Abstract
Acyl-CoA:cholesterol O-acyl transferase 2 (ACAT2) promotes cholesterol absorption by the intestine and the secretion of cholesteryl ester-enriched very low density lipoproteins by the liver. Paradoxically, mice lacking ACAT2 also exhibit mild hypertriglyceridemia. The present study addresses the unexpected role of ACAT2 in regulation of hepatic triglyceride (TG) metabolism. Mouse models of either complete genetic deficiency or pharmacological inhibition of ACAT2 were fed low fat diets containing various amounts of cholesterol to induce hepatic steatosis. Mice genetically lacking ACAT2 in both the intestine and the liver were dramatically protected against hepatic neutral lipid (TG and cholesteryl ester) accumulation, with the greatest differences occurring in situations where dietary cholesterol was elevated. Further studies demonstrated that liver-specific depletion of ACAT2 with antisense oligonucleotides prevents dietary cholesterol-associated hepatic steatosis both in an inbred mouse model of non-alcoholic fatty liver disease (SJL/J) and in a humanized hyperlipidemic mouse model (LDLr−/−, apoB100/100). All mouse models of diminished ACAT2 function showed lowered hepatic triglyceride concentrations and higher plasma triglycerides secondary to increased hepatic secretion of TG into nascent very low density lipoproteins. This work demonstrates that inhibition of hepatic ACAT2 can prevent dietary cholesterol-driven hepatic steatosis in mice. These data provide the first evidence to suggest that ACAT2-specific inhibitors may hold unexpected therapeutic potential to treat both atherosclerosis and non-alcoholic fatty liver disease.
Keywords: Cholesterol, Cholesterol Metabolism, Diet, Enzymes, Liver Injury, Triglyceride, Cholesteryl Ester, NAFLD, Steatosis
Introduction
Non-alcoholic fatty liver disease (NAFLD)4 is characterized by neutral lipid (triglyceride and cholesteryl ester (TG and CE)) accumulation in hepatocytes of patients with no history of chronic alcohol use (1, 2). Lipid droplets consisting of neutral lipids (triglyceride and cholesteryl ester) accumulate within the hepatocyte and can contribute 5–10% or more of the total mass of the liver (3). The causes of the lipid accumulation are not always clear, although a popular hypothesis is that excess free fatty acid availability to the liver in situations where energy excess prevails can lead to the condition (4). NAFLD is often asymptomatic and not regularly detected until progression into diseased fibrotic and/or inflamed non-alcoholic steatohepatitis and cirrhosis has occurred (5, 6). NAFLD is estimated to affect at least 20 and 5% of the general adult and child populations, respectively; it affects greater than 50% of the obese population in both age groups (3, 7). It is expected that as the prevalence of obesity and metabolic syndrome increases, NAFLD-associated diseases will be an increasing healthcare concern (8). Currently, there are no known effective treatments for NAFLD, although it is suggested that major changes in diet and exercise programs to reduce obesity could also diminish the severity and progression of NAFLD into non-alcoholic steatohepatitis and cirrhosis (2, 3, 5–9).
The major tissue cholesterol-esterifying enzyme, acyl-CoA:cholesterol O-acyl transferase 2 (ACAT2 also known as SOAT2, sterol O-acyltransferase) is found within lipoprotein-producing cells such as intestinal enterocytes and hepatocytes (10, 11). It has been previously documented that ACAT2 plays a critical role in the production of atherogenic apoB-containing lipoproteins (12) and that ACAT2-specific inhibitors are extremely effective in preventing murine atherosclerosis (13). In addition to direct alterations in cholesterol metabolism, mice lacking ACAT2 exhibit unexpected hypertriglyceridemia, indicating that either increased production or decreased clearance of VLDL TG is altered in these mice (15). Despite this, ACAT2 deletion is highly atheroprotective (14), and it is reasonable to assume that much of the atheroprotective benefit of limiting ACAT2 can be explained by the diminished packaging of hepatic CE into atherogenic VLDL particles.
Data from the present studies have uncovered an unexpected role for ACAT2 in hepatic TG metabolism. We have found that inhibition of ACAT2 not only has the expected outcome of lowering hepatic CE but also limits hepatic triglyceride accumulation in several mouse models of dietary cholesterol-induced NAFLD. Based on the rapidly increasing prevalence of obesity in Western societies, an increase in obesity co-morbidities seems likely, particularly including diseases with earlier onset in obesity development such as NAFLD (2). The etiology of development of the NAFLD process is unclear, and currently, the treatment options for NAFLD are few (9). Uncovering clues that could help decipher pathways in the progression of NAFLD could greatly facilitate both the prevention and the treatment of this disease. The present studies in mouse models provide support for the possibility that ACAT2-specific inhibitors could limit both atherosclerosis and NAFLD in humans.
