE increases peripheral blood flow at acute doses of 200 mg and higher (e.g., Koltringer et al., 1989).
Placebo
Both the placebo and GBE capsules were prepared by the University of Texas at Austin Pharmacy and were identical in appearance and texture to ensure double-blind conditions.
Long-term
Sex Therapy
A standardized 8-session sex therapy protocol was administered by an advanced student in clinical psychology supervised by a licensed clinical psychologist with more than 20 years of experience in therapy and clinical trials. A therapist manual was developed to ensure the treatment was standardized across participants and all sessions were videotaped to check for protocol compliance.1 A client manual with definitions, descriptions of aim and goals, treatment agenda, and forms to complete in session and during home assignments was also developed to help clients follow the standardized treatment. Each participant was required to attend the eight 55 min individual sessions with the therapist and to set aside 20 min every two days to complete exercises assigned by the therapist. The primary treatment goal was to increase women’s focus on the sexual pleasure and physiological sexual responses during sexual activity. Cognitive-behavioral techniques were used to identify cognitive distortions that created a distraction from sexual pleasure during sexual activities. Exposure interventions were used to help participants to practice focusing on their bodies and explore different behaviors and different sensations during sexual activities.
Our rationale for selecting this type of sex therapy was that if GBE enhanced genital blood flow, by having women attend to those genital changes and interpret them in a positive sexual manner, psychological levels of arousal might also be enhanced. This hypothesis is rooted in the cognitive model originally proposed by Barlow (for a review, see Wiegel, Scepkowski, & Barlow, 2006). According to this model, people with sexual dysfunction are less aware of how aroused they are. Additionally, individuals with sexual dysfunction are more likely to focus on their performance rather than their sexual responses. We expected the combined effect of, on one hand, increasing women’s genital response with GBE and, on the other hand, helping them to attend their genital sensations and identify variables that increased their sexual arousal to increase sensations of both subjective and physiological sexual arousal.
The first four sessions of therapy used exercises to be conducted individually, while the second 4 sessions provided instructions for exercises to be conducted with the partner. The objectives of the first 4 weeks included identifying distracting thoughts and using cognitive restructuring, becoming familiar with one’s own body, and increasing awareness of the type of physical, mental, and emotional contact that is linked to feelings of sexual arousal. Women were provided with written and visual erotic material to use alone during the home exercises and they were instructed to complete forms developed to increase body and sensation awareness during the exercises. The last four sessions focused on the type of cognitive distractions present during sexual activities with a partner. Modified versions of sensate focus exercises were used to provide participants with an opportunity to practice focusing on their own pleasure during sexual activities with a partner. Issues that were not addressed by the treatment protocol included relationship problems and past history of sexual abuse. Participants who indicated they did not have the time or were not interested in the therapy or in completing the home exercises were dropped from the study after the first assessment visit (see Table 1).
Sex therapy plus GBE
Participants received a combination of GBE plus the standardized 8-session sex therapy protocol described above.
Procedure
Short-term Effects of GBE versus Placebo
After an initial assessment interview, participants who qualified were scheduled for a two-session physiological assessment that took place at the Female Sexual Psychophysiology Laboratory. During the first session, participants were given a detailed informed consent form to complete. The form stated that the purpose of the study was to learn whether ginkgo biloba and/or sexual education could help alleviate sexual difficulties. The form included a detailed description of the two parts of the study (short-term effects; long-term effects), and an extensive list of the potential discomforts and risks associated with GBE use. To this regard, the following statements were included in the informed consent, “You should be aware that ginkgo biloba has been associated with mild gastrointestinal complaints, headaches, and allergic skin reactions. There have been isolated reports of cerebral bleeding, and prolonged bleeding associated with ginkgo biloba use and this may be intensified with antidepressant use. Also, there is a mild risk for bleeding of the eye and increased bleeding during surgery.”
During the two physiological assessments, participants were asked to abstain from caffeine and alcohol, and to refrain from engaging in any strenuous physical activity for 12 h prior to the visits. In addition, because rate of GBE absorption may be influenced by food in the stomach, participants were asked to refrain from eating for 4 h prior to the sessions. These two sessions were conducted over two consecutive days scheduled during the 12th and 28th day of the participant’s menstrual cycle. During the first session, participants completed two psychophysiological assessments: a baseline measure of the participant’s sexual response to the neutral-erotic video sequence and an assessment after the administration of either GBE or placebo.
During all physiological assessments, the participants were in a private internally locked room and they were asked to follow a standardized protocol. First, participants inserted the vaginal photoplethysmograph and relaxed on a recliner chair for 10 min (habituation time). Then, the first video sequence started and during this time participants indicated their level of subjective sexual arousal by moving the arousometer. Shortly after the end of this first video sequence (baseline), a GBE or a placebo pill was administered. The time from GBE/placebo ingestion to the onset of the second film sequence was approximately 90 min. This time period was chosen based on research which indicates the times to peak concentration for ginkgo–flavone glycosides, ginkgolides (A, B), and bilobalide are 1.5–3, 1–2, 1–2 h, respectively (Kleijnen & Knipschild, 1992). Participants were scheduled for a second assessment session in 2–3 days.
During the second physiological assessment session, participants viewed a video sequence after the ingestion of the pill they did not receive during their first session. As in the first session, a 90 min waiting period was used from the ingestion of the pill to the onset of the erotic video. At the end of the video, participants were instructed on how to complete the event logs and asked to do so after each sexual activity occurring in the following 2 weeks. Participants received $50 as compensation for their two physiological assessment sessions. After 2 weeks, participants were contacted and asked to mail in their event logs. Upon receiving the event logs, participants were scheduled for an orientation to the second part of the study that investigated the long-term (8 weeks) effects of GBE.
Long-term Effects of GBE, Placebo, Sex Therapy, Sex Therapy plus GBE
Participants who chose to participate in the long-term GBE treatment portion of the study were randomly assigned to one of four groups: GBE, Sex Therapy, Placebo, and Sex Therapy plus GBE. The Placebo and GBE groups were conducted using a double-blind protocol and the Sex Therapy plus GBE was conducted using a single blind protocol. The treatment lasted for 8 weeks and participants completed a mid-treatment at week 4, and a post-treatment assessment at week 8.
