Abstract
Background
Treatment-resistant depression presents a serious challenge to both patients and clinicians. The anterior and midlateral prefrontal cortices play complementary roles in integrating emotional and cognitive experiences and in modulating subcortical regions. Both regions offer a distinct opportunity for targeted antidepressant treatments. We chose to pilot the safety and therapeutic benefits of chronic and intermittent epidural prefrontal cortical stimulation (EpCS) in patients with treatment-resistant depression.
Methods
We enrolled five adults with an average of 5.8 failed antidepressant treatments in their current depressive episode. All subjects underwent comprehensive clinical assessments, detailed neuropsychological testing, and presurgical magnetic resonance imaging. Four cortical stimulation paddle leads were stereotactically placed bilaterally over the anterior frontal poles and midlateral prefrontal cortex. We also acquired a postsurgical computed tomography scan and repeatedly assessed clinical outcomes over time of EpCS as an adjunctive treatment to constant medications.
Results
All patients tolerated the therapy. At 7-month follow-up, the average improvement from preimplant baseline on the Hamilton Rating Scale for Depression and the Inventory of Depressive Symptoms—Self-Report were 54.9% (± 37.7) and 60.1% (± 34.1), respectively. Three implanted subjects reached remission. One patient's left hemisphere leads were explanted 12 weeks postsurgery because of a scalp infection.
Conclusions
Bilateral EpCS over anterior and midlateral frontal cortex is a promising new technology for treatment-resistant depression. Future double-blind studies are warranted.
Keywords: Anterior poles, brain stimulation, cingulate, depression, epidural cortical stimulation, frontal lobes, medial prefrontal cortex
Treatment-resistant depression (TRD) is a recurrent psychiatric illness and a leading cause of premature mortality due to suicide and associated medical conditions (1,2). It is also associated with high social impairments and direct and indirect health care costs (3,4). In the United States, more than 3.2 million patients are diagnosed with TRD, but only a small percentage are adequately treated following expert guidelines (5–7). Large clinical studies (8) demonstrate the fairly limited success of psychopharmacologic interventions, and new treatments are needed.
Nonpharmacologic brain stimulation therapies (BSTs) have emerged since the mid-1990s as potentially efficacious treatments for TRD (9). They vary widely in their approach, brain targets, and stimulation parameters but have all been guided by the emerging knowledge of mood-regulating systems based on known anatomic interconnections (10). Some have suggested that the anterior and midlateral prefrontal cortices play different but complementary roles in mood regulation. The frontal pole is part of a distributed network extending caudally that is now commonly referred to as the “default-mode” network (11) and has rich connections directed to the anterior cingulate cortex, precuneus, and posterior cingulate (12) and orbitofrontal and dorsolateral prefrontal cortex (13,14). It appears to be an integrative center for cognitive and emotional processing with higher-level mnemonic control operations and control of emotions, memory, and motivation. It is also involved in self-reference (15), reflective self-awareness (16), and in attributing mental states to others (17). Conversely, the midlateral frontal regions maintain preferential bidirectional connections with multimodal temporal areas, on one hand, and paralimbic cortical areas such as the cingulate, the retrosplenial cortex, and the rostral temporal cortex, on the other hand (18). The dorsal regions (of the midlateral or medial parts) are involved in the monitoring of information in working memory and the ventral regions are involved in active judgments concerning information held in posterior cortical association regions that are necessary for active retrieval and encoding of information. The lateral frontal regions also play a critical role in organizing, monitoring, and verifying information; in attending to emotional stimuli; and in reappraisal. Both anterior and midlateral prefrontal regions offer a distinct opportunity for targeted antidepressant treatments.
Epidural cortical stimulation (ECS) (19) is a unique therapeutic approach. Leads are placed through a burr hole in the skull but above the dura mater and thus remain separated from the underlying cortical region by the arachnoid space. ECS is more direct than transcranial magnetic stimulation (TMS) (20,21) or vagus nerve stimulation (VNS) (22,23) and potentially safer (24) than deep brain stimulation (DBS), which involves passing the electrodes through brain tissue (25,26). ECS generally offers a wide range of stimulation configurations that can ultimately affect the size of the induced electrical field, its directionality, and the specificity in activated neuronal elements by varying pulse width, intensity, and frequency parameters. Chronic ECS of the motor or sensory areas has been used over the past 10 years to treat intractable pain syndromes (24,27–29), enhance recovery from stroke (30) and for motor disorders such as Parkinson's disease (19). An industry-sponsored study recently reported on the feasibility of constant stimulation unilateral left dorsolateral prefrontal ECS (EpCS) in TRD with modest but encouraging results (31). We hypothesized that chronic and intermittent anterior frontal poles and midlateral prefrontal cortex EpCS could modulate both medial and lateral networks and lead to improved mood in a treatment resistant depressed cohort.
Methods and Materials
Recruitment and Consent
This study was conducted at the Medical University of South Carolina (MUSC) in compliance with an Investigational Device Exemption issued to Z.N. under US Food and Drug Administration (FDA) guidance. The MUSC Institutional Review Board approved the protocol. To address the ethical concerns of providing an experimental and untested intervention that required surgery, the inclusion criteria limited enrollment to depressed participants with definite histories of substantial treatment resistance. An expert psychiatrist, independent of the study team, evaluated all patients to ensure that they were well informed of their clinical options. Written consents were obtained in the presence of a patient advocate also independent of the study team. All subjects underwent comprehensive assessments including detailed neuropsychological testing at baseline and repeatedly after implantation (details in Supplement 1).
Participants
Eligible participants presented with a nonpsychotic, nonatypical major depressive episode (MDE) as part of either bipolar (I or II) disorder or major depressive disorder (MDD), defined by DSM-IV criteria (32). All participants scored ≥20 on the 24-item Hamilton Rating Scale for Depression (HRSD24) (33,34) before implantation and following the 2- to 3-week postimplantation recovery period, after which time the EpCS leads were activated.
Participants were included if, during their current MDE, they had not benefited sufficiently to adequate trials of at least four classes of antidepressant medications or other somatic treatments as defined by the Antidepressant History Treatment Form (ATHF) criteria (35). Further, a minimum of 6 weeks of prior psychotherapy during any MDE was required.