EXPERIMENTAL PROCEDURES
Mice and Diets
Wild type (ACAT2+/+) and ACAT2 knock-out (ACAT2−/−) mice were generously provided by Dr. Robert Farese, Jr. (Gladstone Institute, San Francisco, CA) and were maintained on a mixed genetic background (62.5% C57BL/6, 25% 129 SvJae, 12.5% SvEv). Female ACAT2+/+ littermates were used as controls in all studies to minimize genetic heterogeneity. At 6 weeks of age, these mice were switched from a diet of rodent chow to a homemade low fat (10% of energy as palm oil) diet containing either 0.001% (w/w) or 0.2% (w/w) cholesterol, and they were maintained on this diet for 6 additional weeks. For antisense oligonucleotide (ASO) studies using inbred mouse strains (C57BL/6, C57L/J, SJL/J, SM/J, SWR/J), male and female mice were purchased from The Jackson Laboratory (Bar Harbor, ME) based on previous comparisons of atherosclerosis and hepatic lipid accumulation susceptibility (16). At 6 weeks of age, the inbred mice were switched from a diet of rodent chow to a low fat (10% of energy as palm oil) diet containing 0.2% (w/w) cholesterol, and they were maintained on this diet for 6 additional weeks. ASO-mediated knockdown in hyperlipidemic low density lipoprotein receptor-deficient (LDLr−/−), apolipoproteinB-100 only (apoB100/100) mice was carried out as described previously in mice consuming a low fat (20% of energy as palm oil) diet containing 0.1% (w/w) cholesterol for 8 weeks (17). All animals used in these studies were housed in an American Association for Accreditation of Laboratory Animals (AAALAC)-approved animal facility. Protocols for all studies were preapproved by the Wake Forest University Animal Care and Use Committee (ACUC).
ASO Treatment
Phosphorothioate-modified ASOs were obtained from ISIS Pharmaceuticals (Carlsbad, CA), and have been described previously (13, 17). Normolipidemic male SJL/J mice were randomly assigned to groups to receive treatment with either a control non-targeting ASO (control ASO; 5′-TCCCATTTCAGGAGACCTGG-3′) or an ASO specifically targeting ACAT2 (ACAT2 ASO; 5′-GCTCTAATCACCTCAGAACT-3′). Biweekly, intraperitoneal injections of 25 mg/kg/week of ASO were started at 6 weeks of age and were continued for 6 weeks in inbred (SJL/J) mice or for 8 weeks in hyperlipidemic (LDLr−/−, apoB100/100) mice.
Hepatic Lipid Measurements
Lipid extraction and biochemical determination of hepatic lipid mass were conducted as described previously (17–19). For histologic evaluation, frozen tissue samples were thawed into neutral buffered formalin for 48 h at 4 °C. Liver samples were then embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
ACAT2 Activity Assays and Western Blot Analysis
Total liver homogenates (17) and hepatic microsomal membranes (20, 21) were prepared as described previously from snap-frozen livers. Assays for ACAT2 activity were performed as described (17) using 50-μg aliquots of total protein from liver microsomal membrane suspensions. For immunoblotting of ACAT2 in SJL/J mouse livers, microsomes were used as a protein source, and Western blotting was conducted as described previously (17–19).
Quantitative Real Time PCR
RNA extraction and quantitative real time PCR were conducted as described previously (19). Primer pairs used are described in supplemental Table 1.
Plasma Lipid Analyses
Mice were fasted for 4 h prior to blood collection by submandibular bleed at the indicated time points. Detailed descriptions of plasma lipid and lipoprotein analyses have been provided previously (12, 17–19). For blood glucose measurements, mice were fasted for 6 h, and blood glucose was monitored using a commercial glucometer (Ascensia Contour, Bayer).
Isolated Liver Perfusion and Liver Lipid Secretion
Isolated recirculating liver perfusion was performed according to the protocol of Lee et al. (22) to estimate direct secretion rates of lipids in nascent apoB-containing lipoproteins.
Isotope Labeling of Hepatic and Secreted Lipids
Approximately 1 h prior to surgery, non-fasted mice were injected with an intraperitoneal 200-μl bolus containing 20 μCi of [3H]oleic acid in 5% bovine serum albumin with 0.75% cold oleic acid as a carrier for delivery of 3H-fatty acid to the liver (23) About 8% of the injected dose was found to be incorporated into liver lipids at 1 h after injection, and >85% of this was in triglycerides. Mice were returned to their cage until their perfusion surgery start time 1 h later, at which point they were anesthetized with isoflurane. After surgery and successful cannulation of the circulation into and out of the liver, perfusion at 1 ml/min with recirculating medium was initiated (22), and a constant infusion of [14C]oleic acid complexed to albumin was pumped into the perfusion medium at a fixed rate of ∼11,000 dpm per minute. Perfusate aliquots (1.5 ml at each time point) were taken at half-hour intervals, and fresh medium was added back to maintain a constant medium volume of 10 ml. The appearance rates of [3H]oleic acid and [14C]oleic acid in perfusate triglyceride was monitored as a function of hepatic triglyceride mobilization ([3H]oleate) versus new triglyceride synthesis ([14C]oleate).
