Abstract
When cells attach to the extracellular matrix (ECM) a proliferation permissive signal is engaged. The mechanism involves activation of the integrin/PI3K/Akt signal pathway. FoxO3a is a transcriptional activator and inhibits cell proliferation via up-regulating the expression of the cell cycle inhibitor p27. Furthermore, it is known that activated Akt can suppress FoxO3a function. However, it is not known whether integrin interaction with the ECM regulates FoxO3a function. We examined whether the β1-integrin-mediated signaling pathway promotes fibroblast proliferation via FoxO3a suppression. We found that when fibroblasts are attached to collagen, PTEN protein expression and activity are inhibited due to promotion of PTEN degradation. This decrease in PTEN function permits FoxO3a suppression via the PI3K/Akt pathway. In contrast, the inhibition of PI3K/Akt or reconstitution of PTEN restores FoxO3a expression on collagen. Furthermore, we found that the serine/threonine phosphatase PP2A also regulates FoxO3a. PP2A expression/activity is low when fibroblasts are attached to collagen, and PP2A overexpression augments FoxO3a levels. Thus the mechanism involves a coordinated decrease in PTEN and PP2A phosphatase activity and increase in PI3K/Akt activity. We show that β1-integrin-ECM interaction decreases FoxO3a protein levels via caspase-3-mediated cleavage. Our novel finding indicates that during fibroblast interaction with ECM, activation of β1-integrin/PI3K/Akt by inhibiting PTEN in combination with low PP2A phosphatase activity synergistically inhibits FoxO3a, promoting fibroblast proliferation.
Keywords: Cell/Fibroblast, Extracellular Matrix, Extracellular Matrix/Collagen, Extracellular Matrix/Integrin, Receptors/Extracellular Matrix, Signal Transduction/Phosphoprotein Phosphatases/PP1/PP2A, Signal Transduction/Protein Kinases/Serine/Threonine
Introduction
Cell attachment to extracellular matrix (ECM)2 regulates cell proliferation and apoptosis (1–3). Cell-ECM interactions are mediated through a large family of cell adhesion receptors termed integrins (4–5). Ligation of integrins and subsequent integrin clustering by the ECM induces signal transduction pathways that can regulate cell proliferation (6). Because precise control of cell growth is required for proper tissue development and repair, a mechanism for activation of major growth pathways must exist. Integrin-ECM interaction promotes activation of the Ser/Thr protein kinase Akt through the upstream lipid kinase, PI3K (7). Activation of the PI3K/Akt pathway promotes cell proliferation by facilitating G1 to S phase cell cycle transition.
FoxO3a belongs to the family of FoxO (forkhead box O) transcription factors, which were identified at chromosomal breakpoints in human tumors and are a direct target of Akt phosphorylation (8, 9). Upon activation, FoxO3a inhibits cell cycle progression by up-regulating expression of the cyclin-dependent kinase inhibitor, p27 (9, 34). Activated FoxO3a also promotes apoptosis by enhancing the expression of pro-apoptotic molecules such as Bim and Fas ligands (10, 11). FoxO3a function is negatively regulated by PI3K/Akt. Activated Akt phosphorylates and inactivates FoxO3a (8, 9). Three FoxO3a phosphorylation sites, Thr-32, Ser-253, and Ser-315, are phosphorylated by Akt and the second regulatory site, Ser-253, has a greater affinity than the first and third sites (21, 30, 31). Phosphorylation of FoxO3a promotes its exit from the nucleus, resulting in inhibition of FoxO3a-dependent transcription thereby promoting cell cycle progression (17, 32, 33). We have found that fibroblast attachment to collagen activates the PI3K/Akt pathway via β1-integrin (19, 20). Activation of the PI3K/Akt signal promotes fibroblast proliferation and protects them from contractile collagen-induced apoptosis (20, 26). However, the role of integrin-matrix interaction in regulating FoxO3a function has not been previously examined. We therefore sought to determine whether integrin interaction with the ECM involved phosphorylation and inactivation of FoxO3a by Akt. Here we show that β1-integrin-extracellular matrix interaction suppresses FoxO3a function by promoting PTEN degradation, facilitating fibroblast proliferation by high Akt activity. We have discovered that during fibroblast adhesion to collagen, FoxO3a protein expression decreases whereas the level of phosphorylated or inactive FoxO3a remains steady. This indicates that FoxO3a activity decreases during fibroblast attachment to collagen.
We demonstrate that the mechanism of suppression of FoxO3a function in response to integrin-collagen interaction is complex and involves net phosphorylation of FoxO3a as a consequence of down-regulation of PP2A and PTEN phosphatase activity plus activation of Akt. Although the FoxO3a regulatory mechanism is not fully understood, experimental evidence suggests that FoxO3a may be degraded by Ser/Thr phosphorylation (17, 18). Interestingly, we have found that FoxO3a contains 2 PEST sequences in its N-terminal domain and that 3 potential caspase-3 cleavage sites are located in the second PEST sequence. We show that caspase-3 activity increases in response to fibroblast attachment to collagen. Furthermore, FoxO3a protein levels are preserved in fibroblasts attaching to type I collagen in the presence of caspase-3 inhibitor. Together these data suggest that integrin-collagen interaction inactivates FoxO3a by a complex mechanism involving net phosphorylation of the molecule and cleavage by caspase-3. Our data show that inhibition of FoxO3a is conducted in a coordinated PTEN/PI3K/Akt and PP2A-dependent fashion via β1-integrin, thereby promoting fibroblast proliferation.
MATERIALS AND METHODS
Cell Culture and Type I Collagen Matrices
For this study, the normal human lung fibroblast line HLF-210 was used (purchased from ATCC). HLF-210 fibroblasts were cultured in high glucose DMEM containing 10% fetal calf serum. The fibroblasts were used between passages 5 and 8 for all experiments. In addition, PTEN−/− and PTEN+/+ cells were provided by Deane F. Mosher (University of Wisconsin, Madison). FoxO3a+/+ and FoxO3a−/− mouse embryonic fibroblasts were kindly obtained from Dr. Noboru Motoyama, National Center for Geriatrics and Gerontology, Japan. These cells were maintained in DMEM + 10% fetal calf serum, 1% penicillin, and 1% streptomycin. Type I collagen solution (Vitrogen 1000) was obtained from Cohesion, Palo Alto, CA. Monomeric collagen matrices were prepared by coating Petri dishes with 100 μg/ml of type I collagen solution. The dishes were washed twice with DMEM prior to plating the cells. Fibroblasts were cultured on the surface of the collagen-coated dishes.
Antibodies and Chemicals
Anti-PTEN antibody was obtained from Santa Cruz Biotechnology. Anti-Akt and anti-phospho-Akt (Ser-473) antibodies were obtained form Cell Signaling Technologies. FoxO3a and phosphorylated FoxO3a (Ser-253) antibodies were obtained from Cell Signaling Technologies. p27 antibody was purchased from Santa Cruz Biotechnology, Inc. FoxO3a antibody against mouse was obtained from Millipore. β1-Integrin activating monoclonal antibody TS2/16 was produced from hybridoma culture (ATCC, HB-243). β1-Integrin blocking antibody P5D2 was also produced from hybridoma culture. α1 to α5 and αv integrin blocking antibodies were purchased from Chemicon (Billerica, MA). PI3K inhibitor (wortmannin), Akt inhibitor, caspase-3 inhibitor, ERK inhibitor, and okadaic acid were purchased from Calbiochem.
Adenovirus Constructs, PP2A, and FoxO3a Constructs
For PTEN adenovirus construction, wild type PTEN cDNA was generated from normal human lung fibroblasts (HLF-210) by RT-PCR. The primers used for wild type PTEN, which span the entire coding region of PTEN are 5′-CTA CTC GAG GCT CCC AGA CAT GAC-3′ and 5′-ACG CTC GAG ATA AAA AAA AAT TCA G-3′. The amplified products were cloned into MIGR1-IRES-GFP followed by SalI and EcoRI digestion. Wild type PTEN MIGR1-IRES-GFP fragments were then cloned into the adenoviral vector, pAxCAwt (purchased from Dakara Biol Inc., Japan), and adenovirus titer was measured by plaque method in 0.5% soft agar. The PP2A adenovirus expressing HA-tagged PP2A catalytic subunit was kindly provided by Dr. Alexander Verin (University of Chicago). The cells were infected with adenoviral vectors at a multiplicity of infection of 1:20. Adenovirus expressing HA-tagged Akt with the c-Src myristoylation sequence fused in-frame to the N terminus (Hyperactive Akt), HA-tagged Akt dominant mutant (T308A, S473A), and wild type FoxO3a were purchased from Vector Biolabs (Eagleville, PA). Adenovirus expressing GFP-tagged wild type FoxO3a, dominant-negative FoxO3a with the deletion of the transactivation domain from the C terminus and empty vector were purchased from Vector BioLabs (PA).
PTEN, PP2A, and Caspase-3 Activity Assay
For PTEN activity assay, PTEN was immunoprecipitated from lysates containing equal amounts of protein by incubation with 2 μg of anti-PTEN antibody overnight at 4 °C. The immunoprecipitates were washed twice with TBS buffer (25 mm Tris, pH 7.4, 100 mm NaCl, 10 mm KCl) followed by phosphatase reaction buffer. PTEN activity was assayed using a Malachite green phosphatase kit (Echelon) according to the manufacturer's instructions. For PP2A activity assay, fibroblasts were lysed in lysis buffer containing 2 mm imidazole (pH 7.0), 2 mm EDTA, 2 mm EGTA, 2% Nonidet P-40, and 1× protease inhibitor mixture (Calbiochem, La Jolla, CA). Total protein levels were measured from the resulting lysates. PP2A catalytic subunit protein was then immunoprecipitated from lysates containing equal amounts of protein by incubation with 2 μg of anti-PP2A antibody overnight (4 °C). The immunoprecipitates were washed twice with TBS buffer followed by two additional washes with phosphatase reaction buffer (50 mm Tris, pH 7.4, 10 mm NaCl, 1 mm EDTA, 1 mm dithiothreitol). PP2A activity was assayed using a Malachite green phosphatase kit (Echelon) according to the manufacturer's instructions. For the caspase-3 activity assay, a caspase-3 colorimetric activity assay kit was used (Millipore). Briefly, serum-starved lung fibroblasts were incubated in the presence or absence of β1-integrin blocking antibody followed by plating on type I collagen-coated plates. Cells were lysed and incubated with the caspase-3 substrate (3 mg/ml, Ac-DEVD-p-nitroanilide) for 2 h at 37 °C. Caspase-3 activity was measured using a microtiter plate reader at 405 nm. For pulse-chase analysis, human lung fibroblasts were incubated for 45 min in methionine-free DMEM. The cells were then incubated for 1 h with methionine-free DMEM containing [35S]methionine (150 μ/Ci/ml) (Life Sciences). The cells were trypsinized and replated on type I collagen-coated dishes (100 mg/ml) or degraded collagen and the medium was then replaced with DMEM + 10% fetal calf serum. At the indicated times the cells were lysed, PTEN was immunoprecipitated from labeled cell extracts, and resolved on a 7.5% polyacrylamide gel. Radiolabeled PTEN was detected by autoradiography.
