SUMMARY
Recent work on the mechanisms of DNA damage and replication cell cycle checkpoints has revealed great similarity between the checkpoint pathways of organisms as diverse as yeasts, flies and humans. However, there are differences in the ways these organisms regulate their cell cycles. To connect the conserved checkpoint pathways with various cell cycle targets requires an adaptable link that can target different cell cycle components in different organisms. The Chk1 and Cds1 protein kinases, downstream effectors in the checkpoint pathways, seem to play just such roles. Perhaps more surprisingly, the two kinases not only have different targets in different organisms but also seem to respond to different signals in different organisms. So, whereas in fission yeast Chk1 is required for the DNA damage checkpoint and Cds1 is specifically involved in the replication checkpoint, their roles seem to be shuffled in metazoans.
Keywords: Chk1, Cds1, Rad53, Chk2, Grp, DNA damage checkpoint, Replication checkpoint
INTRODUCTION
When a cell encounters a problem such as damaged DNA or a block to replication, it can call upon a variety of mechanisms to fix the problem. But these mechanisms can take time, and it is often crucial that the cell does not continue through the cell cycle until the problem is fixed. That is where cell cycle checkpoints come in. The checkpoints recognize the problem and delay cell cycle progression by inhibiting the basic cell cycle machinery until the problem is fixed (Hartwell and Weinert, 1989; Elledge, 1996; Rhind and Russell, 1998a). These checkpoints can also regulate transcription and may directly regulate repair machinery, but these functions are beyond the scope of this Commentary. Many, if not all, of the major cell cycle transitions are regulated by one or another checkpoint. However, we will concern ourselves here with the DNA damage and replication checkpoints, which have served as the prototypic checkpoint pathways. These checkpoints are triggered by various forms of DNA damage and various treatments that block replication, respectively.
It is useful to think of checkpoints as divided into three parts: a sensor, a transduction pathway and a target. The transduction pathways for the DNA damage and replication checkpoints are composed of a shared group of conserved proteins that may also serve as the sensors. The pathways have been recently reviewed and will only be briefly addressed here (Elledge, 1996; Longhese et al., 1998; Rhind and Russell, 1998a; Dasika et al., 1999). Of the proteins known to be involved, the most interesting is a large protein kinase of the DNA-PK family, known as Rad3 in the fission yeast Schizosaccharomyces pombe, Mec1 in budding yeast Saccharomyces cerevisiae, MEI-41 in the fruit fly Drosophila melanogaster, and X-ATM in the frog Xenopus laevis (Zakian, 1995). Two homologs, ATM and ATR, have been identified in humans and mice (Westphal, 1997). For convenience, we will refer to these homologs generically as ATMs but use the specific name when referring to a specific organism. By analogy with DNA-PK, a kinase that is activated by binding to DNA ends, it is proposed that ATM acts to recognize the DNA damage or stalled replication forks and initiate the checkpoint signal (Hartley et al., 1995; Bentley et al., 1996). The other members of this pathway could serve as regulatory subunits of a complex that has ATM as its core (Longhese et al., 1998). Although it is appealing, there is little direct evidence for this model. However, the fact that the checkpoints share many upstream proteins suggests that they may recognize a similar or overlapping set of DNA structures. What is known is that downstream of ATM in each species are homologs of the Chk1 and Cds1 protein kinases (Fig. 1; Weinert et al., 1994; Murakami and Okayama, 1995; Walworth and Bernards, 1996; Sanchez et al., 1997; Kumagai et al., 1998; Matsuoka et al., 1998; Blasina et al., 1999; Brown et al., 1999; Chaturvedi et al., 1999; Sanchez et al., 1999; Sibon et al., 1999; Tominaga et al., 1999; Guo and Dunphy, 2000; Liu et al., 2000b).
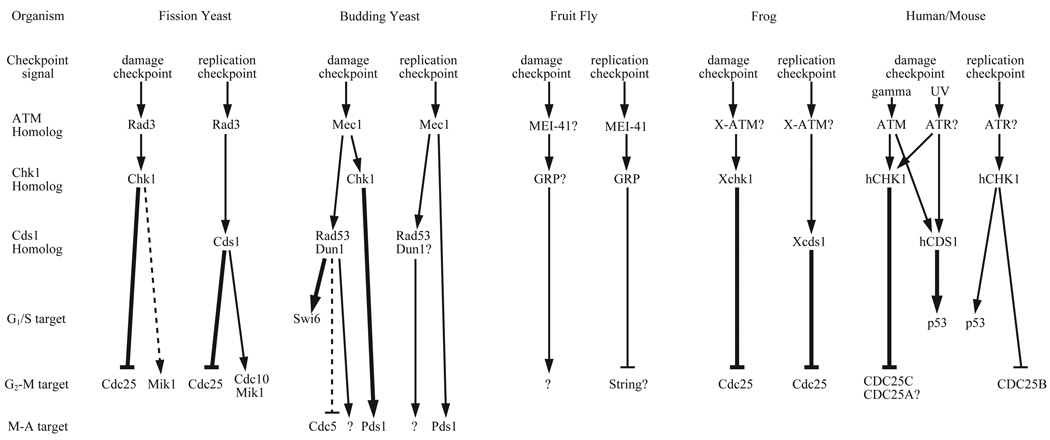
Fig. 1.
A schematic representation of the DNA damage and replication checkpoints in various organisms. The human/mouse pathway is based on evidence from both organisms. Bold arrows indicate some evidence for direct biochemical regulation, although this evidence may only be in vitro phosphorylation. Dashed arrows indicate that the regulation has been shown in vivo not to be sufficient for establishment of cell cycle arrest. In both cases the regulation seems to be more important for maintenance of the arrest. A question mark after a protein indicates that there is only circumstantial evidence for its involvement. A question mark without a protein indicates that there is evidence for some protein at that point in the pathway, but no evidence as to its identity.
Chk1 was originally identified in fission yeast as a kinase required for the DNA damage checkpoint but not the replication checkpoint (Walworth et al., 1993). Genetic experiments place fission yeast Chk1 as the most downstream member in the DNA damage checkpoint pathway and specifically downstream of Rad3 (Walworth and Bernards, 1996). Chk1 homologs have been identified in all other eukaryotes examined, and, where the experiments have been done, the function of Chk1 as a downstream checkpoint effector seems to be conserved (Fogarty et al., 1997; Sanchez et al., 1997; Kumagai et al., 1998; Sanchez et al., 1999; Liu et al., 2000b). The Chk1 homologs share a similar N-terminal kinase domain as well as scattered similarity throughout their C-termini (Sanchez et al., 1997).
