Abstract
Background
Mild cognitive impairment is increasingly recognized as an important public health problem associated with increased risk of developing dementia. Annual conversion rates, however, vary across different studies with clinic samples showing higher rates of conversion than community-based samples.
Objectives
To establish whether the rates of conversion from mild cognitive impairment to dementia differed according to recruitment source and, if so, to investigate factors that might explain this discrepancy.
Design
Rates and predictors of conversion were examined in a prospective longitudinal study at a single center.
Setting
Among the participants, 46% were recruited from a clinical setting and 54% were recruited directly through community outreach.
Participants
One hundred eleven individuals with mild cognitive impairment were followed up longitudinally for an average of 2.4 years (range, 0.5–4.0 years).
Main Outcome Measures
Conversion from mild cognitive impairment to dementia.
Results
During the follow-up period, 28 individuals progressed to dementia with a mean (SD) time to conversion of 2.19 (0.72) years. The clinic sample had an annual conversion rate of 13%, whereas the community sample had an annual conversion rate of 3%. In a Cox proportional hazards model, clinic recruitment source alone was associated with an increased hazard of incident dementia (hazard ratio=3.50; 95% confidence interval, 1.31–9.18; P=.01). When other variables were added to the model, only baseline functional impairment as measured by the Clinical Dementia Rating Scale (and no demographic, cognitive, or neuroimaging variables or mild cognitive impairment subtype) accounted for the differences in conversion rates across the 2 cohorts.
Conclusions
These findings add to the growing literature to suggest that the degree of functional impairment at baseline is an important predictor of conversion to dementia and may help explain differences in findings between epidemiological and clinic-based studies.
The term mild cognitive impairment (MCI) has been used to describe the transition between normal cognition and Alzheimer dementia. 1,2 Mild cognitive impairment is recognized as an important public health problem as a dementia risk. Annual conversion rates often range from 10% to 15% in clinic samples.3–5 Conversion rates in community-based studies are often substantially lower (ie, 3.8%–6.3% per year6–9). Mild cognitive impairment is also less stable in community samples.8,10,11 Clearly patients with MCI compose a heterogeneous group, of whom not all rapidly convert to dementia. As such, it is important to identify risk factors for progressing rapidly among individuals diagnosed with MCI.
The question of whether conversion rates in community samples are comparable to those in clinic samples is important. Clinic samples are convenient and provide the opportunity to extensively and carefully characterize a disorder such as MCI. The composition of clinic samples, however, is shaped by various factors (ie, the demographics of individuals studied, patterns of self-referrals and provider referrals, differential access to health care) that make generalizability and hence implications for public health uncertain. Conversely, community-based studies are necessary to establish prevalence and incidence rates of a disorder, but these studies often use simplified diagnostic assessment methods. In these circumstances, it is difficult to know whether differences in conversion rates between community and clinic samples reflect differences in diagnostic practices (a methodological problem) or differences in population sampling (the results of which reveal something about the disorder itself). To our knowledge, no previous study has directly examined whether the conversion rate differs among individuals who were recruited into the study by different routes but underwent identical diagnostic evaluations.
To directly address this question, we examined conversion rates in individuals prospectively enrolled in a longitudinal study at a single center who were recruited either through a memory disorders clinic or via community outreach. Both groups were diagnosed using the same diagnostic methods. The first purpose of this study was to establish whether the conversion rates differed according to recruitment source. If conversion did differ by recruitment source, the second purpose was to investigate factors that might explain this discrepancy.
A variety of predictors have been examined to improve identification of individuals with MCI who will convert to dementia. Baseline memory performance and other cognitive impairments are robust predictors of incident dementia.12–15 Evidence from quantitative magnetic resonance imaging studies suggests that hippocampal atrophy16 and white matter abnormalities17 are also associated with conversion. While the issue of how much impairment in everyday function is consistent with the syndrome of MCI is an area of ongoing controversy, several studies have documented the presence of mild problems in everyday function in MCI groups.18–20 Recent evidence suggests that mild problems in functional abilities at baseline are associated with more rapid decline21 and greater risk of conversion to dementia.9,22
The first hypothesis of this study was that conversion to dementia occurs at a lower rate in the community sample as compared with the clinic sample. Second, because the diagnostic criteria for MCI are explicit about cognitive criteria but are indistinct about impairment in daily function, it was hypothesized that the baseline level of functional impairment as measured by the Clinical Dementia Rating Scale (CDR) sum of boxes score would explain the effects of recruitment source and independently predict progression to dementia longitudinally.