Cell Proliferation Assay
The number of viable proliferating cells was determined using the Cell Titer 96 Aqueous One solution Cell Proliferation Assay (MTS assay, Promega). Briefly, human lung fibroblasts were transfected with FoxO3a or control siRNA (10 nm, each) for 24 h. 2 × 103 human lung fibroblasts were then grown in 100 μl of DMEM on 96-well plates overnight. 20 μl of Cell Titer 96 Aqueous One solution reagent was added to each well of the 96-well plate followed by incubation for 2 h at 37 °C in a humidified 5% CO2 atmosphere. The absorbance at 490 nm using a 96-well plate reader was measured. For the proliferation assay, 3,000 PTEN+/+ or PTEN−/− cells were incubated in DMEM for 72 h. Cell numbers were then counted using the Coulter counter. For the collagen attachment assay, 5,000 serum-starved lung fibroblast cells were preincubated with α2 or β1 or both integrin blocking antibodies (1 μg/ml, respectively) for 45 min followed by attachment to type I collagen for 30 min. Attached cells were then trypsinized, collected, and counted.
RT-PCR and Transfection
HLF-210 cells were serum-starved for 2 days followed by plating on a collagen-coated plate for the indicated times as described. Cells were then collected and total RNA was isolated using TRIzol. cDNA was prepared using oligo(dT) primer with purified total RNA (1 μg) from each sample, and PCR was carried out with FoxO3a primers (sense FoxO3a primer, 5′-AAA TGT TCG TCG CGG CGG AAC-3′; antisense FoxO3a primer, 5′-GTC GCC CTT ATC CTT GAA GTA-3′) as follows: 95 °C for 5 min for 1 cycle, 95 °C for 45 s, 54 °C for 45 s, and 72 °C for 30 s for 25 cycles. RT-PCR for PTEN was also carried out with PTEN primers (sense PTEN primer, 5′-ATG ACA GCC ATC ATC AAA GAG-3′; antisense PTEN primer, 5′-TGT GGT GGG TTA TGG TCT TC-3′) as follows: 95 °C for 5 min for 1 cycle, 95 °C for 45 s, 60 °C for 45 s, and 72 °C for 50 s for 28 cycles. Amplified products were analyzed using 1.2% agarose gel. For siRNA transfection experiments, human lung or mouse embryonic fibroblasts were transfected with pre-made human or mouse FoxO3a siRNA or a control non-silencing siRNA obtained from Santa Cruz Biotechnology, Inc. using X-tremeGENE siRNA transfection reagent (Roche Applied Science) according to the manufacturer's protocol. At 24 h post-transfection, cells were then serum-starved for 1 day, followed by either plating on type I collagen-coated plates (100 μg/ml) or ligation with 3 μg of integrin activating antibody (TS2/16) as described.
Statistical Analysis
Data are expressed as the mean ± S.D. Experiments were performed three times. Paired evaluations were made for experimental and control conditions within each experiment, and significance was determined by Student's t test. Significance level was set at p < 0.05.
RESULTS
Fibroblast Attachment to the Type I Collagen Increases Akt Activity and Suppresses FoxO3a
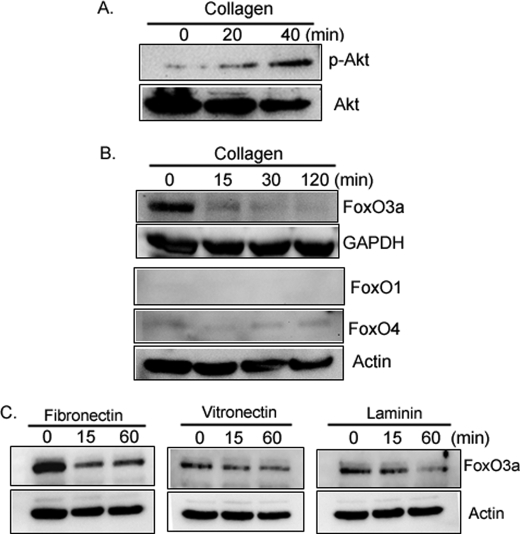
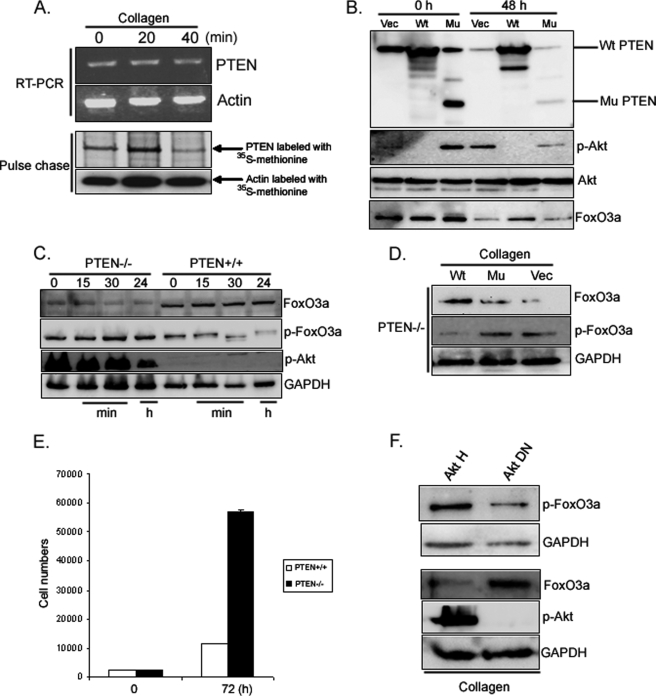
We have previously found that Akt activity (Ser-473 phosphorylation) increases when normal fibroblasts attach to type I collagen via integrin (19, 20). Activated Akt phosphorylates and inactivates FoxO3a, however, whether integrin-matrix interaction regulates FoxO3a function remains unknown. To begin to examine this issue, we first measured Akt activity in lung fibroblasts on collagen-coated plates as a function of time. Phosphorylated Akt levels increased on collagen (Fig. 1A), suggesting that fibroblast attachment to type I collagen can increase Akt activity. We then measured FoxO3a protein expression and activity on collagen as a function of time. The level of FoxO3a protein expression decreased in response to fibroblast attachment to type I collagen-coated plates (Fig. 1B, upper panel). We also examined whether FoxO family members other than FoxO3a are regulated on collagen. The expression levels of FoxO1 were not detectable and FoxO4 expression was low as a function of time on collagen (Fig. 1B, lower panel). This indicates that FoxO3a may be the major FoxO family protein expressed by fibroblasts during interaction to type I collagen. We further examined FoxO3a protein expression in response to fibroblast interaction with other ECMs. Similar to type I collagen, FoxO3a expression decreased in response to fibroblast attachment to fibronectin or laminin as a function of time (Fig. 1C). In contrast, FoxO3a levels modestly decreased when fibroblasts were attached to vitronectin. These data indicate that fibroblast attachment to specific extracellular matrix such as type I collagen and fibronectin promotes a significant decrease in FoxO3a protein expression.
FIGURE 1.
FoxO3a expression decreases on collagen. A, serum-starved human lung fibroblasts were attached to collagen (100 μg/ml, upper panel)-coated plates as a function of time. Akt and Ser-473-phosphorylated Akt levels were then measured. B, human lung fibroblasts were serum-starved for 2 days followed by attachment to collagen-coated plates as a function of time. FoxO3a, FoxO1, and FoxO4 expression levels were measured (upper and lower panels, respectively). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and actin were used as a loading control. C, serum-starved human lung fibroblasts were attached to fibronectin- (10 μg/ml), vitronectin- (10 μg/ml), and laminin (10 μg/ml)-coated plates as a function of time. FoxO3a expression levels were measured. Actin was used as a loading control.
