Abstract
There is an important need for non-medication interventions for depressed youth. The aim of this study is to evaluate the feasibility of using a standardized aerobic exercise regime to treat non-medicated clinically depressed adolescents based on adherence and completion rates, including 1) establishing effect sizes for the primary outcomes including the Chidren’s Depression Rating Scale – Revised (CDRS-R) and Actical (energy expenditure data) as well as selected secondary outcomes; (e.g., Clinical Global Improvement, depression rating scales, exercise logs, attitudes), and 2) determining whether moderate to strenuous exercise (12 kcal/kg/week [KKW]) versus a control stretching activity (<4 KKW) for 12 weeks leads to a clinically meaningful reduction in depressive symptoms and/or improved psychosocial functioning. The challenge is to develop an exercise intervention that can motivate a typically sedentary depressed adolescent to exercise on a regular basis. The goal is to demonstrate that exercise alone can provide an important and effective non-medication intervention for adolescent depression. This paper reports on the rationale and design of a pilot study which aims to inform the design of a larger trial to evaluate the efficacy of aerobic exercise to treat adolescent depression. After describing the case for exercise within the broader context of the prevalence of adolescent depression and other treatments, the paper describes the intervention and procedures for data collection.
Keywords: Childhood depression, Exercise, Exercise and depression, Pediatric depression, Adolescent depression, MDD
1. Introduction
1.1. Adolescent depression and treatments
Major depressive disorder (MDD) is common in adolescents and results in significant morbidity and mortality (Shaffer et al., 1996). In fact most adolescent suicides, the third leading cause of death for this age group, are accounted for by depression (Brent, Kolko, Birmaher, Baugher, & Bridge, 1999). Early onset depression leads to long-term psychosocial impairment and continued depressive episodes in adulthood (Emslie, Weinberg, & Mayes, 1998). With recent concerns about short and long-term safety with selective serotonin reuptake inhibitors (SSRIs) and other antidepressants, and poor overall response even when efficacy has been proven (Emslie et al., 1997, 2002; Hughes et al., 2007), it is essential to determine whether reduction in depressive symptoms can be accomplished with a non-medication treatment. It is also essential to evaluate whether such a non-medication treatment can provide further improvements in social, school, cognitive, and family functioning.
Failure to fully respond to depression treatment is associated with a greater likelihood of relapse (Birmaher et al., 2000; Emslie, Rush, et al., 1998; Emslie, Weinberg, et al., 1998), and continuing impairment in home, school and interpersonal function (Rao et al., 1995; Rohde, Lewinsohn, & Seeley,1994). Symptoms that persist (also called residual symptoms) include low energy and inability to concentrate with likely poor school performance, inactivity, and social withdrawal (e.g., from sports and other organized activities). Alternative non-medication treatment modalities need to be evaluated, and exercise as such an intervention for depressed adolescents shows promise for improving many of these residual symptoms.
Over the past few years, controversies over whether antidepressants increase suicidality have also emerged. In September 2004, the FDA conducted a re-analysis of suicide-related events in youth from antidepressant trials for MDD and for all indications compared to placebo. The overall relative risk of suicide-related events (attempts or ideation) was significantly higher with medication at 1.95 (95% CI = 1.28–2.98) for the trials (all indications). The average risk of such events in patients receiving antidepressants was 4%, twice the placebo risk of 2%. Because of the relative rarity of these events, the difference is only significant when data from all the trials are pooled. As such, the FDA recommended a black box warning on all antidepressants warning of potential worsening of depression or emergence of suicidality. Contrary to the FDA’s conclusions on childhood suicidality, epidemiological studies have found an inverse relationship between suicides in the community and prescription rates of SSRI antidepressants for both youth and adults (Gibbons et al., 2007; Olfson, Marcus, & Shaffer, 2006).
There are an increasing number of efficacy studies reporting positive results for SSRI antidepressant treatment but the medications are not without risk and their use has declined, (Kratochvil, Vitiello, & Walkup, 2006). Many patients do not respond oronly partially respond to treatment, so alternatives are needed. And in terms of alternative treatments, the largest multisite study in adolescents to date with cognitive behavioral therapy (CBT) found that CBT was not significantly different from placebo (March, Silva,& Petrycki,2004), but did enhance clinical response when combined with medication. CBT remains a promising treatment nonetheless, but unlike exercise, access may be limited by the need for specialized training, it is not readily available in all places, can be expensive, and also does not necessarily improve general physical health (Curry, 2001). It may also be associated with concerns about the stigma of psychotherapy and disclosure of personal information.
1.2. The case for exercise as a therapy
Recent reviews of randomized control trials of exercise for the prevention and/or treatment of depression in children and young people (Jorm et al., 2006), including meta analyses (Larun, Nordheim, Ekeland, Hagen, & Helan, 2006), conclude that the small numbers of studies to date and methodological concerns limit any conclusions that can be drawn about the effectiveness of treating depressed youth with exercise. The major limitations of the studies to date, as well as the reviews, include failure to adequately diagnose depression (e.g., do not include a diagnostic process), use of overly inclusive subject groups that are not depressed, have anxiety or conduct disorders, rely on self-reports as opposed to blinded clinical evaluators and standardized measures of depression and severity of symptoms at baseline and outcome, poor randomization strategies, do not typically include a standardized measure that quantifies the frequency, intensity, duration or type of exercise, lack of or poorly matched control groups, and include samples primarily consisting of college age students rather than children and adolescents (Dunn & Weintraub, 2008). Because of the methodological flaws it is hard to know if the lack of effect for exercise is due to method or confounded treatment. Nonetheless, the various authors of these reviews do agree in the importance of further studies that are better designed to address the various methodological issues of previous work. Further, the recent meta-analytic review of exercise to treat depression in adults finds many studies (some with the same methodological issues as found for the pediatric studies) where the exercise intervention has been as effective as pharmaceutical and CBT interventions (Mead et al., 2009). These latter authors concluded that exercise did seem to improve the symptoms of depression, but suggest that exactly how effective and what is the best type of exercise remain to be determined.
There is little empirical data on the relative efficacy of different doses of exercise (most of the studies in the reviews above have not reported on measured energy expenditure – intensity) for the treatment of depression and other mental health problems. The Mead et al. (2009) review reports only two studies that have demonstrated that higher intensity exercise is more effective than low intensity (Dunn, Trivedi, Kampert, Clark, & Chambliss, 2002; Singh et al., 2005). For instance, the Dunn et al. (2002) randomized controlled trial, DOSE (Depression Outcomes Study of Exercise), demonstrated that a high weekly dose (17.5 KKW) of individually-conducted aerobic exercise was significantly more effective than a low-dose (7.0 KKW) as a monotherapy for moderate MDD (Dunn et al., 2002; Dunn, Trivedi, Kampert, Clark, & Chambliss, 2005). As opposed to low intensity exercise, it has been argued that a stretch condition (<4 KWW) provides a placebo equivalent to a low-dose exercise intervention (Trivedi et al., 2006). Clearly it is necessary to identify a dose of exercise that will be effective and acceptable. Also, a low-cost exercise intervention which is sustainable for the individual is likely to be most desirable.