METHODS
SUBJECT RECRUITMENT
All of the participants were part of a longitudinal study of cognition in educationally and ethnically diverse older adults. While participants in the larger longitudinal study varied in terms of cognitive impairment (ie, cognitively normal, having MCI, or having dementia), the present study focuses on only those who were diagnosed with MCI at their baseline evaluation. Subject recruitment for this longitudinal cohort occurred through 2 routes: (1) clinic referrals and (2) community outreach.23
Participants in the clinic referral group were seen through a university-based memory disorders clinic, having been referred for a clinical evaluation by a physician owing to suspected cognitive decline. Healthy control subjects recruited through this route included those determined to have no cognitive impairment and those who were family members of impaired clinical recruits. The community outreach program was developed to further maximize demographic and cognitive diversity. Bicultural (and bilingual) recruiters approached elderly persons in community settings to elicit participation in research. Recruitment settings were designed to be inclusive of individuals with all forms of cognitive ability and included community health care facilities, church groups, community agencies, and senior centers and groups. Ethnic groups specifically targeted for recruitment included African American, Hispanic, and white individuals. On average, of those approached about participating in research, 61% were eventually enrolled.
Regardless of recruitment route, the inclusion criterion was being older than 60 years and the exclusion criteria included unstable major medical illness or major primary psychiatric disorder. All of the participants signed informed consent, and all human subject involvement was overseen by institutional review boards at the University of California, Davis, the Veterans Administration Northern California Health Care System, Mather, and San Joaquin General Hospital, Stockton, California. Within the larger parent sample, those recruited from the clinic setting had a mean (SD) age of 75.9 (6.8) years and a mean (SD) education of 13.5 (4.0) years, with 70% being white and 71% being female. Also within the larger parent sample, participants recruited from the community had a mean (SD) age of 73.9 (7.2) years and a mean (SD) education of 11.6 (5.2) years, with 25% being white and 66% being female.
CLINICAL EVALUATION
All of the participants regardless of recruitment source received annual multidisciplinary clinical evaluations through the University of California, Davis, Alzheimer’s Disease Center. The same group of clinicians (S.T.F., D.M., B.R.R., and C.D.) performed all of the evaluations and established the diagnoses according to the same protocol. Evaluations included a detailed medical history, a physical examination, and a neurological examination. A physician fluent in Spanish (William Seavey, MD) examined subjects who spoke only Spanish. A family member or informant was interviewed to obtain information about independent functioning. All of the subjects received diagnostic neuroimaging according to the American Academy of Neurology guidelines24 and routine laboratory tests.
Each participant underwent a clinical neuropsychological evaluation using a standard battery of tests comprising the Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological battery25 (Mini-Mental State Examination, list learning, animal fluency, constructional praxis, Boston Naming Test) supplemented by the Wechsler Adult Intelligence Scale Revised digit symbol subscale,26 the Trail Making Test, and the American version of the National Adult Reading Test.27
Diagnosis of cognitive syndrome (healthy, MCI, or dementia) was made according to standardized criteria at a consensus conference. Cognitive impairment was clinically identified when a participant’s performance fell approximately 1.5 SDs below age-corrected norms and in reference to their education and occupational background. Dementia was diagnosed using Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised)28 criteria for dementia. Individuals with cognitive changes not meeting criteria for dementia were diagnosed with MCI. Individuals with MCI could have the following: (1) a single memory impairment; (2) a single non-memory impairment; (3) multiple cognitive impairments including memory; or (4) multiple impairments without memory impairment. Individuals with MCI could not have impairments in basic activities of daily living or be dependent on others in any instrumental activities of daily living, but they could have lesser degrees of functional problems. Importantly, all diagnoses were made blind to research neuropsychological testing, quantitative brain image analysis, and the CDR (one of the predictor variables in the current study).
