Abstract
Neonates respond poorly to conventional vaccines. This has been attributed, in part, to the immaturity of neonatal dendritic cells (DC) that lack full capacity for Ag presentation and T cell stimulation. We engineered an attenuated Salmonella Typhi strain to express and export the F1 Ag of Y. pestis (S. Typhi(F1)) and investigated its immunogenicity early in life using a heterologous prime-boost regimen. Newborn mice primed intranasally with a single dose of S. Typhi(F1) elicited mucosal Ab- and IFN-γ secreting cells 1 wk after immunization. They also developed a potent and fast anamnestic response to a subsequent parenteral boost with F1-alum, which surpassed those of mice primed and boosted with S. Typhi(F1) or F1-alum. Neonatal priming with S. Typhi(F1), as opposed to priming with F1-alum, resulted in a more balanced IgG2a/IgG1 profile, enhanced avidity maturation and stimulation of B memory cells, and strong Th1-type cell-mediated immunity. S. Typhi(F1) enhanced the activation and maturation of neonatal CD11c+ dendritic cells, shown by increased expression of CD80, CD86, CD40 and MHC-II cell surface markers and production of pro-inflammatory cytokines IL-12, TNF-α, IL-6 and MCP-1. S. Typhi(F1)-stimulated neonatal DC had improved capacity for Ag presentation and T cell stimulation in vitro and induced F1-specific CD4+ and CD8+ T cell responses when adoptively transferred to newborn mice. Mucosal immunization with S. Typhi expressing a foreign Ag effectively primes the neonatal immune system for potent, fast, and broader responses to a parenteral Ag boost. Such a strategy can prevent infectious diseases, including those considered biowarfare threats, early in life.
Keywords: Prime-boost immunization, S. Typhi live vector vaccines, newborn mice
Introduction
Vaccines against organisms that could be used as biological weapons have gained considerable interest. Yersinia pestis is one such pathogen, a gram-negative bacterium that causes bubonic and pneumonic plague. Regardless of the route of infection, the disease results in high mortality (50-90%) if left untreated (1).
Interest in a prophylactic vaccine extends beyond biodefense, as isolated plague outbreaks occur sporadically and antibiotic-resistant strains have been described (2). There is no commercially available vaccine against plague. Live attenuated strains and more recently formalin-killed whole cell vaccines were developed, but proved highly reactogenic in humans (reviewed in Refs. 3 and 4). A killed whole-cell vaccine was licensed in the U.S., but was withdrawn from use because it required multiple doses, was highly reactogenic, and did not protect against pneumonic plague (5,6). The F1 capsular Ag and the V Ag (LcrV, a component of the Y. pestis type-III secretion system) have been evaluated as subunit vaccines, and shown to induce protection against bubonic and pneumonic plague in several animal models (7-9). These Ags also elicited Abs when given to humans (10).
In the current climate of biowarfare threat, there has been renewed interest in the development of safe and effective biodefense vaccines to protect all segments of the population, including children, the elderly, and the growing number of immunocompromised individuals. Emphasis has also been placed in identifying immunization strategies that would address their particular needs. A subunit alum-adjuvanted plague vaccine tailored for healthy adults would not be suitable for immunization of infants and young children who mount feeble Th2-biased responses to purified proteins in the absence of immunostimulatory signals (11). This has been attributed, at least in part, to the presence of immature APC that lack full capacity for Ag presentation and T cell stimulation (12). The use of alum poses an additional safety concern for pediatric immunization as this adjuvant favors Ab responses, further polarizing the Th2-type biased neonatal immunity and thereby increasing the risk of allergic reactions.
An approach that would enable using Y. pestis protective Ags such as F1 and/or LcrV for early life immunization would be through mucosal delivery via live vectors. Besides targeting Ags to professional APC (13), an attenuated bacterial-vector vaccine would have the capacity to activate innate immune cells and trigger proinflammatory signals that will promote adaptive responses, especially Th1-type cell-mediated immunity. Mucosally delivered live vectors could also stimulate local immune responses to enhance protection against aerosol infection.
Several studies have demonstrated the feasibility of using Salmonella as a live vector expressing Ags from different pathogens, including Y. pestis (reviewed in Refs. 3 and 14). Most of the work on Salmonella plague vaccines has been based on S. Typhimurium constructs expressing Y. pestis F1 and LcrV that were tested in mice (15-19). For humans, however, a Salmonella-based vaccine expressing F1 and/or LcrV would be more useful if engineered in the S. Typhi background, to protect against both plague and typhoid fever. Attenuated S. Typhi strains delivering foreign Ag have been evaluated in animal models (reviewed in 14), and a handful of these recombinant strains have been successfully administered to humans (20-22).
There is mounting evidence that protective immunity can be improved when vaccine Ags are administered in different forms or routes in so-called prime-boost regimens (23). Londoño-Arcila et al. (24) first demonstrated that priming with S. Typhi expressing Helicobacter pylori urease and boosting with urease-alum protected mice against challenge with virulent organisms, whereas neither immunization with S. Typhi expressing urease nor urease-alum conferred protection. Vindurampulle et al. (25) found increased levels of tetanus antitoxin in mice primed with S. Typhi expressing tetanus toxin Fragment C (Frag C) and boosted with tetanus toxoid compared with mice immunized with Frag C-expressing S. Typhi.
Our group showed for the first time that newborn mice immunized intranasally (i.n.) with S. Typhi expressing tetanus toxin Frag C developed remarkable mucosal and systemic responses during the neonatal period (26).
In this study, we developed a S. Typhi-based live vector vaccine that can express and export the Y. pestis F1 capsular Ag and evaluated its use for early life immunization. Borrowing from the success of prime-boost immunization in adult mice we examined whether neonatal responses to F1 expressed by S. Typhi could be further improved by a subsequent parenteral boost with F1-alum. We explored the characteristics of the prime-boost responses and the mechanisms underlying live vector priming during the neonatal period, specifically the capacity of S. Typhi to activate and enhance maturation of dendritic cells (DC) and its influence on Ag presentation, cytokine secretion and T cell stimulation.
Material and Methods
Construction of a recombinant S. Typhi vaccine strain expressing Y. pestis F1; bacterial strains and culture conditions
The F1-expressing operon from Y. pestis strain EV76 (pgm−) genomic DNA was PCR amplified using Pfx DNA polymerase (Invitrogen Life Technologies) and the caf3 (5′- GCC GAA TTC CGA ACA TAA ATC GGT TCA GTG GCC TCA ACG CTG-3′) and cafAvr2 (5′- GGC CCT AGG TGA ACC TAT TAT ATT GCT TCG CGC-3′) primers. The resulting PCR product containing the caf1R transcriptional regulator, the chaperone caf1M and the usher caf1A, and the F1-encoding gene caf1 was digested with EcoRI and AvrII and cloned into plasmid pSEC10 (27). Plasmid pSEC10 was digested with EcoRI and NheI and the ompC promoter and clyA gene were replaced with the caf operon resulting in plasmid pSL74. To reduce the expression of F1, which was found to be mildly toxic to the bacteria, the start codon of the caf1 gene in pSL74 was changed from ATG to GTG using the QuikChange mutagenesis kit (Stratagene) and the primers F1GTG1 (5′-GAT AGA GGT AAT ATG TGA AAA AAA TCA GTT CCG-3)′ and F1GTG2 (5′-CGG AAC TGA TTT TTT TCA CAT ATT ACC TCT ATC-3′). The sequence of the resulting plasmid pSL74-GTG was verified by DNA sequence analysis. Plasmid pSL74-GTG and pSEC10 were electroporated following standard techniques into S. Typhi vaccine strain ACAM948CVD (Acambis), which is a strain derived by growing S. Typhi CVD 908-htrA, an aroC, aroD, htrA mutant (28) in animal free medium to comply with regulatory requirements. The selected vaccine strain, ACAM948CVD(pSL74-GTG), is referred to hereafter as S. Typhi(F1). The live vector strains were grown at 37°C in Luria-Bertani medium containing dihydroxy-benzoic acid (Sigma-Aldrich) and kanamycin (50 μg/ml) when needed; expression of F1 from plasmid pSL74-GTG was constitutive under these conditions.
Analysis of F1 expression
S. Typhi carrying pSEC10 (empty plasmid) and pSL74-GTG were grown overnight at 37°C as described above. F1 expression was detected by immunoblot analysis of whole bacterial lysates using an anti-F1 mAb (Research Diagnostics) and HRP-labeled goat anti-mouse IgG (Amersham Biosciences) as previously described (29). Surface expression of F1 was also investigated by immunofluorescence. S. Typhi(F1) and S. Typhi carrying pSEC10 were stained with mouse anti-F1 mAb (RDI) in PBS containing 0.1% BSA and 0.01% NaN3 (PBA) for 1 h at room temperature, washed and incubated with FITC-labeled anti-mouse IgG (Invitrogen) for 30 min. Stained bacteria were mounted in Prolong Gold (Invitrogen) and visualized using a Nikon Eclipse 2000-E UV fluorescent microscope. Images were acquired using MetaVue software, version 6.1 (Universal Imaging Corp). To demonstrate F1 capsule formation, S. Typhi(F1) and S. Typhi carrying pSEC10 were stained with India ink (BD Biosciences) as previously described (30).
Expression of S. Typhi flagella and motility
The motility of S. Typhi carrying pSEC10 and pSL74-GTG was measured as described (31) with modifications. Briefly, overnight cultures (0.5 μl) were spotted in swarm agar plates, allowed to dry for 1 h at room temperature, and incubated for 30 h at 37°C. Surface expression of flagellar Ag was assessed in overnight cultures using Ryu staining (Remel; Thermo Fisher Scientifics) and images were obtained as described above.
