Abstract
Neuropsychological and imaging studies indicate that attention deficit hyperactivity disorder (ADHD) is associated with alterations in prefrontal cortex (PFC) and its connections to striatum and cerebellum. Research in animals, in combination with observations of patients with cortical lesions, has shown that the PFC is critical for the regulation of behavior, attention, and affect using representational knowledge. The PFC is important for sustaining attention over a delay, inhibiting distraction, and dividing attention, while more posterior cortical areas are essential for perception and the allocation of attentional resources. The PFC in the right hemisphere is especially important for behavioral inhibition. Lesions to the PFC produce a profile of distractibility, forgetfulness, impulsivity, poor planning, and locomotor hyperactivity. The PFC is very sensitive to its neurochemical environment, and either too little (drowsiness) or too much (stress) catecholamine release in PFC weakens cognitive control of behavior and attention. Recent electrophysiological studies in animals suggest that norepinephrine enhances “signals” through postsynaptic α2A adrenoceptors in PFC, while dopamine decreases “noise” through modest levels of D1 receptor stimulation. α2A-Adrenoceptor stimulation strengthens the functional connectivity of PFC networks, while blockade of α2 receptors in the monkey PFC recreates the symptoms of ADHD, resulting in impaired working memory, increased impulsivity, and locomotor hyperactivity. Genetic alterations in catecholamine pathways may contribute to dysregulation of PFC circuits in this disorder. Medications may have many of their therapeutic effects by optimizing stimulation of α2A adrenoceptors and D1 receptors in the PFC, thus strengthening PFC regulation of behavior and attention.
Keywords: prefrontal cortex, norepinephrine, dopamine, stress, attention, cognition
Introduction
The hallmark symptoms of attention deficit hyperactivity disorder (ADHD)—impaired behavioral inhibition, increased motor activity, and inattention—arise from disruptions in circuits regulating attention and action. Findings from imaging studies of ADHD indicate volumetric differences in prefrontal cortex (PFC), cerebellum, and possibly striatum (reviewed by Giedd1), consistent with the role of these regions in the cognitive operations that are impaired in ADHD patients. The PFC is of central relevance to the neuronal pathways of ADHD, as the PFC has extensive connections to sensory and motor cortices, as well as to the cerebellum and basal ganglia. Furthermore, in animals, the cardinal symptoms of ADHD are produced by lesions to the PFC, particularly, the right PFC.2 Catecholamines exert powerful effects on the local circuits of the PFC and associated cognitive operations in animals. The most effective treatments for ADHD enhance catecholamine actions, and recent research indicates that genetic changes in catecholamine pathways are associated with ADHD symptomology. The following chapter briefly reviews the anatomy and function of the higher association cortices relevant to ADHD, the connections of these cortical areas to the basal ganglia and cerebellum, and the powerful modulation of PFC circuits by dopamine (DA) and norepinephrine (NE).
The Functional Contributions of Higher Association Cortices
Association cortices make distinct contributions to our attentional experience. For example, the inferior temporal cortex processes sensory features and can focus resources on a particular detail, for example, the color red; the posterior parietal association cortex allocates attentional resources, allowing us to orient attention in time and space. The PFC receives inputs from these and other sensory association areas, and thus is well-positioned to inhibit processing of irrelevant stimuli and to regulate attention, that is, to be sustained, divided, or coordinated over a period of time via local and distal or reciprocal connections. These intricately interconnected cortical areas3 create both feedforward and feedback loops4 that together create a unified attentional experience. The PFC is a critical area of such integration and plays a prominent role in regulating movement and inhibiting inappropriate behavioral responses. The following is a brief review of these cortical systems.
Inferior Temporal Cortex
The inferior temporal cortex is specialized for processing visual features, determining “what” things are based on their color and shape. (In contrast, the superior temporal cortex processes auditory information, performing both featural and spatial analysis. This work has proceeded more recently,5 and thus will not be discussed in this review). Processing of a visual stimulus by inferior temporal neurons can either be diminished or enhanced, depending on sensory conditions and internal directions from regions such as the PFC or parietal association area (reviewed in Refs. 6 and 7). Neuronal activity is increased by salient or novel stimuli, but repeated experience with the same visual stimulus leads to decreased firing.6 This habituation may account for the boredom of repetition, for example, in a school setting. Processing of visual stimuli is also diminished by interference from nearby stimuli in the same visual field.6 Suppression of neuronal firing can result from intrinsic properties of inferior temporal neurons. Firing properties of inferior temporal neurons are also modulated by afferents arising in the PFC or parietal association cortex. These “top-down” projections can override the intrinsic habituation of inferior temporal neuronal firing, and thus allow for sustained, directed selective attention of visual feature processing.
Posterior Parietal Association Cortex
The posterior parietal association cortex (PAC) is specialized for the analysis of movement and spatial relationships, for analyzing quantity and constructing spatial maps, and for orienting attention in time and space.8 Lesions to the right PAC can result in contralateral neglect: the loss of perception for the left side of visual space. The symptoms of contralateral neglect may present as inattention; that is, the inability to allocate sufficient resources to stimuli for adequate perception. Indeed, the PAC is critical to conscious attention, and is considered to be particularly relevant to the act of “paying attention.”9 Recordings from parietal neurons in monkeys are consistent with a role in the allocation of attention, including covert shifts of attention.10 Neurons in area 7a also appear to create world-referenced maps of visual space,11 and they project this information to the PFC, which uses this information to guide behavior during spatial working-memory tasks (see below).