1.3. Aims of the study
The first aim of our current study is to develop a pragmatic exercise treatment intervention, with a procedural manual, to reduce depressive symptoms in adolescents who present with major depressive disorder, and evaluate the intervention and response with a small sample of 6 subjects. The findings from the first six will guide revisions to the procedures and manual. The second aim of this study is to conduct a pilot study to test the feasibility and acceptability of exercise intervention (and manual) to reduce depressive symptoms in 60 non-medicated depressed youth ages 12–17 years 11 months, as well as the randomization and data collection procedures. Participants will be randomized to either a 12-week aerobic exercise (12 KKW) group (EXER) or to a stretch (<4 KKW) group (STRETCH –non-strenuous exercise group). Since there are no depressed adolescent RCT exercise trials with the CDRS-R as an outcome measure or for Actical to measure ongoing energy expenditure, this feasibility study will gather preliminary data for estimating an effect size for the key outcomes, and conducting future sample size calculations for a fully powered trial. If the results from this pilot trial are comparable to prior child/adolescent fluoxetine vs. placebo trials (e.g. Emslie et al., 1997, 2002), with an n = 30 per group, we will be able to detect as little as a 7.0 point mean difference (standard deviation of 9.0 on the CDRS total score at outcome with 84.2% power, with an alpha = .05). A study with this outcome would enable us to report the mean difference with a precision (95.0% confidence level) of plus/minus 4.62 points. A random regression analysis will allow us to use all available data, including from participants with missing data at follow-up, to enhance the power of the test.
The primary outcome measures will be the Children’s Depression Rating Scale (CDRS-R) and Actical accelerometry data, both treated as continuous variables. We are hypothesizing that 1) EXER will have a greater mean reduction in depressive symptoms as measured by the CDRS-R than STRETCH by week 12, 2) those in the EXER group will be more physically active than those in STRETCH by week 12, and 3) increases in energy expenditure will be associated with reductions in depression severity. Further aims will be to compare the effectiveness of EXER versus STRETCH in improving process outcomes including psychosocial functioning and attitudes toward exercise which are described below.
2. Study procedures
2.1. Participant recruitment
Participants are recruited from the routine intake assessments of various outpatient clinics, and referrals from community clinics and physicians and various website listings. We have found the latter to have become good recruitment mechanisms for young people and they are more likely to self refer when in the past referrals have come from clinicians and parents. Potential participants are given a study contact number that they need to call to speak to our study coordinator. A caller is screened by a phone interview made by the project coordinator who explains the protocol, answers any questions about the study, and does a brief phone screening to determine if the adolescent may be eligible. At the enrollment visit, the principal investigator or a designee obtains consent/assent for research participation from the potential subject’s parent or legal guardians as well as the subject prior to conducting the research assessment.
2.1.1. Inclusion and exclusion criteria
Inclusion criteria focus on individuals with mild to moderate depression. Both genders are included and the adolescent needs to be between the ages of 12–17 years 11 months and in school. They must meet a diagnosis of non-psychotic major depressive disorder (MDD) based on a semi-structured clinical interview for DSM-IV Axis I disorders (described below) and confirmed by a licensed clinician. While MDD must be the primary disorder, other concurrent disorders (anxiety, attention deficit [ADHD], or conduct) are not excluded. Children with ADHD and on medication will be included if clinically stable on existing nonantidepressant medications. Participants must meet a baseline score on the Children’s Depression Rating Scale – Revised, (CDRS-R) 35 ≤ 70; and a baseline Clinical Global Impression – Severity (CGI-S CGI-E ≥ 44). They must be of normal intelligence (i.e., IQ > 70 based on the short WISC-III testing if uncertain) and English language and reading will be required as the majority of the assessment instruments are only available in English. In addition they need to provide a letter from a family physician approving participation in an exercise study. The Cooper Institute (CI) site exercise physician reviews the information and approves the participant for exercise consistent with the American College of Sports Medicine [ACSM] guidelines. The Physical Activity Readiness Questionnaire (PAR-Q) must indicate that no conditions are present that would render exercise inappropriate. Further physical tests are scheduled as needed to assure safety. The participant and a parent(s) need to be capable and willing to provide written informed consent, to accept a randomized group assignment, to attend all study related visits, and to comply with the study protocol.
Individuals are excluded if they have a chronic medical illness requiring regular medications that contraindicate intensive exercise or unstable medical conditions requiring medication(s) with psychotropic effects (anticonvulsants, steroids, etc.). Psychiatric diagnoses of lifetime history of any psychotic disorder, including psychotic depression, bipolar I and II disorder, schizophrenia, alcohol or substance abuse or dependence within the past six months, lifetime anorexia nervosa or bulimia are excluded. Anyone with severe suicidal ideation or previous history of serious suicide attempts, or severe depression (CDRS > 70) that clinically supports the need for immediate alternative treatment intervention will be excluded as will those who participate in current exercise defined as 30 min of vigorous physical activity, 5 times per week or more.
Supportive clinical management is provided by clinical research staff during each weekly study visit and progress review and is based on good clinical practice routine to clinical research protocol interactions. Therapy per se will not be provided. Patients will not be entered into adjunctive structured CBT or individual psychotherapy or receive antidepressant medication unless there is a significant clinical worsening in which case they are appropriately referred. Such individuals will be encouraged to continue in the exercise protocol however.
2.2. Baseline screening and clinical management procedures
At the initial office visit, all participants and their parents are informed of the study procedures, risks, benefits, and alternative treatments, and provide written informed consent and assent prior to any study procedures being conducted. Following consent/assent, a licensed study clinician conducts separate semi-structured psychiatric diagnostic interview (Schedule for Affective Disorders and Schizophrenia for school-age children – present and lifetime [KSADS-PL]; Kaufman et al., 1997) with the adolescent and then the parent to confirm the diagnosis and current severity, reviews all inclusion and exclusion criteria and assures that all of the baseline self-report instruments are completed (Table 1). This initial assessment takes on average 3 h. Upon completion of all the baseline evaluations they remain unmedicated and are then ready to begin the study.
Table 1.