Mice and immunizations
BALB/c mice (8-10 wk old) purchased from Charles River Laboratories were bred to produce pups as previously described (26,29). Newborn (7-day old) mice were immunized i.n. with 1×109 CFU of either S. Typhi(F1) or S. Typhi alone (2.5 μl were delivered in each nare, without anesthesia) and/or i.m. with 5 μg of F1 adsorbed to 0.5% alhydrogel (Accurate Chemical & Scientific Corp) on days 7 and 22 after birth. Pre-immune serum samples were collected by cardiac puncture from age-matched naïve pups. Additional bleedings were performed from the retro-orbital sinus. Mice were euthanized 15 or 70 days after birth. All animal studies were approved by the University of Maryland Institutional Animal Care and Use Committee.
F1-specific IgG, IgG subclasses, and IgG avidity
Serum IgG, IgG1, and IgG2a against Y. pestis F1 were measured by ELISA. Immunlon II plates (Thermo Scientific) were coated with F1 at 0.5 μg/ml in PBS, for 3 h at 37°C, and blocked overnight with 10% dry milk (Nestle) in PBS. Plates were washed six times with PBS containing 0.05% Tween 20 (PBST). Serum samples diluted 2-fold in 10% milk-PBST were incubated for 1 h at 37°C. F1-specific antibodies were detected with HRP-labeled anti-mouse IgG, IgG1, and IgG2a (Roche) diluted 1:1,000 in 10% milk-PBST for 1 h at 37°C. TMB Microwell Peroxidase (Kirkegaard & Perry Laboratories) was used as substrate. After 15 min of incubation, the reaction was stopped by the addition of 100 μl of 1M H3PO4. Sera were run in duplicate; negative and positive calibrated controls were included in each assay. Titers were calculated from linear regression curves as the inverse of the dilution that produces an absorbance value of 0.2 above the blank (ELISA U/ml). The avidity of F1-specific IgG antibodies was measured by ELISA with an additional 10-min 6 M urea elution step after incubation with the samples (29). The avidity index was calculated as the percentage of residual activity (end point titer) after treatment with urea.
Ab-secreting cells (ASC) ELISPOT
The frequency of F1-specific ASC was measured in the lung and nasal tissue, as previously described (26,29). Plates were coated with 5 μg/ml of F1 and blocked with complete medium: RPMI 1640 supplemented with 10% FCS, 200 mM glutamine, and gentamicin 50 μg/ml (Invitrogen Life Technologies). Fresh cells were added in 2-fold dilutions (5×105 to 6.25×104) and incubated overnight at 37°C, 5% CO2. F1-specific Abs were revealed with HRP-labeled goat anti-mouse IgA or IgG (Roche) diluted in PBS 1% BSA. Spots were developed using True Blue substrate (Kirkegaard & Perry Laboratories) in agarose overlay. In all ELISPOT assays spots from control wells were subtracted from experimental wells and the threshold for a positive response was defined as 4 spots per 106 cells.
IFN-γ ELISPOT
Cryopreserved splenocytes (1×106 cells/well in 24-well plates) were allowed to recover in complete RPMI 1640 supplemented with 5 IU/ml of IL-2 (Preprotech) for 2-3 days at 37°C, 5% CO2. Cells (1.25×105/well) were added to MultiScreen-HA nitrocellulose plates (Millipore) coated with 5 μg/ml anti-mouse IFN-γ (BD Pharmingen), and incubated in the presence of F1 (10 μg/ml) for 36 h at 37°C, 5% CO2. Cells in complete RPMI 1640 or stimulated with PHA (2 μg/ml; Sigma-Aldrich) were included as control. IFN-γ spots were revealed with a biotin-labeled anti-mouse IFN-γ at 5 μg/ml (BD Pharmingen) followed by streptavidin-HRP (Sigma-Aldrich) and True Blue (Kirkegaard & Perry Laboratories) substrate.
F1-specific T cell proliferation
Fresh spleen cells (2×105 cells/well in 96-well plates) were incubated with 5 μg/ml F1 or BSA (control) for 6 days at 37°C, 5% CO2. Cell proliferation was measured by incorporation of [3H] thymidine as previously described (26). The cpm in control wells were subtracted from cpm in F1-stimulated wells.
Memory B cells
F1-specific IgG memory B cells were measured using a method described by Crotty et al. (32) adapted for mouse cells. Briefly, splenocytes were stimulated for 6 days with polyclonal mitogens: Phytolacca americana lectin at 2 μg/ml (Sigma-Aldrich), E. coli O55:B5 LPS (Sigma-Aldrich) at 50 μg/ml and CpG ODN 1826 (5′-TCC ATG ACG TTC CTG ACG TT-3′) at 0.3 μg/ml. Expanded memory B cells were transferred to 96-well plates (5×105 to 3.25×104 cells/well) coated with F1 (5 μg/ml in PBS) or with rabbit anti-mouse IgG (Zymed Laboratories) (10 μg/ml in PBS) and blocked with complete RPMI 1640 for 2-4 h at 37°C. Following overnight incubation at 37°C and 5% CO2, total and F1-specific IgG antibodies were revealed with HRP-labeled goat anti-mouse IgG (5 μg/ml, Roche) followed by True Blue substrate (Kirkegaard & Perry Laboratories) in agarose overlay as described above.
Y. pestis challenge
Mice were challenged i.v. with 180 MLD50 (10,000 CFU) of Y. pestis EV76 in 0.2 ml of sterile PBS. FeCl2 (40 μg/mouse) was administered i.p. immediately before challenge to enhance bacterial virulence (33). Health status was monitored for 13 days using a scale from 1 to 4. A score of 1 (normal) was assigned to healthy mice with normal posture and no signs of dehydration; score 2 to early stage of piloerection with normal posture and mild dehydration; score 3 for mild piloerection, dull, hunched and moderate dehydration; and score 4 for severe piloerection, dull, hunched and squinting with severe tenting. All survivors were humanely euthanized at the end of the monitoring period.
Generation of neonatal and adult immature DC
Cells from bone marrow (BM) were collected from the tibias of 7 day-old (neonates) and 8 week-old (adult) mice, as previously described (29). Cells (1-3×107 cells in 25-ml flask) were cultured with mouse GM-CSF (10 ng/ml, BD Pharmingen) and IL-4 (10 ng/ml, BD Pharmingen) for 2 h at 37°C, 5% CO2. Non-adherent cells were transferred to a new tissue culture flask and incubated with complete RPMI 1640 in the presence of GM-CSF and IL-4 for 6 days. Cells were then fed with fresh medium and harvested on day 8. Cell differentiation and viability was monitored by phase light microscopy.
Stimulation of DC with S. Typhi strains and flow cytometric analysis
BM-derived DC resuspended in RPMI 1640 without antibiotics (2.5×105 cells/well in 24-well plates) were incubated with S. Typhi(F1) (multiplicity of infection (m.o.i)=30), S. Typhi alone (m.o.i.=30), F1 (5 μg/ml), or mock stimulated (negative control) for 2 h at 37°C, 5% CO2. CD11c+ DC were also incubated for 24 h with E. coli LPS (Sigma-Aldrich, 10 ng/ml) as a positive control. Cells were then washed 6 times with PBS containing 100 μg/ml of gentamicin to eliminate extracellular bacteria, allowed to recover overnight in complete medium at 37°C, 5% CO2 , and stained with FITC-, PE-, or allophycocyanin-labeled Abs specific for CD11c, CD40, CD80, CD86 and IAd (BD Pharmingen) for 30 min at 4°C. To reduce staining background, cells were pre-incubated with Fc-Block (anti-mouse CD16/32; BD Pharmingen)/PBA buffer for 30 min at 4°C. Viability was confirmed by staining with 5 μg/ml of ethidium monoazide (80-90%). Stained cells were fixed with 4% paraformaldehyde and run in a DakoCytomation MoFlo flow cytometer. Data were collected from 10,000 to 30,000 cells and analyzed using WinList 6.0 3D software (Verity Software House).
Laser confocal microscopy
Magentically sorted CD11c+ DC (95% purity, Miltenyi Biotec) were treated with S. Typhi as described above, fixed and permeabilized for 20 min with Cytofix/Cytoperm (BD Pharmingen) at room temperature. They were subsequently washed and blocked for 30 min with goat anti-mouse IgG and mouse Fc-Block at 4°C. For S. Typhi and MHC-II staining, DC were incubated with FITC-labeled goat anti-Common Salmonella Ag-1 (Kirkegaard & Perry Laboratories) and with purified anti-mouse IAd (BD Pharmingen) (both in PBA, for 1 h at room temperature) followed by Alexa Fluor 546-labeled anti-mouse IgG (Invitrogen), respectively. To visualize the nuclei, cells were incubated with 4′,6′-diamidino-2-phenylindole dihydrochloride (Invitrogen) in PBA for 5 min. Prolong Gold (Invitrogen) was used as a mounting solution. Stained DC were visualized using a Zeiss LSM510 META laser scanning confocal microscope. Images were acquired in the mid-plane of the cells. Z-stack sections were collected at 0.5 μm intervals. Images were analyzed with Adobe Photoshop 7.0 and AxioVision 4.6 software.
FITC-dextran uptake
BM-derived DC stimulated with S. Typhi(F1), F1 or mock-stimulated were resuspended in complete RPMI 1640 and incubated with 1 mg/ml of FITC-dextran (Sigma-Aldrich) for 2 h at 37°C or 4°C. Cells were washed six times with ice-cold PBA and analyzed by flow cytometry. Dextran uptake was measured as the increase of FITC-positive cells incubated at 37°C vs. 4°C.