Prefrontal Cortex
Findings from lesion studies are consistent with the role of the PFC in coordinating, controlling, and executing cognitive and emotional processes, which allow for the regulation of impulses, language, attention, decision-making, and error correction. These “executive” functions require representational knowledge, that is, working memory, to guide overt responses (movement), covert responses (attention), and to inhibit inappropriate responding. The ventromedial PFC performs these same functions in the affective realm. Electrophysiological studies in monkeys have illuminated the cellular basis of representation knowledge. Single-unit recording studies have shown that PFC neurons are able to hold modality-specific information “on-line” over a delay and use this represented information to guide behavior in the absence of environmental cues.12 Importantly, PFC neurons can maintain delay-related firing in the presence of distracting stimuli, protecting performance from interference. 13 Delay-related firing also serves as the basis for behavioral inhibition, that is, the ability to suppress a prepotent response.14 The electrophysiological and cognitive operations of the PFC are greatly influenced by the neurochemical environment of the PFC. The dorsolateral PFC is particularly sensitive to the catecholamines DA and NE. The consequences of insufficient or excessive catecholamine release in the PFC, and the relevance of altered catecholamine regulation to ADHD, are discussed in a later section of this chapter. Disruptions to the intrinsic circuits that underlie working memory can impact the processing of information arriving at the PFC from “bottom-up” sources, including the inferior temporal cortex and PAC. Impairments in delay-related firing or working memory can also affect the execution of “top-down” commands to modulate subsequent processing, and compromise processes inherent to attention and inhibition.
The PFC has particular relevance to ADHD, as imaging studies indicate that ADHD patients often have smaller PFC volume, particularly on the right side.15–17 Lesions of the PFC produce symptoms similar to ADHD, for example, impairment in tasks of behavioral inhibition, working memory, and reward reversal.18–20 Like ADHD patients, patients with PFC lesions are easily distracted, have poor concentration and organization, are more vulnerable to disruption from proactive interference, and can be impulsive, and may have difficulty controlling overt behaviors, especially when the lesions involve the right hemisphere.21 PFC lesions impair the ability to sustain attention, particularly over a delay, and reduce the ability to gate sensory input.22 Deficits in divided and focused attention have been associated with lesions in the left, superior PFC.23 PFC lesions similarly impair attentional function in monkeys and rats, rendering animals more vulnerable to distraction or other types of interference, and impairing attentional regulation on set-shifting tasks.24,25
Cortical Projections to Basal Ganglia and Cerebellum
Imaging studies have reported reduced volume of the caudate nucleus, and particularly parts of the cerebellum in ADHD.26–28 The association cortices project down to both the basal ganglia and cerebellum in a series of parallel, closed-loop circuits.29 The PFC, parietal, and temporal association cortices all project to the caudate nucleus as part of the “cognitive circuit” through the basal ganglia, which in turn projects back to the PFC and premotor cortices. Similarly, the PFC and parietal association cortices project to the cerebellum by way of the pontine nuclei, and the cerebellum in turn projects back to the association cortices by way of dentate projections to thalamus. Although the basal ganglia and cerebellum have long been known to be important for the regulation of movement, their role in higher cognitive function is just beginning to be researched. If these structures influence cognition in a manner similar to their influences on movement, the basal ganglia may be important for the planning, selection, initiation, and execution of thoughts, while the cerebellum may serve as a “biological gyroscope,” correcting cognitive function on a faster timescale. Recent data show that cerebellar circuits interact with basal ganglia circuits via projections through the intralaminar thalamic nuclei to the striatum.30 The functioning of all of these circuits is intricately modulated by the arousal systems.
Modulation of Cortical Circuits
Many arousal systems project to the cortical mantle, including the ascending monoamine systems, acetylcholine neurons from the basal forebrain, and the more recently discovered orexins. The present chapter focuses on DA and NE actions in PFC, as these actions have been studied most extensively. Limited research suggests that NE may enhance “signal/noise” processing in posterior cortices via actions at β and α1-adrenoceptors, and possibly impair them via α2-adrenoceptors.31–33 There has been more extensive research on catecholamine influences on PFC, initiated by the landmark findings of Brozoski et al., who showed that catecholamine depletion in PFC is as destructive as ablation of the tissue itself.34 The following is a brief summary of this work (for more detailed reviews, see Refs. 35 and 36).
