Abstract
The purpose was to determine the relation between visual feedback gain and variability in force and whether visual gain-induced changes in force variability were associated with frequency-specific force oscillations and changes in the neural activation of the agonist muscle. Fourteen young adults (19–29 years) were instructed to accurately match the target force at 2 and 10% of their maximal voluntary contraction with abduction of the index finger. Force was maintained at specific visual feedback gain levels that varied across trials. Each trial lasted 20 s and the amount of visual feedback was varied by changing the visual gain from 0.5 to 1,474 pixels/N (13 levels; equals ~0.001–4.57°). Force variability was quantified as the standard deviation of the detrended force data. The neural activation of the first dorsal interosseus (FDI) was measured with surface electromyography. The mean force did not vary significantly with the amount of visual feedback. In contrast, force variability decreased from low gains compared to moderate gains (0.5–4 pixels/N: 0.09 ± 0.04 vs. 64–1,424 pixels/N: 0.06 ± 0.02 N). The decrease in variability was predicted by a decrease in the power of force oscillations from 0–1 Hz (~50%) and 3–7 Hz (~20%). The activity of the FDI muscle did not vary across the visual feedback gains. These findings demonstrate that in young adults force variability can be decreased with increased visual feedback gain (>64 pixels/N vs. 0.5–4 pixels/N) due to a decrease in the power of oscillations in the force from 0–1 and 3–7 Hz.
Keywords: Visual feedback, Visual gain, Force variability, EMG, Force oscillations, First dorsal interosseus
Introduction
The amount of visual feedback has been associated with influencing the amplitude of force variability (Slifkin et al. 2000; Vaillancourt and Russell 2002; Sosnoff and Newell 2006b; Tracy et al. 2007) and the structure of force output (Baweja et al. 2009b; Prodoehl and Vaillancourt 2009) during constant isometric contractions. Manipulations that have been used to alter the amount of visual feedback and examine its effects on force variability are the following: (1) gain of visual feedback (Sosnoff et al. 2006; Baweja et al. 2009b; Prodoehl and Vaillancourt 2009); (2) frequency of visual feedback (Slifkin et al. 2000; Sosnoff and Newell 2006a) and (3) removal of visual feedback for segments of the contraction (Vaillancourt and Russell 2002; Christou 2005; Tracy 2007; Welsh et al. 2007; Baweja et al. 2009b).
In this study, the amount of visual feedback was manipulated by changing the visual feedback gain across the widest range to-date to examine its effects on force variability. An important goal was to examine the relation between visual feedback gain and the amplitude of force variability, which is controversial in the literature. Previous studies have shown that when the amount of visual feedback (gain) increased from 2 to 64 pixels/N force variability decreased, whereas when the visual feedback increased beyond 256 pixels/N force variability increased (Sosnoff and Newell 2006b). The authors argued that the relation between the gain of visual feedback and force variability can be explained with a U-shape function. Partial support for the U-shape function can be argued from a study which compared moderate (15 pixels/N) and very high visual gains (3,000 pixels/N) and found similar force variability in young adults (Baweja et al. 2009b). Nonetheless, there are at least two findings that contradict the U-shape relation between the gain of visual feedback and force variability. First, recent findings by Prodoehl and Vaillancourt (2009) have shown that force variability decreased with increased amount of visual feedback at low visual gains (in angular dimension from 0.008° to 0.05°) and remained reduced at higher visual gains (0.125–1.95°). Second, there is evidence that removal of visual feedback which can be considered as 0 pixels/N reduced force variability relative to 12 and 51 pixels/N (Baweja et al. 2009b). Therefore, the relation between visual feedback gain and force variability may not be adequately described with a U-shape function when very low (e.g., <2 pixels/N) and very high (>512 pixels/N) visual gains are included.
Independent of whether the relation between visual feedback gain and variability of force is U-shaped (Sosnoff and Newell 2006b) or an exponentially decreasing function to a plateau (Prodoehl and Vaillancourt 2009), force variability is expected to vary with the gain of visual feedback. An additional goal was to determine whether the decrease in force variability with visual gain would be associated with changes in the structure of force and the amplitude of activation of the agonist muscle. Such measurements can provide additional information regarding the physiological mechanisms that can change force variability with amplified visual feedback. The purpose of this study, therefore, was twofold: (1) to determine the relation between gain of the visual feedback and variability in force and (2) to determine whether visual feedback gain-induced changes in force variability were associated with changes in frequency-specific force oscillations and changes in the average neural activation of the agonist muscle. Consistent with previous work (Baweja et al. 2009b), the effects of visual feedback gain on force variability were examined while subjects exerted a constant isometric force with abduction of the index finger because it involves a single agonist muscle [first dorsal interosseus (FDI) muscle during abduction of the index finger; see Chao et al. 1989; Li et al. 2003]. Parts of the findings have been reported in abstract form (Baweja et al. 2009a).
Methods
Fourteen young adults (19–29 years, 7 men and 7 women) volunteered to participate in the experiment. All subjects reported being healthy without any known neurological problems, were right-handed according to a standardized survey (Oldfield 1971), and had normal or corrected vision. The Institutional Review Board at the Texas A&M University approved the procedures, and subjects provided written informed consent before participation in the study. This study, therefore, has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Experimental arrangement
The subjects were seated comfortably in an upright position facing a 27 in. computer screen (SyncMaster™ 275T+, Samsung Electronics America, NJ, USA) that was located 1.25 m away at eye level. The monitor had 925 pixels vertically, and 1,900 pixels horizontally. The amount of visual information varied with the gain of the visual feedback for each subject and across subjects. The monitor was used to display the force produced by the abduction of the index finger. All subjects affirmed that they could see the display clearly. The left arm was abducted by 45° and flexed to ~90° at the elbow. The left forearm was pronated and secured in a specialized padding (Versa form™, AB Germa, Sweden). The thumb, middle, ring, and fifth fingers of the left hand were restrained with metal plates and there was approximately a right angle between the index finger and thumb. Only the left index finger was free to move. The left index finger was placed in an adjustable finger orthosis to maintain extension of the middle and distal interphalangeal joints (for a schematic see Taylor et al. 2003). The left hand (non-dominant) was used so the results could be compared with previous studies (Enoka et al. 2003). This arrangement allowed abduction of the index finger about the metacarpophalangeal joint in the horizontal plane, a movement produced almost exclusively by contraction of the FDI muscle (Chao et al. 1989; Li et al. 2003).
