Abstract
Forkhead Transcription Factors: Vital Elements in Biology and Medicine provides a unique platform for the presentation of novel work and new insights into the vital role that forkhead transcription factors play in both cellular physiology as well as clinical medicine. Internationally recognized investigators provide their insights and perspectives for a number of forkhead genes and proteins that may have the greatest impact for the development of new strategies for a broad array of disorders that can involve aging, cancer, cardiac function, neurovascular integrity, fertility, stem cell differentiation, cellular metabolism, and immune system regulation. Yet, the work clearly sets a precedent for the necessity to understand the cellular and molecular function of forkhead proteins since this family of transcription factors can limit as well as foster disease progression depending upon the cellular environment.
With this in mind, our concluding chapter for Forkhead Transcription Factors: Vital Elements in Biology and Medicine offers to highlight both the diversity and complexity of the forkhead transcription family by focusing upon the mammalian forkhead transcription factors of the O class (FoxOs) that include FoxO1, FoxO3, FoxO4, and FoxO6. FoxO proteins are increasingly considered to represent unique cellular targets that can control numerous processes such as angiogenesis, cardiovascular development, vascular tone, oxidative stress, stem cell proliferation, fertility, and immune surveillance. Furthermore, FoxO transcription factors are exciting considerations for disorders such as cancer in light of their pro-apoptotic and inhibitory cell cycle effects as well as diabetes mellitus given the close association FoxOs hold with cellular metabolism. In addition, these transcription factors are closely integrated with several novel signal transduction pathways, such as erythropoietin and Wnt proteins, that may influence the ability of FoxOs to lead to cell survival or cell injury. Further understanding of both the function and intricate nature of the forkhead transcription factor family, and in particular the FoxO proteins, should allow selective regulation of cellular development or cellular demise for the generation of successful future clinical strategies and patient well-being.
Introduction
Clinical care for many disease entities requires new therapeutic strategies that focus upon a number of pathways and systems in the body to modulate cellular proliferation, metabolism, inflammation, and longevity. In this respect, members of the mammalian forkhead transcription factors of the O class (FoxOs) that include FoxO1, FoxO3, FoxO4, and FoxO6 have been identified as important regulators of cellular proliferation, function, and demise. These transcription factors are increasing considered as potential clinical targets for multiple disorders since they control processes associated with angiogenesis, stem cell proliferation, cardiovascular injury, neurodegeneration, tumorigenesis, and cell longevity. More than 100 forkhead genes and 19 human subgroups that range from FOXA to FOXS are now known to exist since the initial discovery of the fly Drosophila melanogaster gene forkhead.1 The prior nomenclature for these proteins, such as forkhead in rhabdomyosarcoma (FKHR), the Drosophila gene fork head (fkh), and Forkhead RElated ACtivator (FREAC)-1 and -2, has been replaced. The current nomenclature for human Fox proteins places all letters in uppercase, otherwise only the initial letter is listed as uppercase for the mouse, and for all other chordates the initial and subclass letters are in uppercase.2 Initially, the FoxOs were first reported in fusion genes in human soft-tissue tumors and leukemias. FOXO1, termed forkhead in rhabdomyosarcoma (FKHR), and FOXO3a, also known as FKHRL1 (forkhead in rhabdomyosarcoma like protein 1), and their genes were identified through chromosomal translocations in alveolar rhabdomyosarcoma tumors.3 The acute leukemia fusion gene located in chromosome X (AFX), also known as the FOXO4 gene, was described as a gene that fused to MLL transcription factor as a result of the t(X; 11) chromosomal translocation in acute lymphoblastic leukemia.4 A fusion between FOXO2 and MLL also occurs in some cases of acute myeloid leukemia that also is believed to be identical to FOXO3a.5
FoxO Protein Expression
FoxO proteins are found throughout the body and are expressed in tissues of the reproductive system of males and females, skeletal muscle, the cardiovascular system, lung, liver, pancreas, spleen, thymus, and the nervous system.6–11 Since FoxO proteins are not equally expressed in all tissues, it is possible that individual FoxO proteins may have specificity in regards to cellular function. For example, FoxO6 expression is found in several regions of the brain that play a significant role in cognitive function and emotion, such as the hippocampus, the amygdala, and the nucleus accumbens.9 In contrast, FoxO1 may be more suited for the control of motor function and memory formation, since the expression of this protein is primarily in the striatum and sub-regions of the hippocampus.9 In addition, FoxO3 is more diffusely represented in the hippocampus, cortex, and cerebellum, suggesting a complementary role for this FoxO protein to control cognitive and motor function. FoxO expression can be variable in other tissues. Although studies in mice have shown that the mRNA distribution of Foxo1, Foxo3a, and Foxo4 is similar in the embryo and adult,7 Foxo1 expression was highest in adipose tissue, Foxo3a expression was greatest in the liver, and Foxo4 expression was strongest in muscle.7 Subsequent work in mice has described Foxo1 expression in all tissues with high levels in the ovaries.12 Foxo3a also was found to be expressed in all tissues and Foxo4 expression was considered to be more tissue specific in skeletal muscle.12
FoxO Protein Structure and Function as Transcription Factors
Forkhead proteins function as transcription factors to either inhibit or activate target gene expression.13 As a result, these proteins must bind to DNA through the forkhead domain that relies upon fourteen protein-DNA contacts. The forkhead domain in Fox proteins consists of three α-helices, three β-sheets, and two loops that are referred to as the wings,14 but not all winged helix domains are considered to be Fox proteins.15 On X-ray crystallography14 or nuclear magnetic resonance imaging,16 the forkhead domain is described as a “winged helix” as a result of a butterfly-like appearance. High sequence homology is present in the α-helices and β-sheets with variations described in either absent β-sheets and loops or additional α-helices. Although both the first and second loops make contact with DNA, it is the second loop that can influence the stability of DNA binding. In addition, posttranslational modification of FoxO proteins, such as phosphorylation or acetylation that block FoxO activity, alter the binding of the C-terminal basic region to DNA to prevent transcriptional activity.17 However, other mechanisms may influence DNA binding of forkhead proteins, such as variations in the N-terminal region of the DNA recognition helix, changes in electrostatic distribution, and the ability of forkhead proteins to be shuttled to the cell nucleus.10,18
FoxO Proteins, Posttranslational Modulation, Novel Signal Transduction Pathways, and Cell Cycle Regulation
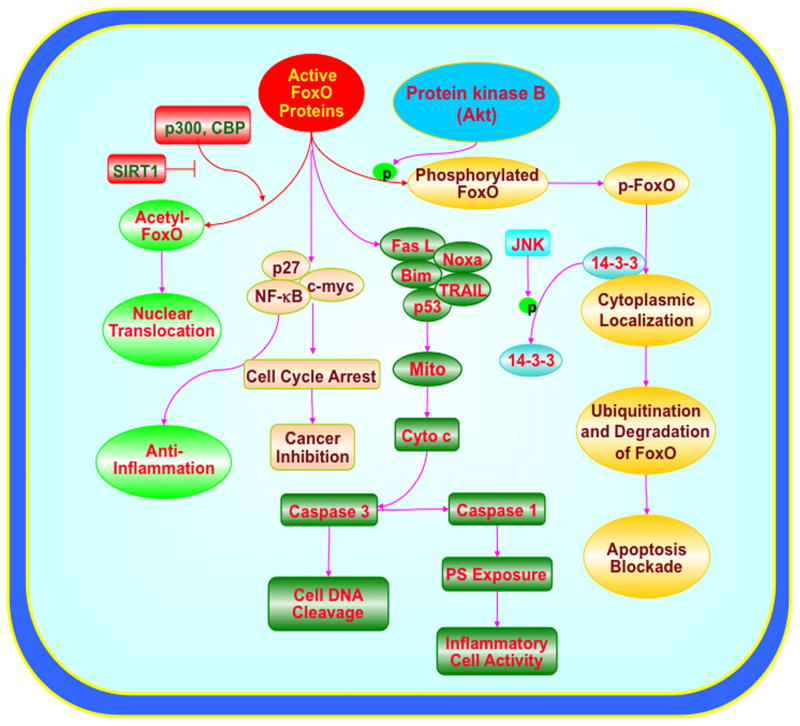
Posttranslational modulation of FoxO proteins involves pathways associated with phosphorylation, acetylation, and ubiquitylation (Fig. 1).3,10,19–21 The serine-threonine kinase protein kinase B (Akt) is a primary mediator of phosphorylation of FoxO1, FoxO3a, and FoxO4 that can block activity of these proteins.3, 22 Activation of Akt is usually cytoprotective, such as during hyperglycemia,23 hypoxia,24 β-amyloid (Aβ) toxicity,25 cardiomyopathy,26 cellular aging,27 and oxidative stress.28–30 Akt can prevent cellular apoptosis through the phosphorylation of FoxO proteins.31 Posttranslational phosphorylation of FoxO proteins will maintain FoxO transcription factors in the cytoplasm by association with 14-3-3 proteins and prevent the transcription of pro-apoptotic target genes.32,33 An exception to these observations involving the subcellular trafficking of FoxO proteins involves FoxO6. This FoxO protein usually resides in the nucleus of cells and is phosphorylated by Akt in the nucleus. FoxO6 does not contain a conserved C-terminal Akt motif which limits nuclear shuttling of this protein, but FoxO6 transcriptional activity can be blocked by growth factors independent of shuttling to the cytosol through a FoxO6 N-terminal Akt site.34
Figure 1.
