Abstract
We present a liquid chromatography-mass spectrometry (LC-MS) method that capitalizes on the mass-resolving power of the orbitrap to enable sensitive and specific measurement of known and unanticipated metabolites in parallel, with a focus on water soluble species involved in core metabolism. The reversed phase LC method, with a cycle time 25 min, involves a water-methanol gradient on a C18 column with tributylamine as the ion pairing agent. The MS portion involves full scans from 85 – 800 m/z at 1 Hz and 100,000 resolution in negative ion mode on a stand alone orbitrap (“Exactive”). The median limit of detection, across 80 metabolite standards, was 5 ng/mL with linear range typically ≥ 100-fold. For both standards and a cellular extract from Saccharomyces cerevisiae (Baker’s yeast), the median inter-run relative standard deviation in peak intensity was 8%. In yeast exact, we detected 137 known compounds, whose 13C-labeling patterns could also be tracked to probe metabolic flux. In yeast engineered to lack a gene of unknown function (YKL215C), we observed accumulation of an ion of m/z 128.0351, which we subsequently confirmed to be oxoproline, resulting in annotation of YKL215C as an oxoprolinase. These examples demonstrate the suitability of the present method for quantitative metabolomics, fluxomics, and discovery metabolite profiling.
Introduction
A key driver of the use of liquid chromatography-mass spectrometry (LC-MS) for metabolomics has been technological advances in instrumentation. On the chromatography side, these include the use of ultra performance liquid chromatography (UPLC) to drive mobile phase through columns packed with small particles 1–3. On the mass spectrometry side, particularly significant progress been in capabilities for affordable, high resolution full scan MS analysis using time-of-flight 4 and orbitrap-based mass spectrometers 5.
The orbitrap is a mass analyzer that dynamically traps ions in an electric field formed between a central spindle electrode and an outer barrel electrode 6, 7. Ions circulate around the inner electrode with an axial frequency that is directly related to mass-to-charge ratio (m/z). Time domain transients are detected and converted to mass spectra through Fourier transformation, analogous to Fourier Transfer Ion Cyclotron Resonance (FTICR) but without a magnet. The orbitrap mass analyzer typically has a working resolution of ~ 100,000 at m/z 200. Resolution is scan-time dependent, with longer scan times yielding higher resolution. The resolution is higher than that of most time of flight instruments (~ 10,000 resolution) 4, 8 and approaches that of FTICR (~ 100,000 – 1,000,000 resolution) 9, 10.
The first commercial instrument involving an orbitrap mass analyzer was a hybrid linear ion trap-orbitrap 11. This instrument has proven to be a powerful tool in proteomics 12. Its typical operation mode involves acquiring high resolution full scan spectra in the orbitrap, while simultaneously conducting data-dependent MS/MS in the ion trap. The full scan data are used for peptide quantitation, with the MS/MS data used for peptide identification. A related strategy can be applied to small molecules, involving (1) quantitation of targeted compounds by selection of their masses from high resolution full scan data, combined with (2) full scan screening in search of unknown compounds, followed by MSn analysis to facilitate their identification 13. Unlike for peptides, however, MSn analysis alone is generally not adequate for identification of unknown metabolites. Areas of application of the hydrid linear ion trap-orbitrap in small molecule analysis have included metabolites from microbes 14, plants 15, and animals16, 17.
The scientific community continues pushing for instruments with high performance but modest cost. Recently a benchtop orbitrap mass spectrometer, marketed as “Exactive”, was introduced by ThermoFisher Scientific 18–20. This stand-alone mass analyzer, without the ion trap in the front, was designed mainly for high accuracy, high resolution full scans. The cost is roughly one-half that of its hybrid counterpart, rendering the instrument more accessible for ordinary laboratories. Here we couple this stand-alone orbitrap instrument to reversed-phase, ion-pairing liquid chromatography via electrospray ionization to analyze the cellular metabolome. Key characteristics of the resulting LC-MS method include fast analysis due to the use of a small particle column, effective quantitation of a broad range of known cellular metabolites, and simultaneous detection also of unanticipated metabolites via untargeted analysis. We evaluate the quantitative performance of the method using both metabolite standards and microbial extracts, including in the presence of isotopic labeling. We also highlight the method’s potential to identify unanticipated metabolites and thereby to assign metabolic roles to genes of unknown function.
Experimental
Chemicals, reagents and media components
HPLC-grade water and methanol (OmniSolv, EMD Chemical) were obtained through VWR International (West Chester, PA). The majority of the metabolite standards (see Supporting Information, Table S-1), as well as tributylamine, acetic acid and all media components, were obtained through Sigma-Aldrich (St. Louis, MO). Additional metabolite standards were obtained from Human Metabolome Database 21. 13C-D-Glucose (99%) and 15N-NH4CI were obtained from Cambridge Isotope Laboratories (Andover, MA). Polytyrosine calibration solution was obtained from ThermoFisher Scientific (San Jose, CA). (S)-(−)-2-Pyrrolidone-5-carboxylic acid (98%) was purchased through ACROS Organics, New Jersey, USA.
Escherichia coli were grown in Gutnick minimal salt media 22: KH2PO4, 4.7 g/L; K2HPO4, 0.5 g/L; K2SO4, 1 g/L; MgSO4-7H2O, 0.1 g/L; NH4Cl, 10 mM; and glucose, 4 g/L. The Baker’s yeast Saccharomyces cerevisiae were grown in yeast nitrogen base (YNB) without amino acids (Difco, Detroit, MI) with 20 g/L glucose.
LC-MS instrumentation and method development
The complete LC-MS platform consists of Accela U-HPLC system with quaternary pumps, HTC PAL autosampler (CTC Analytics AG, Zwingen, Switchland), Keystone hot pocket column heater, and Exactive orbitrap mass spectrometer (all from ThermoFisher Scientific, San Jose, CA, unless otherwise noted), controlled by Xcalibur 2.1 software. Liquid chromatography separation was achieved on a Synergy Hydro-RP column (100 × 2 mm, 2.5 µm particle size, Phenomenex, Torrance, CA), using reversed-phase chromatography with the ion pairing agent tributylamine in the aqueous mobile phase to enhance retention and separation 23–25. The present LC method is a modified version of a method originally described in 23, with the present method employing a smaller particle size column (2.5 µm instead of 4 µm) to reduce peak widths and expedite analysis. The total run time is 25 min (versus 80 min for the original method). Flow rate is 200 µl/min. Solvent A is 97:3 water:methanol with 10 mM tributylamine and 15 mM acetic acid; solvent B is methanol. The gradient is: 0 min, 0% B; 2.5 min, 0% B; 5 min, 20% B;7.5 min, 20% B; 13 min, 55% B; 15.5 min, 95% B; 18.5 min, 95% B; 19 min, 0% B; 25 min, 0% B. Other LC parameters are autosampler temperature 4°C, injection volume 10 µl, and column temperature 25°C.
An electrospray ionization interface was used to direct column eluent to the mass spectrometer. Because the ion pairing agent tributylamine will cause ion suppression in positive mode, the instrument was operated in negative mode only. Initial instrument optimization (tuning) was done by infusing a mixture of malate (m/z 133.0142), ATP (m/z 505.9885), and coenzyme A (m/z 766.1079), each at 1 µg/ml at a flow rate of 200 µg/ml, using a 11Plus syringe pump (Harvard Apparatus, Boston, MA). Various instrumental settings were optimized to maximize the signal with the final parameters as follows: sheath gas flow rate 25 (arbitrary units), aux gas flow rate 8 (arbitrary units), sweep gas flow rate 3 (arbitrary units), spray voltage 3 kV, capillary temperature 325°C, capillary voltage −50 V, tube lens voltage −100 V. The instrument was mass calibrated using the polytyrosine-1,3,6 standards every three days.
The Exactive mass spectrometer has a maximum scan range of m/z 50–4000. For the study of small molecule metabolites, of particular interest is the low mass range (m/z 85–800). In preliminary experiments, we found that the high amount of phosphate and sulfate in typical cellular media adversely impact analysis, apparently due to a combination of ion suppression at the ion source and space-charge effects inside the orbitrap. To mitigate the latter, during the LC segments at which phosphate (H2PO4−, m/z 96.9696, ~ 6 min) and sulfate (HSO4−, m/z 96.9601, ~ 13 min) elute, we chose a lower scan limit of ≥ m/z 100 (rather than m/z 85) to reduce the accumulation of phosphate and sulfate ions in the orbitrap. Although the scan limit does not provide high resolution mass filtration, ions falling outside of the scan range are selected against. Empirically, we found that this selection was adequate to improve substantially analytical results. Later in the LC run, the lower m/z limit was raised yet higher, as low molecular weight metabolites elute early in this reversed phase LC method. The final MS scan method is thus made in the following with segments: 0–5 min, m/z 85–800; 5–6.7 min, m/z 100–800; 6.7–9 min, m/z 85–800; 9–16 min, m/z 110–1000; 16–24 min, m/z 220–1000. The last minute in the LC run is for column equilibrium only and is not scanned. Other MS method settings are, resolution 100,000 at 1 Hz (1 scan per second), AGC (automatic gain control) target 3E6, maximum injection time 100 µS.
