Abstract
Objective
Stimulating the human cortex using transcranial magnetic stimulation (TMS) temporarily reduces clinical and experimental pain, however, it is unclear which cortical targets are the most effective. The motor cortex has been a popular target for managing neuropathic pain, while the prefrontal cortex has been investigated for an array of nociceptive pain conditions. It is unclear whether the motor cortex is the only effective cortical target for managing neuropathic pain, and no published studies to date have investigated the effects of prefrontal stimulation on neuropathic pain.
Design
This preliminary pilot trial employed a sham-controlled, within-subject, cross-over design to evaluate clinical pain as well as laboratory pain thresholds among four patients with chronic neuropathic pain. Each participant underwent three real and three sham 20-minute sessions of 10Hz left prefrontal rTMS. Daily pain diaries were collected for 3-weeks before and after each treatment phase along with a battery of self-report pain and mood questionnaires.
Results
Time-series analysis at the individual patient level indicated that real TMS was associated with significant improvements in average daily pain in 3 of the 4 participants. These effects were independent of changes in mood in 2 of the participants. At the group level, a decrease of 19% in daily pain on average, pain at its worst, and pain at its least was observed while controlling for changes in mood, activity-level and sleep. The effects of real TMS were significantly greater than sham. Real TMS was associated with increases in thermal and mechanical pain thresholds whereas sham was not. No statistically significant effects were observed across the questionnaire data.
Conclusions
The prefrontal cortex may be an important TMS cortical target for managing certain types of pain, including certain neuropathic pain syndromes.
Introduction
Transcranial magnetic stimulation (TMS) is a relatively noninvasive brain stimulation technology that can focally stimulate an awake individual's brain (1,2) via a localized pulsed magnetic field (3). TMS is capable of focally stimulating the cortex by depolarizing superficial neurons (4,5). TMS at different intensities, frequencies and coil angles can excite several elements (e.g., cell bodies, axons) of various neuronal groups (e.g., interneurons, neurons projecting into other cortical areas; 6-8). When TMS pulses are delivered repeatedly, it is referred to as repetitive transcranial magnetic stimulation (rTMS).
To date most studies of TMS effects on pain perception have focused on stimulation of either the motor cortex or the prefrontal cortex. Several studies have found that rTMS delivered over motor cortex can affect the perception of laboratory-induced pain in healthy adults, as well as chronic neuropathic pain in clinical samples (9-20). A recent study demonstrated that the analgesic effects can be sustained for at least 15 days following 3 days of motor cortex rTMS (21) in patients with chronic neuropathic pain. There is some evidence that slow rTMS (1Hz or less) is not as effective as fast rTMS (e.g., 20Hz) in reducing neuropathic pain (22,23). Further, response to 20Hz rTMS has been found to be predictive of response to implanted electrical stimulators over the motor cortex. In addition to neurpoathic pain, there is some evidence that motor rTMS can reduce pain and improve quality of life in patients with fribromyalgia (24).
A few studies have demonstrated analgesic effects with prefrontal cortex rTMS as well. In a published case report, Reid and Pridmore (25) used fast rTMS over the left prefrontal cortex to significantly reduce reported pain experience in a single patient with chronic facial pain. A recent large-scale controlled study investigated the effects of rTMS over the dorsolateral prefrontal cortex on pain tolerance in healthy adults. Slow rTMS (1Hz) over the right prefrontal cortex has been associated with increased cold presser tolerance (26). Recently, Borckardt et al (27) found that 15 minutes of fast, left prefrontal TMS increased thermal pain thresholds in healthy adults. There is some evidence that fast prefrontal rTMS can significantly reduced migraine frequency, headache pain, and amount of medication used during and in the month after rTMS (28). Sampson et al (29) reported a significant reduction in pain among 4 patients with fibromyalgia following slow rTMS over the right prefrontal cortex, and Avery et al (30) found that fast rTMS over the left prefrontal cortex was associated with a significant decrease in muscle, bone, and joint pain among patients undergoing rTMS for depression. Lastly, It has also been shown that fast left-prefrontal rTMS, when delivered immediately following gastric-bypass surgery, can reduce pain reports as well as the amount of patient controlled analgesia use (31,32).
The role of the left prefrontal cortex in pain control is unclear, however there is evidence to support the concept that left prefrontal activation is negatively correlated with pain unpleasantness (33) suggesting a potential governing role of the prefrontal cortex on pain perception. Chronic back pain has been associated with decreased prefrontal gray matter density (34), and diffusion tensor imaging technology has been used to uncover anatomical circuitry connecting the prefrontal cortex with both the nucleus cuneiformis and periaquaductal gray supporting the potential role of the prefrontal cortex in the functionally characterized top-down pain inhibitory system (35). Thus, prefrontal activation might result in analgesic effects, presumably by modulating limbic response to pain. These findings, along with some preliminary work demonstrating analgesic effects of prefrontal TMS in healthy adults and in patients with nonneuropathic pain conditions, seem to warrant an investigation as to whether the prefrontal cortex is a reasonable TMS target for controlling neuropathic pain.
To date, no controlled studies have been published on the effects of fast left-prefrontal rTMS on chronic neuropathic pain, and with respect to laboratory pain measures, most studies of TMS have been conducted on healthy adults. There is little information available on the effects of prefrontal rTMS on thermal or mechanical pain thresholds among patients with chronic neuropathic pain. Because prefrontal TMS may affect cortical and subcortical structures involved in pain perception independent of pain etiology (neuropathic or nociceptive), it was hypothesized that stimulation of the prefrontal cortex would significantly reduce clinical pain as well as increase both thermal and mechanical pain thresholds among patients with chronic neuropathic pain. The present single-blind, sham-controlled, within-subject cross-over pilot study evaluates the effects of 3-sessions of 10Hz left prefrontal rTMS on pain, mood, functioning, and laboratory pain thresholds among four patients with chronic neuropathic pain.