Prior to the beginning of treatment, participants were asked to attend an orientation visit. The orientation lasted 30 min and participants were asked to bring their partners. Of the women in the Placebo, GBE, Sex Therapy, and Sex Therapy plus GBE conditions, 13, 17, 15, and 10 partners, respectively, participated in the orientations. During this orientation, participants were shown a video explaining the rationale for using GBE and/or sex therapy for the treatment of sexual problems. Participants receiving GBE or placebo were told that GBE was expected to increase blood flow in the vaginal walls, which was expected to facilitate sexual arousal. Participants assigned to a sex therapy condition were told that the therapy was designed to increase one’s comfort and knowledge with one’s body and to decrease thoughts that distracted one from attending to pleasurable sexual sensations. Both rationales were given to women in the Sex Therapy plus GBE condition. The orientation was also used to assess participants’ motivation, to clarify the requirements of the study, and to answer any study-related questions. An additional goal of the orientation visit was to increase participants’ commitment and minimize potential drop out rates. At the end of orientation, participants in the GBE, Sex therapy plus GBE, or the Placebo conditions were given a bottle of 28 pills and instructed to take one pill orally once per day approximately 1 h before they would normally engage in sexual activities. Participants in the Sex Therapy and in the Sex Therapy plus GBE conditions met with the therapist to arrange their first session of individual sex therapy. Women in the Sex Therapy plus GBE group were told that their pill may be either placebo or GBE. Participants were instructed to complete the event logs after every sexual activity and mail it in every week in the self-addressed, stamped envelopes that were provided. Telephone calls were made every week to remind participants to mail in their event logs.
After 4 weeks, participants were scheduled for a mid-treatment visit during which they completed the FSFI, the SSS-W, the sexual function interview, and laboratory measures of subjective and physiological sexual responses to the neutral/erotic video sequence. This visit lasted approximately 50 min and participants received $50 upon completion. After 8 weeks from the beginning of treatment, a post-treatment assessment was conducted identical to the 4 week mid-treatment assessment. Participants were compensated $50.00 for this visit. In order to check treatment compliance, for both the 4 week mid-treatment and 8 week post-treatment assessment visits, participants were asked to bring all of the pills that they had not used to their session. Participants who used less than 50% of the pills (>28 returned pills) on either visit were excluded from further analyses; this included three women in the GBE condition.
Results
Short-term Effects of GBE versus Placebo
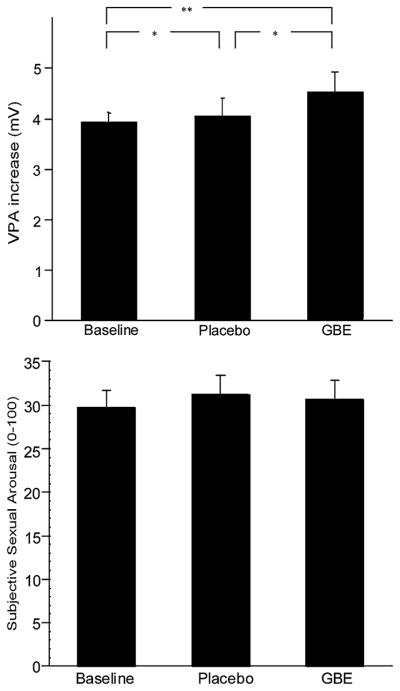
Figure 1 shows the means and SDs of the differences between neutral and erotic videos in VPA responses during the baseline, placebo, and GBE conditions. Women ranged between minus 0.11% to plus 61.57% of increase in VPA from neutral to erotic videos. A repeated-measures ANOVA on Condition (baseline, GBE, placebo) was conducted to assess whether there were differences among baseline, GBE, and placebo in VPA percentage increase in responses to erotic videos. There was a significant main effect of Condition, F(2, 196) = 3.44, p < .05. Two-way contrasts revealed significant differences between GBE and baseline, F(1, 97) = 5.99, p < .01, placebo and baseline, F(1, 97) = 4.32, p < .05, and GBE and placebo, F(1, 97) = 4.33, p < .05, with GBE being the condition associated with the highest VPA response (M = 4.84), followed by placebo (M = 4.34), and by baseline (M = 4.20). The effect sizes computed by using the original SDs of the two means (Dunlop, Cortina, Vaslow, & Burke, 1996) revealed that, although significant, the difference was of questionable clinical significance. The effect size between baseline and GBE (d = −0.19) was below what Cohen defined as a small effect size. Only approximately 14.7% of the VPA values in the GBE condition did not overlap with the VPA values in the baseline condition. The effect size between baseline and placebo was even smaller (d = −0.04) indicating that <7.7% of the VPA values in the placebo condition did not overlap with the VPA values of the baseline condition (Fig. 1).
Fig. 1.
VPA and subjective sexual responses to the sexual video during Baseline, Placebo, and GBE conditions. * p < .05, ** p < .01
Figure 1 shows the means and SDs of the subjective sexual responses to the erotic videos during the baseline, placebo, and GBE conditions. The average subjective rating of sexual arousal during the erotic film varied from plus 0.14 to 90.43 points (possible range, 0–100).
A repeated-measures ANOVA was used to assess differences in average scores of continuous subjective sexual arousal during the erotic video (measured using the arousometer) between baseline, placebo and GBE. No significant differences between baseline, placebo and GBE were detected with a repeated-measures ANOVA, F(2, 128) < 1. Women in each of the conditions showed an increase in subjective sexual arousal during exposure to the erotic video with the overall average increase being approximately 30 units (Fig. 1).
Exploratory analyses were conducted to examine whether a single dose of 300 mg GBE had a differential impact on sexual arousal between women with sexual problems attributable and not attributable to antidepressant medication. Results of two between groups (Antidepressant, No Antidepressant) repeated-measures (baseline, placebo, GBE) ANOVAs revealed women on antidepressants (n = 36) showed a trend towards lower physiological sexual responses to the erotic videos, F(1, 97) = 3.45, p = .066 compared to women not taking anti-depressants (n = 63). There were no significant differences between women receiving and not receiving antidepressants in levels of subjective arousal to the erotic videos, F(1, 97) < 1. There were no significant interactions between Group and Condition for either VPA, F(2, 96) < 1, or subjective arousal, F(2, 96) < 1, suggesting that GBE did not have a differential impact on sexual arousal responses between women with sexual dysfunction secondary to, and not secondary to, antidepressant medication.
Long-term Effects of GBE, Placebo, Sex Therapy, and Sex Therapy plus GBE
Laboratory Measures of Sexual Arousal to Erotic Videos
Three women in the GBE condition were noncompliant in taking the GBE daily and returned more than 50% of their pills to the mid-treatment or post-treatment assessment visits. In an attempt to standardize amount of GBE in the bloodstream, data from these participants were excluded from further analyses. As per other comparable treatment-outcome clinical studies (e.g., Foa et al., 2005), we used an intent-to-treat approach to the outcome analyses, meaning that people who dropped out after the mid-treatment assessment (4 weeks) were assumed to have maintained the same level of functioning and physiological response at the post-treatment assessment visit. Therefore, mid-treatment data were considered representative of the post-treatment level of function and physiological response.
