Abstract
Background
Cannabis is one of the most widely used illicit substances, and there is growing interest in the therapeutic applications of cannabinoids. While known to modulate neuroendocrine function, the precise acute and chronic dose-related effects of cannabinoids in humans are not well-known. Furthermore, the existing literature on the neuroendocrine effects of cannabinoids is limited by small sample sizes (n=6–22), heterogeneous samples with regard to cannabis exposure (lumping users and nonusers), lack of controlling for chronic cannabis exposure, differing methodologies, and limited dose–response data. Delta-9-tetrahydrocannabinol (Δ-9-THC) was hypothesized to produce dose-related increases in plasma cortisol levels and decreases in plasma prolactin levels. Furthermore, relative to controls, frequent users of cannabis were hypothesized to show altered baseline levels of these hormones and blunted Δ-9-THC-induced changes of these hormones.
Materials and methods
Pooled data from a series of laboratory studies with multiple doses of intravenous Δ-9-THC in healthy control subjects (n=36) and frequent users of cannabis (n=40) was examined to characterize the acute, chronic, and acute on chronic effects of cannabinoids on plasma cortisol and prolactin levels. Hormone levels were measured before (baseline) and 70 min after administration of each dose of Δ-9-THC. Data were analyzed using linear mixed models with +70 min hormonal levels as the dependant variable and baseline hormonal level as the covariate.
Results
At socially relevant doses, Δ-9-THC raised plasma cortisol levels in a dose-dependent manner but frequent users showed blunted increases relative to healthy controls. Frequent users also had lower baseline plasma prolactin levels relative to healthy controls.
Conclusions
These group differences may be related to the development of tolerance to the neuroendocrine effects of cannabinoids. Alternatively, these results may reflect inherent differences in neuroendocrine function in frequent users of cannabis and not a consequence of cannabis use.
Keywords: Cannabis, Cannabinoids, Delta-9-tetrahydrocannabinol, Prolactin, Cortisol, Hormones
Introduction
Cannabis is one of the most widely used illicit substances, and recent evidence suggests an increase in the prevalence of cannabis use, abuse, and dependence (Compton et al. 2004; SAMHSA 2004; Stinson et al. 2006). There is growing interest in both the therapeutic and harmful effects of cannabinoid compounds. Amongst their many effects, exogenous cannabinoids have been reported to modulate cortisol release. However, the precise acute and chronic dose-related effects of these compounds in humans are not well-known. Furthermore, recent preclinical studies suggest that endogenous cannabinoids or endocannabinoids appear to modulate neuroendocrine function.
Cortisol
Preclinical studies suggest that the acute administration of delta-9-tetrahydrocannabinol (Δ-9-THC), the principal active constituent of cannabis, is associated with a dose-dependant increase in cortisol levels (Brown and Dobs 2002). However, in humans, the acute effects of cannabinoids on cortisol release are less clear with some (Cone et al. 1986), but not other studies (Dax et al. 1989) reporting increased cortisol levels associated with acute administration of cannabinoids.
Prolactin
The human and preclinical literature is mixed with reports of a decrease, increase, or no change in prolactin levels following the administration of cannabinoids (Cone et al. 1986; Dax et al. 1989; Lemberger et al. 1975; Markianos and Stefanis 1982; Mendelson et al. 1984; Mendelson et al. 1985; Murphy et al. 1990; Rettori et al. 1988; Wenger et al. 1987).
Most of the existing literature on the acute effects of cannabinoids does not control for possible effects of chronic exposure to cannabis. The latter is important given increasing preclinical and clinical evidence suggesting the development of tolerance to several cannabinoid effects in association with chronic exposure. Studies of baseline prolactin levels in individuals with chronic exposure to cannabinoids have also been mixed with some (Dax et al. 1989; Harmon and Aliapoulios 1972; Olusi 1980) but not other evidence (Block et al. 1991) suggesting that chronic cannabis exposure is associated with lower baseline prolactin levels. Finally, in the only study examining baseline cortisol levels, there were no differences between cannabis users and controls (Block et al. 1991).