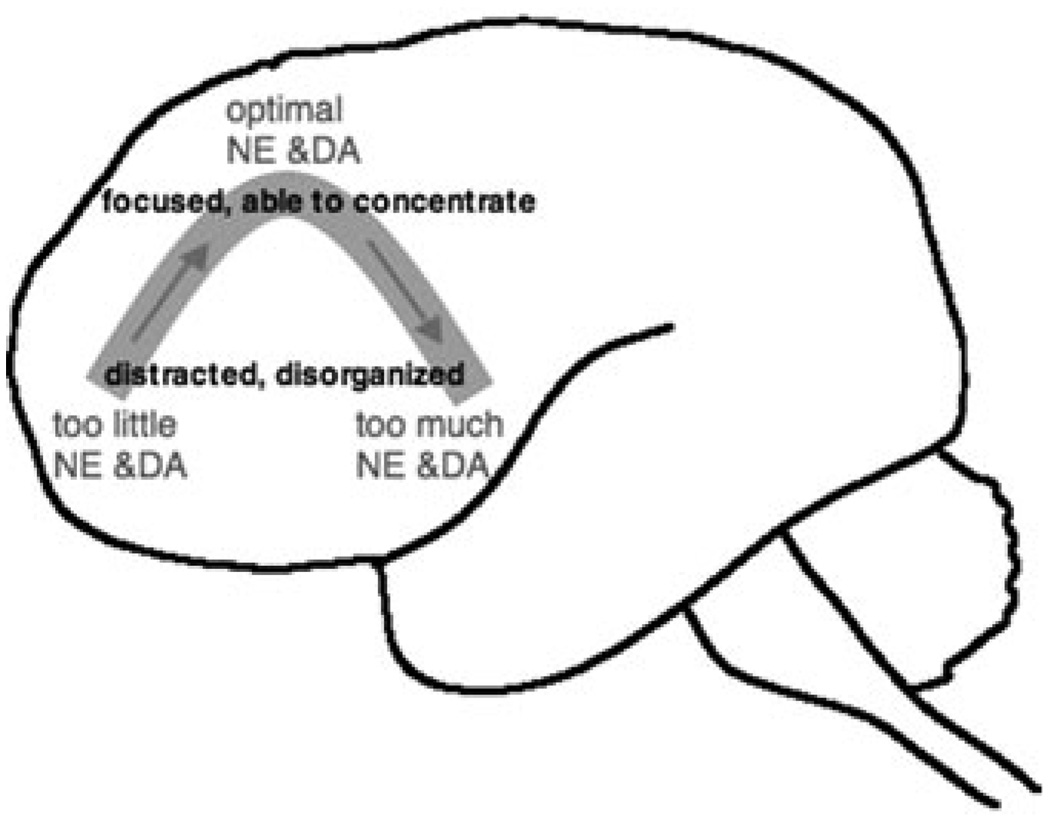
Both DA and NE exhibit an inverted U influence on PFC cognitive functions, where either too little or too much impairs PFC function (FIG. 1). The studies of Aston-Jones have shown that NE cells of the locus coeruleus fire according to levels of arousal, with low levels of both tonic and phasic firing under drowsy conditions, moderate tonic firing and clear phasic firing in response to relevant stimuli when animals are alert, and high tonic firing and poor phasic firing when animals are stressed (see Aston-Jones, this volume). Similarly, DA cells in the midbrain fire phasic responses related to the expectation of reward.37 Thus, the patterns of catecholamine release in PFC are directly related to arousal state and environmental conditions.
FIGURE 1.
Catecholamines have an inverted U influence on prefrontal cortex (PFC) function, whereby either too little or too much norepinephrine (NE) or dopamine (DA) impairs PFC cognitive abilities. Since the PFC is essential for attention regulation, optimal catecholamine actions in PFC are needed for focused, organized attention. Poor concentration and distractibility are common symptoms of weakened PFC function.
Norepinephrine
NE acts at three general families of adrenoceptors: α1, α2, and β(1,2,3). NE has distinct actions at these receptors, with modest levels of NE improving PFC function through actions at postsynaptic α2A receptors, while high levels of NE, such as are released during stress, impairing working memory through actions at α1 and β1 receptors.
α2A Adrenoceptor-Enhancing Actions
NE improves both behavioral and cellular measures of working memory (reviewed in Ref. 36) through actions at postsynaptic38,39 α2A receptors40 on PFC dendritic spines. The α2A agonist, guanfacine, improves working memory, attention regulation, conditional associations, behavioral inhibition, and/or planning in rodents,40,41 monkeys,42–45 and humans.46 Electrophysiological studies have shown that α2A receptor stimulation increases delay-related firing,47 the cellular measure of working memory and behavioral inhibition, that depends on recurrent activity in PFC networks. These enhancing effects are mediated through Gi-mediated suppression of cAMP intracellular signaling. cAMP opens hyperpolarization-activated cyclic nucleotide-gated cation channels (HCN channels) that are localized on PFC dendritic spines, often on the spine necks where they are ideally situated to gate synaptic inputs onto the spine.48 When the HCN channels open, membrane resistance lowers, and inputs to the spine are shunted. Conversely, α2A receptors inhibit cAMP, closing nearby HCN channels and allowing the network to connect.48 Thus, α2A-receptor stimulation increases delay-related firing for the preferred direction, that is, it increases “signals,” likely by strengthening network connectivity with neurons that have similar stimulus characteristics (schematically illustrated in FIG. 2). Conversely, blocking α2 receptors in PFC with yohimbine markedly reduces delay-related cell firing,47 and impairs working memory49 as well as impulse control.50 Yohimbine infusions into PFC have also been shown to induce locomotor hyperactivity.51 Thus, insufficient α2-receptor stimulation in monkey PFC can recreate the profile of ADHD. In this regard it is of interest that ADHD has been associated with genetic alterations in dopamine beta hydroxylase (DBH), the enzyme that synthesizes NE. It is possible that weaker DBH would lead to insufficient endogenous stimulation of α2A receptors in PFC, resulting in a profile similar to yohimbine-treated monkeys. Stimulant or α2A-agonist medications might correct this condition. Guanfacine is currently used for treating ADHD,52–54 especially in patients who cannot take stimulants, for example, those with tics or at risk of drug abuse.