To examine the physiological effects of 8 weeks daily treatment with GBE, a 3-level hierarchical linear modeling (HLM) analysis was used to test whether VPA increased with the erotic video (Level 1), if this change increased from baseline to post-treatment (Level 2), and if there was a condition effect (i.e., GBE, Placebo, Sex Therapy, or Sex Therapy plus GBE) (Level 3). We selected HLM to analyze the VPA data because of the high between participants variance in VPA responses (Prause & Janssen, 2005) known to affect statistical analyses and because of the ability of HLM to provide robust results in spite of this variability (for a review, see Rellini, McCall, Randall, & Meston, 2005). Given that we compared more than two groups, dummy coding was used to compare GBE, Sex Therapy, and Sex Therapy plus GBE to Placebo. No other comparisons were tested in the following HLM analyses.
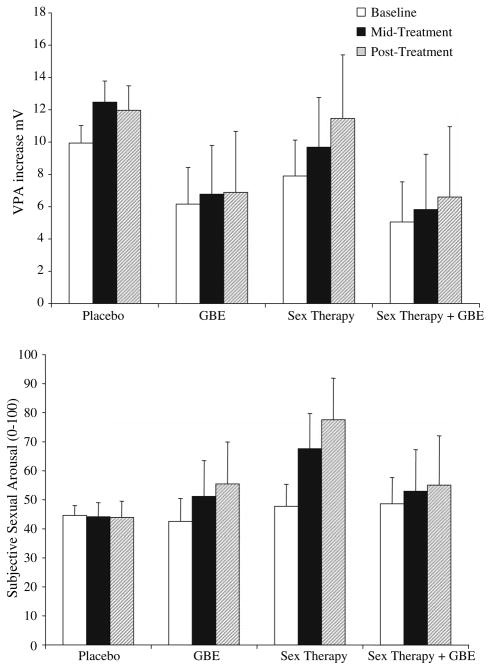
Figure 2 shows the average changes in VPA from neutral to erotic films during baseline, mid-treatment, and post-treatment for each of the treatment conditions (Placebo, GBE, Sex Therapy, Sex Therapy plus GBE). There were no significant differences between conditions in VPA responses to erotic films when measured at baseline. Specifically, women in the placebo condition showed a significant increase in VPA between neutral and erotic videos during baseline, β100 = 3.62, t(55) = 2.41, p < .05, and this increase was not significantly different from the increases in VPA between neutral and erotic films noted among women in the GBE, Sex Therapy, and Sex Therapy plus GBE when measured at baseline. During post-treatment, women in the placebo condition did not show a significant change in VPA responses to erotic films from measures taken at baseline, β110 = 1.01, t(55) = 1.17. When comparing sexual arousal at post-treatment with sexual arousal at baseline, GBE, Sex Therapy, and Sex Therapy plus GBE were not significantly different from placebo (β range, −0.76 to −0.22, t range, −0.86 to −0.22, p > .05). It is worth noting that the direction of the difference between placebo and the other conditions was opposite to predictions such that treatment-induced increases in VPA responses were higher in the Placebo condition as compared to the GBE, Sex Therapy, and Sex Therapy plus GBE conditions.
Fig. 2.
VPA and subjective sexual response at baseline, mid-treatment, and post-treatment for women in the Placebo, GBE, Sex Therapy, and Sex Therapy plus GBE Conditions
Figure 2 shows the average increases in VPA to erotic films during baseline, mid-treatment, and post-treatment for each of the treatment conditions (Placebo, GBE, Sex Therapy, Sex Therapy plus GBE). Changes in subjective sexual arousal as measured with the arousometer were also assessed with a 3-level HLM analysis. Women in the placebo condition showed an increase in subjective sexual arousal during baseline of approximately 44.61 units by the end of the erotic video, β100 = 0.081, t(55) = 7.32, p < .001. No significant differences in subjective sexual arousal during baseline were observed between women in the Placebo, GBE, Sex Therapy, or Sex Therapy plus GBE conditions.
After 4 weeks (mid-treatment) and 8 weeks (post-treatment) of treatment, women in the placebo condition showed no significant changes in their subjective sexual arousal response to the erotic video compared to their baseline, β110 = −0.000, t(55) < 1. At post-treatment, compared to baseline, women in the Sex Therapy condition reported a significantly greater increase in subjective sexual arousal to the erotic videos than did women in the placebo condition, β112 = 0.016, t(55) = 2.65, p < .01. There were no significant changes in subjective sexual arousal from baseline to post-treatment in women in the GBE condition, β111 = −0.0004, t(55) < 1, or in the Sex Therapy plus GBE condition, β113 = 0.0026, t(55) < 1, meaning that they did not show significantly greater subjective sexual arousal responses as treatment progressed.
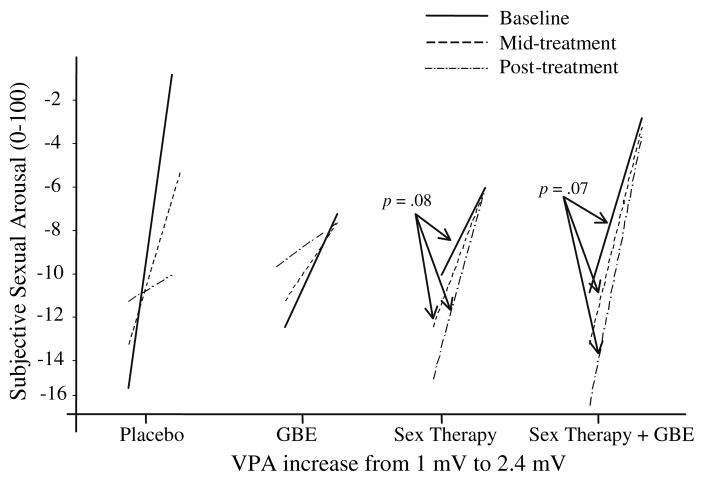
A 3-level HLM analysis was computed to assess the relationship between VPA and subjective sexual arousal and potential differences in this relationship during baseline, GBE, and placebo conditions. This analysis computed at Level 1 a regression line between VPA and continuous subjective sexual arousal for each participant during each of the three conditions. The slope and intercept coefficients were then used as outcome variables for the Level 2 analysis used to test whether there was a difference between conditions. Level 3 is usually used to conduct between participants analyses but, because in this part of the study, the research question was exclusively about within participants’ differences, we left Level 3 empty. In order to compare the three conditions, we used dummy coding to compare both placebo and GBE to baseline, leaving untested differences between placebo and GBE.