In summary, the existing literature, while suggestive of an acute effect of cannabinoids on cortisol and prolactin release in humans, is limited by small sample sizes (n=6–22), heterogeneous samples with regard to cannabis exposure, lack of controlling for chronic cannabis exposure, differing methodologies, and limited dose–response data. Related to dose–response, some cannabinoid effects, e.g., on anxiety are biphasic, with low and high doses producing divergent effects. Whether cannabinoids have biphasic hormonal effects in humans is unknown. Finally, while there are some studies that have separately examined the acute and chronic endocrine effects of cannabinoids in humans, we are unaware of any studies comparing the acute, chronic, and acute on chronic effects. Chronic and acute on chronic effects of Δ-9-THC on endocrine function may reflect clinically relevant evidence of long-term adaptation of the cannabinoid receptor system associated with chronic cannabis use.
A series of studies with several doses of Δ-9-THC at our center have yielded a large database of the endocrine effects of Δ-9-THC in humans. This database provides a unique opportunity to examine the dose-related acute, chronic, and acute on chronic effects of Δ-9-THC in a large sample of frequent users of cannabis and healthy control subjects. We hypothesized firstly that Δ-9-THC would produce dose-related increases in plasma cortisol levels and decreases in plasma prolactin levels. Secondly, relative to controls, frequent users of cannabis would show altered baseline levels of these hormones and blunted Δ-9-THC-induced changes of these hormones.
Materials and methods
Data from two Δ-9-THC studies involving healthy control subjects and frequent users of cannabis were pooled for analysis (Table 1). Some of the data have been published or presented in part (D’Souza et al. 2004, 2008a) and other data have not been published. Furthermore, none of these data have been analyzed collectively. The studies were conducted at the Neurobiological Studies Unit (VA Connecticut Healthcare System, West Haven, CT, USA) with the approval of the Human Subjects Subcommittee of the Veterans Affairs Connecticut Healthcare System and the Human Investigations Committee of the Yale University School of Medicine, New Haven, Connecticut, USA.
Table 1.
Study designs
| Study | Subjects | Δ-9-THC doses (mg/kg) | Duration of infusion (min) | Total dose (mg) |
|---|---|---|---|---|
| 1A | Healthy control subjects | 0, 0.0357, 0.0714 | 2 | 0, 2.5, 5 |
| 1B | Frequent users of cannabis | 0, 0.0357, 0.0714 | 0, 2.5, 5 | |
| 2A | Healthy control subjects | 0, 0.0286 | 20 | 0, 1.5 |
| 2B | Frequent users of cannabis | 0, 0.0286 | 0, 1.5 |
Participants
Two groups of subjects, current frequent users of cannabis and control subjects, were studied in parallel. Current frequent users of cannabis, heretofore referred to as frequent users (D’Souza et al. 2008), were defined as having (1) a positive urine toxicological test for cannabis at screening, and (2) at least ten exposures to cannabis within the past month as quantified by a timeline follow-back approach (Sobell and Sobell 1992). These subjects also met the criteria for current DSM-IV cannabis abuse disorder but not cannabis dependence while none of the controls did. Healthy controls were required to have (1) a negative urine toxicological test at screening, (2) no exposure to cannabis in the past week, (3) and absence of lifetime cannabis use disorder.
Screening
After obtaining written informed consent, subjects (18–55 years) underwent a structured psychiatric interview for DSM-IIIR or DSM-IV (First et al. 2002) and were carefully screened for any DSM axis I or axis II lifetime psychiatric or substance use disorder (except for cannabis in the case of frequent users) and family history of major axis I disorder. All subjects were asked to estimate their lifetime cannabis exposure (number of times), heaviest exposure, and last exposure to cannabis. Subjects were excluded for recent abuse (3 months) or dependence (1 year) to alcohol or any substances other than nicotine in both groups and cannabis in the frequent user group. Cannabis-naïve individuals were excluded to minimize any risk of promoting future cannabis use/abuse. The history provided by subjects was confirmed by a telephone interview conducted with an individual (spouse or family member) identified by the subject prior to screening. A general physical and neurological examination, electrocardiogram, and laboratory tests (serum electrolytes, liver function tests, complete blood count with differential and urine toxicology) were also conducted. Both groups were instructed to refrain from alcohol, illicit drugs, or prescription drugs not approved by the research team for 2 weeks before the study and throughout study participation. Frequent users were permitted to use cannabis until 24 h prior to each test day to minimize cannabis withdrawal effects.