Liver biopsies were taken at 1- and 2-h time points, and a sample was collected at the end of perfusion at 3 h. During surgical isolation of the liver, 4.0 suture silks were looped around two separate liver lobes and secured. At 1 h, one of the loops was tightened around the liver lobe (∼0.1 g) to constrict blood flow into the lobe, which was then removed with a pair of surgical scissors, and surgical glue was immediately applied to the cut site to prevent leakage. At 2 h of perfusion, a second lobe was isolated and removed. The timed liver biopsies were immediately weighed and snap-frozen in liquid nitrogen for later analyses to determine the extent of triglyceride labeling with [14C]oleate to estimate hepatic triglyceride synthesis rate during the experiment. The rate of TG mobilization was calculated from the [3H]oleate appearance in perfusate TG, whereas the rate of new synthesis and deposition was calculated from the [14C]oleate kinetics of appearance in hepatic TG. At the completion of the 3-h experiment, all perfusion medium was collected, and the liver was weighed and snap-frozen in liquid nitrogen. Liver and perfusate lipids were extracted and analyzed as above. Recovery of isotope-labeled lipids was determined after separation of lipid extracts on thin layer chromatography (TLC) plates using a solvent system of hexane:ethyl ether:acetic acid (70:30:1) with quantification of the amount of isotopically labeled lipid in a liquid scintillation counter.
RESULTS
In Addition to Lowering Hepatic Cholesteryl Ester Accumulation, Disruption of ACAT2 Lowers Hepatic Triglyceride Accumulation while Raising Plasma Triglyceride in a Dietary Cholesterol-dependent Manner
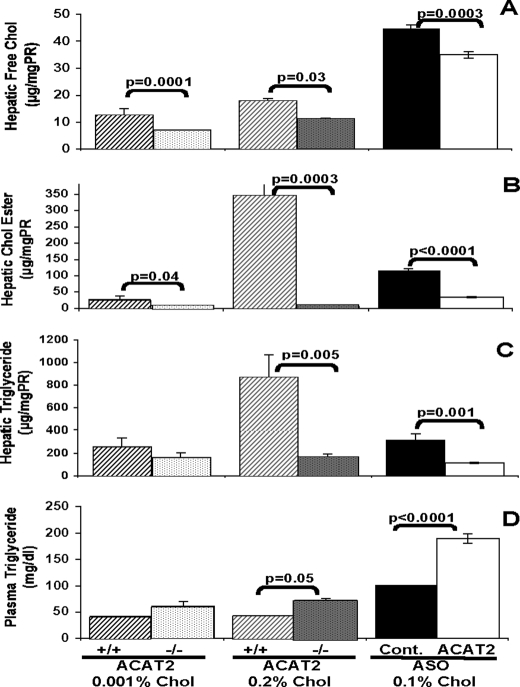
ACAT2 is the main cholesterol-esterifying enzyme in the liver and intestine, and genetic deletion of this enzyme in mice has been shown to lower both intestinal cholesterol absorption and hepatic cholesteryl ester accumulation when mice are challenged with cholesterol in the diet (15, 21, 22). As seen in previous studies (17, 19), 6 weeks of feeding low cholesterol (0.001% w/w) or high cholesterol (0.2% w/w) diets resulted in lower hepatic free cholesterol in ACAT2−/− mice when compared with ACAT2+/+ mice (Fig. 1A). Hepatic CEs were at least 10-fold higher when wild type mice were fed the higher cholesterol diet (Fig. 1B), whereas livers from ACAT2-deficient mice (ACAT2−/−) and mice treated with ACAT2 ASO were protected from accumulation of CE when either diet was fed. The levels of hepatic triglyceride closely followed the cholesteryl ester levels so that the ACAT2+/+ mice fed the 0.2% cholesterol diet had significantly higher hepatic triglyceride concentrations (Fig. 1C) as well as elevated CE concentrations. Intriguingly, plasma triglyceride concentrations were inversely affected and consistently were lower when hepatic triglycerides were higher (Fig. 1D).
FIGURE 1.
Mice lacking ACAT2 or treated with ACAT2 ASO are protected against dietary cholesterol-driven hepatic steatosis. Liver lipids from female mixed strain mice fed a very low fat (10% of energy as fat) diet containing either low (0.001% w/w) or high (0.2% w/w) cholesterol (Chol) for 6 weeks were compared. Livers from littermates with ACAT2 intact (ACAT2+/+) (dark cross-hatched bars and gray cross-hatched bars) were compared with ACAT2 knock-out (ACAT2−/−) mouse livers (open shaded bars and gray shaded bars). Livers from male LDLr−/−, apoB100/100 mice fed a low fat (20% of energy as palm oil), moderate cholesterol (0.1% w/w) diet for 8 weeks and treated biweekly with a non-targeting ASO (ASO control (Cont.)) (black bars) or an ASO targeting ACAT2 (ACAT2 ASO) (open bars) were also compared. A, hepatic free (unesterified) cholesterol concentration. PR, protein. B, hepatic cholesteryl ester (Chol Ester) concentration. C, hepatic triglyceride concentration. D, plasma triglyceride concentrations at the end of the treatment periods. Data represent the average (±S.E.) of individual samples (n = 5–8) per group; p values obtained by Student's t test with p ≤ 0.05 being considered statistically significant.