Posttranslational modulation of FoxO proteins is associated with intricate cellular signal transduction pathways. Posttranslational modulation of FoxO proteins involves pathways associated with phosphorylation, acetylation, and ubiquitylation. Protein kinase B (Akt) can prevent cellular apoptosis through the phosphorylation of FoxO proteins and phosphorylation (p) of FoxO proteins will inhibit FoxO transcription factors through cytoplasmic localization by association with 14-3-3 proteins and prevent the transcription of target genes that lead to apoptosis. If activated, FoxOs can prevent inflammatory cell activation through the inhibition of nuclear factor-κB (NF-κB) and may control inflammatory cell activation through membrane phosphatidylserine (PS) externalization. FoxO proteins can lead to apoptotic death pathways that involve mitochondrial (Mito) release of cytochrome c (Cyto c) and caspase activation through a Fas-mediated ligand (Fas L) death pathway, tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL), BH3-only proteins Noxa and Bim, or p53. Cell cycle inhibition that blocks tumor growth through FoxO protein activation may require c-myc, p27, and NF-κB.
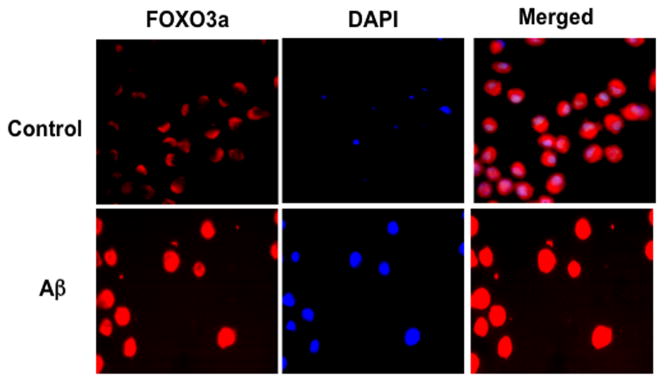
Modulation of Akt activity also oversees apoptotic pathways of caspases that may offer an alternative mechanism to regulate FoxO proteins (Fig. 1).35 Caspases are a family of cysteine proteases that are synthesized as inactive zymogens that are proteolytically cleaved into subunits at the onset of apoptosis.36–38 The caspases 1 and 3 have been linked to the apoptotic pathways of genomic DNA cleavage, cellular membrane PS exposure, and activation of inflammatory cells.39–41 Caspase pathways may be tied to the forkhead transcription factor FoxO3a since increased activity of FoxO3a can result in cytochrome c release and caspase-induced apoptotic death.32,42–44 Pathways that can inhibit caspase 3 activity appear to offer a unique regulatory mechanism. For example, cell death pathways that rely upon FoxO3a also appear to involve caspase 3 activation. Prior studies suggest that not only does FoxO3a activity promote caspase-induced apoptotic death,32,42–44 but also demonstrate that inhibition of caspase 3 has been shown to maintain the phosphorylated “inactive” state of FoxO3a to prevent cell injury.32,42,43 Other work has shown that caspase 3 activity and cleavage is promoted during transfection of a triple mutant FoxO3a expression in which three phosphorylation sites have been altered to prevent inactivation of FoxO3a.45 Recent work adds further insight to these studies by illustrating that FoxO3a may control early activation and subsequent apoptotic injury in microglia during amyloid (Aβ) exposure through caspase 3 (Fig. 2).46 Since Aβ exposure can facilitate the cellular trafficking of FoxO3a from the cytoplasm to the cell nucleus to potentially lead to “pro-apoptotic” programs by this transcription factor,46 one program in particular that may be vital for apoptotic injury appears to involve the activation of caspase 3. Aβ exposure leads to a rapid and significant increases in caspase 3 activity with 6 hours following Aβ administration, but that this induction of caspase 3 activity by Aβ requires FoxO3a, since loss of FoxO3a through gene silencing prevents the induction of caspase 3 activity by Aβ.
Figure 2.
During amyloid (Aβ1-42) exposure in inflammatory microglial cells, FoxO3a translocates to the cell nucleus to govern an initial activation and proliferation of microglial cells. Microglia were followed at 6 hours after Aβ1-42 (10 μM) (Aβ) administration with immunofluorescent staining for FoxO3a (Texas-red). Nuclei of microglia were counterstained with DAPI. In merged images, control cells have readily visible nuclei (white in color) that illustrate absence of FoxO3a in the nucleus. In contrast, merged images after Aβ1-42 (10 μM) exposure are not visible (red in color) and demonstrate translocation of FoxO3a to the nucleus. Control = untreated microglia.
Posttranslational modulation of FoxO proteins also requires pathways associated with ubiquitylation and acetylation.47,48 Akt phosphorylation of FoxO proteins not only retains these transcription factors in the cytoplasm, but also leads to ubiquitination and degradation through the 26S proteasome 19, 48. In the absence of Akt, IκB kinase (IKK) also can directly phosphorylate and block the activity of FoxO proteins, such as FoxO3a.3,10 This leads to the proteolysis of FoxO3a via the Ub-dependent proteasome pathway.3,10,19–21 The serum- and glucocorticoid-inducible protein kinase (Sgk), a member of a family of kinases termed AGC (protein kinase A/protein kinase G/protein kinase C) kinases which includes Akt, also can phosphorylate and retain FoxO3a in the cytoplasm.49 Knowledge that Sgk and Akt can phosphorylate FoxO3a at different sites may offer new opportunities to more effectively prevent apoptotic cell injury that may be mediated by FoxO3a activity. Yet, phosphorylation of FoxO proteins does not always lead to negative regulation. The protein kinase mammalian sterile 20-like kinase-1 also can phosphorylate FoxO proteins directly and lead to their activation.50 The ability of sterile 20-like kinase-1 to activate FoxO proteins may be linked to c-Jun N-terminal kinase ( JNK), since sterile 20-like kinase-1 can increase JNK activation.51 FoxO proteins also are acetylated by histone acetyltransferases that include p300, the CREB-binding protein (CBP), and the CBP-associated factor and are deacetylated by histone deacetylases, such as Sirt1, a NAD+-dependent deacetylase and the mammalian ortholog of the silent information regulator 2 (Sir2) protein (Fig. 1).10 Acetylation of FoxO proteins provides another avenue for the control of these proteins. Once acetylated such as by CBP, FoxO proteins may translocate to the cell nucleus but have diminished activity since acetylation of lysine residues on FoxO proteins has been shown to limit the ability of FoxO proteins to bind to DNA.52 In addition, acetylation can increase phosphorylation of FoxO proteins by Akt.52
FoxO proteins are also tied to other unique signal transduction pathways that involve proteins derived from the Drosophila Wingless (Wg) and the mouse Int-1 genes.20 The Wnt proteins are secreted cysteine-rich glycosylated proteins that can control cell proliferation, differentiation, survival, and tumorigenesis.53,54 More than eighty target genes of Wnt signaling pathways have been demonstrated in human, mouse, Drosophila, Xenopus, and zebrafish. These genes are present in several cellular populations, such as neurons, cardiomyocytes, endothelial cells, cancer cells, and pre-adipocytes.55 At least nineteen of twenty-four Wnt genes that express Wnt proteins have been identified in the human.53,54,56
The canonical Wnt pathway controls target gene transcription through β-catenin.53,54 It is the β-catenin pathway that appears to tie FoxO proteins and Wnt signaling together.57 For example, in relation to Alzheimer’s disease, Aβ is toxic to cells,25,58 and is associated with the phosphorylation of FoxO1 and FoxO3a that can be blocked with ROS scavengers.59 A common denominator in the pathways linked to Ab toxicity involves Wnt signaling through β-catenin. β-catenin may increase FoxO transcriptional activity and competitively limit β-catenin interaction with members of the lymphoid enhancer factor/T cell factor family60 and β-catenin also has been demonstrated to be necessary for protection against Aβ toxicity in neuronal cells.58
Additional shared signal transduction pathways between Wnt and FoxO proteins involve Akt. Processes that involve cellular proliferation, injury, and immune system modulation with FoxO proteins61 also have parallel cellular pathways with Wnt and Akt. For example, Wnt relies upon Akt for the proliferation and differentiation of cardiomyocytes.62 In addition, reduction in tissue injury during pressure overload cardiac hypertrophy and the cytoprotective benefits of cardiac ischemic preconditioning also appear to depend upon Akt.53,54 Furthermore, Wnt over-expression can independently increase the phosphorylation and the activation of Akt to promote cellular protection and control microglial activation.58
Yet, other members of the forkhead family in addition to FoxOs also rely upon Wnt signaling in several scenarios that involve regulated as well as unchecked cell proliferation.53,54,63 For example, FoxD3 is activated by the Wnt pathway to control neural plate development64 and Foxl1 activates the Wnt/β-catenin pathway to increase extracellular proteoglycans, promote gastrointestinal cell proliferation, and possibly foster carcinogenesis.65 The Wnt pathway also utilizes forkhead members to modulate endocrine activity and can activate Foxn1 for regulatory control of thymic function.66 In other examples of cell development, Wnt signaling has been shown to rely upon Foxf1 and Foxf2 during intestinal maturation in murine models.67 In addition, Foxa2 in mice may be a significant component in early anterior-posterior axis polarization.68 Deregulation of Wnt alone also promotes activation of β-catenin that has been associated with the proliferation of medulloblastoma tumors.69 In addition, reduced expression of inhibitors of the Wnt pathway, such as axin, may foster lung cancer cell invasion.70 Multiple other studies also point to the activation of the Wnt pathway during gastric cancer. For example, Wnt5a expression has been correlated with advanced gastric cancer stages and a poor prognosis71 while experimental activation of the β-catenin pathway leads to the development of gastric tumors.72 In conjunction with forkhead proteins, loss of Foxl1 that can regulate the Wnt pathway and prevent β-catenin nuclear accumulation is believed to be a significant etiology for gastrointestinal tumorigenesis.65