Cytokine measurement
The levels of IL-12p70, TNF-α, IL-10, IL-6 and MCP-1 were quantified in overnight culture supernatants from S. Typhi-, S. Typhi(F1)-, F1- and mock-stimulated neonatal and adult BM-derived DC using Cytometric Bead Array (CBA) Mouse Inflammation kit (BD Pharmingen). Samples were run in a Beckman Coulter Epics Elite flow cytometer. Cytokine concentrations were determined by extrapolating fluorescence intensities of individual samples in standard reference curves (CellQuest Pro and CBA software; BD Pharmingen) using BD CBA software (BD Biosciences).
F1-specific in vitro T cell stimulation by S. Typhi-stimulated neonatal DC
CD3+ T cells (90-95% purity) were purified from spleens of naïve or F1-immune mice by negative magnetic sorting (Dynal Biothech). Increasing numbers of S. Typhi(F1)-, F1- or mock-stimulated CD11c+ DC purified by magnetic cell sorting (BD Pharmingen) were co-cultured with 1×105 F1-specific CD3+ T cells. T cell stimulation was assessed by measuring the frequency of IFN-γ production and T cell proliferation as described above. Negative controls included wells containing F1-specific CD3+ T cells alone, CD11c+ DC alone and CD11c+ DC co-cultured with naïve CD3+ T cells. CD3+ T cells incubated with ConA (2 μg/ml, Sigma-Aldrich) or with F1 (5 μg/ml) served as positive controls. Responses in negative control wells were subtracted from experimental wells.
Adoptive transfer of S. Typhi-treated DC
S. Typhi(F1)-, F1- or mock-treated magnetically sorted CD11c+ DC derived from adult (8 wk-old) or newborn (7 days-old) donors were injected into adult (2×105 cells/mouse, i.v.) or newborn (1×106 cells/mouse, i.p.) recipient mice. Spleens were collected seven days later and CD4+ and CD8+ magnetically sorted T cells (90-95% purity) were stimulated in vitro with F1 (5 μg/ml). The frequency of IFN-γ secreting cells and T cell proliferation were measured as described above.
Statistical analysis
All measurements were compared using Student's t test or Mann-Whitney U (if normality failed). Kruskal-Wallis nonparametric ANOVA, followed by Dunn's test, was used for multiple comparisons. Differences with p <0.05 were considered significant at the 95% confidence interval. Statistical analysis was performed using SigmaStat 3.5 (Systat software).
Results
Construction of an attenuated S. Typhi vaccine strain expressing Y. pestis F1
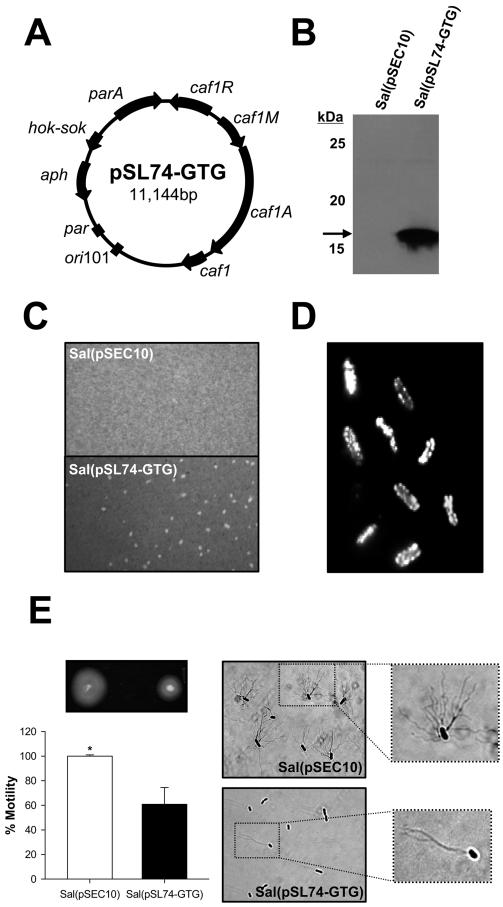
We engineered an attenuated S. Typhi vaccine strain capable of expressing the Y. pestis F1 capsule from the Y. pestis F1 operon encoded by plasmid pSL74-GTG (Fig. 1A). Ag expression was confirmed by Western immunoblot analysis of bacterial lysates (Fig. 1B); S. Typhi(pSL74-GTG), henceforth referred as S. Typhi(F1), produced a single band of the expected molecular mass ∼18 kDa recognized by F1-specific antibodies, whereas no protein expression was produced by S. Typhi carrying the empty plasmid pSEC10. The encapsulated phenotype of S. Typhi(F1) was demonstrated by India ink staining (Fig. 1C); S. Typhi(pSL74-GTG) colonies exhibited visible halos of dye exclusion (F1 capsule) that were absent in S. Typhi(pSEC10). Abundant F1 surface expression was confirmed by immunofluorescence microscopy (Fig. 1D). Both S. Typhi(pSL74-GTG) and S. Typhi(pSEC10) exhibited a flagellated phenotype, although flagellation and motility were markedly reduced in the F1-expressing strain (Fig. 1E).
FIGURE 1.
Expression of Y. pestis F1 by S. Typhi. A, Plasmid map of pSL74-GTG encoding the Y. pestis F1 operon. B, Western blot analysis of F1 expression. Bacterial lysates from S. Typhi strain ACAM948CVD carrying pSL74-GTG or empty plasmid pSEC10 were separated by SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes, and F1 was revealed with F1-specific mAb. C, The formation of an external F1 capsule in S. Typhi(pSL74-GTG) was demonstrated by india ink staining; cultures were photographed under a light microscope with a Nikon Coolpix 4300 digital camera. D, Fluorescent microscopy confirming abundant F1 expression on the surface of S. Typhi(pSL74-GTG) upon incubation with F1-specific mAb and FITC-labeled anti-mouse IgG. No fluorescent staining was observed in S. Typhi(pSEC10) and S. Typhi(pSL74-GTG) incubated with normal mouse serum (data not shown). E,. Differential motility of S. Typhi(pSL74-GTG) and S. Typhi(pSEC10) and flagellar Ag expression shown by Ryu staining. *, p <0.05 indicates significant difference in motility between strains. Images were obtained with a ×100 objective.
Ab responses in newborn mice primed with S. Typhi(F1) and boosted with F1-alum
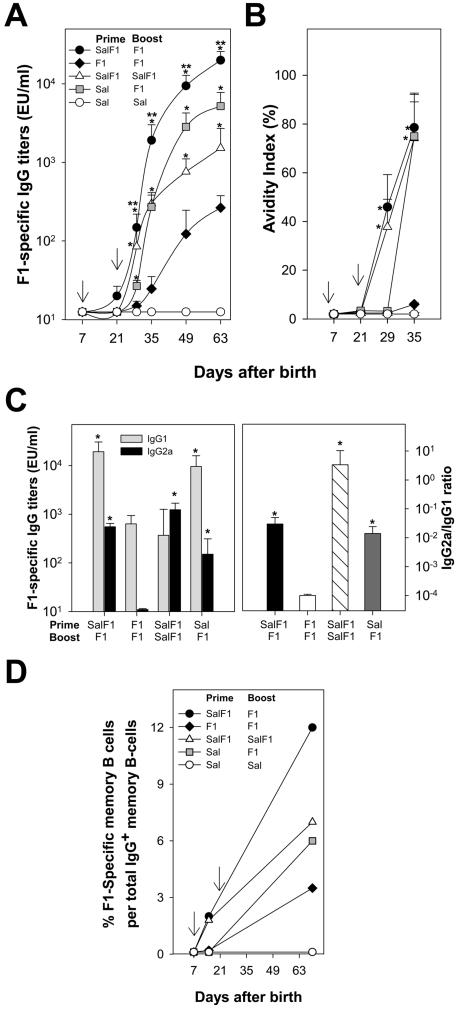
We have shown that mucosal immunization of newborn mice with S. Typhi expressing a foreign Ag generates potent immune responses during the neonatal period (26). In adult mice, responses to Salmonella-expressed Ags were further improved when mucosal live vector priming was followed by parenteral Ag boost (24,25). In this study, we investigated the capacity of S. Typhi(F1) to mucosally prime the neonatal immune system for a vigorous and accelerated anamnestic response to a subsequent parenteral boost with F1-alum. Newborn mice were primed i.n. on day 7 after birth with 1×109 CFU of S. Typhi(F1) or S. Typhi alone (control of priming) and boosted i.m. on day 22 with 5 μg of F1 adsorbed to alum. Additional groups received two consecutive doses of S. Typhi(F1) or F1-alum to compare heterologous vs homologous prime-boost, and S. Typhi empty live vector or PBS as negative controls. Kinetics of F1-specific serum IgG measured by ELISA are shown in Fig. 2A. Newborn mice primed with S. Typhi(F1) and boosted with F1-alum developed the highest levels of F1-specific serum IgG (p <0.05; peak geometric mean titer (GMT), 11,439.1 EU/ml), surpassing those of mice primed with S. Typhi alone or with F1-alum, (peak GMT 5215.3 and GMT 265.1 EU/ml, respectively) and those of mice primed and boosted with S. Typhi(F1) (p <0.05, peak GMT 1522 EU/ml). Mice primed as newborns with F1 had the lowest Ab responses of all groups throughout the experiment. Newborns primed with S. Typhi(F1) and boosted with F1 exhibited the fastest anamnestic responses; titers increased 100-fold within the first 2 wk after the boost (20 to 1915.2 EU/ml on days 21 and 35 respectively, p <0.05). Meanwhile, Ab levels in mice primed and boosted parenterally with F1 remained undetectable (GMT 12.5 to 25 EU/ml on days 21 and 35). Interestingly, mice primed with S. Typhi empty vector exhibited a sharp increase in Ab levels in response to the F1 boost, which exceeded those of mice primed and boosted with S. Typhi(F1) or F1-alum. No responses were observed in mice that received S. Typhi alone or PBS.
FIGURE 2.