Method Validation for Purified Metabolites
Method performance was evaluated for 87 metabolite standards (Supporting Information, Table S-1) with respect to limit of detection (LOD), linearity, reproducibility and mass accuracy. For each purified metabolite, ≥ 0.1 mg/mL stock solution was prepared in 50:50 methanol:water and stored at −80°C. From these stock solutions, mixtures of ~ 10 metabolites dissolved in water were prepared at 1, 2, 5, 10, 20, 50, 100, 200, 500, 1000, 2000 ng/mL and analyzed. LOD was defined as the lowest concentration at which the signal is at least 3-fold of the corresponding background. Linearity was evaluated using the linear regression of the observed signal with respect to concentration, with the lower limit always being the LOD. In case that LOD was > 100 ng/mL, secondary experiments were done using standards at 200, 500, 1000, 2000, 5000, 10000 ng/mL to determine the linear range. In addition, standards at 100 ng/mL were run four times to determine inter-run, intra-day quantitative reproducibility, as measured by the relative standard deviation (RSD). Mass accuracy was determined based on ppm error, defined as [(measured mass – theoretical mass) / theoretical mass] * 106, was evaluated at 100 ng/mL (or 1000 ng/ml for compounds with LOD > 100 ng/mL).
For LC-MS-based targeted metabolomics, it is desirable to detect as many metabolites as possible using their retention times and accurate masses. After verifying that the current method works well for 87 metabolites, we determined the retention time for additional 127 metabolites using their standards at a concentration of ~ 1 µg/mL. This resulted in a list of 214 known metabolites that can be detected by this method (Table S-1 and Table S-2). The number of known metabolites that can be detected is largely limited by the availability of the purified standards.
Yeast culture and extraction
S. cerevisiae strains used were the prototrophs FY4 (Mat a) and FY5 (Mat α), as well as the yeast deletion mutant ykl215cΔ∷kanMX in both backgrounds 26. The deletion mutant was freshly created by homologous recombination using the ykl215cΔ∷kanMX allele amplified by PCR from the systematic yeast deletion collection (Open Biosystems). A saturated overnight culture was set back to an optical density at 600 nm (OD600) of 0.1 in 25 mL of liquid culture media. After approximately two doublings (OD600=0.4), the 25 mL culture was rapidly filtered and the filter-bound cells quenched by immediately dropping them into 0.8 mL −20°C extraction solvent (40:40:20 acetonitrile:methanol:water). The quenched cells (with the filter still present) were then extracted at −20°C for 15 min, at which time any residual cells were washed off of the filter and the filter discarded. The resulting mixture of cell debris and extraction solvent was transferred to a 1.5 mL eppendorf tube and centrifuged at 13,200 rpm for 5 minutes at 4°C. The supernatant was removed and stored at 4°C. The pellet was resuspended in 0.2 mL extraction solvent and allowed to sit at 4°C for 15 min, followed by centrifugation at 13,200 rpm for 5 minutes at 5°C. The two supernatants were combined, dried under nitrogen flow on an N-EVAP Nitrogen Evaporator (Organomation Associates, Berlin, MA), and resuspended in 1 mL of water to yield a sample ready for LC-MS analysis. In all cases, data shown are for FY4, with FY5 yielding similar results.
For experiments involving determining the carbon- and nitrogen-counts in unknown metabolites, yeast were grown in unlabeled media, as well as in [U-13C]-glucose, 15N-ammonia, and [U-13C]-glucose plus 15N-ammonia labeled media for a minimum of twelve doublings, yielding ‘heavy’ metabolites. The difference in mass between the ‘heavy’ and ‘light’ metabolite correlates with the number of carbons or nitrogens in the molecule.
13C-Isotope labeling of metabolites in Escherichia coli
To test the suitability of the current method for the study of metabolic flux, we studied the 13C-isotope labeling pattern of selected metabolites from E. coli, when switched from normal glucose (with natural isotope contribution of 98.9% of 12C and 1.1% of 13C) to uniformly 13C-labeled glucose as the carbon source. The E. coli strain used was NCM3722. For its culture, we used a filter-based approach that allows for rapid switching of media, as described previously 27, 28. In brief, the cells are transferred by filtration to the surface of a filter, which is placed on an agarose support. The cells grow on the filter surface at a rate similar to that in liquid media, fed by diffusion of nutrients through the agarose and filter. Media composition (in this case from unlabeled to uniformly 13C–labeled glucose media) can then be quickly varied by moving the filter-bound cells from one agarose plate to another. All experimental details are as per Yuan et al. 29, with the following exceptions: filter size was reduced from 82 mm to 47 mm; all liquid volumes (for filter loading and extraction) were correspondingly reduced 3-fold; and the final supernatants were dried and re-dissolved in 300 µL water prior to analysis by LC-MS. The signals of the 13C-labeled forms were corrected for the natural isotope abundance of the unlabeled glucose as described previously 30. Similar results to those shown for E. coli have also been obtained from S. cerevisiae.
Data analysis
High resolution mass spectrometers have the potential to quantitate known analytes (targeted analysis) while simultaneously collecting untargeted data on all ions present, including ones arising from unanticipated compounds (untargeted analysis). The raw data files can be analyzed using the standard data analysis software provided by the vendor (Xcalibur), looking at the extracted ion chromatograms for every mass of interest; however, this is a slow process when analyzing a large number of samples and compounds. To facilitate data analysis, in-house software, mzROLL, was developed for semi-automated data processing. The software involves many of the same principles as the widely used XCMS code 31. A brief description is presented here, with details to be published elsewhere.
Targeted analysis
Thermo Fisher mass spectrometry RAW files were converted from profile mode into centroid mode using the ReAdW program 32. Centroided files were loaded into mzROLL, and aligned by fitting a high degree polynomial to the retention times of the highest intensity ions across the samples. Extracted ion chromatograms (EICs) were extracted using a 5 ppm window centered on the expected m/z of each known compound and smoothed by applying a Gaussian filter to the signal intensity. Within each EIC, peaks were detected and the quality of all peaks was evaluated by a Random Forest based classification model that takes into account peak height, peak width, peak area, the signal to noise ratio, and the peak shape 33. Peaks were grouped across samples and matched to the expected retention time of each compound. The peak closest to the expected retention time with an acceptable quality score was used for quantitation. All peaks used for quantitation were hand-checked after automated extraction and assignment. The isotopically labeled forms of compounds were extracted in a similar manner. Quantitation of labeled forms was based on the highest intensity peak within a 5 ppm window around the expected m/z of the 13C- and/or 15N-labeled form of the compound.
Untargeted analysis
Ion-specific chromatograms are generated for all observed ion signals, using a 5 ppm m/z window, resulting in a large matrix with peaks as rows, samples as columns, and peak intensities as the entries. Statistically significant differences between mutant and wild type metabolite profiles are determined using a type III t-test and log2 fold changes in peak area. Peaks are refined to remove those corresponding to different adduct ions, isotopic variants, and in-source fragmentation. A first-pass approach to identification of the resulting list of significantly different [M-H] peaks involves searching of known metabolite databases, such as KEGG 34, Metacyc 35, and Human Metabolome Database 36 for compounds of the correct exact mass, with further constraints provided by carbon- and nitrogen-atom counts obtained via labeling experiments.