HLM coefficients of a 3-level HLM model were computed using subjective sexual arousal as the outcome variable, VPA as the within-participants predictor (Level 1), time as the covariate (Level 1), session (baseline versus post-treatment) as the Level 2 predictor (within-participants), and Condition as the Level 3 predictor (between-participants). The OLS analysis of the coefficients revealed that, during baseline, women in the placebo group showed a significant relationship between subjective sexual arousal and VPA, β100 = 10.97, t(56) = 2.45, p < .05, indicating that for each increase in 1 mV in VPA women overall responded with an increase in 10.97 subjective units of sexual arousal. The VPA/subjective sexual arousal relationship noticed during baseline in women from the placebo group was not significantly different from the VPA/subjective sexual arousal in women from the other groups (Fig. 3). At post-treatment, there was a trend for women in the Sex Therapy, β112 = 6.70, t(56) = 1.78, p = .08, and in the Sex Therapy plus GBE, β111 = 6.55, t(56) = 1.84, p = .07, conditions towards a stronger VPA/subjective sexual arousal relationship as compared to women in the placebo group. By contrast, at post-treatment, women in the placebo group showed a trend towards a weaker VPA/subjective sexual arousal relationship, β110 = −4.91, t(56) = −1.77, p = .08, compared to baseline.
Fig. 3.
Subjective sexual responses estimated when VPA increased from 1 mV to 2.4 mV during baseline, mid-treatment, and post-treatment visits for women in the Placebo, GBE, Sex Therapy, and Sex Therapy plus GBE conditions
Sexual Function Measures
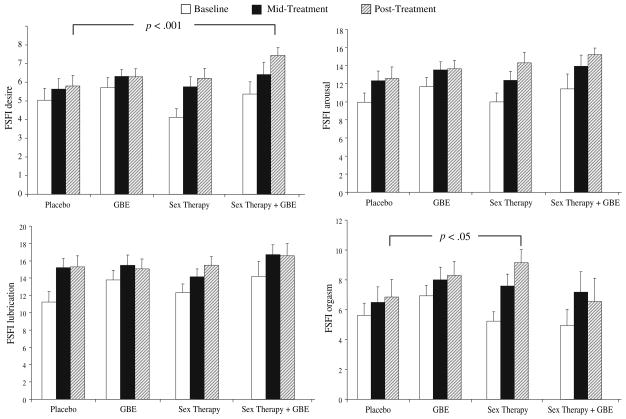
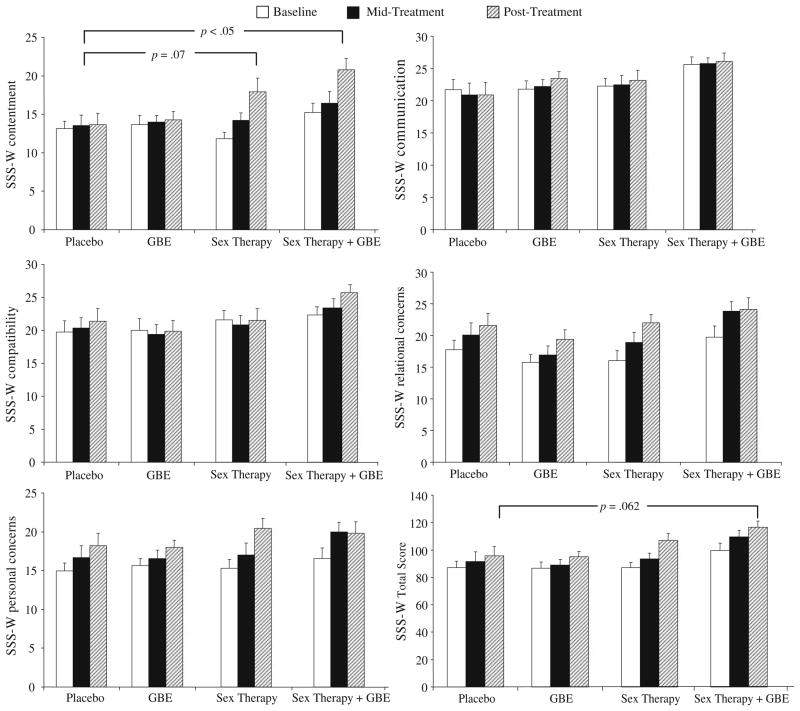
Figures 4 and 5 show the mean and SDs for several of the FSFI and SSS-W subscales measured at baseline, mid-treatment, and post-treatment for each of the treatment conditions (Placebo, GBE, Sex Therapy, Sex Therapy plus GBE). Analyses of sexual function were conducted using data from the FSFI, the SSS-W, and event log entries to compare the impact of Placebo, Sex Therapy, GBE, and Sex Therapy plus GBE on levels of sexual function in the domains of sexual desire, arousal, orgasm, and satisfaction.
Fig. 4.
Scores on the FSFI domains (Desire, Arousal, Lubrication, Orgasm) at baseline, mid-treatment, and post-treatment for women in the Placebo, GBE, Sex Therapy, and Sex Therapy plus GBE conditions.
Note: p values refer to contrast differences calculated for post-treatment scores when controlling for baseline scores as part of univariate ANOVAs computed separately for each of the FSFI factors indicated
Fig. 5.
Scores on the SSS-W factors (Contentment, Communication, Compatibility, Relational Concern, and Personal Concern) and full scale at baseline, mid-treatment, and post-treatment for women in the Placebo, GBE, Sex Therapy, and Sex Therapy plus GBE conditions.
Note: p values refer to contrast differences calculated for post-treatment scores when controlling for baseline scores as part of univariate ANOVAs computed for each SSS-W factors and the full score
A univariate ANOVA was used to test group differences in levels of sexual desire (FSFI desire domain) in the intent-to-treat sample while controlling for baseline levels of sexual desire. A significant condition effect on sexual desire at post-treatment was observed, F(4, 59) = 6.36, p < .001. A two-way comparison indicated that women in the Sex Therapy plus GBE condition showed a significantly greater level of sexual desire at post-treatment compared to placebo (contrast estimate = 1.52, p < .05). This difference was characterized by a large effect size (d = −0.88), indicating that approximately 50% of the desire scores of women in the Sex Therapy plus GBE group did not overlap with the desire scores of the placebo condition. Sexual desire at post-treatment was higher in women in the Sex Therapy condition as compared to placebo but it was not statistically significant (contrast estimate = .881) (see Fig. 4). The lack of significance despite the large effect size may be a consequence of the small sample size. Also, there was no interaction effect between condition and time (baseline and post-treatment, F(3, 59) = 1.82.
The same model was used to test the arousal, lubrication, and orgasm domains of the FSFI (see Fig. 4). When controlling for baseline scores of sexual arousal, post-treatment levels of psychological arousal measured with the FSFI (arousal domain) were higher for women in the Sex Therapy plus GBE condition (M = 14.90), followed by Sex Therapy (M = 14.35), GBE (M = 13.56), and placebo (M = 12.81), but these differences were not statistically significant, F(3, 60) < 1. Physiological arousal measured with the FSFI Lubrication subscale indicated that women in the Sex Therapy plus GBE condition showed the highest scores at post-treatment (M = 15.58), followed by Sex Therapy (M = 15.18), placebo (M = 15.96), and GBE (M = 14.92), F(3, 59) < 1.