Mucosal priming of newborn mice with Salmonella-expressing Y. pestis F1 enhances Ab responses to a subsequent dose of F1 given parenterally. Newborn mice (8-12/group) were immunized i.n. with 1×109 CFU of S. Typhi expressing F1 (SalF1), S. Typhi alone (Sal) or with 5 μg of F1 (F1) i.m. on days 7 and 22 after birth (arrows) in different prime-boost combinations as indicated in the figure legends. A, Kinetics of F1-specific serum IgG; B, kinetics of avidity maturation; and C, IgG subclass distribution (IgG1 and IgG2a titer and IgG2a/IgG1 ratio measured on day 49 after birth). Results shown are mean IgG titers, IgG avidity indices and IgG2a/IgG1 ratio ± SEM. Significant differences (*, p <0.05) compared with the F1 prime-F1 boost or S. Typhi prime-F1 boost (**, p<0.05) groups are indicated. D, Kinetics of F1-specific B memory cells measured by ELISPOT in spleen cells after 6-day mitogen expansion; results indicate percent of mean F1 specific IgG ASC per total IgG+ memory B cells ± SEM.
Neonatal priming with S. Typhi promotes avidity maturation, a balanced IgG2a/IgG1 subclass profile and F1-specific B memory cells
The avidity of Abs is related to their functional capacity, and impaired avidity maturation early in life has been associated with vaccine failure and increased susceptibility to disease (34). Although the capacity for avidity maturation is not fully developed in very young infants, vaccines that support Th1-type responses appear to favor this process. We examined the kinetics of avidity maturation of F1 IgG antibodies in newborn mice primed with S. Typhi(F1) and boosted with F1-alum, as well as in mice primed and boosted with S. Typhi(F1) or F1-alum, and control groups (Fig. 2B). Priming with S. Typhi(F1) followed by F1 boost elicited high-avidity antibodies (p <0.05), as did priming and boosting with S. Typhi(F1); in fact, curves of avidity maturation overlapped for these two groups. Newborns that had been primed with S. Typhi empty live vector and boosted with F1 also produced high avidity Abs, although these Abs appeared later. In contrast, no avidity maturation was seen in mice primed and boosted with F1-alum.
We next investigated the IgG subclass profile in newborns primed with S. Typhi(F1) and boosted with F1-alum, primed and boosted with S. Typhi(F1) or F1-alum, and controls (Fig. 2C). Immunization with S. Typhi(F1) resulted in increased levels of F1-specific IgG2a. In fact, priming and boosting with S. Typhi(F1) produced the highest proportion of IgG2a and the uppermost IgG2a/IgG1 ratio (Fig. 2C, right; p <0.05). In contrast, priming and boosting with F1-alum produced exclusively IgG1; this group had the lowest IgG2a/IgG1 ratio of all treatments. Newborns primed with S. Typhi(F1) and boosted with F1-alum produced high levels of both IgG1 and IgG2a and exhibited an intermediate mean IgG2a/IgG1 ratio compared with those primed and boosted with S. Typhi(F1) or F1-alum. Similar IgG1 and IgG2a levels and mean IgG2a/IgG1 ratio were observed in mice primed with S. Typhi empty vector after the F1 boost.
Because Ab responses to T-dependent Ags rapidly decline during early infancy (35), an effective vaccine for this age group is expected to generate memory B cells to maintain the pool of ASC and circulating Abs. This prompted us to examined the appearance of F1-specific memory B cells in spleen of mice primed as newborns with S. Typhi(F1) and boosted with F1-alum (Fig. 2D), primed and boosted with S. Typhi(F1) or F1, and controls. Newborns primed with S. Typhi(F1) had a detectable pool of F1-specific memory B cells on day 15, during the true neonatal period. This group displayed the highest frequency of memory B cells in response to the F1-alum boost. B memory cells, although at a much lower frequency, were also seen in mice primed and boosted with S. Typhi(F1) and in mice primed with S. Typhi empty live vector and boosted with F1-alum. Mice primed and boosted with F1-alum had the lowest memory B cell responses of all treatments.
Priming of newborn mice with S. Typhi(F1) elicits F1-specific cellular and mucosal immunity
An essential attribute sought in any new vaccine for young infants is the capacity to engender T cell responses, usually absent in the neonatal period, and yet critical to protect against intracellular pathogens. We investigated the F1-specific T cell responses in newborns primed with S. Typhi(F1) and boosted with F1-alum by measuring the frequency of IFN-γ secreting and proliferative T cells on days 15 and 70 after birth (Fig. 3). Newborns primed with S. Typhi(F1) developed potent F1-specific IFN-γ responses after a single immunization, during the true neonatal period (day 15) and these responses exceeded those of mice primed with F1-alum (p <0.05) (Fig. 3A). IFN-γ-secreting T cells were also observed on day 70, after the F1 boost; S. Typhi(F1)-primed mice exhibited higher spot-forming cell (SFC) frequencies than mice primed with S. Typhi alone (although not reaching statistical significance) and mice primed and boosted with S. Typhi(F1) or F1-alum (p <0.05). Newborns primed with S. Typhi(F1) also developed F1-specific T cells that proliferated in vitro upon F1 stimulation (Fig. 3B); proliferative T cells were also seen after boosting with either S. Typhi(F1) or F1-alum. Significant responses were detected in mice primed with S. Typhi alone and boosted with F1. Mice primed and boosted with F1-alum were the lowest responders of all vaccine groups.
FIGURE 3.
Mucosal priming with S. Typhi(F1), followed by parenteral F1 boost, elicits potent F1-specific T cell-mediated immunity. Newborn mice were immunized as described in Fig. 2. IFN-γ production and T cell proliferation were measured in spleen collected on days 15 (neonatal period) and 70 after birth. A, Frequencies of F1-specific IFN-γ secreting T cells were measured by ELISPOT upon ex-vivo stimulation with F1; results are expressed as mean SFC per 1×106 cells ± SEM of replicate cultures. B, Proliferative responses in spleen cells stimulated in vitro with F1 were measured by [3H] thymidine incorporation; results are expressed as mean cpm ×103 ± SEM from replicate wells. Significant differences (*, p <0.05) compared with the F1 prime-boost group are indicated.
An advantage of using bacterial vector vaccines is the potential for oral delivery and their ability to induce mucosal as well as systemic immunity. We investigated the presence of F1-specific IgA and IgG ASC in lungs and nasal tissue of newborn mice primed with S. Typhi(F1). High levels of F1-specific IgA were found in both tissues on day 15 after birth (Fig. 4; p <0.05). F1-specific IgG ASC were also detected on day 15, in response to S. Typhi(F1) priming, and on day 70, after boosting with S. Typhi(F1) or F1-alum. Priming and boosting with F1-alum consistently failed to elicit mucosal responses; no ASC were detected in either tissue, at any time point in repeated experiments. No responses were detected in mice primed with S. Typhi empty vector and boosted with F1 or in the negative control group.
FIGURE 4.
Neonatal priming with S. Typhi(F1) elicits potent mucosal immunity. Newborn mice were immunized as described in Fig. 2. F1-specific IgA or IgG ASC responses were measured in lung (A) and nasal tissue (B) on days 15 and 70 after birth. Results shown are mean IgA or IgG ASC per 1×106 cells ± SEM of replicate wells. Significant differences (*, p <0.05) compared with the F1 prime-boost group are indicated.
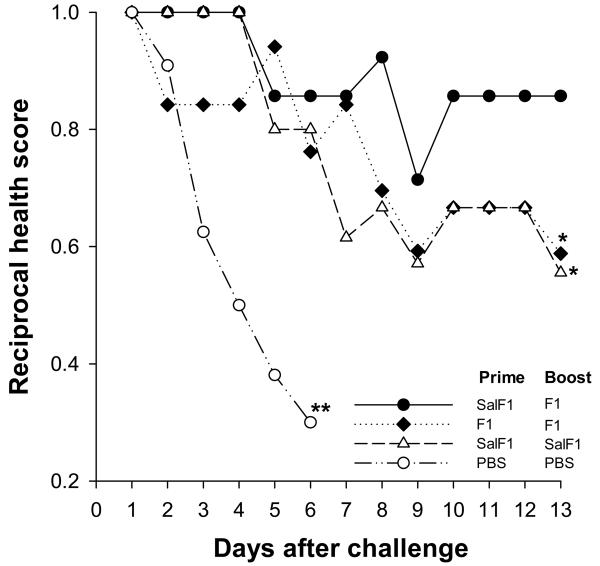
S. Typhi(F1) followed by F1 boost protects mice against lethal plague challenge
We demonstrated the protective efficacy of the neonatal S. Typhi(F1)-prime F1-boost regimen in a model of systemic plague infection. Mice were subjected to lethal i.v. challenge with Y. pestis EV76 and subsequently monitored for signs of disease and mortality (Fig. 5). Unvaccinated mice died shortly after challenge. In contrast, newborns that had been primed with S. Typhi(F1) and boosted with F1-alum remained healthy throughout the monitoring period. Significantly lower protection (p<0.02) was observed in mice primed and boosted with S. Typhi(F1) or F1-alum; these animals showed progressive signs of disease.
FIGURE 5.
Neonatal priming with S. Typhi(F1), followed by F1 boost, protects mice against lethal plague infection. Mice were immunized as described in Fig. 2, and challenged ∼2 mo after the boost with Y. pestis EV76 (180 MLD50) in the presence of FeCl2. Curves show health scores (reciprocal of mean values) during the 13-day monitoring period. Significant differences (*, p <0.02) compared with the heterologous prime-boost are indicated. **, Unvaccinated PBS controls died on day 6 after challenge.
