Abstract
Kappa opioid receptor (KOR) agonists such as U-50488H and bremazocine are analgesics and diuretics. In monkeys, the selective KOR antagonist, nor-binaltorphimine (nor-BNI), produces a long-lasting antagonism of the antinociceptive effects of U-50488H but not those of bremazocine, suggesting that KOR-mediated antinociception may occur through two distinct KORs. The aim of this study was to characterize the antagonist effect of nor-BNI against the diuretic effects of U-50488H and bremazocine in monkeys. Urine outputs were collected over 3 h subsequent to i.m. administration of KOR agonists. Both U-50488H (0.032–1 mg/kg) and bremazocine (0.00032–0.01 mg/kg) dose-dependently increased urine output and the diuretic effect reached a plateau at higher doses. The maximum effect of either U-50488H or bremazocine was approximately 15 ml/kg/3 h of urine. Pretreatment with intracisternal nor-BNI 0.32 mg significantly blocked both U-50488H (0.18 mg/kg)- and bremazocine (0.0032 mg/kg)-induced diuresis for 20 weeks. However, the same dose of nor-BNI 0.32 mg given subcutaneously was not effective. These results demonstrate that central KOR mediate KOR agonist-induced diuresis in monkeys. More important, this study provides functional evidence for a homogenous population of KOR underlying KOR-mediated diuresis and illustrates a unique pharmacological profile of nor-BNI-induced ultra-long KOR antagonism in vivo.
Theme: Neurotransmitters, modulators, transporters, receptors
Topic: Opioids, anatomy, physiology, physiology
Keywords: Diuresis, Kappa opioid receptor, Cisterna magna
1. Introduction
Activation of kappa opioid receptors (KORs) produces several physiological functions such as antinociception [9,24], diuresis [8,21,22], hormonal regulation [6,17,20], and neuroprotection [35]. The function of KORs is particularly intriguing because, like mu opioid receptors (MORs), KOR agonists have antinociceptive effects [8,9,14], but KOR agonists possess several actions opposite to MOR agonists [26]. For example, MOR agonists stimulate dopamine release, whereas KOR agonists decrease dopamine release [33,36]. MOR agonists provoke itch sensation, whereas KOR agonists inhibit itch sensation [18]. In addition, selective KOR agonists do not have MOR-mediated side effects such as constipation and respiratory depression. KOR agonists have their own side effects (e.g., dysphoria) [27], although their relevance in actual therapeutic conditions has not been widely studied in primates.
Studies of KOR-mediated antinociception in non-human primates have raised the possibility that there are KOR subtypes that modulate this effect. Based on antagonist studies in monkeys, it has been suggested that KOR agonists such as U-50488H produce antinociception through KOR-1, whereas other KOR agonists such as bremazocine produce this effect through KOR-2 [4,5,14]. The distinction between KOR-1 and KOR-2 agonists is drawn primarily from in vivo observations of a differential potency of a KOR antagonist, nor-binaltorphimine (nor-BNI), and other opioid antagonists such as naltrexone. For example, subcutaneous (s.c.) administration of nor-BNI antagonizes the antinociceptive effect of U-50488H, but not bremazocine [4]. Both nor-BNI and naltrexone have differential affinities for KOR-1 versus KOR-2 binding sites in the cortical membranes of monkeys [5,14]. However, some studies have shown that nor-BNI is equally effective in antagonizing effects produced by various KOR agonists and suggest that U-50488H and bremazocine activate G-proteins through the same receptor [7,10,29]. Currently, only one type of KOR has been cloned and it appears to resemble the KOR-1 [31,38]. Thus, additional functional studies are needed to understand the possible relevance in vivo of apparent KOR subtypes. In particular, it is not clear whether the differential ability of nor-BNI to antagonize KOR agonists is retained across different in vivo assays in monkeys.
KOR agonists are the only opioid receptor agonists that produce diuresis [21,22]. Several studies in rodents have reported that the KOR-mediated diuretic effect is due mainly to inhibition of vasopressin release from the neurohypophysis [22,25,37]. Studies in primates also suggest the involvement of vasopressin in the diuretic effects of KOR agonists [1,19] as well as the presence of KOR in relevant hypothalamic nuclei [28,32]. Although one study suggests that KOR agonist-induced diuresis is mediated partially in the periphery [30], most studies indicate that the diuretic effect of KOR agonists is mediated primarily in the central nervous system (CNS) [3,12,21]. However, it is not known whether there are KOR subtypes involved in KOR-mediated diuresis or whether the effect is mediated through central KORs in primates.