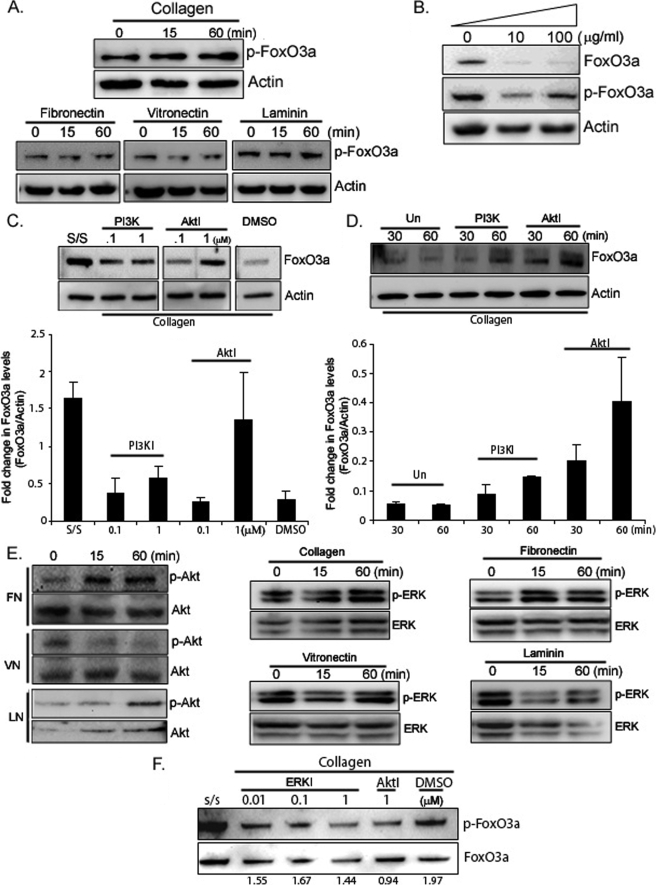
The serine/threonine kinase Akt phosphorylates FoxO3a, regulating its activity. It preferentially phosphorylates Ser-253. This promotes FoxO3a translocation from the nucleus to the cytoplasm thereby inhibiting its function (21). Therefore, we examined the phosphorylation status of Ser-253 as a surrogate marker of FoxO3a activity. Unlike low FoxO3a expression levels on collagen (Fig. 1B), phosphorylated FoxO3a levels were unchanged on collagen-coated plates as a function of time (Fig. 2A, upper panel), indicating that FoxO3a activity was suppressed when fibroblasts were attached to collagen. We further measured phosphorylated FoxO3a levels on fibronectin-, vitronectin-, and laminin-coated plates (Fig. 2A, lower panel). Phosphorylated FoxO3a (inactive FoxO3a) levels were barely changed on fibronectin or laminin. Because FoxO3a levels were significantly low on these ECM (Fig. 1, B and C), these data suggested that FoxO3a activity was also decreased on fibronectin- and laminin-coated plates. Likewise, phosphorylated FoxO3a levels did not alter notably on vitronectin (Fig. 2A, lower panel). However, unlike other ECM we tested, as FoxO3a expression was modestly decreased on vitronectin (Fig. 1C, middle panel), these results indicate that FoxO3a activity does not alter significantly on vitronectin compared with that on collagen and fibronectin. We further measured FoxO3a activity on various concentrations of type I collagen and found that FoxO3a levels were decreased, whereas phosphorylated FoxO3a levels were unaltered on collagen (Fig. 2B). Taken together, these data demonstrated that fibroblast attachment to ECM such as collagen, fibronectin, and laminin promotes significant inhibition of FoxO3a activity and cell attachment to specific ECM is crucial in determining FoxO3a activity. Because Akt is known to inactivate FoxO3a we sought to determine whether modulation of FoxO3a is dependent on the PI3K/Akt pathway in our model. Fibroblasts were pre-treated with different doses of PI3K or Akt inhibitor (0.1 and 1 μm, respectively) and plated on collagen-coated plates for 30 min. We found that FoxO3a protein levels were preserved when fibroblasts were pre-treated with PI3K or Akt inhibitor (Fig. 2C). In contrast, FoxO3a expression decreased when DMSO-preincubated cells attached to collagen. Likewise, FoxO3a expression remained relatively high when fibroblasts were cultured in the presence of PI3K or Akt inhibitor on collagen-coated plates as a function of time (Fig. 2D). These data indicate that FoxO3a protein expression is regulated by the PI3K/Akt pathway. Because Akt phosphorylates FoxO3a, thereby regulating its activity, we further measured phosphorylated Akt levels in lung fibroblasts in response to ECM. Like phosphorylated Akt levels on collagen (Fig. 1A), Akt activity (Ser-473 phosphorylated Akt) was increased when fibroblasts were placed on fibronectin (p-Akt/Akt signal ratio is provided in supplemental Fig. S1A). In contrast, phosphorylated Akt levels were low and remained unaltered on vitronectin as a function of time (Fig. 2E, left panel). Phosphorylated Akt levels also did not change significantly on laminin. These data showed that Akt activity can vary in response to fibroblast attachment to specific ECM. A prior study showed that FoxO3a is also regulated by ERK and its activity is dependent on cell type (36). To address whether ERK activity is also regulated by fibroblast attachment to ECM, cells were placed on type I collagen-, fibronectin-, vitronectin-, and laminin-coated plates and phosphorylated ERK1/2 levels (p-p44/p42) were measured as a function of time. Phosphorylated ERK levels were increased on collagen- and fibronectin-coated plates (Fig. 2E, middle and right panels). In contrast, ERK activity did not change significantly on the vitronectin- and laminin-coated plates (Fig. 2E, middle and right panels, p-ERK/ERK signal ratio is provided in supplemental Fig. S1B). To examine whether ERK activity is crucial in regulating FoxO3a activity on the collagen matrix, we measured the phosphorylated and total FoxO3a levels in the presence of various doses of the ERK inhibitor on collagen. The signal ratio of phosphorylated FoxO3a/FoxO3a did not change significantly even at the highest dose of the ERK inhibitor on collagen (Fig. 2F). In contrast, the phosphorylated FoxO3a/FoxO3a signal ratio was low in the presence of Akt inhibitor (lane 5), indicating that Akt may play a major role in regulating FoxO3a activity in response to fibroblast attachment to type I collagen.
FIGURE 2.
FoxO3a activity decreases in response to fibroblast attachment to collagen. A, serum-starved human lung fibroblasts were attached to collagen-coated plates as a function of time and phosphorylated FoxO3a (p-FoxO3a, Ser-253) and actin levels were measured (upper panel). Cells were placed on fibronectin-, vitronectin-, and laminin-coated plates as a function of time and phosphorylated FoxO3a levels were measured (lower panel). Actin was used as a loading control. B, serum-starved human lung fibroblasts were attached at 0 to 100 μg/ml of collagen-coated plates for 30 min. FoxO3a and phosphorylated FoxO3a levels were then measured. Actin was used as a loading control. C, upper panel, human lung fibroblasts were serum-starved followed by preincubation with PI3K or Akt inhibitor as indicated for 45 min. Cells were then allowed to attach to collagen-coated plates for 30 min. FoxO3a expression levels were then measured. Actin was used as a loading control. PI3KI, PI3K inhibitor; AktI, Akt Inhibitor; s/s, serum-starved fibroblasts. DMSO, dimethyl sulfoxide control. Lower panel, fold-changes in FoxO3a expression (FoxO3a/actin) in the presence of PI3K or Akt inhibitor on collagen. The results are representative of three different experiments. D, upper panel, serum-starved lung fibroblasts were preincubated with PI3K (200 nm) or Akt inhibitor (1 μm) for 45 min followed by attachment to collagen-coated plates as a function of time. FoxO3a and Actin expression levels were then measured. Un, untreated cells. Lower panel, fold-changes in FoxO3a expression (FoxO3a/actin) on collagen matrix in the presence or absence of PI3K or Akt inhibitor. The results are representative of three different experiments. E, left panel, serum-starved human lung fibroblasts were placed on fibronectin (FN)-, vitronectin (VN)-, or laminin (LN)-coated plates as a function of time and phosphorylated (p-Akt) and total Akt (Akt) levels were measured. Middle and right panels, cells were attached to collagen (100 μg/ml)-, fibronectin (10 μg/ml)-, vitronectin (10 μg/ml)-, or laminin (10 μg/ml)-coated plates as a function of time. Phosphorylated ERK (p-ERK, p-44/p-42) and ERK levels were then measured. F, human lung fibroblasts were preincubated with different doses of ERK inhibitor (ERKI) or Akt inhibitor (AktI, 1 μm) as indicated and phosphorylated FoxO3a (p-FoxO3a) and total FoxO3a (FoxO3a) levels were then measured. Numbers represent p-FoxO3a versus FoxO3a signal ratio.
β1-Integrin/PI3K/Akt Pathway Suppresses FoxO3a in Response to Fibroblast Attachment to Collagen
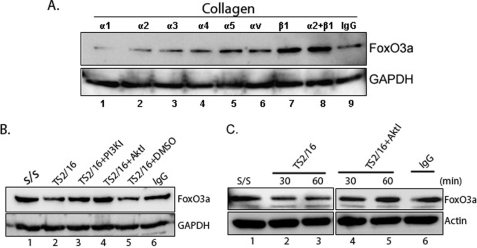
β1-Integrin is a crucial integrin subunit that facilitates attachment to type I collagen (19, 22, 23). We therefore examined whether the integrin-ECM interaction regulates FoxO3a function. For this assay, serum-starved lung fibroblasts were seeded on type I collagen in the presence or absence of α or β integrin subunit blocking antibodies and FoxO3a protein expression was examined. FoxO3a protein expression was preserved when fibroblasts were pretreated with β1-integrin blocking antibody (Fig. 3A, upper panel, lane 7, FoxO3a/GAPDH signal ratio is provided in supplemental Fig. S1C). In contrast, FoxO3a protein expression was lower when fibroblasts were pretreated with α subunit blocking antibodies. Our data suggest that the β1-integrin-collagen interaction plays a crucial role in regulating FoxO3a.
FIGURE 3.
β1-Integrin-collagen interaction suppresses the FoxO3a via PI3K/Akt pathway. A, human lung fibroblasts were preincubated with α1 to α5 and αv-integrin and/or β1-integrin blocking antibodies (1 μg/ml, respectively) for 45 min followed by the attachment to type I collagen for 30 min. FoxO3a levels were then measured. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. B, serum-starved human lung fibroblasts were preincubated with PI3K (0.2 μm) or Akt inhibitor (1 μm) for 45 min. Cells were then ligated with β1-integrin activating antibody (3 μg/ml) for 60 min. FoxO3a expression levels were measured. TS2/16, β1-integrin activating antibody; PI3KI, PI3K inhibitor; AktI, Akt inhibitor; IgG, isotype control. C, serum-starved human lung fibroblasts were preincubated with Akt inhibitor (1 μm) for 45 min. Cells were then stimulated with β1-integrin activating antibody TS2/16 (3 μg/ml) as a function of time. FoxO3a and actin expression levels were then measured. IgG, isotype control.