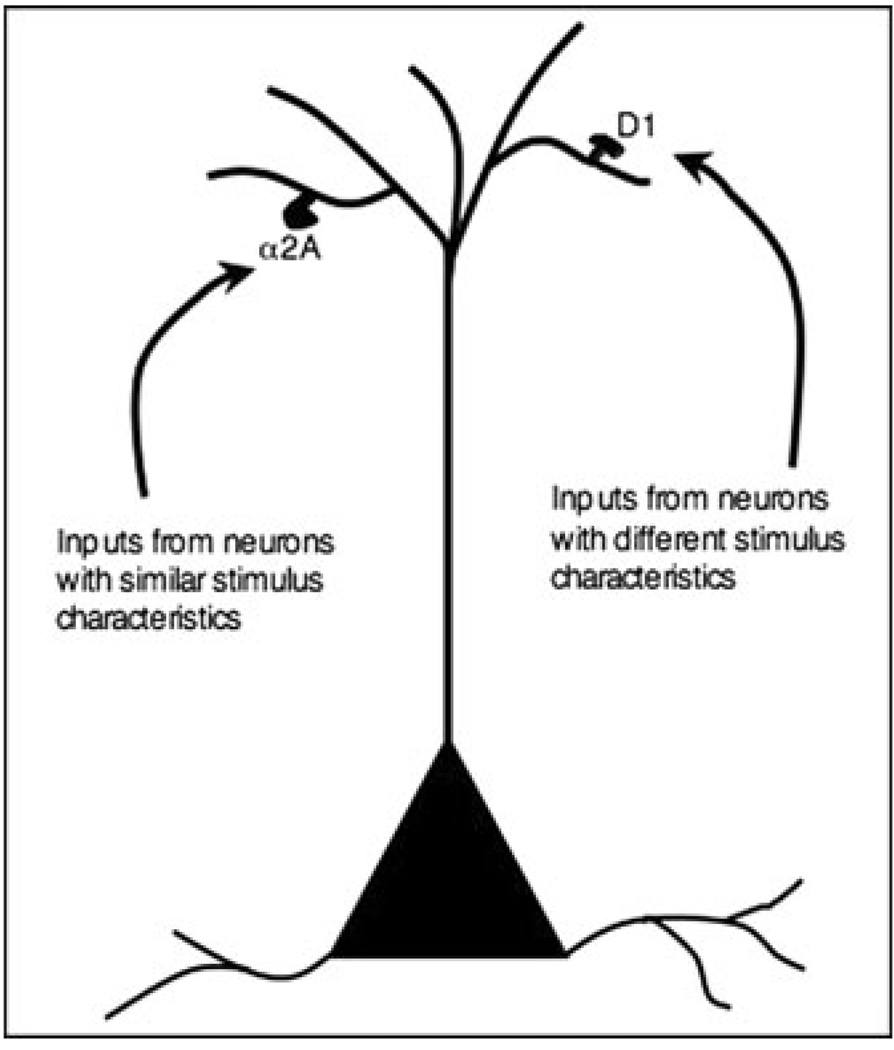
FIGURE 2.
A working model of how catecholamines may gate inputs onto prefrontal cortex (PFC) neurons. Electrophysiological and anatomical data suggest that norepinephrine (NE) acts at α2A-adrenoceptors on dendritic spines to enhance connectivity with inputs from other neurons with shared characteristics, and thus increase the “signal” of that neuron. For example, a neuron spatial tuned for 90° needs to connect with other 90° neurons in order to generate network firing to represent this spatial location in the absence of environmental stimulation. α2A-Adrenoceptors increase this connectivity with other members of the network by inhibiting cAMP production in the dendritic spine, thus closing nearby hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, increasing membrane resistance and strengthening inputs onto the spine.48 In contrast, D1 receptors on separate spines appear to gate out inputs from neurons with dissimilar properties (e.g., 45°), thus decreasing “noise.”77 This appears to be accomplished, at least in part, by increasing the production of cAMP within the spine, opening HCN channels, and shunting inputs onto that spine. This may occur in a dynamic fashion, to narrow or broaden the tuning of the neuron according to task demands.
α1-Adrenoceptor Actions
High levels of NE release (e.g., during stress) impair PFC function through actions at α1 receptors.55 The Gq-coupled α1 receptor activates the phosphotidyl inositol intracellular signaling cascade, leading to the activation of protein kinase C.55 Agonists such as phenylephrine (similar to Sudafed), impair working memory when infused into the PFC in rats56 or monkeys. 44 Similar effects are observed at the cellular level, where α1-receptor stimulation suppresses delay-related neuronal firing.55 Conversely, α1 antagonists such as urapidil and prazosin prevent stress-induced PFC impairment.57,58 The protein kinase C inhibitor, chelerythrine, also protects PFC function at the behavioral and cellular levels.55 Based on this research in animals, prazosin is being successfully used to treat patients with posttraumatic stress disorder, including veterans returning from the Iraq war.59 Interestingly, most effective antipsychotic medications, including the “atypical” neuroleptics, have potent α1 blocking properties, and overactivity of the protein kinase C signaling pathway has been associated with mania60 and possibly schizophrenia.61 Indeed, the recent publication of the bipolar genome showed that mutations in the gene-encoding diacylglycerol (DAG) kinase is linked with bipolar disorder. DAG kinase normally metabolizes DAG, which is a necessary cofactor for activation of protein kinase C.62 These mechanisms may be particularly relevant to children with symptoms resembling ADHD that worsen with stimulant treatment, and are found to have childhood bipolar disorder.63
β-Adrenoceptor Actions
Recent studies suggest that stimulation of β1 adrenoceptors impairs PFC function.64 Thus, systemic or local application of the β1 antagonist, betaxolol, improved working memory in rats and monkeys. However, this treatment appeared to be associated with serious pancreatic side effects, and thus may not be appropriate for human use.