Force measurement
The constant isometric force produced by the abduction of the index finger was recorded with a one-dimensional force transducer (FORT 250 rigid-lever force transducer, World Precision Instruments Inc., FL, USA). The force signal was sampled at 200 Hz with a Power 1401 A/D board (Cambridge Electronic Design, UK) and stored on a personal computer.
EMG measurement
Abduction of the index finger is produced almost exclusively by the contraction of the FDI muscle (Chao et al. 1989; Li et al. 2003). The FDI muscle activity was recorded with Ag–AgCl sintered fixed-wire electrodes (4 mm diameter, model E220N-LS, In Vivo Metric, Healdsburg, CA, USA) and taped on the skin distally to the innervation zone (Homma and Sakai 1991). The recording electrodes were placed in line with the muscle fibers. The center-to-center distance between the two electrodes was 5 mm. The reference electrode was placed over the ulnar styloid process. The electromyography (EMG) signal was amplified (×2,000) and band pass filtered at 3–6,000 Hz (Grass Model 15LT system; Grass Technologies, West Warwick, RI, USA). The EMG signal was sampled at 2 kHz with a Power 1401 A/D board (Cambridge Electronic Design, UK) and stored on a personal computer.
MVC task
All subjects performed a maximal voluntary contraction (MVC) task before and after the constant isometric tasks. Subjects were instructed to increase the force from baseline to maximum over a 3-s period and maintain the maximal force for about 4–7 s. Five such recordings were made or until two of the maximal trials were within 5% of each other. The MVC force was quantified as the average force over the 3–6 s (constant part) of the highest trial. This procedure allows the identification of a more conservative MVC that reflects the capability of a person to perform constant isometric contractions.
Constant isometric force task
A custom-written program in Matlab® (Math Works™ Inc., Natick, MA, USA) manipulated the targeted force level, and gain of visual feedback. The target force was provided as a red horizontal line in the middle of the monitor (background) and the force exerted by the subjects as a blue line progressing with time from left to right. Each subject was presented with two constant force targets at 2 and 10% MVC, which were counterbalanced across subjects. The subjects were instructed to gradually push against the force transducer and increase their force to match the red line (target force) within 3 s. When the target was reached, subjects were instructed to maintain their force (blue line) on the target (red line). The whole trial lasted 20 s and the subjects were instructed to match the target as accurately and consistently as possible for the last 10 s of each trial which was marked by a black vertical line in the center of the screen (Fig. 1). The gain of visual feedback was manipulated by changing the ordinate scale, while the abscissa remained the same. Because the resolution (number of pixels) of the computer screen remained the same throughout the experiment, manipulation of the ordinate scale resulted in 13 distinct visual feedback gains equal to 0.5, 1, 2, 3, 4, 8, 16, 32, 64, 128, 356, 712, and 1,424 pixels/N. Subjects performed 39 trials at every force level, 3 at each visual feedback gain. Within each force level (blocked), the rest time between each trial was 15 s and between visual feedback gains 30 s. The rest time between force levels was 1 min. The order for the visual feedback gains was randomized and the force levels were counterbalanced among subjects.
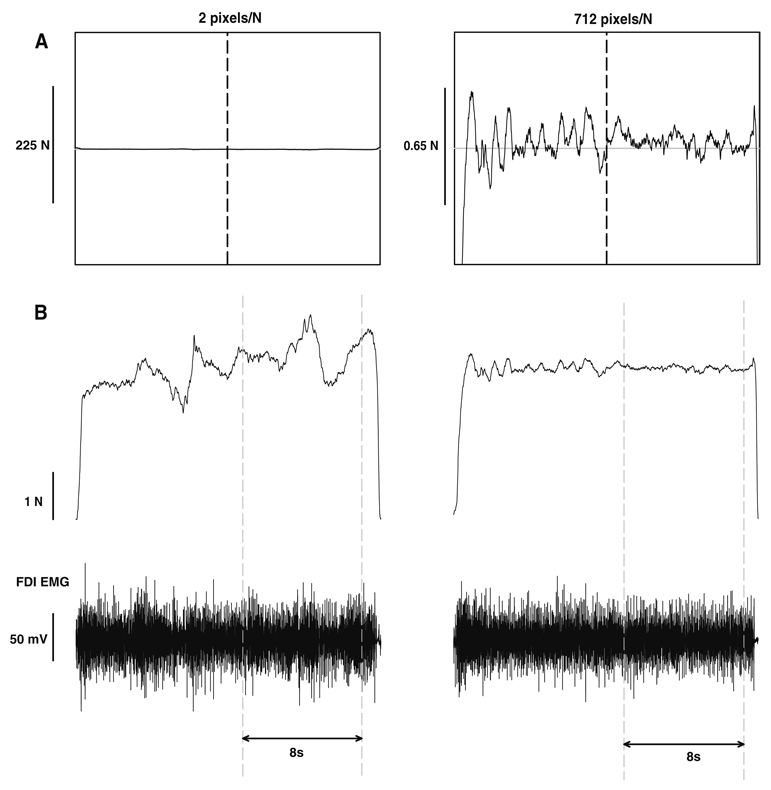
Fig. 1.