Drugs
Subjects received active Δ-9-THC or placebo by intravenous (i.v.) route under double-blind conditions (Table 1). The preparation, formulation, and storage of Δ-9-THC solution are reported elsewhere (D’Souza et al. 2004). For the placebo condition, an equivalent volume (≅2 mL) of ethanol (vehicle) was used which was undetectable in multiple postinjection samples. As reviewed elsewhere (D’Souza et al. 2004), the i.v. route of administration, while not socially relevant, was chosen to standardize the delivery of Δ-9-THC and to reduce interindividual and intra-individual variability in plasma Δ-9-THC levels associated with the inhaled and oral routes.
Study designs
In study I, subjects received one of three doses (placebo, 0.0357 mg/kg, and 0.0714 mg/kg) of Δ-9-THC on each test day. Blood was sampled before and 70 min after placebo and active Δ-9-THC infusion. In study II, subjects received placebo Δ-9-THC followed by Δ-9-THC 0.0286 mg/kg in fixed order on each test day. Blood was sampled before and 70 min after administration of placebo and active Δ-9-THC infusion.
Outcome measures
Blood was sampled from the i.v. line of the arm opposite to the one used for administering study drug (D’Souza et al. 2004) for prolactin and cortisol, Δ-9-THC and its primary inactive metabolite 11-nor-Δ-9-THC-9-COOH (THC-COOH). Immediately after collection, blood samples were placed on ice, centrifuged, and the extracted plasma was aliquoted into vials for storage at −70°C. The samples were assayed at the same time in one batch.
Hormonal analysis
Cortisol and prolactin levels were measured at baseline (i.e., before) and 70 min after administration of i.v. Δ-9-THC. Plasma cortisol was measured by radioimmunoassay after denaturation of the binding proteins by heat. Primary antibodies (raised in rabbit against cortisol-3-0-carboxymethyloxime-BSA) and I125-labeled cortisol were purchased from ICN Biomedicals. The cortisol standard was purchased from Sigma Chemical. Antirabbit globulin serum in conjunction with polyethylene glycol was used for separation of the bound and free fractions. Samples were assayed in duplicate. Plasma prolactin was measured by a double antibody radioimmunoassay. The prolactin standard was purchased from Calbiochem and was calibrated against the National Pituitary Agency (NPA) primary prolactin standard (HPRL-RP-1). The antiserum was donated by the NPA. The labeled PRL-1125 was purchased from New England Nuclear and repurified on the day of the assay on a G-100 Sephadex column. Antirabbit globulin serum was used for separation of bound and free fractions. Samples were assayed in duplicate.
Measurement of Δ-9-THC and THC-COOH are described elsewhere (D’Souza et al. 2004). Δ-9-THC and THC-COOH were assayed in a subsample of subjects (30 frequent users and 22 healthy controls) to ensure that any group endocrine differences are not related to pharmacokinetic differences.
Statistical analysis
Initially, data were examined descriptively using means, standard deviations, and graphs. Each outcome was tested for normality using Kolmogorov–Smirnov test statistics and normal probability plots. Data were transformed (log) as necessary. Analyses included data combined from both studies. Linear mixed models were used to analyze each hormone. In these models, hormone levels at post-70 min represented the dependent variable, while dose (study 1 placebo, study 2 placebo, 1.5 mg [0.0286 mg/kg], 2.5 mg [0.0357 mg/kg], and 5 mg [0.0714 mg/kg]) and group (frequent users vs. healthy controls) were included as fixed effects and subject was included as a random effect. Due to the wide interindividual variability in cortisol levels and because hormonal levels were drawn later in the day for study 2 (1.5 mg [0.0286 mg/kg] Δ-9-THC dose), baseline hormone levels were included as a covariate. The interaction between dose and group was fitted. Significant dose effects were followed by Tukey’s multiple comparisons procedure to determine significant pair-wise differences. All results were considered statistically significant at p<0.05 and data were analyzed using SAS, version 9.1 (SAS Institute, Cary, NC, USA).