In a previous study [16], we used intracisternal (i.c.) administration of nor-BNI to distinguish central from peripheral KOR-mediated antinociception and to distinguish central KOR-1 and KOR-2 antinociception in monkeys. Pretreatment with i.c. nor-BNI 0.32 mg, which is a systemically inactive dose, significantly blocked antinociception induced by systemically administered U-50488H for 5 weeks. In contrast, the same dose of i.c. nor-BNI failed to block bremazocine-induced antinociception [16]. The aim of the current study was to characterize central antagonism of KOR agonist-induced diuresis by using i.c. nor-BNI administration. In this study, we compared the effectiveness of i.c. nor-BNI 0.32 mg in blocking U-50488H- and bremazocine-induced diuresis and measured the duration of any antagonism observed in monkeys.
2. Materials and methods
2.1. Subjects
Twelve adult male and female rhesus monkeys (Macaca mulatta) with body weights ranging between 5.1 and 10.8 kg were used. They were housed individually with free access to water and were fed approximately 25 to 30 biscuits (Purina Monkey Chow; Ralston Purina, St. Louis, MO, USA) and fresh fruit daily. All monkeys were housed in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. The studies were conducted in accordance with the University Committee on the Use and Care of Animals in the University of Michigan and the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington DC, revised 1996).
2.2. Procedure
Urine outputs were collected over 3 h (typically between 9:30 a.m. and 12:30 p.m.) subsequent to the intramuscular (i.m.) administration of KOR agonists. Monkeys were fed after the collection period. Doses of U-50488H (0.032–1 mg/kg) and bremazocine (0.00032–0.01 mg/kg) were chosen based on previous studies indicating that they were behaviorally active doses [8,9,14]. A single dosing procedure was used in all sessions. Urine measurements were performed in the home cage of each monkey with a clean cage pan placed under the grid floor. In agonist dose– effect curve studies, all monkeys (n=8) received the vehicle and doses of i.m. U-50488H and bremazocine that were given in a random order. Each experimental condition/dose was repeated twice except in the antagonist study. Drug doses were typically given once per week. Occasionally, a drug dose was given twice per week with at least a 3-day interval between drug administrations.
In the antagonist studies, one group of six monkeys received i.c. nor-BNI (0.32 mg) and another group of five monkeys received i.c. vehicle (saline 1 ml). The diuretic effects of U-50488H (0.18 mg/kg) and bremazocine (0.0032 mg/kg) were determined before and after the pretreatment. These doses of KOR agonists were chosen because they were the smallest doses that produced maximal diuretic effects in these monkeys. The selected doses of U-50488H and bremazocine were given on alternate weeks. The first injection for both groups was of U-50488H and it was given 24 h after the pretreatment. Urine collection measurements were taken weekly after administration of KOR agonists for 25 weeks. In addition, the centrally effective dose of nor-BNI, 0.32 mg, was given subcutaneously in the scapular region in a group of six monkeys in order to compare the effect of nor-BNI by the i.c. and s.c. routes.
2.3. Data analysis
Individual 3-h cumulative urine volumes for each session were determined as ml/kg/3 h based on each monkey’s body weight and were used to calculate mean values (mean±S.E.M.). Data from all experiments were analyzed by one-way analysis of variance (ANOVA) followed by the Newman–Keuls test for multiple (post hoc) comparisons (P<0.05).
2.4. Drugs
U-50488H HCl (Upjohn Company, Kalamazoo, MI, USA) and bremazocine HCl (Research Biochemicals, Natick, MA, USA) were dissolved in sterile water. For i.m. or s.c. administration, all compounds were administered at a volume of 0.1 ml/kg. For i.c. administration, monkeys were anesthetized with ketamine HCl (10 mg/kg, i.m.) and the dorsal upper neck/lower skull area was shaved and sterilized with Betadine. A spinal needle (22 gauge, 11/2 in. long, Becton Dickinson, Franklin Lakes, NJ, USA; 1 in.=2.54 cm) was inserted into the cisterna magna by puncturing the skin and atlanto-occipital membranes. The position of needle was confirmed by free flow of clear cerebrospinal fluid. A 1 ml solution of either nor-BNI (Sigma, St. Louis, MO, USA) in saline or saline alone was slowly infused through the spinal needle in 30 s and monkeys were returned to their home cages.