Constant isometric force task with the FDI muscle. Each subject was instructed to exert a force with abduction of the index finger against a force transducer and match the horizontal target line for 20 s. The subjects were asked to match the target accurately and consistently for the last 10 s of each trial marked by the vertical black line in the center of the screen at 10 s. a Representative trial from one subject when exerting a constant isometric force at 10% MVC with a visual feedback gain of 2 pixels/N (left column) and 64 pixels/N (right column). b The force and EMG analysis was based on the selected segment of each trial. The top row represents the force trace for the trials represented in a and the bottom row is the corresponding FDI EMG activity. The analysis was performed from 11 to 19 s on each trial
Data analysis
Data were acquired with the Spike2 software (Version 6.02; Cambridge Electronic Design, Cambridge, UK) and analyzed off-line using custom-written programs in Matlab (Math Works Inc., Natick, MA, USA). The force and surface EMG signals were analyzed over a segment of 8 s, between 11 and 19 s of the trial (Fig. 1). Thus, the data analysis did not include the first second after the trial initiation and the last second of the constant isometric contraction. Prior to data analysis, the force output was filtered with a fourth-order (bi-directional) Butterworth filter using a 20-Hz low-pass cut-off. The standard deviation of force was quantified from the detrended force output of the 8 s because any drift from the targeted force could influence the force variability. This was achieved by removing the linear trend from the force data. The dependent variables were the mean force, standard deviation of force, and the amplitude of the EMG signal [root mean square (RMS) of interference signal (Farina et al. 2004)].
In addition, a Fourier analysis was performed on the force and EMG signals (Christou 2005). Autospectral analysis of the force and EMG signals were obtained using Welch’s average periodogram method with a non-overlapping Hanning window (Matlab). The length of the data segment was 8 s and the sampling frequency was 1 kHz. The window size was 4,096, which gave a resolution of 0.244 Hz. For statistical comparisons, the frequency data of the force signal were divided into 0–1, 1–3, 3–7, and 7–10 Hz frequency bands (see Slifkin and Newell 2000 for bands of interest in force). The dependent variable for the spectral analysis of the force signal was the absolute peak power in the above bins. The frequency data of the EMG signal were divided into 0–7, 7–13, 13–30, 30–50, and 50–100 Hz frequency bands (see Brown 2000 for frequency bands of interest in EMG). The dependent variable for the spectral analysis of the EMG signal was the absolute peak power in the above bins.
Statistical analysis
A two-way ANOVA (2 forces × 13 visual gains) with repeated measures on all factors compared mean force, and SD of force for the different force levels and visual feedback gains. Because there were only significant main effects for force and visual feedback gain, we compared two distinct visual gains (2 and 64 pixels/N) with a two-way ANOVA (2 forces × 2 visual feedback gains) to determine the changes in the force power spectrum and muscle activity (see “Results” section). A three-way ANOVA (2 forces × 2 visual feedback gains × 4 frequency bins) with repeated measures on all factors compared the absolute power in the force spectrum for the different visual feedback gains and frequency bins. A similar model (2 forces × 2 visual feedback gains × 5 frequency bins) compared the absolute power in the EMG spectrum for the different visual feedback gains and frequency bins. Multiple linear regression analysis (stepwise) was used to examine the contribution of the change in power at each frequency bin from the force spectrum to the change in the SD of force between visual feedback gains. Any subjects that exhibited values outside ±3SD were excluded as outliers (all the subjects in this manuscript exhibited values within ±3SD). The change for each variable was quantified as follows: [(values at 64 pixels/N − values at 2 pixels/N)/values at 2 pixels/N] × 100. The relative importance of the predictors was estimated with the part correlations (part r), which provide the correlation between a predictor and the criterion, that were used to examine the unique contribution of each of the frequency bins in the force spectrum to the SD of force.
Analyses were performed with the SPSS 16.0 statistical package (SPSS Inc., Chicago, IL, USA). Significant interactions from the ANOVA models were followed by appropriate post hoc analyses. For example, differences among force levels and visual feedback gains were followed with paired t tests. The alpha level for all statistical tests was 0.05. Data are reported as mean ± SD within the text and as mean ± SEM in the figures. Only the significant main effects and interactions are presented, unless otherwise noted.
Results
MVC force
To determine whether the experimental procedures induced muscle fatigue to the subjects, the MVC force was compared before and immediately after the experimental session. The MVC force did not change significantly (t = 0.051, P = 0.96). Specifically, the MVC force was 23.02 ± 8.5 N prior to the experimental protocol and 22.96 ± 9.05 N after the experimental protocol. These findings demonstrate that the experimental protocol did not induce any fatigue.
Mean force and force variability
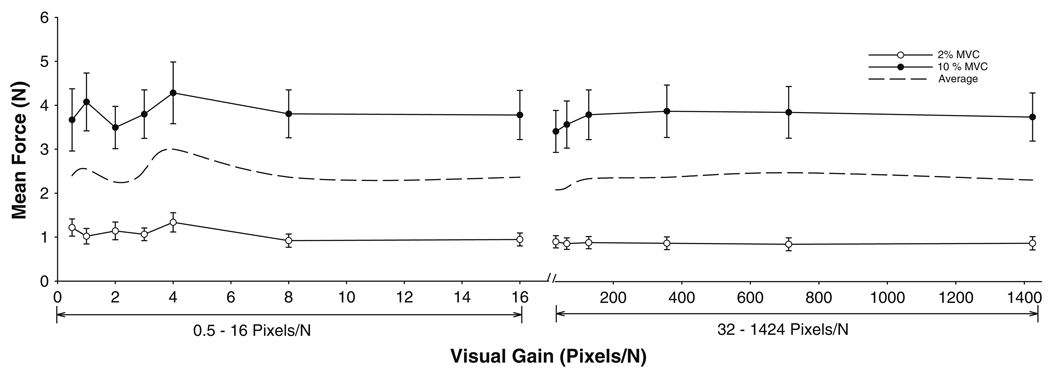
As expected, the mean force increased significantly with force level (F1,9 = 22.2, P < 0.001; Fig. 2) and the visual feedback gains were different from each other (visual gain main effect: F12,108 = 3.7, P = 0.038). The force × visual feedback gain interaction was not significant, indicating that the mean force did not vary differently for the 2 and 10% with a change in the visual feedback gain (Fig. 2).
Fig. 2.