Results
There were 40 frequent users and 36 healthy controls in the sample. Frequent users were older than healthy control subjects (p=0.05) but were not significantly different for gender (p=0.39; Table 2), education, socioeconomic status, concomitant medications including hormonal contraception (three in each group), smoking status, or other drug/alcohol exposure. Relative to healthy controls, frequent users had greater lifetime exposure to cannabis and heavier cannabis use. In the month prior to study participation, all frequent users had used cannabis compared to 23% of the healthy controls. Furthermore, within the past week, 85% of the frequent cannabis users but none of the healthy controls had used cannabis (Table 2). Those frequent users who reported having used cannabis in the past week had used it sometime within 72 h prior to, but not within, the 24 h preceding each test day. None of the women in the sample were pregnant. There was no main effect of gender or smoking status on the baseline or the change from baseline hormonal levels and, therefore, both were dropped from the analysis.
Table 2.
Subject characteristics
| Frequent users |
Healthy controls |
|
|---|---|---|
| Number of subjects | 40 | 36 |
| Age, mean (SD) | 28.28 (10.2) | 24.58 (4.9) |
| Gender (male/female) | 30/10 | 27/9 |
| Smoking status (no. of smokers)a | 13 | 9 |
| Last exposure to cannabis (%) | ||
| Cannabis use in past week | 85 | 0 |
| Cannabis use in past month | 100 | 23 |
| Lifetime cannabis use (%) | ||
| <5 times | 0 | 23 |
| 5–10 times | 0 | 10 |
| 11–20 times | 0 | 13 |
| 21–50 times | 0 | 15 |
| 51–100 times | 2 | 18 |
| >100 times | 98 | 20 |
| Heaviest cannabis use | ||
| Daily | 53 | 0 |
| 1–6 times per week | 46 | 0 |
| 1–3 times per month | 0 | 0 |
| 1–11 times per year | 0 | 100 |
| <Once per year | 0 | 0 |
There was no main effect of smoking status on the baseline or the change from baseline hormonal levels and, therefore, it was dropped from the analysis
Cortisol
Cortisol levels were approximately normally distributed. Baseline cortisol levels were similar between the two groups. Δ-9-THC administration raised cortisol levels significantly from baseline in a dose-dependent manner (F4,78=17.4, p<0.0001; Fig. 1). There was a group effect (F1,78=3.954, p=0.05) with frequent users of cannabis showing blunted Δ-9-THC-induced cortisol increases relative to healthy controls. Post hoc analysis revealed that the group effect was driven by the highest dose (0.0714 mg/kg; F1,78=11.37, p=0.0012).
Fig. 1.
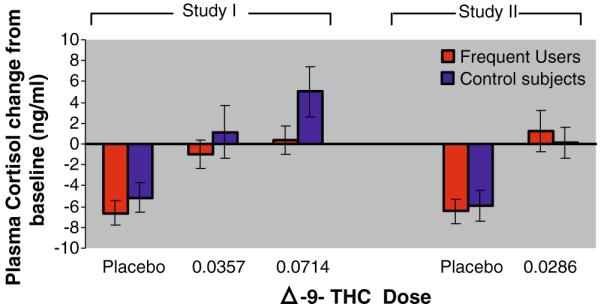
Δ-9 THC-induced plasma cortisol. The X-axis denotes the Δ-9-THC dose and the Y-axis denotes the change in mean (±SEM) plasma cortisol levels at +70 min post-Δ-9-THC administration. Δ-9-THC increased plasma cortisol in both frequent users and healthy controls (p<0.0001). Compared to healthy controls, frequent users had “blunted” increases in plasma cortisol levels (p=0.05)
Prolactin
Prolactin levels were approximately normally distributed after log transformation, and thus, these values were used for analysis. For baseline prolactin levels, there was a main effect of group such that cannabis frequent users had significantly lower baseline or pre-Δ-9-THC prolactin levels compared to healthy controls (F1,83=7.7, p=0.007; Fig. 2).
Fig. 2.
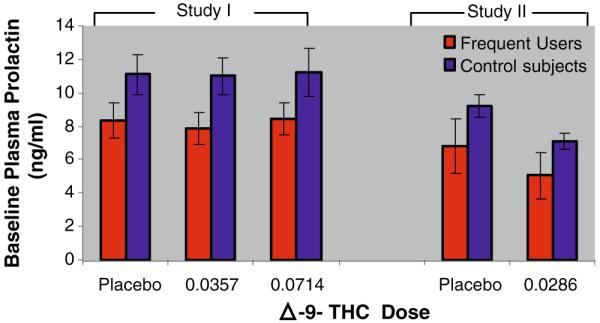
Baseline plasma prolactin. The X-axis denotes Δ-9-THC dose and the Y-axis denotes the baseline plasma prolactin levels mean (±SEM). Compared to healthy controls, frequent users had lower baseline plasma prolactin levels (p=0.007)
Overall, Δ-9-THC administration did not alter prolactin levels in a significant manner. There was a significant effect of group with the frequent users of cannabis having lower post-Δ-9-THC prolactin levels compared to healthy controls (F1,71=9.8, p=0.0025).