FoxO proteins also appear to be ideal to cellular proliferation not only through Wnt mediated pathways, but also through the blockade of cell cycle progression. For example, FoxO3a and FoxO4 can promote cell cycle arrest in mouse myoblastic cell lines through modulation of growth-arrest and DNA-damage-response protein 45.10,73 Treatment of chronic myelogenous leukemia cell lines with the Bcr-Abl tyrosine kinase inhibitor imatinib requires FoxO3a activation to antagonize cell proliferation and promote apoptotic cell death through increased TRAIL production.74 In addition, the transcription factor E2F-1 that controls the induction of the cell cycle has been reported in cell lines to increase the endogenous expression of FoxO1 and FoxO3a to lead to cell cycle arrest.75 In contrast, the loss of FoxO3a activity in association with c-myc, p27, and nuclear factor-κB (NF-κB) can result in cell cycle induction and malignant transformation of mouse cells in the presence of oncogene activation (Fig. 1).3,10 Other work suggests that FoxO proteins utilize the p53 upstream regulator p19(Arf ) through myc to block cell cycle induction and lymphoma progression.76
FoxO Proteins, Apoptosis, and Oxidative Stress
Although genes linked to apoptosis sometimes foster cellular proliferation rather than cell death, cellular apoptosis can become a significant component for pathology in diseases such as neurodegenerative disease, diabetes mellitus (DM), and cardiovascular injury.77 More importantly, regulation of apoptotic pathways appears to serve a critical juncture for the control of tumor growth and unregulated cell proliferation.10,57 Apoptotic cell death is considered to be a dynamic process that involves both early and late events. Membrane phosphatidylserine (PS) externalization is an early event during cell apoptosis that assists microglia to target cells for phagocytosis.29,78 This process occurs with the expression of the phosphatidylserine receptor (PSR) on microglia during oxidative stress,79–81 since blockade of PSR function in microglia prevents the activation of microglia.30,40 As an example, externalization of membrane PS residues occur in cells during periods of oxidative stress that involve anoxia,58 reactive oxygen species (ROS) exposure,82 and with agents that produce ROS, such as 6-hydroxydopamine.83 In contrast to cells with PS exposure, the cleavage of genomic DNA into fragments is considered to be a later event during apoptotic injury.77,84 Endonucleases responsible for DNA degradation have been identified and include the acidic, cation independent endonuclease (DNase II), cyclophilins, and the 97 kDa magnesium—dependent endonuclease. In the nervous system, endonucleases include a constitutive acidic cation-independent endonuclease, a constitutive calcium/magnesium-dependent endonuclease, and an inducible magnesium-dependent endonuclease.77,84
Interestingly, the induction of apoptosis in cells through FoxO proteins may require pathways aligned with oxidative stress. Oxidative stress is a result of the release of reactive oxygen species (ROS) that consist of oxygen free radicals and other chemical entities. Oxygen free radicals and mitochondrial DNA mutations have become associated with tissue injury, aging, and accumulated toxicity for an organism.77 ROS include superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide, and peroxynitrite.84 Most reactive species are produced at low levels during normal physiological conditions and are scavenged by endogenous antioxidant systems that include superoxide dismutase, glutathione peroxidase, catalase, and small molecules, such as vitamins C, E, D3 and nicotinamide, the amide form of niacin or vitamin B3.78,85,86 During periods of oxidative stress, FoxO transcription factors can lead to apoptosis,31 since forkhead transcription factors such as FoxO1 and FoxO3a must be present for oxidative stress to result in apoptotic cell injury.87 Under other conditions of oxidative stress, FoxO3a in conjunction with JNK have been shown to modulate an apoptotic ligand activating a Fas-mediated death pathway in cultured motoneurons,88 to lead to apoptosis through tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) and BH3-only proteins Noxa and Bim in neuroblastoma cells,44 and to promote pro-apoptotic activity of p53.89 Additional work shows that loss of FoxO expression during oxidative stress is protective to cells. For example, protein inhibition or gene knockdown of FoxO1 or FoxO3a can lead to reduction in ischemic infarct size in the brain,90 mediate protection of metabotropic glutamate receptors during vascular injury,42 enhance pancreatic β-cell or neuronal survival through NAD+ precursors during oxidative stress,43 and provide trophic factor protection with erythropoietin (EPO)32 and neurotrophins.91 Yet, it should be noted that some studies suggest that the loss of FoxO1, FoxO3a, and FoxO4 protein expression may actually lead to an increase in free radical release that can be responsible for oxidative stress.92 In addition, FoxO proteins may be protective during aging and exercise, since FoxO3a activity may enhance vascular smooth muscle antioxidant properties in aged animals and be beneficial to the cardiovascular system during physical exertion.93
FoxO Proteins, Metabolism and Cell Longevity
Clinical and experimental studies highlight the role of FoxO proteins during cellular metabolism and cellular longevity. When one considers DM, this disorder is a significant health concern for both young and older populations.94,95 Approximately 16 million individuals in the United States and more than 165 million individuals worldwide suffer from DM. By the year 2030, it is predicted that more than 360 million individuals will be afflicted with DM and its debilitating conditions. Type 2 DM represents at least 80% of all diabetics and is dramatically increasing in incidence as a result of changes in human behavior and increased body mass index.85,94 Type 1 insulin-dependent DM is present in 5–10% of all diabetics, but is increasing in adolescent minority groups.85,94 Furthermore, the incidence of undiagnosed diabetes and impaired glucose tolerance in the population raises additional concerns.
Patients with DM can develop significant neurodegenerative55,85,96 and cardiovascular disease.85,97 Interestingly, the development of insulin resistance and the complications of DM can be the result of cellular oxidative stress.85,94 Hyperglycemia can lead to increased production of ROS in endothelial cells, liver cells, and pancreatic β-cells.85,94,95 Recent clinical correlates support these experimental studies to show that elevated levels of ceruloplasmin are suggestive of increased ROS.85,94,95 Furthermore, acute glucose swings in addition to chronic hyperglycemia can trigger oxidative stress mechanisms, illustrating the importance for therapeutic interventions during acute and sustained hyperglycemic episodes.85,94
Early work with FoxO proteins has shown that metabolic signaling with these transcription factors is conserved among multiple species including Caenorhabditis elegans, Drosophila melanogaster, and mammals. FoxO proteins are homologous to the transcription factor DAuer Formation-16 (DAF-16) in the worm Caenorhabditis elegans that can determine metabolic insulin signaling and lead to lifespan extension,98,99 suggesting a significant role for FoxO proteins in relation to mammalian cell function.3,10 In fact, FoxO proteins can stimulate the insulin-like growth factor binding protein-1 (IGFBP1) promoter by binding to the insulin-responsive sequence (IRS).100 Both insulin and insulin-like growth factor-1 (IGF-1) can suppress this activity through activation of Akt.100,101