Dopamine
There are two families of DA receptors: the D1 (D1 and D5) and D2 (D2, D3, and D4) receptor families. (It should be noted that NE has very high affinity for D4 receptors,65 and thus should really be considered a catecholamine receptor rather than a DA receptor.) Currently, there are no pharmacological agents that distinguish D1 from D5 receptors; thus, these receptors are discussed as one entity. As there is little information on D3-receptor actions in PFC at this time, this receptor is not reviewed.
D1-Receptor Actions
The PFC has a bilaminar distribution of D1 receptors in both upper and lower layers.66 The D1 subtype is especially focused on spines, while the D5 subtype is found more on shafts of pyramidal neurons.67 In vitro recordings from PFC slices have revealed many important influences of D1 receptors on mechanisms governing the excitability of PFC neurons (see, e.g., Refs. 68–70). In vivo studies of both cognitive performance and neuronal firing have shown that stimulation of D1-like receptors in the PFC produces an inverted U-shaped dose-response influence on the working memory and attention regulation processes of the PFC.71,72 Similar to NE, modest levels of D1 receptor stimulation are essential to PFC function, while high levels of DA release, for example, during exposure to stress, impair working memory. Thus, high doses of D1 agonist impair working-memory performance. This inverted U has been observed in mice,73 rats,74 monkeys, 75 and humans.76 A similar inverted U has been described at the cellular level,77 where moderate levels of D1-receptor stimulation suppress neuronal processing of irrelevant information (i.e., reduce “noise”), by sharpening tuning and rendering PFC firing more selective. These D1 actions arise from increased cAMP production,77 and preliminary data suggest that D1 may shunt inputs onto spines by opening HCN channels (schematically depicted in FIG. 2). In contrast to the enhancing effects of moderate levels, high levels of D1 receptor stimulation reduce the relevant signals as well as noise, thus producing a nonspecific suppression of cell firing.77 High levels of D1-receptor stimulation also impair PFC cognitive function and produce a per-severative profile of responding.74 These impairing effects are reversed by inhibiting cAMP actions at the behavioral and cellular levels.77
Human experiments also note an inverted U, with the more D1-like compounds being most effective in improving working memory.78 Genetic studies in humans indicate similar results.76 Substitutions of methionine for valine in the DA catalytic enzyme, COMT, results in weaker enzyme activity, and thus more DA. Under basal conditions, subjects with this substitution have better working memory and more efficient PFC activation than those with the native enzyme.76 However, following amphetamine and/or stress exposure, those with the methionine substitutions become markedly impaired (presumably due to excessive DA stimulation), while those with the native enzyme show improved PFC cognitive performance (presumably due to more optimal DA levels).76
D2-Receptor Actions
There has been much less research on the influence of D2-receptor stimulation on PFC function. D2 receptors are concentrated on neurons in layer 5 (the output layer that projects to striatum), and overall show lower levels of binding than the D1-receptor family.66 Early studies showed that blockade of D2 receptors in the PFC of monkeys performing a working-memory task had no effect on performance.79 However, this task had ceiling effects, and studies in rats suggest that excessive D2-receptor stimulation impairs working memory abilities.80 Recent electrophysiological studies have shown that D2-receptor stimulation increases the response-related firing of PFC neurons in monkeys performing a working-memory task,81 consistent with the localization of these receptors on cells projecting to areas guiding movement. Some of the response-related firing appears to be a form of corollary discharge, informing the brain that a response has taken place. Accumulating evidence indicates that alterations in corollary discharge contribute to auditory hallucinations in schizophrenia.82 As D2-receptor blockade plays a key role in antipsychotic medications, these findings may have special relevance to schizophrenia.
D4-Receptor Actions
D4 receptors are concentrated on GABAergic interneurons, 83 and appear to inhibit GABA transmission via Gi-mediated reduction of cAMP signaling.84 Weaker D4-receptor actions thus lead to excessive GABA transmission and suppression of pyramidal cell firing (Ref. 84; Wang and Arnsten, unpublished data). ADHD is associated with the increased incidence of the 7 repeat polymorphism of the D4 receptor, which weakens D4 receptor efficacy.85,86 Thus, the basic physiology in animals would suggest that subjects with this polymorphism would have insufficient D4 inhibition of GABA, and thus insufficient PFC pyramidal cell firing. Stimulant medication may increase endogenous DA (and NE) stimulation of D4 receptors, thus normalizing GABAergic inhibition. However, there is also some evidence from basic physiological studies that D4 receptors can inhibit pyramidal cells,84 so the actions of these receptors are not entirely straightforward.
Summary
The PFC appears to thrive under conditions of moderate catecholamine release, when NE α2A-receptor stimulation increases “signals,” and optimal DA D1-receptor stimulation decreases “noise.” In contrast, PFC working-memory functions are impaired under conditions of high catecholamine release that engage α1 and β receptors, and excessive D1-receptor stimulation. These neurochemical needs are opposite to those of sensory cortex and subcortical structures such as the amygdala.87 Thus, catecholamines may act as a chemical switch, turning on PFC during normal waking, and turning it off during drowsiness or stress. In contrast, high levels of catecholamines may turn on more primitive brain structures such as the amygdala for more automatic control of behavior under conditions of danger.88
Relevance to Genetic Alterations in Attention Deficit Hyperactive Disorder
A large number of studies have found that ADHD symptoms are often associated with alterations in genes involved with catecholamine transmission.89 Original research focused on associations with genes involved with DA transmission, and found associations with the DA D1, D4, and D5 receptors85,90–92 and the DA transporter. 90 More recent research has also found associations of ADHD with NE genes, including the synthetic enzyme for NE, DBH,90,93 the NE transporter,92,94 and the α2A receptor,95–98 which is the site of NE’s beneficial actions in the PFC. Thus, suboptimal catecholamine regulation of PFC may contribute to impaired regulation of attention and behavior in patients with ADHD.