Results and discussion
“Exactive” overview
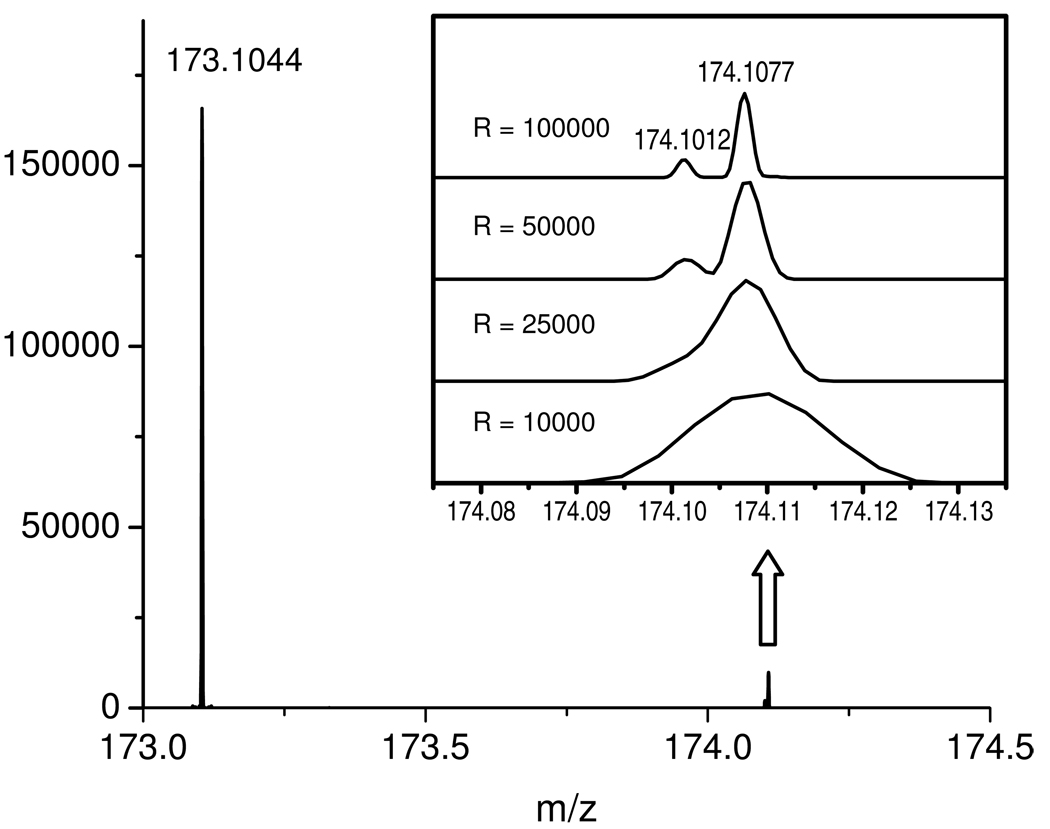
The “Exactive” is a stand-alone orbitrap mass spectrometer that is designed for high resolution full scans. Ions formed by electrospray ionization are transferred to a curved linear trap (C-Trap), from which they are injected into the orbitrap for mass analysis. Mass resolution depends on scan speed. At m/z 200, resolution is 10,000 at 10 Hz; 25,000 at 4 Hz; 50,000 at 2 Hz; and 100,000 at 1 Hz. The effect of the Exactive’s resolving power on analytical results has been demonstrated recently 20. We find that the higher resolution settings offer important benefits, as they frequently help to separate overlapping ion peaks. As an example, Fig. 1 shows the mass spectrum of arginine (purified standard), with a focus on the natural isotope peaks containing one 15N nucleus (m/z 174.1012) versus one 13C nucleus (m/z 174.1077). These two peaks merge at a resolution setting of 10,000 and 25,000, partially resolve at 50,000, and separate fully at 100,000. The Exactive’s ability to separate 15N- and 13C-isotopomer peaks is useful for isotope tracer experiments.
Figure 1.
Negative ion mass spectrum of arginine standard at 10 µg/mL showing the base peak at m/z 173.1044, and its natural isotope peaks with one 15N (m/z 174.1012) and one 13C (m/z 174.1077). The latter two were not resolved at a resolution setting of 10,000 or 25,000, partially separated at 50,000, and well separated at 100,000.
Like any trap instrument, the performance of Exactive is affected by the space-charge effect 37. If there are too many ions inside the trap, these will lead to distortion of the electric field and compromise the instrument performance. The Exactive has an Automatic gain Control (AGC) function which controls the duration of ion injection into the orbitrap to maintain an optimum total number of ions (the AGC target value). When using AGC, the instrument alternates between pre-scans and analytical scans. When the pre-scan finds a large total ion current (TIC), the injection time for the subsequent analytical scan is reduced. The user selects between three AGC target values: 3e6 for a high dynamic range scan, 1e6 for a balanced scan, and 5e5 for the best mass accuracy. Here we used the 3e6 target value to maximize quantitative performance, with the 5e5 target value useful for improving mass accuracy when aiming to identify unanticipated metabolites. To mitigate further the space-charge effect caused by anions in the cell culture media (phosphate and sulfate), we modulated the instrument’s molecular weight scan range to reduce accumulation of these ions in the orbitrap during the chromatographic internals where they elute (see Materials and Methods for details).
LC-MS method development and validation
Luo et al. originally described an LC-MS/MS method for metabolomics that coupled reversed phase HPLC with tributylamine as an ion-pairing reagent via electrospray ionization to triple quadrupole MS/MS 23. Run time was 80 min. We subsequently modified the gradient to reduce the running time to 50 min 24. The column particle size was 4 µm and the flow rate 200 µL/min. Although briefer than the original method of Luo et al., this method was still undesirably slow. Efforts to expedite analysis, however, ran up against the need to for relatively slow compound elution to accommodate a large number of selected reaction monitoring scan events while retaining adequate coverage of individual chromatogram peaks. This is a typical problem when using triple-quadrupole instruments for “omic” analysis.
In the current method, we aimed to take advantage of the full scan capability of the Exactive orbitrap to expedite analysis. To this end, we selected a column particle size of 2.5 µm, which, without changing flow rate, enabled us to reduce the total running time to 25 min. Most of the resulting chromatogram peaks have a width of ~ 15 s, resulting in 15 data points across the peaks when scanning at 1 Hz (100,000 resolution). The maximum pump pressure during the LC run is ~ 300 bar, which can be obtained on high quality HPLC systems, as well as UPLC systems.
In Supporting Information, Table S-1 we summarize the results of LC-MS method validation for 87 metabolite standards. The median limit-of-detection (LOD) is 5 ng/mL with 63 compounds (72%) having a LOD ≤ 10 ng/mL. The compounds with a higher LOD are mostly nucleotide di- and triphosphates and coenzyme A derivatives. All the compounds gave a linear response (R2 > 0.98) over a concentration range of at least 10-fold, with 68 compounds (78%) showing an R2 > 0.98 over at least a 100-fold concentration range. The quantitative reproducibility (intra-day) was determined at a compound concentration of 100 ng/ml. For the 82 metabolites with an LOD ≤ 100 ng/mL, median RSD is 8.2% with 74 compounds (90%) showing a RSD < 20%.
We also evaluated mass accuracy using external calibration. For the 87 metabolites studied, 86 (99%) showed a ppm error of < 5 ppm with a median of −0.29. Mass accuracy is particularly good at the low mass region where most of compounds of interest appear. Mass accuracy can likely be further improved by using internal calibration.
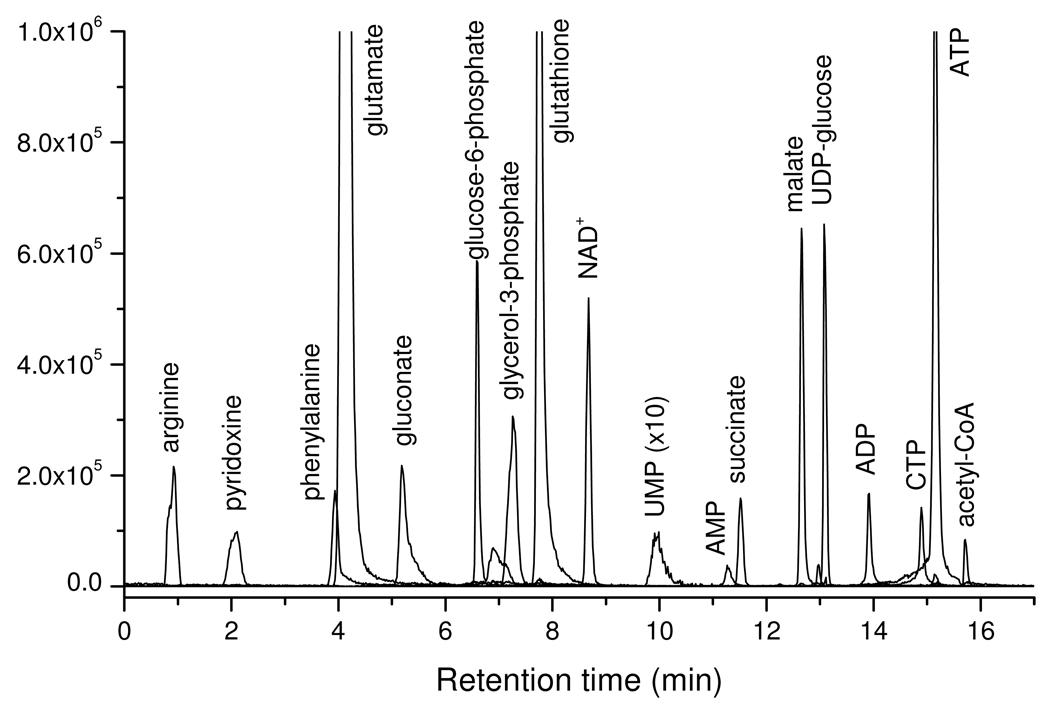
Finally, we tested the method on an extract of Baker’s yeast, Saccharomyces cerevisiae. We detected 137 known metabolites, with the observed signals (peak heights), as well as RSDs (intra-day) listed in Table 1. The median RSD is 7.6%, indicating good reproducibility in real samples. Metabolites detected include amino acids, carboxylic acids, sugar phosphates, nucleotides, and coenzyme A derivatives, indicating the ability of the method to quantitate much of the core metabolome. Ion-specific chromatograms corresponding to selected metabolites are shown in Figure 2.
Table 1.