S. Typhi enhances neonatal DC maturation and production of proinflammatory cytokines
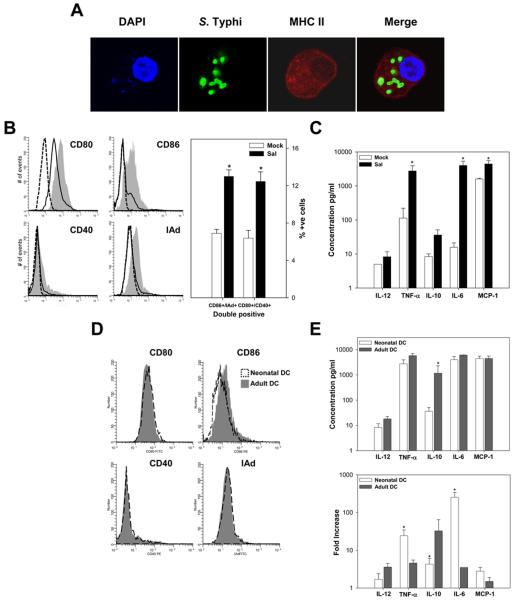
One of the major hurdles to overcome for successful immunization in the neonatal setting is the presence of immature DC that have not yet developed full capacity for Ag presentation and T cell stimulation (36). We hypothesized that a Salmonella-based vaccine, due to its adjuvant properties, would activate and enhance maturation of neonatal DC, thereby allowing for a more efficient Ag presentation and T cell stimulation in the early life setting. To test this hypothesis, BM-derived immature neonatal DC were generated by treatment of nonadherent cells with IL-4 and GM-CSF; CD11c+ cells were sorted and stimulated in vitro with S. Typhi using increasing m.o.i. Bacterial infection was examined by confocal microscopy (Fig. 6A). Cell activation and maturation were first assessed through the expression of cell surface markers by flow cytometry (Fig. 6B). S. Typhi-stimulated neonatal CD11c+ DC exhibited a clear and consistent up-regulation of CD80 (74.7% vs. 65.2%), CD86 (70.7% vs. 55%), CD40 (19.4% vs. 11.8%) and MHC class II (24.2% vs. 8.5%) molecules compared with mock-treated cells (Fig. 6B, gray histograms). A large proportion of the S. Typhi-stimulated CD11c+ DC showed simultaneous expression of at least two of these activation markers (i.e. CD86 and MHC II, or CD80 and CD40 cells) as opposed to the mock-treated control (Fig. 6B, right). Abundant expression of MHC class II molecules was also seen in confocal images of S. Typhi-infected DC (Fig. 6A). To assess the improved functionality of activated/mature S. Typhi-stimulated DC, we investigated their capacity to produce pro-inflammatory cytokines. The levels of IL-12p70, IL-10, TNF-α, IL-6, and MCP-1 were measured in culture supernatants of S. Typhi-stimulated CD11c+ DC by CBA and flow cytometry (Fig. 6C). Increased levels of TNF-α, IL-6, and MCP-1 were produced by S. Typhi-activated, but not mock-treated DC.
FIGURE 6.
S. Typhi induces activation and maturation of neonatal DC. A, Confocal laser microscopy images of CD11c+ DC treated with S. Typhi for 2 h. FITC anti-common Salmonella Ag-1 mAb was used to detect S. Typhi; DC were stained with a purified MHC class II (I-Ad) mAb followed by Alexa Fluor 546 anti-mouse IgG; 4′,6′-diamidino-2-phenylindole dihydrochloride was used to show the nuclei. Images shown are representative from the mid-plane of the cell. B, Expression of costimulatory molecules (CD80, CD86, CD40, and MHC class II (I-Ad)) on CD11c+ BM-derived neonatal DC after S. Typhi (gray-filled histogram) or mock-infection (solid line); dashed line indicates isotype control staining. Right panel, Shows percent of CD11c+ simultaneously expressing CD86/I-Ad and CD80/CD40 ± SEM; results are mean from three independent experiments. C, Cytokines produced by neonatal BM-derived DC upon S. Typhi or mock-infection measured in culture supernatants. Data represent mean cytokine concentration ± SEM from three independent experiments. Significant differences (*, p <0.05) compared with the mock-infected cells. D and E, Comparison on surface markers levels (D) and cytokine levels and fold increase (E) between S. Typhi treated neonatal and adult DC. Significant differences (*, p <0.05) compared with the adult-infected cells. Both adult and newborn CD11c+ stimulated with LPS (control) showed increased expression of activation and maturation markers and enhanced cytokine production (data not shown).
There is an ongoing debate as to whether neonatal immune cells can achieve adult-like levels of activation and protective immunity. Increasing evidence, however, suggests that this largely depends on the nature and potency of the stimulatory and maturation signals (37-39). We asked, therefore, whether neonatal and adult S. Typhi-activated DC could reach similar levels of activation when stimulated alike. To this end, we compared the expression of activation and maturation cell surface markers in neonatal vs. adult DC exposed to S. Typhi (Fig. 6D). As expected, BM-derived immature DC from adult mice also acquired a mature phenotype when exposed to S. Typhi. Remarkably, the levels of expression of cell surface molecules were similar for both neonatal and adult DC upon bacterial exposure; a slightly higher mean fluorescent intensity was observed for CD86 in adult DC. We also compared the capacity of neonatal vs adult CD11c+ DC to produce cytokines upon S. Typhi stimulation (Fig. 6E) and found that except for IL-10, which was scarcely produced by neonatal cells, the same cytokines were produced and similar levels were detected in culture supernatants. The baseline production of IL-6 and TNFα was significantly lower in the neonates, and yet both neonatal and adult DC produced similar levels of IL-6 and TNFα. It can be argued, therefore, that neonatal responses for these cytokines were even stronger than those of their adult counterparts, as shown by the higher fold-increases after S. Typhi stimulation (Fig. 6E, bottom).
S. Typhi-stimulated DC have increased capacity for Ag presentation and T cell stimulation
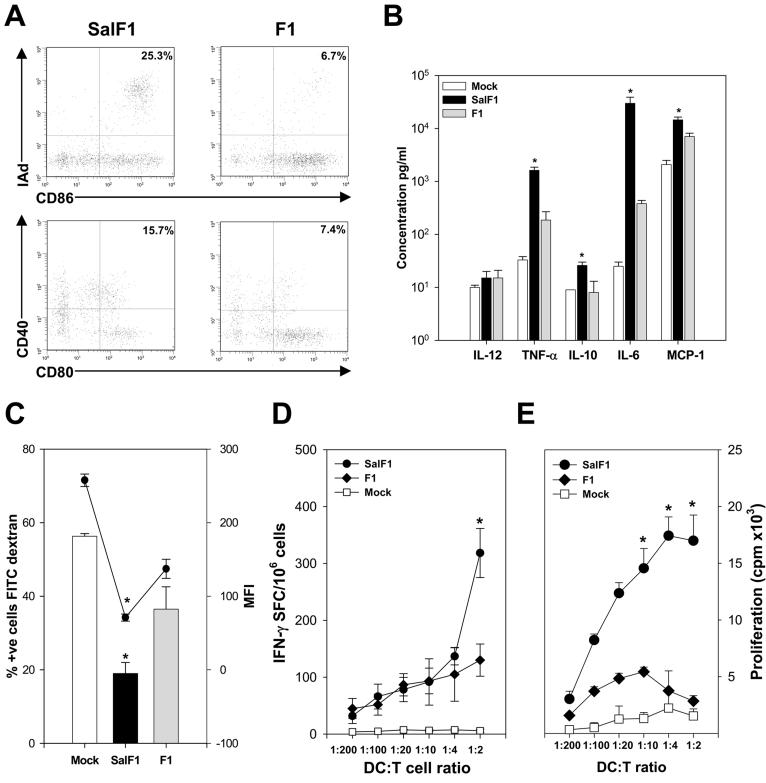
Next, we investigated the capacity of S. Typhi(F1)-stimulated neonatal DC to present the F1 Ag expressed by S. Typhi and to stimulate F1-specific T cells. We first confirmed that DC exposed to S. Typhi(F1) also acquired an activated/mature phenotype, as we had shown for DC stimulated with the S. Typhi empty vector (Fig. 6). We also included DC treated with F1 as controls. As shown in Fig. 7A, whereas the DC population stimulated with S. Typhi(F1) exhibited a large percent of cells expressing activation and maturation markers (i.e. both MHC II and CD86 as well CD80 and CD40), only a small proportion of DC stimulated with F1 expressed those markers (25.3% and 15.7% vs. 6.7 % and 7.4%, respectively). Furthermore, we examined the profile of cytokines secreted by S. Typhi(F1) and F1-treated neonatal DC and found that upon stimulation with S. Typhi(F1), neonatal DC produced significantly higher levels of TNF-α, IL-10, IL-6 and MCP-1 compared with F1- and mock-treated cells (Fig. 7B). This pattern of response is very similar to that described for DC exposed to S. Typhi empty vector (Fig. 6C). Although the cytokine response prompted by F1 treatment was much lower compared with the bacterial treatment, neonatal DC stimulated with F1 produced levels of TNF-α and IL-6 that surpassed those of mock treated DC.
FIGURE 7.