The founding member of the Cds1 family is budding yeast Rad53 (Allen et al., 1994). Like Chk1, Rad53 is widely conserved (Murakami and Okayama, 1995; Matsuoka et al., 1998; Blasina et al., 1999; Brown et al., 1999; Chaturvedi et al., 1999; Tominaga et al., 1999; Guo and Dunphy, 2000). Since these homologs are generally called Cds1, we will refer to them generically as Cds1 but use the specific name when referring to a specific organism. The Cds1 homologs are recognizable by a similar kinase domain and an N-terminal forkhead associated (FHA) domain (Blasina et al., 1999). FHA domains, originally recognized in the forkhead transcription factor, are believed to act as protein-protein interaction domains, and in some instances bind specifically to phosphorylated partners (Hofmann and Bucher, 1995; Sun et al., 1998; Durocher et al., 1999). Rad53 is unique among the Cds1 homologs in possessing a second C-terminal FHA domain.
The wide conservation of Chk1, Cds1 and the upstream checkpoint proteins at the amino acid level suggested that the DNA damage and replication checkpoints function similarly in all eukaryotes*. Although this idea holds in general, it is becoming increasingly clear that there are significant differences in the details. One area in which differences were inevitable is the targets of the checkpoint effectors, Chk1 and Cds1. Although the basic cell cycle machinery is well conserved, there are important differences in the points of the cell cycle at which organisms impose regulation (Murray and Hunt, 1993). Thus the checkpoints need to target different points in the cell cycle, and it could have been predicted that the targets of the effector kinases would vary. More surprising is the fact that the checkpoints in which each effector kinase acts seem not to be conserved – for example, Chk1 being required for the DNA damage checkpoint in fission yeast and the replication checkpoint in frogs and flies (Walworth et al., 1993; Kumagai et al., 1998; Sibon et al., 1999). Here, we focus on the various targets of Chk1 and Cds1 and on which checkpoints activate them to regulate these targets.
THE VARIOUS TARGETS OF CHK1 AND CDS1
Targets in yeast
Fission yeast cells spend most of their time in G2, and the DNA damage and replication checkpoints prevent cell cycle progression by blocking the G2-M transition (Rhind and Russell, 1998a). They do this by inhibiting Cdc2, the kinase that drives mitosis, through Tyr15 phosphorylation (Rhind et al., 1997; Rhind and Russell, 1998b). The tyrosine phosphorylation of Cdc2 is regulated by the Wee1 and Mik1 tyrosine kinases and the Cdc25 tyrosine phosphatase (Coleman and Dunphy, 1994). Thus, the checkpoints could act to upregulate Wee1 and/or Mik1, or inhibit Cdc25. In fact, the checkpoints seem to both upregulate Mik1 and inhibit Cdc25.
In fission yeast, Chk1 is the effector of the DNA damage checkpoint pathway (Walworth et al., 1993). Chk1 is phosphorylated in a Rad3-dependent manner in response to activation of the DNA damage checkpoint, but not the replication checkpoint, and this phosphorylation correlates with its ability to arrest cells in G2 (Walworth and Bernards, 1996). Of its targets, Cdc25 is the best understood. In vivo experiments show that Cdc25 is strongly inhibited in response to activation of the DNA damage checkpoint, and this inhibition requires Chk1 (Furnari et al., 1997; Rhind et al., 1997). This inhibition is presumed to be due to direct regulation by Chk1, which binds to Cdc25 in vivo and phosphorylates it in vitro (Furnari et al., 1997; Zeng et al., 1998). The phosphorylation of Cdc25 by Chk1 has two effects. First, in vitro phosphorylation by Chk1 inhibits its phosphatase activity (Blasina et al., 1999; Furnari et al., 1999). Second, activation of the DNA damage checkpoint results in reduced nuclear localization of Cdc25 (Lopez-Girona et al., 1999). This relocalization of Cdc25 is blocked by mutation of Chk1 phosphorylation sites in Cdc25, which suggests that it is due to Chk1 phosphorylation (Zeng and Piwnica-Worms, 1999). The phosphorylation of Cdc25 on these sites promotes the binding of Rad24, a 14-3-3 protein, and the binding of Rad24 is believed to sequester Cdc25 in the cytoplasm, away from its substrate, Cdc2 (Zeng et al., 1998; Lopez-Girona et al., 1999). It is not clear to what extent each of these two modes of regulation contributes to the inhibition of Cdc25 in vivo. Part of the problem is that Cdc25 is phosphorylated in vitro on at least 12 sites (Zeng and Piwnica-Worms, 1999). Mutation of nine of these sites impairs the 14-3-3 binding of Cdc25, its relocalization after damage, and the DNA damage checkpoint (Zeng and Piwnica-Worms, 1999). However, the mutations may also impair the regulation of Cdc25 phosphatase activity, and thus do not distinguish between the two possible mechanisms of regulation.
The other cell cycle target of the DNA damage checkpoint in fission yeast is Mik1 (Baber-Furnari et al., 2000; Christensen et al., 2000; Rhind and Russell, unpublished data). Although Cdc25 regulation is alone sufficient to arrest cells in response to activation of the DNA damage checkpoint, regulation of Mik1 alone suffices only to produce an attenuated DNA damage checkpoint delay (Rhind and Russell, unpublished data). It appears that Mik1 is regulated at two levels by the DNA damage checkpoint. Mik1 is able to contribute to the establishment of a DNA damage checkpoint delay, in a manner that does not require increased Mik1 accumulation (Rhind and Russell, unpublished data). In addition, after a prolonged activation of the DNA damage checkpoint, Mik1 accumulates through a post-transcriptional mechanism (Baber-Furnari et al., 2000; Christensen et al., 2000). This accumulation correlates with its requirement for maintenance of an extended checkpoint arrest (Baber-Furnari et al., 2000). It is not known whether Chk1 directly phosphorylates Mik1 to effect its regulation or whether other substrates are involved.