STUDY SAMPLE
This study focused on 111 individuals diagnosed as having MCI at the baseline assessment; 46% were recruited through a memory disorders clinic (University of California, Davis, Alzheimer’s Disease Center) and 54% were recruited through the community. The distribution of MCI subtypes across the 2 samples was not significantly different (P=.10) (Table 1). Participants had a mean (SD) age of 75.3 (7.5) years and a mean (SD) education of 12.2 (5.2) years, and 51% were female. Of the entire sample, 52% were self-identified as belonging to an ethnic minority (27 individuals were African American, 25 were Hispanic, 3 were Asian, and 6 were of other ethnicities). Length of follow-up ranged from 0.5 to 4.0 years, with a mean of 2.4 years.
Table 1.
Mild Cognitive Impairment Subtype Distributions by Recruitment Source
| Recruitment Source, % | ||
|---|---|---|
| MCI Subtype | Clinic (n=51) |
Community (n=60) |
| Memory | ||
| Single domain | 53 | 38 |
| Multiple domain | 32 | 26 |
| Nonmemory | ||
| Single domain | 4 | 16 |
| Multiple domain | 11 | 20 |
Abbreviation: MCI, mild cognitive impairment.
INSTRUMENTS
Neuropsychological Measures
The Spanish and English Neuropsychological Assessment Scales (SENAS)29,30 was used to measure 2 specific domains of cognitive functioning: episodic memory and executive function. The SENAS is the result of an extensive development process that has used Item Response Theory methods to create English and Spanish language measures of cognitive domains. Episodic memory is a composite variable composed of indices from a supraspan word list learning task. The executive functioning variable comprises several working memory and fluency tests. The SENAS is unique among neuropsychological test batteries in that it measures multiple cognitive domains using a set of tests that have equivalent (unbiased) versions in Spanish and English, have large-sample normative data in both Spanish and English, use subscales that are psychometrically matched so that they have linear measurement properties, and use subscales that have similar reliability of measurement across a 4-SD range of function. The SENAS measures are also equally sensitive to clinical diagnosis (healthy, MCI, or dementia) in Hispanic and white individuals.31 These 2 cognitive measures served as primary cognitive risk factors for conversion and were not used in the diagnostic process.
Quantitative Magnetic Resonance Imaging Measures
Brain imaging was obtained at the University of California, Davis, Research Imaging Center on a 1.5-T GE Signa Horizon LX Echo-speed system (GE Healthcare, Milwaukee, Wisconsin) or at the Martinez Outpatient Clinic, Veterans Affairs Northern California Health Care System, Martinez, on a 1.5-T Marconi system (Marconi Medical Systems, Cleveland, Ohio). Comparable imaging parameters were used at each site. Volumetric measures of white matter hyperintensity (WMH), total brain matter, and total intracranial volume were derived from previously described automated image segmentation methods32 based on a fluid-attenuated inversion recovery sequence designed to enhance WMH segmentation.32 Volume of the hippocampus was also quantitated using a computer algorithm applied to manually traced hippocampal boundaries on 3-dimensional T1-weighted images. Magnetic resonance imaging measures of WMH volume, total brain volume, and hippocampal volume were normalized to total intracranial volume and multiplied by 100.
Functional Assessment
The CDR33 was used as a global measure of everyday functioning. The CDR is based on a structured caregiver interview. Scores are obtained in 6 different functional domains (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care). The sum of boxes score is the arithmetic sum of the 6 subscores9 and was used in the primary analysis. The CDR was not used to determine diagnosis, and scores could diverge from clinical diagnosis (eg, patients with a CDR score of 0.5 were not necessarily diagnosed as having MCI).
DATA ANALYSIS
We used t test and analysis of covariance to analyze the differences between the groups with regard to the demographic and clinical variables for continuous variables, and the distributions of categorical variables were compared with χ2 test. Time from study entry to either the last assessment or the assessment at which a diagnosis of dementia was made was considered the time to the event. A person was considered censored if he or she was no longer being followed up and had not yet received a dementia diagnosis or had been followed up for 4 years without receiving a diagnosis of dementia. Kaplan-Meier curves with conversion to dementia diagnosis considered an event were used to illustrate differences in conversion patterns between the recruitment sources. Cox proportional hazards models were used to assess risk for incident dementia. The baseline model examined recruitment source as a potential risk factor. Subsequent hazards models included recruitment source along with demographic, cognitive, imaging, and functional variables as potential risks for conversion. To reduce differences in follow-up time and provide a fair comparison between recruitment sources, analyses were restricted to a maximum of 4 years of follow-up. Survival models that accounted for interval censoring (owing to the uncertainty of the timing of the conversion between assessments) were also run.