3. Results
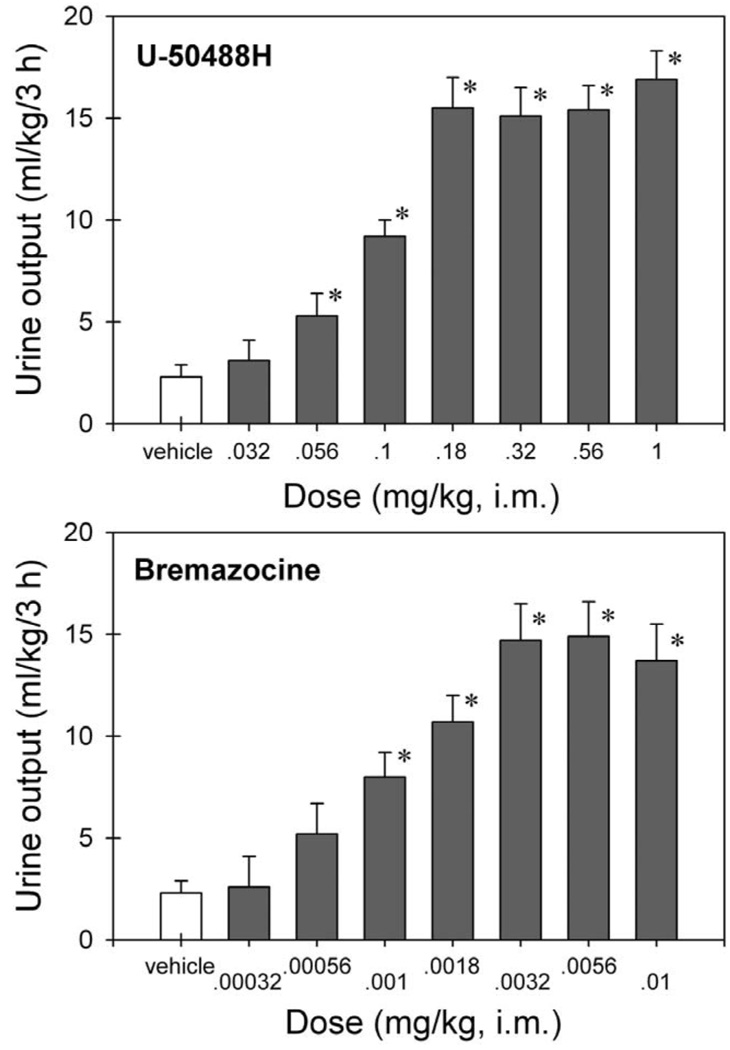
Fig. 1 illustrates the dose–response of the two KOR agonists for diuresis in monkeys. Vehicle-treated monkeys produced approximately 6 to 23 ml of urine (i.e., 2.3±0.6 ml/kg) during the 3-h measurement period. U-50488H dose-dependently increased urine output [F(7,56)=27.8; P<0.05]. Post hoc comparisons indicated that i.m. U-50488H (0.056–1 mg/kg) significantly increased urine output. Bremazocine also dose-dependently increased urine output [F(7,56)=13.7; P<0.05]. Post hoc comparisons indicated that i.m. bremazocine (0.001–0.01 mg/kg) significantly increased urine output. The maximum urine output for the two compounds appeared comparable at approximately 15 ml/kg/3 h. The smallest dose of U-50488H to produce this maximum urine output was 0.18 mg/kg, and the smallest dose of bremazocine to produce a maximum urine output was 0.0032 mg/kg. Male and female monkeys produced similar amounts of urine under these conditions. The mean values of i.m. U-50488H (0.18 mg/kg)-evoked urine output were 16.2 (mean)±2.6 (S.E.M.) ml/kg/3 h in male monkeys (n=4) and 14.9±1.6 ml/kg/3 h in female monkeys (n=4). The mean values of i.m. bremazocine (0.0032 mg/kg)-evoked urine output were 14.7±1.7 ml/kg/3 h in male monkeys (n=4) and 14.8±3.6 ml/kg/3 h in female monkeys (n=4).