Plasma Δ-9-THC and 11-nor-Δ-9-THC-9-COOH levels
Plasma Δ-9-THC and THC-COOH levels were analyzed in 52 of 76 subjects (30 frequent users and 22 healthy controls). Plasma Δ-9-THC levels increased in a dose-dependent manner without significant group differences (ATS=0.82, df=1, p=0.36) or group by dose interactive effects on plasma Δ-9-THC levels (ATS=0.29, df=1.43, p=0.67). As expected, relative to controls, frequent users had higher baseline plasma levels of THC-COOH, the principal inactive metabolite of Δ-9-THC (ATS=105.56, df=1, p<0.0001). However, there were no significant group by dose interactive effects on plasma THC-COOH levels (ATS=1.14, df=1.53, p=0.52).
The hormonal effects reported above were accompanied by a wide range of behavioral, subjective, cognitive, and physiological effects consistent with the known effects of Δ-9-THC as reported elsewhere (D’Souza et al. 2004, 2008a,b).
Discussion
This is the first report that we are aware of demonstrating acute, chronic, and acute on chronic endocrine effects of cannabinoids in a large human sample. As expected, Δ-9-THC, in a dose-related manner, increased plasma cortisol levels in both healthy controls and frequent users. However, the novel finding of this study is that, relative to healthy controls, frequent users showed blunted Δ-9-THC-induced cortisol and lower baseline plasma prolactin levels.
Cortisol
Δ-9-THC-induced cortisol responses
Δ-9-THC, in a dose-related manner, increased plasma cortisol levels in both healthy controls and frequent users. Placebo Δ-9-THC administration did not interfere with the normal diurnal rhythm of cortisol. In contrast, with all the active doses of Δ-9-THC, the normal diurnal decline was reduced resulting in cortisol levels that were either the same as or higher than baseline. Interestingly, the rate of drug administration (2 vs. 20 min) did not appear to significantly affect the response.
Mechanism of Δ-9-THC effects on cortisol
In preclinical studies, exogenous cannabinoids have been shown to robustly affect the hypothalamic–pituitary–adrenal (HPA) axis causing increases in levels of corticotrophin-releasing hormone (CRH), adrenocorticotrophic hormone, and cortisol (Pagotto et al. 2006). Stimulation of the HPA axis is believed to occur via brain cannabinoid receptor (CB-1R) activation primarily in the paraventricular nuclei of the hypothalamus where these receptors and CRH mRNA are co-expressed and also in the pituitary (Pagotto et al. 2006). Little is known about the effects of exogenous cannabinoids on the adrenals. The normal diurnal rhythm of cortisol is such that levels peak early in the morning soon after awakening with a progressive decline subsequently. Cortisol levels with placebo administration in our sample followed this pattern, while Δ-9-THC clearly interfered with the normal decline resulting in similar or higher levels 70 min post Δ-9-THC compared to baseline.
Blunted Δ-9-THC-induced cortisol responses in frequent users
Although, Δ-9-THC induced cortisol release in both groups, we show, for the first time to our knowledge, that frequent users had blunted increases in cortisol release compared to healthy controls. We propose two possible explanations for the blunted Δ-9-THC-induced cortisol responses in frequent users: effects of chronic cannabis exposure and inherent differences between the two groups.
Tolerance to Δ-9-THC effects on cortisol
The blunted cortisol response to Δ-9-THC in frequent users is consistent with other published and unpublished data from our laboratory demonstrating that frequent users show blunted behavioral, subjective, and cognitive effects of Δ-9-THC relative to healthy controls (D’Souza et al. 2004, 2008a,b). There is preclinical evidence of a rapid development of tolerance in the hypothalamus following chronic exposure to cannabinoid agonists (Brown and Dobs 2002) resulting in a blunted cortisol response to subsequent acute exposure (Murphy et al. 1998; Pagotto et al. 2006). The mechanisms underlying tolerance to cannabinoids include the downregulation due to receptor internalization and desensitization of receptors (reviewed in Gonzalez et al. 2005; Lichtman and Martin 2005; Romero et al. 1997).