Methods
Participants
This pilot study was approved by the Institutional Review Board in the Office of Research Integrity at the Medical University of South Carolina. Four patients (3 women) with chronic neuropathic pain of various etiologies volunteered to participate in the study. Participant information is provided in table 1. Participants were diagnosed and referred for the study by their outpatient treating Anesthesiologist (ARS) at MUSC. Participants were eligible to participate if they had any type of chronic neuropathic pain (1-year or longer), no family history of epilepsy, no history of seizures, no implanted medical devices, no history of tumors or brain tissue abnormalities, no implanted metal in the head, neck or chest, and were not taking any medications known to lower seizure threshold.
Table 1.
Characteristics of patients enrolled in this trial.
| Subject Number | Age | Sex | Diagnosis | Duration | Medications | Randomization |
|---|---|---|---|---|---|---|
| 1 | 58 | F | -Mandibular | 12 Years | Duloxetine | Sham First |
| |
|
|
Neuropathy |
|
Pregabalin |
Real Second |
| 2 | 41 | F | -Ilioinguinal | 5 Years | Morphine | Real First |
| Neuropathy | Sulfate | Sham Second | ||||
| Pregabalin | ||||||
| Tramadol | ||||||
| |
|
|
|
|
Hydrocodone |
|
| 3 | 52 | F | -Diabetic | 12 Years | Carisoprodol | Sham First |
| Peripheral | Pregabalin | Real Second | ||||
| Neuropathy | Meperidine | |||||
| -Atypical Facial | Promethazine | |||||
| |
|
|
Pain |
|
|
|
| 4 | 33 | M | -Mandibular | 12 Years | Methadose | Real First |
| Neuropathy | Sham Second |
Dependent Measures
DAILY PAIN DIARY RATINGS
Participants completed paper-and-pencil pain diaries at bedtime every day for 3-weeks prior to the first TMS session, throughout all real and sham TMS treatment phases, and for 3-weeks following the last TMS session. Each day, participants were asked to indicate their “Pain on average,” “Pain at its least,” and “Pain at its worst” using an 11-point numeric rating scale where “0=no pain at all” and “10=worst pain imaginable.” Additionally they were asked to indicate their average mood rating each day using an 11-point Likert scale where “0=extremely agitated/depressed” and “10=no depression/agitation.” Participants were also asked to record the number of hours of sleep they had the previous night, and the number of pain medications (pills; prn's and daily medications) taken each day. Lastly participants were asked to rate their level of activity each day using an 11-point Likert where “0=not active at all” and “10=extremely active day.”
QUESTIONNAIRE DATA
Participants completed the Neuropathy Pain Scale (NPS; 36), the Brief Pain Inventory (BPI; 37-39), the Beck Depression Inventory (BDI; 40-44), and the Center For Epidemiological Studies 10-Item Depression Scale (CESD; 45) at baseline, before the first TMS session, after the 3rd TMS session, before the 4th TMS-session, after the 6th TMS session, and at 3-week follow-up. Mean total scores were used from each scale for statistical analyses. For the BPI, the mean of the functional impairment items was used.
THERMAL PAIN THRESHOLD ASSESSMENT
Cutaneous heat stimuli were delivered via a TSA-II Neurosensory Analyzer (Medoc, USA) using a 30mm × 30mm contact thermode attached to the volar forearm of each subject's left arm 5 cm from the wrist. The Method of Limits was used (46). Each Subject's skin was allowed to adapt to a temperature of 32° Celsius for 1 minute. The thermode was then programmed to heat at the rate of 1° C per second until the subject indicated that the “sensation reached a level that they considered to be painful” by pressing a button attached to the heating device (stopping the stimulus). The first trial of 10 was discarded and the average of the subsequent 10 trials was used to represent thermal pain threshold. A minimum of 30 seconds elapsed between trials.
MECHANICAL PAIN THRESHOLD ASSESSMENT
An Electronic von Frey Anesthesiometer (IITC Life Science; Woodland Hills, CA, USA) with rigid tips was used to assess mechanical pain threshold. Increasing pressure was applied to the distal phalanx of the digiti minimi of the left hand at the rate of 10 grams per second until participants verbally indicated that the sensation had “reached the level they considered to be painful.” The Anesthesiometer displayed the maximum pressure exerted (point at which participants indicated pain threshold had been reached) during each trial, and this value was recorded to a database. The first trial (of 10) was discarded and the average of the subsequent 9 trials was used to represent mechanical threshold for each subject. For each trial, the point of stimulation was systematically varied within a 1cm × 1cm square so that stimulation never occurred in the same spot more than once.
Procedures
DESIGN AND VISITS
A single-blind, sham-controlled, within-subject, cross-over design was employed. All participants received a series of 3 real rTMS treatments and a series of 3 sham rTMS treatments separated by 3 weeks. The order (real first or sham first) was randomized and participants were blinded to whether they were receiving real or sham rTMS.
Participants made 8 visits to the Medical University of South Carolina during the study. Visit-1 involved a screening interview that was conducted to determine eligibility to receive rTMS, and collection of a high-resolution, T1-weighted structural MRI scan (to be used for prefrontal cortex location). Also at visit-1, participants completed all of the questionnaires. Participants then completed daily pain diaries for 3 weeks. The next three visits (visits 2, 3, and 4) were made within a 5-day period and participants received either 3 real or 3 sham rTMS sessions (randomly determined). Prior to the first rTMS treatment and after the third, participants completed the questionnaires and underwent laboratory pain testing. Participants completed the pain diaries daily during the treatment periods, and then continued to complete daily pain diaries for 3 weeks.