RESULTS
BASELINE DIFFERENCES BETWEEN GROUPS
Table 2 presents comparisons in demographic and clinical variables across the recruitment groups. The groups differed across all of the demographic variables. The clinic sample was slightly older, was more educated, and included a higher percentage of men. Community-recruited participants were more likely to be of an ethnic minority. Because performance on most cognitive tests is associated with education, comparisons across the 2 groups included education as a covariate. Analysis showed that the 2 groups did not differ on any baseline cognitive measures. Of the neuroimaging variables, the groups did not differ in WMH volumes. However, the community group had larger fractional total brain matter and hippocampal volumes. Finally, clinic-recruited participants showed more functional impairment than the community-recruited participants. Adjusting for education did not change the results of the group comparison for the CDR.
Table 2.
Differences Between Demographic and Clinical Variables Across the Recruitment Source Groups
| Recruitment Source | |||
|---|---|---|---|
| Variable | Clinic | Community | P Value |
| Demographic | |||
| Age, mean (SD), y | 76.9 (7.3) | 73.8 (6.8) | .02 |
| Education, mean (SD), y | 13.5 (3.7) | 10.2 (6.1) | <.001 |
| Female, % | 47 | 56 | .007 |
| Ethnic minority, % | 18 | 82 | <.001 |
| Baseline clinical, mean (SD) | |||
| MMSE score | 26.5 (2.7) | 25.5 (10.8) | .52 |
| Memory scorea | −0.84 (0.65) | −0.73 (0.66) | .15 |
| Executive scorea | −0.18 (0.52) | −0.58 (0.61) | .13 |
| Total brain volumeb | 77.4 (4.1) | 79.9 (5.1) | .006 |
| Hippocampus volumeb | 0.28 (0.05) | 0.31 (0.05) | .004 |
| WMH volumeb | 1.07 (1.47) | 0.72 (0.72) | .11 |
| CDR sum of boxes | 2.24 (1.82) | 1.12 (1.41) | <.001 |
Abbreviations: CDR, Clinical Dementia Rating Scale; MMSE, Mini-Mental State Examination; WMH, white matter hyperintensity.
These values are in reference to a mean of 0.0 and an SD of 1.0; P values are from between-group analyses that covaried education.
Imaging variables are expressed as the percentage of total cranial volume.
CONVERSION RATES
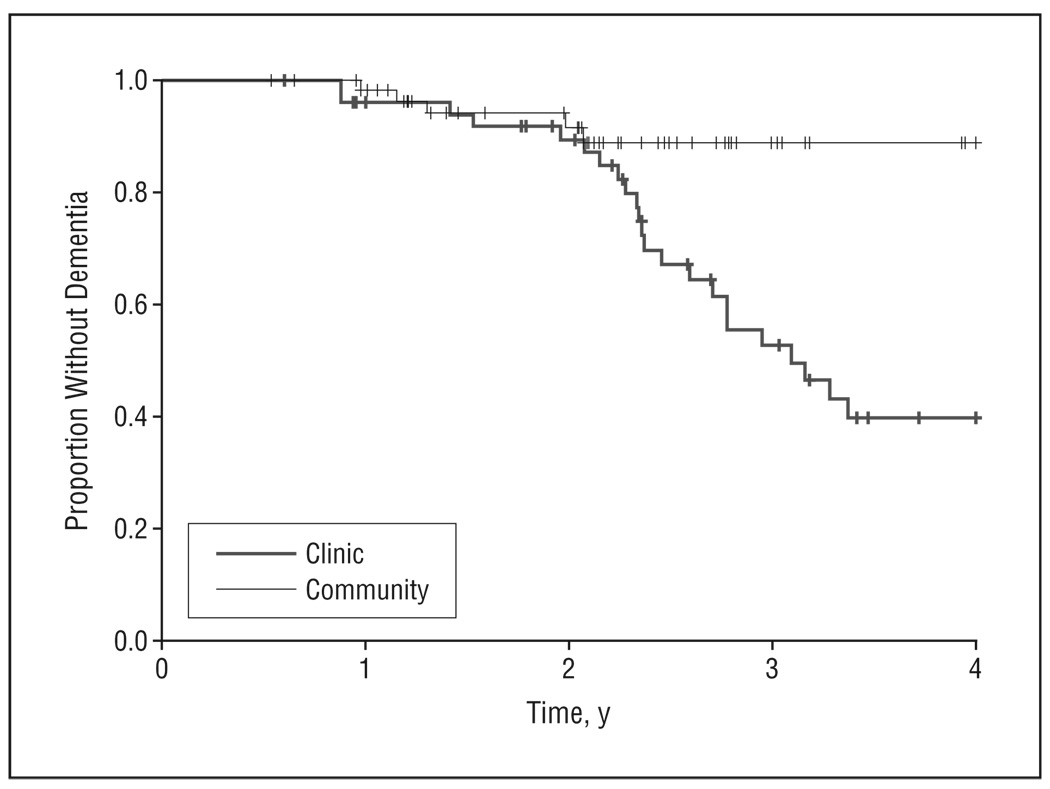
Of the entire sample, 28 individuals (23 from the clinic and 5 from the community) progressed to dementia with a mean (SD) time to conversion of 2.19 (0.72) years (range, 0.88–3.40 years), resulting in a dementia incidence of 10% per year. However, when examined separately, the 2 subsamples had substantially different rates of conversion. The clinic sample showed an annual rate of conversion of 13%, whereas the community group had a conversion rate of 3% per year. The corresponding incidence rates were 0.17 per person-year in the clinic and 0.04 per person-year in the community. In a Cox proportional hazards model, clinic recruitment source was associated with an increased hazard of incident dementia (hazard ratio [HR]=3.50;95%confidence interval [CI], 1.31–9.18; P=.01). The Figure shows the survival curves associated with developing dementia over time in the 2 samples.