The role of Cds1 in fission yeast is as the effector of the replication checkpoint pathway (Murakami and Okayama, 1995; Boddy and Russell, 1999). Cds1 is activated by the checkpoint and is required for cells to survive treatments that block replication (Boddy et al., 1998; Lindsay et al., 1998). However, the role of Cds1 as effector of the replication checkpoint is complicated by the fact that, in the absence of Cds1, Chk1 can act to impose a checkpoint delay (Boddy et al., 1998; Zeng et al., 1998; Brondello et al., 1999). Although Chk1 may be involved in the replication checkpoint in wild-type cells, it does not appear to play an important role (Walworth et al., 1993; Boddy and Russell, 1999; Brondello et al., 1999). In vivo, Cdc25 is inhibited by the replication checkpoint (Rhind and Russell, 1998b). Cds1 seems to regulate Cdc25 in a similar manner to Chk1. Cds1 phosphorylates Cdc25 in vitro on sites similar to Chk1, and inhibits Cdc25 in vitro (Zeng et al., 1998; Furnari et al., 1999). The effect of the replication checkpoint on Cdc25 localization has yet to be described.
In addition to Cdc25, Mik1 is also an important target of Cds1 (Boddy et al., 1998; Christensen et al., 2000; Rhind and Russell, unpublished data). Like Cdc25, regulation of Mik1 alone is sufficient to arrest cells in response to activation of the replication checkpoint (Rhind and Russell, unpublished data). In a Cds1-dependent manner, Mik1 accumulates in replication checkpoint arrested cells (Boddy et al., 1998; Christensen et al., 2000). The accumulation of Mik1 correlates with the accumulation of its mRNA. The upregulation of several other S-phase-specific transcripts in response to activation of the replication checkpoint requires the Cdc10-dependent S-phase transcription factor (Baum et al., 1997). So it seems plausible that Cds1 acts through Cdc10 machinery to maintain the S-phase transcription program during a replication checkpoint arrest, although its direct targets are unknown.
Although both Cdc25 and Mik1 are in vivo targets of the checkpoints, Wee1 does not seem to be. This conclusion is drawn from genetic experiments showing that Wee1 is neither necessary nor sufficient for checkpoint function in vivo (Barbet and Carr, 1993; Rhind and Russell, unpublished data, but see also Raleigh and O’Connell, 2000). This conclusion was somewhat surprising given previous in vitro results implicating Wee1 as a target in both checkpoints. In response to activation of the replication checkpoint, Cds1 binds to and phosphorylates exogenous Wee1 in cell lysates (Boddy et al., 1998). Furthermore, Chk1 can phosphorylate Wee1 in vitro (O’Connell et al., 1997). These phosphorylations do not appear to be important for the establishment of either checkpoint. However, it is possible that they play a role in maintenance of, or adaptation to, the checkpoints.
The targets of the checkpoints in budding yeast are dictated by the unusual organization of the cell cycle in this yeast. Specifically, budding yeast has no well-defined G2 phase (Lew et al., 1997). Rather, events traditionally defined as mitotic and requiring the tyrosine dephosphorylation of Cdc28 (the budding yeast Cdc2 homolog), such as spindle formation, occur before S phase is complete (Lim et al., 1996; Lew et al., 1997). Thus, regulating the tyrosine phosphorylation of Cdc28 is not practical for the budding yeast checkpoints* (Elledge, 1996). Instead, they prevent cell cycle progression by blocking the metaphase to anaphase (M-A) transition (Yamamoto et al., 1996).
In response to DNA damage, both Rad53 and Chk1 are required to arrest fully cells in metaphase. One, and possibly the major, target of Rad53 in the DNA damage pathway is thought to be Dun1, another homolog in the Cds1 family (Zhou and Elledge, 1993). Dun1 is downstream of Rad53, and appears to be required for many of the functions of Rad53 (Elledge, 1996; Pati et al., 1997; Gardner et al., 1999). The role of Rad53 and Dun1 in the DNA damage checkpoint seems to be to maintain the activity of Cdc28, possibly by inhibiting Cdc5, a polo-like kinase that is required for mitotic-cyclin degradation (Charles et al., 1998; Sanchez et al., 1999). This conclusion fits nicely with earlier work showing that inactivation of Cdc28 could override a metaphase checkpoint arrest (Minshull et al., 1996; Li and Cai, 1997). How Rad53 and Dun1 might regulate Cdc5 remains to be addressed. Since cells lacking Cdc5 arrest in telophase, not metaphase, it is likely that the inhibition of Cdc5 is required not for establishment of the arrest but rather its maintenance (Charles et al., 1998). The fact that Cdc5 has been shown to be involved in the adaptation of budding yeast to prolonged DNA damage arrests is consistent with this idea (Toczyski et al., 1997).
One likely target of Chk1 is the anaphase inhibitor Pds1 (Sanchez et al., 1999; Liu et al., 2000b). Pds1 is required for the DNA damage metaphase arrest, and is phosphorylated and stabilized during the checkpoint arrest in a Chk1-dependent manner (Yamamoto et al., 1996; Cohen-Fix and Koshland, 1997; Sanchez et al., 1999). Furthermore, Chk1 can bind to and phosphorylate Pds1 in vitro (Sanchez et al., 1999). The fact that cells lacking Chk1 or Pds1 exhibit a partial DNA-damage-induced metaphase arrest, whereas cells lacking both Chk1 and Rad53, Pds1 and Rad53 or Pds1 and Dun1 show no metaphase arrest, suggests that there is another Rad53-dependent target regulating establishment of the arrest (Gardner et al., 1999; Sanchez et al., 1999; Liu et al., 2000b). Alternatively, in the absence of Chk1, inhibition of Cdc5 may be sufficient to delay anaphase, although this would not be predicted by the known phenotypes of cdc5 mutants.
The replication checkpoint in budding yeast seems to be separated into two parts. When cells are blocked in early replication, they arrest in a Rad53-dependent manner (Allen et al., 1994; Weinert et al., 1994). Although Rad53 regulates Dun1 in the transcriptional response to blocked replication, it is not known whether Dun1 is required for the metaphase arrest, or what other targets Rad53 might have. In the absence of Rad53, cells blocked in replication proceed with anaphase, but arrest in telophase (Allen et al., 1994). This telophase arrest indicates that there are other targets in the replication checkpoint. One of those other targets appears to be Pds1 (Clarke et al., 1999). There is no evidence that Chk1 plays a role in the budding yeast replication checkpoint, and it is unknown how Pds1 might be regulated by the replication checkpoint.