Retention time, mass accuracy, signal intensity, and quantitative reproducibility for 137 metabolites detected from Saccharomyces cerevisiae extract
| Metabolite | Neutral formula |
Theoretical mass |
Observed mass |
Mass error (ppm) |
Retention time (min) |
Signal (peak height) |
RSD (%) |
|---|---|---|---|---|---|---|---|
| pyruvate | C3H4O3 | 87.0088 | 87.0088 | 0.0 | 8.3 | 746625 | 3.3 |
| alanine | C3H7NO2 | 88.0404 | 88.0404 | 0.0 | 1 | 67324 | 13.3 |
| 4-aminobutyrate | C4H9NO2 | 102.0561 | 102.0561 | 0.0 | 4.5 | 931637 | 2.1 |
| serine | C3H7NO3 | 104.0353 | 104.0353 | 0.0 | 1 | 334496 | 5.7 |
| glycerate | C3H6O4 | 105.0193 | 105.0194 | 1.0 | 6.3 | 23659 | 28.4 |
| proline | C5H9NO2 | 114.0561 | 114.0561 | 0.0 | 1.12 | 68602 | 9.4 |
| fumarate | C4H4O4 | 115.0037 | 115.0039 | 1.7 | 13.4 | 431531 | 1.7 |
| 2-keto-isovalerate | C5H8O3 | 115.0401 | 115.0402 | 0.9 | 13.06 | 482462 | 1.9 |
| indole | C8H7N | 116.0506 | 116.0506 | 0.0 | 7.5 | 2428 | 8.6 |
| valine | C5H11NO2 | 116.0717 | 116.0718 | 0.9 | 1 | 732170 | 3.6 |
| succinate | C4H6O4 | 117.0193 | 117.0195 | 1.7 | 11.6 | 159916 | 1.5 |
| threonine/homoserine* | C4H9NO3 | 118.051 | 118.051 | 0.0 | 1 | 625574 | 10.8 |
| cysteine | C3H7NO2S | 120.0125 | 120.0124 | −0.8 | 1.1 | 2531 | 14.3 |
| nicotinate | C6H5NO2 | 122.0248 | 122.0249 | 0.8 | 11.2 | 479925 | 1.8 |
| citraconic acid | C5H6O4 | 129.0193 | 129.0196 | 2.3 | 12.95 | 501333 | 4.1 |
| hydroxyproline | C5H9NO3 | 130.051 | 130.0509 | −0.8 | 1.1 | 4612 | 16.6 |
| N-acetyl-L-alanine | C5H9NO3 | 130.051 | 130.0511 | 0.8 | 7.97 | 36010 | 3.1 |
| leucine/isoleucine* | C6H13NO2 | 130.0874 | 130.0874 | 0.0 | 1.9 | 459288 | 9.4 |
| oxaloacetate | C4H4O5 | 130.9986 | 130.9986 | 0.0 | 13.64 | 999 | 11.9 |
| asparagine | C4H8N2O3 | 131.0462 | 131.0462 | 0.0 | 1 | 345435 | 7.7 |
| hydroxyisocaproic acid | C6H12O3 | 131.0714 | 131.0715 | 0.8 | 14.35 | 77875 | 2.9 |
| ornithine | C5H12N2O2 | 131.0826 | 131.0825 | −0.8 | 1 | 1799867 | 10.4 |
| aspartate | C10H16N2O3S | 132.0302 | 132.0302 | 0.0 | 4.4 | 1064408 | 4.4 |
| malate | C4H6O5 | 133.0143 | 133.0145 | 1.5 | 12.7 | 638679 | 1.1 |
| homocysteine | C4H9NO2S | 134.0281 | 134.028 | −0.7 | 1 | 5919 | 8.8 |
| p-aminobenzoate | C7H7NO2 | 136.0404 | 136.0403 | −0.7 | 8.7 | 1756 | 21.3 |
| p-hydroxybenzoate | C7H6O3 | 137.0244 | 137.0245 | 0.7 | 10.88 | 3153 | 8.7 |
| acetylphosphate | C2H5O5P | 138.9802 | 138.9803 | 0.7 | 12.38 | 2487 | 7 |
| histidinol | C6H11N3O | 140.0829 | 140.0831 | 1.4 | 0.8 | 2851 | 14.1 |
| αεταρατυλγoτεκ– | C5H6O5 | 145.0143 | 145.0144 | 0.7 | 13.1 | 242708 | 4 |
| glutamine | C5H10N2O3 | 145.0619 | 145.0618 | −0.7 | 1 | 7112816 | 6.3 |
| lysine | C6H14N2O2 | 145.0983 | 145.0984 | 0.7 | 0.8 | 88482 | 5.3 |
| O-acetyl-L-serine | C5H9NO4 | 146.0459 | 146.0456 | −2.1 | 1.1 | 2992 | 16.3 |
| glutamate | C5H9NO4 | 146.0459 | 146.0459 | 0.0 | 4.6 | 2337096 | 1 |
| 2-hydroxy-2-methylbutanedioic acid | C5H8O5 | 147.0299 | 147.0302 | 2.0 | 12.6 | 440951 | 6.3 |
| methionine | C5H11NO2S | 148.0438 | 148.0438 | 0.0 | 1.6 | 158639 | 6.4 |
| hydroxyphenylacetic acid | C8H8O3 | 151.0401 | 151.0403 | 1.3 | 14.8 | 1541 | 9.1 |
| histidine | C6H9N3O2 | 154.0622 | 154.0623 | 0.6 | 0.8 | 250706 | 9.7 |
| orotate | C5H4N2O4 | 155.0098 | 155.0101 | 1.9 | 8.1 | 8437 | 13.6 |
| dihydroorotate | C5H6N2O4 | 157.0255 | 157.0255 | 0.0 | 6.8 | 7360 | 9.9 |
| allantoin | C4H6N4O3 | 157.0367 | 157.0358 | −5.7 | 0.82 | 32477 | 5.2 |
| indole-3-carboxylic acid | C9H7NO2 | 160.0404 | 160.0406 | 1.2 | 14.4 | 8789 | 6.4 |
| aminoadipic acid | C6H11NO4 | 160.0615 | 160.0615 | 0.0 | 4.33 | 43651 | 3.3 |
| phenylpyruvate | C9H8O3 | 163.0401 | 163.0402 | 0.6 | 15.1 | 13865 | 7.3 |
| phenylalanine | C9H11NO2 | 164.0717 | 164.0717 | 0.0 | 4.1 | 159499 | 6.5 |
| phenyllactic acid | C9H10O3 | 165.0557 | 165.0559 | 1.2 | 14.82 | 20423 | 7.4 |
| quinolinate | C7H5NO4 | 166.0146 | 166.0146 | 0.0 | 13.58 | 5908 | 10.7 |
| phosphoenolpyruvate | C3H5O6P | 166.9751 | 166.9752 | 0.6 | 13.7 | 6117 | 1.8 |
| pyridoxine | C8H11NO3 | 168.0666 | 168.0667 | 0.6 | 1.8 | 83996 | 15.1 |
| D-glyceraldehdye-3-phosphate | C3H7O6P | 168.9908 | 168.9907 | −0.6 | 7.3 | 7245 | 5.7 |
| dihydroxyacetone phosphate (DHAP) | C3H7O6P | 168.9908 | 168.9908 | 0.0 | 9.3 | 69141 | 3 |
| sn-glycerol-3-phosphate | C3H9O6P | 171.0064 | 171.0065 | 0.6 | 7.3 | 291568 | 3.9 |
| aconitate | C6H6O6 | 173.0092 | 173.0092 | 0.0 | 13.8 | 160846 | 6.8 |
| N-acetyl-L-ornithine | C7H14N2O3 | 173.0932 | 173.093 | −1.2 | 1 | 249359 | 9.9 |
| arginine | C10H14N5O7P | 173.1044 | 173.1045 | 0.6 | 0.8 | 240015 | 13 |
| citrulline | C6H13N3O3 | 174.0884 | 174.0882 | −1.1 | 0.9 | 2308219 | 9.4 |
| N-carbamoyl-L-aspartate | C5H8N2O5 | 175.036 | 175.0363 | 1.7 | 12.6 | 34300 | 2.7 |
| 2-isopropylmalic acid | C7H12O5 | 175.0612 | 175.0613 | 0.6 | 13.94 | 2251182 | 1.9 |
| glucono-δ-lactone | C6H10O6 | 177.0405 | 177.0406 | 0.6 | 6.59 | 54726 | 13.9 |
| hydroxyphenylpyruvate | C9H8O4 | 179.035 | 179.0351 | 0.6 | 13.58 | 5208 | 7.7 |
| tyrosine | C9H11NO3 | 180.0666 | 180.0667 | 0.6 | 2 | 23290 | 11.4 |
| 3-phospho-serine | C3H8NO6P | 184.0017 | 184.0017 | 0.0 | 8.2 | 2789 | 10.1 |
| 3-phosphoglycerate | C3H7O7P | 184.9857 | 184.9859 | 1.1 | 13.58 | 113737 | 30.2 |
| N-acetyl-glutamine | C7H12N2O4 | 187.0724 | 187.0725 | 0.5 | 7 | 374275 | 1.9 |
| acetyllysine | C8H16N2O3 | 187.1088 | 187.1089 | 0.5 | 1.2 | 120124 | 4.2 |
| kynurenic acid | C10H7NO3 | 188.0353 | 188.0353 | 0.0 | 14.22 | 5987 | 9.8 |
| N-acetyl-glutamate | C7H11NO5 | 188.0564 | 188.0567 | 1.6 | 13 | 956618 | 3.3 |
| citrate/isocitrate* | C6H8O7 | 191.0197 | 191.0198 | 0.5 | 13.6 | 1350132 | 0.7 |
| 2-dehydro-D-gluconate | C6H10O7 | 193.0354 | 193.0354 | 0.0 | 5 | 3770 | 4.8 |
| D-gluconate | C6H12O7 | 195.051 | 195.0511 | 0.5 | 5.8 | 210301 | 4.8 |
| D-erythrose-4-phosphate | C4H9O7P | 199.0013 | 199.0014 | 0.5 | 7.45 | 23954 | 6.7 |
| tryptophan | C11H12N2O2 | 203.0826 | 203.0826 | 0.0 | 7.6 | 47267 | 3.2 |