Mean force as a function of visual feedback gains. The mean force was similar across the feedback gains for 2% (open circles) and 10% (filled circles) MVC. The dashed line is the averaged function of the two forces, indicating that the mean force was not significantly different across the 13 visual gains
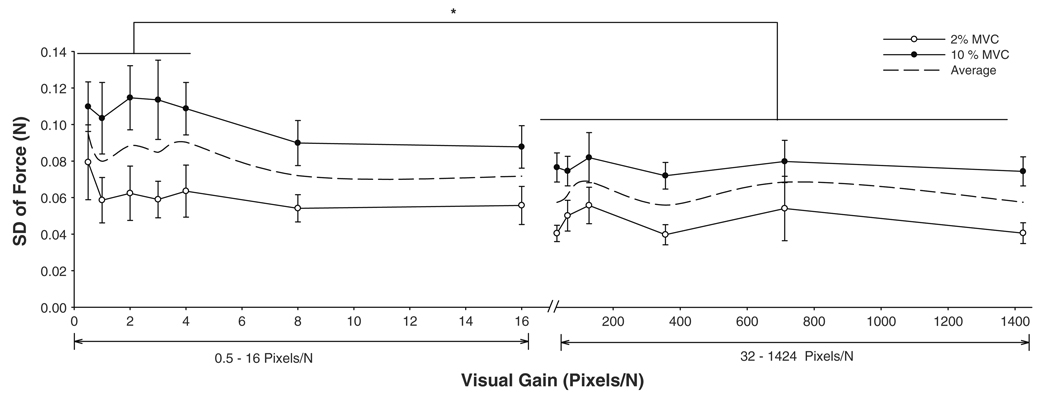
Force variability was quantified as the SD of force. The SD of force increased significantly with the force level (F1,9 = 19.8, P = 0.002; Fig. 3) and the SD of force was lower with greater visual feedback gains (visual feedback gain main effect: F12,108 = 2.5, P = 0.006). Pair-wise comparisons of the SD of force among visual feedback gains revealed that the variability of force at the first five visual feedback gains (0.5–4 pixels/N) was not significantly different across these visual conditions. Similarly, the SD of force was not significantly different across the last six visual feedback gains (64–1,424 pixels/N). However, the SD of force was significantly lower at the higher visual feedback gains (64–1,424 pixels/N) compared with lower visual feedback gains (0.5–4 pixels/N; e.g., 2 vs. 64 pixels/N: 0.089 ± 0.05 vs. 0.061 ± 0.02 N; t = 2.4, P = 0.027). Therefore, all further analyses compared the power spectrum of force, muscle activity and the power spectrum of EMG at visual feedback gains of 2 and 64 pixels/N. The 2 pixels/N was selected because it was in the middle of the low visual feedback gains, whereas the 64 pixels/N was selected because it was the first visual feedback gain that significantly reduced force variability from the low visual feedback gains. All other main effects and interactions were not significant.
Fig. 3.
Force variability as a function of visual feedback gains. The SD of force was lower at 2% MVC (open circles) compared with 10% MVC (filled circles). On average, it was higher (asterisk) at smaller visual gains (0.5–4 pixels/N) compared with greater visual gains (32–1,424 pixels/N). The dashed line is the averaged function of the two forces, indicating the decrease of force variability after 32 pixels/N across the 13 visual gains
Force power spectrum
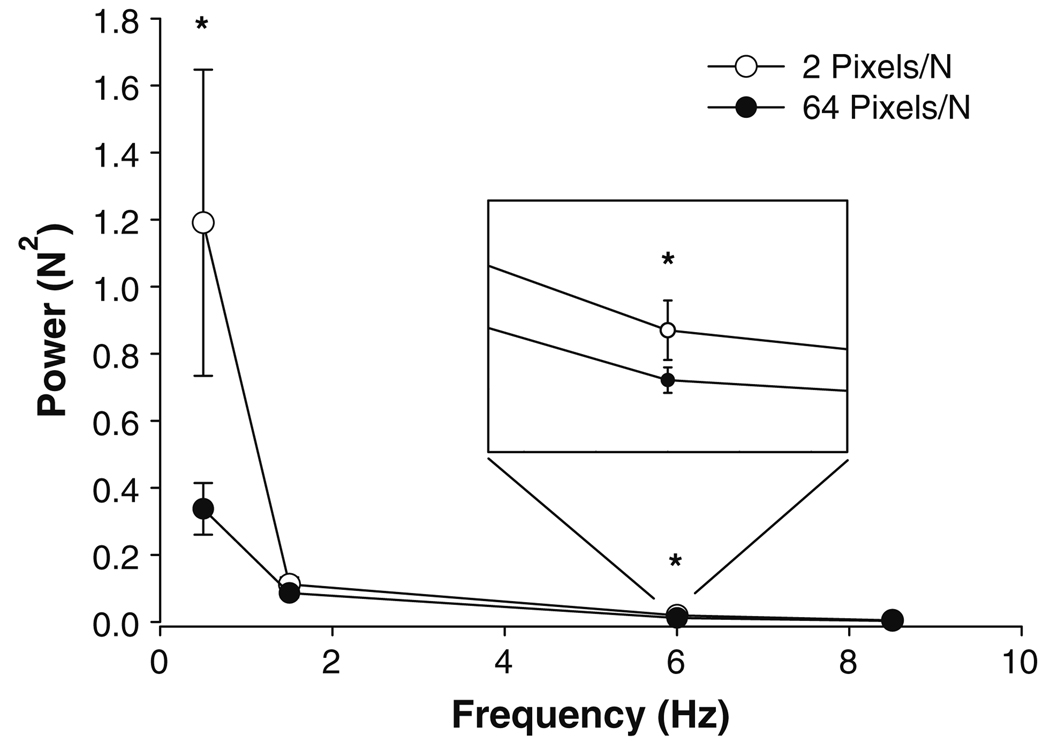
The absolute power from 0–1 Hz increased with force level, whereas the absolute power in higher frequencies (3–10 Hz) decreased with force level (frequency band × force interaction: F12,192 = 3.3, P = 0.01). There was a significant visual feedback gain × frequency band interaction (F12,192 = 3.5, P = 0.009) and based on post hoc analyses indicated the following: (1) the power from 0–1 Hz increased with force; (2) the 0–1 Hz power was greater at 2 pixels/N compared with 64 pixels/N (Fig. 4); (3) the power from 3–7 Hz decreased with force and was greater at 2 pixels/N compared with 64 pixels/N. All other main effects and interactions were not significant.