A minimum 2-week recovery period followed implantation. The surgical procedure (Figure 1) is detailed in Supplement 1. The EpCS paddle leads (Medtronic, Minneapolis, MN) were then activated and adjusted over 2 more weeks, after which the stimulation parameters were fixed for the subsequent 15 weeks. Participants were on stable medication regimens for at least 4 weeks before and for 5 months following implantation, except that dose reductions were allowed during this period (primary outcome). If patients were already implanted with VNS therapy, their device needed to be off for a minimum of 6 months before and 1 year after their enrollment. During the longer-term follow-up study, which followed the acute phase, stimulation parameters and medications could be changed in type or dose. ECT, TMS, and DBS could not be provided. In effect, the bulk of treatment during changes during the study consisted of EpCS dose modifications (e.g., current, frequency, duty cycle, different leads stimulated) with minimal changes in medications.
Figure 1.
Surgical planning and electrodes placement. Before surgery, the region of interests (Brodmann's areas [BA] 10 and 46 bilaterally) were identified and highlighted on each subject's magnetic resonance imaging (MRI) scan (A and B). In the operating room, the patient's head was coregistered with the MRI image using a stereotactic frameless system (BrainLab, Westchester, Illinois). A three-dimensional rendering displayed the head surface anatomy in native space with bilateral BA 10 and 46 in distinctive colors. An external wand helped identify landmarks of interest for accurate position of the leads. (A–C) The external wand pointing at the right midlateral region of interest (BA 46). The surgical team used intraoperative fluoroscopy (D) in conjunction with the stereotactic frameless system to optimize targeting accuracy.
Intraoperative Testing
After all four leads were in place (see Supplement 1 for details), they were, by extension wires, connected to external stimulators. The moderate sedation was then lifted by discontinuing the propofol infusion. After the patient was fully alert, the surgical table was adjusted with head and back elevation for better patient interaction, and testing began. We conducted a patient masked sham-controlled parametric testing of bilateral anterior or midlateral leads at 0, 2, and 4–5 V and 60 Hz. Order was randomized, with “no stimulation condition” (0 V) always occurring first and at least once more during testing. Two patients were also tested at 6 V, but their data are not included here for consistency across sessions. Each train of stimulation lasted approximately 2 to 3 min, and patients were instructed to record their subjective experience by responding to 13 questions in the form of a visual analogue scale. Questions were presented on a laptop computer placed on the patient's abdomen and were typically phrased “I feel SAD,” “I feel ANXIOUS,” “I feel SETTLED emotionally,” “I feel ATTENTIVE to my surroundings,” and so on. All patients had the opportunity to familiarize themselves with the format and content of these subjective inquiries during their preoperative baseline visits. They were also encouraged to express any perceived changes in mood, attention, or cognition.
Postoperative Testing and Stimulation Optimization Period
The EpCS lead activation to determine stimulation parameters for chronic therapy took place on average 2 to 3 weeks postimplantation. Participants underwent a brief psychological screening 20 min before device activation. The screening measures were designed to assess general cognitive functioning quickly, including memory and executive functioning. Patients also underwent a series of experiments to investigate the psychological (mood, attention, and social relatedness) and biological correlates (electroencephalogram) of various stimulation parameters of the different leads to determine the optimal settings for clinical follow-up. These challenge results of this baseline acute stimulation are reported separately. After optimal settings were determined, patients underwent a repeat of the neuropsychologic testing with the EpCS leads active for at least 1 hour. In general, all paddle leads with their corresponding four contact electrodes were set to “–” (the most distal contact), “+,” “–” and “+” (the most proximal contact). Patients were discharged with a chronic and intermittent bilateral stimulation of all four paddle leads at 60 Hz, 2–4 V, 30 min on/ 2.5 hours off from 8 am to 10 pm (stimulation was “off” during nighttime). The following 2 weeks, parameters were adjusted according to patients’ input of what appeared to help the most in alleviating depressive symptoms or increasing attention and energy and then were held constant for 17 weeks. On average, patients received a cumulative 36,000 stimuli per day, as opposed to 345,600 if stimulation were continuous for 24 hours per day. (Note that these intermittent settings [30 min on, 2.5 hours off] are radically different from other DBS or ECS protocols, which have employed constant stimulation 24 hours/day.)
Data Analyses
Response was defined a priori as a 50% or greater reduction in mean HRSD24 scores relative to the average of the baseline (preimplantation) visits or, for secondary analyses, a 50% or greater reduction in the mean of baseline Montgomery-Asberg Depression Rating Scale (MADRS) score, or a 50% or greater reduction in the mean of baseline Inventory of Depressive Symptoms—Self-Report (IDS-SR) score. Remission was defined a priori as an HRSD24 score of 10 or lower. We employed a last observation carried forward analysis for missing data.
All data were quality checked and queries clarified before final analyses were conducted. The intraoperative subjective visual analogue scales were specifically scrutinized and matched to notes taken during testing. Certain values were reversed when there was a documentation that the patient intended to rate the opposite immediately after pressing the mouse pad key. Several responses were deleted when there was an indication in the output files that the questions skipped rapidly before the patient was able to respond accordingly. More than 80% of the values remained unchanged. Subject 2 was dysphoric and psychomotorically slowed during testing and was unable to complete the computerized VAS. His responses were conservatively entered as 0% change from baseline for all 13 questions.
The following techniques were used: descriptive statistics (including mean and standard deviation), Shapiro-Wilks Test for normality, Kruksal-Wallis Test, one-sample t test for normed intraoperative subjective visual analogue scales, repeated-measure analysis of variance (ANOVA) with repeated contrasts for clinical outcomes with post hoc analyses contrasting baseline to 4-month scores and baseline to 7-month scores, and graphical displays. For cognitive and neuropsychologic testing data, paired-sample t tests were used to assess for change over time. All statistical tests were two-tailed at .05 alpha level.