In addition to regulating the M-A transition, the budding yeast DNA damage checkpoint also delays cells in G1 in a Mec1- and Rad53-dependent manner (Siede et al., 1994; Sidorova and Breeden, 1997). One target of Rad53 in this checkpoint appears to be the transcription factor Swi6. Swi6 is phosphorylated in vivo in a DNA-damage-induced and Rad53-dependent manner, and it is directly phosphorylated in vitro by Rad53 (Sidorova and Breeden, 1997). Furthermore, the transcription of CLN1 and CLN2, two G1 cyclin genes that regulate the G1-S transition, is reduced during the G1 delay, as would be predicted following inhibition of Swi6. However, there must be other targets, since ectopic expression of Cln1 and Cln2 does not override the checkpoint.
Targets in metazoans
The cloning of ATM from humans and its identification as a homolog of Rad3 and Mec1 was the first clue that the yeast DNA damage and replication checkpoints are conserved in humans (Savitsky et al., 1995). Since then, homologs of most of the fission yeast checkpoint genes have been found in humans, including hCHK1 and hCDS1, also known as CHK2 (Matsuoka et al., 1998; Rhind and Russell, 1998a). Furthermore, the human checkpoints seem to regulate the G2-M transition in the same way as fission yeast ones, through ATM to the tyrosine phosphorylation of CDC2 (Blasina et al., 1997; Westphal, 1997). Although the checkpoint regulation of the G2-M transition appears to be similar between fission yeast and humans, the predominant DNA damage checkpoint in humans is the p53-dependent G1 arrest pathway (Wahl et al., 1997). ATM is required for p53 activation in response to gamma-ray-induced DNA damage, but not other types of damage, such as that induced by UV radiation, which is speculated to act through ATR (Kastan et al., 1992; Wright et al., 1998). Neither ATM nor ATR is thought to be involved with the DNA-damage-independent activation of p53 of the kind induced by growth factor withdrawal. These results indicate that the ATM-dependent DNA damage checkpoint pathway regulates both the G1-S and G2-M transitions in humans in response to ionizing radiation, and suggest that ATR may serve a similar role in response to UV radiation. It is proposed that hCHK1 and hCDS1 act downstream of ATM and ATR in these pathways.
In vitro, hCHK1 phosphorylates the three isoforms of human CDC25: CDC25A, CDC25B, and CDC25C (Sanchez et al., 1997). It phosphorylates CDC25C on Ser216 and other sites and inhibits its in vitro phosphatase activity (Sanchez et al., 1997; Blasina et al., 1999). Ser216 is a major site of in vivo phosphorylation of CDC25C. It is phosphorylated to high stoichiometry during a normal cell cycle by processes other than the DNA damage checkpoint (Ogg et al., 1994). Phosphorylation of Ser216 leads to 14-3-3 binding (Peng et al., 1998). It is proposed that the binding of 14-3-3 to this site leads to the inhibition of CDC25, either directly or through nuclear exclusion, although inactivation of CDC25C by in vitro phosphorylation does not correlate with 14-3-3 binding (Peng et al., 1997; Weinert, 1997; Blasina et al., 1999). Furthermore, expression of CDC25C-S216A, which cannot be phosphorylated on Ser216, causes only a minor disruption of either the DNA damage or replication checkpoint in human cells (Peng et al., 1997). Thus it is unlikely that Ser216 is the only site of DNA-damaged-induced phosphorylation on CDC25C. hCHK1 has also been implicated in the regulation of CDC25A stability in response to DNA damage (Mailand et al., 2000). In addition to phosphorylating CDC25, hCHK1 phosphorylates p53 in vitro on Ser20, a site of damage-inducible phosphorylation in vivo (Shieh et al., 2000).
An in vivo role for CHK1 in the mammalian G2 DNA damage checkpoint is supported by analysis of Chk1-mutant mouse cells. These analyses are complicated by the fact that CHK1 appears to be required for cellular viability, at least in embryonic cells. However, two strategies have been employed in which Chk1-mutant cells can be tested for checkpoint defects before they die (Liu et al., 2000a; Takai et al., 2000). Chk1−/− blastocysts fail to proliferate, and die between 3.5 and 6.5 days post-fertilization. If wild-type blastocysts are irradiated at day 3.5, the percentage of cells in mitosis drops substantially, which is consistent with activation of a G2 checkpoint. In contrast, Chk1−/− blastocysts contain many mitotic cells after irradiation, which suggests that CHK1 is required to prevent mitosis in response to DNA damage (Takai et al., 2000). Another approach to study the role of CHK1 has been to delete Chk1 by induced recombination in embryonic stem (ES) cell cultures. ES cells lacking Chk1 die after about 48 hours. During those 48 hours, the cells continue to enter mitosis in spite of radiation-induced DNA damage (Liu et al., 2000a). An important caveat for both sets of experiments is that the Chk1−/− cells are destined to die during or shortly after the time course of the experiments, and it is not clear whether the accumulation of mitotic cells is due to a specific DNA damage checkpoint defect or a general mitotic-catastrophe phenotype. Atr−/− embryos display similar phenotypes, which suggests that ATR and CHK1 may operate in the same pathway (Brown and Baltimore, 2000; Liu et al., 2000a). The analysis of the Chk1 mutant blastocysts also suggest a role for CHK1 in the replication checkpoint. The possible targets of CHK1 in the mammalian replication checkpoint include p53 and CDC25B (Nishijima et al., 1997; Wahl et al., 1997).
Additional evidence that hCHK1 plays a role in the human DNA damage checkpoint is that the CHK1 inhibitors UCN01 and SB-218078 abrogate the DNA-damage-induced G2 arrest in human cell lines (Busby et al., 2000; Graves et al., 2000; Jackson et al., 2000). It should be noted, however, that both inhibitors are structurally related to staurosporine, a competitive inhibitor of ATP binding, and may well inhibit other kinases (Jackson et al., 2000). Specifically, UCN01 also inhibits C-TAK1, a kinase that can phosphorylate CDC25C on Ser216 (Busby et al., 2000). Since C-TAK1 has been proposed to be a major CDC25C-inhibitory kinase, it is conceivable that a C-TAK1 inhibitor could activate CDC25C and override the checkpoint (Ogg et al., 1994; Peng et al., 1998). Curiously, neither UCN01 nor SB-218078 causes cell lethality, despite the fact that they are both strong inhibitors of CHK1. These observations raise the possibility that CHK1 is essential for embryonic cell growth but not for somatic cell viability.