Schedule and types of DATE assessments.
| WEEK (Visit Code) |
88 |
77 |
00 |
01 |
02 |
03 |
04 |
05 |
06 |
07 |
08 |
09 |
10 |
11 |
12 |
26 |
52 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assessment | SV1 | SV2 | BL | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | FU1 | FU2 |
| General/Psychiatric Screening Measures (UTSW) | |||||||||||||||||
| Phone Screen (Initial) | |||||||||||||||||
| Demographic Form | × | ||||||||||||||||
| Medical/Psych Hx Form | × | ||||||||||||||||
| KSADS-PL (DSM-IV) | × | × | × | × | |||||||||||||
| CDRS-R/QIDS-A | × | ||||||||||||||||
| PAR-Q | × | ||||||||||||||||
| Letter from Physician | × | ||||||||||||||||
| Medical Screening & Physiological Measures (CI) | |||||||||||||||||
| Blood Pressure | × | × | |||||||||||||||
| BMI/Body Comp | × | × | |||||||||||||||
| Depression Outcome Measures (UTSW) | |||||||||||||||||
| Blind Evaluations | |||||||||||||||||
| CDRS-R | × | × | × | × | × | × | × | × | |||||||||
| QIDS-A17-C | × | × | × | × | × | × | × | × | |||||||||
| QIDS-A17 –SR, P | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| CGI-S; CGI-I | × | × | × | × | × | × | × | × | |||||||||
| CGAS-FGAS | × | × | × | × | × | ||||||||||||
| SSRI Compliance | × | × | × | × | × | × | × | ||||||||||
| Psychosocial/Functional Outcome Measures (Self-report) | |||||||||||||||||
| Health Habits- Adult | × | × | × | × | × | ||||||||||||
| Health Habits – Student | × | × | × | × | × | ||||||||||||
| SAS-SR (HRQOL) | × | × | × | × | × | ||||||||||||
| SCQ | × | × | × | × | × | ||||||||||||
| CSQ-8 | × | × | × | × | × | ||||||||||||
| POMS – SF | × | × | × | × | × | ||||||||||||
| SSPAS | × | × | × | × | × | ||||||||||||
| MPAM-R | × | × | × | × | × | ||||||||||||
| ESE | × | × | × | × | × | ||||||||||||
| DB | × | × | × | × | × | ||||||||||||
| OEE | × | × | × | × | × | ||||||||||||
| PACES | × | × | × | × | × | ||||||||||||
| Actical monitor data | × | × | × | × | × | × | × | × | × | × | × | × | |||||
The choice of including school, exercise clubs, or home-based sessions in this study, instead of all sessions conducted at the CI, is to address both the patient and parent burden of transportation to the CI 3–4 times a week that would be required if we did not. It also provides a generalization strategy to “real world” exercise sessions that would continue following completion of this study. For home-based sessions, individuals will also be permitted to perform aerobic walks outside or at malls, recreational centers or parks, YW(M)CAs, or commercial exercise facilities. Participants are provided either an Exercise or Stretch Guide (group randomized to) to assist with the web-based information for use throughout the study for documenting their exercise sessions. The guide contains informational documents to provide knowledge about how the exercise or stretch sessions are conducted and the exercise prescription (specific to the participant).
2.3. Measures
The primary outcome measure of depression is the CDRS-R total score and CGI-I, and the Actical monitor data (total activity, energy expenditure, and adherence). Adherence measures assess feasibility; completed at baseline and then every week during the 12 weeks of the study or until early termination (Table 1). The other measures (the SAS-SR and exercise evaluation measures) are completed at baseline, Week 6, and after the study is completed at Week 12 (12 weeks of EXER or STRETCH) or at early exit (Section 3). The secondary outcome measures will be used as potential predictors of clinical response and to better characterize feasibility issues as well as different functional domain outcomes besides depression symptom severity.
The measures selected to assess depressive symptomatology in DATE are widely accepted and used in clinical trials and the exercise measures are well known to exercise researchers. A range of depressive symptoms and overall response outcomes are gathered using three types of instruments: 1) blind interviewer-rated –Independent Evaluator and clinician review, 2) parent-rated, and 3) patient self-report.
2.3.1. Childhood Depression Rating Scale – Revised [CDRS-R]
The Children’s Depression Rating Scale – Revised [CDRS-R] (Poznanski & Mokros,1996) is a clinician-rated instrument, modeled after the Hamilton Depression Rating Scale for adults, and is used to measure the presence and severity of depressive symptomatology in children and adolescents. The CDRS-R has become the industry standard for childhood depression studies and has been a major outcome variable in all large-scale double blind studies to date. Each item is rated on a 1–5 or 1–7 point scale, with a 1 describing absence of the given symptom. The CDRS-R yields a total score from 17 to 113 with a score of 36 or greater considered being compatible with a diagnosis of depression. Good test-retest reliability (.80), internal consistency (Cronbach Alpha = .85) and interrater reliability (.92) have been demonstrated for the instrument. Administration can be done in 20 min or less and is often integrated with the affective section of KSADS to minimize redundancy and time required to obtain the information.
2.3.2. Quick Inventory for Depressive Symptomatology – Adolescent Version; Clinician-Rated & Self-Report (17 item; QIDS-A-C17, QIDS-A-P17 and QIDS-A-SR17)
The Quick Inventory of Depressive Symptoms – Adolescent version (Clinician report, self-report and parent versions) consists of 17 items on each instrument designed to assess both the core criterion symptoms and the commonly associated symptoms of depression (Rush, Bernstein, & Trivedi, 2006). The QIDS correlates highly with the CDRS (Jain et al., 2007), HAM-D and the BDI (Rush, Carmody, & Ibrahim, 2006). The QIDS-A17- C is included to allow comparison with adult studies, and because it contains items relating to atypical depression, such as reactivity of mood, rejection sensitivity, hyper-somnia, and weight gain, which may be more common in adolescents and may potentially, change with exercise augmentation. Administration requires 20 min or less and is often integrated with the affective section of the KSADS and the CDRS to minimize patient/parent burden.
2.3.3. Clinical Global Impression scale (improvement and severity) (CGI)
The Clinical Global Improvement scale (CGI-I) is used to assess overall clinical severity and improvement, each with a seven point scale, with lower values being more favorable. At intake, only severity can be rated. In subsequent assessments, both severity and improvement will be rated (Guy, 1976). This is a standard scale for psychopharmacological research, and a CGI-Improvement of 1 (very much), or 2 (much) improved, is considered to be an acceptable response to acute treatment as is a clinical severity rating of less than or equal to 3.
2.3.4. Profile of Mood State (POMS)
The POMS has been the most consistent measure of mood in exercise settings. It has six subscales representing vigor, confusion, anxiety, tension, anger and fatigue with internal consistency reliability estimates of approximately .90. As one of the most frequently used measures of mood, it provides a link between physical activity and mental health (McNair, Lorr, & Droppleman, 1992; Terry, 2000) and can assess both state and trait characteristics. We will use the POMS-Short Form (Curran, Andrykowski, & Studts, 1995). This will be the first comparison of the POMS for exercise with the CDRS-R for clinical depression.