Results
Sample Characteristics
Of six initially enrolled participants, five received EpCS and participated in this study. One participant withdrew consent before implantation. Table 1 summarizes the sample characteristics. The mean age was 44.2 (± 9.4) years. Four were women, and three were diagnosed with recurrent MDD; two others had bipolar affective disorder I, depressed type. All were unemployed, and three were receiving disability. The average length of depressive illness was 25.6 (± 8.3) years. The average length of the current depressive episode was 3 years, 7 months (± 38 months). It is noteworthy that participants had received an average of 9.8 (± 5.3) unsuccessful clinical treatments during the current MDE. Of these central nervous system–active compounds, an average of 6.2 (± 2.1) were classic antidepressant treatments of which 5.8 (± 2.05) met ATHF criteria for trial adequacy (7). Two patients had failed a trial of a monoamine oxydase inhibitor or buprenorphine augmentation (36); one failed intravenous ketamine infusion (37), a trial of clozapine (38), and another trial of mifepristone for nonpsychotic depressive symptoms (39). Four of the patients had received prior treatments with ECT, TMS, or VNS. They enrolled in the study taking on average 6 (± 2.3) psychotropic drugs.
Table 1.
Sample Demographics
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Group | |
|---|---|---|---|---|---|---|
| Gender | F | M | F | F | F | 4 F/1 M |
| Diagnosis | Recurrent MDD | BPAD Depressed | BPAD Depressed | Recurrent MDD | Recurrent MDD | 3 MDD/2 BPAD |
| Current Age | 42 | 57 | 47 | 31 | 45 | 44.4 (± 9.7) |
| Length of Illness (years) | 17 | 32 | 31 | 16 | 32 | 25.6 (± 8.3) |
| Current Depressive Episode (months) | 31 | 83 | 84 | 8 | 8 | 42.8 (± 38.3) |
| HRSD Score (24 item) | 23 | 33 | 33 | 29 | 24 | 28.4(± 8) |
| Previous Brain Stimulation Therapies | ECT, VNS, TMS | ECT, VNS, TMS | ECT | VNS, TMS | None | 4 Yes/1 No |
| Past Psychotherapy | Yes | Yes | Yes | Yes | Yes | All |
| Family History of Depression | Yes | Yes | No | Yes | Yes | 4 Yes/1 No |
| Number of Psychiatric Treatments in Current Depressive Episodea | 12 | 18 | 6 | 8 | 5 | 9.8 (± 5.3) |
| Current ATHF | 8 | 8 | 4 | 5 | 4 | 5.8 (± 2.05) |
| Number of Psychotropics at Baseline | 9 | 5 | 6 | 3 | 7 | 6 (± 2.23) |
ATHF, Antidepressant History Treatment Form; BPAD, bipolar affective disorder; ECT, electroconvulsive therapy; F, female; HRSD, Hamilton Rating Scale for Depression; M, male; MDD, major depressive disorder; TMS, transcranial magnetic stimulation; VNS, vagus nerve stimulation.
Excluding psychotherapy.
Accuracy of Lead Placement
The postoperative computed tomography scan coregistered with a presurgical magnetic imaging resonance scan identified four contact electrodes for each paddle lead. In all subjects, contact electrodes were over the anterior frontal pole or midlateral prefrontal cortex (Table 2). Note that Subject 4 had both anterior paddle leads extending more ventrally to overlap Brodmann's area (BA) 11, and Subject 5's ventral portion of the left lateral lead was directed slightly more posterior and arching over BA 45.
Table 2.
Spiral Computed Tomography Scan Acquired Postoperatively Allowed the Identification of Each Paddle Lead and Its Four Contact Electrodes: Individual Correspondence Between Contact Electrode and Underlying Cortical Brodmann Area in All Five Subjects
| Left Midlateral Frontal Cortex | Left Anterior Frontal Pole | Right Anterior Frontal Pole | Right Midlateral Frontal Cortex | |
|---|---|---|---|---|
| Subject 1 | ||||
| Contact 4 | 9 | 9 | 9 | 9 |
| Contact 3 | 46 | 10 | 10 | 46 |
| Contact 2 | 46 | 10 | 10 | 46 |
| Contact 1 | 46 | 10 | 10 | 46 |
| Subject 2 | ||||
| Contact 4 | 46 | 10 | 10 | 46 |
| Contact 3 | 46 | 10 | 10 | 46 |
| Contact 2 | 46 | 10 | 10 | 46 |
| Contact 1 | 46 | 10 | 11 | 45 |
| Subject 3 | ||||
| Contact 4 | 46 | 10 | 10 | 46 |
| Contact 3 | 46 | 10 | 10 | 46 |
| Contact 2 | 46 | 11 | 11 | 46 |
| Contact 1 | 46 | 11 | 11 | 46 |
| Subject 4 | ||||
| Contact 4 | 46 | 9 | 9 | 46 |
| Contact 3 | 45 | 10 | 10 | 46 |
| Contact 2 | 45 | 10 | 10 | 46 |
| Contact 1 | 45 | 10 | 10 | 45 |
| Subject 5 | ||||
| Contact 4 | 46 | 10 | 10 | 46 |
| Contact 3 | 46 | 10 | 10 | 46 |
| Contact 2 | 46 | 10 | 10 | 46 |
| Contact 1 | 46 | 10 | 11 | 46 |
Intraoperative Testing
In this single-blind sham-controlled experiment, which lasted on average 30 min, active EpCS was associated with significant decreases compared with baseline in patients’ subjective sadness (p = .05) and anxiety levels (p = .034) despite this small sample size (Table 3). Active stimulation was also associated with more than 100% improvement in self-ratings of “improved pleasure,” “sense of involvement,” and “attention,” but these effects were specifically pronounced in two subjects, which led to a large standard deviation and were not statistically significant. Of note is “I feel ANGRY,” and the average percent change from baseline is primarily driven by one subject whose rating increased from 4/100 to 15/100, representing a 300% increase despite its relative small absolute change. Patients’ trust was high at baseline and was not changed throughout the session. None of the sham conditions led to significant changes from baseline. The changes attributable to specific active leads were not exclusive, although two patients distinctly noted improved attention, increased brightness, and alertness with lateral stimulation. They commented: “I feel attentive,” “feel better and I can talk now,” “I can think clearer.” A patient noted during anterior frontal pole stimulation feeling as if a “weight [was] lifting off my shoulder,” “I feel calm”; another stated, “and although I am worried, I feel dissociated from it. I can think back at my worry.” Interestingly, this last comment of subjective dissociation was observed at 6 V. The same patient would later experience similar effects during postoperative stimulation optimization period. Subject 2 failed to notice or report any positive changes across all tested parameters and became disappointed, discouraged, and dysphoric. His testing was interrupted early.