Fig. 1.
Effects of several doses of U-50488H and bremazocine on urine output in monkeys. Each value represents the mean±S.E.M. (n=8). Abscissae: dose (mg/kg, i.m.). Ordinates: cumulative urine output (ml/kg) over a 3-h period. The asterisk represents a significant difference from the vehicle condition (* P<0.05).
Fig. 2 illustrates the time–course of the antagonist effects of i.c. nor-BNI on U-50488H- and bremazocine-induced diuresis. Pretreatment with i.c. vehicle did not change either U-50488H- or bremazocine-induced diuretic effects (P>0.05). In contrast, pretreatment with i.c. nor-BNI 0.32 mg significantly blocked U-50488H-increased urine output [F(13,70)=8.5; P<0.05]. Post hoc comparisons indicated that i.c. nor-BNI 0.32 mg blocked U-50488H-induced diuresis for 20 weeks (P<0.05). U-50488H-induced diuresis (4.3±0.7 ml/kg/3 h) on day 1 after nor-BNI pretreatment is not shown in Fig. 2 for the sake of clarity.
Fig. 2.
Time–course of the antagonist effect of i.c. nor-BNI on the diuretic effect of KOR agonists. Each value represents the mean±S.E.M. (n=5–6). Left panels show the effect of i.c. vehicle (saline) pretreatment on the diuretic effects of U-50488H and bremazocine. Right panels show the effect of i.c. nor-BNI 0.32 mg pretreatment on the diuretic effect of U-50488H and bremazocine. BL (baseline) represents the diuretic effects of U-50488H (0.18 mg/kg, i.m.) or bremazocine (0.0032 mg/kg, i.m.) before each group of monkeys received either vehicle or nor-BNI pretreatment. The asterisk represents a significant difference from the BL condition (* P<0.05).
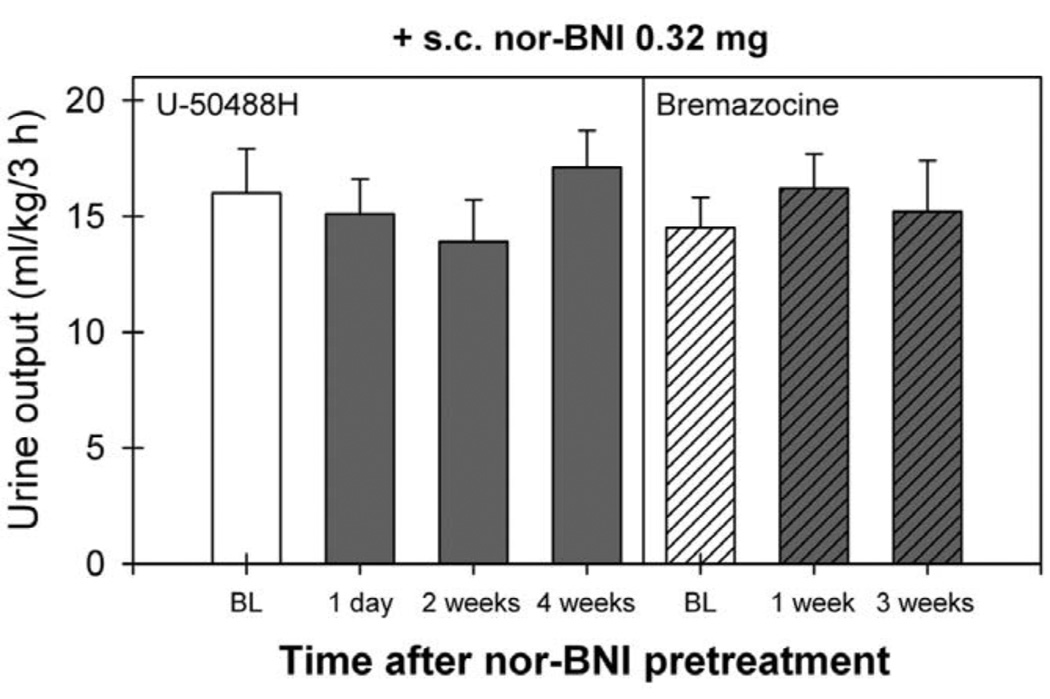
Pretreatment with nor-BNI also significantly blocked bremazocine-increased urine output [F(14,75)=7.7; P<0.05]. Post hoc comparisons indicated that i.c. nor-BNI 0.32 mg blocked bremazocine-induced diuresis for 19 weeks (P<0.05). The average body weight of monkeys did not significantly change throughout the study. The range of average body weights was from 7.9±0.7 to 8.7±0.8 kg during the course of the study. Fig. 3 illustrates a lack of antagonist effect of s.c. nor-BNI on KOR agonist-induced diuresis. Pretreatment with s.c. nor-BNI 0.32 mg did not change either U-50488H- or bremazocine-induced diuretic effects (P>0.05). Therefore, the study was terminated 4 weeks following nor-BNI administration.