Participants then returned for another 3 treatment sessions (visits 5, 6, and 7) within a 5-day period (3 real or 3 sham treatments; cross-over from previous treatment period). Prior to the fourth rTMS treatment and after the sixth, participants completed the questionnaires and underwent laboratory pain testing just as before. They completed daily pain diaries for an additional 3 week-period, and then had one follow-up visit (visit 8) during which they completed the questionnaires and laboratory pain measures one last time. At the 8th visit, participants were forced to guess which treatment period was real and which was sham.
TMS PROCEDURES
Subjects underwent a standard resting motor threshold assessment at each visit using a Neotonus Neopulse TMS machine with a figure-8 coil. The TMS machine was set initially to 40% of maximal output and 0.5Hz frequency and the TMS administrator located the area of the scalp that produced visible thumb movement upon TMS stimulation by systematically moving the coil around the scalp while making adjustments to the TMS intensity. Next, adaptive Parameter Estimation by Sequential Testing (PEST;47) procedures were conducted with the aid of custom-developed software to determine the amount of machine output necessary to produce visible thumb movement 50% of the time (resting motor threshold; rMT) (48,49).
LOCATION OF LEFT PREFRONTAL CORTEX
The Brainsight TMS frameless sterotaxic system (v1.7b6; Rogue Research Inc, USA) was used to locate the left prefrontal cortex for each participant. Each participant's structural MRI image was imported into Brainsight and registered to standard (MNI) space. A custom-developed program (by the first author) was used to determine the center of mass of left BA9 as well as the shortest distance from the center of BA9 to the surface of the cortex. Each participant's structural image was marked with trajectories within Brainsight at the point corresponding with the cortical surface target with the shortest distance to the center of BA9. The Brainsight headband was attached to each participant and they were registered to the system. The Brainsight pointer and targeting system was used to identify each participants scalp location corresponding with the appropriate marked trajectory to BA9.
TREATMENT PARAMETERS
TMS was delivered at 10Hz, 100% of resting motor threshold, 10 seconds-on, 20 seconds-off for 20 minutes (4000 pulses) for each of the TMS sessions. Participants underwent the same motor threshold assessment and treatment procedures on each of the 3 separate visits within 5 consecutive days (treatment-period). Questionnaires and laboratory pain measures were administered prior to TMS on the first day of each treatment-period, and after TMS on the last day of each treatment-period. All participants underwent both a real and a sham treatment-period separated by 3 weeks and the order of treatments (sham versus real) was randomized (2 participants received the 3 real TMS treatments first followed by the 3 sham TMS treatments 3-weeks later, and the other 2 participants received the 3 sham TMS treatments first, followed by the real treatments 3-weeks later).
Sham rTMS was conducted with a specially-designed Neuronetics sham-coil that looks and sounds identical to the active coil except that a hidden aluminum plate blocks actual stimulation from occurring.
Statistical Analyses
For laboratory data analyses, Hierarchical Linear Modeling was used and the model error-structure was first-order autoregressive (AR1). All participants’ intercepts were entered into the model as random effects at level-1. This technique is a powerful approach to handling time-series data when observations are serially dependent over time (see Singer (50) for details on the use of HLM).
For pain diary data, time-series analyses were conducted at the individual patient level using Autoregressive Integrated Moving Average (ARIMA: 1,0,0) models to partial-out the influence of lag-1 autocorrelation. Average daily pain ratings were modeled against a numeric phase variable representing the 3 different phases of the study (baseline=0, real TMS=1, and sham TMS=0). This independent variable was designed to determine if real TMS was associated with a change in average daily pain relative to both baseline and sham TMS phases. Further, daily mood ratings were included in the model such that any observed changes in pain perception would be observed over and above potential TMS effects on mood. Lastly, HLM was used to examine overall effects of real and sham TMS on all diary variables at the group level.
For questionnaire data, paired-sample t-tests were used to compare pre and post TMS scores for both real and sham conditions.
Results
INTEGRITY OF THE BLIND
Two of the four participants (50%) correctly guessed which treatment periods were real and sham, which is equal to chance. All 4 of the participants initially said that they did not know which was which, and it was not until they were pushed to “make a guess” that they were able to offer an opinion about which sessions were real and which were sham.