The role of Chk1 in the replication checkpoint in metazoans is supported by studies in frogs and flies. In a Xenopus cell-free egg extract system, XChk1 is phosphorylated in response to a replication block but not DNA damage (Kumagai et al., 1998). Furthermore, depletion of XChk1 reduces the mitotic delay in response to unreplicated DNA but not DNA damage. Cdc25 is thought to be at least one of the targets of XChk1 in response to unreplicated DNA. In vitro, XChk1 phosphorylates Cdc25 on several sites, including Ser287, a 14-3-3 binding site thought to be analogous to Ser216 of human CDC25C (Kumagai et al., 1998). But, again, there are other Ser287 kinases in Xenopus. And, whereas Cdc25-S287A does impair the DNA damage and replication checkpoints in the egg extract system, it also advances mitosis in untreated extracts and thereby may override other modes of checkpoint control (Kumagai et al., 1998).
Studies in flies tell a similar story. MEI-41 and Grapes (GRP), the ATM/ATR and Chk1 homologs in flies, are required for the replication-block-induced delay of mitosis in embryonic cells (Hari et al., 1995; Fogarty et al., 1997; Sibon et al., 1997; Sibon et al., 1999). In the absence of either protein, embryos fail to maintain String, a Cdc25 homolog, or tyrosine phosphorylation of Cdc2, although it has yet to be determined whether these changes are the cause or the effect of the failure of the checkpoint (Sibon et al., 1999). As in mice, both GRP and MEI-41 are required for viability (Hari et al., 1995; Fogarty et al., 1997). However, the requirement is limited to early embryogenesis and does not appear to affect the function of somatic cells, providing a precedent for the suggestion that CHK1 may be required for embryonic, but not somatic, cell growth in mammals. Nevertheless, the fly studies provide strong in vivo evidence that, in metazoans, Chk1 can function in the replication checkpoint and suggest that it may target Cdc25. GRP is also required for the regulation of the abundance of cyclin A (Su et al., 1999). However, flies lacking the homolog of Wee1 appear to have phenotypes similar to those lacking GRP, which suggests that a major role of GRP is to regulate the tyrosine phosphorylation of CDC2 (Price et al., 2000). The requirement for GRP in the DNA damage checkpoint has not been directly addressed.
A strong case can be made for the involvement of hCDS1 in the human DNA damage checkpoint. Like hCHK1, hCDS1 can phosphorylate CDC25C on Ser216 and other sites, and this phosphorylation leads to the inhibition of CDC25 in vitro (Matsuoka et al., 1998; Blasina et al., 1999; Brown et al., 1999; Chaturvedi et al., 1999). Likewise hCDS1 can phosphorylate p53 on Ser20 (Chehab et al., 2000; Hirao et al., 2000; Shieh et al., 2000). hCDS1 is also phosphorylated and activated in response to DNA damage and replication arrest (Matsuoka et al., 1998; Brown et al., 1999; Chaturvedi et al., 1999; Tominaga et al., 1999). This regulation requires ATM in the case of gamma-radiation-induced DNA damage but not in the case of UV-radiation-induced DNA damage or replication arrest. Mouse thymocytes lacking CHK2, the mouse homolog of CDS1, fail to upregulate p53 in response to gamma radiation but not UV radiation (Hirao et al., 2000). Moreover, mutations in hCDS1 have been associated with Li-Fraumeni syndrome, a syndrome usually caused by mutations in the gene encoding p53 (Bell et al., 1999). Taken together, these result show that in mammals, CDS1 is required for the p53-dependent G1 DNA damage response and suggest that ATM acts through CDS1, instead of directly regulating p53.
Although CDS1 seems to play an important role in the DNA damage checkpoint pathway in humans, two results suggest that hCDS1 is not required for the regulation of G2-M transition. First, UCN01, which strongly inhibits the G2 DNA damage checkpoint response, does not inhibit hCDS1 (Busby et al., 2000; Graves et al., 2000). Second, mouse embryonic stem cells lacking the mouse homolog of CDS1 arrest properly in response in G2 to DNA damage, although they leak through the arrest at later timepoints (Hirao et al., 2000). Similarly, although Xcds1 is activated by DNA ends in a cell-free Xenopus egg extract, Xcds1 is not required for the delay of mitosis induced by DNA ends in that system (Guo and Dunphy, 2000).
Other targets
In addition to the targets discussed above, there are certainly other targets of Chk1 and Cds1. For instance, in fission yeast, Cds1 is required to maintain cell viability during a replication arrest, which is independent of its role in preventing mitosis (Murakami and Okayama, 1995). Rad53 is also required for budding yeast to survive replicative stress (Desany et al., 1998). It probably has several targets, which may be indirectly regulated through Dun1, including induction of gene expression through regulation of the transcriptional repressor Rfx1/Crt1 (Zhou and Elledge, 1993; Huang et al., 1998). These functions may be related to a checkpoint that we have not discussed, the intra-S checkpoint, which slows DNA replication in response to DNA damage. This checkpoint has been studied in humans, budding yeast and fission yeast, and requires ATM and Cds1, although the role of hCDS1 has yet to be determined (Dasika et al., 1999). In budding yeast this checkpoint inhibits origin firing and regulates primase phosphorylation in a Rad53-dependent manner, implicating Rad53, and possibly other Cds1s, in the regulation of origin function (Santocanale and Diffley, 1998; Shirahige et al., 1998; Pellicioli et al., 1999). In addition, hCDS1 binds to and phosphorylates BRCA1, a protein implicated in DNA damage repair and recombination that may function in the intra-S and other DNA damage checkpoint pathways (Dasika et al., 1999; Lee et al., 2000).
CONCLUDING REMARKS
Studies in a variety of organisms have led to the discovery of a largely conserved pathway for the DNA damage and replication checkpoints. These pathways are best understood in fission and budding yeast. It is thought that most of the players have been identified, and the biochemical mechanisms of many of the steps are beginning to be understood.
Although the studies in fission and budding yeast provide a useful framework for understanding the human checkpoints, it is clear that much work remains to be done in humans. In particular it is still unclear what proteins act downstream of ATM in the G2 checkpoints. Since most human cancer cells lack the p53-dependent G1 DNA damage checkpoint, the G2 DNA damage checkpoint is particularly important for cancer cell survival. As such, it is a promising target for chemotherapy, because drugs that specifically inhibit the G2 checkpoints should sensitize cancer cells to DNA damage. Understanding the molecular details of this pathway is crucial for both design and assay of new checkpoint inhibiting drugs. Structural studies, such as the recent crystal structure of CHK1, may well help (Chen et al., 2000). It is also an open question as to why CHK1 should be required for cell viability in mice. The development of more in vivo techniques will provide clearer understanding of the roles of the yeast homologs in mammalian cells. In this respect, establishment and analysis of further mouse mutant strains will certainly play a key role.