2.4. Psychosocial and functional outcome measures
The psychosocial and functional measures (see Table 1) are completed at baseline, Week 6, and after the study is completed at Week 12 (12 weeks of EXER or STRETCH). The secondary outcome measures are used for potential mediator measures of acute response and to better characterize different functional domain outcomes besides depression symptom severity during the acute treatment.
2.4.1. Children’s Global Assessment Scale (C-GAS) and Family Global Assessment Scale (FGAS)
The Children’s Global Assessment Scale (C-GAS) was adapted from the Global Assessment Scale for Adults (Shaffer et al., 1983). The C-GAS is included since it provides a measure of the overall level of functioning, not limited to impairment from depression. The Child’s Family Global Assessment Scale (FGAS) rates the child’s family’s most impaired level of general functioning in the past year. There are four areas of functioning to be considered when rating: social functioning of parents as related to economic and social goals; marital/parental teamwork; parent understanding and provision for the developmental needs of the child; integrity and stability of family relationships.
2.4.2. Social Adjustment Scale-Self-Report [SAS-SR]
The adolescent versions of the Social Adjustment Scale-Self Report [SAS-SR] (Weissman & Bothwell, 1976; Weissman, Orvaschel, & Padian, 1980) are used to document the subject’s perception of functioning. The adolescent version of the SAS-SR is a 23-item scale that covers the patient’s role performance, interpersonal relationships, friction, feelings and satisfaction in work, and social and leisure activities with the extended family, as a member of a family unit. The instrument has good discriminative and predictive qualities, and is sensitive to treatment effects (Reinherz et al., 1995).
2.5. Physical activity
2.5.1. Actical kilocalories (kcal)
Total energy expenditure from exercise for each exercise session and each week of study is gathered and is used to calculate % adherence (e.g., kcal prescribed/kcal completed × 100). Activity is monitored with the Actical (Mini-Mitter, Bend, OR) 24 h per day, seven days per week for the duration of the 12 week study for each participant. The Actical provides calculated energy expenditure values for Active Energy Expenditure (KKW) in kilocalories and total energy expenditure in Metabolic Equivalents per Time (METs) in kilocalories/min/kg. It is the smallest accelerometer available (28 × 27 × 10 mm, 17 g.), and is also water resistant making it well suited for adolescents. The devise is a compact, battery-operated, physical activity monitor with physical characteristics similar to a small wristwatch. The Actical has enough memory to store data for twenty-two days at thirty-second sampling epochs or forty-four days at 1 min sampling epochs. Most importantly it provides an objective measure of exercise adherence and total energy consumed on a daily, 24 h basis and is not dependent on self or parent report. It also provides important information related to exercise and sleep patterns which are often symptomatic of depression. It is predicted that the sleep patterns will become more normalized over time directly proportional to the amount of exercise.
2.6. Exercise measures
We recognize that the current pilot project is significantly under-powered (too few subjects, two active treatments) to adequately address mediators of treatment improvement and exercise adherence. Our goal in collecting this additional information only at baseline, 6 weeks, and exit is to minimize burden while identifying measures that have potential to be used in a larger project. The following measures are being used:
2.6.1. Social Support for Physical Activity Scale: child Version (SSPAS-C/P)
Adolescents indicate on a 5-point scale how often over the past 3 months their family and friends provided instrumental and emotional support for their exercise, such as offering to do physical activities with the adolescent. This measure has demonstrated acceptable test–retest reliability and concurrent criterion-related validity (Sallis, Grossman, Pinski, Patterson, & Nader, 1987) and is the most widely accepted and utilized measure of social support for exercise (Marcus & Forsyth, 2003). Parents complete a parent version of this questionnaire assessing their perceptions of the degree to which they provide emotional and instrumental support for their child’s exercise as well as assessing parents’ exercise attitudes and habits.
2.6.2. Outcome Expectations for Exercise (OEE)
Adolescents’ expectations about how they may benefit from physical activities are evaluated using the OEE (Resnick, Zimmerman, Orwig, Furstenberg, & Magaziner, 2000). The OEE asks about both the physical and mental benefits of exercise. This scale has been utilized in several exercise trials for adults and has demonstrated adequate reliability and validity (Resnick et al., 2000).
2.6.3. Motivation for Physical Activity Measure-revised (MPAM-R)
The 30-item MPAM-R assesses five general motives for activity participation, including enjoyment, competence, appearance, fitness and social. Each item is rated on a 7-point Likert scale. The MPAM-R is an expansion of the MPAM that has demonstrated adequate reliability, validity and internal consistency (Frederick & Ryan, 1993).
2.6.4. Exercise Self-Efficacy (ESE)
Adolescents complete a 5-item measure of exercise-specific self-efficacy (Marcus, Selby, Niaura, & Rossi, 1992) that assesses their confidence in their ability to engage in physical activity in a variety of compromising situations (e.g., when I am in a bad mood). The ESE measure has demonstrated acceptable validity and reliability.
2.6.5. Decisional Balance (DB)
Decision making about exercise is evaluated using the 16-item Decisional Balance measure that assesses how important potential benefits or barriers are with regard to the adolescent’s decision to exercise. The DM measure has demonstrated acceptable validity and reliability (Marcus et al., 1992).
2.6.6. Physical Activity Enjoyment Scale (PACES)
The PACES is an 18-item measure that assesses adolescents’ enjoyment of physical activity (Kendzierski & DeCarlo, 1991). Using a 7-point scale, adolescents rate how they feel about physical activity using a series of statements representing opposite ideas (I enjoy it; I hate it). The PACES has demonstrated good test–retest reliability and high internal consistency (Kendzierski & DeCarlo, 1991).
2.6.7. Stages of Change Questionnaire (SCQ)
The SCQ has been adapted for use with exercise by Marcus and colleagues (Marcus et al., 1992). For the SCQ, participants indicate which of four statements best applies to his or her current exercise status. This instrument has demonstrated good internal consistency and reliability (Marcus et al., 1992).
2.6.8. Barriers to Physical Activity
After each week, when the participant logs in to the website, they must fill out a short questionnaire on the previous week’s activity. We developed a simple measurement of self-reported exercise adherence and perceived exercise barriers. This 24-item scale uses a 5-point Likert scale to assess patient perception of barriers to participation in physical activity (Sallis et al., 1996) and gives the participant some tips to avoid said barriers and informs trainer/clinical staff regarding various issues to help the participant problem solve when needed.
2.6.9. Rating of Perceived Exertion (RPE)
The Borg Scale (Borg, 1982) patient self-report rating (6–20) is used for perceived exertion during Weekly visits (1–12) and measures patient self-monitoring of intensity at the end of each exercise session.