We have previously shown that β1-integrin activating antibody TS216 activates PI3K/Akt function (19, 22). Therefore, we next examined the effect of β1-integrin on FoxO3a modulation via PI3K/Akt. FoxO3a expression decreased when fibroblasts were treated with β1-integrin activating antibody TS2/16 (Fig. 3B, lane 2, FoxO3a/GAPDH signal ratio is provided in supplemental Fig. S1D), whereas FoxO3a levels were unaltered in the presence of PI3K or Akt inhibitor or the IgG isotype control antibody (Fig. 3B, lanes 3, 4, and 6, respectively). These data support the concept that β1-integrin activation inhibits FoxO3a function via PI3K/Akt. We further measured FoxO3a expression levels by the β1-integrin activating antibody (TS2/16) as a function of time. FoxO3a expression was also high when fibroblasts were treated with the β1-integrin activating antibody in the presence of the Akt inhibitor as a function of time (Fig. 3C, lanes 4 and 5, FoxO3a/actin ratio is provided in supplemental Fig. S1E). Taken together, our data suggest that FoxO3a activity is suppressed via the β1-integrin/PI3K/Akt pathway.
Integrin-Collagen Interaction Suppresses PTEN
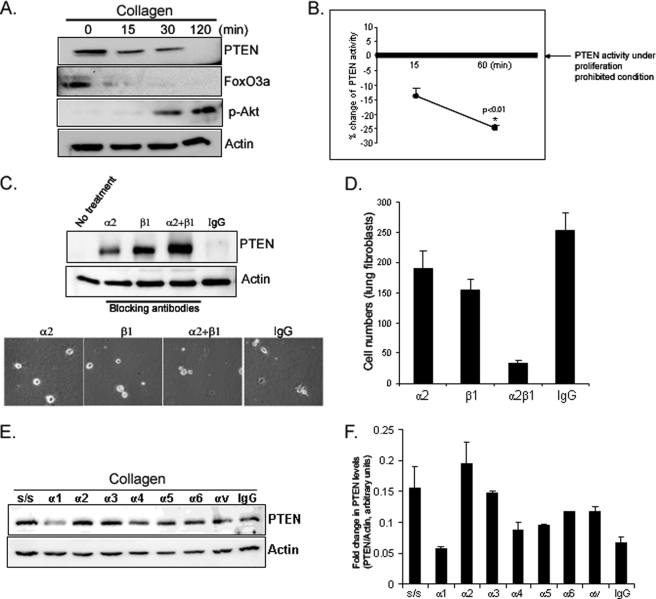
Because integrin-ECM interaction results in activation of PI3K/Akt, we sought to determine whether the integrin inhibitory function of PTEN relaxes during cell attachment to collagen. Because cell attachment to collagen promotes Akt activation and cell proliferation, we examined the PTEN expression on collagen-coated plates as a function of time. Like FoxO3a levels, PTEN protein expression markedly decreased as fibroblasts adhered to collagen, whereas phosphorylated Akt levels increased (Fig. 4A). We further measured PTEN activity on collagen as a function of time. Like PTEN expression, PTEN activity was also decreased on collagen-coated plates as a function of time, demonstrating that fibroblast attachment to collagen suppresses PTEN function (Fig. 4B). The major integrin mediating attachment to type I collagen is α2β1. Both α2- and β1-integrin blocking antibodies inhibited the decrease in PTEN expression seen in response to fibroblast adhesion to collagen (Fig. 4C, upper and lower panels). The combination of α2- and β1-integrin blocking antibodies had a synergistic effect in preventing this decrease in PTEN protein expression. Because integrin blocking antibodies inhibit fibroblast attachment to collagen, we further analyzed cell numbers attached to collagen in the presence or absence of these antibodies. When 5,000 cells were preincubated with IgG isotype control antibody, ∼260 cells attached to collagen after 30 min. However, when fibroblasts were incubated with α2-integrin blocking antibody, ∼190 cells were able to attach to collagen (Fig. 4D, a 27% decrease compared with that of isotype control). The attached cell numbers on the collagen matrix decreased to 155 (40% decrease) when fibroblasts were preincubated with β1-integrin blocking antibody, and when fibroblasts were preincubated with α2- and β1-integrin blocking antibodies together, most cells failed to attach to collagen with only 40 cells able to attach to collagen matrix (85% decrease). These data demonstrated that α2β1-integrins are crucial in fibroblast attachment to collagen and β1-integrin plays a major role in the interaction with collagen matrix. Because α-integrin subunits other than α2 have been implicated in mediating adhesion to collagen, we examined a panel of α-integrin blocking antibodies on PTEN expression in response to fibroblast attachment to collagen. In addition to α2, the α3 blocking antibody inhibited the decrease in PTEN expression during fibroblast adhesion to collagen-coated plates (Fig. 4, E and F). The other α blocking antibodies including α1 had minimal effect on PTEN expression. These data indicate that fibroblast attachment to collagen via α2β1-integrin mainly triggers a decrease in PTEN protein expression and activity.
FIGURE 4.
Fibroblast adhesion to collagen decreases PTEN expression and activity. A, serum-starved fibroblasts were plated on dishes coated with type I collagen (100 μg/ml) and PTEN, FoxO3a, phosphorylated Akt (Ser-473), and actin levels were measured by Western analysis. B, serum-starved fibroblasts were plated on collagen for 15 and 60 min. p < 0.01 versus a reference value. Cells lysates were immunoprecipitated with anti-PTEN antibody, incubated with phosphatidylinositol (3,4,5)-trisphosphate substrate, and PTEN activity was quantified. To provide a reference value for PTEN activity under proliferation prohibitive conditions, PTEN activity was assessed in serum-starved fibroblasts cultured on tissue culture plastic plates under contact-inhibited conditions. C, upper panel, serum-starved fibroblasts were preincubated with α2- or β1-integrin blocking antibody (1 μg/ml), both (α2+β1), or isotype control antibody (IgG, 1 μg/ml) for 45 min and plated on collagen-coated plates for 30 min. PTEN levels were then measured. Actin was used as a loading control. Lower panel, shown are the phase-contrast microscopic cell morphologies at 30 min after plating the cells on collagen-coated plates. α2 and β1 represent α2 and β1 blocking antibody, respectively. IgG, isotype control. D, 5,000 cells were preincubated with integrin blocking antibodies for 45 min as indicated and the attached cells were counted at 30 min after plating the cells on collagen. E, serum-starved fibroblasts were pretreated with the indicated integrin blocking antibodies (1 μg/ml) and then plated on collagen-coated plates for 30 min. Total PTEN and actin levels were determined by Western analysis. As a reference, serum-starved (S/S) fibroblasts were trypsinized but not plated on collagen. F, PTEN expression was quantified by densitometry, normalized to actin.
PTEN Restores FoxO3a Activity in Response to Fibroblast Attachment to Type I Collagen
We next sought to determine the mechanism by which PTEN protein levels decrease when fibroblasts attach to the ECM. For these studies we examined fibroblast interaction with type I collagen. We first examined the effect of attachment to collagen as a function of time on PTEN mRNA levels using RT-PCR. PTEN mRNA levels remained stable during the same time period when PTEN protein expression was decreasing (Fig. 5A, upper panel). This indicates that changes in PTEN mRNA transcription could not account for the decrease in PTEN protein expression seen when fibroblasts attach to type I collagen. It has previously been reported that in response to modulation of PTEN phosphorylation, PTEN protein stability is altered (37). To determine whether the changes in PTEN protein levels in response to attachment of collagen matrix were related to changes in protein stability, pulse-chase experiments using fibroblasts labeled with [35S]methionine were performed. In these experiments we found that the half-life of PTEN was significantly reduced when fibroblasts were cultured on collagen (Fig. 5A, lower panel). Our data indicate that the decrease in PTEN protein levels in response to attachment to type I collagen are not due to changes in PTEN mRNA transcription but are secondary to increased protein degradation. Because integrin-collagen interaction suppresses PTEN, we next examined whether PTEN can regulate FoxO3a activity via Akt. We first examined the effect of overexpression of PTEN on FoxO3a function when fibroblasts were plated on collagen as a function of time. Serum-starved lung fibroblasts infected with adenovirus expressing wild type or mutant PTEN were seeded on type I collagen-coated plates, and FoxO3a and Akt activity were measured at 0 and 48 h. Akt activity was suppressed when wild type PTEN was overexpressed, whereas FoxO3a expression remained high at 48 h (Fig. 5B). In contrast, Akt activity was high when fibroblasts were infected with adenovirus expressing mutant PTEN or empty vector, whereas the FoxO3a expression was low. This data suggests that elevated PTEN levels suppress Akt and maintain FoxO3a function.
FIGURE 5.
PTEN inhibition increases fibroblast proliferation via high Akt activity. A, upper panel, RT-PCR. Serum-starved fibroblasts were plated on collagen-coated dishes for the indicated times. The cells were lysed and total RNA was isolated by TRIzol. RT-PCR was performed as described under “Materials and Methods.” Lower panel, pulse-chase analysis. Fibroblasts were incubated in methionine-free medium followed by DMEM containing [35S]methionine as outlined under “Materials and Methods.” The cells were then plated on collagen-coated dishes in DMEM + 10% fetal calf serum for the indicated times. The cells were lysed, PTEN was immunoprecipitated from labeled cell extracts and resolved by SDS-PAGE. Radiolabeled PTEN was visualized by autoradiography. B, human lung fibroblasts were infected with adenovirus expressing wild type PTEN or mutant PTEN followed by serum starvation for 1 day. Cells were then attached to collagen-coated plates (100 μg/ml) for 48 h. Total Akt, phosphorylated Akt, and FoxO3a expression levels were then measured. Wt, wild type PTEN; Mu, phosphatase-dead mutant PTEN; Vec, empty vector. C, PTEN−/− and PTEN+/+ cells were serum-starved for 24 h followed by attachment to collagen-coated plates as a function of time. FoxO3a, Ser-253-phosphorylated FoxO3a, and Ser-473-phosphorylated Akt levels were measured. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. D, PTEN−/− fibroblasts infected with adenovirus expressing wild type PTEN (Wt), phosphatase-dead mutant PTEN (Mu), or empty vector (Vec) were attached to collagen-coated plates for 60 min. FoxO3a and Ser-253-phosphorylated FoxO3a expression levels were then measured. GAPDH was used as a loading control. E, 3,000 PTEN−/− and PTEN+/+ cells were incubated in DMEM and cell numbers were counted at 72 h as described under “Materials and Methods.” The results are representative of three different experiments. F, serum-starved lung fibroblasts infected with adenovirus expressing hyperactive Akt (Akt H) or dominant Akt (Akt DN, T308A, S473A) were seeded on collagen-coated plates for 30 min. FoxO3a, Ser-253 phosphorylated FoxO3a (p-FoxO3a), and Ser-473 phosphorylated Akt (p-Akt) levels were measured. GAPDH was used as a loading control.