In clinical studies, analysis of the genetic variance in FOXO1a and FOXO3a on metabolic profiles, age-related diseases, fertility, fecundity, and mortality have observed higher HbA1c levels and increased mortality risk associated with specific haplotypes of FOXO1a.102 These clinical observations may coincide with the demonstration in human endothelial progenitor cells that elevated glucose levels can reduce posttranslational phosphorylation of FOXO1, FOXO3a, and FOXO4 and allow for the nuclear translocation of these proteins to initiate an apoptotic program in endothelial progenitor cells.103 In experimental models, FoxO proteins may prevent the toxic effects of high serum glucose levels. Interferon-gamma driven expression of tryptophan catabolism by cytotoxic T lymphocyte antigen 4 may activate Foxo3a to protect dendritic cells from injury in nonobese diabetic mice.104 Additional studies have demonstrated that adipose tissue-specific expression of Foxo1 in mice improved glucose tolerance and sensitivity to insulin during an elevated fat diet.105 FoxO proteins also may protect against diminished mitochondrial energy levels known to occur during insulin resistance such as in the elderly populations.85,94,95 In caloric restricted mice that have decreased energy reserves, Foxo1, Foxo3a, and Foxo4 mRNA levels were noted to progressively increase over a two-year course.8 These observations complement studies in Drosophila and mammalian cells that demonstrate an increase in insulin signaling to regulate cellular metabolism during the up-regulation of FoxO1 expression.106
However, the ability for FoxO proteins to maintain proper physiologic controls over cellular metabolism may be limited and occur only during specific circumstances. For example, mice with a constitutively active Foxo1 transgene have increased microsomal triglyceride transfer protein and elevated plasma triglyceride levels.107 Studies in cardiomyocytes also suggest detrimental results with enhanced FoxO activity. Increased transcriptional activity of FoxO1, such as by the Sirt1 activator resveratrol, can diminish insulin mediated glucose uptake and result in insulin resistance.108 In addition, over-expression of Foxo1 in skeletal muscles of mice can lead to reduced skeletal muscle mass and poor glycemic control,109 illustrating that activation of FoxO proteins also may impair cellular energy reserves. Additional investigations that block the expression of Foxo1 in normal and cachectic mice110 or reduce FoxO3 expression111 show the reverse with an increase in skeletal muscle mass or resistance to muscle atrophy. These results become especially relevant in patients with cancer and cachexia, since FoxO protein expression may further muscle wasting for these individuals. Given these concerns, one potential agent to consider for the maintenance of cellular metabolism in cancer patients is nicotinamide,36,78 an agent that also can inhibit FoxO protein activity.43 In patients with DM, oral nicotinamide protects β-cell function, prevents clinical disease in islet-cell antibody-positive first-degree relatives of Type-1 DM, and can reduce HbA1c levels.36,78,94 Nicotinamide, which is closely linked to cell longevity pathways,112,113 may derive its protective capacity through two separate mechanisms of posttranslational modification of FoxO3a. Nicotinamide not only can maintain phosphorylation of FoxO3a and inhibit its activity, but also can preserve the integrity of the FoxO3a protein to block FoxO3a proteolysis that can yield pro-apoptotic amino-terminal fragments.43
As an extension to the work with cellular metabolism, FoxO proteins also have been linked to cell longevity and aging as shown by early studies linking DAF-16 in Caenorhabditis elegans to increased longevity.3,19,21,114 However, the relationship between FoxO transcription factors and proteins that increased cellular lifespan has been met with controversy. Sirt1 is a NAD+-dependent deacetylase and the mammalian ortholog of the silent information regulator 2 (Sir2) protein associated with increased lifespan in yeast. Some studies suggest that stimulation of Sirt1 during starvation is dependent upon FoxO3a activity as well as p53.115 In contrast, other work has shown in cell culture that Sirt1 may repress the activity of FoxO1, FoxO3a, and FoxO4, suggesting that cellular longevity may benefit from reduction in FoxO protein generated apoptosis.116 Additional studies offer alternative views to illustrate that Sirt1 binds to FoxO proteins, such as FoxO4, to catalyze its deacetylation and enhance FoxO4 activity while acetylation of FoxO4 by cyclic-AMP responsive element binding (CREB)-binding protein serves to inhibit FoxO4 transcriptional activity.3,19,21,114
FoxO proteins also may be protective during aging, cell senescence, and exercise. In cultured human dermal fibroblasts, gene silencing of FoxO3a protein results in cell morphology consistent with cell senescence, cell population doubling times, and the generation of ROS, suggesting that FoxO protein activity may be required to extend cell longevity and limit oxidative stress.117 Additional work in animal models of aging demonstrates a reduction in Sirt1 in the heart, but no significant change in FoxO3a expression with advanced age. However, during exercise training, an up-regulation of FoxO3a and Sirt1 activity is observed in the heart,93 suggesting that the benefits of physical activity for the cardiovascular system may be associated with FoxO proteins. Interestingly, increased levels of Sirt1 less than 7.5-fold can be associated with expression of catalase, an anti-oxidant that is controlled by FoxO1a to possibly reduce cell injury during oxidative stress. Yet, elevated levels of Sirt1 at 12.5-fold can result in cardiomyocyte apoptosis and decreased cardiac function.118 In addition, FoxO proteins may be protective during aging, since loss of FoxO3a activity in explanted vascular smooth muscle of aged animals may limit tissue antioxidant properties through decreased manganese-superoxide dismutase and lead to enhanced cell injury with aging.119 Extension of cellular lifespan that depends upon the prevention of cell senescence at least in primary human cultured vascular cells also may require the negative regulation of Akt to allow for the activation of FoxO3a.120
FoxO Proteins, Stem Cells and Cardiovascular Development
FoxO proteins represent important targets for several disorders, but high on the list may be therapies to block cancer growth since FoxO proteins can modulate stem cell proliferation and new vessel growth. The initial identification of FoxO proteins in soft-tissue tumors and leukemias, neoplasms now believed to harbor cancer stem cells for tumor self-renewal,69 suggests that FoxO proteins may be closely associated with the oversight of stem cell proliferation and differentiation. For example, either simultaneous deletion of Foxo1, Foxo3a, and Foxo4 or single deletion of Foxo3a in mice prevents the repopulation of hematopoietic stem cells and leads to apoptosis in these stem cell populations.92,121 Furthermore, vascular cytoprotective agents, such as the growth factor EPO,33,122,123 also may be required to modulate FoxO protein activity such as during erythroid progenitor cell development,73,124 suggesting that current clinical use of agents such as EPO during anemia or cancer may have less defined treatment implications for patients than originally anticipated.33,124 In cell culture and animal studies, EPO is cytoprotective in vascular cells and can stimulate postnatal neovascularization by increasing endothelial progenitor cell mobilization from the bone marrow.73,124,125 Interestingly, the ability of EPO to foster eythroid progenitor cell development is dependent upon the inhibition of FoxO3a activity,33,124 but also may require regulation of specific gene expression through an EPO-FoxO3a association to promote erythropoiesis in cultured cells.126 In relation to the reproductive potential of an organism, deletion of the FoxO3a gene results in the depletion of oocytes and subsequent infertility.127 Other work using a mouse model of FoxO3a over-expression in oocytes further suggests that FoxO3a retards oocyte growth and follicular development and leads to anovulation and luteinization of unruptured follicles.128 These studies may suggest a role for FoxO proteins, and specifically FoxO3a, in relation to not only the development of cancer stem cell niches, but also in regards to oocyte and follicular cell maturation. For example, in a small percentage of women who suffer from premature ovarian failure mutations in FOXO3a and FOXO1a have been observed.129
In addition to the modulation of stem cell development, FoxO proteins play a significant role to govern new vessel growth that can impact upon tumor cell growth and dispersion. New capillary formation from pre-existing vessels into an avascular area is a process known as angiogenesis that is present during embryogenesis, during menstruation, and during pathological processes that involve wound healing, chronic inflammation, and tumor growth.54,124 FoxO proteins are intimately involved in endothelial cell development and angiogenesis. For example, Foxo3a −/minus; and Foxo4 minus;/minus; mice develop without incidence and are indistinguishable from control littermates. However, mice that are singly deficient in Foxo1 die by embryonic day eleven and lack development of the vascular system.130 Additional studies illustrate that endothelial cell colonies in Foxo1-deficient mice fail to respond to vascular endothelial growth factor in a manner similar to wild-type endothelial cells,131 suggesting that FoxOs are necessary for the development of vascular cells as well as for the biological response to cellular mediators.