LABORATORY PAIN DATA
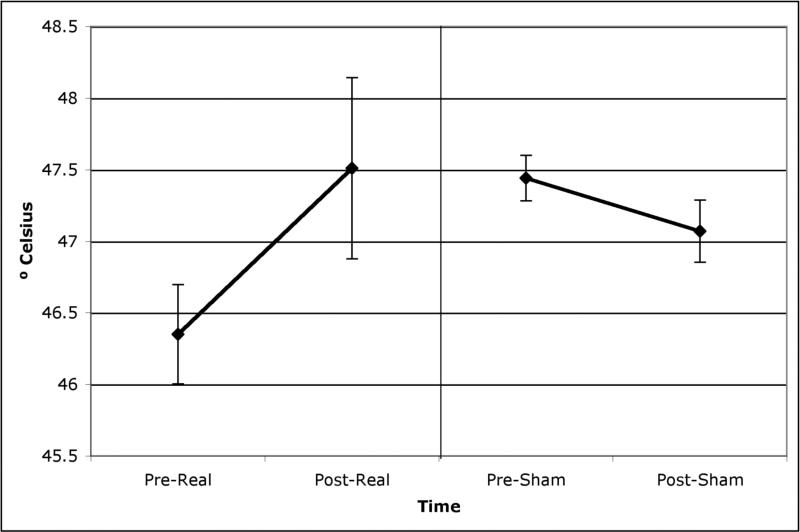
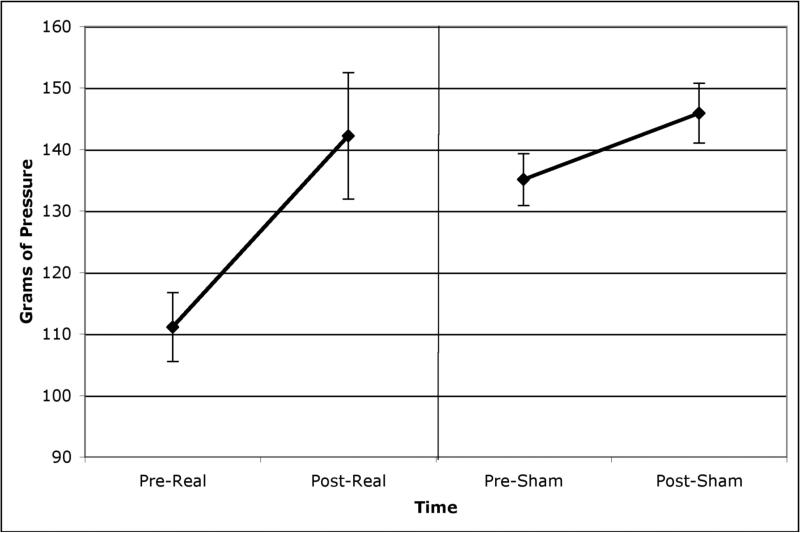
Figures 1 and 2 show the mean (and 95% confidence intervals) thermal and mechanical pain thresholds for participants pre and post both real and sham rTMS. Real TMS was associated with a significant increase in thermal pain thresholds (t(73)=2.85, p=.006) and in mechanical pain thresholds (t(72)=2.35, p=.02). Sham TMS was not associated with any significant change in thermal or mechanical thresholds (t(73)=1.32, p=.19; t(73)=1.78, p=.08).
Figure 1.
Thermal pain thresholds (mean and 95% confidence intervals) pre and post both real and sham rTMS.
Figure 2.
Mechanical pain thresholds (mean and 95% confidence intervals) pre and post both real and sham rTMS.
PAIN DIARY DATA
Patient 1 - Mandibular Neuropathy
A significant effect for active prefrontal TMS was observed on average daily pain ratings (model estimate=-1.42, SE=.45, p=.002) over and above effects of autocorrelation (model estimate=.34, SE=.12, p=.006). However the effects of active TMS on pain were not independent of changes in daily mood. For this patient, when mood-ratings were included in the model, the effects of rTMS on pain ratings were rendered statistically insignificant (model estimate=-.76, SE=.46, p=.10). The mean of the patient's daily pain ratings during the baseline phase was 5.62 out of 10. During the active TMS phase the mean was 3.93 (a 30% reduction) and during the sham TMS phase it was 4.95 (a 12% reduction from baseline).
Patient 2 - Ilioinguinal Neuropathy
A significant effect for active prefrontal TMS was observed on average daily pain (model estimate=-1.15, SE=.39, p=.005) over and above autocorrelation (model estimate=.71, SE=.09, p<.001) and effects on mood (model estimate=.46, SE=.15, p=.005). Mean daily pain ratings during baseline was 7.52 out of 10. The mean during the active TMS phase was 5.41 (a 28% reduction) and during the sham TMS phase it was 5.74 (a 24% reduction from baseline).
Patient 3: Diabetic Peripheral Neuropathy and Atypical Facial Pain
A significant effect was observed for active prefrontal TMS on average daily pain ratings (model estimate=-1.64, SE=.37, p<.001) over and above autocorrelation (model estimate=.46, SE=.12, p<.001) and effects on daily mood ratings (model estimate=.01, SE=2.31, p>.99) which was not related to average daily pain. Mean of daily pain ratings during the baseline phase was 5.29 out of 10. During the active TMS phase the mean was 4.05 (a 23% reduction), and during the sham TMS phase it was 6.57 (a 24% increase over baseline).
Patient 4: Mandibular Neuropathy
There was no variability in this patients pain or mood ratings during the entire trial. Thus, no effects were observed for either active or sham TMS on pain or mood.
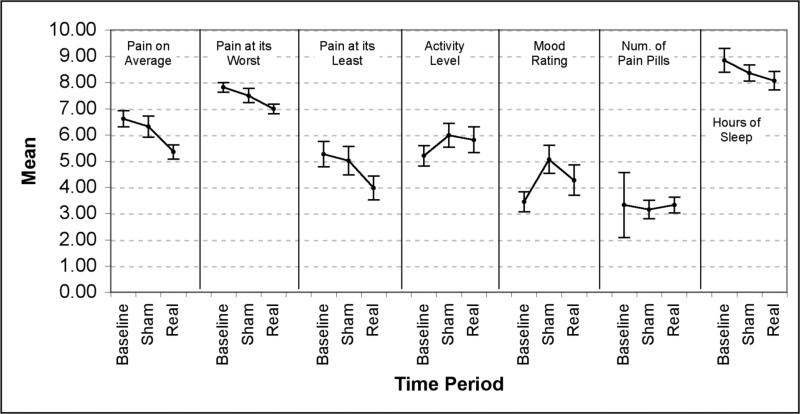
For the HLM analyses of diary data at the group level, Bonferroni correction was used to adjust critical alpha multiple comparisons (alpha=.002). Table 2 shows the means and standard errors for each diary variable and for each study period along with the p-values from the hierarchical linear models. Figure 3 shows the mean (and 95% CI) ratings for each of the diary variables across each phase of the study. Real TMS was associated with significant decreases in average pain, pain at its worst and pain at its least (t(158)=12.34, p<.0001; t(159)=7.86, p<.0001; and t(158)=10.22, p<.0001 respectively) over and above effects on activity, mood and sleep. Sham TMS was also associated with decreases in average pain (t(159)=2.92, p=.004) and pain at its worst (t(160)=3.04, p=.003), but not pain at its least (t(158)=1.33, p=.19) over and above effects on activity, mood and sleep. However, real TMS had significantly larger effects on average pain (t(161)=5.79, p<.0001), pain at its worst (t(162)=2.97, p=.003), and pain at its least (t(160)=6.68, p<.0001) than sham TMS.