Table 3.
Intraoperative Testing
| Subjective | t | df | p Value (2-tailed) | Mean Change from Baseline | 95% CI of the Difference |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Sad | –2.768 | 4 | .050 | –34.65% | –69.41 | .10 |
| Secure | 1.564 | 4 | .193 | 31.01% | –24.05 | 86.08 |
| Tense | –.481 | 4 | .656 | –9.26% | –62.72 | 44.20 |
| Angry | .761 | 4 | .489 | 103.04% | –272.70 | 478.78 |
| Anxious | –3.082 | 4 | .037 | –45.81% | –87.08 | –4.54 |
| Comfortable | 1.857 | 4 | .137 | 58.38% | –28.89 | 145.66 |
| Worried | –2.376 | 4 | .076 | –36% | –78.05 | 6.06 |
| Confused | –2.600 | 4 | .060 | –45.72% | –94.55 | 3.11 |
| Trustful | –.373 | 4 | .728 | –1.712% | –14.47 | 11.05 |
| Settled | 1.845 | 4 | .139 | 73.34% | –37.05 | 183.74 |
| Pleasure | 1.015 | 4 | .367 | 102.22% | –177.31 | 381.75 |
| Involved | 1.611 | 4 | .182 | 137.73% | –99.60 | 375.06 |
| Attentive | 1.573 | 4 | .191 | 103.85% | –79.44 | 287.14 |
CI, confidence interval.
Clinical Outcomes
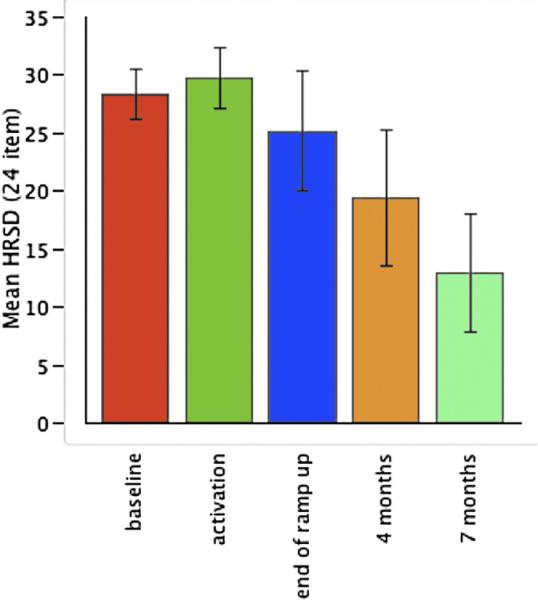
Table 4 details the individual scores at study landmarks following device activation. Patients did not improve clinically 2 to 3 weeks postoperatively with the EpCS leads turned off but rather showed a 5% worsening of symptoms. However, after the leads were activated, patients showed a significant improvement over time (df = 4, F = 4.867, p = .009). After 4 months of active stimulation, the group showed a mean HRSD24 improvement of 36% (± 39), which increased to 55% (± 38) at 7 months (Figure 2, Table 5). Two patients achieved remission at 4 months, and three of five were remitted at 7 months.
Table 4.
Individual 24-Item Hamilton Rating Scale for Depression Scores over Time
| Time | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 |
|---|---|---|---|---|---|
| Preoperative Baseline | 23 | 33 | 33 | 29 | 24 |
| Week of EpCS Activation | 22 | 32 | 38 | 29 | 28 |
| 2 Weeks post-EpCS Activation | 14 | 36 | 38 | 24 | 14 |
| 4 Months | 2 | 33 | 23 | 29 | 10 |
| 7 Months | 4 | 3 | 19 | 30 | 9 |
EpCS, epidural prefrontal cortical electrical stimulation.
Figure 2.
Mean and standard error in 24-item Hamilton Rating Scale for Depression patients over time in five treatment-resistant depression-treated with adjunctive anterior pole and midlateral epidural prefrontal cortical electrical stimulation.
Table 5.
Changes in Average Group Scores over Time for Primary (HRSD24) and Secondary (MADRS, IDS-SR30, and CGI-C) Outcome Measures with Corresponding Statistical Values, Functional Measures (Q-LES and MOS-SF36), and Average Psychotropic Drugsa
| Patients (n = 5) Mean Scores (SD) |
Repeated-Measure ANOVA |
Paired t Test |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop | Activation | 2 Weeks | 4 Months | 7 Months | df | F | p Value | Observed Power | Preop vs. 4 Months p Value | Preop vs. 7 Months p Value | |
| HRSD (24 Item) | 28.4 (4.8) | 29.8 (5.8) | 25.2 (11.5) | 19.4 (13) | 13 (11.4) | 4 | 4.867 | .009 | .879 | .092 | .037 |
| MADRS | 32 (7.6) | 31.2 (9.6) | 27 (6.2) | 22 (6.6) | 14.6 (6.1) | 4 | 3.886 | .022 | .683 | .075 | .043 |
| IDS-SR (30 Item) | 45.8 (16.2) | 43 (12.9) | 36.8 (19.1) | 31.4 (25.3) | 19 (18.48)a | 4 | 4.419 | .014 | .842 | .1 | .023 |
| CGI | 5.6 (1.14) | 5.2 (.8) | 4.8 (1.3) | 3 (1.2) | 3.4 (1.67) | 4 | 7.647 | .0001 | .981 | .075 | .043 |
| Q-LES | 40 (7.1) | — | — | 52 (20.9) | 50.4 (16.9) | ||||||
| MOS-SF36 | |||||||||||
| Physical functioning | 62 (21.96) | — | — | 71 (25.83) | 81 (12.94) | ||||||
| Role limit due to physical health | 0 | — | — | 0 | 0 | ||||||
| Role limit due to emotional problem | 0 | — | — | 0 | 0 | ||||||
| Energy/fatigue | 13 (10.36) | — | — | 32 (19.55) | 40 (22.63) | ||||||
| Emotional well-being | 36.8 (22.16) | — | — | 56.8 (38.09) | 67.2 (25.2) | ||||||
| Social functioning | 22.5 (18.54) | — | — | 50 (47.59) | 60 (45.41) | ||||||
| Pain | 67 (31.29) | — | — | 65.5 (23.41) | 69.5 (30.68) | ||||||
| General health | 50 (28.06) | — | — | 50 (31.62) | 66 (22.74) | ||||||
| Average Psychotropics | 6.23 | 6.4b (2.6) | 6.2b (2.28) | 6.4 (2.6) | 6.6 (2.3) | ||||||
ANOVA, analysis of variance; MADRS, Montgomery-Asberg Depression Scale; IDS-SR, Inventory of Depression Symptoms–Self-Report; CGI, Clinical Global Impression; Q-LES, Quality of Life, Enjoyment and Satisfaction Questionnaire; MOS_SF36, Medical Outcomes Study Short Form-36.