During cardiac development, FoxO proteins also appear to be necessary to modulate cardiomyocyte proliferation. Both FoxO1 and FoxO3 are expressed during embryonic through prenatal stages in the developing myocardium. The expression of these FoxO proteins is believed to negatively regulate cardiomyocyte growth, since overexpression of FoxO1 blocks cardiomyocyte proliferation but expression of dominant negative FoxO1 leads to enhanced cardiomyocyte growth.132 These observations may provide clues into the roles of FoxO proteins during cardiac hypertrophy. Atrogin-1, a protein that can block cardiac hypertrophy, may rely upon the up-regulation of Foxo1 and Foxo3a to disrupt cardiac hypertrophy, since mice lacking atrogin-1 are susceptible to cardiac hypertrophy and do not yield increased expression of Foxo1 and Foxo3a.133
In regards to smooth muscle cell growth, Foxo3a has been demonstrated to block vascular smooth muscle proliferation and may lessen the effects from disorders such as atherosclerosis and hypertension. In a rat balloon carotid arterial injury model, gene transfer of FoxO3a can inhibit neointimal hyperplasia through the prevention of vascular smooth muscle growth.134 However, not all FoxO proteins may exert an inhibitory effect upon vascular smooth muscle cells. FoxO4 may inhibit smooth muscle cell differentiation through the repression of the transcriptional coactivator of smooth muscle genes myocardin,135 but other work suggests that FoxO4 also can increase matrix metalloproteinase 9 expression to promote vascular smooth muscle migration and foster neointimal hyperplasia.136
In light of the ability of FoxO proteins to regulate vascular smooth muscle cell proliferation, these transcription factors may have a significant clinical role in regards to disorders that involve hypertension and cardiac failure. Vascular smooth muscle cells are vital for the regulation of vascular tone and systemic arterial blood pressure. For example, high flow states in vessels can reduce FoxO1 activity, resulting in the potential proliferation of vascular smooth muscle cells, vascular neointimal hyperplasia, and subsequent pathological states such as hypertension.137 In fact, α1-adrenergic agonists that increase systemic blood pressure can have the reverse effect and stimulate the expression of FoxO1 and its nuclear translocation that ultimately may lead to apoptotic endothelial cell injury.138 In addition, more than moderate levels of vessel cyclic stretch that can occur during hypertension may lead to the phosphorylation and inhibition of Foxo1 and Foxo3a in smooth muscle cells to further contribute to pathological smooth muscle cell proliferation.139 Furthermore, in human as well as murine models of cardiac failure, increased expression of Fox transcription factors, such as FoxO1a, have been observed to suggest a potential association of FoxO proteins with imminent cardiac failure.140
FoxO Proteins and the Immune System
Forkhead transcription factors have a vital role in maintaining immune system function. For example, the forkhead family member FoxP3 can control the development and function of thymic-derived CD4(+)CD25(+) regulatory T cells (Treg) that impart autoimmunity. Loss of FoxP3 can result in autoimmune disorders.141 In addition, recent work identifies the expression of FoxP3 in tumor cells, such as melanoma,142 as well as in Tregs which may significantly affect patient mortality since the increased presence of Tregs in cancer patients combined with FoxP3 expression in tumors may impair antitumor autoimmune responses and lead to high mortality.143
In regards to FoxO proteins, these forkhead transcription factors also may impact upon neoplastic progression since they lead to the induction of apoptotic pathways and may influence early apoptotic membrane PS externalization (Fig. 1). The ability to regulate early apoptotic membrane PS exposure40 and inflammatory cell activity29 can ultimately impact upon cell survival since activated immune cells can lead to the phagocytic removal of tumor cells.79,84 Inflammatory cells, such as macrophages or microglia, require the activation of intracellular cytoprotective pathways to proliferate and remove injured cells.80,144 At times, this can be a beneficial process and form a barrier for the removal of foreign micro-organisms and promote tissue repair during cell injury.73,85 However, inflammatory cells also may lead to cellular damage through the generation of ROS and through the production of cytokines.73 Interestingly, in mice deficient for Foxo3a, lymphoproliferation, organ inflammation of the salivary glands, lung, and kidney, and increased activity of helper T cells results, supporting an important role for FoxO3a in preventing T cell hyperactivity.145 FoxO3a also appears to be necessary for neutrophil activity, since Foxo3a null mice are resistant to models of neutrophilic inflammation that involve immune complex-mediated inflammatory arthritis.146
In clinical studies, patients with rheumatoid arthritis and osteoarthritis show phosphorylation of FOXO3a in T lymphocytes as well as FOXO1 and FOXO4 in synovial macrophages, suggesting that loss of functional FOXO family members may lead to inflammatory cell activation in these disorders.147 FOXO1 gene transcript levels also are down-regulated in peripheral blood mononuclear cells of patients with systemic lupus erythematosus and rheumatoid arthritis,148 illustrating a potential etiology through the loss of functional FOXO proteins for these disorders and possibly providing a biomarker of disease activity. Other work has demonstrated that FOXO1 protein regulates L-selectin expression that can regulate human T lymphocyte trafficking.149 More importantly, studies suggest a relationship between the regulation of immune system activity and the induction of apoptotic pathways that are dependent upon FoxO proteins. Prevention of inflammatory activation and apoptosis in the nervous system such as in systemic lupus erythematosus in animal models may require the up-regulation of different Fox proteins, such as FoxJ1 and FoxO3a, that can block NF-κB activation and interferon-gamma secretion.150 FoxO proteins also may work in concert with Fas signaling to clear activated T cells following a decrease in cytokine stimulation in patients with autoimmune lymphoproliferative syndromes,151 suggesting that activation of specific FoxO proteins may be beneficial for autoimmune disorders but may impair treatments designed to target tumor cells through immune mediated pathways.
FoxO Proteins and Cancer
As previously mentioned, one of the most important treatment possibilities for FoxO proteins involves strategies designed to control human cancer progression in light of the ability of FoxO proteins to lead to apoptosis and block cell cycle progression. For example, studies with prostate cancer have shown that the tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is mutated in approximately 80% of tumors with the loss of FOXO1 and FOXO3a activity. In cell cultures, over-expression of FoxO1 and FoxO3a in prostrate tumor cell lines also leads to apoptosis, suggesting that FoxO1 and FoxO3a are necessary for limiting prostate cell tumor growth.11 In addition, it has been shown that inhibition of FoxO3a activity can result in enhanced prostate tumor cell growth152 while agents that increase FoxO3a activity in both androgen sensitive and androgen insensitive prostate cell lines prevent prostate cancer cell progression.153 Furthermore, therapeutic strategies that rely upon the over-expression of a nonphosphorylatable form of FoxO3a that cannot be inactivated can sensitize prostate cancer cells to androgen-withdrawal-induced apoptosis.154 Yet, it should be noted that in prostate cell lines FoxO3a can be a positive regulator of androgen receptor expression and therefore may play a complex role in prostate cancer cell proliferation and growth inhibition.155 Other factors that control FoxO protein function also may play a role during prostate tumor progression. In prostate cancer cells, cyclin-dependent kinase 1 (CDK1) can become over-expressed and subsequently phosphorylate FOXO1 to block its transcriptional activity and contribute to prostate tumorigenesis.156 In a similar manner, it has been shown that astrocyte-elevated gene-1 (AEG-1) can be upregulated in clinical prostate cancer,157 possibly lead to activation of Akt that suppresses FOXO3a158 and apoptosis in prostate tumor cells.
Initial investigations of FOXO3a in clinical breast cancer suggested that activation of FOXO3a was associated with lymph nodal metastasis and a poor prognosis.159 In contrast to these observations, other studies reported that FOXO3a was inactivated by IKK and that inactivation of FOXO3a was associated with a poor prognosis in breast cancer,160 suggesting that FOXO3a sub-cellular localization and pathways that enhance its activity could be used not only as prognostic assays but also as therapeutic targets. Other work in breast cancer cells demonstrate the tumor repressive ability of FoxOs by illustrating that increased activity of FoxO3a in association with JNK in breast cancer cell lines161 or in association with cyclin-dependent kinase inhibitor p27 in isolated human breast cancer cells can prevent breast cancer growth.162 In addition, FoxO proteins may be able to modulate estrogen function and indirectly block breast cancer growth. Over-expression of FoxO3a in breast cancer cell lines can decrease the expression of estrogen receptor regulated genes and inhibits 17beta-estradiol (E2)-dependent breast cancer growth.163
In addition to the ability to inhibit prostate and breast tumor growth, FoxO proteins may represent a viable option to control tumor progression in other tissues. FoxO proteins can function as redundant repressors of tumor growth. For example, somatic deletion in mice of Foxo1, Foxo3a, and Foxo4 results in the growth of thymic lymphomas and hemangiomas.164 Other work illustrates that FoxO3a activation in colon carcinoma cell lines prevents tumor proliferation through Myc target genes that involve the Mad/Mxd family of transcriptional repressors.165 In addition, the loss of FoxO3a activity may participate in oncogenic transformation in B-chronic lymphocytic leukemia166 and in the progression of chronic myelogenous leukemia cell lines.74 Furthermore, studies suggest that some proteins, such as the Kaposi’s sarcoma-associated herpes virus latent protein LANA2, may specifically block the transcriptional activity of FoxO3a to lead to tumor growth.167 In cell models of endometrial cancer, presensitization of cells to block Akt activation and foster transcription activity of FoxO1 enhances the effect of chemotherapy to limit tumor growth.168
Conclusions and Future Perspectives for Clinical Care
The potential translation of FoxO proteins and their signal transduction pathways into viable therapeutic strategies offer exciting prospects for the future. FoxO proteins control several vital cellular pathways in relation to cell proliferation, metabolism, inflammation, and survival. For example, the known mutations in FoxO proteins that exist in several disease entities may provide novel insights for therapeutic strategies that can address a broad range of disorders. Further analysis in larger populations of patients with premature ovarian failure, diabetes, or stroke could enhance our understanding of the role of FoxO proteins in these disorders. When one considers the role of FoxO proteins at the cellular level such as in cardiac and endothelial cells, targeting the activity of FoxO1, FoxO3a, or FoxO4 may prevent the onset of pathological cardiac hypertrophy and neointimal hyperplasia that may result in atherosclerosis. Interestingly, new work suggests that the utilization and combination of multiple biomarkers may improve risk assessment for patients suffering from cardiovascular disorders.169 These studies illustrate that FoxO proteins may serve as biomarkers of disease activity such as in individuals with imminent cardiac failure.140
In regards to potential treatments directed against cancer, the ability of FoxO proteins to control cell cycle progression and promote apoptosis highlights the potential of FoxOs to become an important component for new strategies directed against tumorigenesis. For example, use of triple mutant FoxO1 or FoxO3a expression in which three phosphorylation sites have been altered to prevent inactivation of this protein has been proposed as a potential therapeutic agent against melanoma tumors45 and endometrial cancer.170 Other work also offers additional support for the use of FoxO proteins as biomarkers of cancer progression. As an example, down regulation of the phosphatidylinositol 3 kinase and Akt pathways have been associated with increased transcript levels for FOXO1a and FOXO3a in clinical prostate cancer samples and may indicate the onset of precancerous changes or the progression of on-going tumor growth.171 Although loss of Akt activity in prostate cancer cells can result in enhanced FoxO3a activity and subsequent apoptosis of tumor cells,157 it is conceivable that early stages of cancer may lead to reduced Akt activity with insufficient levels of active forkhead transcription factors to limit tumor progression. In addition, the early and persistent expression of phosphorylated FOXO1a in gastric tumors may not only indicate the onset of cancer, but also suggest an improved prognosis for patients.172
Despite the presently known attributes of FoxO proteins to potentially treat a number of disorders, FoxO transcription factors also may limit clinical utility. Further investigations are required since FoxO protein inhibition of cell cycle progression may not consistently lead to apoptotic cell death. Some investigations suggest that during oxidative stress, FoxO3a activation in association with Sirt1 can lead to cell cycle arrest, but not result in apoptotic cell injury.173 Furthermore, during hypoxic stress, forkhead transcription factors, such as FOXO3a, may potentiate anti-apoptotic pathways in breast cancer cells to further tumor growth.174 FoxO proteins also have been linked to potential chemotherapy drug resistance. Increased expression of MDR1 (P-glycoprotein) has been associated with chemotherapy drug resistance in breast cancer cells and recent work shows that FoxO1 can stimulate the transcriptional activity of MDR1 that may promote increased tolerance of tumor cells.175 In addition, the common pathways shared between Wnt and forkhead proteins may have another side that impacts upon the ability to control tumor growth.53,63 FoxO proteins may assist with β-catenin activation in the Wnt pathway and lead to tumor cell proliferation.54 In the presence of Wnt deregulation and increased β-catenin activity, tumorigenesis may ensue, such as with the proliferation of medulloblastoma tumors.69 Therefore, prediction of biological outcomes during FoxO protein involvement may be uncertain and may be influenced by a host of factors such as tissue characteristics, cellular metabolic state, and the age of an individual. Given these circumstances, further basic and clinical investigations will be required to continue to elucidate the immense potential of FoxO proteins as well as to understand the potential limits of these transcription factors.