2.7. Intervention acceptability
2.7.1. Client Satisfaction questionnaire-8 (CSQ-8)
Subject satisfaction and acceptability of the interventions is based on a self-report exit interview. The CSQ-8 (Nguyen, Attkisson, & Stegner,1983) has been used in other adolescent treatment studies demonstrating adequate internal consistency.
2.7.2. Assessment of patient satisfaction and acceptability of the intervention
An exit interview will also ask the adolescents and their parents separately about their perceptions of the exercise/stretch intervention, those aspects of the protocol that they found most helpful, the least, the skills or techniques that were most and least used, and suggestions they have for improving the intervention. Every attempt is made to obtain information from subjects who drop out of treatment regarding their reasons for dropping out and “barriers to treatment.”
2.8. Randomization procedures
Patients that meet entry criteria for the study are randomized to a standardized aerobic exercise regime [EXER] or [STRETCH] condition. A stratified randomization procedure is used. Separate randomization lists were constructed for gender and diagnostic categories of MDD only or MDD and ADHD. Each randomization list uses random permuted blocks with a block size determined by the statistician. Block sizes vary and are only known to the statistician so that treatment assignments cannot be predicted. Subjects who withdraw (early termination) from the study are not denied treatment.
2.9. Timing of assessments
Clinical research staff closely monitor all study patients weekly for the duration of the study for any significant clinical worsening or concerns related to suicidality as part of the weekly outcome measures along with any adverse events related to the intervention. Subjects who worsen and need adjunct therapy will be referred to appropriate resources. Careful documentation of duration and type of adjunct treatment will occur. Ongoing clinical assessment is sustained by the QIDS-A outcome measure collected at weekly intervals and the CDRS tri-weekly for the 12 weeks of exercise treatment. Comprehensive state of the art processes for monitoring adherence with web-based tools and Actical 24/7 monitoring are coupled with motivational techniques for depressed youth and their parents to assure a successful completion rate.
At the end of 12 weeks of exercise, all subjects will have the option to continue exercise of their choice, but no supervised intervention will be provided by the study. All of the additional functioning measures will be based on the repeated outcome measures at the end of the 12 weeks of exercise or gathered at the time of early termination. All participants will also be evaluated at 6 and 12 months. The adolescent participants will receive a cash equivalent of $25 upon completion of each weekly exercise visit at CI resulting in a possible total payment of $300 if all 12 weekly exercise assignments are completed. We will also ask the patients to come in for a 6 and 12 month follow-up evaluation to monitor how they have been doing. They will receive $50 for each follow-up visit completed.
A follow-up interview at 6 and 12 months requires about 2 h each. At these visits, participants will be asked about the status of their depressive symptoms, functioning (e.g. school grades, social functioning, family environment), ongoing interventions for depression, and whether or not they have continued exercising.
3. Intervention (EXERcise or STRETCHing) design
The amount of time required for participating in the exercise activities is the same for the EXER group and the STRETCH group. The only difference is the amount of energy expended during the activity. At the first session, the exercise trainer explains the procedures for the respective intervention (EXER or STRETCH), shows them the equipment available for the exercise or stretch sessions, and familiarizes the participant with the Actical device (similar size to a watch), while supporting the participant in becoming completely independent in instigating and recording his or her exercise sessions and general use of the internet website for entering data. The Actical data is downloaded at the weekly sessions at the Cooper Institute (CI). The first two weeks requires a minimum of 3 sessions at CI for the trainer to teach them how to use the equipment and the exercise or stretch routines. Following the first 2 weeks, participants can begin doing their exercise program at home or other location (gym, etc.), and only need to come to CI once a week for an exercise session and to allow recording of Actical information, review of upcoming week dosage calibration and a brief clinical interview. A combined supervised and home-based approach to exercise is used to minimize time barriers and maximize flexibility. The patient receives his/her exercise prescription, conducts exercise planning, and monitors and records completed exercise via the Exercise website (Fig. 1). Each exercise/stretch session is approximately 30 min to an hour depending on the energy expending requirements of the condition (Table 2). A couple of early participants in the exercise group have realized that by increasing the intensity of their work out they can shorten the time required to complete the session (i.e., burning more calories per unit time). By contrast, one mother needed reminding that it is not the time required to complete the session, but the total amount of calories consumed and this will vary as a function of intensity. She was concerned that her daughter was not moving fast enough so the trainer went over it with her again and let her know that the important thing was that she was completing her assigned session, not the length of time it took.
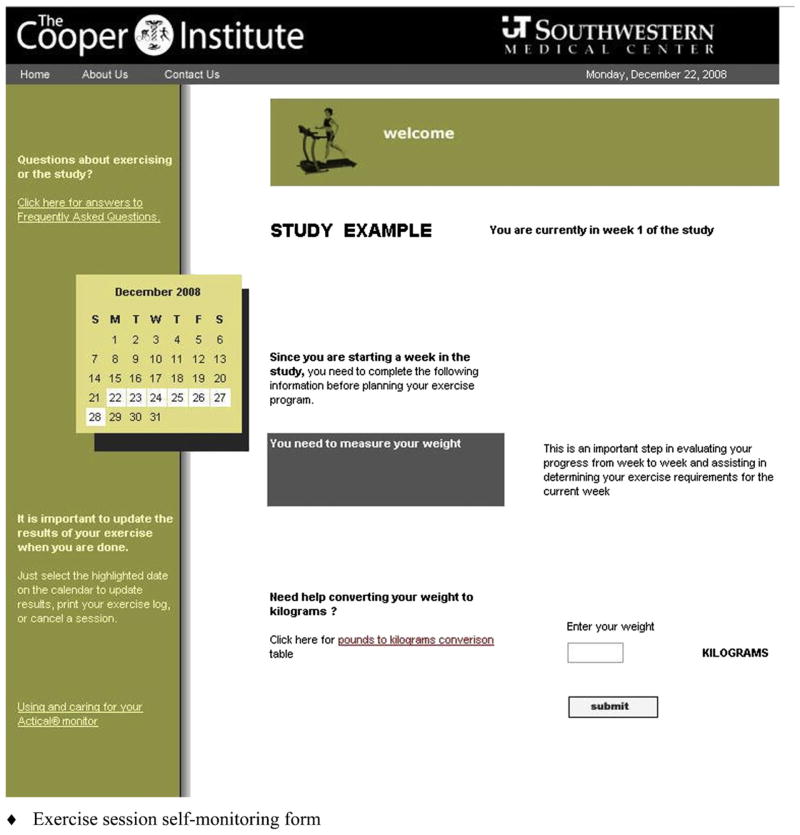
Fig. 1.
DATE Website. Patients use the DATE Website from The Cooper Institute, home, or work to: 1. REVIEW their weekly exercise prescription (and exercise left to complete) 2. PLAN next exercise session within the current week 3. UPDATE and track progress day-to-day.
Table 2.