Fig. 4.
Power spectrum of the force output. The force spectrum was analyzed from 0–1, 1–3, 3–7, and 7–10 Hz bins. The x-axis is presented as the mean frequency of each band (0.5, 2, 5, and 8.5 Hz). Although the overall shape of the power spectrum was similar for 2 pixels/N (open circles) and 64 pixels/N (filled circles), there was a significant difference between the visual gains at 0–1 and 3–7 Hz (asterisk). Specifically, the higher visual gain (64 pixels/N) decreased power from 0–1 and 3–7 Hz (inset) compared with the lower visual gain (2 pixels/N)
Associations between force variability and the force power spectrum
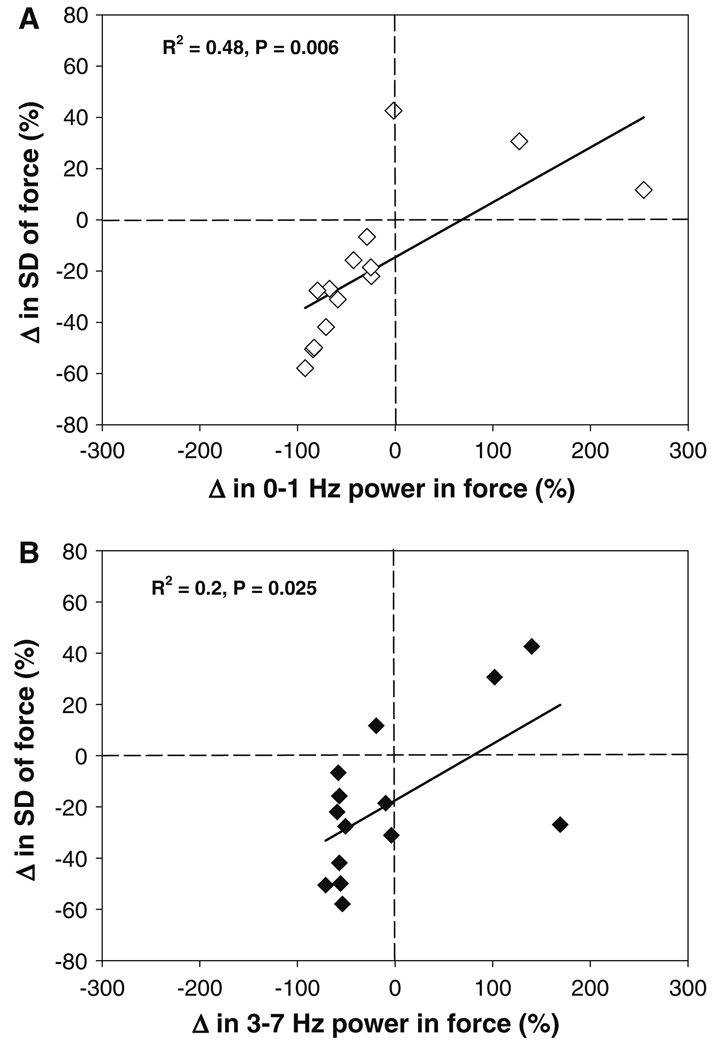
Multiple regression analysis indicated that there was a significant relation between the change in SD of force and the change of power in the force power spectrum with visual feedback gain (adjusted R2 = 0.68, P = 0.002). The decrease in the SD of force was due to a decrease in power from 0–1 Hz (R2 = 0.48, P = 0.006; Fig. 5a) and a decrease in power from 3–7 Hz (R2 = 0.2, P = 0.025; Fig. 5b). Therefore, subjects decreased force variability at higher gains by reducing the oscillations from 0–1 and 3–7 Hz in their force output.
Fig. 5.
Prediction of the change in SD of force. The decrease in SD of force with greater visual gain was associated with a decrease in power from 0–1 and 3–7 Hz. a The change of power from 0–1 Hz in the force power spectrum predicted the change in the SD of force. The relation indicates that modulation of force at 0–1 Hz accounted for ~48% of the decrease in the variability of force (R2 = 0.48, P = 0.006). b The change of power from 3–7 Hz in the force power spectrum predicted the change in the SD of force. The relation indicates that modulation of force at 3–7 Hz accounted for ~20% of the decrease in the variability of force (R2 = 0.2, P = 0.025)
EMG amplitude and power spectrum
The amplitude of FDI EMG was quantified as the RMS of the interference signal. As expected, the amplitude of the EMG increased significantly with force level (F1,11 = 19.85, P < 0.001), but was not significantly affected by the visual feedback gains (P > 0.5). All other main effects and interactions were not significant (P > 0.45).