Fig. 3.
Lack of antagonist effect of s.c. nor-BNI on the diuretic effect of KOR agonists. Each value represents the mean±S.E.M. (n=6). BL (baseline) represents the diuretic effects of U-50488H (0.18 mg/kg, i.m.) or bremazocine (0.0032 mg/kg, i.m.) before monkeys received s.c. nor-BNI 0.32 mg pretreatment. There was no significant difference between each value.
4. Discussion
The present study showed that both U-50488H and bremazocine dose-dependently produced diuresis in monkeys. Pretreatment with i.c. nor-BNI significantly blocked both U-50488H- and bremazocine-induced diuretic effects for approximately 20 weeks. However, the centrally effective dose of nor-BNI, when given by s.c. route, was not effective in blocking the diuretic effects of U-50488H and bremazocine. This study provides functional evidence for a homogeneous population of central KOR-mediated diuresis in primates.
Highly detailed i.m. U-50488H and bremazocine dose– effect curves were established in the diuresis endpoint in rhesus monkeys (Fig. 1). These doses of both compounds were active in other behavioral assays in monkeys including drug discrimination, antinociception, and sedation [4,9,14,16]. Both U-50488H and bremazocine produced diuresis in a dose-dependent manner and that diuresis reached a plateau at higher doses. This suggests that U-50488H and bremazocine are ‘pure’ KOR agonists and agrees with earlier reports in rats showing that KOR agonists without MOR agonist activity had sigmoidal dose–effect curves, whereas KOR agonists with MOR agonist activity had inverted U-shaped dose–effect curves for diuresis [21–23]. Both U-50488H and bremazocine were characterized as full KOR agonists in terms of stimulation of [35S]GTPγS binding in a cell line expressing the human KOR [29,39]. In addition, both compounds were able to produce full antinociception against a higher intensity of noxious stimulus, 55 °C water, in which partial opioid agonists were not effective [4,9]. Taken together, these results indicate that U-50488H and bremazocine are full KOR agonists with little or no MOR activation and both compounds produce comparable diuresis.
Pretreatment with i.c. nor-BNI 0.32 mg blocked U-50488H- and bremazocine-induced diuresis for approximately 20 weeks (Fig. 2). To our knowledge, this is the longest duration of nor-BNI-induced KOR antagonism measured in vivo. The results of the vehicle group show that the factor of time and weekly agonist administration did not change the diuretic effects of the KOR agonists in monkeys and that tolerance did not develop. It is well known that systemic or central administration of nor-BNI produces a long-lasting KOR antagonism across different in vivo assays and species [2,4,10,11]. For example, pretreatment with i.c.v. nor-BNI 1 nmol antagonized KOR agonist-induced antinociception for 4 weeks in mice [10]. There are few studies that elucidate the mechanisms underlying this unique pharmacological profile of nor-BNI-induced long-term antagonism. It has been speculated that either nor-BNI is resistant to metabolism or nor-BNI may produce conformational changes in KOR. A recent study reported that nor-BNI was detected in mouse brain homogenates for 21 days using high-performance liquid chromatography (HPLC) following a single injection of s.c. nor-BNI 30 mg/kg [13].