Table 2.
Means (and StdErr) along with p-values from the Hierarchical Linear Models evaluating the effects of real and sham rTMS on daily pain diary data. Critical alpha was set to .002 (Bonferroni corrected) to correct for type-I error inflation from multiple comparisons.
| Mean(StdErr) | Pre-Post Real | Pre-Post Sham | Real-Sham | |||
|---|---|---|---|---|---|---|
| Dependent Measure | Baseline | Sham | Real | p-value | p-value | p-value |
| Pain on Average | 6.61(.16) | 6.32(.21) | 5.35(.14) | p<.0001* | p=.040 | p<.0001* |
| Pain at its Worst | 7.81(.10) | 7.50(.14) | 6.99(.09) | p<.0001* | p=.007 | p<.0001* |
| Pain at its Least | 5.26(.25) | 5.01(.28) | 3.98(.23) | p<.0001* | p=.131 | p<.0001* |
| Activity Rating | 5.20(.20) | 5.99(.23) | 5.81(.25) | p=.006 | p<.0001* | p=.3587 |
| Average Mood Rating | 3.45(.20) | 5.06(.27) | 4.27(.30) | p=.007 | p<.0001* | p=.001* |
| Number of Pain Pills Taken | 3.32(.63) | 3.15(.18) | 3.32(.16) | p=1.00 | p=.785 | p=.022 |
| Hours of Sleep | 8.84(.23) | 8.36(.16) | 8.06(.18) | p=.0004* | p=.029 | p=.099 |
p<.002
Figure 3.
Summary of effects (means and 95% confidence intervals) of real and sham TMS across all daily diary measures employed.
QUESTIONNAIRE DATA
No statistically significant effects were seen on any of the questionnaire data across any of the treatment conditions. Table 3 shows the means (and 95% confidence intervals) for each scale at each measurement time-point in the study. Real TMS was associated with a 14% reduction in NPS scores, a 43% reduction in CESD scores, and a 44% reduction in BDI scores. These were all greater than the reductions associated with sham TMS (4%, 10%, and 19%, respectively). Sham TMS was associated with a 12% improvement on BPI functionality scores whereas real TMS was only associated with 6% improvement.
Table 3.
Mean (and 1.96 standard error values i.e., 95% confidence intervals) for all questionnaire data collected at each assessment point during the study.
| Baseline | Pre-Real | Post-Real | Pre-Sham | Post-Sham | Follow-Up | |
|---|---|---|---|---|---|---|
| Mean 95%CI | Mean 95%CI | Mean 95%CI | Mean 95%CI | Mean 95%CI | Mean 95%CI | |
| BPI | 6.0 +/-1.8 | 6.1 +/-1.1 | 5.7 +/-1.5 | 6.0 +/-1.1 | 5.3 +/-0.8 | 5.2 +/-2.5 |
| NPS | 6.0 +/-0.7 | 5.8 +/-0.9 | 5.0 +/-1.3 | 5.2 +/-1.4 | 4.9 +/-1.9 | 5.1 +/-1.2 |
| CESD | 14.8 +/-4.9 | 21.5 +/-13.2 | 12.3 +/-3.5 | 13.3 +/-4.6 | 12.0 +/-5.5 | 12.8 +/-4.5 |
| BDI | 20.5 +/-12.1 | 29.0 +/-2.8 | 16.3 +/-9.3 | 21.0 +/-9.9 | 17.0 +/-8.3 | 16.0 +/-9.2 |
Brief Pain Inventory (BPI); Neuropathy Pain Scale (NPS); Center for Epidemiological Studies 10-item Depression Scale (CESD); Beck Depression Inventory (BDI)
Discussion
A series of three 20-minute sessions of 10 Hz rTMS over the left prefrontal cortex within one week (12000 pulses) was associated with a 19% decrease in daily average pain on average lasting ~ 2 weeks in 4 participants with chronic neuropathic pain. In 2 participants, prefrontal TMS was associated with a significant reduction in average daily pain over and above effects on mood. In 1 participant, the significant decrease in average daily pain was not independent of changes in mood. In these 3 participants, real rTMS was superior to sham TMS stimulation with respect to improving daily pain and mood ratings. One participant experienced no change in pain or mood during the trial. Overall, real TMS was associated with increased thermal and mechanical pain thresholds, whereas sham was not, however, no significant effects were observed on the NPS, BPI, BDI or CESD scales.
While much research has been done on the effects of motor cortex stimulation via TMS on neuropathic pain, little work has focused on the potential role of prefrontal TMS in the management of neuropathic pain conditions. The present findings, taken together, are interesting and novel inasmuch as they suggest that future work is warranted to develop a better understanding of the role of the prefrontal cortex in pain perception as well as to determine optimal cortical targets and stimulation parameters for managing chronic neuropathic pain via TMS.