S. Typhi(F1) enhances neonatal DC maturation, Ag presentation and T cell stimulation. A, Expression of surface markers CD80, CD86, CD40, and MHC class II on CD11c+ neonatal BM-derived DC stimulated with SalF1 (m.o.i.=30) or F1 (5 μg/ml). Percentage of I-Ad+ and CD86+ cells and CD40+ and CD80+ after SalF1 or F1-stimulation are indicated in the upper right quadrant; data are representative of three independent experiments. B, Cytokines produced by neonatal BM-derived DC upon SalF1, F1, or mock-infection measured in culture supernatants by CBA. Data represent mean cytokine concentration ± SEM from three independent experiments. C, BM-derived neonatal DC exposed to SalF1 or F1 were incubated with dextran FITC and Ag uptake was examined by flow cytometry; bars show percent of positive cells and the circles indicate mean fluorescent intensity ± SEM of three independent experiments. D, Neonatal BM-derived magnetic sorted CD11c+ DC were stimulated with SalF1, F1 or mock-stimulated, irradiated and incubated with F1-specific CD3+ T cells in different DC:T cell ratios. IFN-γ production was measured by ELISPOT, data show the frequency of IFN-γ secreting T cells ± SEM from replicate cultures. E, Neonatal DC stimulated SalF1, F1 or mock-stimulated were incubated with F1-specific T cells as described above and cell proliferation was measured by [3H] thymidine incorporation, data represent mean cpm ± SEM from replicate cultures. Significant differences (*, p <0.05) compared with the F1-stimulated cells.
In the process of maturation, DC progressively lose their capacity for Ag uptake and become more efficient APC. To provide further evidence for the described S. Typhi(F1)-mediated DC maturation, we examined the capacity of S. Typhi(F1) and F1-treated neonatal CD11c+ to phagocytose dextran-FITC. A significantly lower percent of S. Typhi(F1)-exposed mature DC were stained with FITC compared with F1-treated immature DC; the former cells also displayed lower mean fluorescent intensity compared with F1-treated DC (Fig. 7C).
Furthermore, we examined the capacity of S. Typhi(F1) and F1-stimulated DC to present Y. pestis F1 and to stimulate F1-specific T cells using an in vitro Ag presentation assay. F1-specific purified CD3+ T lymphocytes were incubated with neonatal CD11c+ DC previously stimulated with S. Typhi(F1) or F1, and IFN-γ production and lymphocyte proliferation were measured as a surrogate of T cell stimulation. Higher frequency of IFN-γ secreting cells and proliferative T cell responses were observed when T cells were cultured with S. Typhi(F1)-treated DC, as opposed to F1- or mock-treated cells. Responses increased when larger numbers of Ag-loaded DC were available for T cell stimulation (i.e. increasing DC:T cell ratio) (Fig. 7, D and E).
Neonatal and adult S. Typhi(F1)-stimulated DC induce F1-specific T cell responses in vivo
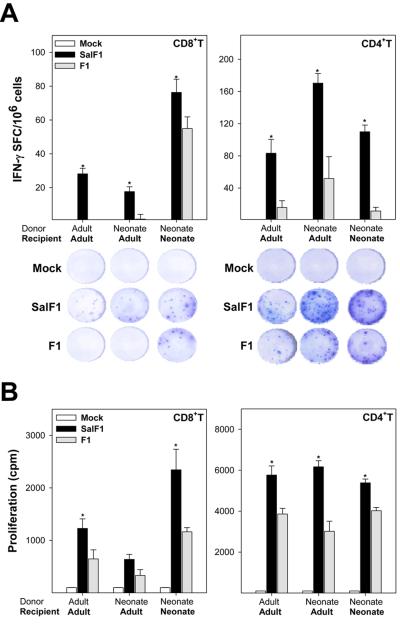
Finally, to confirm our hypothesis that the strong immunogenicity seen in vivo following live vector priming was related to the capacity of S. Typhi to activate DC, adult and neonatal BM-derived sorted CD11c+ DC stimulated in vitro with S. Typhi(F1), F1 or mock stimulated were adoptively transferred to adult (8-wk-old) and newborn (1-wk-old) mice. The frequency of F1-specific IFN-γ secreting CD8+ and CD4+ T cells and their proliferative responses measured in spleens 7 days after DC transfer are shown in Fig. 8. Consistently, S. Typhi(F1)-stimulated DC induced higher frequencies of IFN-γ secreting CD4+ and CD8+ T cells compared with F1-stimulated DC; responses were detected in adult mice that received either adult or neonatal S. Typhi(F1)-stimulated DC, but most remarkably in newborns upon transfer of S. Typhi(F1)-stimulated neonatal DC. Similarly, F1-specific CD4+ and CD8+ T cells with the capacity to proliferate in vitro in the presence of F1 were observed in adult mice after adoptive transfer of S. Typhi-treated adult and neonatal DC as well as in newborns that received S. Typhi-treated neonatal DC. An interesting finding was the strong F1-specific CD8+-mediated T cell response in the newborns, which even surpassed those of adult mice both for IFN-γ secretion and cell proliferation.
FIGURE 8.
Neonatal DC stimulated in vitro with S. Typhi(F1) prime F1-specific T cell responses in newborn and adult mice. Adult and neonatal BM-derived CD11c+ DC stimulated in vitro with S. Typhi(F1), F1 or mock-stimulated were transferred to adult (2×105 cells, i.v.) and newborn (1×106 cells, i.p.) recipient mice and spleens were collected 1 wk post-transfer. A, Frequencies of F1-specific IFN-γ secreting CD4+ and CD8+ T cells were measured by ELISPOT; results are expressed as mean SFC per 1×106 cells ± SEM of replicate cultures. Pictures show representative wells for the different DC treatments and recipient groups. B, Proliferative responses in spleen cells stimulated in vitro with F1 were measured by [3H] thymidine incorporation; results are expressed as mean cpm ± SEM from replicate wells. Significant differences (*, p <0.05) compared with F1-stimulated DC are indicated.
Discussion
The goal of this study was 2-fold, as follows: 1) to evaluate the potential utility of a newly developed S. Typhi vaccine strain expressing Y. pestis F1 for early life immunization using a mucosal prime-parenteral boost regimen and, 2) to dissect the mechanisms involved in the priming of immune responses, particularly the capacity of S. Typhi to reverse the immature phenotype of neonatal DC facilitating the processes of Ag presentation and T cell stimulation.
The S. Typhi(F1) strain used in our studies was the first of a series of F1- and LcrV-expressing constructs produced at the CVD. It was highly immunogenic in adult mice, producing higher F1 IgG levels than several other constructs, including bivalent F1- and LcrV-expressing strains.
In this study, we show that neonatal intranasal priming with S. Typhi(F1) produced high frequencies of F1-specific IgA- and IgG-secreting cells in mucosal tissues and systemic IFN-γ-secreting T cells 1 wk after immunization, during the neonatal period. This is remarkable, considering the difficulty in generating such responses at very early life stages and the importance of these effector mechanisms to protect against pneumonic and bubonic plague. Mucosal immunization with S. Typhi(F1) was also able to overcome the limited neonatal immune functions successfully priming the immune system for a rapid and vigorous anamnestic Ab response to a parenteral F1 boost.
The superiority of S. Typhi(F1) prime-F1 boost regimen over priming and boosting with F1-alum was astonishing in all the immunological outcomes evaluated. Besides the magnitude, the quality of the humoral responses notably improved, as indicated by a more balanced IgG2a/IgG1 profile, clearly distinct from the polarized IgG1 response generated by F1 alum, the accelerated kinetics of avidity maturation and the capacity to elicit memory B cells.
Although correlates of protection against plague have not been identified, Abs play a critical role in host defense. Increased levels of systemic F1 IgG have been correlated with survival upon Y. pestis challenge in animal models (19,40,41). The production of high levels of long-lasting, high avidity Abs, especially IgG2a, which are highly efficient opsonins (42), would be beneficial to facilitate bacterial clearance through capsule-mediated phagocytosis, among other mechanisms. Immunization of adult mice with S. Typhimurium expressing F1 has been shown to confer better protection than immunization with F1-alum and it was suggested that it might be due to increased production of IgG2a by the F1-expressing strain (19).
Another important attribute of the S. Typhi(F1)-prime F1-boost regimen was the capacity to elicit a strong and persisting cell-mediated immunity, including F1-specific T cells with the capacity to produce IFN-γ, which appears to be necessary to resolve infection with Y. pestis (reviewed in Refs. 3 and 4). The overall enhancement of immune responses using the prime-boost regimen in newborn mice is consistent with that described for other Salmonella live vector vaccines in adult mice (24,25).
Mucosal immunity was also generated when newborns were immunized i.n. with S. Typhi(F1) but not when primed and boosted i.m. with F1-lum. Adaptive immunity in the respiratory mucosa is deemed essential to protect against aerosolized Y. pestis. The advantages of mucosal (as opposed to parenteral) delivery of plague Ags to enhance the quality of immune responses and to promote mucosal immunity have been shown by others in adult mice (43-45).
The immune responses generated in newborns primed with S. Typhi(F1) and boosted with F1 were sufficient to protect these animals against systemic lethal plague infection, whereas groups primed and boosted with S. Typhi(F1) or F1 exhibited partial protection with overt signs of disease. Morton et al. (46) had previously reported 65% protection in adult mice immunized with an attenuated (aroA, aroC, htrA) S. Typhi strain expressing F1, upon s.c. lethal pestis challenge. Because our primary goal was to demonstrate the suitability of S. Typhi to deliver an Ag of biodefense interest for early life immunization and to dissect the immunological intricacies of a neonatal mucosal prime parenteral boost regimen, we did not pursue more elaborated protection studies such as inhalational challenge with wild type organisms. Considering the robust mucosal responses were elicited, a Salmonella-based immunization strategy is likely to be effective against pneumonic plague.