Pharmacological Treatments for Attention Deficit Hyperactive Disorder Optimize Catecholamine Actions in Prefrontal Cortex
Medications for the treatment of ADHD all enhance catecholamine transmission. Drugs such as amphetamine and methylphenidate act to enhance the release and/or inhibit the reuptake of both DA and NE. Methylphenidate can improve PFC working-memory function and enhance the efficiency of PFC activation in patients with ADHD99 as well as in healthy adults.100,101 Recent studies in rats show that low, oral doses of methylphenidate, which reduce locomotor activity and produce plasma levels similar to therapeutic doses in humans,102 increase NE and DA release in PFC103 and improve performance of working-memory and attention tasks dependent on the PFC.103,104 The cognitive enhancing effects of methylphenidate in rodents depend on DA D1- and NE α2A-receptor stimulation. 104 Although methylphenidate is often (falsely) referred to as a selective DA drug, NE is increased more than DA in the PFC.103 Similarly, atomoxetine increases both NE and DA in the PFC.105 It is likely that both DA and NE actions contribute to the therapeutic effects of stimulants in patients with ADHD. However, excessive doses of stimulant medication may produce cognitive inflexibility through excessive α1-, β1-, and D1-receptor stimulation.
Environmental Factors Can Impair Prefrontal Cortex Function and Mimic the Effects of Attention Deficit Hyperactive Disorder
Attention deficit hyperactive disorder is the default diagnosis for children with attention and behavior problems; neurobiological studies demonstrating the great sensitivity of the PFC to its neurochemical environment may explain why so many conditions can mimic this disorder. PFC regulation of attention and behavior requires an optimal catecholamine environment. Thus, either fatigue (insufficient catecholamines) or stress (excessive catecholamines) may produce changes in attention and behavioral regulation that resemble ADHD symptoms resulting from genetic alterations. This may be particularly problematic in children exposed to stressors such as families experiencing divorce, illness, or death, or to social stressors at school. These common environmental factors may also contribute to aggressive or oppositional behaviors that can arise from inadequate ventral PFC regulation of emotion. If the stressors have not been identified, children may be thought to have ADHD and be treated inappropriately with stimulant medications that worsen the condition.
In summary, catecholamines have powerful influences on the brain circuits, and genetic studies have often linked alterations in catecholamine genes with ADHD. Medications that optimize catecholamine transmission may normalize the function of these circuits and ameliorate ADHD symptomology.
Acknowledgments
This work was supported by PHS grants R37 AG060636 and P50MH068789 to AFTA.
Footnotes
Competing Interest
Dr. Arnsten and Yale University have license agreements with Shire Pharmaceuticals for the development of guanfacine for the treatment of ADHD, and with Marinus Pharmaceuticals for the development of chelerythrine for the treatment of bipolar disorder.
References
- 1.Giedd JN, et al. Brain imaging of attention deficit/hyperactivity disorder. Ann. N. Y. Acad. Sci. 2001;931:33–49. doi: 10.1111/j.1749-6632.2001.tb05772.x. [DOI] [PubMed] [Google Scholar]
- 2.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Goldman-Rakic PS. Circuitry of the primate prefrontal cortex and the regulation of behavior by representational memory. In: Plum VF, editor. Handbook of Physiology, The Nervous System, Higher Functions of the Brain. Vol. Bethesda, MD: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- 4.Barbas H, et al. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb. Cortex. 2005;15:1356–1370. doi: 10.1093/cercor/bhi018. [DOI] [PubMed] [Google Scholar]
- 5.Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc. Natl. Acad. Sci. USA. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desimone R. Neural mechanisms for visual memory and their role in attention. Proc. Natl. Acad. Sci. USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kastner S, et al. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- 8.Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J. Neurosci. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesulam M-M. Principles of Behavioral and Cognitive Neurology. New York: Oxford University Press; 2000. [Google Scholar]
- 10.Crowe DA, et al. Neural activity in primate parietal area 7a related to spatial analysis of visual mazes. Cereb. Cortex. 2004;14:23–34. doi: 10.1093/cercor/bhg088. [DOI] [PubMed] [Google Scholar]
- 11.Snyder LH, et al. Separate body- and world-referenced representations of visual space in parietal cortex. Nature. 1998;394:887–891. doi: 10.1038/29777. [DOI] [PubMed] [Google Scholar]
- 12.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 13.Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J. Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 15.Castellanos FX, et al. Quantitative brain magnetic resonance imaging in attention deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 16.Casey BJ, et al. Implication of right fronto-striatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Sowell ER, et al. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 18.Itami S, Uno H. Orbitofrontal cortex dysfunction in attention-deficit hyperactivity disorder revealed by reversal and extinction tasks. Neuroreport. 2002;13:2453–2457. doi: 10.1097/00001756-200212200-00016. [DOI] [PubMed] [Google Scholar]
- 19.Bedard AC, et al. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. J. Abnorm. Child Psychol. 2003;31:315–327. doi: 10.1023/a:1023285614844. [DOI] [PubMed] [Google Scholar]
- 20.McLean A, et al. Characteristic neurocognitive profile associated with adult attention-deficit/hyperactivity disorder. Psychol. Med. 2004;34:681–692. doi: 10.1017/S0033291703001296. [DOI] [PubMed] [Google Scholar]
- 21.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–365. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- 23.Godefroy O, Rousseaux M. Divided and focused attention in patients with lesion of the prefrontal cortex. Brain Cogn. 1996;30:155–174. doi: 10.1006/brcg.1996.0010. [DOI] [PubMed] [Google Scholar]
- 24.Bartus RT, Levere TE. Frontal decortication in rhesus monkeys: a test of the interference hypothesis. Brain Res. 1977;119:233–248. doi: 10.1016/0006-8993(77)90103-2. [DOI] [PubMed] [Google Scholar]