Patient 4 self-rating was 25.
Postoperative pain medications.
Secondary Outcomes
Mean scores of the MADRS and IDS-SR are provided in Table 5. MADRS percent change from baseline significantly improved over time (ANOVA: df = 4, F = 3.886, p = .022). The IDS-SR scores at baseline, 4 months, and 7 months were also significantly improved (ANOVA: df = 4, F = 4.419 p = .014). One patient reached remission after 2 weeks of stimulation based on IDS-SR of 15 or less and remained well until her 7-month follow-up. Two additional patients reached remission, one at 4-month and the second at 7-month follow-up.
Functional Measures
Participants completed the Quality of Life, Enjoyment and Satisfaction Questionnaire (40) at baseline and at 4 and 7 months (Table 5). They also completed the Medical Outcomes Study Short Form-36 (41). At 4 months after implant, no changes were noted as a group in physical functioning, pain or general health.
Before and After First Day of Activation
Scores for each of the brief screening measures are presented in Table 6. Significant improvement was observed on the Stroop Color–Word Test and the Sentence Repetition Test after the devices were turned on [t(4) = 3.81, p = .019, and t(4) = 3.16, p = .03, respectively]. No significant changes were observed across any of the other brief screening measures (Table 6).
Table 6.
Mean (SD) Neuropsychological Testing Scores Before and During EpCS (Testing Performed 2–3 Weeks After Implantation)
| Mean and (SD) |
|||||||
|---|---|---|---|---|---|---|---|
| Cognitive Estimation |
Stroop Color–Word |
Sentence Repetition |
Oral Trails B |
Digit Span |
Logical Memory I |
Logical Memory II |
|
| Condition | Number Correct | Time (s) | Percentile | Secs Converted | Scaled Score | Scaled Score | Scaled Score |
| Before EpCS Activation | 8.40 (1.517) | 8.20 (2.05) | 6.00 (2.236) | 86.85 (66.48) | 9.40 (2.41) | 10.40 (2.96) | 10.80 (1.92) |
| During EpCS | 9.00 (.71) | 11.00 (3.67) | 11.00 (4.183) | 71.8 (46.04) | 10.00 (3.94) | 10.40 (2.88) | 12.00 (2.24) |
EpCS, epidural prefrontal cortical electrical stimulation.
Baseline and Postacute Phase
No significant changes were seen after 20 (± 9) weeks of bilateral EpCS compared with baseline (Table 7).
Table 7.
Mean (SD) Neuropsychological Testing Scores Before and After 20 (± 9) Weeks of Epidural Prefrontal Cortical Electrical Stimulation Therapy
| Baseline Mean Score (SD) | Follow-up Mean Score (SD) | t | df | p | |
|---|---|---|---|---|---|
| Choice Reaction Test (No. Correct) | 58.80 (1.30) | 50.80 (20.02) | .86 | 4 | .437 |
| Choice Reaction Test (msec) | 836.08 (432.68) | 508.72 (322.89) | 1.22 | 4 | .288 |
| Choice Reaction Test (No. Incorrect) | 1.20 (1.30) | 9.20 (20.02) | –.86 | 4 | .437 |
| Simple Reaction Time (msec) | 402.40 (138.80) | 361.82 (96.53) | 1.50 | 4 | .208 |
| Continuous Performance Task (No. False Alarms) | 3.00 (2.45) | 3.75 (.96) | –.57 | 3 | .608 |
| Continuous Performance Task (False Alarm Time—msec) | 602 (90.39) | 625.37 (183.61) | –.23 | 2 | .839 |
| Continuous Performance Task (No. Hits) | 18.75 (7.09) | 21.50 (6.03) | –2.48 | 3 | .089 |
| Continuous Performance Task (Hit Time—msec) | 536.21 (30.23) | 519.59 (45.65) | .52 | 3 | .640 |
| Cognitive Failures Test | 51.25 (10.50) | 37.75 (18.37) | 1.61 | 3 | .206 |
| Modified Mini-Mental Status Examination (Total Score) | 60.25 (6.95) | 63.50 (.58) | –.89 | 3 | .437 |
Adverse Events
In one subject, on surgically passing one of the paddles down the epidural space over the left frontal pole, a small rent in the dura was caused. The lead paddle was successfully placed epidurally and covering the slit defect. There was no leakage or subgaleal cerebrospinal fluid collection postoperatively. Between the initiation of stimulation (turning on the device) and the 7-month follow-up, one serious adverse event occurred that required a rehospitalization at Week 12 postimplant. The patient failed to notify the research team until 1 week after noticing oozing of pus at the site of the left scalp incision. She immediately underwent debridement and antibiotic therapy for 1 week before the team elected to act conservatively and explant the patient's ipsilateral leads. She was asymptomatic otherwise. Her clinical depressive symptoms worsened temporarily for 2 to 3 weeks but began to show gradual signs of improvement afterward. At 7-month follow-up, she demonstrated a 42% drop from her study-entry HRSD24 score. Two patients with a history of migraines continued to have periodic attacks without any documented changes in the frequency of their occurrences compared with baseline. One patient experienced urinary incontinence and was placed on oxybutynin chloride extended-release and had a worsening of essential tremor. One patient experienced a mild dissociative state when stimulated at 6 V over the anterior frontal poles, which was reversible upon reducing the EpCS intensity. There were no seizures, incidents of hypomania, impulsivity, or disinhibition, nor were there reports of emergent cognitive deficits compared with baseline. Of note is that the detailed cognitive testing did not reveal any deficits attributable to the stimulation itself.