Estimated number of minutes required by a 70-kg participant exercising at a moderate intensity to achieve an energy expenditure of 12 kcal per kg−1 per week−1. The placebo stretch condition is less than 4 kcal per kg−1 per week−1.
| Exercise Activity Options: Stretching <4 kcal per kg−1 per/wk−1 Versus Moderate Exercise ≥12 kcal | 4 kcal per kg−1 per week−1 |
12 kcal per kg−1 per week−1 |
||||
|---|---|---|---|---|---|---|
| Minutes per session |
Minutes per session |
|||||
| 3 x/wk | 4 x/wk | 5 x/wk | 3 x/wk | 4 x/wk | 5 x/wk | |
| Walking (4 mph, 0% grade) | 19 | 14 | 11 | 56 | 42 | 34 |
| Walking (3 mph, 2% grade) | 18 | 14 | 11 | 55 | 41 | 33 |
| Cycling (lkp, 70 rpm) | 17 | 13 | 10 | 51 | 39 | 31 |
3.1. EXERcise intervention
Supervised exercise sessions at the Cooper Institute (CI) for the participants begin by using the treadmills, stationary cycles, or both. Additional exercise options that adolescents may enjoy (e.g., Wii Sports and Fit, jazzercise, jogging, weight training) are also allowed. Our manual is designed for depressed adolescents to move from a sedentary life style to one with regular exercise. The weekly CI visit is scheduled to generally be the patient’s first exercise session each week. Training intensity for each patient is self-selected (the greater the intensity selected the less time required but is also related to individual conditioning – Table 2). Exercise sessions include a brief (10-minute) exercise planning and behavioral contracting intervention designed to optimize each patient’s exercise adherence. The trainer provides brief encouraging feedback and helps the patient develop strategies to address exercise barriers. Home-based exercise sessions are unsupervised workouts completed at the patient’s home or in the community – parents are encouraged to participate and become involved. The CI trainers teach patients how to complete home-based exercise.
Participants exercise at a self-selected power output for the designated time to meet their weekly caloric expenditure. The duration of each session generally is that time required to reach 1/3 or 1/4 of the total weekly caloric expenditure but can be modified occasionally if the participant has scheduling issues or problems that require adjustment. Weight is obtained at the weekly CI visits to assess the need for modification of the exercise prescription. The Actical and web-based data obtained are reviewed to ensure that participants are complying with their exercise or stretch prescription and meeting their prescribed dose of exercise or stretch. Percent body fat is determined at baseline and then again at the exit visit.
Although we want each participant to expend the total number of kcal per week (KKW) required by the EXER group assignment, all participants may not be immediately capable of exercising at their required dose. For this reason, there is a progression to the assigned exercise dose group in the first few weeks that gets them up to their kcal energy expenditure per week (e.g., 8 KKW first week, 10 KKW second and 12 KKW by the third week). This gradual increase in total energy expenditure makes it easier for participants to reach their goal and may minimize fatigue, soreness, injuries, and dropouts. Based on previous pilot work prior to this study and anecdotal reports from others, most participants tend to have a baseline fitness level that results in completing 3–4 exercise sessions per week of about 20–40 min within the first month. Participants should not exercise less than twice per week or more than six times per week. In addition, if needed, participants are allowed to split each daily session into 2 bouts each day to help them achieve their weekly caloric expenditure and increase exercise adherence. Total energy expenditure per week remains the same. In all cases, the duration of the training program can be modified, as the computer displays a running tally of the total number of kilocalories expended.
3.2. STRETCH intervention
The stretch group spends approximately the same amount of time, but at energy expenditures of less than 4 KKW whereas the exercise group will be expending 12 KKW. A 5–10 min stretching warm-up period includes stretches that exercise the major muscle groups of the body. The series includes such traditional “warm-up” stretches as: stretches of the gluts, inner thigh, calves and ankles, Achilles tendon, hamstring stretches, shoulder rolls forward and back, shoulder shrugs, isometrics for the neck hugging knees into the chest, moving forehead to right knee, then to left, then to both, and use of the pelvic tilt. An additional 10–15 min consists of moving on to right and left calf stretches, quad stretches, and then to a series for the arms, hands, fingers, wrist, biceps/triceps, shoulders and back. All of the exercises are designed to be done slowly, emphasizing proper alignment, and rest periods to minimize overall physical exertion while obtaining general flexibility, and most importantly controlling for contact time with trainers and any social facilitation from participating in such activities. We have selected a different set of low level/low intensity routines for each of the 12 weeks to minimize boredom with the routines.
These routines were developed as a control group in prior adult studies at CI and have been adapted for the adolescents and manualized. The stretch dose has not been seen as effective as the higher dose when used in adult studies in treating depression and serves as an important comparison group to the more active exercise condition (Dunn et al., 2005).
3.3. Web-based training and monitoring
We have designed an online activity management system interfaced with a website [http://www.exerciseanddepression.org/date] that facilitates the CI exercise and stretch interventions for participants’ activity sessions online based on the system designed by Trivedi et al. (2006). The website is linked to a database that records exercise session times, perceived exertion (RPE), and overall adherence to weekly exercise assignments (Fig. 1). The system allows for input of each participant’s relevant data (study ID#, group assignment, and exercise/stretch schedule) while maintaining secure confidentiality. The participant chooses a comfortable exercise intensity to determine power output (PO) for the cycle ergometer, speed and grade for the treadmill, or their personal choice of exercise that meets KCAL energy expenditure requirement (Galper, Trivedi, Barlow, Dunn, & Kampert, 2006; Trivedi et al., 2006). The website will then indicate the number of minutes the participant must exercise to achieve his or her exercise dose in KKW for that session. The website also provides KKW expenditure totals for the week (progress made and progress needed through all sessions), exercise session logs for past weeks, and overall progress throughout the 12-week intervention.
3.4. Adherence to the exercise protocol
Exercise adherence is a concern in a study such as this one (Biddle, 2001; O’Neal & Blair, 2001; Trivedi et al., 2006). A major developmental difference in working with younger patients is the expectation that the parents will help encourage and monitor the regular exercise sessions. It is also critical that they endorse the importance of regular activity for their child and provide lots of reinforcement for participating and completing non-CI exercise sessions. Parent(s) are encouraged to exercise/stretch with their teenager and reinforce regular sessions by doing it with them. Additionally, staff regularly call and remind participants of pending appointments and follow-up on missed exercise sessions. The web-based training and exercise log system helps with this monitoring. CI has a well-designed and tested process for scheduling make-up sessions to sustain the goals of kcal expenditure for the study. Improved adherence is facilitated by developing and maintaining an excellent therapeutic alliance with patients and parents early on and by weekly monitoring of adherence to intervene with problem solving and support as needed. Given that it is unrealistic to expect 100% adherence to the exercise protocol in adolescents, acceptable adherence is defined as completing 70% or more of the total amount of exercise prescribed (Morss et al., 2004).