- 25.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berquin PC, et al. Cerebellum in attention-deficit hyperactivity disorder: a morphometric MRI study. Neurology. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- 27.Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Castellanos FX, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 29.Dum RP, Li C, Strick PL. Motor and nonmotor domains in the monkey dentate. Ann. N.Y. Acad. Sci. 2002;978:289–301. doi: 10.1111/j.1749-6632.2002.tb07575.x. [DOI] [PubMed] [Google Scholar]
- 30.Hoshi E, et al. The cerebellum communicates with the basal ganglia. Nat. Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 31.Mouradian RD, Seller FM, Waterhouse BD. Noradrenergic potentiation of excitatory transmitter action in cerebrocortical slices: evidence of mediation by an alpha1-receptor-linked second messenger pathway. Brain Res. 1991;546:83–95. doi: 10.1016/0006-8993(91)91162-t. [DOI] [PubMed] [Google Scholar]
- 32.Witte EA, Marrocco RT. Alterations of brain noradrenergic activity in rhesus monkeys affects the alerting component of covert orienting. Psychopharmacology. 1997;132:315–323. doi: 10.1007/s002130050351. [DOI] [PubMed] [Google Scholar]
- 33.Coull JT, Nobre AC, Frith CD. The noradrenergic alpha2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cereb. Cortex. 2001;11:73–84. doi: 10.1093/cercor/11.1.73. [DOI] [PubMed] [Google Scholar]
- 34.Brozoski T, et al. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 35.Arnsten AFT, Robbins TW. Neurochemical modulation of prefrontal cortical function in humans and animals. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 51–84. [Google Scholar]
- 36.Arnsten AFT, Li B-M. Neurobiology of executive functions: catecholamine influences on prefrontal cortical function. Biol. Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Schultz W. The phasic reward signal of primate dopamine neurons. Adv. Pharmacol. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- 38.Arnsten AFT, Goldman-Rakic PS. Alpha-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- 39.Cai JX, et al. Reserpine impairs spatial working memory performance in monkeys: reversal by the alpha-2 adrenergic agonist clonidine. Brain Res. 1993;614:191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- 40.Franowicz JS, et al. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J. Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birnbaum SG, Arnsten AFT. The alpha-2A noradrenergic agonist, guanfacine, reverses the working memory deficits induced by pharmacological stress (FG7142) Soc. Neurosci. Abstr. 1996;22:1126. [Google Scholar]
- 42.Arnsten AFT, Contant TA. Alpha-2 adrenergic agonists decrease distractability in aged monkeys performing a delayed response task. Psychopharmacology. 1992;108:159–169. doi: 10.1007/BF02245302. [DOI] [PubMed] [Google Scholar]
- 43.Steere JC, Arnsten AFT. The alpha-2A noradrenergic agonist, guanfacine, improves visual object discrimination reversal performance in rhesus monkeys. Behav. Neurosci. 1997;111:1–9. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- 44.Mao Z-M, Arnsten AFT, Li B-M. Local infusion of alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol. Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- 45.Wang M, Ji JZ, Li BM. The alpha(2A)-adrenergic agonist guanfacine improves visuomotor associative learning in monkeys. Neuropsychopharmacology. 2004;29:86–92. doi: 10.1038/sj.npp.1300278. [DOI] [PubMed] [Google Scholar]
- 46.Jakala P, et al. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 47.Li B-M, et al. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 48.Wang M, et al. Alpha2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 49.Li B-M, Mei Z-T. Delayed response deficit induced by local injection of the alpha-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav. Neural. Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 50.Ma C-L, et al. Selective deficit in no-go performance induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–1016. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- 51.Ma C-L, Arnsten AFT, Li B-M. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha-2-adrenoceptors in monkeys. Biol. Psychiatry. 2005;57:192–195. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Hunt RD, Arnsten AFT, Asbell MD. An open trial of guanfacine in the treatment of attention deficit hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Scahill L, et al. Guanfacine in the treatment of children with tic disorders and ADHD: a placebo-controlled study. Am. J. Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- 54.Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit-hyperactivity disorder. J. Clin. Psychopharm. 2001;21:223–228. doi: 10.1097/00004714-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Birnbaum SB et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 56.Arnsten AFT, et al. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol. Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 57.Arnsten AFT, Jentsch JD. The alpha-1 adrenergic agonist, cirazoline, impairs spatial working memory performance in aged monkeys. Pharmacol. Biochem. Behav. 1997;58:55–59. doi: 10.1016/s0091-3057(96)00477-7. [DOI] [PubMed] [Google Scholar]
- 58.Birnbaum SG, et al. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in prefrontal cortex. Biol. Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 59.Raskind MA, et al. Prazosin reduces nightmares and other PTSD symptoms in combat veterans: a placebo-controlled study. Am. J. Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 60.Manji HK, Lenox RH. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol. Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- 61.Williams NM, et al. Support for RGS4 as a susceptibility gene for schizophrenia. Biol. Psychiatry. 2004;55:192–195. doi: 10.1016/j.biopsych.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Baum AE, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol. Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biederman J, et al. Pediatric mania: a developmental subtype of bipolar disorder? Biol. Psychiatry. 2000;48:458–466. doi: 10.1016/s0006-3223(00)00911-2. [DOI] [PubMed] [Google Scholar]