Figure.
Kaplan-Meier curves comparing conversion patterns from mild cognitive impairment to dementia between the clinic- and community-based samples.
PREDICTORS OF CONVERSION
Next, we examined specific variables that may account for the recruitment source effect. We first examined demographic variables (age, education, and sex). Because ethnicity was highly correlated with recruitment source, these 2 variables could not be included together as risk factors for conversion. In a proportional hazards model including recruitment source and the demographic variables, recruitment source remained the only variable independently associated with an increased hazard of conversion (HR=3.22; 95% CI, 1.18–8.84; P=.02). No demographic variables were significantly associated with conversion independent of recruitment source, including age (P=.74), education (P=.57), and sex (P=.16).
We then evaluated baseline cognitive variables. In a model that included recruitment source, Mini-Mental State Examination score, age, and education, only recruitment source was a significant predictor of future conversion (HR=3.54; 95% CI, 1.22–10.29; P=.02). The specific domains of (baseline) episodic memory and executive function were examined next. In a model that included recruitment source, episodic memory, age, and education, both clinic recruitment source (HR=3.01; 95% CI, 1.07–8.44; P=.04) and worse baseline episodic memory (HR=0.50; 95% CI, 0.26–0.97; P=.04) were associated with an increased hazard of future conversion. In a model that included recruitment source, baseline executive function, age, and education, clinic recruitment source (HR=3.39; 95% CI, 1.21–9.50; P=.02), but not executive function (HR=0.63; 95% CI, 0.27–1.50; P=.30), was associated with an increased risk hazard of conversion. An alternate approach to examining the relationship between cognition and risk of conversion is to examine MCI subtype in relation to conversion to dementia. In this analysis, the MCI subtype was not related to conversion to dementia (P=.75).
Baseline brain imaging variables were also examined as predictors of conversion. In a model containing recruitment source, WMH volume, and demographic variables, both WMH volume (HR=1.23; 95% CI, 1.02–1.48; P=.03) and recruitment source (HR=3.09; 95% CI, 1.09–8.74; P=.03) were significantly associated with conversion. In similar models, neither hippocampal volume (P=.12) nor total brain matter volume (P=.14) was significantly associated with conversion, independent of recruitment source.