Innate differences
Alternatively, frequent users of cannabis may have innate differences in response to Δ-9-THC. Lower Δ-9-THC-induced cortisol levels may reflect HPA axis responsivity which has been associated with sensation-seeking behaviors. In rats, lower HPA axis activity is reported to be associated with heightened acquisition of drug self-administration (Kosten and Ambrosio 2002). These findings suggest that individuals with low responsiveness to stress may use drugs to increase arousal and evoke stronger sensations that are essential for physiological comfort (Majewska 2002). Most studies report an inverse relationship between lower cortisol levels and sensation-seeking in humans (Rosenblitt et al. 2001). Interestingly, adolescents with early onset of cannabis use had lower basal cortisol levels (Huizink et al. 2006) which the authors interpreted as evidence of higher sensation-seeking. While there were no group differences in baseline cortisol levels in this sample, this may have been because of the known substantial interindividual variability in morning cortisol levels. In contrast, the Δ-9-THC-induced changes in cortisol levels observed in this study, being covaried for baseline cortisol levels, may reflect HPA axis responsivity more accurately.
Prolactin
Δ-9-THC-induced prolactin responses
Δ-9-THC did not affect plasma prolactin levels across a wide range of socially relevant doses in this study involving the largest published sample that we are aware of. These data are consistent with some of the literature demonstrating a lack of acute Δ-9-THC effects on plasma prolactin levels in humans. In contrast, preclinical studies suggest a late and predominantly inhibitory effect of cannabinoids (Murphy et al. 1998). Perhaps, the higher doses of Δ-9-THC in animal studies and the short sampling period in this study may account for the differences between our study and the preclinical literature.
Lower baseline and post-Δ-9-THC prolactin levels in frequent users
Frequent users of cannabis had significantly lower baseline as well as post-Δ-9-THC prolactin levels compared to healthy controls. Our data are consistent with a smaller study (n=17) reporting lower prolactin levels in heavy cannabis users (Dax et al. 1989). However, the acute administration of Δ-9-THC did not appear to significantly alter prolactin secretion.
Mechanism of Δ-9-THC effects on prolactin
Prolactin is secreted in a pulsatile manner from the anterior pituitary under a tonic inhibitory hypothalamic influence mediated by dopamine (DA). CB-1R are colocalized with DA receptors in hypothalamic DA projections and Δ-9-THC acutely increases the release of DA (Rodriguez De Fonseca et al. 2001). DA exerts feedback inhibition by stimulating endocannabinoid secretion which then inhibits further DA release (Rodriguez De Fonseca et al. 2001). Chronic exposure to cannabis, which is associated with a downregulation of CB-1R, may interfere with this feedback process resulting in an enhanced DA tone. For example, Rodriguez De Fonseca et al. (2001) have attributed an enhanced amphetamine response in Δ-9-THC-tolerant animals to a disruption of the feedback loop described above. If this also occurs within the tuberoinfundibular DA–CB system, it may result in a greater tonic suppression of prolactin and hence lower baseline levels and enhanced Δ-9-THC-induced suppression or prolactin release in frequent users. Alternatively, reduced baseline prolactin levels may reflect higher DA tone which in turn may reflect increased CB-1R function. This seems unlikely given extensive preclinical evidence of a CB-1R downregulation associated with chronic cannabinoid exposure.
Although lower than in healthy controls, baseline prolactin levels in frequent users were within the normal physiological range for prolactin. Whether the lower prolactin levels in this study were clinically significant was not evaluated.
Further studies should be directed to clarify the specific mechanisms of the acute and chronic endocrine effects of cannabinoids in humans and the consequences if any of these effects.