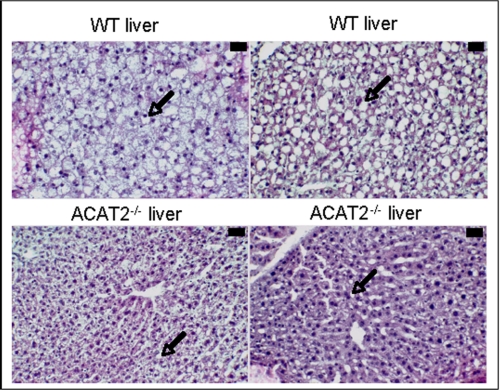
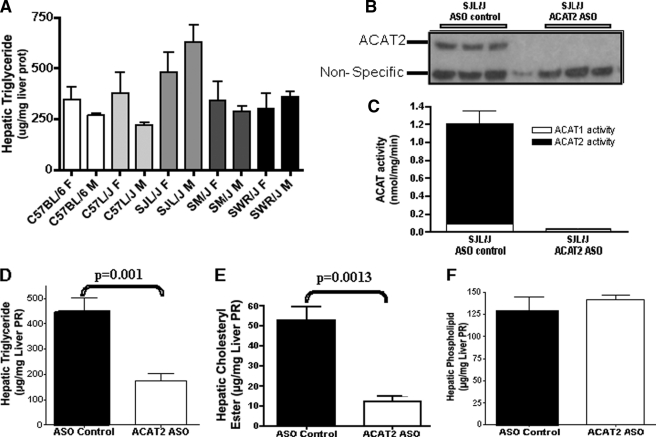
Consistent with the biochemistry of liver lipids, after hematoxylin and eosin staining, histological analysis of ACAT2+/+ mouse livers showed several large lipid droplets within most hepatocytes, typical of NAFLD, whereas ACAT2−/− livers had fewer and smaller lipid droplets (Fig. 2). To make sure that this outcome was not an unusual consequence of the mixed strain background of these mice, male and female mice of several inbred strains were purchased from The Jackson Laboratory based on previous studies on the susceptibility among these strains of mice to develop liver steatosis (16). After 6 weeks of feeding the high cholesterol diets (0.2% w/w cholesterol), mice were fasted for 4 h, livers were collected, and lipid concentrations were determined. Hepatic triglyceride was highest in the SJL/J male mice and lowest in the C57L/J male mice (Fig. 3A), with all other strains and genders having intermediate values. ACAT2 was not disrupted in any of these inbred strains of mice so that hepatic cholesteryl ester was present in all livers (data not shown). SJL/J male mice were selected for further studies with an ASO targeted to ACAT2 to see whether depletion of hepatic ACAT2 activity would alter the cholesteryl ester as well as the triglyceride response in the liver of SJL mice.
FIGURE 2.
Histological appearance consistent with NAFLD in ACAT2+/+ mice. Liver sections stained with hematoxylin and eosin from two ACAT2+/+ mice and two ACAT2−/− mice fed high cholesterol diets are shown. ACAT2+/+ livers had many large lipid droplets within the hepatocytes, whereas ACAT2−/− livers had fewer and smaller lipid droplets (arrows). The black bar in the upper right corner indicates 30 μm. WT, wild type.
FIGURE 3.
ASO-mediated knockdown of ACAT2 in adult inbred SJL mice protects against dietary cholesterol-driven hepatic steatosis. A, hepatic triglyceride concentrations in female (F) and male (M) mice of five different inbred strains purchased from The Jackson Laboratory and fed a low fat (10% of energy as palm oil) diet containing 0.2% (w/w) cholesterol for 6 weeks. liver prot, liver proteins. B–F, ACAT2 and lipid values in livers of SJL/J male mice fed the high cholesterol diet for 6 weeks and treated biweekly with injections (25 mg/kg) of either an ASO targeting the knockdown of ACAT2 (ACAT2 ASO) (open bars) or a non-targeting ASO (ASO control) (black bars). B, ACAT2 protein in three individual mouse liver microsomal preparations by Western blotting. C, ACAT2 activity. D, hepatic triglyceride concentration. Liver PR, liver proteins. E, hepatic cholesteryl ester concentration. F, hepatic phospholipid concentration. Data in panels A (n = 3) and C–F (n = 6) represent the mean ± S.E.; p values obtained by Student's t test with p ≤ 0.05 being considered statistically significant.