To further examine the role of PTEN in regulating FoxO3a via Akt, we examined FoxO3a and Akt activity as a function of time in PTEN+/+ and PTEN−/− cells on collagen. Phosphorylated FoxO3a and phosphorylated Akt expression levels were high and remained relatively unaltered, whereas FoxO3a levels were low at all time points in PTEN−/− fibroblasts (Fig. 5C). In contrast, the expression levels of inactive FoxO3a were low and further decreased at 24 h in response to PTEN+/+ cell attachment to collagen. Likewise, phosphorylated Akt levels were also very low at all time points in PTEN+/+ cells. To confirm these results, we examined phosphorylated levels of FoxO3a in PTEN−/− fibroblasts infected with adenovirus expressing wild type PTEN, phosphatase-dead PTEN, or empty vector on collagen matrices. Phosphorylated FoxO3a expression levels were suppressed when wild type PTEN was expressed, whereas phosphorylated FoxO3a levels were high when mutant PTEN or empty vector was expressed (Fig. 5D), showing that PTEN increases FoxO3a activity by suppressing FoxO3a phosphorylation. Our data strongly suggest that the absence of PTEN increases phosphorylated FoxO3a via Akt on collagen. Because the absence of PTEN increases Akt activity, which in turn suppresses FoxO3a function, we conducted a fibroblast proliferation assay by counting PTEN−/− and PTEN+/+ cells at 72 h in DMEM. PTEN−/− cells were increased more than 5-fold compared with that of PTEN+/+ cells, showing that the absence of PTEN greatly promoted fibroblast proliferation (24, 25) (Fig. 5E). To prove that Akt increases FoxO3a phosphorylation in our model, FoxO3a activity was examined in lung fibroblasts infected with adenovirus expressing hyperactive wild type Akt (Akt H) or dominant-negative Akt (Akt DN) on collagen matrix. Phosphorylated FoxO3a levels were high when hyperactive Akt was expressed, whereas dominant-negative Akt suppressed FoxO3a phosphorylation (Fig. 5F, upper panel). In contrast, the FoxO3a level was low when hyperactive Akt was expressed, whereas the FoxO3a level was high when the Akt dominant-negative protein was expressed (Fig. 5F, lower panel). These data demonstrate that Akt modulates FoxO3a activity on collagen.
PP2A Modulates FoxO3a
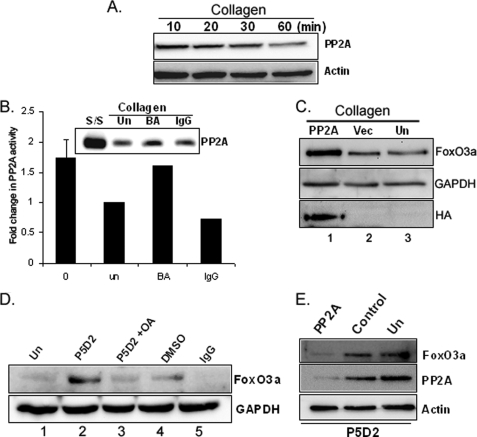
Our data indicates that the integrin/PI3K/Akt signaling pathway inhibits FoxO3a. Several prior studies suggest that Ser/Thr phosphatase PP2A can activate FoxO3a (14–16). To begin to explore whether PP2A also regulates FoxO3a function in response to fibroblast interaction with collagen, we first measured the PP2A catalytic subunit expression levels and activity in response to fibroblast attachment to collagen-coated plates as a function of time. We found that PP2A expression was decreased in response to lung fibroblast attachment to collagen as a function of time (Fig. 6A), suggesting that collagen-β1-integrin interaction suppresses PP2A function. To confirm these results, we further examined PP2A expression levels and activity in the presence or absence of the β1-integrin blocking antibody P5D2 on collagen-coated plates. PP2A levels and activity decreased when fibroblasts were attached to collagen-coated plates or in the presence of isotype control antibody (Fig. 6B, un and IgG lanes). However, when fibroblasts were preincubated with β1-integrin blocking antibody, PP2A expression and activity were high on collagen matrices (Fig. 6B, BA lane). These data demonstrate that during fibroblast interaction with collagen via β1-integrin, PP2A expression and activity decrease.
FIGURE 6.
PP2A regulates FoxO3a on collagen matrix. A, serum-starved human lung fibroblasts were attached to collagen-coated plates (100 μg/ml) as a function of time and PP2A catalytic subunit expression levels were measured. Actin was used as a loading control. B, serum-starved lung fibroblasts were preincubated in the presence or absence of β1-integrin blocking antibody (1 μg/ml, BA) for 45 min. Cells were then attached to collagen-coated plates for 30 min. Western analysis was carried out to measure PP2A catalytic subunit expression (upper panel). PP2A activity was measured as described under “Materials and Methods” (lower panel). s/s, serum-starved cells; Un, untreated cells; BA, cells preincubated with β1-integrin blocking antibody; IgG, isotype control. C, lung fibroblasts infected with adenovirus expressing PP2A catalytic subunit or empty vector were serum-starved for 1 day. Cells were then attached to collagen-coated plates for 60 min. FoxO3a and HA-tagged proteins were then measured. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. PP2A, adenovirus expressing HA-tagged PP2A catalytic subunit; Vec, adenovirus expressing empty vector; Un, untreated cells; HA, HA-tagged antibody. D, serum-starved lung fibroblasts were preincubated with β1-integrin blocking antibody (1 μg/ml) in the presence or absence of 100 nm okadaic acid for 1 h. Cells were then attached to type I collagen for 30 min. FoxO3a expression levels were measured. GAPDH was used as a loading control. Un, untreated cells; P5D2, β1-integrin blocking antibody; DMSO, dimethyl sulfoxide control; OA, okadaic acid; IgG, isotype control. E, human lung fibroblasts were transfected with 10 nm PP2A siRNA (PP2A) or control siRNA (Control) along with untransfected (Un) cells followed by serum starvation for 1 day. Cells were then preincubated with β1-integrin blocking antibody (P5D2, 1 μg/ml) for 45 min and attached to collagen-coated plates for 30 min. FoxO3a and PP2A expression were then measured. Actin was used as a loading control.
To determine whether FoxO3a expression is regulated by PP2A in response to fibroblast interaction with type I collagen, lung fibroblasts were infected with adenovirus expressing HA-tagged PP2A catalytic subunit and FoxO3a protein levels were measured. FoxO3a levels were high in fibroblasts overexpressing the PP2A catalytic subunit (Fig. 6C, lane 1). In contrast, FoxO3a protein was lower in fibroblasts uninfected or infected with virus expressing empty vector (lanes 2 and 3). We have shown that PP2A expression and activity increase when fibroblast interaction with collagen is inhibited by β1-integrin blocking antibody. We next sought to determine whether FoxO3a expression is regulated by PP2A in response to fibroblast interaction with collagen via β1-integrin. To address this question, we utilized β1-integrin blocking antibody to inhibit the β1-integrin-collagen interaction in the presence of the PP2A inhibitor, okadaic acid. Serum-starved lung fibroblasts were preincubated with β1-integrin blocking antibody in the presence or absence of okadaic acid and FoxO3a expression was measured on collagen. FoxO3a expression was high in the presence of β1-integrin blocking antibody on collagen (Fig. 6D, lane 2). However, FoxO3a expression was low when fibroblasts were preincubated with β1-integrin blocking antibody (P5D2) in the presence of the PP2A inhibitor, okadaic acid (Fig. 6D, lane 3). These data suggest that PP2A modulates FoxO3a function via β1-integrin. To confirm that PP2A inhibition suppresses FoxO3a via β1-integrin, PP2A was silenced by the catalytic subunit siRNA and FoxO3a expression was measured in the presence of β1-integrin blocking antibody (P5D2) on collagen (Fig. 6E). The FoxO3a level was high when control siRNA or untransfected cells were attached to collagen in the presence of the β1-integrin blocking antibody. In contrast, the FoxO3a level was low when PP2A protein was suppressed by PP2A siRNA in the presence of the β1-integrin blocking antibody. Taken together, these data demonstrate that PP2A regulates FoxO3a protein expression via β1-integrin.