| xanthurenic acid | C10H7NO4 | 204.0302 | 204.0304 | 1.0 | 13.9 | 9495 | 15.5 |
| D-glucarate | C6H10O8 | 209.0303 | 209.0305 | 1.0 | 13 | 627 | 28.7 |
| pantothenate | C9H17NO5 | 218.1034 | 218.1035 | 0.5 | 11 | 504663 | 3.1 |
| cystathionine | C7H14N2O4S | 221.0602 | 221.0598 | −1.8 | 1 | 29616 | 4.4 |
| ribose-5-phosphate | C5H11O8P | 229.0119 | 229.0119 | 0.0 | 7.2 | 103275 | 3.7 |
| cytidine | C9H13N3O5 | 242.0783 | 242.0782 | −0.4 | 1.2 | 1704 | 27 |
| uridine | C9H12N2O6 | 243.0623 | 243.0622 | −0.4 | 2.1 | 79656 | 3.7 |
| biotin | C10H16N2O3S | 243.0809 | 243.0811 | 0.8 | 12.6 | 3822 | 9 |
| shikimate-3-phosphate | C7H11O8P | 253.0119 | 253.0121 | 0.8 | 13.44 | 25262 | 7.2 |
| D-glucono-δ-lactone-6-phosphate | C6H11O9P | 257.0068 | 257.0068 | 0.0 | 6.17 | 4570 | 14 |
| D-glucosamine-1/6-phosphate* | C6H14NO8P | 258.0384 | 258.0387 | 1.2 | 1.5 | 6274 | 29.8 |
| fructose-6-phosphate | C6H13O9P | 259.0224 | 259.0225 | 0.4 | 7.3 | 67097 | 4.6 |
| glucose-6-phosphate | C6H13O9P | 259.0224 | 259.0225 | 0.4 | 6.9 | 594878 | 1.9 |
| thiamine | C12H16N4OS | 263.0972 | 263.0973 | 0.4 | 0.8 | 404390 | 9.9 |
| 2,3-diphosphoglycerate | C3H8O10P2 | 264.952 | 264.9522 | 0.8 | 14.68 | 48631 | 3.3 |
| inosine | C10H12N4O5 | 267.0735 | 267.0736 | 0.4 | 3.6 | 5219 | 14.1 |
| 6-phospho-D-gluconate | C6H13O10P | 275.0174 | 275.0176 | 0.7 | 13.38 | 249507 | 2.8 |
| 1-methyladenosine | C11H15N5O4 | 280.1051 | 280.104 | −3.9 | 1 | 1031 | 32.1 |
| guanosine | C10H13N5O5 | 282.0844 | 282.0845 | 0.4 | 4.2 | 1155 | 2 |
| S-methyl-5'-thioadenosine | C11H15N5O3S | 296.0823 | 296.0828 | 1.7 | 11.6 | 1123 | 21.9 |
| 7-methylguanosine | C11H16N5O5 | 297.1079 | 297.1068 | −3.7 | 1.16 | 1104 | 19.2 |
| N-acetyl-D-glucosamine-1/6-phosphate* | C8H16NO9P | 300.049 | 300.0492 | 0.7 | 7.3 | 27244 | 1.4 |
| glutathione | C10H17N3O6S | 306.0765 | 306.0766 | 0.3 | 8 | 2002776 | 2.9 |
| thymidine-5'-phosphate (dTMP) | C10H15N2O8P | 321.0493 | 321.0496 | 0.9 | 11.27 | 1640 | 12.9 |
| cytidine-5'-phosphate (CMP) | C9H14N3O8P | 322.0446 | 322.0451 | 1.6 | 8.7 | 1944 | 17.1 |
| Uridine-5'-monophosphate (UMP) | C9H13N2O9P | 323.0286 | 323.0291 | 1.5 | 10.2 | 8410 | 13.1 |
| 3',5'-cyclic AMP | C10H12N5O6P | 328.0453 | 328.0458 | 1.5 | 12.6 | 3287 | 10.6 |
| fructose-1,6-bisphosphate | C6H14O12P2 | 338.9888 | 338.9891 | 0.9 | 13.5 | 1405676 | 5.6 |
| trehalose/sucrose/cellobiose* | C12H22O11 | 341.109 | 341.1089 | −0.3 | 1.18 | 80239 | 8.8 |
| adenosine-5'-phosphate (AMP) | C10H14N5O7P | 346.0558 | 346.0562 | 1.2 | 11.3 | 35081 | 7.6 |
| inosine-5'-phosphate (IMP) | C10H13N4O8P | 347.0398 | 347.0403 | 1.4 | 10.7 | 13453 | 9.2 |
| guanosine-5'-phosphate (GMP) | C10H14N5O8P | 362.0507 | 362.0512 | 1.4 | 10.7 | 10006 | 4.8 |
| xanthosine-5-phosphate | C10H13N4O9P | 363.0347 | 363.0356 | 2.5 | 12.7 | 2887 | 8.6 |
| orotidine-5'-phosphate | C10H13N2O11P | 367.0184 | 367.0188 | 1.1 | 13.58 | 1278 | 25.8 |
| riboflavin | C17H20N4O6 | 375.131 | 375.1319 | 2.4 | 12.2 | 885 | 6.2 |
| S-adenosyl-L-homocysteine | C10H12N5O6P | 383.1143 | 383.1149 | 1.6 | 6.1 | 10200 | 11 |
| 5-phosphoribosyl-1-pyrophosphate | C5H13O14P3 | 388.9446 | 388.9452 | 1.5 | 14.94 | 27112 | 7.7 |
| thymidine-5'-diphosphate (dTDP) | C10H16N2O11P2 | 401.0157 | 401.0161 | 1.0 | 13.58 | 1430 | 40.7 |
| cytidine-5'-diphosphate (CDP) | C9H15N3O11P2 | 402.0109 | 402.0115 | 1.5 | 13.3 | 7945 | 12.3 |
| uridine-5'-diphosphate (UDP) | C9H14N2O12P2 | 402.9949 | 402.9952 | 0.7 | 13.5 | 31368 | 4 |
| trehalose-6-Phosphate | C12H23O14P | 421.0753 | 421.0757 | 0.9 | 7.2 | 21367 | 12.1 |
| adenosine-5'-diphosphate (ADP) | C10H15N5O10P2 | 426.0221 | 426.0227 | 1.4 | 13.7 | 170373 | 4.5 |
| guanosine-5'-diphosphate (GDP) | C10H15N5O11P2 | 442.0171 | 442.0174 | 0.7 | 13.5 | 28920 | 7 |
| CDP-ethanolamine | C11H20N4O11P2 | 445.0531 | 445.0536 | 1.1 | 6.38 | 3180 | 12 |
| flavin mononucleotide (FMN) | C17H21N4O9P | 455.0974 | 455.0981 | 1.5 | 14.2 | 13753 | 6.6 |
| dCTP | C9H16N3O13P3 | 465.9823 | 465.9829 | 1.3 | 14.8 | 6439 | 7.6 |
| thymidine 5'-triphosphate (dTTP) | C10H17N2O14P3 | 480.982 | 480.9824 | 0.8 | 14.94 | 38064 | 9.2 |
| cytidine-5'-triphosphate (CTP) | C9H16N3O14P3 | 481.9772 | 481.9779 | 1.5 | 14.8 | 160273 | 7.7 |
| uridine-5'-triphosphate (UTP) | C9H15N2O15P3 | 482.9612 | 482.9619 | 1.4 | 15 | 989523 | 6.8 |
| dATP | C10H16N5O12P3 | 489.9936 | 489.9941 | 1.0 | 14.94 | 9053 | 10.2 |
| adenosine-5'-triphosphate (ATP) | C10H16N5O13P3 | 505.9885 | 505.9889 | 0.8 | 15.1 | 1449520 | 5 |
| guanosine-5'-triphosphate (GTP) | C10H16N5O14P3 | 521.9834 | 521.9839 | 1.0 | 15 | 170050 | 8.2 |
| UDP-D-glucose | C15H24N2O17P2 | 565.0477 | 565.0482 | 0.9 | 13.25 | 670930 | 3.1 |
| UDP-N-acetyl-glucosamine | C17H27N3O17P2 | 606.0743 | 606.0748 | 0.8 | 13.27 | 1159364 | 2.9 |
| glutathione disulfide | C20H32N6O12S2 | 611.1447 | 611.1458 | 1.8 | 12.8 | 142342 | 8.6 |
| nicotinamide adenine dinucleotide (NAD+) | C21H27N7O14P2 | 662.1019 | 662.1024 | 0.8 | 8.6 | 529138 | 2.4 |
| nicotinamide adenine dinucleotide reduced (NADH) |
C21H29N7O14P2 | 664.1175 | 664.1187 | 1.8 | 13.9 | 5125 | 10.3 |
| dephospho-CoA | C21H35N7O13P2S | 686.1416 | 686.1451 | 5.1 | 14.8 | 623 | 18.9 |
| nicotinamide adenine dinucleotide phosphate (NADP+) |
C21H28N7O17P3 | 742.0682 | 742.0687 | 0.7 | 13.5 | 20534 | 8.3 |
| nicotinamide adenine dinucleotide phosphate reduced (NADPH) |
C21H30N7O17P3 | 744.0838 | 744.0845 | 0.9 | 14.87 | 57597 | 7.3 |
| coenzyme A | C21H36N7O16P3S | 766.1079 | 766.1079 | 0.0 | 15.5 | 3194 | 28.1 |
| flavin adenine dinucleotide (FAD) | C27H33N9O15P2 | 784.1498 | 784.1503 | 0.6 | 15 | 16629 | 9.5 |
| acetyl-CoA | C23H38N7O17P3S | 808.1185 | 808.119 | 0.6 | 15.6 | 103422 | 13.9 |
| malonyl-coA | C24H38N7O19P3S | 852.1083 | 852.1105 | 2.6 | 15.6 | 2979 | 11.8 |
| 3-hydroxy-3-methylglutaryl-CoA | C27H44N7O20P3S | 910.1502 | 910.1516 | 1.5 | 15.72 | 5296 | 4.9 |
indicates structural isomers that are not separated with current method.