Discussion
Our primary objective was to test the potential safety, tolerability, and potential therapeutic benefits of bilateral anterior and lateral EpCS in TRD patients. We developed a unique presurgical planning method that rendered our stereotactic surgical implant accurate and safe. In this small sample, acute stimulation during surgery of anterior and midlateral leads was associated with significant mood changes under blinded conditions. By 7 months, adjunctive intermittent open-label EpCS was well tolerated and was associated with marked and sustained improvement in three of the five severe TRD patients.
Despite the invasive nature of EpCS and the functional impairment of these patients at the time of enrollment, patients tolerated the surgical procedure and chronic stimulation with no worsening of cognition, especially tasks that involved the frontal lobes. The infection of the left surgical site at 3 months in one patient was unexpected, especially that late after implant. In one recent large study of neurosurgical postoperative infections, the mean latency between surgery and infection was 34 days, ranging from a minimum of 8 days (cerebral spinal fluid infection) to a maximum of 4 months (42). Nevertheless, the subsequent removal of the leads is a reminder of the risks associated with device implantations and craniotomies. In a recently published series, 3 of 20 (15%) DBS-implanted depressed patients had to be explanted because of infection (43), and depressed patients do have abnormal immune system function (44,45). Detailed neuropsychologic examination showed that the patients did not exhibit any cognitive impairment in attention, working memory, or problem solving that could have been possible with targeted prefrontal cortical regions. There was nonspecific increased subjective alertness and attention with both targets. The reversible dissociative experience with high-intensity stimulation in one patient was only seen with anterior pole EpCS. This is intriguing given this cortical region's role in self-reference (15), reflective self-awareness (16), and abstract integration of affective and cognitive inputs (13). All patients were continuing with the therapy at last follow-up.
Over the past decade, there have been several studies that explored subconvulsive brain stimulation for TRD. VNS therapy was one of the first to be systematically tested and is now U.S. FDA approved for TRD. In early reports on its feasibility and putative efficacy (n = 30), adjunctive antidepressant responses at 3 and 6 months were 40% and 55%, respectively. Remission rates were up to 37% at 6 months (46). A subsequent double-blind placebo-controlled study in a larger sample and 21 clinical sites showed more modest effects with 15% response and 9% remission at 3 months (47). Six months open data were associated with 18% response and 9% remission (48). It is noteworthy that experience has shown that VNS therapy appears to have a slow onset, with response and remission rates achieving 27.2% and 15.8% at 1 year (49,50). Longitudinal imaging studies also support the gradual modulation of critical brain regions implicated in mood regulation, including medial prefrontal cortex and insula (23). In general, studies investigating VNS therapy have included 10% (51) to 27% (52) of patients with bipolar affective disorder and showed comparable response rates to the general cohort (22,47,53). There are several uncontrolled reports with small samples of successfully treating TRD using high-frequency DBS to the caudate nucleus (54), anterior thalamic nuclei (55), subgenual cingulate (26,43), the anterior limb of the internal capsule (56), or the nucleus accumbens (25). The clinical effects range between 20% and 35% remission rates at 6-month open-label follow-up (43). To our knowledge, the only other EpCS study to date involved 12 TRD patients who were randomized to active or sham single-blind treatment for 8 weeks with an adaptive open design follow-up (31). No significant difference across conditions was noted after 2 months. At 4 months, active left dorsolateral prefrontal cortex EpCS demonstrated a 21% ± 23% improvement in depressive symptoms. One of 12 patients reached remission at 4 months. Additional follow-up will provide critical information on further changes over time in this cohort. Importantly, this study had two major differences compared with our report: the Dougherty 2008 study involved only stimulation of the left prefrontal region, not bilaterally with four paddle leads, and the stimulation was applied in a constant manner, not intermittently.
Assuming these results are more than merely placebo, the antidepressant mechanisms of action of EpCS are yet to be elucidated. ECS over the motor cortex appears to induce its therapeutic effects at stimulation intensities below the threshold for overt motor responses. Stimulated axons could be short fibers of small inhibitory interneurons within the frontal cortex, as well as afferent or efferent fibers connected with distant cortical or subcortical structures (57). We approached stimulation targets and parameters guided by known functional neuroanatomy and previous experience in intermittent brain stimulation therapies with TMS (21,58–60) and VNS (61). It is possible that EpCS, when applied at 60 Hz, exerts strong inhibition on the underlying cellular network in the prefrontal cortex itself, locally modulating its output. The duty cycle (on/off trains of stimulation) is a key parameter because the pathophysiology of depression may benefit from intermittent stimulation to optimize durable long-term changes that re not subject to immediate relapse upon the interruption of treatment (i.e., when battery expires). In some applications (e.g., DBS for tremor), the clinical changes occur immediately after switching the stimulator on or off. This argues for stimulus-locked activation or inhibition of neural cells or modification of a pattern of desynchronization within specific neural pathways or loops (62). In contrast, other applications (e.g., DBS for dystonia or depression, ECS for chronic pain, or the unilateral EpCS study listed earlier) show delayed and gradual clinical improvements. In these cases, time-consuming processes are needed to alter synaptic plasticity critical for long-term treatment of major depression. Similarly, we have observed longer antidepressant onset with VNS applied intermittently with on/off duty cycles. Our current study does not yet fully address this critical theoretical hypothesis, but to the degree that we may have found some efficacy, we provide evidence that intermittent stimulation may work.
Our study has clear limitations owing to its small sample size and open design. The patients were motivated, but their treatment resistance was unquestionable. Use parameters (which combination of leads, intensity, frequency) were guided by acute subjective changes, but it is not clear whether these will predict later responses. We are using electroencepalogram and diffusion tensor imaging to understand better the structural and functional connectivity associated with EpCS (63). At this stage in the development, it is not clear why the functional measure did not show significant improvements. This is likely related to the relatively small sample size and baseline variability in functional disability. It is also not known whether a bilateral approach is always necessary to optimize treatment outcomes.
In summary, bilateral anterior pole and midlateral EpCS for TRD appears relatively safe, feasible, and, most important, has shown open-label improvement consistent with other functional neurosurgical approaches in a treatment resistant cohort. More studies are needed.