Caspase-3 Promotes FoxO3a Cleavage
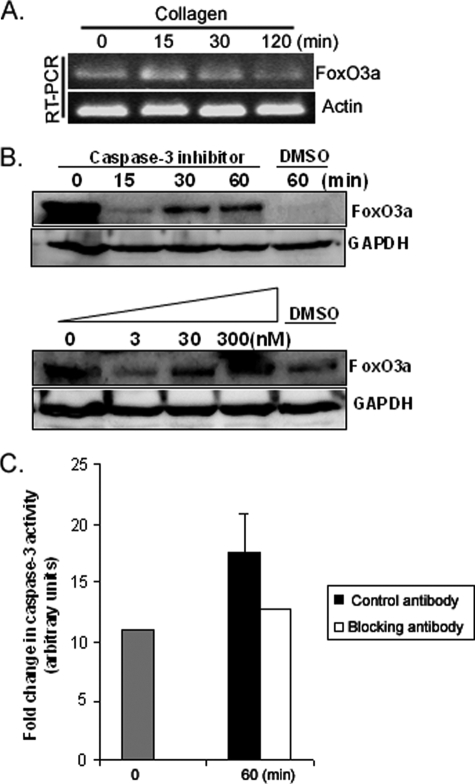
We have demonstrated that FoxO3a protein expression decreases when fibroblasts adhere to type I collagen. To begin to examine the mechanism for this decrease in FoxO3a protein levels, we examined FoxO3a mRNA levels. RT-PCR analysis showed that FoxO3a mRNA levels were unaltered (Fig. 7A), suggesting that the decrease of FoxO3a protein expression was not due to the alteration of mRNA expression. We next performed an amino acid sequence analysis of FoxO3a using the web-based PEST-FIND program. We have determined that FoxO3a contains two PEST sequences in its N-terminal domain (amino acids 1–26 and 46–72). Furthermore, we have determined that FoxO3a contains six potential caspase-3 recognition sites (DXXD), three of which are located in the second FoxO3a PEST sequence (62–65, 63–66, and 65–68). This suggests that cleavage of FoxO3a by caspase-3 may be responsible for the fall in FoxO3a protein levels that occur when fibroblasts attach to collagen. To test if this was the case, we pretreated fibroblasts with the caspase-3 inhibitor followed by seeding fibroblasts on type I collagen. FoxO3a protein expression was modestly stabilized by pretreatment with the caspase-3 inhibitor, whereas the FoxO3a protein level was undetectable in control DMSO-treated cells (Fig. 7B, upper panel). Moreover FoxO3a protein expression was preserved with the highest dose of caspase-3 inhibitor (300 nm, Fig. 7B, lower panel). We next measured caspase-3 activity in response to fibroblast adhesion to collagen in the presence or absence of the β1-integrin blocking antibody. Caspase-3 activity increased when fibroblasts were attached to collagen in the presence of mouse IgG control antibody (Fig. 7C). In contrast, caspase-3 activity was unaltered when fibroblasts were attached to collagen matrix in the presence of the β1-integrin blocking antibody. Our data suggest that fibroblast attachment to collagen inhibits FoxO3a protein expression via a caspase-3-dependent mechanism.
FIGURE 7.
Cell attachment to collagen promotes caspase-3-dependent cleavage of FoxO3a. A, RT-PCR was carried out with primers designed for FoxO3a as described under “Materials and Methods.” Actin was used as a loading control. B, upper panel, serum-starved human lung fibroblasts were pretreated with caspase-3 inhibitor (30 nm) for 60 min. Cells were then attached to type I collagen as a function of time. FoxO3a levels were measured. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. DMSO, control. Lower panel, serum-starved human lung fibroblasts were pretreated with different doses of caspase-3 inhibitor (3 to 300 nm) for 60 min. Cells were then attached to type I collagen for 30 min. FoxO3a levels were then measured. GAPDH was used as a loading control. DMSO, control. C, serum-starved human lung fibroblasts were pretreated with β1-integrin blocking antibody (1 μg/ml) or isotype control antibody IgG (1 μg/ml) for 45 min. Cells were then attached to type I collagen (100 μg/ml) for 60 min. Caspase-3 activity assay was performed as described under “Materials and Methods.”
Activation of Integrin Inhibits FoxO3a, thereby Suppressing p27
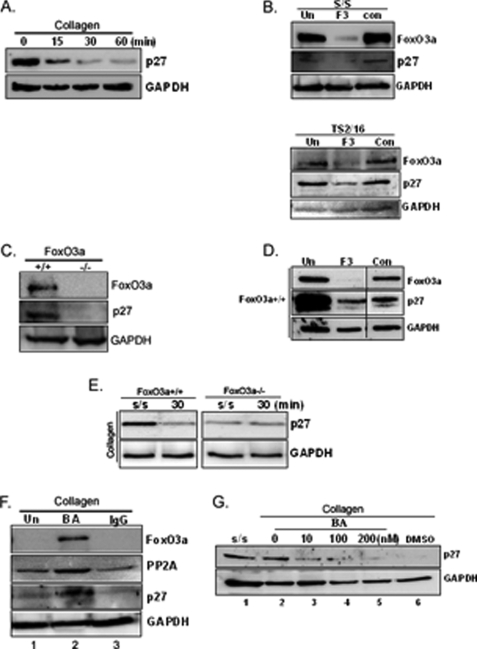
Activated FoxO3a inhibits G1 to S cell cycle progression by up-regulating the expression of p27, a cyclin-dependent kinase inhibitor protein (12, 13). Because FoxO3a expression decreases when fibroblasts attach to collagen, these data suggested that p27 expression may also be suppressed, facilitating cell cycle progression. To examine this issue, we measured the p27 expression level in fibroblasts on collagen. Like the expression levels of FoxO3a and PP2A, p27 expression progressively decreased when fibroblasts were attached to collagen-coated plates as a function of time (Fig. 8A). To confirm that p27 is a downstream target of FoxO3a, FoxO3a was silenced in serum-starved fibroblasts by FoxO3a siRNA. The p27 expression level was low when FoxO3a expression was suppressed by human FoxO3a siRNA (Fig. 8B, upper panel). The p27 expression level was also low when fibroblasts were ligated with β1-integrin activating antibody in the presence of FoxO3a siRNA (lower panel). To confirm this, we utilized FoxO3a−/− and FoxO3a+/+ mouse embryonic fibroblasts. The absence of FoxO3a conferred very low p27 expression in FoxO3a−/− cells compared with FoxO3a+/+ cells (Fig. 8C), demonstrating that the presence or absence of FoxO3a affects p27 expression. Similar to the result of p27 levels in FoxO3a−/− cells, knockdown of FoxO3a in FoxO3a+/+ fibroblasts decreased p27 expression (Fig. 8D). These data demonstrated that FoxO3a regulates p27. To verify that fibroblast attachment to collagen modulates p27 via FoxO3a, we further explored p27 expression in FoxO3a−/− and FoxO3a+/+ cells on collagen matrices as a function of time. Because FoxO3a regulates p27 expression, the absence of FoxO3a in null cells may result in a loss of regulation of p27 expression. As we expected, p27 expression was decreased in the attachment of FoxO3a+/+ cells to collagen, whereas the level of p27 was low and unaltered in FoxO3a−/− cells as a function of time (Fig. 8E).
FIGURE 8.
FoxO3a increases p27 on the collagen matrix. A, serum-starved human lung fibroblasts were attached to collagen-coated plates as a function of time and p27 expression levels were measured. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. B, upper, FoxO3a siRNA (10 nm) transfected lung fibroblasts were serum-starved (s/s), and Western analysis was carried out to measure FoxO3a and p27 levels. GAPDH was used as a loading control. Lower, lung fibroblasts were ligated with β1-integrin activating antibody (TS2/16, 3 μg/ml) for 30 min in the presence or absence of FoxO3a siRNA. Western analysis was then carried out with antibodies as indicated. F3, FoxO3a siRNA; con, control siRNA; Un, untransfected cells. C, FoxO3a+/+ and Foxo3−/− cells were serum-starved for 1 day, and FoxO3a and p27 expression levels were measured using Western analysis. GAPDH was used as a loading control. D, FoxO3a+/+ cells were transfected with mouse FoxO3a siRNA (F3, 10 nm) or control siRNA (Con, 10 nm) and Western analysis was then carried out to measure FoxO3a and p27 expression levels. GAPDH was used as a loading control. Un, untransfected cells. E, serum-starved FoxO3a+/+ and FoxO3a−/− cells were attached to type I collagen for 30 min. p27 expression levels were then measured. GAPDH was used as a loading control. s/s, serum-starved fibroblasts. F, serum-starved human lung fibroblasts were preincubated with either β1-integrin blocking antibody (1 μg/ml) or isotype control mouse IgG (1 μg/ml) for 45 min. Cells were then attached to collagen-coated plates for 30 min. FoxO3a, PP2A, and p27 expression levels were then measured. GAPDH was used as a loading control. Un, untreated cells; BA, β1-integrin blocking antibody pretreated cells. G, serum-starved human lung fibroblasts were preincubated with β1-integrin blocking antibody (1 μg/ml) in the presence of 10, 100, or 200 nm or absence of okadaic acid for 60 min. Cells were then attached to type I collagen for 30 min. Western analysis was carried out to measure p27 expression levels. GAPDH was used as a loading control. s/s, serum-starved human lung fibroblasts; DMSO, control.
We have found that when fibroblasts attach to collagen via β1-integrin, PP2A activity decreases and this facilitates the decrease in FoxO3a expression. We next sought to determine whether this also facilitates a decrease in p27 levels. To address this issue, serum-starved human lung fibroblasts were preincubated with β1-integrin blocking antibody and seeded on collagen-coated plates. As shown, PP2A expression decreased when lung fibroblasts were attached to collagen in the absence of β1-integrin blocking antibody (Fig. 8F, lane 1). In contrast, PP2A levels remained high when cells preincubated with β1-integrin blocking antibody (BA) were plated on collagen (lane 2). Likewise, FoxO3a and p27 expression levels were also increased in the presence of β1-integrin blocking antibody. These results suggested that the inhibition of cell attachment to collagen by β1-integrin blocking antibody up-regulates p27 via enhanced PP2A/FoxO3a function. To confirm that PP2A facilitates activation of p27 via FoxO3a, we utilized β1-integrin blocking antibody and okadaic acid. We measured p27 levels in fibroblasts seeded on collagen in the presence of the β1-integrin blocking antibody and varying doses of okadaic acid. Similar to FoxO3a expression, we found that p27 levels were high when fibroblasts were serum-starved or plated on collagen in the presence of β1-integrin blocking antibody (Fig. 8G, lanes 1 and 2). However, p27 expression was progressively decreased when fibroblasts attached to collagen in the presence of increasing doses of okadaic acid (Fig. 8G, lanes 3–5). To confirm this result, we measured p27 expression when PP2A siRNA was utilized in the presence of β1-integrin blocking antibody on collagen (supplemental Fig. S1F). Like the case of okadaic acid, p27 expression was low when PP2A was silenced, whereas p27 levels were high when control siRNA was used. These results showed that β1-integrin plays a crucial role in modulating p27 via PP2A/FoxO3a. Our data suggest that the maximum inhibition of FoxO3a requires the coordination of PP2A inhibition and Akt activation in a synergistic fashion in response to fibroblast attachment to type I collagen.