When controlling for baseline measures, orgasm (measured using the FSFI Orgasm domain) at post-treatment showed a significant difference between the Sex Therapy and Placebo conditions (contrast = 2.48, p < .05), although no overall difference was detected in the between-participants analysis, F(3, 57) = 1.77. The effect size for orgasm difference was medium (d = 0.76), indicating that, at post-treatment, approximately 43% of scores on orgasm in the placebo condition did not overlap with orgasm score in the Sex Therapy condition (see Fig. 4).
The overall SSS-W score for sexual satisfaction at post-treatment was used as an outcome variable in a univariate ANOVA, where baseline sexual satisfaction was used as a covariate. The two-way contrasts compared Placebo to GBE, Sex Therapy, and Sex Therapy plus GBE (Fig. 5). Women in the Sex Therapy plus GBE condition showed a trend towards a higher score in overall sexual satisfaction as compared to women in the placebo condition (contrast estimate = 15.78, p = .062). Follow-up analyses were conducted to investigate whether the difference observed between women in the Sex Therapy plus GBE condition and Placebo was due to specific subfactors of the SSS-W. Indeed, only Factor 1, Contentment, was significantly different between conditions at post-treatment, F(3, 57) = 3.22, p < .05, when accounting for baseline scores (Fig. 5). In particular, women in the Sex Therapy plus GBE condition showed a significantly higher level of sexual contentment as compared with women in the placebo condition (estimated contrast = 4.94, p < .05). This difference was characterized by a large effect size (d = −0.93), indicating that approximately 51.6% of the scores on the Contentment factor in the Sex Therapy plus GBE condition did not overlap with contentment scores in the placebo condition. Women in the Sex Therapy condition also had higher levels of sexual contentment at post-treatment as compared to placebo, but the difference was not statistically significant (estimated contrast = 3.74, p =.07). The effect size of this difference was medium (d = −0.63), indicating that approximately 39% of the scores in contentment among women in the Sex Therapy condition did not overlap with the scores on the Contentment factor for women in the placebo condition.
Event log entries were returned by 47 women: nine women in the placebo condition, 15 women in the GBE group, 13 in the Sex Therapy group, and nine in the Sex Therapy plus GBE group. A set of four 2-level HLM analyses was used to test for treatment-induced improvements in the ratio of (1) satisfying sexual encounters, (2) frequency of orgasm, (3) the ability to become physically sexually aroused, and (4) the ability to become mentally sexually aroused. Given that there were missing data, HLM provided an optimal alternative to repeated measures ANOVA since HLM results are known to be robust to missing data (Bryk, Raudenbush, & Congdon, 1996).
Results indicated a trend towards an increase in percentage of satisfying sexual encounters for women in the Placebo group, β01 = 0.03, t(41) = 1.93, p = .061. No significant differences were observed between women in the GBE, β11 = −0.001, t(41) < 1, Sex Therapy, β21 = −0.022, t(41) < 1, and Sex Therapy plus GBE, β31 = 0.014, t(41) < 1, conditions compared to placebo, indicating that all women showed a tendency towards improvement in frequency of satisfying sexual behaviors at post-treatment independent of the condition to which they were assigned.
Discussion
This study provided a comprehensive examination of the short-term and long-term effects of GBE for treating women’s sexual dysfunction. The short-term effects of GBE were examined using a double-blind, placebo-controlled, within subjects design in which laboratory measures of subjective and physiological sexual arousal to neutral and erotic film stimuli were measured 90 min after ingestion of either 300 mg GBE or placebo. It was predicted that women would attain higher levels of sexual arousal in response to the erotic videos after having received GBE versus placebo. This was based, in part, on speculation that GBE could enhance vascular flow to the genitals in a similar manner to the mechanism by which it enhances cerebral perfusion, and by having a direct effect on prostaglandins which are known to enhance sexual arousal in men (Cohen & Bartlik, 1998). Consistent with prediction, VPA responses to erotic stimuli were significantly higher in women who received GBE compared with women who received placebo. The effect size was small, however, and, combined with the fact that, contrary to prediction, subjective reports of arousal were not significantly higher with GBE versus placebo, this leads one to question the clinical significance of this finding. Whether the positive impact of GBE on sexual arousal would have been greater among sexually dysfunctional women with subnormal capillary vascular function is a topic for future study.
GBE did not differentially impact physiological or subjective sexual arousal responses among women with sexual difficulties secondary to and not secondary to antidepressant medication. Interestingly, VPA responses to erotic videos showed a trend towards being lower among women receiving versus not receiving antidepressant medication. To our knowledge, this is the first study to compare physiological levels of arousal between these groups of women. The finding that levels were lower among women receiving antidepressants is consistent with the literature indicating a high incidence of arousal difficulties reported among women on antidepressant treatment (e.g., Meston & Gorzalka, 1992; Rosen et al., 1999). While speculative, the fact that levels were lower among women on antidepressant medication, compared with women not on antidepressants but who also reported arousal dysfunction, suggests a potential physiological difference between these subgroups of women who both self-report arousal concerns. This finding should, of course, be interpreted with caution given that it is well known that wide variability exists between women on laboratory measures of VPA responses and between groups comparisons may not be especially reliable (Janssen, 2001). Future research that examines sexual responses before, during, and even after the termination of antidepressant treatment would provide valuable insight into the impact of antidepressant mediation on sexual responding in women.
It was predicted that, independent of any potential short term effects of GBE on arousal, long-term treatment with GBE would enhance sexual function in women. This was based on research showing chronic GBE has a relaxant effect on vascular smooth muscle (e.g., Auguet & Clostre, 1983), and has NO scavenging properties (e.g., Marcocci et al., 1994), both of which are known to play a role in the female sexual arousal response, and on limited findings from past uncontrolled studies indicating a decrease in antidepressant-induced sexual side effects among women after four weeks of GBE treatment (Cohen & Bartlik, 1998). In the present study, the long-term effects of GBE were examined by comparing the effects of 8 weeks treatment with 300 mg/day GBE to 8 weeks of either Placebo, Sex Therapy, or Sex Therapy plus GBE. Outcome measures included laboratory measures of sexual arousal to erotic videos, validated measures of sexual function and satisfaction, and daily event log entries.