Pretreatment with i.c. nor-BNI 0.32 mg was equally effective in blocking U-50488H- and bremazocine-induced diuresis (Fig. 2). This finding agrees with other reports demonstrating that nor-BNI suppressed the diuretic activity of various KOR agonists including U-50488H and bremazocine in rats [34]. More important, this finding is consistent with other studies indicating that effects mediated by either U-50488H or bremazocine can be blocked by nor-BNI [2,7,10,11]. As reported in a previous study, the same dose of i.c. nor-BNI blocked only U-50488H-and not bremazocine-induced antinociception and sedation in monkeys [16]. The insensitivity of KOR-2 agonists, such as bremazocine and GR89,696, to nor-BNI antagonism in vivo was observed only in monkeys [4,6,16] and not in other species [2,10,11]. However, we recently found that nor-BNI had the same potency in blocking the [35S]GTPγS binding stimulated by both KOR-1 and KOR-2 agonists in the cortical membranes of monkeys (unpublished observation). The present study is the first in primates showing lack of relative nor-BNI selectivity for U-50488H versus bremazocine in vivo. Although KOR-1 and proposed KOR-2 agonists may have differential sensitivity to opioid antagonists depending on assays and species, both types of compounds are equally active in a variety of behavioral assays in monkeys [4,6,8,9,15,16].
It is worth noting that 0.32 mg of i.c. nor-BNI used in this study is a very small dose compared with systemically effective doses (e.g., 3.2–10 mg/kg) in monkeys [4,5]. In particular, this centrally effective dose of nor-BNI, when given subcutaneously, was not effective in blocking either U-50488H- or bremazocine-induced diuresis (Fig. 3). This observation supports the notion that systemic administration of KOR agonists produces diuresis mainly through central KOR activation [3,12,21]. One intriguing finding is that in monkeys the same dose of i.c. nor-BNI 0.32 mg produced KOR antagonism of diuresis for 20 weeks, but only 5 weeks of KOR antagonism of antinociception and sedation [16]. It is possible that the KOR population required for producing antinociception and sedation is greater than that for diuresis. As noted, the dose of U-50488H producing full antinociception is between approximately 1 and 3.2 mg/kg [4,9,14,16] and the dose of U-50488H producing a maximum diuretic effect is 0.18 mg/kg. In addition, the site of action of KOR-mediated diuresis (i.e., hypothalamus) may be more localized and anatomically close to the cisternal injection of nor-BNI, whereas the site of KOR related to antinociception may be more diffuse throughout the CNS. It will be interesting to characterize the time course of KOR changes in different neural substrates following a single dose of i.c. nor-BNI in monkeys by using brain imaging techniques such as a positron emission tomography (PET) scan.
In summary, the results of this study demonstrated central KOR-mediated diuresis of KOR agonists in monkeys. Both U-50488H, a KOR-1 agonist, and bremazocine, a proposed KOR-2 agonist, produced diuresis through the same KOR population. The technique of i.c. nor-BNI administration can be used further to study the role of central KOR in drug discrimination and endocrine assays [6,9,17]. These studies will establish the pharmacological basis of central KOR antagonism and facilitate our understanding of KOR in vivo pharmacology in primates.
Acknowledgements
The authors thank Dr. Gail Winger for her assistance with the editing of manuscript and Michael Song, Ryan Sherriff, and Chun-Yan Dou for excellent technical assistance. This study was supported by US Public Health Service grants DA-00254 and DA-13685.