One important consideration dictating the potential for TMS to be used as a bonafide treatment for chronic pain is the duration of any analgesic effects. TMS effects on pain are generally short lived. Findings from this trial and from the Kehdr et al (18) trial suggest that 3 to 5 TMS sessions are associated with about 2 weeks of pain relief. These findings are in contrast to earlier work suggesting very short-lived response (< 1 hour) following a single session. Given these findings, it would seem that more TMS sessions might lead to longer-lasting analgesic effects. However, Avery et al (30) delivered 15 sessions of TMS but noted that analgesic effects began to disappear 1 week after the last TMS treatment. In the Avery et al (30) study though, participants were receiving TMS primarily as a treatment for depression. The patients with co-morbid chronic pain were identified retrospectively from the sample of depressed patients, and methods used to measure pain were secondary to depression measures. It may be that longer-term analgesic TMS effects can be achieved with longer treatment courses among patients presenting with chronic pain as a primary complaint, and longer-term effects may be detected when more conventional pain measurement techniques are employed.
Although the results from this pilot trial are promising, this study has a number of limitations. First, the sample size is small, and thus the accuracy of any estimates of the true effect-size of prefrontal rTMS on neuropathic pain drawn from this study may be limited. Additionally, questions about generalizability are often unanswerable when sample-sizes are small.
There is emerging evidence that many people experience repetitive TMS as mildly to moderately painful, whereas sham TMS (when administered in the manner employed in this study) is typically less bothersome to participants. It is possible that the painfulness of real TMS may lead to changes in pain perception due to activation of central and peripheral endogenous pain regulating mechanisms. While, it seems highly unlikely that such mechanisms could explain a 2-week long 19% reduction in pain, it is possible that the painfulness of real TMS could have influenced the more immediate laboratory measures of pain threshold. However, participants in this study had a difficult time determining which sessions were real and which were sham. Future studies should employ sham conditions that are matched to real TMS with respect to painfulness and unpleasantness in order to rule out a potentially problematic confound.
No statistically significant effects were noted across any of the questionnaire data. Real TMS was associated with a 14% reduction in NPS scores, a 43% reduction in CESD scores, and a 44% reduction in BDI scores. These were all greater than the reductions associated with sham TMS. There was likely not enough power due to limited n-size for traditional paired sample t-tests to detect differences.
Because of the nature of the within-subject cross-over design, the gradual nature of clinical improvement and return to baseline functioning, and the largely unknown duration of TMS effects on pain perception, it is possible for carry-over effects to have muddied the available inferences in this study. It is unclear why there were apparent differences in mechanical and thermal thresholds before both the real and sham TMS treatment-periods, however, it is possible that carryover effects contributed to this phenomenon. Future studies employing this type of design for evaluating the effects of rTMS on chronic pain should consider allowing more time to pass between treatment phases in order to ensure all participants ample time to return to baseline functioning (both clinically and with respect to laboratory measures) before initiating a new treatment.
Another limitation of the present study is that the patient population was heterogenous with respect to the type of neuropathic pain disorder. This makes it difficult to generalize the findings in a clinically useful fashion. However, the goal of this preliminary pilot was to determine if prefrontal rTMS would have any impact at all on neuropathic pain, and it appears to be associated with a noticeable effect. Nonetheless, future investigations would do well to focus on specific types of neuropathic pain in order to begin to develop a list of disorders for which TMS might be beneficial.
The prefrontal cortex may be a promising TMS target for reducing pain in neuropathic, rheumatologic and post-surgical populations although the mechanisms by which it might work remain unclear. Future studies are needed to determine TMS treatment parameters and cortical targets that can optimize the effect and its duration, as well as begin to elucidate potential mechanisms of action.
Acknowledgments
Disclosures: Dr. Borckardt receives research funding from the National Institute for Neurological Disorders and Stroke at NIH, Cyberonics Inc, the Neurosciences Institute at MUSC, and is a consultant for Neuropace, however he has no equity ownership in any device or pharmaceutical company. Dr. George receives research funding from the National Institute for Mental Health, NIDA, and NIAAA at NIH, Jazz Pharmaceuticals, GlaxoSmithKline, and Cyberonics Inc. He is a consultant for Aspect Biomedical, Argolyn, Aventis, Abbott, Bristol-Meyers Squibb, Cephos, Cyberonics, and Neuropace, and , however he has no equity ownership in any device or pharmaceutical company. Dr. Nahas receives research funding from the National Institute for Mental Health at NIH, and Cyberonics Ind, and is a consultant for Neuropace. Dr. Kozel receives research funding from the National Institute for Mental Health at NIH and the US Department of Defense. MUSC has filed 6 patents or invention disclosures in one or more of the authors’ names regarding brain imaging and stimulation.