Contrary to prediction, 8 weeks daily treatment with GBE did not significantly increase VPA or subjective sexual arousal responses to erotic stimuli. The finding that a one time dose of 300 mg GBE (90 min prior to viewing the videos) enhanced VPA responses compared with placebo, albeit a small effect, and long-term GBE (daily for 8 weeks) did not, suggests that any facilitatory effect of GBE on vasocongestion occurs shortly after GBE is absorbed into the bloodstream. In other words, the tendency for long term treatment with GBE to increase overall peripheral blood flow (Smith et al., 1996) does not seem to translate into a sexual arousal specific increase in genital engorgement. This finding may, of course, be limited to a laboratory setting and not generalizable to a real life sexual context.
Women in the Sex Therapy condition showed a significant increase in subjective reports of sexual arousal to the erotic videos compared with placebo. Women in the Sex Therapy plus GBE condition also showed an increase in subjective responses to the erotic films compared with placebo but the increase did not reach significance. The fact that GBE did not have an additive effect on outcome measures suggests that any beneficial impact of these interventions on laboratory measures of subjective sexual arousal was due to the sex therapy component of treatment. In both conditions, the primary focus of the sex therapy was on training women to attend to genital changes and to interpret them in a positive sexual manner. Whether due to an increased comfort with experiencing and/or expressing feelings of sexual arousal, being less distracted by negative thoughts, or feeling more “in tune with” sexual sensations, the type of sex therapy intervention used in the present study seems to have enhanced the women’s psychological experience of becoming aroused to the erotic videos.
We predicted that a higher concordance between laboratory measures of subjective and physiological arousal would be seen in the Sex Therapy plus GBE condition compared with GBE, Placebo, or Sex Therapy alone conditions. This was based on speculation that GBE would increase women’s genital sensations of arousal and the sex therapy would train women to attend to those sensations, together resulting in an increased concordance between measures. Findings did not reach significance, but several interesting trends warrant discussion. As predicted, the relationship between subjective and physiological measures of sexual arousal increased from baseline in women who received sex therapy plus GBE. Relations also strengthened compared with baseline in women who received sex therapy alone. A similar positive trend was not seen among women in the Placebo or GBE conditions.
Recently, a great deal of attention has been placed on the issue of concordance between subjective and physiological measures of sexual arousal in women (for review, see Prause & Janssen, 2005). Chivers, Seto, Lalumiere, Laan, and Grimbos (2005) reported the results of a meta-analysis indicating an average correlation of only .32 between subjective and physiological laboratory measures of sexual arousal in women. Rellini et al. (2005) proposed that, rather than using single data points to conduct such analyses, as has been done in the past, a more appropriate way to analyze the relationship between VPA and subjective sexual arousal is to continuously and simultaneously measure the two variables throughout exposure to sexual and non-sexual films and to use HLM for the statistical analysis (for discussion of advantages to using HLM with VPA data, see Rellini et al., 2005).
Using this technique, Rellini et al. (2005) reported significant relations between VPA and subjective sexual arousal in sexually healthy women, and Meston, Rellini, and McCall (2005) found significant relations between measures in women with Sexual Arousal Disorder, albeit significantly less strong than correlations noted among sexually functional women. Although only a trend, the present results add to these earlier findings in that they suggest that correlations between measures can be increased in women who are trained to attend to genital sensations. The question of clinical relevance is to what degree is the increase in subjective reports of sexual arousal, both in the laboratory and in real life scenarios (FSFI arousal), related to an increase in synchrony between psychological and physiological sexual arousal? In other words, does increasing the synchrony between genital and psychological arousal increase a woman’s experience of feeling sexually aroused? Unfortunately, power limitations rendered us unable to test this hypothesis using the present data.
In addition to increasing laboratory measures of sexual arousal, it was predicted that 8 weeks daily treatment with GBE would increase more long term indices of sexual functioning, as measured by the FSFI domains of desire, arousal, lubrication, and orgasm, and by the SSS-W satisfaction domains. Partly consistent with predictions, women who received sex therapy plus GBE showed significant and substantial increases in sexual desire and contentment, and non significant increases in arousal and lubrication from baseline measures compared with women in the Placebo condition. Inconsistent with predictions, women who received GBE alone, did not show a significant increase in these measures beyond placebo. Women receiving sex therapy alone showed a trend towards increased desire, arousal, lubrication and contentment, but not of the same magnitude as that seen by women receiving a combination of treatments. Orgasm function was significantly and substantially improved from baseline only in the Sex Therapy compared with Placebo condition. Together, these findings suggest that sex therapy contributed substantially to enhancing desire, arousal, orgasm, and contentment in women, and combining GBE with therapy had an additive effect on all but one (i.e., orgasm) of these outcome measures. The fact that orgasm function was significantly higher in the Sex Therapy versus Placebo condition, but not in the Sex Therapy plus GBE versus Placebo condition is noteworthy and may suggest that long-term treatment with GBE had a negative impact women’s orgasm ability. Because women receiving Sex Therapy plus GBE were told the pill was either GBE or Placebo, we were, unfortunately, unable to determine whether the additive effect of GBE with sex therapy was the result of specific pharmacological properties of GBE or, alternatively, the result of a placebo effect.
In the present study, a placebo effect was noted with short-term administration on subjective measures of sexual arousal in the laboratory, and with long-term administration on validated measures of desire, arousal, lubrication, and orgasm function, and on event log records of satisfying sexual encounters. Several studies examining the impact of sildenafil on women’s sexual dysfunction have also noted substantial placebo effects (e.g., Basson & Brotto, 2003; Basson et al., 2002), and it is now well known that the placebo effect is a strong agent in managing sexual dysfunction (for a discussion of the mechanisms by which placebos enhance treatment outcome effects, see Kirsch, 1997; Straus & von Ammon, 1996). Future research that attempts to parse out the potential components of placebo treatment on sexual function in women would provide enormous insight into developing effective treatments for women’s sexual dysfunction.
Three overall conclusions can be drawn from this study. First, neither short- or long-term administration of GBE alone substantially impacts sexual function in women with sexual desire, arousal, and/or orgasm difficulties beyond the improvements seen with placebo. Second, the results confirm past findings that indicate a substantial placebo effect on sexual function exists in women with sexual concerns, and suggest the placebo effect is operative with both short term and long term treatment. Third, the present findings indicate that sex therapy, designed to train women to attend to genital cues, has a positive impact on women’s sexual function, including measures of desire, arousal, orgasm and contentment. Limitations of the present study include sample sizes that limited the ability to examine potential predictor variables of outcome success (e.g., coexistent sexual dysfunction), and the absence of a Sex Therapy plus Placebo condition which made interpretation of the differences between women in the Sex Therapy plus GBE and Sex Therapy alone conditions speculative.
Acknowledgments
This publication was made possible by Grant Number 5 RO1 AT00224-02 from the National Center for Complementary and Alternative Medicine to Cindy Meston. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine. The authors wish to thank Jessica Davis for her assistance in all phases of this investigation.