DC play a key role in the immune responses against Salmonella and passenger Ags in vivo (13,47). The ability of S. Typhimurium to infect and activate DC from adult mice has been demonstrated in several studies (48,49). S. Typhimurium-derived membrane vesicles were also recently shown to promote DC maturation and stimulation of protective adaptive immunity (50). Our results demonstrate, for the first time, that neonatal immature DC become activated and acquire a mature phenotype upon stimulation with a S. Typhi live vector vaccine and these mature cells exhibit an improved functional capacity, shown by increased production of proinflammatory cytokines and efficient Ag presentation and stimulation of T cells. This phenomenon is expected to take place in vivo as well. In fact, we demonstrate that neonatal DC stimulated in vitro with S. Typhi expressing F1 primed strong Ag-specific CD4+ and CD8+ T cell when adoptively transferred to newborns that lacked mature and fully competent DC. The enhanced capacity of Salmonella-activated DC for Ag presentation and T cell stimulation can explain the potent Th1-type responses observed in vivo, as well as the improved quality of Abs, which probably result from a more efficient T-B cell cooperation. In contrast, neonatal DC exposed to F1 showed marginal activation and reduced capacity for Ag presentation and T cell stimulation concurring with the poor neonatal responses to F1 in vivo.
Neonatal and adult DC reached similar levels of activation and cytokine production after S. Typhi stimulation, further supporting the possibility of inducing adult-like immune responses during the neonatal period. Kingston et al. (51) have shown in vitro that mature DC can prime naive T cells to respond to F1 and V Ags, and suggested that these cells may play an important role in the induction of protective immunity to plague in vivo.
An interesting finding in our study was that newborns primed with S. Typhi alone responded more efficiently to the F1-boost than mice primed and boosted with F1-alum, suggesting a non-specific live vector-mediated activation of the neonatal immune system. Salmonella expresses a variety of pattern-recognition-receptor ligands, primarily LPS and flagellin, which can activate innate immunity through TLR4 and TLR5 and trigger proinflammatory cytokines and chemokines that recruit and activate DC(reviewed in Ref. 52). Interestingly, flagella abound in nonrecombinant S. Typhi, but are scarce in the F1-expressing strain, which may help explain the potent immune stimulation of S. Typhi alone. Conceivably, the bacteria increase the level of activation of neonatal DC and educate these cells for a more efficient response to an Ag given later in life. Our findings underscore the notion that vaccine components capable of activating DC can dramatically influence the immunization outcome in the early life setting. We also provide evidence that upregulation of DC early in life and a proinflammatory Th1-type cytokine milieu are critical not only to improve immune responses in the short-term, but to enhance responses to Ags coming in contact with the host later in life. Several studies have shown that virulent strains of S. Typhimurium can interfere with the capacity of DC to prime adaptive immunity (53-56). We did not observe any dampening of responses when using an attenuated S. Typhi vaccine strain in our neonatal mouse model; on the contrary, adaptive immunity was enhanced in comparison with a subunit vaccine.
Current biodefense vaccine initiatives have been primarily focused on healthy adults, whereas little consideration has been given to strategies that can be safe and effective in special groups, such as infants and young children, who would be at higher risk of infection in the event of bioterror attack. In fact, vaccines with proven efficacy in adults may confer poor protection in early life (35). Children will perhaps be the most difficult group to treat post-exposure due to the limitations in the use of antibiotics in this group. Our results show for the first time that mucosal priming with S. Typhi expressing a foreign Ag, followed by a protein Ag boost, appears to be a highly efficient and novel vaccine approach for early life immunization. A S. Typhi-based plague vaccine would be an efficacious and practical prophylactic tool for protection of infants and young children. We envision the use of such a vaccine to prime young hosts early in life for a rapid and heightened response to a parenteral subunit vaccine boost administered later if needed. Improved Salmonella constructs expressing both F1 and LcrV from highly stabilized plasmids without antibiotic markers are under development for use in humans. Our studies warrant further assessment of protective efficacy of improved S. Typhi-based plague vaccines in this model. They also pave the way for further investigation of biodefense vaccine strategies and heterologous prime-boost immunization for early life immunization.
Acknowledgements
We thank Dr. Helen Atkins (Defence Science and Technology Laboratory, Porton Down, UK) and Dr. Wendy Picking (University of Kansas, Lawrence, KS), for providing F1 Ag; Ana Liliana Rodriguez for assistance in challenge and adoptive transfer experiments; James Galen and Magaly Chinchilla for critical reading of this manuscript; and personnel from the Applied Immunology Section for exceptional technical support. We dedicate this work to the memory of Susana Albornoz de Pasetti (1942-2007).
This work was supported by National Institute of Health grants U19-AI-56578 (to J. P. N) and R01-AI065760 (to M.F. P).
Abbreviations used in this paper
- Frag C
Fragment C
- ASC
Ab-secreting cells
- BM
bone marrow
- CBA
Cytometric Bead Array
- DC
dendritic cell
- GMT
geometric mean titer
- i.n.
intranasally
- m.o.i.
multiplicity of infection
- PBA
PBS containing 0.1% BSA and 0.01% NaN3
- PBST
PBS containing 0.05% Tween 20
- SFC
Spot-forming cell
Footnotes
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org”
References
- 1.Centers for Disease Control and Prevention Plague Information: CDC Division of Vector-Borne Infectious Diseases. 2005 http://www.cdc.gov/ncidod/dvbid/plague/index.htm.
- 2.Cornelius C, Quenee L, Anderson D, Schneewind O. Protective immunity against plague. Adv. Exp. Med. Biol. 2007;603:415–424. doi: 10.1007/978-0-387-72124-8_38. [DOI] [PubMed] [Google Scholar]
- 3.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert. Rev. Vaccines. 2008;7:209–221. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Titball RW, Williamson ED. Yersinia pestis (plague) vaccines. Expert. Opin. Biol. Ther. 2004;4:965–973. doi: 10.1517/14712598.4.6.965. [DOI] [PubMed] [Google Scholar]
- 5.Cohen RJ, Stockard JL. Pneumonic plague in an untreated plague-vaccinated individual. JAMA. 1967;202:365–366. [PubMed] [Google Scholar]
- 6.Meyer KF, Cavanaugh DC, Bartelloni PJ, Marshall JD., Jr Plague immunization. I. Past and present trends. J Infect. Dis. 1974;129(Suppl.):S13–S18. doi: 10.1093/infdis/129.supplement_1.s13. [DOI] [PubMed] [Google Scholar]
- 7.Williamson ED, Stagg AJ, Eley SM, Taylor R, Green M, Jones SM, Titball RW. Kinetics of the immune response to the (F1+V) vaccine in models of bubonic and pneumonic plague. Vaccine. 2007;25:1142–1148. doi: 10.1016/j.vaccine.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 8.Williamson ED, Eley SM, Stagg AJ, Green M, Russell P, Titball RW. A single dose sub-unit vaccine protects against pneumonic plague. Vaccine. 2000;19:566–571. doi: 10.1016/s0264-410x(00)00159-6. [DOI] [PubMed] [Google Scholar]
- 9.Deparment of Health and Human Services. U.S. Food Drug Administration. Center for Biologics Evaluation and Research Public workshop on animal models and correlates of protection for plague vaccines. 2004 http://www.fda.gov/cber/minutes/plague101304T.pdf.
- 10.Williamson ED, Flick-Smith HC, Lebutt C, Rowland CA, Jones SM, Waters EL, Gwyther RJ, Miller J, Packer PJ, Irving M. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 2005;73:3598–3608. doi: 10.1128/IAI.73.6.3598-3608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegrist CA. Vaccination in the neonatal period and early infancy. Int. Rev. Immunol. 2000;19:195–219. doi: 10.3109/08830180009088505. [DOI] [PubMed] [Google Scholar]
- 12.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 13.Wick MJ. The role of dendritic cells during Salmonella infection. Curr. Opin. Immunol. 2002;14:437–443. doi: 10.1016/s0952-7915(02)00364-3. [DOI] [PubMed] [Google Scholar]
- 14.Pasetti MF, Levine MM, Sztein MB. Animal models paving the way for clinical trials of attenuated Salmonella enterica serovar Typhi live oral vaccines and live vectors. Vaccine. 2003;21:401–418. doi: 10.1016/s0264-410x(02)00472-3. [DOI] [PubMed] [Google Scholar]
- 15.Branger CG, Fetherston JD, Perry RD, Curtiss R., III Oral vaccination with different antigens from Yersinia pestis KIM delivered by live attenuated Salmonella typhimurium elicits a protective immune response against plague. Adv. Exp. Med. Biol. 2007;603:387–399. doi: 10.1007/978-0-387-72124-8_36. [DOI] [PubMed] [Google Scholar]
- 16.Leary SE, Griffin KF, Garmory HS, Williamson ED, Titball RW. Expression of an F1/V fusion protein in attenuated Salmonella typhimurium and protection of mice against plague. Microb. Pathog. 1997;23:167–179. doi: 10.1006/mpat.1997.0141. [DOI] [PubMed] [Google Scholar]