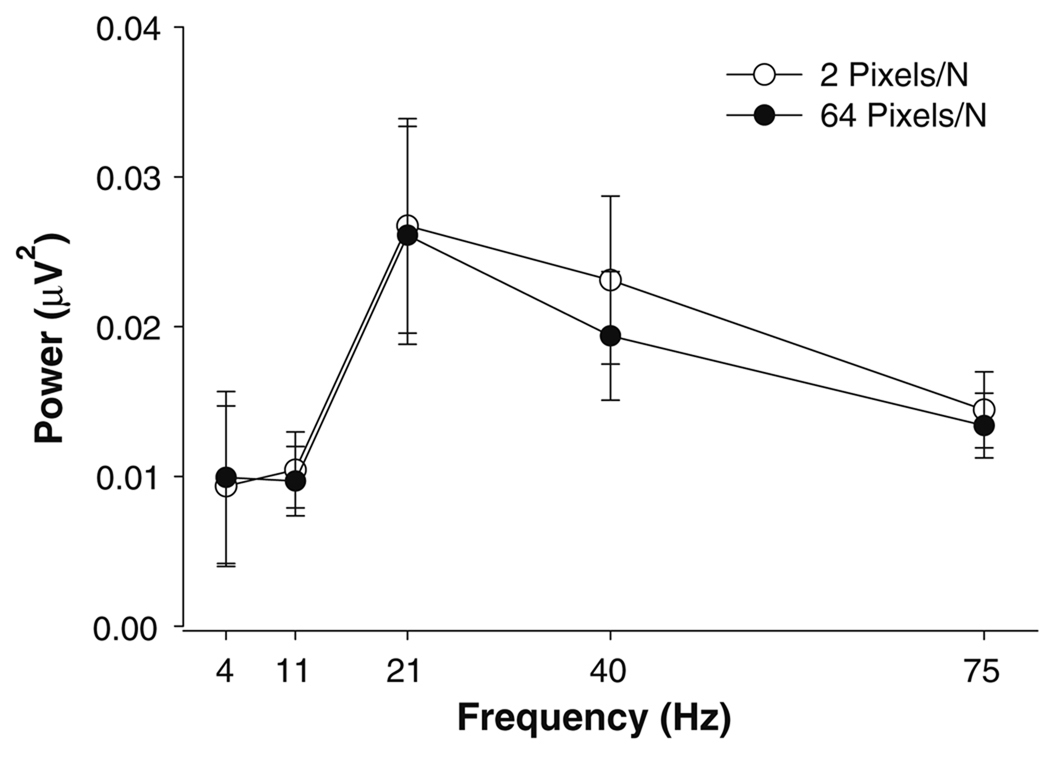
The organization of the EMG signal during constant isometric contractions is typically evaluated with its power spectrum. Although the power in the EMG signal varied with the frequency band (frequency band main effect: F4,48 = 3.65, P = 0.03; Fig. 6), the structure of the EMG spectrum was similar across both the visual feedback gains and forces. All other main effects and interactions were not significant.
Fig. 6.
Power spectrum of FDI EMG. The EMG power spectrum was analyzed from 0–7, 7–13, 13–30, 30–50, and 50–100 Hz bins. Although the EMG power spectrum varied with frequency, the structure of the FDI EMG was similar at 2 pixels/N (open circles) and 64 pixels/N (closed circles). No significant differences were observed between the two gains
Discussion
The relation between the amount of visual feedback of force (gain) and variability of force is unclear in the literature. Some studies suggest that there is a U-shape function describing the relation between visual feedback gain of force and force variability (Sosnoff and Newell 2006b; Sosnoff et al. 2006), whereas other studies suggest that visual feedback gain of force can decrease force variability up to a point and beyond that force variability plateaus (Prodoehl and Vaillancourt 2009). In addition, the physiological mechanism that may cause this reduction in force variability with greater visual feedback gain remains unknown. The purpose of this study, therefore, was to determine the relation between visual feedback gain and force variability and the associated changes to force oscillations and agonist muscle activity. Our findings suggest the following: (1) Differences in force variability occur only between very low visual gains (0.5–4 pixels/N) and moderate-to-high visual gains (>64 pixels/N) and thus support previous findings which demonstrate a plateau in force variability with increased visual feedback gain (Prodoehl and Vaillancourt 2009); (2) decreased force variability with increased visual feedback gain was due to reductions in the power of force oscillations from 0–1 and 3–7 Hz demonstrating that visuomotor corrections can occur at multiple frequency bands; and (3) the amplitude of neural activation of the FDI in this task was not influenced by the amount of visual feedback and thus cannot explain the reductions in force variability.
Relation between the amount of visual feedback and force variability
Our results suggest that moderate-to-high visual feedback gains (>64 pixels/N) can reduce force variability compared with very low visual feedback gains (0.5–4 pixels/N) and that force variability does not vary significantly from moderate-to-high visual feedback gains (64–1,424 pixels/N). Therefore, our results support the recent findings by Prodoehl and Vaillancourt (2009), which demonstrated that force variability varies only at very low visual feedback gains during elbow flexion and dorsiflexion. We extend these findings by providing similar results during abduction of the index finger, an action that is exclusively controlled by a single agonist muscle. In contrast, our results (and those of Prodoehl and Vaillancourt 2009) challenge previous findings, which suggest that force variability would increase at higher visual feedback gains (U-shape function; Sosnoff and Newell 2006a, b). Sosnoff and Newell (2006a, b) suggested that the reason they found increased force variability at higher feedback gains compared with previous studies was because they used a range of gains well beyond those in previous studies. The current findings contradict that explanation because the current study used the largest range of feedback gains to date. For example, the variation of visual gain was almost twice as large in our paper (0.5–1,424 pixels/N or ~0.001–4.57° visual angle) compared with that of Sosnoff and Newell (2006a, b) (2–512 pixels/N or ~0.01–2.7° visual angle as approximated based on description in methodology). The differences in results between the two studies cannot be explained due to differences in force levels (2 and 10% MVC vs. 5 and 25% MVC) or the action used (index finger abduction vs. index finger flexion) because of the experiment by Prodoehl and Vaillancourt (2009). Their results were similar to ours despite the fact that they used higher force levels than our study (5 and 40% MVC) and different effectors (elbow flexion and dorsiflexion). One potential difference between the current study and Sosnoff and Newell (2006a, b) was the monitor size. We used a 27 in. display, whereas Sosnoff and Newell used a 17 in. display. It is possible that the visual feedback could have temporarily gone off the screen on a 17 in. display such that subjects received less information at higher gains. Hong and Newell (2008) examined this issue and showed that at high feedback gain levels the visual feedback can exceed the screen height when the display is small (Hong and Newell 2008). Nonetheless, all these studies agree that increases in visual feedback gain from low (e.g., 2 pixels/N) to moderate visual feedback gains (e.g., 64 pixels/N) will significantly reduce force variability.