Knockdown of ACAT2 with ASO Provides Protection from Dietary Cholesterol-dependent Hepatic Triglyceride Accumulation
Treatment of male SJL/J mice with an ACAT2-targeted ASO lowered hepatic ACAT2 mRNA (data not shown), protein (Fig. 3B), and ACAT2 activity (Fig. 3C) when compared with treatment with a non-targeting control ASO. Targeted knockdown of ACAT2 in SJL mice lowered hepatic triglyceride accumulation (Fig. 3D) and hepatic cholesteryl ester accumulation (Fig. 3E) (as was the case for the ACAT2 ASO-treated hyperlipidemic mice of Fig. 1), without altering hepatic phospholipid concentration (Fig. 3F).
Previous studies have shown that ASO-mediated knockdown of ACAT2 can lower hepatic cholesteryl ester when compared with control ASO-treated mice on a hyperlipidemic (apoB100/100,LDLr−/−) background (11, 12). In the data shown here, responses in livers from ASO-treated apoB100/100, LDLr−/− mice fed a 0.1% (w/w) cholesterol diet for 8 weeks (Fig. 1) were consistent with the effect in normolipidemic SJL mice (Fig. 3) so that knockdown of ACAT2 in mice of either background resulted in lower hepatic triglyceride concentrations when compared with their ASO-treated controls. At the same time, consistent with previous studies and the expected role of ACAT2, ASO-mediated knockdown of ACAT2 also significantly lowered hepatic cholesteryl ester (Figs. 1B and 3E).
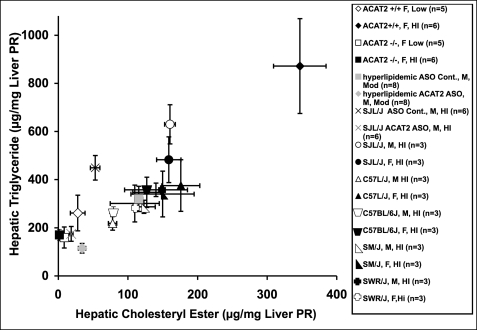
Consistency in the Association between Hepatic CE and Triglyceride Concentrations
Our data suggest that ACAT2 depletion is consistently associated with a major decrease in hepatic cholesteryl ester and also with a decrease in liver triglycerides. Values of hepatic cholesteryl ester concentrations in a variety of experimental mice with and without ACAT2 fed different dietary cholesterol levels were plotted against hepatic triglyceride concentrations (Fig. 4), and a strongly significant and positive association with a correlation coefficient of r = 0.87 (p < 0.0001) was defined by these data. This finding suggests that the two hepatic lipids are metabolically linked across a wide range of concentrations through a mechanism that remains to be defined. Because ACAT2 KO leads to lower triglycerides and dietary cholesterol leads to higher hepatic triglycerides, we made the assumption that cholesteryl ester levels are of key importance.
FIGURE 4.
Relationship between hepatic cholesteryl ester and triglyceride mass. Hepatic cholesteryl ester and triglyceride concentrations were quantified by group as mean ± S.E. and correlated among several groups of animals with and without ACAT2 fed for at least 6 weeks of low (0.001%), moderate (0.1%), or high (0.2%) cholesterol diets. Several groups of animals were compared, as shown in the legend. ASO treatment was with either ASO control or ACAT2 ASO. Dietary cholesterol is indicated as a percentage (w/w) followed by the number of animals in each group (n = #). All points together describe a regression line with a correlation coefficient of r = 0.87, p < 0.0001). Liver PR, liver proteins.
Nascent Lipoprotein Secretion Rate during Liver Perfusion Depends on ACAT2 Status
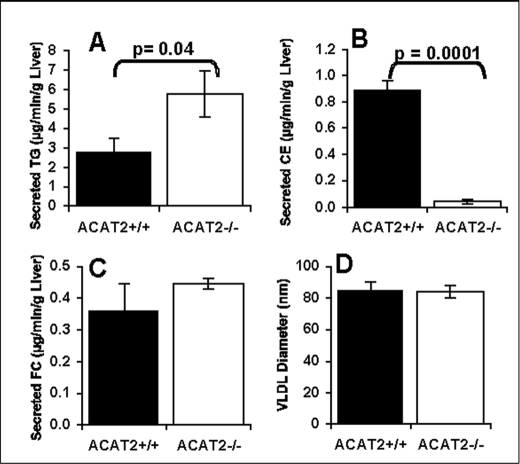
Liver perfusion experiments were done to examine possible mechanisms for the neutral lipid association. During isolated liver perfusion, the livers from mice deficient in ACAT2 secreted lipoprotein particles with a neutral lipid core that was essentially only triglyceride (Fig. 5A) and depleted in cholesteryl ester (Fig. 5B). Secretion of free cholesterol was not significantly different (Fig. 5C). Further, the diameter (∼85 nm) of the isolated perfusate VLDL did not differ based on ACAT2 status, as determined using a dynamic light scatter instrument (Fig. 5D). In other work, these differences were reproduced in a second set of liver perfusion experiments where pure strain C57Bl6 mice with and without ACAT2 were studied (data not shown). Thus, the data in two separate sets of perfusion experiments (using mixed strain mice (Fig. 5) and pure C57Bl6/J mice) demonstrated that an important effect of ACAT2 deletion is to promote a significant increase in VLDL triglyceride secretion in the form of increased numbers of VLDL particles of similar size to those secreted by livers from ACAT2+/+ mice.