Footnotes
The therapist manual is available from the corresponding author upon request.
References
- Auguet M, Clostre F. Effects of an extract of ginkgo biloba and diverse substances on the phasic and tonic components of the contraction of an isolated rabbit aorta. General Pharmacology. 1983;14:277–280. doi: 10.1016/0306-3623(83)90010-1. [DOI] [PubMed] [Google Scholar]
- Auguet M, DeFeudis FV, Clostre F. Effects of ginkgo biloba on arterial smooth muscle responses to vasoactive stimuli. General Pharmacology. 1982;13:169–171. doi: 10.1016/0306-3623(82)90075-1. [DOI] [PubMed] [Google Scholar]
- Azadzoi KM, Kim N, Brown ML, Goldstein I, Cohen RA, Saenz de Tejada I. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. Journal of Urology. 1992;147:220–225. doi: 10.1016/s0022-5347(17)37201-4. [DOI] [PubMed] [Google Scholar]
- Basson R, Brotto LA. Sexual psychophysiology and effects of sildenafil citrate in oestrogenised women with acquired genital arousal disorder and impaired orgasm: A randomized controlled trial. British Journal of Obstetrics and Gynecology. 2003;100:1014–1024. [PubMed] [Google Scholar]
- Basson R, McInnes R, Smith MD, Hodgson G, Kippiker N. Efficacy and safety of sildenafil citrate in women with sexual dysfunction associated with Female Sexual Arousal Disorder. Journal of Women’s Health & Gender-Based Medicine. 2002;11:367–377. doi: 10.1089/152460902317586001. [DOI] [PubMed] [Google Scholar]
- Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: An orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. International Journal of Impotence Research. 1996;8:47–52. [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW, Congdon RT. Hierarchical linear and nonlinear modeling with the HLM/2L and HLM/3L programs. 2. Chicago: Scientific Software International Inc; 1996. [Google Scholar]
- Burnett AL. Nitric oxide in the penis: Physiology and pathology. Journal of Urology. 1997;157:320–324. [PubMed] [Google Scholar]
- Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: A double-blind, cross-over, placebo-controlled study. British Journal of Obstetrics and Gynaecology. 2001;108:623–628. doi: 10.1111/j.1471-0528.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- Chivers ML, Seto MC, Lalumiere ML, Laan E, Grimbos T. The agreement of genital and subjective measures of sexual arousal: A meta-analysis. Poster presentation to the annual meeting of the International Academy of Sex Research; Ottawa, Canada. 2005. Jul, [Google Scholar]
- Christen Y, Courtois Y, Droy-Lefaix MT. Effects of ginkgo biloba extract (EGB 761) on aging and age-related disorders. Paris, France: Elsevier; 1995. [Google Scholar]
- Cohen AJ, Bartlik B. Ginkgo biloba for antidepressant-induced sexual dysfunction. Journal of Sex and Marital Therapy. 1998;24:139–143. doi: 10.1080/00926239808404927. [DOI] [PubMed] [Google Scholar]
- DeFeudis FV. Ginkgo biloba extract (GBE 761): Pharmacological activities and clinical applications. Paris, France: Elsevier; 1991. [Google Scholar]
- Dinsmore WW, Hodges M, Hargreaves C, Osterloh IH, Smith MD, Rosen RC. Sildenafil citrate (Viagra) in erectile dysfunction: Near normalization in men with broad-spectrum erectile dysfunction compared with age-matched healthy control subjects. Urology. 1999;53:800–805. doi: 10.1016/s0090-4295(98)00586-x. [Erratum in: Urology, 1999, 53, 1072] [DOI] [PubMed] [Google Scholar]
- Dunlop WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods. 1996;1:170–177. [Google Scholar]
- Ellison JM, DeLuca P. Fluoxetine-induced genital anesthesia relieved by ginkgo biloba extract. Journal of Clinical Psychiatry. 1998;59:199–200. doi: 10.4088/jcp.v59n0409f. [DOI] [PubMed] [Google Scholar]
- FDA Guidance document. Guidance for industry: Female sexual dysfunction: Clinical development of drug products for treatment. 2000 http://www.fda.gov/cder/guidance/3312dft.htm.
- Foa EB, Hembree EA, Cahill SP, Rauch SAM, Riggs DS, Feeny NC, et al. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: Outcome at academic and community clinics. Journal of Consulting and Clinical Psychology. 2005;73:953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. New England Journal of Medicine. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- Janssen E. Psychophysiological measurement of sexual arousal. In: Wiederman MW, Whitley BE, editors. Handbook for conducting research on human sexuality. Mahwah, NJ: Erlbaum; 2001. pp. 139–171. [Google Scholar]
- Jung F, Morowietz C, Kiesewetter H, Wenzel E. Effect of ginkgo-biloba on fluidity of blood an peripheral microcirculation in volunteers. Drug Research. 1990;40:589–593. [PubMed] [Google Scholar]
- Kang B, Lee S, Kim M, Cho M. A placebo-controlled, double-blind trial of ginkgo biloba for antidepressant-induced sexual dysfunction. Human Psychopharmacology. 2002;17:279–284. doi: 10.1002/hup.409. [DOI] [PubMed] [Google Scholar]
- Kirsch I. Specifying nonspecifics: Psychological mechanisms of placebo effects. In: Harrington A, editor. The placebo effect: An interdisciplinary exploration. Cambridge, MA: Harvard University Press; 1997. pp. 166–186. [Google Scholar]
- Kleijnen J, Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136–1139. doi: 10.1016/0140-6736(92)93158-j. [DOI] [PubMed] [Google Scholar]
- Koltringer P, Eber O, Lind P, Langsteger W, Wakonig P. Mikrozirkulation und Viskoelastizitat des Vollblutes unter Ginkgo-biloba-extrakt. Eine plazebokontrollierte, randomisierte Doppelblind-Studie. Perfusion. 1989;1:28–30. [Google Scholar]
- Maitra I, Marcocci L, Droy-Lefaix MT, Packer L. Peroxyl radical scavenging activity of ginkgo biloba extract EGB 761. Biochemical Pharmacology. 1995;49:1649–1655. doi: 10.1016/0006-2952(95)00089-i. [DOI] [PubMed] [Google Scholar]
- Marcocci L, Maguire JJ, Droy-Lefaix MT, Paker L. The nitric oxide-scavenging properties of ginkgo biloba extract Egb 761. Biochemical and Biophysical Research Communications. 1994;201:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- Marks LS, Duda C, Dorey FJ, Macairan ML, Santos PB. Treatment of erectile dysfunction with Sildenafil. Urology. 1999;53:19–24. doi: 10.1016/s0090-4295(98)00525-1. [DOI] [PubMed] [Google Scholar]