Finally, we tested our hypothesis that baseline functional status would help account for differences in conversion rates across the 2 recruitment source groups. In a model that included recruitment source, CDR score, age, and education, recruitment source was no longer associated with conversion (P=.40), whereas greater functional impairment as measured by the CDR was significantly associated with hazard of conversion (HR=1.30; 95% CI, 1.01–1.66; P=.03). Additionally, in a joint model including all of the variables that were independently associated with risk of conversion in previous models (ie, recruitment source, episodic memory, WMH volume, and CDR score), only CDR score (HR=1.39; 95% CI, 1.04–1.83; P=.03) was independently associated with risk of conversion to dementia. Results were identical when including demographic variables in the model.
Models incorporating interval censoring showed results essentially identical to those described earlier.
COMMENT
To our knowledge, this study is the first to specifically evaluate source of recruitment as a predictor of conversion in a group of participants identically evaluated from the same research center. These results demonstrate that the rate of conversion differs substantially as a function of the method by which participants are recruited to the study, with the community sample showing a substantially lower annual conversion rate than the clinic sample (3% and 13%, respectively). The low conversion rate within the community sample of this study is similar to those in other population-based studies of conversion that had used similar diagnostic methods.34 We found the difference in conversion rate between the clinic and community MCI groups somewhat surprising given their equivalent baseline cognitive scores. However, the 2 groups did differ on a number of other important variables that could put them at increased risk of conversion to dementia. For example, the clinic sample had more functional impairment and lower total brain matter and hippocampal volumes at baseline. These differences suggest more advanced disease in the clinic sample, thereby indicating a state of illness further along the continuum toward dementia.
Although there were a number of differences between the clinical and community groups, the only variable that accounted for group membership itself as a risk for dementia was baseline functional impairment on the CDR. Thus, regardless of whether an individual was a clinic patient or recruited directly from the community, more functional impairment at baseline was an important risk for future conversion to dementia. The greater functional impairment at baseline within the clinically recruited group appears to account for their increased risk of conversion. When the CDR was replaced with an alternate measure of functional status in the analysis (the Blessed Roth Dementia Rating Scale), identical results were obtained (data not shown); this suggests that findings are not specific to one particular measure of functional status. It may be that functional changes are further downstream in the disease process and so reflect the disease effect in a summative way that has substantial prognostic importance. Consistent with this idea, the CDR is considered a measure of disease severity. Along these lines, it was the CDR sum of boxes rather than any of the specific CDR domains that was most strongly associated with future conversion (data not shown). Measures of functional decline may also be particularly useful measures of disease severity in individuals with low levels of education and/or of an ethnic minority because they are less affected by demographic variables.35
Although the CDR score was the only variable to remove the recruitment source differences in conversion rate, 2 other variables showed evidence of an independent risk of conversion. Greater baseline episodic memory impairment regardless of recruitment source was associated with conversion. Such findings are consistent with a large body of literature that suggests baseline memory to be associated with conversion.12,36 Additionally, higher WMH volume burden was associated with increased risk for conversion to dementia. Given that WMH volume is related to impaired executive function,37 it is possible that WMH volume contributes to additional cognitive impairments necessary for the diagnosis of dementia, particularly when Alzheimer disease pathologic manifestations are relatively mild38 such as in MCI.
Very few longitudinal studies of MCI have been carried out among older adults from diverse racial, ethnic, educational, and linguistic backgrounds34; as such, this study represents an important step toward closing this gap in the literature. The differential ethnic distribution in our 2 samples (80% of the community sample is of an ethnic minority, whereas 80% of the clinic sample is white) is a limitation of the current study. However, it seems extremely unlikely that it is simply the case that conversion rates are lower in Hispanic and African American individuals, given previous evidence that the rate of dementia in Hispanic and African American individuals is similar39–42 or possibly higher than that in white individuals. 43,44 It is possible that the rate of misdiagnosis was higher in the community sample, specifically that more ethnic minorities who were in fact cognitively normal were misdiagnosed as having MCI.45,46 For a number of reasons, we do not believe that to be true here. First, the samples did not differ on psychometrically robust scales of memory and executive function derived from the SENAS. The SENAS is a battery of tests created from the ground up as to assess cognitive function in a cross-cultural context and is the result of an extensive test development process using Item Response Theory methods. Item Response Theory provides powerful methods for examining the issue of item and scale bias; validation data have shown that the SENAS scales perform very similarly in ethnic minority and majority samples.31 We have also recently shown that brain volumes differ similarly between healthy persons, those with MCI, and those with dementia regardless of ethnicity,23 providing further validation of these diagnostic syndromes.