- 64.Ramos B, et al. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol. Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 65.Van Tol HHM, et al. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- 66.Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotonergic receptors and complementarity of their subtypes in primate prefrontal cortex. J. Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smiley JF, et al. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc. Natl. Acad. Sci. USA. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J, O’Donnell P. D(1) dopamine receptors potentiate nmda-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb. Cortex. 2001;11:452–462. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- 69.Seamans JK, et al. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc. Natl. Acad. Sci. USA. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Young CE, Yang CR. Dopamine D1/D5 receptor modulates state-dependent switching of somadendritic Ca2+ potentials via differential protein kinase A and C activation in rat prefrontal cortical neurons. J. Neurosci. 2004;24:8–23. doi: 10.1523/JNEUROSCI.1650-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnsten AFT, et al. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 72.Granon S, et al. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J. Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lidow MS, Koh P-O, Arnsten AFT. D1 dopamine receptors in the mouse prefrontal cortex: immunocytochemical and cognitive neuropharmacological analyses. Synapse. 2003;47:101–108. doi: 10.1002/syn.10143. [DOI] [PubMed] [Google Scholar]
- 74.Zahrt J, et al. Supranormal stimulation of dopamine D1receptors in the rodent prefrontal cortex impairs spatial working memory performance. J. Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arnsten AFT, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch.Gen. Psychiatry. 1998;55:362–369. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 76.Mattay VS, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc. Natl. Acad. Sci. USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vijayraghavan S, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 78.Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- 79.Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptors in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed response task. J. Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 80.Druzin MY, et al. The effects of local application of D2 selective dopaminergic drugs into the medial prefrontal cortex of rats in a delayed spatial choice task. Behav. Brain Res. 2000;109:99–111. doi: 10.1016/s0166-4328(99)00166-7. [DOI] [PubMed] [Google Scholar]
- 81.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 82.Ford JM, et al. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol. Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 83.Mrzljak L, et al. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 84.Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J. Neurosci. 2002;22:9185–9193. doi: 10.1523/JNEUROSCI.22-21-09185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tahir E, et al. Association and linkage of DRD4 and DRD5 with attention deficit hyperactivity disorder (ADHD) in a sample of Turkish children. Mol. Psychiatry. 2000;5:396–404. doi: 10.1038/sj.mp.4000744. [DOI] [PubMed] [Google Scholar]
- 86.Sunohara GA, et al. Linkage of the dopamine D4 receptor gene and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:1537–1542. doi: 10.1097/00004583-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 87.Arnsten AFT. Through the looking glass: differential noradrenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arnsten AFT. Stress impairs PFC function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog. Brain Res. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- 89.Faraone SV, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 90.Daly G, et al. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol. Psychiatry. 1999;4:192–196. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- 91.Durston S, et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol. Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- 92.Bobb AJ, et al. Support for association between ADHD and two candidate genes: NET1 and DRD1. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2005;134:67–72. doi: 10.1002/ajmg.b.30142. [DOI] [PubMed] [Google Scholar]
- 93.Roman T, et al. Further evidence for the association between attention-deficit/hyperactivity disorder and the dopamine-beta-hydroxylase gene. Am. J. Med. Genet. 2002;114:154–158. doi: 10.1002/ajmg.10194. [DOI] [PubMed] [Google Scholar]
- 94.Kim CH, et al. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc. Natl. Acad. Sci. USA. 2006;103:19164–19169. doi: 10.1073/pnas.0510836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Comings DE. Clinical and molecular genetics of ADHD and Tourette syndrome. Two related polygenic disorders. Ann. N. Y. Acad. Sci. 2001;931:50–83. doi: 10.1111/j.1749-6632.2001.tb05773.x. [DOI] [PubMed] [Google Scholar]
- 96.Roman T, et al. Is the alpha-2A adrenergic receptor gene (ADRA2A) associated with attention-deficit/hyperactivity disorder? Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2003;120:116–120. doi: 10.1002/ajmg.b.20018. [DOI] [PubMed] [Google Scholar]
- 97.Park L, et al. Association and linkage of alpha-2A adrenergic receptor gene polymorphisms with childhood ADHD. Mol. Psychiatry. 2005;10:572–580. doi: 10.1038/sj.mp.4001605. [DOI] [PubMed] [Google Scholar]
- 98.Deupree JD, et al. Possible involvement of alpha-2A adrenergic receptors in attention deficit hyperactivity disorder: radioligand binding and polymorphism studies. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2006;141:877–884. doi: 10.1002/ajmg.b.30371. [DOI] [PubMed] [Google Scholar]
- 99.Turner DC, et al. Neurocognitive effects of methylphenidate in adult attention-deficit/hyperactivity disorder. Psychopharmacology. 2005;178:286–295. doi: 10.1007/s00213-004-1993-5. [DOI] [PubMed] [Google Scholar]
- 100.Elliott R, et al. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology. 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- 101.MehtA MA, et al. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J. Neurosci. 2000;20:RC651–RC656. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J. Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berridge CW, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 104.Arnsten AFT, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through a2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in attention deficit hyperactivity disorder. Behav. Brain Funct. (Biomed. Central) 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bymaster FP, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]