Limitations
Several issues should be considered in interpreting the results. First, blood was not sampled at exactly the same time points across the two studies. Second, basal (early morning) cortisol levels were not sampled. However, these studies were not aimed primarily at evaluating basal HPA axis activity. Rather, these studies were aimed at evaluating HPA axis responsivity to the administration of cannabinoids. This was reflected in the change in cortisol levels (pre–post Δ-9-THC administration). In order to address the interindividual and intra-individual variability and because study II cortisol levels were assayed later in the day, analyses were covaried for baseline cortisol levels. Furthermore, the sample consisted of both men and women; the small number of women in the sample was insufficient to determine if gender had significantly affected the hormonal levels. Three subjects in each group were on oral contraceptive medications which could have affected their prolactin response.
Acknowledgements
The authors wish to acknowledge the critical clinical research contributions of the Biological Studies Unit, VA Connecticut Healthcare System including Elizabeth O’Donell, RN; Angelina Genovese, RN; Sonah Yoo, RPh; Robert Sturwold, RPh; and Mr. Willie Ford. This study was supported by the National Institute of Drug Abuse (DA12382-01 to DCD). In addition, the authors acknowledge the support from the (1) Department of Veterans Affairs Schizophrenia Biological Research Center (John Krystal), (2) National Institute of Mental Health (MH61019-02 to DCD), (3) National Institute of Alcohol Abuse and Alcoholism (R03 AA11413-02 to DCD), (4) Stanley Medical Research Institute (DCD), and (5) Donaghue Foundation (DCD).
Abbreviations
- Δ-9-THC
delta-9-tetrahydrocannabinol
- CB
cannabinoid
Footnotes
Declaration of interest There are no direct or indirect conflicts of interest for any of the authors relevant to the subject of this manuscript.
Contributor Information
Mohini Ranganathan, Schizophrenia Biological Research Center, VA Connecticut, Healthcare System, West Haven, CT, USA; Department of Psychiatry, School of Medicine, Yale University, New Haven, CT, USA.
Gabriel Braley, Schizophrenia Biological Research Center, VA Connecticut, Healthcare System, West Haven, CT, USA; Department of Psychiatry, School of Medicine, Yale University, New Haven, CT, USA.
Brian Pittman, Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, New Haven, CT, USA.
Thomas Cooper, Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York, USA; Nathan Kline Institute, Orangeburg, NY, USA.
Edward Perry, Schizophrenia Biological Research Center, VA Connecticut, Healthcare System, West Haven, CT, USA; Department of Psychiatry, School of Medicine, Yale University, New Haven, CT, USA.
John Krystal, Schizophrenia Biological Research Center, VA Connecticut, Healthcare System, West Haven, CT, USA; Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, New Haven, CT, USA; Department of Psychiatry, School of Medicine, Yale University, New Haven, CT, USA.
Deepak Cyril D’Souza, Schizophrenia Biological Research Center, VA Connecticut, Healthcare System, West Haven, CT, USA; Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, New Haven, CT, USA; Department of Psychiatry, School of Medicine, Yale University, New Haven, CT, USA.
References
- Block RI, Farinpour R, Schlechte JA. Effects of chronic marijuana use on testosterone, luteinizing hormone, follicle stimulating hormone, prolactin and cortisol in men and women. Drug Alcohol Depend. 1991;28:121–128. doi: 10.1016/0376-8716(91)90068-a. [DOI] [PubMed] [Google Scholar]
- Brown TT, Dobs AS. Endocrine effects of marijuana. J Clin Pharmacol. 2002;42:90S–96S. doi: 10.1002/j.1552-4604.2002.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Johnson RE, Moore JD, Roache JD. Acute effects of smoking marijuana on hormones, subjective effects and performance in male human subjects. Pharmacol Biochem Behav. 1986;24:1749–1754. doi: 10.1016/0091-3057(86)90515-0. [DOI] [PubMed] [Google Scholar]
- Dax EM, Pilotte NS, Adler WH, Nagel JE, Lange WR. The effects of 9-ene-tetrahydrocannabinol on hormone release and immune function. J Steroid Biochem. 1989;34:263–270. doi: 10.1016/0022-4731(89)90090-3. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008a;33(10):2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman N, Ranganathan M, Bhakta S, Zimolo Z, Cooper T, Perry E. Effects of haloperidol on the behavioral, subjective, cognitive, motor and neuroendocrine effects of D-9-tetrahydrocannabinol in humans. Psychopharmacology. 2008b doi: 10.1007/s00213-007-1042-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders—non-patient edition. American Psychiatric Association; Arlington, VA: 2002. [Google Scholar]
- Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Harmon J, Aliapoulios MA. Gynecomastia in marihuana users. N Engl J Med. 1972;287:936. doi: 10.1056/NEJM197211022871824. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Ferdinand RF, Ormel J, Verhulst FC. Hypothalamic-pituitary-adrenal axis activity and early onset of cannabis use. Addiction. 2006;101:1581–1588. doi: 10.1111/j.1360-0443.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Lemberger L, Crabtree R, Rowe H, Clemens J. Tetrahydrocannabinols and serum prolactin levels in man. Life Sci. 1975;16:1339–1343. doi: 10.1016/0024-3205(75)90319-7. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handb Exp Pharmacol. 2005;168:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- Majewska MD. HPA axis and stimulant dependence: an enigmatic relationship. Psychoneuroendocrinology. 2002;27:5–12. doi: 10.1016/s0306-4530(01)00033-6. [DOI] [PubMed] [Google Scholar]
- Markianos M, Stefanis C. Effects of acute cannabis use and short-term deprivation on plasma prolactin and dopamine-beta-hydroxylase in long-term users. Drug Alcohol Depend. 1982;9:251–255. doi: 10.1016/0376-8716(82)90050-3. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Ellingboe J, Mello NK. Acute effects of natural and synthetic cannabis compounds on prolactin levels in human males. Pharmacol Biochem Behav. 1984;20:103–106. doi: 10.1016/0091-3057(84)90109-6. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Ellingboe J. Acute effects of marihuana smoking on prolactin levels in human females. J Pharmacol Exp Ther. 1985;232:220–222. [PubMed] [Google Scholar]
- Murphy LL, Steger RW, Smith MS, Bartke A. Effects of delta-9-tetrahydrocannabinol, cannabinol and cannabidiol, alone and in combinations, on luteinizing hormone and prolactin release and on hypothalamic neurotransmitters in the male rat. Neuroendocrinology. 1990;52:316–321. doi: 10.1159/000125604. [DOI] [PubMed] [Google Scholar]
- Murphy LL, Munoz RM, Adrian BA, Villanua MA. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis. 1998;5:432–446. doi: 10.1006/nbdi.1998.0224. [DOI] [PubMed] [Google Scholar]
- Olusi SO. Hyperprolactinaemia in patients with suspected cannabis-induced gynaecomastia. Lancet. 1980;1:255. doi: 10.1016/s0140-6736(80)90738-2. [DOI] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- Rettori V, Wenger T, Snyder G, Dalterio S, McCann SM. Hypothalamic action of delta-9-tetrahydrocannabinol to inhibit the release of prolactin and growth hormone in the rat. Neuroendocrinology. 1988;47:498–503. doi: 10.1159/000124961. [DOI] [PubMed] [Google Scholar]
- Rodriguez De Fonseca F, Gorriti MA, Bilbao A, Escuredo L, Garcia-Segura LM, Piomelli D, Navarro M. Role of the endogenous cannabinoid system as a modulator of dopamine transmission: implications for Parkinson’s disease and schizophrenia. Neurotox Res. 2001;3:23–35. doi: 10.1007/BF03033228. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia-Palomero E, Castro JG, Garcia-Gil L, Ramos JA, Fernandez-Ruiz JJ. Effects of chronic exposure to delta9-tetrahydrocannabinol on cannabinoid receptor binding and mRNA levels in several rat brain regions. Brain Res Mol Brain Res. 1997;46:100–108. doi: 10.1016/s0169-328x(96)00277-x. [DOI] [PubMed] [Google Scholar]
- Rosenblitt JC, Soler H, Johnson SE, Quadagno DM. Sensation seeking and hormones in men and women: exploring the link. Horm Behav. 2001;40:396–402. doi: 10.1006/hbeh.2001.1704. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2003 National Survey on Drug Use and Health: national findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2004. [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. Measuring alcohol consumption. Humana; Totowa, NJ: 1992. [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med. 2006;36:1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Wenger T, Rettori V, Snyder GD, Dalterio S, McCann SM. Effects of delta-9-tetrahydrocannabinol on the hypothalamicpituitary control of luteinizing hormone and follicle-stimulating hormone secretion in adult male rats. Neuroendocrinology. 1987;46:488–493. doi: 10.1159/000124870. [DOI] [PubMed] [Google Scholar]