The inclusion of individuals recruited directly from the community who were not actively seeking evaluation likely increased the generalizability of our findings as compared with studies using only clinical samples. However, there is still a self-selection bias operating within the community sample. Participants had to agree to be part of the study and undergo testing. Those who declined to participate may have been in poorer health or may have had more cognitive impairment. Thus, while the conversion rate in the community group is similar to many epidemiological studies, it may underestimate rates of conversion in the general elderly population. As noted in “Methods,” however, our recruitment efforts were designed to maximize involvement of cognitively impaired individuals.
In summary, MCI is a heterogeneous group with variable rates of conversion to dementia. This study confirms that rates of conversion systematically vary depending on the nature of the sample, with a significantly higher conversion rate in a clinical-based sample of individuals actively seeking evaluation and/or treatment. Similarly evaluated and diagnosed persons in the community show substantially lower conversion rates. An important implication of this is that the conversion rate for MCI obtained in clinic samples should not be used to estimate the risk of dementia in population-based samples of persons with MCI. One objective variable that helps to account for this bias is baseline functional status. Thus, in an educationally and ethnically diverse population, those with more functional impairment at their baseline evaluation—regardless of whether they are actively seeking an evaluation for a neurodegenerative disease—are at increased risk for conversion to dementia even within a relatively short follow-up period.
Acknowledgments
Funding/Support: This work was supported by grants AG10129, AG021511, and AG12435 from the National Institute on Aging, by the California Department of Health Services, and by the Veterans Affairs Northern California Health Care System.
Footnotes
Author Contributions: Study concept and design: Farias, Mungas, Reed, and DeCarli. Acquisition of data: Farias, Mungas, Reed, and DeCarli. Analysis and interpretation of data: Farias, Mungas, Reed, Harvey, and DeCarli. Drafting of the manuscript: Farias, Mungas, Reed, and DeCarli. Critical revision of the manuscript for important intellectual content: Farias, Mungas, Harvey, and DeCarli. Statistical analysis: Farias, Mungas, and Harvey. Obtained funding: Farias, Mungas, Reed, and DeCarli. Administrative, technical, and material support: Farias. Study supervision: DeCarli.
Financial Disclosure: None reported.
Additional Contributions: We thank our community recruiters and the volunteers of this study for all of their efforts.
REFERENCES
- 1.Flicker C, Ferris S, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41(7):1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr. 1997;9 suppl 1:65–69. doi: 10.1017/s1041610297004717. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment-clinical characterization and outcome [published correction appears appears in Arch Neurol. 1999;56(6):760] Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 4.Bozoki A, Giordani B, Heidebrink J, Berent S, Foster N. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001;58(3):411–416. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 5.Tierney MC. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2001;57(1):163–164. doi: 10.1212/wnl.57.1.163. [DOI] [PubMed] [Google Scholar]
- 6.Grober E, Lipton R, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54(4):827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 7.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 8.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56(1):37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 9.Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57(5):675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 10.Feldman HH, Jacova C. Mild cognitive impairment. Am J Geriatr Psychiatry. 2005;13(8):645–655. doi: 10.1176/appi.ajgp.13.8.645. [DOI] [PubMed] [Google Scholar]
- 11.Palmer K, Wang HX, Bäckman L, Winblad B, Fratiglioni L. Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen Project. Am J Psychiatry. 2002;159(3):436–442. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- 12.Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63(8):916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 13.Guarch J, Marcos T, Salamero M, Blesa R. Neuropsychological markers of dementia in patients with memory complaints. Int J Geriatr Psychiatry. 2004;19(4):352–358. doi: 10.1002/gps.1074. [DOI] [PubMed] [Google Scholar]
- 14.DeCarli C, Mungas M, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63(2):220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruscoli M, Lovestone S. Is MCI really just early dementia? a systematic review of conversion studies. Int Psychogeriatr. 2004;16(2):129–140. doi: 10.1017/s1041610204000092. [DOI] [PubMed] [Google Scholar]