3.5. Documentation of treatment fidelity
Whether exercising at CI or home, participants are asked to keep an online diary and written exercise/stretch logs of frequency and duration of all exercise/stretch sessions. The DATE interactive website is used to schedule and document each exercise session throughout the 12 weeks of the study. Items recorded during exercise sessions are a part of the website that they log in to for reporting and instructions (Fig. 2). Paper and pencil duplicates of exercise logs and other documentation are provided in case a participant does not have Internet access or experiences temporary lack of access due to unforeseen circumstances. At the weekly CI visit the trainer goes over the prior week’s log and exercise/stretch session to review compliance and that the weekly energy goal has been met, and if in the STRETCH group, not exceeded the 4 KKW limit. Objective energy expenditure is captured with the Actical. Total energy expenditure data is captured via the Actical monitor and downloaded once a week to verify self-reported exercise or stretch sessions. The CI Trainer provides feedback about how each participant is doing, reinforcement for goal attainment, and encouragement for those who need to improve. These exchanges coupled with the clinical assessor’s interactions provide ongoing encouragement.
Fig. 2.
Website exercise log for monitoring patient adherence.
4. Summary
No studies to date have conducted a controlled study of standardized clinic-based exercise intervention compared to a non-vigorous stretch condition in depressed adolescents meeting DSM-IV MDD criteria. Hence, there are no established effect sizes for the outcome measures of such a study and these will be generated with this pilot study. This study will guide clinicians in the effectiveness of exercise in a community setting for adolescents with major depressive disorder and most importantly may provide a new non-medication treatment. Important contributions will be the demonstration of the willingness of depressed adolescents to participate in an exercise program, determining the effect of exercise in reduction of depression symptoms, and in increasing the number of patients that attain full remission of their symptoms without medication. Further, this study provides the basis for determining whether a larger scale study should be undertaken which compares exercise as a treatment alone against known medication interventions similar to what has been demonstrated for depressed adult patients. Overall, our study will advance the translational study of academic models applied for treatment of depressed adolescents in community settings. Data collection is scheduled to end in the Spring of 2011.
Acknowledgments
Funding support
The study is supported by a grant entitled Exercise for Depressed Youth (R34 MH075762-02) from the National Institute of Mental Health (Principal Investigator: Carroll W. Hughes).
The authors express their gratitude to the editor (GF) for his assistance and guidance in the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
Financial disclosure
Carroll Hughes is a consultant for BioBehavioral Diagnostics Inc. He, Graham Emslie, and Beth Kennard receive research support from the National Institute of Mental Health. Graham Emslie also receives research support from Eli Lilly, Forest Laboratories, Somerset, and BioBehavioral Diagnostics Inc.; and is a consultant for Eli Lilly, GlaxoSmithKline, Wyeth–Ayerst, Shire, and BioBehavioral Diagnostics Inc.; and is on the Speaker’s Bureau for McNeil. Tracy Greer receives funding from NARSAD. Joseph Cleaver, Shauna Dorman, Tyson Bain, Judy Dubreuil, and Conrad Barnes report no competing interests. Dr. Trivedi has received research support from the Agency for Healthcare Research and Quality (AHRQ), Corcept Therapeutics, Inc., Cyberonics, Inc., Merck, National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health, National Institute on Drug Abuse, Novartis, Pharmacia & Upjohn, Predix Pharmaceuticals, Solvay Pharmaceuticals, Inc., and Targacept. He has received consulting and speaker fees from Abbott Laboratories, Inc., Abdi Brahim, Akzo (Organon Pharmaceuticals Inc.), AstraZe-neca, Bristol–Myers Squibb Company, Cephalon, Inc., Evotek, Fabre Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica Products, LP, Johnson & Johnson PRD, Eli Lilly & Company, Meade Johnson, Medtronic, Neuronetics, Otsuka Pharmaceuticals, Parke–Davis Pharmaceuticals, Inc., Pfizer Inc., Sepracor, SHIRE Development, VantagePoint, and Wyeth–Ayerst Laboratories.
References
- Biddle SJH. Adherence to sport and physical activity in children and youth. In: Bull S, editor. Adherence issues in sport and exercise. New York: 2001. pp. 111–144. [Google Scholar]
- Birmaher B, Brent DA, Kolko DJ, Baugher M, Bridge J, Iyengar S, et al. Clinical outcome after short-term psychotherapy for adolescents with major depressive disorder. Archives of General Psychiatry. 2000;57:29–36. doi: 10.1001/archpsyc.57.1.29. [DOI] [PubMed] [Google Scholar]
- Borg GAV. Psychophysical bases of perceived exertion. Medical Science and Sports Exercise. 1982;14:377–381. [PubMed] [Google Scholar]
- Brent DA, Kolko DJ, Birmaher B, Baugher M, Bridge J. A clinical trial for adolescent depression: predictors of additional treatment in the acute and follow-up phases of the trial. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:263–270. doi: 10.1097/00004583-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Curran SL, Andrykowski MA, Studts JL. Short form of the profile of mood states (POMS-SF) Psychological Assessment. 1995;7:80–83. [Google Scholar]
- Curry JF. Specific psychotherapies for childhood and adolescent depression. Biological Psychiatry. 2001;49:1091–1100. doi: 10.1016/s0006-3223(01)01130-1. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. The DOSE study: a clinical trial to examine efficacy and dose response of exercise as treatment for depression. Controlled Clinical Trials. 2002;23:584–603. doi: 10.1016/s0197-2456(02)00226-x. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression. American Journal of Preventive Medicine. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Dunn A, Weintraub P. Exercise in the prevention and treatment of adolescent depression: a promising but little researched intervention. American Journal of Lifestyle and Medicine. 2008;2:207–518. [Google Scholar]
- Emslie G, Heiligenstein J, Wagner K, Hoog S, Ernest D, Brown E, et al. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1205–1215. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Emslie G, Rush A, Weinberg W, Kowatch R, Carmody T, Mayes T. Fluoxetine in child and adolescent depression: acute and maintenance treatment. Depression and Anxiety. 1998;7:32–39. doi: 10.1002/(sici)1520-6394(1998)7:1<32::aid-da4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Emslie G, Rush A, Weinberg W, Kowatch R, Hughes CW, Carmody T, et al. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Archives of General Psychiatry. 1997;54:1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Weinberg WA, Mayes TL. Treatment of children with antidepressants: focus on selective serotonin reuptake inhibitors. Depression and Anxiety. 1998;8(Suppl 1):13–17. [PubMed] [Google Scholar]
- Frederick CM, Ryan RM. Differences in motivation for sport and exercise and their relations with participation and mental health. Journal of Sport Behavior. 1993;16:124–146. [Google Scholar]