Figure 2.
Overlay of extracted ion chromatograms of selective metabolites from a S. cerevisiae extract.
Kinetic flux profiling
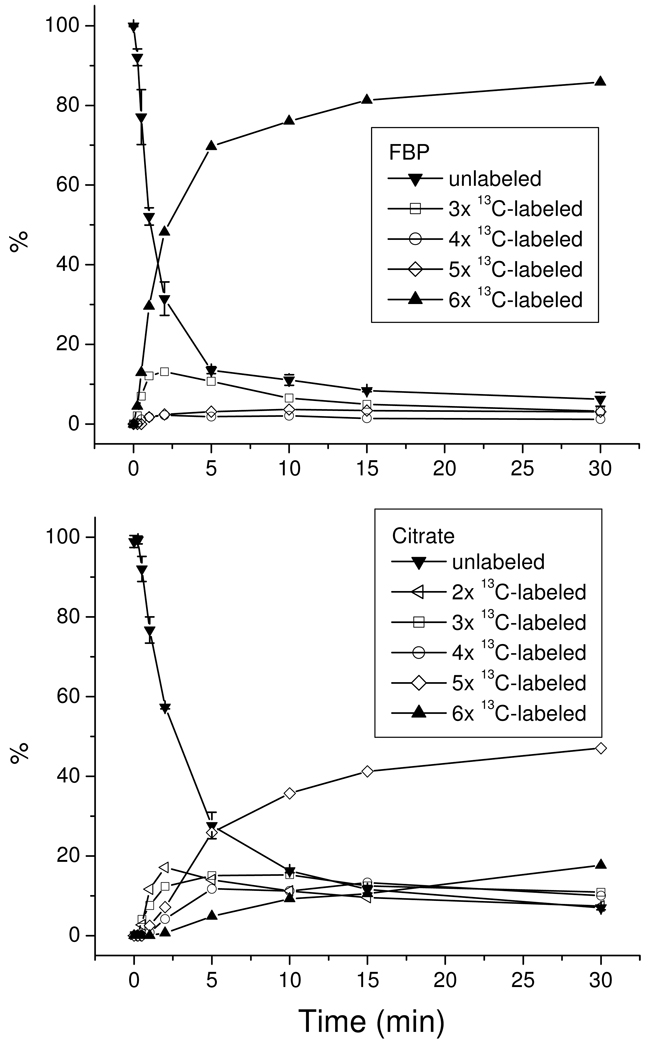
Upon switching from unlabeled to isotope-labeled nutrient, intracellular metabolites becomes labeled, with the rates of labeling depending on the proximity of the metabolite to the labeled nutrient in the metabolic network and the flux through the metabolite. Accordingly, labeling dynamics (kinetic flux profiling) can be used to probe metabolic network structures, as well as to quantitate metabolic fluxes 27, 29 In our previous studies, this was done on a triple quadrupole mass spectrometer operating in multiple reaction monitoring (MRM) mode. When examining many metabolites, the MRM-based approach is cumbersome, especially for metabolites that can be partially labeled in many different ways, as each partially labeled form requires its own MRM scan. Moreover, selecting the appropriate product ion to monitor the partially labeled forms is not always straightforward 27, 38. Using high resolution full scan MS, labeled metabolites can be identified based on their accurate masses. With this approach, we are able to monitor the labeling patterns of a large number of metabolites in parallel. Here we show results obtained upon switching E. coli from unlabeled to uniformly 13C-labeled glucose for two representative compounds: fructose-1,6-bisphosphate (FBP) in glycolysis and citrate in TCA cycle (Fig. 3). Comparable quality of labeling data is obtained for most compounds. The full data, in tandem with relevant network-level analyses, will be described elsewhere.
Figure 3.
Relative labeling percentage of two representative metabolites as a function of time: fructose-1,6-bisphosphate (FBP, top panel), and citrate (bottom panel). For the purpose of simplicity, the following labeled forms were not plotted: 13C1- and 13C2-labeld FBP which has a maximum percentage of 1.7% and 1.4%, respectively, and 13C1-labeled citrate which has a maximum percentage of 1.3%. The standard deviations for N=2 were plotted only for the unlabeled forms.
Consistent with its position just downstream of glucose in the high-flux pathway of glycolysis, FBP labels quickly, with a half-time of ~ 2 min. Initially, FBP accumulates in two labeled forms: fully labeled and 3 × 13C-labeled. The fully labeled form is consistent with passage of glucose down glycolysis, whereas the three-labeled form is consistent with the reverse aldolase reaction joining together a fully labeled with an unlabeled triose phosphate (dihydroxyacetone phosphate or glycealdehyde-3-phosphate). The initial accumulation of three-labeled FBP at ≥ 50% of the rate of fully labeled FBP implies the reverse aldolase flux is a substantial fraction of the net glycolytic flux, i.e., that the aldolase reaction is near to equilibrium in glucose-fed E. coli. This observation is consistent with recent thermodynamic analysis of the glycolytic pathway 39.
Citrate is a six-carbon metabolite formed by the condensation of the acetyl carbons of acetyl-CoA with the four-carbon TCA cycle compound oxaloacetate. Oxaloacetate can be generated in E. coli either by carboxylation of the three-carbon glycolytic compound phosphoenolpyruvate (“anapleurosis”) or by turning of the TCA cycle. These sets of reactions lead to formation of citrate with 2, 3, 4, 5, or 6 labeled carbon atoms. Initially, 2 × 13C-labeling (from acetyl-CoA) and 3 × 13C-labeling (from anapleurosis) dominate, with subsequent rises in 4, 5, and 6 carbon labeling. After 30 min, the most abundant form is 5 × 13C-labeled, consistent with a 3 × 13C-labeled oxaloacetate (formed by the condensation of unlabeled carbon dioxide from the environment with labeled phosphoenolpyruvate) being joined with two labeled carbon atoms from acetyl-CoA. These data indicate that the anapleurotic flux in E. coli is larger than the flux associated with complete turning of the TCA cycle: complete turning of the TCA cycle would lead to 4 × 13C-labeling (from 2 × acetyl-CoA) exceeding 3 × 13C-labeling and 5 × 13C-labeling (from anapleurosis) at early time points; similarly, complete turning of the TCA cycle would lead to complete citrate labeling (rather than five carbon labeling) at late time points.
Discovery metabolite profiling
Even for well-studied organisms like E. coli and yeast, the functions of many genes remain unknown. For metabolic enzymes, comparison of the metabolome of wild-type and gene deletion strains provides a powerful tool for gene function elucidation. Enzyme knockout typically leads to the elevation of the enzyme’s substrates and/or the depletion of its products. This approach has been previously used to assign enzyme functions in mammals 40. Its success relies on having an analytical method for metabolome profiling which is adequate to quantitate the enzyme’s substrates and/or products. The method should preferably be untargeted, to enable discovery also of novel metabolites, enzymatic activities, and pathways.