FIGURE 5.
Liver triglyceride lowering seen with ACAT2 deficiency is linked to elevated VLDL-TG secretion rate. Isolated recirculating liver perfusion was done with livers from female mixed strain ACAT2+/+ and ACAT2−/− mice fed a 0.2% cholesterol, low fat (10% of energy) diet for 6 weeks. The rates of accumulation in perfusate of triglycerides (A), cholesteryl esters (B), and free cholesterol (FC) (C) were measured after collection of perfusate samples every 30 min during 3 h of perfusion. The data in D show the VLDL particle size measurements made in VLDL isolated from several perfusate time point samples to help sort the difference between enrichment (enlargement) of particles with triglycerides versus the increase in the number of particles that transport secreted triglycerides. Data represent the mean ± S.E. (n = 5 liver perfusion experiments). p values obtained by Student's t test with p ≤ 0.05 being considered statistically significant.
Examination of Gene Expression in the Regulation of ACAT2-related Modifications of Hepatic Lipid Metabolism
Mice with a disruption in ACAT2 can become hypertriglyceridemic and have livers that are depleted in cholesteryl ester and reduced in triglyceride concentration but still secrete triglyceride at a higher rate than livers from ACAT2 intact animals. Accordingly, we looked for changes in hepatic gene expression that could control such modifications in liver lipid metabolism. Expression of genes was examined using reverse transcription-PCR. We monitored several groups of genes whose protein products are involved in (a) lipogenesis, including sterol regulatory element-binding protein-1c (SREBP-1c), fatty acid synthase (FAS), stearoyl-CoA desaturase-1 (SCD-1), acetyl-CoA carboxylase-1 (ACC-1), and tribbles-3 (TRB3); (b) triglyceride synthesis and degradation, including diacylglycerol acyltransferase 1 and 2 (DGAT1 and DGAT2), triacylglycerol hydrolase 1 and 2 (TGH1 and TGH2), adipose tissue triacylglycerol lipase (ATGL), comparative gene identification-58 (CGI58), also termed ADHD5, carnitine palmitoyltransferase 1 (CPT1), and mitochondrial glycerol phosphate acyltransferase 1 (mGPAT1); (c) hepatic inflammation including interleukin 6 (IL6) and cluster of differentiation-68 (CD68); and (d) miscellaneous aspects of lipid metabolism, including ATP binding cassette transporter G5 (ABCG5), 3-hydroxy-3-methylglutaryl-CoA synthase (HMGCoA Syn), adipose differentiation-related protein (ADRP), cell death-inducing DNA fragmentation factor α (DFFA)-like effector B (CIDE-B), microsomal triglyceride transfer protein (MTP), fibroblast growth factor 21 (FGF21), fibroblast growth factor receptor 4 (FGFR4), cytochrome P-450 3A11b (CYP3A11B), small heterodimeric partner (SHP), and Lipin1. Data from two separate experiments were compared. Experiment 1 measured the mRNA abundance of genes in livers of normolipidemic ACAT2+/+ and ACAT2−/− mice, whereas the second experiment was done in mice treated with a control or ACAT2 ASO. In both cases, no significant transcriptional changes were identified so that the mechanism(s) for the differences in hepatic lipid accumulation between ACAT2+/+ and ACAT2−/− mice remains unexplained. Statistically significant decreases (>95%) were measured in the expression of ACAT2 in both experiments to confirm that where present, significant differences would be detected. However, no significant differences in gene expression were identified to account for the shift in the hepatic triglyceride accumulation, mobilization, and secretion that was induced by ACAT2 disruption.
Fasting blood glucose was also monitored in subsets of ACAT2+/+ and ACAT2−/− mice to determine whether these mice had altered glucose metabolism that often accompanies hepatic steatosis. However, there were no differences in fasting blood glucose between ACAT2+/+ and ACAT2−/− animals at baseline or after 10 or 20 weeks of high dietary cholesterol feeding. Therefore, the diet-induced NAFLD described here is not apparently associated with insulin resistance and hyperglycemia secondary to steatosis.