- Galper DI, Trivedi MH, Barlow CE, Dunn AL, Kampert JB. Inverse association between physical inactivity and mental health in men and women. Medicine and Science in Sports and Exercise. 2006;38:173–178. doi: 10.1249/01.mss.0000180883.32116.28. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Brown CH, Kur K, Marcus SM, Bhaumik DK, Erkens JA, et al. Early evidence on the effects or regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adoelscents. American Journal of Psychiatry. 2007;164:1356–1363. doi: 10.1176/appi.ajp.2007.07030454. [DOI] [PubMed] [Google Scholar]
- Guy W. Clinical global improvement scale. Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- Hughes CW, Emslie G, Crismon M, Posner K, Birmaher B, Ryan N, et al. Texas children’s medication algorithm project: update from Texas consensus conference panel on medication treatment of childhood major depressive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:667–686. doi: 10.1097/chi.0b013e31804a859b. [DOI] [PubMed] [Google Scholar]
- Jain S, Carmody T, Trivedi MH, Hughes CW, Bernstein I, Morris DW. A psychometric evaluation of the CDRS and MADRS in assessing depressive symptoms in children. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1204–1212. doi: 10.1097/chi.0b013e3180cc2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Allen NB, O’Donnell CP, Parslow RA, Purcell R, Morgan AJ. Effectiveness of complementary and self-help treatments for depression in children and adolescents. Medical Journal of Australia. 2006;185:368–372. doi: 10.5694/j.1326-5377.2006.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendzierski D, DeCarlo KJ. Physical activity enjoyment scale: two validation studies. Journal of Sport & Exercise Psychology. 1991;13:50–64. [Google Scholar]
- Kratochvil CJ, Vitiello B, Walkup J. SSRIs in pediatric depression: is the balance between benefits and risk favorable? Journal of Child and Adolescent Psychopharmacology. 2006;16:11–14. doi: 10.1089/cap.2006.16.11. [DOI] [PubMed] [Google Scholar]
- Larun L, Nordheim LV, Ekeland E, Hagen KB, Helan F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database of Systematic Reviews. 2006;3:CD004691. doi: 10.1002/14651858.CD004691.pub2. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of mood states. San Diego: Education and Industrial Testing Service; 1992. [Google Scholar]
- March JS, Silva S, Petrycki S. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: treatment for adolescents with depression study (TADS) randomized control trial. Journal of the American Medical Association. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Forsyth LH. Motivating people to be physically active. Champaign, IL: Human Kinetics; 2003. [Google Scholar]
- Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Research Quarterly for Exercise & Sport. 1992;63:60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database of Systematic Reviews. 2009;3:CD004366. doi: 10.1002/14651858.CD004366.pub4. [DOI] [PubMed] [Google Scholar]
- Morss GM, Jordan AN, Skinner JS, Dunn AL, Church TS, Earnest CP, et al. Dose response to exercise in women aged 45–75 yr (DREW): design and rationale. Medicine and Science in Sports and Exercise. 2004;36:336–344. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Attkisson C, Stegner B. Assessment of patient satisfaction development and refinement of a service evaluation. Evaluation and Program Planning. 1983;6:299–313. doi: 10.1016/0149-7189(83)90010-1. [DOI] [PubMed] [Google Scholar]
- O’Neal HA, Blair SN. Enhancing adherence in clinical exercise trials. Quest. 2001;53:310–317. [Google Scholar]
- Olfson M, Marcus SC, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults. Archives of General Psychiatry. 2006;63:865–872. doi: 10.1001/archpsyc.63.8.865. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB. Children’s depression rating scale, revised (CDRS-R) manual. Los Angeles: Western Psychological Services Publishers and Distributors; 1996. [Google Scholar]
- Rao U, Neal RD, Birmaher B, Dahl RE, Williamson DE, Kaufman J, et al. Unipolar depression in adolescents: clinical outcome in adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:566–578. doi: 10.1097/00004583-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Reinherz HZ, Giaconia RM, Silverman AB, Friedman A, Pakiz B, Frost AK, et al. Early psychological risks for adolescent suicidal ideation and attempts. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:599–611. doi: 10.1097/00004583-199505000-00012. [DOI] [PubMed] [Google Scholar]
- Resnick B, Zimmerman SI, Orwig D, Furstenberg AL, Magaziner J. Outcome expectations for exercise scale: utility and psychometrics. Journals of Gerontology Series B-Psychological Sciences & Social Sciences. 2000;55:S352–S356. doi: 10.1093/geronb/55.6.s352. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Are adolescents changed by an episode of major depression? Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:1289–1298. doi: 10.1097/00004583-199411000-00010. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH. An evaluation of the quick inventory of depressive symptomatology and the Hamilton rating scale for depression: a STAR*D report. Biological Psychiatry. 2006;59:493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Carmody TJ, Ibrahim HM. Comparison of self-report and clinician ratings on two inventories of depressive symptomatology. Psychiatric Services. 2006;57:829–837. doi: 10.1176/ps.2006.57.6.829. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Preventive Medicine. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Strikmiller PK, Warsha DW, Feldman HA, Ehlinger S, Stone EJ. Validation of interviewer- and self- administered physical activity checklists for fifth grade students. Medical Science and Sports Exercise. 1996;28:840–851. doi: 10.1097/00005768-199607000-00011. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould M, Brasic J, Ambrosini P, Fisher P, Bird H. A children’s global assessment scale (C-GAS) Archives of General Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Fisher P, Tautman P, Moreau D, Kleinman M, et al. Psychiatric diagnosis in child and adolescent suicide. Archives of General Psychiatry. 1996;53:339–348. doi: 10.1001/archpsyc.1996.01830040075012. [DOI] [PubMed] [Google Scholar]
- Singh NA, Stravrikons TM, Scarbek Y, Galambos G, Liber C, Singh MA. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. Journal of Gerontological Series A Biological Sciences Medical Sciences. 2005;60A:768–776. doi: 10.1093/gerona/60.6.768. [DOI] [PubMed] [Google Scholar]
- Terry P. Perspectives on mood in sport and exercise. Journal of Applied Sport Psychology. 2000;12:1–4. [Google Scholar]
- Trivedi MH, Greer TL, Grannemann BD, Church TS, Galper DI, Sunderajan P, et al. TREAD: treatment with exercise augmentation for depression: study rationale and design. Clinical Trials. 2006;3:291–305. doi: 10.1191/1740774506cn151oa. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Archives of General Psychiatry. 1976;33:1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Orvaschel H, Padian N. Children’s symptom and social functioning self-report scales: comparison of mother’s and children’s reports. Journal of Nervous and Mental Diseases. 1980;168:736–740. doi: 10.1097/00005053-198012000-00005. [DOI] [PubMed] [Google Scholar]