- 17.Liu WT, Hsu HL, Liang CC, Chuang CC, Lin HC, Liu YT. A comparison of immunogenicity and protective immunity against experimental plague by intranasal and/or combined with oral immunization of mice with attenuated Salmonella serovar Typhimurium expressing secreted Yersinia pestis F1 and V antigen. FEMS Immunol Med. Microbiol. 2007;51:58–69. doi: 10.1111/j.1574-695X.2007.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Hinnebusch BJ, Trunkle T, Bosio CM, Suo Z, Tighe M, Harmsen A, Becker T, Crist K, Walters N, Avci R, Pascual DW. Oral vaccination with Salmonella simultaneously expressing Yersinia pestis F1 and V antigens protects against bubonic and pneumonic plague. J. Immunol. 2007;178:1059–1067. doi: 10.4049/jimmunol.178.2.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Titball RW, Howells AM, Oyston PC, Williamson ED. Expression of the Yersinia pestis capsular antigen (F1 antigen) on the surface of an aroA mutant of Salmonella typhimurium induces high levels of protection against plague. Infect Immun. 1997;65:1926–1930. doi: 10.1128/iai.65.5.1926-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacket CO, Galen J, Sztein MB, Losonsky G, Wyant TL, Nataro J, Wasserman SS, Edelman R, Chatfield S, Dougan G, Levine MM. Safety and immune responses to attenuated Salmonella enterica serovar typhi oral live vector vaccines expressing tetanus toxin fragment C. Clin. Immunol. 2000;97:146–153. doi: 10.1006/clim.2000.4924. [DOI] [PubMed] [Google Scholar]
- 21.Lewis GK. Live-attenuated Salmonella as a prototype vaccine vector for passenger immunogens in humans: are we there yet? Expert. Rev. Vaccines. 2007;6:431–440. doi: 10.1586/14760584.6.3.431. [DOI] [PubMed] [Google Scholar]
- 22.Bumann D, Metzger WG, Mansouri E, Palme O, Wendland M, Hurwitz R, Haas G, Aebischer T, von Specht B, Meyer TF. Safety and immunogenicity of live recombinant Salmonella enterica serovar Typhi Ty21a expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine. 2001;20:845–852. doi: 10.1016/s0264-410x(01)00391-7. [DOI] [PubMed] [Google Scholar]
- 23.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Londoño-Arcila P, Freeman D, Kleanthous H, O'Dowd AM, Lewis S, Turner AK, Rees EL, Tibbitts T, Greenwood J, Monath TP, Darsley MJ. Attenuated Salmonella enterica serovar Typhi expressing urease effectively immunizes mice against Helicobacter pylori challenge as part of a heterologous mucosal priming-parenteral boosting vaccination regimen. Infect Immun. 2002;70:5096–5106. doi: 10.1128/IAI.70.9.5096-5106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vindurampulle CJ, Cuberos LF, Barry EM, Pasetti MF, Levine MM. Recombinant Salmonella enterica serovar Typhi in a prime-boost strategy. Vaccine. 2004;22:3744–3750. doi: 10.1016/j.vaccine.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Capozzo AV, Cuberos L, Levine MM, Pasetti MF. Mucosally delivered Salmonella live vector vaccines elicit potent immune responses against a foreign antigen in neonatal mice born to naive and immune mothers. Infect. Immun. 2004;72:4637–4646. doi: 10.1128/IAI.72.8.4637-4646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokes MG, Titball RW, Neeson BN, Galen JE, Walker NJ, Stagg AJ, Jenner DC, Thwaite JE, Nataro JP, Baillie LW, Atkins HS. Oral administration of a Salmonella enterica-based vaccine expressing Bacillus anthracis protective antigen confers protection against aerosolized B. anthracis. Infect. Immun. 2007;75:1827–1834. doi: 10.1128/IAI.01242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Nataro JP, Edelman R, Pickard D, Dougan G, Chatfield SN, Levine MM. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect. Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capozzo AV, Ramirez K, Polo JM, Ulmer J, Barry EM, Levine MM, Pasetti MF. Neonatal immunization with a Sindbis virus-DNA measles vaccine induces adult-like neutralizing antibodies and cell-mediated immunity in the presence of maternal antibodies. J. Immunol. 2006;176:5671–5681. doi: 10.4049/jimmunol.176.9.5671. [DOI] [PubMed] [Google Scholar]
- 30.Barrow GI. In: Cowan and Steel's Manual for the Identification of Medical Bacteria. 3rd Ed. Felthan RKA, editor. Cambridge University Press; Cambridge: 2003. [Google Scholar]
- 31.Kim W, Killam T, Sood V, Surette MG. Swarm-cell differentiation in Salmonella enterica serovar typhimurium results in elevated resistance to multiple antibiotics. J Bacteriol. 2003;185:3111–3117. doi: 10.1128/JB.185.10.3111-3117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Brubaker RR, Beesley ED, Surgalla MJ. Pasteurella pestis: Role of Pesticin I and Iron in Experimental Plague. Science. 1965;149:422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- 34.Lee YC, Kelly DF, Yu LM, Slack MP, Booy R, Heath PT, Siegrist CA, Moxon RE, Pollard AJ. Haemophilus influenzae type b vaccine failure in children is associated with inadequate production of high-quality antibody. Clin. Infect. Dis. 2008;46:186–192. doi: 10.1086/524668. [DOI] [PubMed] [Google Scholar]
- 35.Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007;137(Suppl 1):S4–S9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 37.Fadel SA, Cowell LG, Cao S, Ozaki DA, Kepler TB, Steeber DA, Sarzotti M. Neonate-primed CD8+ memory cells rival adult-primed memory cells in antigen-driven expansion and anti-viral protection. Int. Immunol. 2006;18:249–257. doi: 10.1093/intimm/dxh360. [DOI] [PubMed] [Google Scholar]
- 38.Dadaglio G, Sun CM, Lo-Man R, Siegrist CA, Leclerc C. Efficient in vivo priming of specific cytotoxic T cell responses by neonatal dendritic cells. J. Immunol. 2002;168:2219–2224. doi: 10.4049/jimmunol.168.5.2219. [DOI] [PubMed] [Google Scholar]
- 39.Regner M, Martinez X, Belnoue E, Sun CM, Boisgerault F, Lambert PH, Leclerc C, Siegrist CA. Partial activation of neonatal CD11c+ dendritic cells and induction of adult-like CD8+ cytotoxic T cell responses by synthetic microspheres. J. Immunol. 2004;173:2669–2674. doi: 10.4049/jimmunol.173.4.2669. [DOI] [PubMed] [Google Scholar]
- 40.Simpson WJ, Thomas RE, Schwan TG. Recombinant capsular antigen (fraction 1) from Yersinia pestis induces a protective antibody response in BALB/c mice. Am. J Trop. Med. Hyg. 1990;43:389–396. doi: 10.4269/ajtmh.1990.43.389. [DOI] [PubMed] [Google Scholar]
- 41.Oyston PC, Williamson ED, Leary SE, Eley SM, Griffin KF, Titball RW. Immunization with live recombinant Salmonella typhimurium aroA producing F1 antigen protects against plague. Infect Immun. 1995;63:563–568. doi: 10.1128/iai.63.2.563-568.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 43.Tripathi V, Chitralekha KT, Bakshi AR, Tomar D, Deshmukh RA, Baig MA, Rao DN. Inducing systemic and mucosal immune responses to B-T construct of F1 antigen of Yersinia pestis in microsphere delivery. Vaccine. 2006;24:3279–3289. doi: 10.1016/j.vaccine.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Jones T, Adamovicz JJ, Cyr SL, Bolt CR, Bellerose N, Pitt LM, Lowell GH, Burt DS. Intranasal Protollintrade mark/F1-V vaccine elicits respiratory and serum antibody responses and protects mice against lethal aerosolized plague infection. Vaccine. 2005;24:1625–1632. doi: 10.1016/j.vaccine.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 45.Khan AA, Babu JP, Gupta G, Rao DN. Identifying B and T cell epitopes and studying humoral, mucosal and cellular immune responses of peptides derived from V antigen of Yersinia pestis. Vaccine. 2008;26:316–332. doi: 10.1016/j.vaccine.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 46.Morton M, Garmory HS, Perkins SD, O'Dowd AM, Griffin KF, Turner AK, Bennett AM, Titball RW. A Salmonella enterica serovar Typhi vaccine expressing Yersinia pestis F1 antigen on its surface provides protection against plague in mice. Vaccine. 2004;22:2524–2532. doi: 10.1016/j.vaccine.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Sundquist M, Rydstrom A, Wick MJ. Immunity to Salmonella from a dendritic point of view. Cell Microbiol. 2004;6:1–11. doi: 10.1046/j.1462-5822.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 48.Yrlid U, Svensson M, Hakansson A, Chambers BJ, Ljunggren HG, Wick MJ. In vivo activation of dendritic cells and T cells during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2001;69:5726–5735. doi: 10.1128/IAI.69.9.5726-5735.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yrlid U, Svensson M, Johansson C, Wick MJ. Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response. FEMS Immunol. Med. Microbiol. 2000;27:313–320. doi: 10.1111/j.1574-695X.2000.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 50.Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179:7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- 51.Kingston R, Burke F, Robinson JH, Bedford PA, Jones SM, Knight SC, Williamson ED. The fraction 1 and V protein antigens of Yersinia pestis activate dendritic cells to induce primary T cell responses. Clin. Exp. Immunol. 2007;149:561–569. doi: 10.1111/j.1365-2249.2007.03452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasan A, McSorley SJ. Activation of Salmonella-specific immune responses in the intestinal mucosa. Arch. Immunol Ther. Exp. (Warsz. ) 2006;54:25–31. doi: 10.1007/s00005-006-0003-5. [DOI] [PubMed] [Google Scholar]
- 53.Bueno SM, Gonzalez PA, Carreno LJ, Tobar JA, Mora GC, Pereda CJ, Salazar-Onfray F, Kalergis AM. The capacity of Salmonella to survive inside dendritic cells and prevent antigen presentation to T cells is host specific. Immunology. 2008;124:522–533. doi: 10.1111/j.1365-2567.2008.02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alaniz RC, Cummings LA, Bergman MA, Rassoulian-Barrett SL, Cookson BT. Salmonella typhimurium coordinately regulates FliC location and reduces dendritic cell activation and antigen presentation to CD4+ T cells. J Immunol. 2006;177:3983–3993. doi: 10.4049/jimmunol.177.6.3983. [DOI] [PubMed] [Google Scholar]
- 55.Van der Velden AW, Copass MK, Starnbach MN. Salmonella inhibit T cell proliferation by a direct, contact-dependent immunosuppressive effect. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17769–17774. doi: 10.1073/pnas.0504382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tobar JA, Carreno LJ, Bueno SM, Gonzalez PA, Mora JE, Quezada SA, Kalergis AM. Virulent Salmonella enterica serovar typhimurium evades adaptive immunity by preventing dendritic cells from activating T cells. Infect. Immun. 2006;74:6438–6448. doi: 10.1128/IAI.00063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]