Measurement of Rates of Hepatic Triglyceride Synthesis and Mobilization by Liver Perfusion
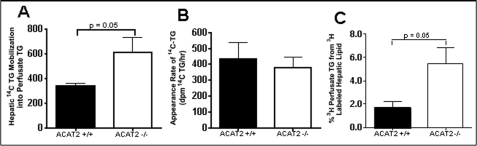
In the absence of data suggesting that ACAT2 exerts major effects on gene expression of enzymes involved in synthesis of hepatic lipids, we designed experiments to test the hypothesis that the presence of cholesteryl ester in the lipid droplet could reduce the rate of triglyceride mobilization assuming that hydrolysis and resynthesis of triglyceride in the lipid droplet are required for its subsequent secretion in VLDL. For these experiments, hepatic lipids were prelabeled 1 h before perfusion with [3H]oleate so that the rate of mobilization of preformed triglyceride could be monitored during liver perfusion. Further, isolated liver perfusion was done with a continuous infusion of [14C]oleate to permit estimation of the rates of nascent hepatic triglyceride synthesis to be estimated. In comparing the apparent secretion rates between the livers of ACAT2+/+ and ACAT2−/− mice, a higher rate of appearance in perfusate for triglyceride mass (Fig. 5A, for [3H]oleate-labeled TG (Fig. 6C) and for [14C]oleate labeled triglyceride (Fig. 6A)) was seen for ACAT2−/− livers. Further, when monitoring accumulation rates of [14C]oleate-labeled triglyceride synthesized during the continuous infusion of [14C]oleate during liver perfusion (Fig. 6B), the data showed no difference between livers from ACAT2−/− when compared with ACAT2+/+ mice. These data provide support for the hypothesis that accumulation of hepatic cholesteryl ester in lipid droplets could be limiting the mobilization of hepatic triglyceride. The specific enzyme(s) that might be responsible for this outcome is uncertain, but a competitive interference by CE with triglyceride hydrolysis has previously been documented for lipoprotein lipase (24). Depending on which hydrolase(s) may be required for hepatic triglyceride mobilization, a similar effect could occur. Most of the candidate enzymes for this step, such as TGH1 (31, 32) apparently hydrolyze both CE and TG in vitro (33), although the relative rates and the degree of competition between each lipid class are as yet undetermined.
FIGURE 6.
ACAT2 deficiency is associated with increased mobilization of stored hepatic triglyceride for VLDL secretion. Hepatic triglycerides were isotopically labeled with [3H]oleic acid before and with [14C]oleic acid during isolated liver perfusion of livers from female C57Bl/6 ACAT2+/+ and ACAT2−/− mice fed the 0.2% cholesterol, low fat diet for 6 weeks. [14C]Oleate on albumin was continuously infused at a constant rate (∼11,000 cpm/min) during recirculating liver perfusion (A and B), and the incorporation into 14C-labeled hepatic triglyceride was monitored in liver samples collected hourly during perfusion (B), whereas the appearance of 14C-lipid in perfusate lipoproteins was also monitored (A). The rate of appearance of [3H]oleate in perfusate lipids (mainly in triglycerides (>85%)) was monitored in perfusate samples with collections made every 30 min (C). Data represent the mean ± S.E. from n = 5–6. p values obtained by Student's t test with p ≤ 0.05 being considered statistically significant.
DISCUSSION
Dietary cholesterol has long been recognized to play a role in the development of cardiovascular disease (25). The findings presented here demonstrate an association between dietary cholesterol and the development of NAFLD in relevant mouse models. When absorbed cholesterol enters the body, it is esterified by ACAT2 and directed to the liver, where some of it can be stored within hepatocytes in lipid droplets as CEs (26). Data presented here show a direct correlation between the concentration of hepatic CE (adjusted upwards by diet or downward by limiting ACAT2 expression) and TG. We have demonstrated that when excess stored CE molecules are present in the liver, the mobilization of hepatic TG is limited and TG secretion is reduced, both resulting in retention of neutral lipids (CE and TG) within the liver in lipid droplets, as is typical of hepatic steatosis. The sequence of events leading from NAFLD to non-alcoholic steatohepatitis and onto cirrhosis is thought to be important in the development of liver disease. In this context, it is important to note that in a study of 9221 participants with 13.3 years of follow-up, 118 diagnoses of cirrhosis and 5 diagnoses of liver cancer were positively linked to consumption of higher levels of dietary cholesterol (27). Clearly, the role of dietary cholesterol, with the subsequent increased hepatic esterification of cholesterol by ACAT2 and its association to hepatic triglyceride accumulation as first demonstrated here (Figs. 1 and 4), is a new paradigm for fatty liver disease that needs further study. The fact that ACAT2 levels can be adjusted downward by treatment with the antisense oligonucleotides, providing an effective treatment to limit hepatic triglyceride accumulation in mice, suggests that a treatment for steatosis using ACAT2 inhibition could also be effective in patients with NAFLD and might prevent more serious complications later on. In addition to the studies presented here, we have also done studies to show that treatment of mice with ACAT2 ASOs will cause reversal of pre-existing NAFLD.5 One advantage to a treatment targeting ACAT2 would be the apparent lack of complications that would be anticipated from other types of treatments, e.g. inhibition of lipogenesis.