References
- 1.Bellissant E, Denolle T, Sinnassamy P, Bichet DG, Giudicelli JF, Lopez F, Gandon JM, Allain H. Systemic and regional hemodynamic and biological effects of a new kappa-opioid agonist, niravoline, in healthy volunteers. J. Pharmacol. Exp. Ther. 1996;278:232–242. [PubMed] [Google Scholar]
- 2.Broadbear JH, Negus SS, Butelman ER, DeCosta BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on k-opioid agonists in the mouse writhing assay. Psychopharmacology. 1994;115:311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- 3.Brooks DP, Giardina G, Gellai M, Dondio G, Edwards RM, Petrone G, DePalma PD, Sbacchi M, Jugus M, Misiano P, Wang YX, Clarke GD. Opiate receptors within the blood–brain barrier mediate kappa agonist-induced water diuresis. J. Pharmacol. Exp. Ther. 1993;266:164–171. [PubMed] [Google Scholar]
- 4.Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J. Pharmacol. Exp. Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- 5.Butelman ER, Ko MC, Sobczyk-kojiko K, Mosberg HI, Van Bemmel B, Zernig G, Woods JH. Kappa-opioid receptor binding populations in rhesus monkey brain: relationship to an assay of thermal antinociception. J. Pharmacol. Exp. Ther. 1998;285:595–601. [PubMed] [Google Scholar]
- 6.Butelman ER, Ko MC, Traynor JR, Vivian JA, Kreek MJ, Woods JH. GR89,696: a potent kappa-opioid agonist with subtype selectivity in rhesus monkeys. J. Pharmacol. Exp. Ther. 2001;298:1049–1059. [PubMed] [Google Scholar]
- 7.Childers SR, Xiao R, Vogt L, Sim LJ. Lack of evidence of k2-selective activation of G-proteins. κ opioid receptor stimulation of [35S]GTPγS binding in guinea pig brain. Biochem. Pharmacol. 1998;56:113–120. doi: 10.1016/s0006-2952(98)00123-3. [DOI] [PubMed] [Google Scholar]
- 8.Dykstra LA, Gmerek DE, Winger G, Woods JH. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J. Pharmacol. Exp. Ther. 1987;242:413–420. [PubMed] [Google Scholar]
- 9.France CP, Medzihradsky F, Woods JH. Comparison of kappa opioids in rhesus monkeys: behavioral effects and receptor binding affinities. J. Pharmacol. Exp. Ther. 1994;268:47–58. [PubMed] [Google Scholar]
- 10.Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J. Pharmacol. Exp. Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- 11.Jewett DC, Woods JH. Nor-binaltorphimine: an ultra-long acting kappa-opioid antagonist in pigeons. Behav. Pharmacol. 1995;6:815–820. [PubMed] [Google Scholar]
- 12.Kapusta DR, Obih JC. Central kappa opioid receptor-evoked changes in renal function in conscious rats: participation of renal nerves. J. Pharmacol. Exp. Ther. 1993;267:197–204. [PubMed] [Google Scholar]
- 13.Kishioka S, Maeda T, Shimizu N, Fukazawa Y, Fan X, Ozaki M, Ko MCH, Woods JH, Yamamoto H. Pharmacokinetics of nor-binaltorphimine: evidence for long-lasting effects; Conference Proceedings of Annual Conference on Opioid Mimetic Analgesics; 2002. pp. 112–113. [Google Scholar]
- 14.Ko MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J. Pharmacol. Exp. Ther. 1998;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- 15.Ko MC, Butelman ER, Woods JH. Activation of peripheral kappa opioid receptors inhibits capsaicin-induced thermal nociception in rhesus monkeys. J. Pharmacol. Exp. Ther. 1999;289:378–385. [PMC free article] [PubMed] [Google Scholar]
- 16.Ko MCH, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, Woods JH. Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J. Pharmacol. Exp. Ther. 1999;291:1113–1120. [PMC free article] [PubMed] [Google Scholar]
- 17.Ko MC, Williams KL, Mukhopadhyay P, Lee H, Ensley AJ, Rice KC, Woods JH. Effects of opioid receptor agonists on the hypothalamic–pituitary–adrenal axis in rhesus monkeys; Conference Proceedings of the 64th Annual Meeting of College on Problems of Drug Dependence, Drug Alcohol Depend; 2002. p. S95. [Google Scholar]
- 18.Ko MCH, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, Woods JH, Naughton NN. Activation of kappa-opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J. Pharmacol. Exp. Ther. 2003;305:173–179. doi: 10.1124/jpet.102.044909. [DOI] [PubMed] [Google Scholar]