Supplementary Material
Acknowledgments
This study was funded primarily by a National Alliance of Research for Depression and Schizophrenia (NARSAD) Independent Investigator Award to ZN. It was also made possible with general funds from the Mood Disorders Program, the Brain Stimulation Laboratory, the General Clinical Research Center, the Center for Advanced Imaging Research at the Medical University of South Carolina. Medtronic, Inc. (Minneapolis, MN) donated the devices but was otherwise not involved in the study, particularly data acquisition, analysis, or drafting the article. We thank Mark Rise (Medtronic, Inc.) for technical assistance in stimulation setups and Sarah Coker (now a postgraduate year I psychiatry resident at Medical University of South Carolina [MUSC]) for assistance in the accuracy of lead placement analysis.
Dr. Nahas reports having received consulting fees from Neuronetics, Inc. and Cyberonics, Inc.; research funding from the National Institute of Mental Health (NIMH), National Alliance of Research for Schizophrenia and Depression, Hope for Depression Research, Neuronetics, Inc., Cyberonics, Inc., Medtronic, Inc. (in the form of device donations for this study), Brainsway, and Integra. Dr. George reports research funding from GlaxoSmithKline, Jazz Pharmaceuticals, NIMH, National Institute on Drug Abuse, National Institute of Neurological Disorders and Stroke, and the Ralph H. Johnson VA Medical Center. Dr. George reported consulting for Bristol-Meyers-Squibb, DarPharma, GlaxoSmithKline, Jazz Pharmaceuticals, Parke Davis, Aspect Medical, Brainsway, Brainsonix (unpaid), Cephos (unpaid), Cyberonics, Inc., Dantex, Medi-Physics/Amersham, Neuronetics, Inc. (unpaid), and Neuropace. Dr. George also reported that the Medical University of South Carolina has filed eight patents or invention disclosures under his name regarding brain imaging and brain stimulation. Dr. Borckardt receives research funding from the National Institute for Neurological Disorders and Stroke and the National Institute of Nursing Research at the National Institutes of Health, Cyberonics, Inc., and the Neurosciences Institute at MUSC. Dr. Borckardt is a consultant for Neuropace. Dr. Reeves, Dr. Tackacs, Mr. Anderson, and Ms. Arana reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Portions of this work were presented at the Annual Meeting of the American College of Neuropsychopharmacology, Scottsdale, Arizona, in December 2008.
Supplementary material cited in this article is available online.
References
- 1.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global burden of disease study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 2.Greden JF. The burden of recurrent depression: Causes, consequences, and future prospects. J Clin Psychiatry. 2001;62(suppl 22):5–9. [PubMed] [Google Scholar]
- 3.Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: How did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 4.Berto P, D'Ilario D, Ruffo P, Di Virgilio R, Rizzo F. Depression: Cost-of-illness studies in the international literature, a review. J Ment Health Policy Econ. 2000;3:3–10. doi: 10.1002/1099-176x(200003)3:1<3::aid-mhp68>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: Results from the national comorbidity survey replication (NCS-R). J Am Med Assoc. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 6.Klerman GL, Weissman MM. Increasing rates of depression. J Am Med Assoc. 1989;261:2229–2235. [PubMed] [Google Scholar]
- 7.Robins LN, Regier DA. Psychiatric Disorders in America: The Epidemiological Catchment Area Study. Free Press; New York: 1991. [Google Scholar]
- 8.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 9.Nahas Z, Lorberbaum JP, Kozel FA, George MS. Somatic treatment in psychiatry. In: Panksepp J, editor. Textbook of Biological Psychiatry. John Wiley & Sons; Hoboken, NJ: 2003. pp. 521–548. [Google Scholar]
- 10.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 11.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1089. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 12.Goldman-Rakic PS. Topography of cognition: Parallel distributed networks in Primate Association Cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 13.Petrides M, Pandya D. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramnani N, Owen A. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 15.Posner MI, et al. Localization of cognitive operations in the human brain. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- 16.Kjaer TW, et al. Increased dopamine tone during meditation-induced change of consciousness. Brain Res Cogn Brain Res. 2002;13:255–259. doi: 10.1016/s0926-6410(01)00106-9. [DOI] [PubMed] [Google Scholar]
- 17.Vogeley K, Bussfeld P, Newen A, Herrmann S, Happé F, Falkai P, et al. Mind reading: Neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- 18.Petrides M. Lateral prefrontal cortex: Architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Priori A, Lefaucheur JP. Chronic epidural motor cortical stimulation for movement disorders. Lancet Neurol. 2007;6:279–286. doi: 10.1016/S1474-4422(07)70056-X. [DOI] [PubMed] [Google Scholar]
- 20.Nahas Z, et al. Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: a pilot study. Depress Anxiety. 2004;19:249–256. doi: 10.1002/da.20015. [DOI] [PubMed] [Google Scholar]
- 21.Nahas Z, et al. Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry. 2001;50:712–720. doi: 10.1016/s0006-3223(01)01199-4. [DOI] [PubMed] [Google Scholar]
- 22.Nahas Z, Li X, Kozel FA, Mirzki D, Memon M, Miller K, et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatry. 2005;66:1097–1104. doi: 10.4088/jcp.v66n0902. [DOI] [PubMed] [Google Scholar]
- 23.Nahas Z, Teneback C, Chae JH, Mu Q, Molnar C, Kozel FA, et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 24.Canavero S, Bonicalzi V. Therapeutic extradural cortical stimulation for central and neuropathic pain: A review. Clin J Pain. 2002;18:48–55. doi: 10.1097/00002508-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 26.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen JP, Lefaucher JP, Le Guerinel C, Eizenbaum JF, Nakano N, Carpentier A, et al. Motor cortex stimulation in the treatment of central and neuropathic pain. Arch Med Res. 2000;31:263–265. doi: 10.1016/s0188-4409(00)00078-3. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen JP, Keravel Y, Feve A, Uchiyama T, Cesaro P, Le Guerinel C, Pollin B. Treatment of deafferentation pain by chronic stimulation of the motor cortex: Report of a series of 20 cases. Acta Neurochir Suppl. 1997;68:54–60. doi: 10.1007/978-3-7091-6513-3_10. [DOI] [PubMed] [Google Scholar]
- 29.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl. 1991;52:137–139. doi: 10.1007/978-3-7091-9160-6_37. [DOI] [PubMed] [Google Scholar]