The results of this paper also support recent findings (Baweja et al. 2009b), which demonstrated that force variability did not vary significantly when comparing 12.8 with 51.2 pixels/N and 15 with 3,000 pixels/N. In this paper, we examined 13 visual feedback gains per subject, and show that significant differences in force variability reliably occur only when the comparison is between very low (0.5–4 pixels/N) and moderate-to-high visual feedback gains (>64 pixels/N). An interesting finding from the Baweja et al. (2009a, b) paper was that force variability was significantly lower when visual feedback of the force was removed. Therefore, this could have indicated that at very low visual gains (close to 0 pixels/N) force variability would decrease. The current manuscript clearly demonstrates that even with extremely low visual feedback gains (0.5 pixels/N) force variability is significantly higher compared with moderate visual feedback gains. It is possible that complete removal of visual feedback changes the strategies used by the subject to maintain the force constant. For example, the subject may rely more on proprioceptive information rather than searching for visual feedback to perform visuomotor corrections.
Amount of visual feedback and force oscillations
It is well accepted that the variability in force during constant isometric contractions is primarily due to oscillations in force from 0–2 Hz (Slifkin and Newell 2000; Christou et al. 2004; Christou 2005; Baweja et al. 2009b). Our findings clearly demonstrate that when the visual feedback gain increased from low to moderate visual feedback gains force variability decreased due to decreased power in the 0–1 and 3–7 Hz bands. The reduction in power from 0–1 Hz explained ~50% of the decrease in force variability, whereas the reduction in power from 3–7 Hz explained ~20% of the decrease in force variability. These results, therefore, demonstrate that visuomotor corrections can alter the oscillations in force via modulation of the 0–1 Hz and 3–7 Hz bands. Although other studies have shown that visuomotor corrections can change the oscillations in force (Miall et al. 1993; Slifkin and Newell 2000; Baweja et al. 2009b), these results demonstrate that low-frequency oscillations in force (0–1 Hz) cannot be entirely explained from visuomotor corrections. This is evident because force output at very low visual feedback gains (e.g., 0.5 Hz) also contained low-frequency oscillations (0–1 Hz). Low-frequency oscillations have been attributed to the coherent modulation of motor unit discharge at low frequencies (De Luca and Erim 1994; Brown 2000; Vaillancourt et al. 2003), variability in motor unit discharge due to synaptic noise (Taylor et al. 2003; Moritz et al. 2005), intrinsic neuronal properties such as active calcium conductance (Falcke 2003), heart rate (Hunter et al. 2007), and breathing (Turner 2002; Li and Yasuda 2007).
Furthermore, the exact physiological mechanism that induced the modulation (decrease in power) within these frequency bands remains unclear. One possibility is that the change in low-frequency oscillations (0–1 Hz) occurred by changing the breathing amplitude (Fulks et al. 2008). The 3–7 Hz modulation can be potentially explained by decreasing the number of newly recruited motor units (initial rates of newly recruited motor units range from 5–7 Hz and contribute the most to the variability of force; Enoka and Fuglevand 2001). Further research is needed to clarify the origins of low-frequency oscillations in force (0–1 Hz) and the mechanisms that modulate the 0–1 and 3–7 Hz frequency bands in force with changes in visual feedback gain.
Amount of visual feedback and neural activation of agonist muscle
One advantage of using the abduction of the index finger as a model to understand changes in muscle activation with visual feedback gain is that it is controlled by a single agonist muscle, namely, the FDI muscle (Chao et al. 1989; Li et al. 2003). Despite the simplicity of the model, we found that the amplitude of neural activation of the FDI muscle was not influenced by the changes in visual feedback gain and thus cannot explain the reductions in force variability. We demonstrated this by comparing the amplitude (RMS) and structure (power spectrum density) of the recorded EMG signal. The non-significant changes in the muscle activity of the FDI muscle may be due to the following. (1) Changes in the amplitude of neural activation occur at the antagonist and not to the agonist muscle (FDI in this task). In this study, we have measured only the agonist muscle activity. (2) The surface EMG signal is insensitive to changes in motor unit activity including motor unit synchronization and discharge rate variability (Farina et al. 2002). Therefore, additional research is needed to examine the concurrent activation of antagonistic muscles and the activation of motor units in agonist and antagonist muscles with variations in visual feedback gain.
In conclusion, our results demonstrate that increased amount of visual feedback reduced force variability. This improvement in force variability, however, occurred only when comparing very low visual feedback gains (0.5–4 pixels/N) to moderate-to-high visual feedback gains (>64 pixels/N). Variation of the gain at higher levels (e.g., >64 pixels/N) did not change force variability and thus challenge the proposition that the relation between visual feedback gain and force variability is a U-shape (Sosnoff and Newell 2006b). In addition, we showed that the improvements in the variability of force were due to decreased force oscillations from 0–1 and 3–7 Hz. Nonetheless, the changes in force oscillations (and thus force variability) could not be explained by changes in the neural activation amplitude of the single agonist muscle involved in this task. Future studies should attempt to identify the physiological mechanisms that modulate force oscillations from 0–1 and 3–7 Hz and reduce force variability when the amount of visual feedback increases.
Acknowledgments
This study was supported by R01 NS52318 and R01 NS58487 to David E. Vaillancourt and R01 AG031769 to Evangelos A. Christou.
Contributor Information
Harsimran S. Baweja, Department of Health and Kinesiology, Texas A&M University, College Station, TX 77843-4243, USA
Deanna M. Kennedy, Department of Health and Kinesiology, Texas A&M University, College Station, TX 77843-4243, USA
Julie Vu, Department of Health and Kinesiology, Texas A&M University, College Station, TX 77843-4243, USA.
David E. Vaillancourt, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, IL 60612, USA
Evangelos A. Christou, Email: eachristou@hlkn.tamu.edu, Department of Health and Kinesiology, Texas A&M University, College Station, TX 77843-4243, USA.