FoxO3a Suppresses Fibroblast Proliferation
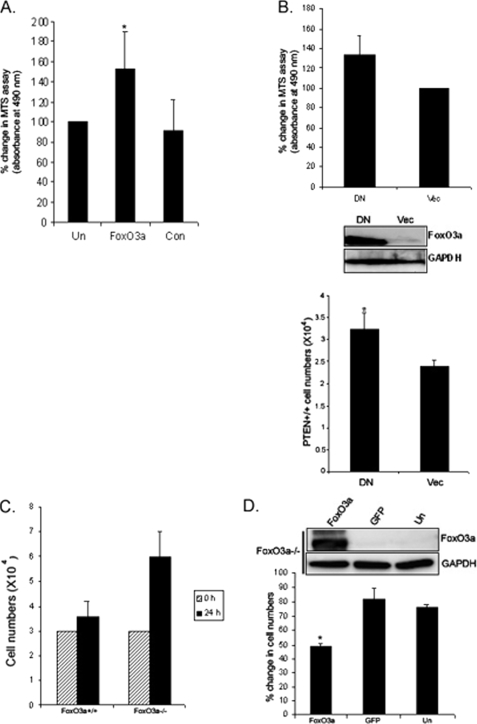
We have previously shown that fibroblast attachment to collagen promotes fibroblast proliferation (26, 27). In this study, we showed that β1-integrin-collagen interaction suppresses FoxO3a, thereby inhibiting p27. These data suggest that FoxO3a inhibition promotes fibroblast proliferation on type I collagen. To address this issue, we first examined fibroblast proliferation on type I collagen in the presence or absence of FoxO3a siRNA. Human lung fibroblasts were transfected with FoxO3a siRNA or control siRNA and an MTS assay was conducted as described under “Materials and Methods.” Fibroblasts transfected with FoxO3a siRNA showed an ∼50% increase in fibroblast proliferation compared with that of control siRNA-transfected cells (Fig. 9A). Our data showed that FoxO3a inhibits fibroblast proliferation. To examine whether the precise modulation of FoxO3a can alter proliferation, we utilized adenovirus expressing dominant-negative FoxO3a in PTEN+/+ cells. Although PTEN+/+ cells had high FoxO3a expression, the inhibition of FoxO3a in this experimental setting can demonstrate the role of FoxO3a in modulating fibroblast proliferation. An MTS assay showed that there was a 33% increase of absorbance at 490 nm in cells infected with dominant-negative FoxO3a, indicating that inhibition of FoxO3a promotes cell proliferation (Fig. 9B, upper graph). We further measured PTEN+/+ cell numbers at 48 h postinfection of FoxO3a dominant-negative and empty vector. PTEN+/+ cell numbers increased (∼27%) when cells were infected with dominant-negative FoxO3a compared with that of empty vector (Fig. 9B, lower graph). These data demonstrated that the alteration of FoxO3a can affect fibroblast proliferation. We also analyzed the proliferation of FoxO3a−/− and FoxO3a+/+ cells on collagen matrices. After a 24-h culture on collagen-coated plates, FoxO3a−/− cell numbers were substantially higher than wild type cells (Fig. 9C). We further analyzed FoxO3a function in modulation of fibroblast proliferation by overexpression of FoxO3a using an adenovirus vector containing FoxO3a protein in FoxO3a−/− cells. FoxO3a−/− cells were infected with adenovirus expressing wild type FoxO3a or empty vector plated on type I collagen, and cell numbers were determined. Overexpression of FoxO3a substantially decreased fibroblast proliferation on type I collagen compared with control (empty vector or uninfected cells, Fig. 9D). Taken together, these data demonstrated that FoxO3a function is crucial in promoting fibroblast proliferation on collagen matrix and integrin-collagen interaction suppresses FoxO3a, thereby promoting fibroblast proliferation.
FIGURE 9.
FoxO3a suppresses cell proliferation. A, FoxO3a siRNA or control siRNA-transfected human lung fibroblasts were grown in 96-well plates and an MTS assay was carried out as described under “Materials and Methods.” Note that FoxO3a siRNA-transfected fibroblasts had an enhanced proliferation rate compared with that of control siRNA or untransfected cells. *, p < 0.03 versus control siRNA. B, PTEN+/+ cells infected with adenovirus expressing dominant-negative FoxO3a (DN) or empty vector (Vec) were grown in 96-well plates and an MTS assay was carried out (upper panel). Dominant-negative FoxO3a expression was evaluated using Western analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control (middle panel). PTEN+/+ cells infected with adenovirus expressing dominant-negative FoxO3a or empty vector were incubated with DMEM and cells numbers were measured at 24 h (lower panel). *, p < 0.05 versus empty vector. C, 3 × 104 of FoxO3a+/+ and FoxO3a−/− cells were serum-starved for 1 day followed by attachment to type I collagen-coated plates for 24 h. Cells numbers were then measured. D, FoxO3a−/− cells were infected with adenovirus expressing wild type FoxO3a (FoxO3a) or empty vector (GFP) along with uninfected (Un) cells for 24 h. 1.2 × 104 of Foxo3a−/− fibroblasts were then plated on tissue culture plates in the presence of 0.5% serum for 72 h. Cell numbers were then measured using a Coulter counter. Upper, Western analysis of FoxO3a protein expression in FoxO3a−/− cells infected with adenovirus expressing FoxO3a wild type or empty vector or untransfected cells. GAPDH was used as a loading control. Lower, % change in cell numbers in FoxO3a−/− cells infected with adenovirus expressing FoxO3a wild type or empty vector or untransfected cells. *, p < 0.03 versus GFP empty vector.
DISCUSSION
Integrin-ECM interaction promotes fibroblast proliferation via activation of the PI3K/pathway (19, 20). The FoxO3a transcriptional activator inhibits cell proliferation by up-regulating the expression of the cyclin-dependent kinase inhibitor p27. Moreover, activated Akt can phosphorylate and inactivate FoxO3a. However, it is not known whether integrin-ECM interaction can modulate FoxO3a function. Here we demonstrate that integrin-ECM interaction suppresses FoxO3a function via high Akt and the inhibition of PTEN and PP2A. The mechanism is complex and involves the coordinated activation of PI3K/Akt with suppression of PTEN and PP2A phosphatase. We found that fibroblast attachment to collagen results in the promotion of PTEN degradation. This results in the preferential phosphorylation and inactivation of FoxO3a via high Akt activity. We also demonstrate that integrin-ECM interaction promotes a decrease in FoxO3a protein expression. We have found that 2 PEST sequences are located in the FoxO3a N terminus. 3 putative caspsase-3 cleavage sites are located in the second PEST sequence. We show that inhibition of caspase 3 attenuates the decrease in FoxO3a protein expression in response to the fibroblast-matrix interaction. Thus we have identified a novel basic mechanism by which the integrin-matrix interaction is capable of down-regulation of the FoxO3a activity, permitting fibroblast proliferation via the PTEN/PI3K/Akt and PP2A-dependent pathways.
Our studies demonstrate that cell attachment to specific extracellular matrices promotes different degrees of FoxO3a suppression. For example, we have found that when fibroblasts attach to type I collagen or fibronectin, FoxO3a levels rapidly decrease. To a lesser extent, FoxO3a protein expression was rather slightly decreased as a function of time on vitronectin-coated plates. These data suggest that FoxO3a activity is dependent upon cell attachment to specific ECM. Cells preferentially use certain integrins to attach to specific matrices. Cells bearing the α2β1-integrin attach and spread on type I collagen. We found that blockage of β1-integrin function by β1-integrin blocking antibody preferentially inhibits FoxO3a suppression in response to fibroblast attachment to type I collagen, showing that β1-integrin-type I collagen interaction is required for FoxO3a inhibition. FoxO3a inhibitory mechanisms have previously been examined and suggested that Akt suppresses FoxO3a by phosphorylating several Ser/Thr residues (29). Among them Ser-253 is known to be a surrogate marker of FoxO3a activity and studies showed that the Ser-253 residue is preferentially phosphorylated by Akt (21) and its phosphorylation promotes FoxO3a translocation to the cytoplasm from the nucleus, thereby inhibiting its function. We have found that the PI3K inhibitor, wortmannin, and Akt inhibitor stabilize FoxO3a expression levels in response to fibroblast attachment to type I collagen, suggesting that FoxO3a is regulated by PI3K/Akt in our model. Utilizing adenovirus expressing hyperactive and dominant Akt in fibroblasts demonstrated that Akt suppresses FoxO3a function on collagen. Our data also showed that the modulation of FoxO3a is not due to the transcriptional change but rather protein stability is the mechanism of FoxO3a suppression.
Our results suggest a model where integrin-ECM interaction promotes the rapid inhibition of FoxO3a by Akt via PTEN. We have previously found that low PTEN activity increases Akt phosphorylation, promoting cell proliferation. In this experiment, we showed that integrin-collagen interaction in lung fibroblasts suppresses PTEN function, thereby inhibiting FoxO3a via high Akt activity. PTEN−/− fibroblasts further demonstrated that the absence of PTEN suppressed FoxO3a expression and activity via Akt. Because fibroblast attachment to collagen activates Akt via low PTEN, we also examined FoxO3a phosphorylation levels in response to lung fibroblast attachment to collagen. We have found that Ser-253 levels remained unaltered, whereas total FoxO3a expression levels decreased when fibroblasts were attached to collagen-coated plates. These data demonstrated that FoxO3a activity decreases when fibroblasts are attached to collagen. Utilizing β1-integrin activating or β1-integrin blocking antibody along with a PI3K or Akt inhibitor also showed that β1-integrin plays a crucial role in modulating FoxO3a activity via the PI3K/Akt pathway. Because p27 is a direct transcriptional target of FoxO3a, β1-integrin-ECM interaction facilitates fibroblast proliferation at least in part via the PTEN/PI3K/Akt/FoxO3a/p27 regulatory pathway.