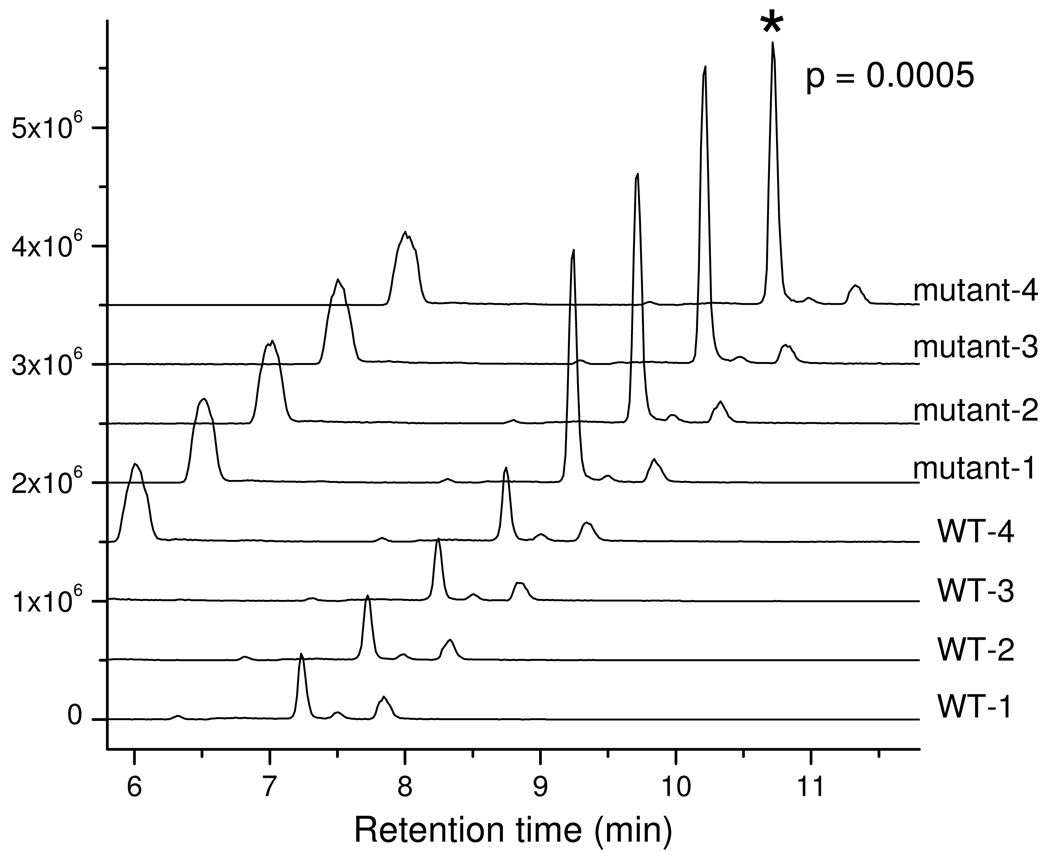
Here we used the present LC-MS method to investigate the function of the gene YKL215C, whose role in yeast was previously unknown. Untargeted metabolite profiling of extracts of the wild type and knockout strains revealed >10,000 mass spectral features. Three m/z features with ≥ 3-fold differences between all mutant and control strains were identified, each of which appeared at the same retention time, 7.2 min, with the strongest signal associated with m/z 128.0351 (Fig. 4). The other two features are at m/z 279.0593 and m/z 355.0454, with a relative signal intensity of 10% and 0.7% of the feature at m/z 128.0353. When searched against the KEGG database, the compound with m/z 128.0351 in negative ion mode matched the exact mass of 5-oxoproline (neutral formula C5H6NO3, m/z 128.0353 in negative mode) with < 2 ppm mass accuracy.
Figure 4.
Chromatogram traces of m/z slice 128.0347–128.0360 (128.0353 ± 5 ppm) from extracts of wild type yeast and ykl215c (oxp1) deletion mutant yeast (each with four biological replicates). The replicates of the same strain give similar results, while there is a > 3-fold difference for the features at 7.2 min between the WT and mutant strains (p=0.0005 by T-test). The feature was identified as 5-oxoproline (see text for details).
Additional experiments were performed to confirm the compound’s identity. First, when fed [U-13C]-glucose or 15N-ammonia, we observed the disappearance of ion of m/z 128.0351. Instead, at the same retention time, we observed the accumulation of ions with m/z 133.0516, and m/z 129.0317, respectively. The masses match those of 5 × 13C oxoproline (expected m/z 133.0521, 3.8 ppm error), and 1 × 15N oxoproline (expected m/z 129.0323, 4.6 ppm error), consistent with 5-oxoproline containing five carbons and one nitrogen. Secondly, we spiked the cellular extracts with authentic standard of 5-oxoproline at concentrations of 0.5 and 2.5 µg/mL. The peak signal corresponding to m/z 128.0351 with a retention time of 7.2 minute increased with the concentration of standard added.
The other compounds that were elevated in the YKL215C deletion strain had exact masses of m/z 279.0593, and m/z 355.0454, and both co-eluted with 5-oxoproline, but with smaller ion intensity. Both also increase upon the addition of the 5-oxoproline standard to a metabolite extract, consistent with their being adducts of 5-oxoproline, although we remain unsure of their precise identities.
Based on these data, we hypothesized that Ykl215c is an oxoprolinase. Consistent with this, subsequent investigation revealed that YKL215C shares 48% sequence identity to the verified M. musculus ATP-hydrolyzing 5-oxoprolinase (gene name Oplah). Based on these results, and following the nomenclature of the oxoprolinase genes in plants, we have registered the gene name in the Saccharomyces Genome Database (SGD) as OXP1. Thus, the present method has been successfully employed, without the need for MS/MS, for untargeted metabolite profiling, resulting in improved annotation of the genome of Baker’s yeast.
Comparison with triple-quadrupole instrument
The market price of the “Exactive” benchtop orbitrap instrument is comparable to that of modern triple-quadrupole instruments. Accordingly, a practical question regards their relative advantages and disadvantages for metabolomic analysis (Table 2). Both techniques are suitable for targeted analysis. Triple quadrupole instruments use MS/MS (multiple reaction monitoring, MRM) to achieve a high degree of analyte specificity, even in complex biological samples. On the downside, pre-optimization is required to determine appropriate MRM parameters. The measured compounds are limited to those targeted by MRM events programmed in the method. In addition, quantitative performance decreases with increasing number of MRM scan events, since each scan event takes a fixed time (so-called “scan time” or “dwell time”).
Table 2.
A comparison of triple quadrupole MS/MS and “Exactive” orbitrap full scan MS for metabolomics.
| Features | Triple quadrupole MS | “Exactive” Orbitrap MS |
|---|---|---|
| Detection mechanism | Multiple reaction monitoring (MS/MS) | High resolution accurate mass |
| Pre-optimization | Required to find the product ions and best collision energy |
Not required |
| Number of detectable compounds | Limited | Virtually unlimited |
| Untargeted analysis | No | Yes |
| Unknown metabolite identification | Possible, through MS/MS fragmentation | Possible, through accurate mass |
| UPLC compatibility | Sometimes challenging due to requirement for adequate chromatographic time for MRM scans |
Yes |
| Sensitivity * | Typical LOD in low ng/mL range | Typical LOD in low ng/mL range |
| Dynamic range * | Mostly over 2–3 orders of magnitude | Mostly over 2–3 orders of magnitude |
| Reproducibility * | Typical RSD of 10% | Typical RSD of 10% |
Based on results obtained using Thermo instruments in our laboratory; results with other instruments and methods may vary
The “Exactive” orbitrap mass analyzer, on the other hand, detects ions solely using high resolution accurate mass. The high resolution is critical to obtaining adequate analyte specificity in complex biological samples without MS/MS. Pre-optimization is not required. Rather a generic full scan method can be used, looking for everything in the appropriate scan range. The number of compounds that can be detected is virtually unlimited. This is particularly useful when following many partially labeled metabolites in isotope tracer experiments. Quantitative performance is generally not affected by the number of metabolites to be detected, as long as the total number of ions entering orbitrap at any given moment does not induce space charge effects 37. A major advantage of the orbitrap is its usefulness also for untargeted analysis. While accurate mass alone is generally not sufficient to identify an unknown compound, it is a critical first step towards such a goal.
In our hands, the quantitative performance of both instrument types is quite similar. Both show sensitivity in the ng/mL range, linear response over 2–3 orders of magnitude, and reasonable reproducibility. Our finding that both instrument types offer similar quantitative performance for metabolomics is consistent with recent literature reaching a similar conclusion in the areas of pharmacokinetic analysis 19 and small molecule quantitation 17.
In terms of specificity, each instrument type has its strengths and weaknesses. The orbitrap’s high resolving power offers certain advantages compared to triple quadruple MS/MS. For example, lysine (m/z 145.0983) and glutamine (m/z 145.0619), which have very similar MS/MS spectra, can be distinguished without the need for LC separation based on their exact masses. Similarly, IMP (m/z 347.0398) can also be distinguished from 13C1-labeled AMP (m/z 347.0592); as AMP is typically more abundant than IMP in cell extracts, this proves a practical virtue in metabolomic analysis even in the absence of isotope labeling. On the other hand, full scan alone can never distinguish isomers; a second dimension separation such as LC is needed. In contrast, it may be possible to distinguish isomers by MRM alone. For example, citrate can be detected using selected reaction monitoring (SRM) transition m/z 191 (C6H7O7−) ➔ 87 (C3O3H3−) at 18 eV, and isocitrate can be detected using SRM m/z 191 (C6H7O7−) ➔ 117 (C4O4H5−)at 18 eV. Also, certain interferences can be better separated by MS/MS than exact mass. For example, in the present method, an unknown interference at m/z 89.0244 typically masks the signal for lactate (m/z 89.0244). Although uncommon, such cases highlight the utility of having multiple different MS techniques available.