- Meston CM. Validation of the Female Sexual Function Index (FSFI) in women with female orgasmic disorder and in women with hypoactive sexual desire disorder. Journal of Sex & Marital Therapy. 2003;29:39–46. doi: 10.1080/713847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meston CM, Gorzalka BB. Psychoactive drugs and human sexual behavior: The role of serotonergic activity. Journal of Psychoactive Drugs. 1992;24:1–40. doi: 10.1080/02791072.1992.10471616. [DOI] [PubMed] [Google Scholar]
- Meston CM, Heiman JR. Ephedrine-activated physiological sexual arousal in women. Archives of General Psychiatry. 1998;55:652–656. doi: 10.1001/archpsyc.55.7.652. [DOI] [PubMed] [Google Scholar]
- Meston CM, Rellini AH, McCall KM. The sensitivity of continuous laboratory measures of physiological and subjective sexual arousal for diagnosing women’s sexual dysfunction. Poster presentation to the annual meeting of the International Society for the Study of Women’s Sexual Health; Las Vegas, NV. 2005. Oct, [Google Scholar]
- Meston CM, Trapnell PD. Development and validation of a five factor’ sexual satisfaction and distress scale: The Sexual Satisfaction Scale for Women (SSS-W) Journal of Sexual Medicine. 2005;2:66–81. doi: 10.1111/j.1743-6109.2005.20107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montorsi F, McDermott TE, Morgan R, Olsson A, Schultz A, Kirkeby HJ, et al. Efficacy and safety of fixed-dose oral sildenafil in the treatment of erectile dysfunction of various etiologies. Urology. 1999;53:1011–1018. doi: 10.1016/s0090-4295(98)00643-8. [DOI] [PubMed] [Google Scholar]
- Paick JS, Lee JH. An experimental study of the effect of ginkgo biloba extract on the human and rabbit corpus cavernosum tissue. Journal of Urology. 1996;156:1876–1880. [PubMed] [Google Scholar]
- Park K, Goldstein I, Andry C, Siroky M, Krane RJ, Azadzoi K. Vasculogenic female sexual dysfunction: The hemodynamic basis for vaginal engorgement insufficiency and clitoral erectile insufficiency. International Journal of Impotence Research. 1997;9:27–37. doi: 10.1038/sj.ijir.3900258. [DOI] [PubMed] [Google Scholar]
- Park K, Moreland RB, Goldstein I, Atala A, Traish A. Sildenafil inhibits phosphodiesterase type 5 in human clitoral corpus cavernosum smooth muscle. Biochemical and Biophysical Research Communications. 1998;249:612–617. doi: 10.1006/bbrc.1998.9206. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Martin P, Lenegere A, Fromage S, Drieu K. Effects of an extract of ginkgo biloba (GBE 761) on “learned helplessness” and other models of stress in rodents. Pharmacology, Biochemistry and Behavior. 1990;36:963–971. doi: 10.1016/0091-3057(90)90107-s. [DOI] [PubMed] [Google Scholar]
- Prause N, Janssen E. Blood flow: Vaginal photoplethysmography. In: Goldstein I, Meston CM, Davis SR, Traish AM, editors. Women’s sexual function and dysfunction: Study, diagnosis, and treatment. London: Taylor & Francis; 2005. pp. 368–382. [Google Scholar]
- Rapin JR, Lamproglou I, Drieu K, DeFeudis FV. Demonstration of the “anti-stress” activity of an extract of ginkgo biloba (GBE 761) using a discrimination learning task. General Pharmacology. 1994;25:1009–1016. doi: 10.1016/0306-3623(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Rellini AH, McCall KM, Randall PK, Meston CM. The relationship between self-reported and physiological measures of female sexual arousal. Psychophysiology. 2005;42:116–124. doi: 10.1111/j.1469-8986.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Rellini AH, Meston CM. The sensitivity of events logs, self-administered questionnaires and photoplethysmography to detect treatment-induced changes in female sexual arousal disorder (FSAD) diagnosis. Journal of Sexual Medicine. 2006;3:283–291. doi: 10.1111/j.1743-6109.2005.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen R, D’Agostino R, Brown C, Heiman J, Leiblum S, Meston CM, et al. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. Journal of Sex and Marital Therapy. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- Rosen R, Lane R, Menza M. Effects of SSRIs on sexual function: A critical review. Journal of Clinical Pharmacology. 1999;19:67–85. doi: 10.1097/00004714-199902000-00013. [DOI] [PubMed] [Google Scholar]
- Sintchak G, Geer JH. A vaginal plethysmograph system. Psychophysiology. 1975;12:113–115. doi: 10.1111/j.1469-8986.1975.tb03074.x. [DOI] [PubMed] [Google Scholar]
- Smith PF, Maclennan K, Darlington CL. The neuroprotective properties of the ginkgo biloba leaf: A review of the possible relationship to platelet-activating factor (PAF) Journal of Ethnopharmacology. 1996;50:131–139. doi: 10.1016/0378-8741(96)01379-7. [DOI] [PubMed] [Google Scholar]
- Stimmel GL, Dopheide JA, Stahl SM. Mirtazapine: An antidepressant with noradrenergic and specific serotonergic effects. Pharmacotherapy. 1997;17:10–21. [PubMed] [Google Scholar]
- Straus J, von Ammon C. Placebo effects: Issues for clinical practice in psychiatry and medicine. Psychosomatics. 1996;37:315–326. doi: 10.1016/S0033-3182(96)71544-X. [DOI] [PubMed] [Google Scholar]
- Toesca A, Stolfi VM, Cocchia D. Immunohistochemical study of the corpora cavernosa of the human clitoris. Journal of Anatomy. 1996;188:513–520. [PMC free article] [PubMed] [Google Scholar]
- Tyler VE. Ginkgo. In: Tyler VE, editor. The honest herbal. New York: Pharmaceutical Products Press; 1993. pp. 149–151. [Google Scholar]
- van Beek TA, van Scheeren HA, Rantio T, Melger WCh, Lelyveld GP. Determination of ginkgolides and bilobalide in ginkgo biloba leaves and phytopharmaceuticals. Journal of Chromatography. 1991;543:375–387. [Google Scholar]
- Wiegel M, Meston CM, Rosen RC. The Female Sexual Function Index (FSFI): Cross-validation and development of clinical cutoff scores. Journal of Sex and Marital Therapy. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- Wiegel M, Scepkowski LA, Barlow DH. Cognitive and affective processes in female sexual dysfunction. In: Goldstein I, Meston CM, Davis SR, Traish AM, editors. Women’s sexual function and dysfunction: Study, diagnosis, and treatment. London: Taylor & Francis; 2006. pp. 85–92. [Google Scholar]