At a more theoretical level, it is interesting to speculate as to why Chk1 and Cds1 seem to play different roles in different organisms. One interesting observation is that all of the known or suspected targets of Chk1 are cell cycle regulatory proteins. Cds1, by contrast, seems to be also involved in regulating DNA repair, DNA recombination and transcription. It may be easiest to posit an ancestral situation in which, in response to both DNA damage and replication blocks, Chk1-regulated cell cycle progression and Cds1 regulated repair. From there, it is possible to imagine how Cds1 could acquire cell cycle roles and come to be associated with checkpoints that most require DNA repair, such as those before and during replication. However these systems evolved, it appears that much of the plasticity centers on the functions of Chk1 and Cds1. Thus, these kinases act as linchpins that link the checkpoint signal transduction pathways with the basic cell cycle and DNA repair machinery.
Acknowledgments
We would like to thank the members of the Scripps cell cycle group, in particular Clare McGowan and Duncan Clarke, for many useful and interesting discussions on these subjects, and for critical reading of this manuscript.
Footnotes
Although little is known about these checkpoints in plants, plant homologs of Chk1 and ATM exist, and recent work suggests that the replication checkpoint functions in plants (Corellou et al., 2000; Garcia et al., 2000).
However, the bud morphogenesis checkpoint, which acts before spindle formation, does use tyrosine phosphorylation of Cdc28 (Lew and Reed, 1995).
REFERENCES
- Allen JB, Zhou Z, Siede W, Friedberg EC, Elledge SJ. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev. 1994;8:2401–2415. doi: 10.1101/gad.8.20.2401. [DOI] [PubMed] [Google Scholar]
- Baber-Furnari BA, Rhind N, Boddy MN, Shanahan P, Lopez-Girona A, Russell P. Regulation of mitotic inhibitor mik1 helps to enforce the DNA damage checkpoint. Mol. Biol. Cell. 2000;11:1–11. doi: 10.1091/mbc.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet NC, Carr AM. Fission yeast wee1 protein kinase is not required for DNA damage-dependent mitotic arrest. Nature. 1993;364:824–827. doi: 10.1038/364824a0. [DOI] [PubMed] [Google Scholar]
- Baum B, Wuarin J, Nurse P. Control of S-phase periodic transcription in the fission yeast mitotic cycle. EMBO J. 1997;16:4676–4688. doi: 10.1093/emboj/16.15.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DW, Varley JM, Szydlo TE, Kang DH, Wahrer DC, Shannon KE, Lubratovich M, Verselis SJ, Isselbacher KJ, Fraumeni JF, et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science. 1999;286:2528–2531. doi: 10.1126/science.286.5449.2528. [DOI] [PubMed] [Google Scholar]
- Bentley NJ, Holtzman DA, Flaggs G, Keegan KS, DeMaggio A, Ford JC, Hoekstra M, Carr AM. The Schizosaccharomyces pombe Rad3 checkpoint gene. EMBO J. 1996;15:6641–6651. [PMC free article] [PubMed] [Google Scholar]
- Blasina A, de Weyer IV, Laus MC, Luyten WH, Parker AE, McGowan CH. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr. Biol. 1999;9:1–10. doi: 10.1016/s0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- Blasina A, Paegle ES, McGowan CH. The role of inhibitory phosphorylation of Cdc2 following DNA replication block and radiation-induced damage in human cells. Mol. Biol. Cell. 1997;8:1013–1023. doi: 10.1091/mbc.8.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy MN, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- Boddy MN, Russell P. DNA replication checkpoint control. Front. Biosci. 1999;4:D841–D848. doi: 10.2741/boddy. [DOI] [PubMed] [Google Scholar]
- Brondello JM, Boddy MN, Furnari B, Russell P. Basis for the checkpoint signal specificity that regulates Chk1 and Cds1 protein kinases. Mol. Cell Biol. 1999;19:4262–42629. doi: 10.1128/mcb.19.6.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AL, Lee CH, Schwarz JK, Mitiku N, Piwnica-Worms H, Chung JH. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc. Natl. Acad. Sci. USA. 1999;96:3745–3750. doi: 10.1073/pnas.96.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- Busby EC, Leistritz DF, Abraham RT, Karnitz LM, Sarkaria JN. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 2000;60:2108–21012. [PubMed] [Google Scholar]
- Charles JF, Jaspersen SL, Tinker-Kulberg RL, Hwang L, Szidon A, Morgan DO. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr. Biol. 1998;8:497–507. doi: 10.1016/s0960-9822(98)70201-5. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, Zhang X, Annan RS, Lu Q, Faucette LF, et al. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- Chen P, Luo C, Deng Y, Ryan K, Register J, Margosiak S, Tempczyk-Russell A, Nguyen B, Myers P, Lundgren K, et al. The 1.7 A crystal structure of human cell cycle checkpoint kinase Chk1: implications for Chk1 regulation. Cell. 2000;100:681–692. doi: 10.1016/s0092-8674(00)80704-7. [DOI] [PubMed] [Google Scholar]
- Christensen PU, Bentley NJ, Martinho RG, Nielsen O, Carr AM. Mik1 levels accumulate in S phase and may mediate an intrinsic link between S phase and mitosis. Proc. Natl. Acad. Sci. USA. 2000;97:2579–25684. doi: 10.1073/pnas.97.6.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DJ, Segal M, Mondesert G, Reed SI. The Pds1 anaphase inhibitor and Mec1 kinase define distinct checkpoints coupling S phase with mitosis in budding yeast. Curr. Biol. 1999;9:365–368. doi: 10.1016/s0960-9822(99)80163-8. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O, Koshland D. The anaphase inhibitor of Saccharomyces cerevisiae Pds1 is a target of the DNA damage checkpoint pathway. Proc. Natl. Acad. Sci. USA. 1997;94:14361–14366. doi: 10.1073/pnas.94.26.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr. Opin. Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Corellou F, Bisgrove SR, Kropf DL, Meijer L, Kloareg B, Bouget F. A S/M DNA replication checkpoint prevents nuclear and cytoplasmic events of cell division including centrosomal axis alignment and inhibits activation of cyclin-dependent kinase-like proteins in fucoid zygotes. Development. 2000;127:1651–1660. doi: 10.1242/dev.127.8.1651. [DOI] [PubMed] [Google Scholar]