Acknowledgments
This research was supported by the following grants (KM): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), and NIH NINDS/NIA.
Abbreviations
- Aβ
β-amyloid
- Akt
protein kinase B
- AFX
acute leukemia fusion gene located in chromosome X
- AGC
protein kinase A/protein kinase G/protein kinase C
- CBP
CREB-binding protein
- DAF-16
DAuer Formation-16
- DM
diabetes mellitus
- EPO
erythropoietin
- FKHR
forkhead in rhabdomyosarcoma
- FKHRL1
forkhead in rhabdomyosarcoma like protein 1
- IKK
IκB kinase
- IGF-1
insulin-like growth factor-1
- IGFBP1
insulin-like growth factor binding protein-1
- IRS
insulin-responsive sequence
- JNK
Jun N-terminal kinase
- NF-κB
nuclear factor-κB
- PCP
planar cell polarity
- PS
phosphatidylserine
- PTEN
tumor suppressor phosphatase and tensin homolog deleted on chromosome ten
- PSR
phosphatidylserine receptor
- ROS
reactive oxygen species
- Wg
Drosophila Wingless
Footnotes
Forkhead Transcription Factors: Vital Elements in Biology and Medicine, edited by Kenneth Maiese.
References
- 1.Weigel D, Jurgens G, Kuttner F, et al. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the drosophila embryo. Cell. 1989;57(4):645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14(2):142–146. [PubMed] [Google Scholar]
- 3.Maiese K, Chong ZZ, Shang YC. “Sly as a FOXO”: new paths with forkhead signaling in the brain. Curr Neurovasc Res. 2007;4(4):295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parry P, Wei Y, Evans G. Cloning and characterization of the t(X;11) breakpoint from a leukemic cell line identify a new member of the forkhead gene family. Genes Chromosomes Cancer. 1994;11(2):79–84. doi: 10.1002/gcc.2870110203. [DOI] [PubMed] [Google Scholar]
- 5.Hillion J, Le Coniat M, Jonveaux P, et al. AF6q21, a novel partner of the MLL gene in t(6;11)(q21;q23), defines a forkhead transcriptional factor subfamily. Blood. 1997;90(9):3714–3719. [PubMed] [Google Scholar]
- 6.Castrillon DH, Miao L, Kollipara R, et al. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301(5630):215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 7.Furuyama T, Nakazawa T, Nakano I, et al. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem. 2000;349(Pt 2):629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuyama T, Yamashita H, Kitayama K, et al. Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech. 2002;59(4):331–334. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- 9.Hoekman MF, Jacobs FM, Smidt MP, et al. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns. 2006;6(2):134–140. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14(5):219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modur V, Nagarajan R, Evers BM, et al. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277(49):47928–47937. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- 12.Biggs WH, Cavenee WK, Arden KC. Identification and characterization of members of the FKHR (FOX O) subclass of winged-helix transcription factors in the mouse. Mamm Genome. 2001;12(6):416–425. doi: 10.1007/s003350020002. [DOI] [PubMed] [Google Scholar]
- 13.Maiese K, Chong ZZ, Shang YC, et al. A “FOXO” in sight: Targeting Foxo proteins from conception to cancer. Med Res Rev. 2009;29(3):395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark KL, Halay ED, Lai E, et al. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364(6436):412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 15.Larson ET, Eilers B, Menon S, et al. A winged-helix protein from Sulfolobus turreted icosahedral virus points toward stabilizing disulfide bonds in the intracellular proteins of a hyperthermophilic virus. Virology. 2007;368(2):249–261. doi: 10.1016/j.virol.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Jin C, Marsden I, Chen X, et al. Sequence specific collective motions in a winged helix DNA binding domain detected by 15N relaxation NMR. Biochemistry. 1998;37(17):6179–6187. doi: 10.1021/bi980031v. [DOI] [PubMed] [Google Scholar]
- 17.Tsai KL, Sun YJ, Huang CY, et al. Crystal structure of the human FOXO3a-DBD/DNA complex suggests the effects of post-translational modification. Nucleic Acids Res. 2007;35(20):6984–6994. doi: 10.1093/nar/gkm703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wijchers PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem J. 2006;397(2):233–246. doi: 10.1042/BJ20060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagani Z, Singh A, Khosravi-Far R. FoxO tumor suppressors and BCR-ABL-induced leukemia: a matter of evasion of apoptosis. Biochim Biophys Acta. 2008;1785(1):63–84. doi: 10.1016/j.bbcan.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiese K, Chong ZZ, Shang YC, et al. Clever cancer strategies with FoxO transcription factors. Cell Cycle. 2008;7(24):3829–3839. doi: 10.4161/cc.7.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8(6):440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 22.Chong ZZ, Li F, Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20(1):299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116(2):344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106(23):2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 25.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2(5):387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008;324(1):160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- 27.Tajes M, Yeste-Velasco M, Zhu X, et al. Activation of Akt by lithium: pro-survival pathways in aging. Mech Ageing Dev. 2009;130(4):253–261. doi: 10.1016/j.mad.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3, and 9. Exp Cell Res. 2004;296(2):196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003;64(3):557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- 30.Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003;74(1):37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- 31.Maiese K, Chong ZZ, Hou J, et al. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5(2):125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150(7):839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293(1):90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Heide LP, Jacobs FM, Burbach JP, et al. FoxO6 transcriptional activity is regulated by Thr26 and Ser184, independent of nucleo-cytoplasmic shuttling. Biochem J. 2005;391(Pt 3):623–629. doi: 10.1042/BJ20050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiese K, Chong ZZ, Shang YC, et al. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond) 2009;116(3):191–203. doi: 10.1042/CS20080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006;13(8):883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res. 2005;2(5):425–446. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi: 10.1007/978-1-4020-6554-5_2. [DOI] [PubMed] [Google Scholar]
- 39.Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and caspase-9 form the critical elements for cerebral vascular protection by erythropoietin. J Cereb Blood Flow Metab. 2003;23(3):320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- 40.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138(6):1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid Redox Signal. 2004;6(2):277–287. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- 42.Chong ZZ, Li F, Maiese K. Group I metabotropic receptor neuroprotection requires Akt and its substrates that govern FOXO3a, Bim, and beta-catenin during oxidative stress. Curr Neurovasc Res. 2006;3(2):107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24(7):728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 44.Obexer P, Geiger K, Ambros PF, et al. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14(3):534–547. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Gutierrez JG, Souza V, Hao HY, et al. Adenovirus-mediated gene transfer of FKHRL1 triple mutant efficiently induces apoptosis in melanoma cells. Cancer Biol Ther. 2006;5(7):875–883. doi: 10.4161/cbt.5.7.2911. [DOI] [PubMed] [Google Scholar]
- 46.Shang YC, Chong ZZ, Hou J, et al. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009;6(1):20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuzaki H, Daitoku H, Hatta M, et al. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA. 2003;100(20):11285–11290. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278(14):12361–12366. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 49.Leong ML, Maiyar AC, Kim B, et al. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem. 2003;278(8):5871–5882. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- 50.Lehtinen MK, Yuan Z, Boag PR, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125(5):987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 51.Song JJ, Lee YJ. Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily. Cell Signal. 2008;20(5):892–906. doi: 10.1016/j.cellsig.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuzaki H, Daitoku H, Hatta M, et al. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci USA. 2005;102(32):11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006;21(1):103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maiese K, Li F, Chong ZZ, et al. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008;118(1):58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maiese K. Triple play: promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62(4):218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li F, Chong ZZ, Maiese K. Vital elements of the wnt-frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2(4):331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maiese K, Chong ZZ, Shang YC, et al. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert Opin Ther Targets. 2008;12(7):905–916. doi: 10.1517/14728222.12.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19(6):1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith WW, Norton DD, Gorospe M, et al. Phosphorylation of p66Shc and forkhead proteins mediates Abeta toxicity. J Cell Biol. 2005;169(2):331–339. doi: 10.1083/jcb.200410041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoogeboom D, Essers MA, Polderman PE, et al. Interaction of FOXO with {beta}-catenin inhibits {beta}-catenin/T cell factor activity. J Biol Chem. 2008;283(14):9224–9230. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 61.Kerdiles YM, Beisner DR, Tinoco R, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10(2):176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naito AT, Akazawa H, Takano H, et al. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97(2):144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- 63.Emami KH, Corey E. When prostate cancer meets bone: control by wnts. Cancer Lett. 2007;253(2):170–179. doi: 10.1016/j.canlet.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 64.Pohl BS, Knochel W. Overexpression of the transcriptional repressor FoxD3 prevents neural crest formation in xenopus embryos. Mech Dev. 2001;103(1–2):93–106. doi: 10.1016/s0925-4773(01)00334-3. [DOI] [PubMed] [Google Scholar]