- 16.Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer’s disease: a longitudinal MRI study. Brain. 1996;119(pt 6):2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 17.Wolf H, Ecke GM, Bettin S, Dietrich J, Gertz HJ. Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? Int J Geriatr Psychiatry. 2000;15(9):803–812. doi: 10.1002/1099-1166(200009)15:9<803::aid-gps190>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Jefferson AL, Byerly LK, Vanderhill S, et al. Characterization of activities of daily living in individuals with mild cognitive impairment. Am J Geriatr Psychiatry. 2008;16(5):375–383. doi: 10.1097/JGP.0b013e318162f197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, DeCarli C. MCI is associated with deficits in everyday functioning. Alzheimer Dis Assoc Disord. 2006;20(4):217–223. doi: 10.1097/01.wad.0000213849.51495.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam CW, Lam LC, Chiu HF, Lui VW. Characteristic profiles of instrumental activities of daily living in Chinese older persons with mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2007;22(3):211–217. doi: 10.1177/1533317507301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purser JL, Fillenbaum GG, Wallace RB. Memory complaint is not necessary for diagnosis of mild cognitive impairment and does not predict 10-year trajectories of functional disability, word recall, or short portable mental status questionnaire limitations. J Am Geriatr Soc. 2006;54(2):335–338. doi: 10.1111/j.1532-5415.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 22.Pérès K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology. 2006;67(3):461–466. doi: 10.1212/01.wnl.0000228228.70065.f1. [DOI] [PubMed] [Google Scholar]
- 23.DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D. Brain behavior relationships among African Americans, whites, and Hispanics. Alzheimer Dis Assoc Disord. 2008;22(4):382–391. doi: 10.1097/wad.0b013e318185e7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 25.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part V: a normative study of the neuropsychological battery. Neurology. 1994;44(4):609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Adult Intelligence Scale Revised. New York, NY: Psychological Corp; 1981. [Google Scholar]
- 27.Grober E, Sliwinski M. Development and validation of a model for estimating pre-morbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 29.Mungas D, Reed BR, Marshall SC, González HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology. 2000;14(2):209–223. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- 30.Mungas D, Reed BR, Crane PK, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16(4):347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- 31.Mungas D, Reed BR, Tomaszewski Farias S, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: equivalent performance in elderly Hispanic and non-Hispanic whites. J Int Neuropsychol Soc. 2005;11(5):620–630. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack CR, Jr, O’Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging. 2001;14(6):668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 34.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artero S, Tierney MC, Touchon J, Ritchie K. Prediction of transition from cognitive impairment to senile dementia: a prospective, longitudinal study. Acta Psychiatr Scand. 2003;107(5):390–393. doi: 10.1034/j.1600-0447.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 37.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63(2):246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 38.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62(7):1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 40.Fillenbaum G, Heyman A, Huber M, et al. The prevalence and 3-year incidence of dementia in older black and white community residents. J Clin Epidemiol. 1998;51:587–595. doi: 10.1016/s0895-4356(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 41.Sandberg G, Stewart W, Smialek J, Troncoso JC. The prevalence of the neuro-pathological lesions of Alzheimer’s disease is independent of race and gender. Neurobiol Aging. 2001;22(2):169–175. doi: 10.1016/s0197-4580(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 42.Wilkins CH, Grant EA, Schmitt SE, McKeel DW, Morris JC. The neuropathology of Alzheimer disease in African American and white individuals. Arch Neurol. 2006;63(1):87–90. doi: 10.1001/archneur.63.1.87. [DOI] [PubMed] [Google Scholar]
- 43.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 44.Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14(6):481–493. [PubMed] [Google Scholar]
- 45.Gasquoine PG. Variables moderating cultural and ethnic differences in neuropsychological assessment: the case of Hispanic Americans. Clin Neuropsychol. 1999;13(3):376–383. doi: 10.1076/clin.13.3.376.1735. [DOI] [PubMed] [Google Scholar]
- 46.Manly JJ, Jacobs DM, Sano M, et al. Cognitive test performance among nondemented elderly African Americans and whites. Neurology. 1998;50(5):1238–1245. doi: 10.1212/wnl.50.5.1238. [DOI] [PubMed] [Google Scholar]