Conclusion
A primary challenge in analytical method development in metabolomics is balancing comprehensiveness of metabolome coverage with quantitative performance. The present method, which couples reversed phase ion pairing chromatography on a small particle column with high resolution full scan MS, is a step in this direction. Although limited to negative ion mode, the method is nevertheless suitable for the analysis of a broad range of core, water-soluble cellular metabolites. Its performance relies on the resolving power of the orbitrap mass analyzer, used here in stand-alone form, to separate targeted compounds from interfering peaks in complex cellular samples. This resolving power is also critical for enabling reliable analysis of specific isotopomers in samples rendered yet more complex by isotope labeling, and for facilitating identification of untargeted metabolites. Applications of the method include quantitative metabolomics, fluxomics, and discovery metabolite profiling.
Supplementary Material
Acknowledgements
This research was supported by NIH grant GM071508 for Center of Quantitative Biology at Princeton University. Additional support came from the Beckman Foundation, American Heart Association grant 0635188N, NSF Career Award MCB-0643859, NIH grant AI078063, and the DOE Biohydrogen program (to J.D.R.).
Footnotes
Supporting Information Available:
This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Wilson ID, Nicholson JK, Castro-Perez J, Granger JH, Johnson KA, Smith BW, Plumb RSJ. Proteome Res. 2005;4:591–598. doi: 10.1021/pr049769r. [DOI] [PubMed] [Google Scholar]
- 2.Yu K, Little D, Plumb R, Smith B. Rapid Commu. Mass Spectrom. 2006;20:544–552. doi: 10.1002/rcm.2336. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DTT, Guillarme D, Rudaz S, Veuthey JL. J. Sep. Sci. 2006;29:1836–1848. doi: 10.1002/jssc.200600189. [DOI] [PubMed] [Google Scholar]
- 4.Stroh JG, Petucci CJ, Brecker SJ, Huang N, Lau JM. J. Am. Soc. Mass Spectrom. 2007;18:1612–1616. doi: 10.1016/j.jasms.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Breitling R, Pitt AR, Barrett MP. Trends Biotechnol. 2006;24:543–548. doi: 10.1016/j.tibtech.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Makarov A. Anal. Chem. 2000;72:1156–1162. doi: 10.1021/ac991131p. [DOI] [PubMed] [Google Scholar]
- 7.Hu QZ, Noll RJ, Li HY, Makarov A, Hardman M, Cooks RG. J. Mass Spectrom. 2005;40:430–443. doi: 10.1002/jms.856. [DOI] [PubMed] [Google Scholar]
- 8.van der Heeft E, Bolck YJC, Beumer B, Nijrolder A, Stolker AAM, Nielen MWF. J. Amer. Soc. Mass Spectrom. 2009;20:451–463. doi: 10.1016/j.jasms.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Feng X, Siegel MM. Anal. Bioanal. Chem. 2007;389:1341–1363. doi: 10.1007/s00216-007-1468-8. [DOI] [PubMed] [Google Scholar]
- 10.Schaub TM, Hendrickson CL, Horning S, Quinn JP, Senko MW, Marshall AG. Anal. Chem. 2008;80:3985–3990. doi: 10.1021/ac800386h. [DOI] [PubMed] [Google Scholar]
- 11.Makarov A, Denisov E, Kholomeev A, Baischun W, Lange O, Strupat K, Horning S. Anal. Chem. 2006;78:2113–2120. doi: 10.1021/ac0518811. [DOI] [PubMed] [Google Scholar]
- 12.Han XM, Aslanian A, Yates JR. Curr. Opin. Chem. Biol. 2008;12:483–490. doi: 10.1016/j.cbpa.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogenboom AC, van Leerdam JA, de Voogt P. J. Chromatogr. A. 2009;1216:510–519. doi: 10.1016/j.chroma.2008.08.053. [DOI] [PubMed] [Google Scholar]
- 14.Herebian D, Zuhlke S, Lamshoft M, Spiteller M. J. Sep. Sci. 2009;32:939–948. doi: 10.1002/jssc.200800589. [DOI] [PubMed] [Google Scholar]
- 15.Herebian D, Choi JH, Abd El-Aty AM, Shim JH, Spiteller M. Biomed. Chromatogr. 2009;23:951–965. doi: 10.1002/bmc.1207. [DOI] [PubMed] [Google Scholar]
- 16.Dunn WB, Broadhurst D, Brown M, Baker PN, Redman CWG, Kenny LC, Kell DB. J. Chromatogr. B. 2008;871:288–298. doi: 10.1016/j.jchromb.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Zhang NR, Yu S, Tiller P, Yeh S, Mahan E, Emary WB. Rapid Commun. Mass Spectrom. 2009;23:1085–1094. doi: 10.1002/rcm.3975. [DOI] [PubMed] [Google Scholar]
- 18.Koulman A, Woffendin G, Narayana VK, Welchman H, Crone C, Volmer DA. Rapid Commun. Mass Spectrom. 2009;23:1411–1418. doi: 10.1002/rcm.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bateman KP, Kellmann M, Muenster H, Papp R, Taylor L. J. Am. Soc. Mass Spectrom. 2009;20:1441–1450. doi: 10.1016/j.jasms.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Kellmann M, Muenster H, Zomer P, Mol H. J. Am. Soc. Mass Spectrom. 2009;20:1464–1476. doi: 10.1016/j.jasms.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, Fung C, Nikolai L, Lewis M, Coutouly MA, Forsythe I, Tang P, Shrivastava S, Jeroncic K, Stothard P, Amegbey G, Block D, Hau DD, Wagner J, Miniaci J, Clements M, Gebremedhin M, Guo N, Zhang Y, Duggan GE, MacInnis GD, Weljie AM, Dowlatabadi R, Bamforth F, Clive D, Greiner R, Li L, Marrie T, Sykes BD, Vogel HJ, Querengesser L. Nucleic Acids Res. 2007;35:D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutnick D, Calvo JM, Klopotow T, Ames BN. J. Bacter. 1969;100:215. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo B, Groenke K, Takors R, Wandrey C, Oldiges M. J. Chromatogr A. 2007;1147:153–164. doi: 10.1016/j.chroma.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Bennett BD, Rabinowitz JD. J. Chromatogr. B. 2008;871:236–242. doi: 10.1016/j.jchromb.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buscher JM, Czernik D, Ewald JC, Sauer U, Zamboni N. Anal. Chem. 2009;81:2135–2143. doi: 10.1021/ac8022857. [DOI] [PubMed] [Google Scholar]
- 26.Winston F, Dollard C, Ricupero-Hovasse SL. Yeast. 1995;11:53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]
- 27.Yuan J, Fowler WU, Kimball E, Lu WY, Rabinowitz JD. Nat. Chem. Biol. 2006;2:529–530. doi: 10.1038/nchembio816. [DOI] [PubMed] [Google Scholar]
- 28.Brauer MJ, Yuan J, Bennett BD, Lu WY, Kimball E, Botstein D, Rabinowitz JD. Proc. Natl. Acad. Sci. USA. 2006;103:19302–19307. doi: 10.1073/pnas.0609508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan J, Bennett BD, Rabinowitz JD. Nat. Protoc. 2008;3:1328–1340. doi: 10.1038/nprot.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. Nat. Biotechnol. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G. Anal. Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 32.Keller A, Eng J, Zhang N, Li XJ, Aebersold R. Mol. Syst. Biol. 2005;1:8. doi: 10.1038/msb4100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breiman L. Mach. Learn. 2001;45:5–32. [Google Scholar]
- 34.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y. Nucl. Acids Res. 2008;36:D480–D484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caspi R, Foerster H, Fulcher CA, Kaipa P, Krummenacker M, Latendresse M, Paley S, Rhee SY, Shearer AG, Tissier C, Walk TC, Zhang P, Karp PD. Nucl. Acids Res. 2008;36:D623–D631. doi: 10.1093/nar/gkm900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, Hau DD, Psychogios N, Dong E, Bouatra S, Mandal R, Sinelnikov I, Xia JG, Jia L, Cruz JA, Lim E, Sobsey CA, Shrivastava S, Huang P, Liu P, Fang L, Peng J, Fradette R, Cheng D, Tzur D, Clements M, Lewis A, De Souza A, Zuniga A, Dawe M, Xiong YP, Clive D, Greiner R, Nazyrova A, Shaykhutdinov R, Li L, Vogel HJ, Forsythe I. Nucl. Acids Res. 2009;37:D603–D610. doi: 10.1093/nar/gkn810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox KA, Cleven CD, Cooks RG. Intern. J. Mass Spectrom. Ion Proc. 1995;144:47–65. [Google Scholar]
- 38.Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz J. D.Nat. Biotechn. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD. Nat. Chem. Biol. 2009;5:593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saghatelian A, Cravatt BF. Nat. Chem. Biol. 2005;1:130–142. doi: 10.1038/nchembio0805-130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.