- 30.Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: A prospective, multicenter safety study. Neurosurgery. 2006;58:464–473. doi: 10.1227/01.NEU.0000197100.63931.04. [DOI] [PubMed] [Google Scholar]
- 31.Dougherty DD, Thase ME, Howland RH, Evans KC, Harsch H, Kondziolka D, et al. Feasibility study of an implantable cortical stimulation system for patients with major depressive disorder.. Presented at the Society of Biological Psychiatry 63rd Annual Meeting; Washington, D.C.. 2008. [Google Scholar]
- 32.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th ed. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- 33.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;12:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 35.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10–17. [PubMed] [Google Scholar]
- 36.Nyhuis PW, Gastpar M, Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J Clin Psychopharmacol. 2008;28:593–595. doi: 10.1097/JCP.0b013e31818638a4. [DOI] [PubMed] [Google Scholar]
- 37.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 38.Frye MA, Ketter TA, Altshuler LL, Denicoff K, Dunn RT, Kimbrell TA, et al. Clozapine in bipolar disorder: Treatment implications for other atypical antipsychotics. J Affect Disord. 1998;48:91–104. doi: 10.1016/s0165-0327(97)00160-2. [DOI] [PubMed] [Google Scholar]
- 39.Belanoff JK, Flores BH, Kalezhan M, Sund B, Schatzberg AF. Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol. 2001;21:516–521. doi: 10.1097/00004714-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 41.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 42.Valentini LG, Casali C, Chatenoud L, Chiaffarino F, Uberti-Foppa C, Broggi G. Surgical site infections after elective neurosurgery: A survey of 1747 patients. Neurosurgery. 2008;62:88–95. doi: 10.1227/01.NEU.0000311065.95496.C5. discussion: 95–96. [DOI] [PubMed] [Google Scholar]
- 43.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 44.Anisman H, Merali Z. Cytokines, stress, and depressive illness. Brain Behav Immun. 2002;16:513–524. doi: 10.1016/s0889-1591(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 45.Vedder H, Schreiber W, Schuld A, Kainz M, Lauer CJ, Krieg JC, et al. Immune-endocrine host response to endotoxin in major depression. J Psychiatr Res. 2007;41:280–289. doi: 10.1016/j.jpsychires.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Marangell LB, Rush AJ, George MS, Sackeim HA, Johnson CR, Husain MM, et al. Vagus nerve stimulation (VNS) for major depressive episodes: One year outcomes. Biol Psychiatry. 2002;51:280–287. doi: 10.1016/s0006-3223(01)01343-9. [DOI] [PubMed] [Google Scholar]
- 47.Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, Howland R, et al. Vagus nerve stimulation for treatment-resistant depression: A randomized, controlled acute phase trial. Biol Psychiatry. 2005;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 48.Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM, et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: A naturalistic study. Biol Psychiatry. 2005;58:355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 49.Sackeim HA, Brannan SK, Rush AJ, George MS, Marangell LB, Allen J. Durability of antidepressant response to vagus nerve stimulation (VNSTM). Int J Neuropsychopharmacol. 2007;10:817–826. doi: 10.1017/S1461145706007425. [DOI] [PubMed] [Google Scholar]
- 50.Nahas Z, Marangell LB, Husain MM, Rush AJ, Sackeim HA, Lisanby SH, et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatry. 2005;66:1097–1104. doi: 10.4088/jcp.v66n0902. [DOI] [PubMed] [Google Scholar]
- 51.George M. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005;58:364–373. doi: 10.1016/j.biopsych.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 52.Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: A multicenter study. Biol Psychiatry. 2000;47:276–286. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 53.Nierenberg AA, Alpert JE, Gardner-Schuster EE, Seay S, Mischoulon D. Vagus nerve stimulation: 2-year outcomes for bipolar versus unipolar treatment-resistant depression. Biol Psychiatry. 2008;64:455–460. doi: 10.1016/j.biopsych.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 54.Pool JL. Psychosurgery of older people. J Geriatr Assoc. 1954;2:456–465. doi: 10.1111/j.1532-5415.1954.tb02138.x. [DOI] [PubMed] [Google Scholar]
- 55.Mark VH, Barry H, McLardy T, Ervin FR. The destruction of both anterior thalamic nuclei in a patient with intractable agitated depression. J Nerv Ment Dis. 1970;150:266–272. doi: 10.1097/00005053-197004000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Greenberg BD. Update on deep brain stimulation. J ECT. 2002;18:193–196. doi: 10.1097/00124509-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 57.García-Larrea L, Peyron R, Mertens P, Laurent B, Mauguière F, Sindou M. Functional imaging and neurophysiological assessment of spinal and brain therapeutic modulation in humans. Arch Med Res. 2000;31:248–257. doi: 10.1016/s0188-4409(00)00083-7. [DOI] [PubMed] [Google Scholar]
- 58.Nahas Z, Teneback CC, Kozel A, Speer AM, DeBrux C, Molloy M, et al. Brain effects of TMS delivered over prefrontal cortex in depressed adults: Role of stimulation frequency and coil-cortex distance. J Neuropsychiatr Clin Neurosci. 2001;13:459–470. doi: 10.1176/jnp.13.4.459. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Nahas Z, Kozel FA, Anderson B, Bohning DE, George MS. Acute left prefrontal transcranial magnetic stimulation in depressed patients is associated with immediately increased activity in prefrontal cortical as well as subcortical regions. Biol Psychiatry. 2004;55:882–890. doi: 10.1016/j.biopsych.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 60.Kose S, Lix, Morgan PS, Anderson B, Nahas Z, George MS, et al. Does the effect of prefrontal rTMS on corticolimbic circuits vary as A function of mood state? Interleaved TMS-fMRI scans in treatment resistant depression patients in the NIMH OPT-TMS study.. Presented at the Annual Meeting of the American College of Neuropsychopharmacology; Scottsdale, AZ. 2008. [Google Scholar]
- 61.Nahas Z, Teneback C, Chae JH, Mu Q, Molnar C, Kozel FA, et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 62.Vitek JL. Mechanisms of deep brain stimulation: Excitation or inhibition. Mov Disord. 2002;17(suppl 3):S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- 63.Hajcak G, Takacs S, Anderson B, Borckardt J, Reeves S, Arana A, et al. Direct bilateral epidural prefrontal cortical electrical stimulation (EpCS) down-regulates amygdala-mediated emotional appraisal in treatment-resistant depression.. Poster presentation at the American College of Neuropsychopharmacology; Scottsdale, AZ. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.