- 65.Perreault N, Sackett SD, Katz JP, et al. Foxl1 is a mesenchymal modifier of min in carcinogenesis of stomach and colon. Genes Dev. 2005;19(3):311–315. doi: 10.1101/gad.1260605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balciunaite G, Keller MP, Balciunaite E, et al. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol. 2002;3(11):1102–1108. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- 67.Ormestad M, Astorga J, Landgren H, et al. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development. 2006;133(5):833–843. doi: 10.1242/dev.02252. [DOI] [PubMed] [Google Scholar]
- 68.Kimura-Yoshida C, Tian E, Nakano H, et al. Crucial roles of Foxa2 in mouse anterior-posterior axis polarization via regulation of anterior visceral endoderm-specific genes. Proc Natl Acad Sci USA. 2007;104(14):5919–5924. doi: 10.1073/pnas.0607779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sauvageot CM, Kesari S, Stiles CD. Molecular pathogenesis of adult brain tumors and the role of stem cells. Neurol Clin. 2007;25(4):891–924. vii. doi: 10.1016/j.ncl.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 70.Xu HT, Wei Q, Liu Y, et al. Overexpression of axin downregulates TCF-4 and inhibits the development of lung cancer. Ann Surg Oncol. 2007;14(11):3251–3259. doi: 10.1245/s10434-007-9555-9. [DOI] [PubMed] [Google Scholar]
- 71.Kurayoshi M, Oue N, Yamamoto H, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66(21):10439–10448. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 72.Tomita H, Yamada Y, Oyama T, et al. Development of gastric tumors in Apc(Min/+) mice by the activation of the beta-catenin/Tcf signaling pathway. Cancer Res. 2007;67(9):4079–4087. doi: 10.1158/0008-5472.CAN-06-4025. [DOI] [PubMed] [Google Scholar]
- 73.Maiese K, Chong ZZ, Li F, et al. Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kikuchi S, Nagai T, Kunitama M, et al. Active FKHRL1 overcomes imatinib resistance in chronic myelogenous leukemia-derived cell lines via the production of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Sci. 2007;98(12):1949–1958. doi: 10.1111/j.1349-7006.2007.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nowak K, Killmer K, Gessner C, et al. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim Biophys Acta. 2007;1769(4):244–252. doi: 10.1016/j.bbaexp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Bouchard C, Lee S, Paulus-Hock V, et al. FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes Dev. 2007;21(21):2775–2787. doi: 10.1101/gad.453107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75(3):207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24(5):228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 79.Chong ZZ, Kang J, Li F, et al. mGluRI targets microglial activation and selectively prevents neuronal cell engulfment through Akt and caspase dependent pathways. Curr Neurovasc Res. 2005;2(3):197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006;3(3):187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21(3):262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 82.Chong ZZ, Lin SH, Kang JQ, et al. The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cell Mol Neurobiol. 2003;23(4–5):561–578. doi: 10.1023/A:1025158314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salinas M, Diaz R, Abraham NG, et al. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278(16):13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- 84.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22(11):1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maiese K. Diabetic stress: new triumphs and challenges to maintain vascular longevity. Expert Rev Cardiovasc Ther. 2008;6(3):281–284. doi: 10.1586/14779072.6.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Slomka M, Zieminska E, Lazarewicz J. Nicotinamide and 1-methylnicotinamide reduce homocysteine neurotoxicity in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp. 2008;68(1):1–9. doi: 10.55782/ane-2008-1666. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol. 2007;281(1–2):47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Barthelemy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci. 2004;5(1):48. doi: 10.1186/1471-2202-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci USA. 2006;103(24):9051–9056. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Won CK, Ji HH, Koh PO. Estradiol prevents the focal cerebral ischemic injury-induced decrease of forkhead transcription factors phosphorylation. Neurosci Lett. 2006;398(1–2):39–43. doi: 10.1016/j.neulet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 91.Caporali A, Sala-Newby GB, Meloni M, et al. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15(2):299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 93.Ferrara N, Rinaldi B, Corbi G, et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11(1):139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 94.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14(16):1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4(1):63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maiese K, Chong Z, Li F. Reducing oxidative stress and enhancing neurovascular longevity during diabetes mellitus. In: Maiese K, editor. Neurovascular Medicine: Pursuing Cellular Longevity for Healthy Aging. New York: Oxford University Press; 2009. [Google Scholar]
- 97.Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298(7):765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 98.Lin K, Dorman JB, Rodan A, et al. Daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278(5341):1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 99.Ogg S, Paradis S, Gottlieb S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389(6654):994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 100.Guo S, Rena G, Cichy S, et al. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274(24):17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 101.Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274(23):15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 102.Kim JR, Jung HS, Bae SW, et al. Polymorphisms in FOXO gene family and association analysis with BMI. Obesity (Silver Spring) 2006;14(2):188–193. doi: 10.1038/oby.2006.24. [DOI] [PubMed] [Google Scholar]
- 103.Marchetti V, Menghini R, Rizza S, et al. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes. 2006;55(8):2231–2237. doi: 10.2337/db06-0369. [DOI] [PubMed] [Google Scholar]
- 104.Fallarino F, Bianchi R, Orabona C, et al. CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J Exp Med. 2004;200(8):1051–1062. doi: 10.1084/jem.20040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakae J, Cao Y, Oki M, et al. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57(3):563–576. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- 106.Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005;19(20):2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kamagate A, Dong HH. Foxo1 integrates insulin signaling to VLDL production. Cell Cycle. 2008;7(20):3162–3170. doi: 10.4161/cc.7.20.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ni YG, Wang N, Cao DJ, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci USA. 2007;104(51):20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kamei Y, Miura S, Suzuki M, et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004;279(39):41114–41123. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 110.Liu CM, Yang Z, Liu CW, et al. Effect of RNA oligonucleotide targeting Foxo-1 on muscle growth in normal and cancer cachexia mice. Cancer Gene Ther. 2007;14(12):945–952. doi: 10.1038/sj.cgt.7701091. [DOI] [PubMed] [Google Scholar]
- 111.Sandri M, Lin J, Handschin C, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA. 2006;103(44):16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Balan V, Miller GS, Kaplun L, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283(41):27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chong ZZ, Maiese K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and sirtuin mediated pathways. Curr Neurovasc Res. 2008;5(3):159–170. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7(11):847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 115.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306(5704):2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 116.Motta MC, Divecha N, Lemieux M, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116(4):551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 117.Kyoung Kim H, Kyoung Kim Y, Song IH, et al. Down-regulation of a forkhead transcription factor, FOXO3a, accelerates cellular senescence in human dermal fibroblasts. J Gerontol A Biol Sci Med Sci. 2005;60(1):4–9. doi: 10.1093/gerona/60.1.4. [DOI] [PubMed] [Google Scholar]
- 118.Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100(10):1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 119.Li M, Chiu JF, Mossman BT, et al. Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J Biol Chem. 2006;281(52):40429–40439. doi: 10.1074/jbc.M606596200. [DOI] [PubMed] [Google Scholar]