- Dasika GK, Lin SC, Zhao S, Sung P, Tomkinson A, Lee EY. DNA damage-induced cell cycle checkpoints and DNA strand break repair in development and tumorigenesis. Oncogene. 1999;18:7883–7899. doi: 10.1038/sj.onc.1203283. [DOI] [PubMed] [Google Scholar]
- Desany BA, Alcasabas AA, Bachant JB, Elledge SJ. Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 1998;12:2956–2970. doi: 10.1101/gad.12.18.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher D, Henckel J, Fersht AR, Jackson SP. The FHA domain is a modular phosphopeptide recognition motif. Mol. Cell. 1999;4:387–394. doi: 10.1016/s1097-2765(00)80340-8. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Fogarty P, Campbell SD, Abu-Shumays R, Phalle BS, Yu KR, Uy GL, Goldberg ML, Sullivan W. The Drosophila grapes gene is related to checkpoint gene Chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 1997;7:418–426. doi: 10.1016/s0960-9822(06)00189-8. [DOI] [PubMed] [Google Scholar]
- Furnari B, Blasina A, Boddy MN, McGowan CH, Russell P. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol. Biol. Cell. 1999;10:833–845. doi: 10.1091/mbc.10.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Garcia V, Salanoubat M, Choisne N, Tissier A. An ATM homologue from arabidopsis thaliana: complete genomic organisation and expression analysis. Nucl. Acids Res. 2000;28:1692–1699. doi: 10.1093/nar/28.8.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R, Putnam CW, Weinert T. RAD53, DUN1 and PDS1 define two parallel G2/M checkpoint pathways in budding yeast. EMBO J. 1999;18:3173–3185. doi: 10.1093/emboj/18.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves PR, Yu L, Schwarz JK, Gales J, Sausville EA, O’Connor PM, Piwnica-Worms H. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J. Biol. Chem. 2000;275:5600–5605. doi: 10.1074/jbc.275.8.5600. [DOI] [PubMed] [Google Scholar]
- Guo Z, Dunphy WG. Response of Xenopus cds1 in cell-free extracts to DNA templates with double-stranded ends. Mol. Biol. Cell. 2000;11:1535–1546. doi: 10.1091/mbc.11.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari KL, Santerre A, Sekelsky JJ, McKim KS, Boyd JB, Hawley RS. The mei-41 gene of D. melanogaster is a structural and functional homolog of the human ataxia telangiectasia gene. Cell. 1995;82:815–821. doi: 10.1016/0092-8674(95)90478-6. [DOI] [PubMed] [Google Scholar]
- Hartley KO, Gell D, Smith GC, Zhang H, Divecha N, Connelly MA, Admon A, Lees-Miller SP, Anderson CW, Jackson SP. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell. 1995;82:849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Bucher P. The FHA domain: a putative nuclear signaling domain found in protein kinases and transcription factors. Trends Biochem. Sci. 1995;20:347–349. doi: 10.1016/s0968-0004(00)89072-6. [DOI] [PubMed] [Google Scholar]
- Huang M, Zhou Z, Elledge SJ. The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell. 1998;94:595–605. doi: 10.1016/s0092-8674(00)81601-3. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Gilmartin A, Imburgia C, Winkler JD, Marshall LA, Roshak A. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 2000;60:566–572. [PubMed] [Google Scholar]
- Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Collins KM, Brown AL, Lee CH, Chung JH. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201–204. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- Lew DJ, Reed SI. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J. Cell Biol. 1995;129:739–749. doi: 10.1083/jcb.129.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ, Weinert TA, Pringle JR. Cell cycle control in Saccharomyces cerevisiae. In: Pringle JR, Broach JR, Jones WE, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces: Cell Cycle and Cell Biology. Cold Spring Harbor, New York: Cold Spring Harbor Press; 1997. pp. 607–696. [Google Scholar]
- Li X, Cai M. Inactivation of the cyclin-dependent kinase Cdc28 abrogates cell cycle arrest induced by DNA damage and disassembly of mitotic spindles in Saccharomyces cerevisiae. Mol. Cell Biol. 1997;17:2723–2734. doi: 10.1128/mcb.17.5.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Goh PY, Surana U. Spindle pole body separation in Saccharomyces cerevisiae requires dephosphorylation of the tyrosine 19 residue of Cdc28. Mol. Cell Biol. 1996;16:6385–6397. doi: 10.1128/mcb.16.11.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay HD, Griffiths DJ, Edwards RJ, Christensen PU, Murray JM, Osman F, Walworth N, Carr AM. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000a;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Vidanes G, Lin YC, Mori S, Siede W. Characterization of a Saccharomyces cerevisiae homologue of Schizosaccharomyces pombe Chk1 involved in DNA-damage-induced M-phase arrest. Mol. Gen. Genet. 2000b;262:1132–1146. doi: 10.1007/pl00008656. [DOI] [PubMed] [Google Scholar]
- Longhese MP, Foiani M, Muzi-Falconi M, Lucchini G, Plevani P. DNA damage checkpoint in budding yeast. EMBO J. 1998;17:5525–5528. doi: 10.1093/emboj/17.19.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona A, Furnari B, Mondesert O, Russell P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- Minshull J, Straight A, Rudner AD, Dernburg AF, Belmont A, Murray AW. Protein phosphatase 2A regulates MPF activity and sister chromatid cohesion in budding yeast. Curr. Biol. 1996;6:1609–1620. doi: 10.1016/s0960-9822(02)70784-7. [DOI] [PubMed] [Google Scholar]
- Murakami H, Okayama H. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature. 1995;374:817–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- Murray A, Hunt T. The Cell Cycle: an Introduction. Oxford: Oxford University Press; 1993. [Google Scholar]
- Nishijima H, Nishitani H, Seki T, Nishimoto T. A dual-specificity phosphatase Cdc25B is an unstable protein and triggers p34(Cdc2)/cyclin B activation in hamster BHK21 cells arrested with hydroxyurea. J. Cell Biol. 1997;138:1105–1116. doi: 10.1083/jcb.138.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting Cdc2 by Y15 phosphorylation. EMBO J. 1997;16 doi: 10.1093/emboj/16.3.545. 545-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Gabrielli B, Piwnica-Worms H. Purification of a serine kinase that associates with and phosphorylates human Cdc25C on serine 216. J. Biol. Chem. 1994;269:30461–30469. [PubMed] [Google Scholar]