Prior studies suggest that PP2A dephosphorylates FoxO3a, activating its function by translocating FoxO3a into the nucleus. Because FoxO3a protein expression was rapidly decreased on collagen, we predicted that PP2A function may be abolished in response to fibroblast attachment to collagen. We found that PP2A expression and activity were also suppressed as a function of time via β1-integrin. Likewise, the cell cycle inhibitor protein p27 decreased when fibroblasts were attached to collagen matrices. In contrast, PP2A overexpression or in the presence of β1-integrin blocking antibody on collagen restored FoxO3a and p27 expression, suggesting that PP2A modulates p27 via FoxO3a. Thus, our data indicate that the precise orchestration of PP2A inhibition and Akt activation via low PTEN can confer the maximum inhibition of FoxO3a activity.
We found that FoxO3a contains 2 PEST sequences in the N-terminal domain of FoxO3a. Interestingly, there are 3 caspase-3 recognition sites within the PEST sequences. Dose and time-dependent assays in the presence or absence of FoxO3a inhibitor suggests that FoxO3a function is inhibited by caspase-3. Furthermore, caspase-3 activity increased when fibroblasts were attached to collagen, whereas β1-integrin blocking antibody abolished caspase-3 activity. These data showed that the integrin-ECM interaction may increase caspase-3 activity, promoting the FoxO3a cleavage process. A prior study showed that FoxO3a is inhibited via MDM2 (murine double minute 2)-mediated degradation (28). Fu and Yang (35, 36) also previously reported that MDM2 acts as an ubiquitin E3 ligase to regulate the degradation of FoxO3a. Thus, a plausible scenario is that Ser-253 phosphorylation of FoxO3a promotes its translocation to the cytoplasm from the nucleus and facilitates its cleavage by caspase-3 and/or polyubiquitination by E3 ligase, which promotes its degradation. In summary, we have found a novel FoxO3a inhibitory mechanism utilized by the fibroblast-ECM interaction via β1-integrin/PTEN/PI3K/Akt and a PP2A-dependent pathway. We showed that fibroblast attachment to ECM inhibits FoxO3a function via coordination of PTEN/PI3K/Akt and PP2A, promoting fibroblast proliferation.
Supplementary Material
Acknowledgments
We thank Dr. Noboru Motoyama, National Institute for Longevity Sciences, Japan, for FoxO3a−/− and FoxO3a+/+ mouse embryonic fibroblasts and Dr. Deane Mosher, University of Wisconsin-Madison, for PTEN−/− and PTEN+/+ cells for this research.
This work was supported by Scientist Development and Pulmonary Fibrosis Research grants from the American Heart Association and the American Lung Association (to R. S. N.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- ECM
- extracellular matrix
- FoxO
- forkhead box O
- PI3K
- phosphatidylinositol 3-kinase
- DMSO
- dimethyl sulfoxide
- DMEM
- Dulbecco's modified Eagle's medium
- ERK
- extracellular signal-regulated kinase
- RT
- reverse transcription
- GFP
- green fluorescent protein
- HA
- hemagglutinin
- MTS
- (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
- siRNA
- small interfering RNA
- DN
- dominant-negative
- PTEN
- the phosphatase and tensin homolog deleted on chromosome 10.
REFERENCES
- 1.Aoshiba K., Rennard S. I., Spurzem J. R. (1997) Am. J. Physiol. Lung Cell. Mol. Physiol. 272, L28–L37 [DOI] [PubMed] [Google Scholar]
- 2.Khwaja A., Rodriguez-Viciana P., Wennström S., Warne P. H., Downward J. (1997) EMBO J. 16, 2783–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X., Ye X., Yanoff M., Li W. (1997) Exp. Eye Res. 65, 117–126 [DOI] [PubMed] [Google Scholar]
- 4.Jokinen J., Dadu E., Nykvist P., Käpylä J., White D. J., Ivaska J., Vehviläinen P., Reunanen H., Larjava H., Häkkinen L., Heino J. (2004) J. Biol. Chem. 279, 31956–31963 [DOI] [PubMed] [Google Scholar]
- 5.Wallner E. I., Yang Q., Peterson D. R., Wada J., Kanwar Y. S. (1998) Am. J. Physiol. 275, F467–F477 [DOI] [PubMed] [Google Scholar]
- 6.Upadhyay J., Aitken K. J., Damdar C., Bolduc S., Bagli D. J. (2003) J. Urol. 169, 750–755 [DOI] [PubMed] [Google Scholar]
- 7.Lee J. W., Juliano R. L. (2000) Mol. Biol. Cell 11, 1973–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusuf I., Zhu X., Kharas M. G., Chen J., Fruman D. A. (2004) Blood 104, 784–787 [DOI] [PubMed] [Google Scholar]
- 9.Nakao T., Geddis A. E., Fox N. E., Kaushansky K. (2008) Cell Cycle 7, 257–266 [DOI] [PubMed] [Google Scholar]
- 10.Yang J. Y., Xia W., Hu M. C. (2006) Int. J. Oncol. 29, 643–648 [PMC free article] [PubMed] [Google Scholar]
- 11.Behzad H., Jamil S., Denny T. A., Duronio V. (2007) Cytokine 38, 74–83 [DOI] [PubMed] [Google Scholar]
- 12.Chahdi A., Sorokin A. (2008) Mol. Biol. Cell 19, 2609–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y., Fei M., Wang Y., Lu M., Cheng C., Shen A. (2008) Eur. J. Haematol. 81, 83–93 [DOI] [PubMed] [Google Scholar]
- 14.Singh A., Ye M., Bucur O., Zhu S., Tanya Santos M., Rabinovitz I., Wei W., Gao D., Hahn W. C., Khosravi-Far R. (2010) Mol. Biol. Cell 21, 1140–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertoli C., Copetti T., Lam E. W., Demarchi F., Schneider C. (2009) Oncogene 28, 721–733 [DOI] [PubMed] [Google Scholar]
- 16.Kuo Y. C., Huang K. Y., Yang C. H., Yang Y. S., Lee W. Y., Chiang C. W. (2008) J. Biol. Chem. 283, 1882–1892 [DOI] [PubMed] [Google Scholar]
- 17.Huang H., Tindall D. J. (2007) J. Cell Sci. 120, 2479–2487 [DOI] [PubMed] [Google Scholar]
- 18.Choe G., Horvath S., Cloughesy T. F., Crosby K., Seligson D., Palotie A., Inge L., Smith B. L., Sawyers C. L., Mischel P. S. (2003) Cancer Res. 63, 2742–2746 [PubMed] [Google Scholar]
- 19.Nho R. S., Xia H., Kahm J., Kleidon J., Diebold D., Henke C. A. (2005) J. Biol. Chem. 280, 26630–26639 [DOI] [PubMed] [Google Scholar]
- 20.Nho R. S., Xia H., Diebold D., Kahm J., Kleidon J., White E., Henke C. A. (2006) J. Biol. Chem. 281, 33291–33301 [DOI] [PubMed] [Google Scholar]
- 21.Brunet A., Park J., Tran H., Hu L. S., Hemmings B. A., Greenberg M. E. (2001) Mol. Cell. Biol. 21, 952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian B., Lessan K., Kahm J., Kleidon J., Henke C. A. (2002) J. Biol. Chem. 277, 24667–24675 [DOI] [PubMed] [Google Scholar]
- 23.Depraetere H., Wille C., Gansemans Y., Stanssens P., Lauwereys M., Baruch D., De Reys S., Deckmyn H. (1997) Thromb. Haemost. 77, 981–985 [PubMed] [Google Scholar]
- 24.Diao L., Chen Y. G. (2007) Cell Res. 17, 291–292 [DOI] [PubMed] [Google Scholar]
- 25.Radu A., Neubauer V., Akagi T., Hanafusa H., Georgescu M. M. (2003) Mol. Cell. Biol. 23, 6139–6649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia H., Nho R., Kleidon J., Kahm J., Henke C. A. (2008) J. Biol. Chem. 283, 20350–20360 [DOI] [PubMed] [Google Scholar]
- 27.Xia H., Diebold D., Nho R., Perlman D., Kleidon J., Kahm J., Avdulov S., Peterson M., Nerva J., Bitterman P., Henke C. A. (2008) J. Exp. Med. 205, 1659–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J. Y., Zong C. S., Xia W., Yamaguchi H., Ding Q., Xie X., Lang J. Y., Lai C. C., Chang C. J., Huang W. C., Huang H., Kuo H. P., Lee D. F., Li L. Y., Lien H. C., Cheng X., Chang K. J., Hsiao C. D., Tsai F. J., Tsai C. H., Sahin A. A., Muller W. J., Mills G. B., Yu D., Hortobagyi G. N., Hung M. C. (2008) Nat. Cell. Biol. 10, 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng W. H., Kar S., Quirion R. (2000) J. Biol. Chem. 275, 39152–39158 [DOI] [PubMed] [Google Scholar]
- 30.Nakae J., Barr V., Accili D. (2000) EMBO J. 19, 989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rena G., Guo S., Cichy S. C., Unterman T. G., Cohen P. (1999) J. Biol. Chem. 274, 17179–17183 [DOI] [PubMed] [Google Scholar]
- 32.Nakae J., Park B. C., Accili D. (1999) J. Biol. Chem. 274, 15982–15985 [DOI] [PubMed] [Google Scholar]
- 33.Tang E. D., Nuñez G., Barr F. G., Guan K. L. (1999) J. Biol. Chem. 274, 16741–16746 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt M., Fernandez, de Mattos S., van der Horst A., Klompmaker R., Kops G. J., Lam E. W., Burgering B. M., Medema R. H. (2002) Mol. Cell. Biol. 22, 7842–7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu W., Ma Q., Chen L., Li P., Zhang M., Ramamoorthy S., Nawaz Z., Shimojima T., Wang H., Yang Y., Shen Z., Zhang Y., Zhang X., Nicosia S. V., Zhang Y., Pledger J. W., Chen J., Bai W. (2009) J. Biol. Chem. 284, 13987–14000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang W., Dolloff N. G., El-Deiry W. S. (2008) Nat. Cell Biol. 10, 125–126 [DOI] [PubMed] [Google Scholar]
- 37.Valiente M., Andrés-Pons A., Gomar B., Torres J., Gil A., Tapparel C., Antonarakis S. E., Pulido R. (2005) J. Biol. Chem. 280, 28936–28943 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.