References
- 1.George MS, Nahas Z, Kozel FA, et al. Mechanisms and the current state of transcranial magnetic stimulation. CNS Spectrums. 2003;8(7):496–514. doi: 10.1017/s1092852900018976. [DOI] [PubMed] [Google Scholar]
- 2.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of the human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 3.Barker AT, Freeston IL, Jarratt JA, Jalinous R. Magnetic stimulation of the human nervous system: an introduction and basic principles. In: Chokroverty S, editor. Magnetic Stimulation in Clinical Neurophysiology. Butterworth's; Boston, Mass: 1989. pp. 55–72. [Google Scholar]
- 4.George MS, Belmaker RH. Transcranial magnetic stimulation. In: George MS, Belmaker RH, editors. Neuropsychiatry. 1st ed. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- 5.George MS, Lisanby SH, Sackeim HA. transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry. 1999;56:300–311. doi: 10.1001/archpsyc.56.4.300. [DOI] [PubMed] [Google Scholar]
- 6.Roth BJ, Saypol JM, Hallett M, Cohen LG. A Theoretical calculation of the electric field induced in the cortex during magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:47–56. doi: 10.1016/0168-5597(91)90103-5. [DOI] [PubMed] [Google Scholar]
- 7.Amassian VE, Eberle L, Maccabee PJ, Cracco RQ. Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fiber bending in excitation. Electroencephalogr Clin Neurophysiol. 1992;85:291–301. doi: 10.1016/0168-5597(92)90105-k. [DOI] [PubMed] [Google Scholar]
- 8.Davey KR, Cheng CH, Epstein CM. Prediction of magnetically induced electric fields in biologic tissue. IEEE Trans Biomed Eng. 1991;38:418–422. doi: 10.1109/10.81560. [DOI] [PubMed] [Google Scholar]
- 9.Migita K, Uozumi T, Arita K, Monden S. Transcranial magnetic coil stimulation of motor cortex in patients with central pain. Neurosurgery. 1995;36:1037–9. doi: 10.1227/00006123-199505000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Rollnik JD, Wustefeld S, Dauper J, Karst M, Fink M, Kossev A, Dengler R. Repetitive transcranial magnetic stimulation for the treatment of chronic pain-a pilot study. Eur Neurol. 2002;48:6–10. doi: 10.1159/000064950. [DOI] [PubMed] [Google Scholar]
- 11.Lefaucheur J-P, Drouot X, Keravel Y, Nguyen J-P. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. NeuroReport. 2001;12:2963–2965. doi: 10.1097/00001756-200109170-00041. [DOI] [PubMed] [Google Scholar]
- 12.Topper R, Hfoltys H, Meister IG, Sparing R, Boroojerdi B. Repetitive transcranial magnetic stimulation of the parietal cortex transiently ameliorates phantom limb pain-like syndrome. Clinical Neurophysiology. 2003;114:1521–1530. doi: 10.1016/s1388-2457(03)00117-2. [DOI] [PubMed] [Google Scholar]
- 13.Pleger B, Janssen F, Schwenkreis P, et al. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neuroscience Letters. 2004;356:87–90. doi: 10.1016/j.neulet.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 14.Tamura Y, Okabe S, Ohnishi T, et al. Effects of 1-Hz repetitive transcranial magnetic stimulation on acute pain induced by capsaicin. Pain. 2004;107:107–115. doi: 10.1016/j.pain.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Summers J, Johnson S, Pridemore S, Oberoi G. Changes to cold detection and pain thresholds following low and high frequency transcranial magnetic stimulation of the motor cortex. Neuroscience Letters. 2004;368:197–200. doi: 10.1016/j.neulet.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Lefaucheur JP, Drouot X, Menard-Lefaucher I, Nguyen JP. Neuropathic pain controlled for more than a year by monthly sessions of repetitive transcranial magnetic stimulation of the motor cortex. Neurophys Clin. 2004;34(2):91–95. doi: 10.1016/j.neucli.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Canavero S, Bonicalzi V, Dotta M, et al. Transcranial magnetic cortical stimulation relieves central pain. Stereo Funct Neurosurg. 2002;78(3-4):192–196. doi: 10.1159/000068965. [DOI] [PubMed] [Google Scholar]
- 18.Yoo WK, Kim WH, Doh WS, Lee JH, Jung KI, Park DS, Park ES. Dissociable modulating effect of repetitive transcranial magnetic stimulation on sensory and pain perception. Neuroreport. 2006;17:141–144. doi: 10.1097/01.wnr.0000198438.37012.d6. [DOI] [PubMed] [Google Scholar]
- 19.Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, Kato A, Yoshimine T. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain. 2006;122:22–27. doi: 10.1016/j.pain.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Johnson S, Summers J, Pridmore S. Changes to somatosensory detection and pain thresholds following high frequency repetitive TMS of the motor cortex in individuals suffering from chronic pain. Pain. 2006;123:187–192. doi: 10.1016/j.pain.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76:833–838. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andre-Obadia N, Peyron R, Mertens P, Mauguiere F, Laurent B, Garcia-Larrea L. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clinical Neurophysiology. 2006;117:1536–1544. doi: 10.1016/j.clinph.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Saitoh Y, Hirayama A, Kishima H, Shimokawa T, Oshino S, Hirata M, Tani N, Kato A, Yoshimine T. Reduction of intractable deafferentation pain due to spinal cord or peripheral lesion by high-frequency repetitive transcranial magnetic stimulation of the primary motor cortex. Journal of Neurosurgery. 2007;107:555–559. doi: 10.3171/JNS-07/09/0555. [DOI] [PubMed] [Google Scholar]
- 24.Passard A, Attal N, Benadhira R, Brasseur L, Saba G, Sichere P, Perrot S, Januel D, Bouhassira D. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain. 2007;130:2661–2670. doi: 10.1093/brain/awm189. [DOI] [PubMed] [Google Scholar]
- 25.Reid P, Pridmore S. Improvement in chronic pain with transcranial magnetic stimulation. Australian & New Zealand Journal of Psychiatry. 2001;35(2):252. doi: 10.1046/j.1440-1614.2001.0884e.x. [DOI] [PubMed] [Google Scholar]