- Pati D, Keller C, Groudine M, Plon SE. Reconstitution of a MEC1-independent checkpoint in yeast by expression of a novel human fork head cDNA. Mol. Cell Biol. 1997;17:3037–3046. doi: 10.1128/mcb.17.6.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicioli A, Lucca C, Liberi G, Marini F, Lopes M, Plevani P, Romano A, Di Fiore PP, Foiani M. Activation of Rad53 kinase in response to DNA damage and its effect in modulating phosphorylation of the lagging strand DNA polymerase. EMBO J. 1999;18:6561–6572. doi: 10.1093/emboj/18.22.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Ogg S, Thoma RS, Byrnes MJ, 3rd, Wu Z, Stephenson MT, Piwnica-Worms H. C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ. 1998;9:197–208. [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Price D, Rabinovitch S, O’Farrell PH, Campbell SD. Drosophila wee1 has an essential role in the nuclear divisions of early embryogenesis. Genetics. 2000;155:159–166. doi: 10.1093/genetics/155.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh JM, O’Connell MJ. The G2 DNA damage checkpoint targets both Wee1 and Cdc25. J. Cell Sci. 2000;113:1727–1736. doi: 10.1242/jcs.113.10.1727. [DOI] [PubMed] [Google Scholar]
- Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- Rhind N, Russell P. Mitotic DNA damage and replication checkpoints in yeast. Curr. Opin. Cell Biol. 1998a;10:749–758. doi: 10.1016/s0955-0674(98)80118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N, Russell P. Tyrosine phosphorylation of Cdc2 is required for the replication checkpoint in Schizosaccharomyces pombe. Mol. Cell Biol. 1998b;18:3782–3787. doi: 10.1128/mcb.18.7.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y, Bachant J, Wang H, Hu F, Liu D, Tetzlaff M, Elledge SJ. Control of the DNA damage checkpoint by Chk1 and rad53 protein kinases through distinct mechanisms. Science. 1999;286:1166–1171. doi: 10.1126/science.286.5442.1166. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Santocanale C, Diffley JF. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature. 1998;395:615–618. doi: 10.1038/27001. [DOI] [PubMed] [Google Scholar]
- Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- Shirahige K, Hori Y, Shiraishi K, Yamashita M, Takahashi K, Obuse C, Tsurimoto T, Yoshikawa H. Regulation of DNA-replication origins during cell-cycle progression. Nature. 1998;395:618–621. doi: 10.1038/27007. [DOI] [PubMed] [Google Scholar]
- Sibon OC, Laurencon A, Hawley R, Theurkauf WE. The Drosophila ATM homologue Mei-41 has an essential checkpoint function at the midblastula transition. Curr. Biol. 1999;9:302–312. doi: 10.1016/s0960-9822(99)80138-9. [DOI] [PubMed] [Google Scholar]
- Sibon OC, Stevenson VA, Theurkauf WE. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature. 1997;388:93–97. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]
- Sidorova JM, Breeden LL. Rad53-dependent phosphorylation of Swi6 and down-regulation of CLN1 and CLN2 transcription occur in response to DNA damage in Saccharomyces cerevisiae. Genes Dev. 1997;11:3032–3045. doi: 10.1101/gad.11.22.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siede W, Friedberg AS, Dianova I, Friedberg EC. Characterization of G1 checkpoint control in the yeast Saccharomyces cerevisiae following exposure to DNA-damaging agents. Genetics. 1994;138:271–281. doi: 10.1093/genetics/138.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TT, Campbell SD, O’Farrell PH. Drosophila grapes/Chk1 mutants are defective in cyclin proteolysis and coordination of mitotic events. Curr. Biol. 1999;9:919–922. doi: 10.1016/s0960-9822(99)80399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Hsiao J, Fay DS, Stern DF. Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science. 1998;281:272–274. doi: 10.1126/science.281.5374.272. [DOI] [PubMed] [Google Scholar]
- Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M, Nakayama K. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- Toczyski DP, Galgoczy DJ, Hartwell LH. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell. 1997;90:1097–1106. doi: 10.1016/s0092-8674(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Tominaga K, Morisaki H, Kaneko Y, Fujimoto A, Tanaka T, Ohtsubo M, Hirai M, Okayama H, Ikeda K, Nakanishi M. Role of human Cds1 (Chk2) kinase in DNA damage checkpoint and its regulation by p53. J. Biol. Chem. 1999;274:31463–31467. doi: 10.1074/jbc.274.44.31463. [DOI] [PubMed] [Google Scholar]
- Wahl GM, Linke SP, Paulson TG, Huang LC. Maintaining genetic stability through TP53 mediated checkpoint control. Cancer Surv. 1997;29:183–219. [PubMed] [Google Scholar]
- Walworth N, Davey S, Beach D. Fission yeast Chk1 protein kinase links the rad checkpoint pathway to Cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- Walworth NC, Bernards R. rad-dependent response of the Chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- Weinert T. A DNA damage checkpoint meets the cell cycle engine. Science. 1997;277:1450–1451. doi: 10.1126/science.277.5331.1450. [DOI] [PubMed] [Google Scholar]
- Weinert TA, Kiser GL, Hartwell LH. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- Westphal CH. Cell-cycle signaling: Atm displays its many talents. Curr. Biol. 1997;7:R789–R792. doi: 10.1016/s0960-9822(06)00406-4. [DOI] [PubMed] [Google Scholar]
- Wright JA, Keegan KS, Herendeen DR, Bentley NJ, Carr AM, Hoekstra MF, Concannon P. Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and abrogate cell cycle checkpoint control. Proc. Natl. Acad. Sci. USA. 1998;95:7445–7450. doi: 10.1073/pnas.95.13.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Guacci V, Koshland D. Pds1, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s) J. Cell Biol. 1996;133:99–110. doi: 10.1083/jcb.133.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian VA. ATM-Related Genes: What Do They Tell Us about Functions of the Human Gene? Cell. 1995;82:685–687. doi: 10.1016/0092-8674(95)90463-8. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Piwnica-Worms H. DNA damage and replication checkpoints in fission yeast require nuclear exclusion of the Cdc25 phosphatase via 14-3-3 binding. Mol. Cell Biol. 1999;19:7410–7419. doi: 10.1128/mcb.19.11.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Elledge SJ. DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell. 1993;75:1119–1127. doi: 10.1016/0092-8674(93)90321-g. [DOI] [PubMed] [Google Scholar]