- 120.Miyauchi H, Minamino T, Tateno K, et al. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23(1):212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miyamoto K, Araki KY, Naka K, et al. Foxo3a Is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 122.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141(1):14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 123.Cariou A, Claessens YE, Pene F, et al. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: a matched control study. Resuscitation. 2008;76(3):397–404. doi: 10.1016/j.resuscitation.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 124.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19(2):145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4(3):194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bakker WJ, van Dijk TB, Parren-van Amelsvoort M, et al. Differential regulation of Foxo3a target genes in erythropoiesis. Mol Cell Biol. 2007;27(10):3839–3854. doi: 10.1128/MCB.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, et al. FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress. J Biol Chem. 2002;277(30):26729–26732. doi: 10.1074/jbc.C200256200. [DOI] [PubMed] [Google Scholar]
- 128.Liu L, Rajareddy S, Reddy P, et al. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134(1):199–209. doi: 10.1242/dev.02667. [DOI] [PubMed] [Google Scholar]
- 129.Watkins WJ, Umbers AJ, Woad KJ, et al. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertil Steril. 2006;86(5):1518–1521. doi: 10.1016/j.fertnstert.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 130.Hosaka T, Biggs WH, 3rd, Tieu D, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA. 2004;101(9):2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Furuyama T, Kitayama K, Shimoda Y, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279(33):34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 132.Evans-Anderson HJ, Alfieri CM, Yutzey KE, et al. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res. 2008;102(6):686–694. doi: 10.1161/CIRCRESAHA.107.163428. [DOI] [PubMed] [Google Scholar]
- 133.Li HH, Willis MS, Lockyer P, et al. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of forkhead proteins. J Clin Invest. 2007;117(11):3211–3223. doi: 10.1172/JCI31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abid MR, Yano K, Guo S, et al. Forkhead transcription factors inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. J Biol Chem. 2005;280(33):29864–29873. doi: 10.1074/jbc.M502149200. [DOI] [PubMed] [Google Scholar]
- 135.Liu ZP, Wang Z, Yanagisawa H, et al. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev Cell. 2005;9(2):261–270. doi: 10.1016/j.devcel.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 136.Li H, Liang J, Castrillon DH, DePinho RA, et al. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol Cell Biol. 2007;27(7):2676–2686. doi: 10.1128/MCB.01748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goettsch W, Gryczka C, Korff T, et al. Flow-dependent regulation of angiopoietin-2. J Cell Physiol. 2008;214(2):491–503. doi: 10.1002/jcp.21229. [DOI] [PubMed] [Google Scholar]
- 138.Morris JB, Kenney B, Huynh H, et al. Regulation of the proapoptotic factor FOXO1 (FKHR) in cardiomyocytes by growth factors and alpha1-adrenergic agonists. Endocrinology. 2005;146(10):4370–4376. doi: 10.1210/en.2005-0162. [DOI] [PubMed] [Google Scholar]
- 139.Sedding DG, Seay U, Fink L, et al. Mechanosensitive p27Kip1 regulation and cell cycle entry in vascular smooth muscle cells. Circulation. 2003;108(5):616–622. doi: 10.1161/01.CIR.0000079102.08464.E2. [DOI] [PubMed] [Google Scholar]
- 140.Hannenhalli S, Putt ME, Gilmore JM, et al. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation. 2006;114(12):1269–1276. doi: 10.1161/CIRCULATIONAHA.106.632430. [DOI] [PubMed] [Google Scholar]
- 141.Cools N, Ponsaerts P, Van Tendeloo VF, et al. Regulatory T cells and human disease. Clin Dev Immunol 2007. 2007:89195. doi: 10.1155/2007/89195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ebert LM, Tan BS, Browning J, et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008;68(8):3001–3009. doi: 10.1158/0008-5472.CAN-07-5664. [DOI] [PubMed] [Google Scholar]
- 143.Kono K, Kawaida H, Takahashi A, et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55(9):1064–1071. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19(2):263–272. [PMC free article] [PubMed] [Google Scholar]
- 145.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21(2):203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 146.Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat Med. 2005;11(6):666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- 147.Ludikhuize J, de Launay D, Groot D, et al. Inhibition of forkhead box class O family member transcription factors in rheumatoid synovial tissue. Arthritis Rheum. 2007;56(7):2180–2191. doi: 10.1002/art.22653. [DOI] [PubMed] [Google Scholar]
- 148.Kuo CC, Lin SC. Altered FOXO1 transcript levels in peripheral blood mononuclear cells of systemic lupus erythematosus and rheumatoid arthritis patients. Mol Med. 2007;13(11–12):561–566. doi: 10.2119/2007-00021.Kuo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fabre S, Carrette F, Chen J, et al. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181(5):2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 150.Sela U, Dayan M, Hershkoviz R, et al. The negative regulators Foxj1 and Foxo3a are up-regulated by a peptide that inhibits systemic lupus erythematosus-associated T cell responses. Eur J Immunol. 2006;36(11):2971–2980. doi: 10.1002/eji.200636137. [DOI] [PubMed] [Google Scholar]
- 151.Bosque A, Aguilo JI, Alava MA, et al. The induction of Bim expression in human T-cell blasts is dependent on nonapoptotic Fas/CD95 signaling. Blood. 2007;109(4):1627–1635. doi: 10.1182/blood-2006-05-022319. [DOI] [PubMed] [Google Scholar]
- 152.Lynch RL, Konicek BW, McNulty AM, et al. The progression of LNCaP human prostate cancer cells to androgen independence involves decreased FOXO3a expression and reduced p27KIP1 promoter transactivation. Mol Cancer Res. 2005;3(3):163–169. doi: 10.1158/1541-7786.MCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 153.Li Y, Wang Z, Kong D, et al. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282(29):21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 154.Cornforth AN, Davis JS, Khanifar E, et al. FOXO3a mediates the androgen-dependent regulation of FLIP and contributes to TRAIL-induced apoptosis of LNCaP cells. Oncogene. 2008;27(32):4422–4433. doi: 10.1038/onc.2008.80. [DOI] [PubMed] [Google Scholar]
- 155.Yang L, Xie S, Jamaluddin MS, et al. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J Biol Chem. 2005;280(39):33558–33565. doi: 10.1074/jbc.M504461200. [DOI] [PubMed] [Google Scholar]
- 156.Liu P, Kao TP, Huang H. CDK1 promotes cell proliferation and survival via phosphorylation and inhibition of FOXO1 transcription factor. Oncogene. 2008;27(34):4733–4744. doi: 10.1038/onc.2008.104. [DOI] [PubMed] [Google Scholar]
- 157.Kikuno N, Shiina H, Urakami S, et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26(55):7647–7655. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]
- 158.Trotman LC, Alimonti A, Scaglioni PP, et al. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441(7092):523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jin GS, Kondo E, Miyake T, et al. Expression and intracellular localization of FKHRL1 in mammary gland neoplasms. Acta Med Okayama. 2004;58(4):197–205. doi: 10.18926/AMO/32088. [DOI] [PubMed] [Google Scholar]
- 160.Hu MC, Lee DF, Xia W, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117(2):225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 161.Sunters A, Madureira PA, Pomeranz KM, et al. Paclitaxel-induced nuclear translocation of FOX-O3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66(1):212–220. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 162.Eddy SF, Kane SE, Sonenshein GE. Trastuzumab-resistant HER2-driven breast cancer cells are sensitive to epigallocatechin-3 gallate. Cancer Res. 2007;67(19):9018–9023. doi: 10.1158/0008-5472.CAN-07-1691. [DOI] [PubMed] [Google Scholar]
- 163.Zou Y, Tsai WB, Cheng CJ, et al. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast Cancer Res. 2008;10(1):R21. doi: 10.1186/bcr1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128(2):309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Delpuech O, Griffiths B, East P, et al. Induction of Mxi1-SR{alpha} by FOXO3a Contributes to Repression of Myc-Dependent Gene Expression. Mol Cell Biol. 2007;27(13):4917–4930. doi: 10.1128/MCB.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ticchioni M, Essafi M, Jeandel PY, et al. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007;26(50):7081–7091. doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- 167.Munoz-Fontela C, Marcos-Villar L, Gallego P, et al. Latent protein LANA2 from kaposi’s sarcoma-associated herpesvirus interacts with 14-3-3 proteins and inhibits FOXO3a transcription factor. J Virol. 2007;81(3):1511–1516. doi: 10.1128/JVI.01816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hoekstra AV, Ward EC, Hardt JL, et al. Chemosensitization of endometrial cancer cells through AKT inhibition involves FOXO1. Gynecol Oncol. 2008;108(3):609–618. doi: 10.1016/j.ygyno.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 169.Zethelius B, Berglund L, Sundstrom J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 170.Ward EC, Hoekstra AV, Blok LJ, et al. The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology. 2008;149(4):1942–1950. doi: 10.1210/en.2007-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hellwinkel OJ, Rogmann JP, Asong LE, et al. A comprehensive analysis of transcript signatures of the phosphatidylinositol-3 kinase/protein kinase B signal-transduction pathway in prostate cancer. BJU Int. 2008;101(11):1454–1460. doi: 10.1111/j.1464-410X.2008.07540.x. [DOI] [PubMed] [Google Scholar]
- 172.Kim JH, Kim MK, Lee HE, et al. Constitutive phosphorylation of the FOXO1A transcription factor as a prognostic variable in gastric cancer. Mod Pathol. 2007;20(8):835–842. doi: 10.1038/modpathol.3800789. [DOI] [PubMed] [Google Scholar]
- 173.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 174.Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28(6):941–953. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 175.Han CY, Cho KB, Choi HS, et al. Role of FoxO1 activation in MDR1 expression in adriamycin-resistant breast cancer cells. Carcinogenesis. 2008;29(9):1837–1844. doi: 10.1093/carcin/bgn092. [DOI] [PubMed] [Google Scholar]