- 26.Graff-Guerrero A, Gonzalez-Olivera J, Fresan A, Gomez-Martin D, Mendez-Nunez JC, Pellicer F. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Cognitive Brain Research. 2005;21(1):153–160. doi: 10.1016/j.cogbrainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Borckardt JJ, Smith AR, Reeves ST, Weinstein M, Kozel FA, Shelley N, Branham RK, Thomas KJ, George MS. Fifteen Minutes of Left Prefrontal Repetitive Transcranial Magnetic Stimulation Acutely Increases Thermal Pain Thresholds in Healthy Adults. Pain Research and Management. 2007;12(4):287–290. doi: 10.1155/2007/741897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, Fierro B. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. Journal of the Neurological Sciences. 2004;227:67–71. doi: 10.1016/j.jns.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Sampson SM, Rome JD, Rummans TA. Slow-frequency rTMS reduces fibromyalgia pain. Pain Medicine. 2006;7(2):115–118. doi: 10.1111/j.1526-4637.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 30.Avery DH, Holtzheimer PE, Fawaz W, Russo J, Neumaier J, Dunner DL, Haynor DR, Claypoole KH, Wajdik C, Roy-Byrne P. Transcranial Magnetic Stimulation Reduces Pain in Patients With Major Depression: A Sham-Controlled Study. Journal of Nervous and Mental Disease. 2007;195(5):378–381. doi: 10.1097/NMD.0b013e31802f58d1. [DOI] [PubMed] [Google Scholar]
- 31.Borckardt JJ, Weinstein M, Reeves ST, Kozel FA, Nahas Z, Smith AR, Byrne K, Morgan K, George M. Post-Operative Left Prefrontal Repetitive Transcranial Magnetic Stimulation Reduces Patient-Controlled Analgesia Use. Anesthesiology. 2006;105(3):1–6. doi: 10.1097/00000542-200609000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Borckardt JJ, Reeves ST, Weinstein M, Smith AR, Shelley N, Kozel FA, Byrne KT, Morgan K, George MS. Significant Analgesic Effects of One Session of Postoperative Left Prefrontal Cortex Repetitive Transcranial Magnetic Stimulation: A replication study. Brain Stimulation. 2008;1(2):122–127. doi: 10.1016/j.brs.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 34.Apkarian VA, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. The Journal of Neuroscience. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadjipavlou G, Dunckley P, Behrens TE, Tracey I. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: A diffusion tensor imaging study in healthy controls. Pain. 2006;123:169–178. doi: 10.1016/j.pain.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 36.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: The Neuropathic Pain Scale. Neurology. 1997;48:332–338. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- 37.Daut Randall L, Cleeland Charles S, Flanery Randall C. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983 Oct;17(2):197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 38.Tan Gabriel, Jensen Mark P, Thornby John I, Shanti Bilal F. Validation of the brief pain inventory for chronic nonmalignant pain. Journal of Pain. 2004 Mar;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Caraceni Augusto, Cherny Nathan, Fainsinger Robin, Kaasa Stein, Poulain Philippe, Radbruch Lukas, De Conno Franco. European Assn of Palliative Care (EAPC), Research Network, Milan, Italy; Pain measurement tools and methods in clinical research in palliative care: Recommendations of an Expert Working Group of the European Association of Palliative Care. Journal of Pain & Symptom Management. 2002 Mar;23(3):239–255. doi: 10.1016/s0885-3924(01)00409-2. [DOI] [PubMed] [Google Scholar]
- 40.Gallagher Dolores. The Beck Depression Inventory and older adults: Review of its development and utility. Clinical Gerontologist. 1986 Jun;5(1-2):149–163. [Google Scholar]
- 41.Faravelli Carlo, Albanesi Giorgio, Poli Enrico. Assessment of depression: A comparison of rating scales. Journal of Affective Disorders. 1986 Nov-Dec;11(3):245–253. doi: 10.1016/0165-0327(86)90076-5. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka-Matsumi Junko, Kameoka Velma A. Reliabilities and concurrent validities of popular self-report measures of depression, anxiety, and social desirability. Journal of Consulting & Clinical Psychology. 1986 Jun;54(3):328–333. doi: 10.1037//0022-006x.54.3.328. [DOI] [PubMed] [Google Scholar]
- 43.Boyle Gregory J. Self-report measures of depression: Some psychometric considerations. British Journal of Clinical Psychology. 1985 Feb;24(1):45–59. doi: 10.1111/j.2044-8260.1985.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds William M, Gould Jonathan W. A psychometric investigation of the standard and short form Beck Depression Inventory. Journal of Consulting & Clinical Psychology. 1981 Apr;49(2):306–307. doi: 10.1037//0022-006x.49.2.306. [DOI] [PubMed] [Google Scholar]
- 45.Radloff LS. AppliedPsychologicalMeasurement. West Publishing Co; New York, NY: 1977. The CES-D Scale: a self-report depression scale for research in the general population. pp. 385–401. [Google Scholar]
- 46.Heft MW, Gracely RH, Dubner R, McGrath PA. A validation model for verbal description scaling of human clinical pain. Pain. 1980 Dec;9(3):363–73. doi: 10.1016/0304-3959(80)90050-0. [DOI] [PubMed] [Google Scholar]
- 47.Borckardt JJ, Nahas Z, Koola J, George MS. Estimating Resting Motor Thresholds In TMS Research And Practice: A computer simulation evaluation of best methods. Journal of ECT. 2006;22(3):169–175. doi: 10.1097/01.yct.0000235923.52741.72. [DOI] [PubMed] [Google Scholar]
- 48.Awiszus F. TMS and Threshold Hunting. EEG Clin Neurophys. 2003;(Supplement) doi: 10.1016/s1567-424x(09)70205-3. [DOI] [PubMed] [Google Scholar]
- 49.Mishory A, Molnar C, Koola J, LI X, Kozel FA, Myrick H, Stroud Z, Nahas Z, George MS. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J Ect. 2004;20:160–165. doi: 10.1097/00124509-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models and individual growth curves. Journal of Educational and Behavioral Statistics. 1998;24(4):323–355. [Google Scholar]