References
- Baweja HS, Kennedy DM, Vu JL, Vaillancourt DE, Christou EA. Greater amounts of visual feedback alters muscle activity and reduce force variability during constant isometric contractions. Chicago, IL: Society for Neuroscience; 2009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baweja HS, Patel BK, Martinkewiz JD, Vu J, Christou EA. Removal of visual feedback alters muscle activity and reduces force variability during constant isometric contractions. Exp Brain Res. 2009b;197:35–47. doi: 10.1007/s00221-009-1883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol. 2000;60:97–108. doi: 10.1016/s0301-0082(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Chao EYS, An KN, Cooney WP, Linschied RL. Biomechanics of the hand. A basic research study. Teaneck: World Scientific Publishing; 1989. [Google Scholar]
- Christou EA. Visual feedback attenuates force fluctuations induced by a stressor. Med Sci Sports Exerc. 2005;37:2126–2133. doi: 10.1249/01.mss.0000178103.72988.cd. [DOI] [PubMed] [Google Scholar]
- Christou EA, Jakobi JM, Critchlow A, Fleshner M, Enoka RM. The 1- to 2-Hz oscillations in muscle force are exacerbated by stress, especially in older adults. J Appl Physiol. 2004;97:225–235. doi: 10.1152/japplphysiol.00066.2004. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends Neurosci. 1994;17:299–305. doi: 10.1016/0166-2236(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Fuglevand AJ. Motor unit physiology: some unresolved issues. Muscle Nerve. 2001;24(1):4–17. doi: 10.1002/1097-4598(200101)24:1<4::aid-mus13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol. 2003;13:1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- Falcke M. Buffers and oscillations in intracellular Ca2+ dynamics. Biophys J. 2003;84:28–41. doi: 10.1016/S0006-3495(03)74830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Fattorini L, Felici F, Filligoi G. Nonlinear surface EMG analysis to detect changes of motor unit conduction velocity and synchronization. J Appl Physiol. 2002;93(5):224–227. doi: 10.1152/japplphysiol.00314.2002. [DOI] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Fulks ER, Baweja HS, Patel BK, Martinkewiz JD, Srinivasan D, Christou EA. Breathing amplitude influences force variability but not muscle activity during constant isometric contractions. Washington, DC: Society for Neuroscience; 2008. [Google Scholar]
- Homma T, Sakai T. Ramification pattern of intermetacarpal branches of the deep branch (ramus profundus) of the ulnar nerve in the human hand. Acta Anat (Basel) 1991;141:139–144. doi: 10.1159/000147113. [DOI] [PubMed] [Google Scholar]
- Hong SL, Newell KM. Visual information gain and the regulation of constant force levels. Exp Brain Res. 2008;189:61–69. doi: 10.1007/s00221-008-1403-z. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Taijin T, Patel B, Rodriguez TM, Christou EA. Heart rate contributes to the low-frequency oscillations in force. San Diego: Society for Neuroscience; 2007. [Google Scholar]
- Li S, Yasuda N. Forced ventilation increases variability of isometric finger forces. Neurosci Lett. 2007;412:243–247. doi: 10.1016/j.neulet.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZM, Pfaeffe HJ, Sotereanos DG, Goitz RJ, Woo SL. Multi-directional strength and force envelope of the index finger. Clin Biomech (Bristol, Avon) 2003;18:908–915. doi: 10.1016/s0268-0033(03)00178-5. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Intermittency in human manual tracking tasks. J Mot Behav. 1993;25:53–63. doi: 10.1080/00222895.1993.9941639. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol. 2005;93:2449–2459. doi: 10.1152/jn.01122.2004. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Vaillancourt DE. Effects of visual gain on force control at the elbow and ankle. Exp Brain Res. 2009 doi: 10.1007/s00221-009-1966-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifkin AB, Newell KM. Variability and noise in continuous force production. J Mot Behav. 2000;32:141–150. doi: 10.1080/00222890009601366. [DOI] [PubMed] [Google Scholar]
- Slifkin AB, Vaillancourt DE, Newell KM. Intermittency in the control of continuous force production. J Neurophysiol. 2000;84:1708–1718. doi: 10.1152/jn.2000.84.4.1708. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM. Aging, visual intermittency, and variability in isometric force output. J Gerontol B Psychol Sci Soc Sci. 2006a;61:P117–P124. doi: 10.1093/geronb/61.2.p117. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM. Information processing limitations with aging in the visual scaling of isometric force. Exp Brain Res. 2006b;170:423–432. doi: 10.1007/s00221-005-0225-5. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Valantine AD, Newell KM. Independence between the amount and structure of variability at low force levels. Neurosci Lett. 2006;392:165–169. doi: 10.1016/j.neulet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Christou EA, Enoka RM. Multiple features of motor-unit activity influence force fluctuations during isometric contractions. J Neurophysiol. 2003;90:1350–1361. doi: 10.1152/jn.00056.2003. [DOI] [PubMed] [Google Scholar]
- Tracy BL. Visuomotor contribution to force variability in the plantarflexor and dorsiflexor muscles. Hum Mov Sci. 2007;26:796–807. doi: 10.1016/j.humov.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy BL, Dinenno DV, Jorgensen B, Welsh SJ. Aging, visuomotor correction, and force fluctuations in large muscles. Med Sci Sports Exerc. 2007;39:469–479. doi: 10.1249/mss.0b013e31802d3ad3. [DOI] [PubMed] [Google Scholar]
- Turner DL. Expiratory resistive loaded breathing in humans increases fluctuations of force production in submaximal isometric quadriceps contractions. Neurosci Lett. 2002;328:13–16. doi: 10.1016/s0304-3940(02)00420-2. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Russell DM. Temporal capacity of short-term visuomotor memory in continuous force production. Exp Brain Res. 2002;145:275–285. doi: 10.1007/s00221-002-1081-1. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Larsson L, Newell KM. Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiol Aging. 2003;24:25–35. doi: 10.1016/s0197-4580(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Welsh SJ, Dinenno DV, Tracy BL. Variability of quadriceps femoris motor neuron discharge and muscle force in human aging. Exp Brain Res. 2007;179:219–233. doi: 10.1007/s00221-006-0785-z. [DOI] [PubMed] [Google Scholar]