- 19.Kramer HJ, Uhl W, Ladstetter B, Becker A. Influence of asimadoline, a new kappa-opioid receptor agonist, on tubular water absorption and vasopressin secretion in man. Br. J. Clin. Pharmacol. 2000;50:227–235. doi: 10.1046/j.1365-2125.2000.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krulich L, Koenig JI, Conway S, McCann SM, Mayfield MA. Opioid kappa receptors and the secretion of prolactin (PRL) and growth hormone (GH) in the rat. I. Effects of opioid kappa receptor agonists bremazocine and U-50,488 on secretion of PRL and GH: comparison with morphine. Neuroendocrinology. 1986;42:75–81. doi: 10.1159/000124252. [DOI] [PubMed] [Google Scholar]
- 21.Leander JD. A kappa opioid effect: increased urination in the rat. J. Pharmacol. Exp. Ther. 1983;224:89–94. [PubMed] [Google Scholar]
- 22.Leander JD. Further study of kappa opioids on increased urination. J. Pharmacol. Exp. Ther. 1983;227:35–41. [PubMed] [Google Scholar]
- 23.Leander JD. Effects of full and partial kappa agonists and mu agonists on urine output of normally hydrated rats. Neuropeptides. 1984;5:283–286. doi: 10.1016/0143-4179(84)90083-0. [DOI] [PubMed] [Google Scholar]
- 24.Millan MJ. Kappa-opioid receptor-mediated antinociception in the rat. I. Comparative actions of mu- and kappa-opioids against noxious thermal, pressure and electrical stimuli. J. Pharmacol. Exp. Ther. 1989;251:334–341. [PubMed] [Google Scholar]
- 25.Oiso Y, Iwasaki Y, Kondo K, Takatsuki K, Tomita A. Effect of the opioid kappa-receptor agonist U50488H on the secretion of arginine vasopressin. Study on the mechanism of U50488H-induced diuresis. Neuroendocrinology. 1988;48:658–662. doi: 10.1159/000125078. [DOI] [PubMed] [Google Scholar]
- 26.Pan ZZ. mu-Opposing actions of the kappa-opioid receptor. Trends Pharmacol. Sci. 1998;19:94–98. doi: 10.1016/s0165-6147(98)01169-9. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science (Washington,DC) 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 28.Pfeiffer A, Pasi A, Mehraien P, Herz A. Opiate receptor binding sites in human brain. Brain Res. 1982;248:87–96. doi: 10.1016/0006-8993(82)91150-7. [DOI] [PubMed] [Google Scholar]
- 29.Remmers AE, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. J. Pharmacol. Exp. Ther. 1999;288:827–833. [PubMed] [Google Scholar]
- 30.Salas SP, Roblero J, Ureta H, Huidobro-Toro JP. Diuretic effect of bremazocine, a kappa-opioid with central and peripheral sites of action. J. Pharmacol. Exp. Ther. 1989;250:992–999. [PubMed] [Google Scholar]
- 31.Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei MG, Charron G, Bloch B, Kieffer B. K-opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc. Natl. Acad. Sci. USA. 1995;92:7006–7010. doi: 10.1073/pnas.92.15.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sim-Selley LJ, Daunais JB, Porrino LJ, Childers SR. Mu and kappa1 opioid-stimulated [35S]guanylyl-5′-O-(γ-thio)-triphosphate binding in cynomolgus monkey brain. Neuroscience. 1999;94:651–662. doi: 10.1016/s0306-4522(99)00344-9. [DOI] [PubMed] [Google Scholar]
- 33.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takemori AE, Schwartz MM, Portoghese PS. Suppression by nor-binaltorphimine of kappa opioid-mediated diuresis in rats. J. Pharmacol. Exp. Ther. 1988;247:971–974. [PubMed] [Google Scholar]
- 35.Tortella FC, DeCoster MA. Kappa opioids: therapeutic considerations in epilepsy and CNS injury. Clin. Neuropharmacol. 1994;17:403–416. [PubMed] [Google Scholar]
- 36.Xi Z-X, Fuller SA, Stein EA. Dopamine release in the nucleus accumbens during heroin self-administration is modulated by kappa opioid receptors: an in vivo fast-cyclic voltammetry study. J. Pharmacol. Exp. Ther. 1998;284:151–161. [PubMed] [Google Scholar]
- 37.Yamada K, Imai M, Yoshida S. Mechanism of diuretic action of U-62,066E, a κ opioid receptor agonist. Eur. J. Pharmacol. 1989;160:229–237. doi: 10.1016/0014-2999(89)90495-0. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Chen C, Xue JC, Kunapuli S, DeRiel JK, Liu-Chen LY. Cloning of a human κ opioid receptor from the brain. Life Sci. 1995;56:201–207. doi: 10.1016/0024-3205(94)00507-o. PL. [DOI] [PubMed] [Google Scholar]
- 39.Zhu J, Luo LY, Li JG, Chen C, Liu-Chen LY. Activation of the cloned human kappa opioid receptor by agonists enhances [35S]GTPγS binding to membranes: Determination of potencies and efficacies of ligands. J. Pharmacol. Exp. Ther. 1997;282:676–684. [PubMed] [Google Scholar]