Using the various mouse models fed diets with different amounts of cholesterol, we were able to establish a direct correlation between the accumulation of hepatic cholesteryl esters and hepatic triglycerides. We showed that in normolipidemic mice, the triglyceride-altering effect is dependent on dietary cholesterol. This finding may seem counterintuitive as the level of CE in the liver is typically much lower than the level of TG. However, a previous study in primary rat hepatocytes demonstrated that these cells isolated from rats that had been fed a high fat/high cholesterol diet contained higher cholesteryl esters and secreted less triglyceride than hepatocytes from rats not fed cholesterol (28). This study did not identify ACAT2 and the ACAT2-derived CE as the basis for the TG accumulation as ACAT2 was not identified until several years later (29, 30), but it may be the culprit in these studies as well. When applying the current data to the previous rat hepatocyte model, it can be seen that ACAT2-derived CEs are not apparently an inert molecule in the liver generated only to prevent excess free cholesterol from building up within the cell. Instead, CEs apparently serve a role in regulation of the accumulation and secretion of hepatic triglyceride, perhaps through the effects on mobilization of TG for secretion as suggested here. If hepatic CE were completely inert in the cell, it seems that TG would still accumulate to the same extent in the ACAT2 disrupted animals lacking hepatic CE.
In our studies, we showed that hepatocytes are more limited in TG content when hepatic cholesteryl esters are absent and that despite the differences in the composition of the cores of nascent VLDL secreted from the liver, the VLDL are the same diameter whether they came from an ACAT2-intact or an ACAT2-depleted liver. The data have suggested that in the absence of CE, there is an increased rate of mobilization of TG. This hypothesis is consistent with the idea that there is a consensus domain for neutral lipid binding (NLBD) that is seen in cholesteryl esterases, lipases, triglyceride hydrolases, and perhaps even lecithin cholesterol acyl transferase (31). Rat TGH (32), also known as hepatic neutral cholesteryl ester hydrolase (33), also exhibits hydrolase activity toward both triglycerides and cholesteryl ester.
To explain the highly significant correlation between hepatic CE and TG (Fig. 4), we hypothesize that CE effectively competes with TG for hydrolysis, thereby limiting TG mobilization, perhaps according to a mechanism analogous to that described in phospholipid monolayers by Demel and Jackson (24). In addition to effects that would be represented by solubilities at the surface of lipid droplets, we further speculate that the substrate flexibility of the binding domain in neutral lipid hydrolytic enzymes may allow that when CE are present in cytoplasmic lipid droplets in the liver, albeit at lower concentrations than triglycerides, competition for hydrolysis by the enzyme could occur with subsequent effects to decrease mobilization of TG. In this case, in contrast to when CEs are absent from the liver, the overall rate of mobilization would be slowed, leading to more TG storage. Because there is a linear relationship between hepatic CE and TG concentrations, it would seem that the effect of CE to inhibit mobilization is concentration-dependent. In strong support of this hypothesis is the fact that there is a higher secretion rate of TG directly from the liver to nascent VLDL particles when CEs are absent (ACAT2−/−), compared with when CEs are present (ACAT2+/+) (Fig. 6, A and C). The core lipid in particles synthesized when hepatic ACAT2 activity is absent is essentially only TG, the situation where lipid droplet TG is most rapidly hydrolyzed and resynthesized for packaging and secretion because stored CE is absent and does not interfere with TG hydrolysis.
We performed a series of reverse transcription-PCR experiments to determine whether there were any significant changes in gene expression that would suggest a mechanism for the lower TG in ACAT2-depleted livers. The chemical data consistently showed that the livers of ACAT2−/− mice had lower hepatic TG than ACAT2+/+ livers when mice were fed a diet containing 0.2% cholesterol. It is possible that ACAT2−/− livers could have lower de novo synthesis of TG, and a decrease in TG synthesis could be due to differences in precursor availability or in the TG-synthetic enzymes. However, in our reverse transcription-PCR assays, we found that sterol regulatory element-binding protein isoform 1c (SREBP-1c) had only modestly lower (38%) but not significantly different expression in ACAT2−/− livers when compared with ACAT2+/+ livers. Further, we did not find statistically significant differences between the two genotypes in the expression of downstream transcriptional targets of SREBP-1c involved in lipogenesis (fatty acid synthase (FAS), stearoyl-CoA desaturase-1 (SCD-1), acyl-CoA carboxylase-1 (ACC-1), and tribbles-3 (TRB3)), although it appeared that some levels of these genes may have been slightly lower in liver from ACAT2−/− when compared with ACAT2+/+ mice.
We repeated the gene expression experiment in livers from hyperlipidemic mice treated with the ACAT2 ASO to knock down the enzyme. To varying degrees, there was a slight but insignificantly lower expression of genes associated with lipogenesis in ACAT2 ASO-treated animals when compared with controls. Expression of several genes was repeated in the pooled liver cDNA samples, and no transcriptional changes were seen that could account for the lower hepatic TG in ACAT2 knockdown animals. For all of the genes monitored in the ASO-treated animals, including mGPAT, Cyp3a11b, SHP, and Lipin1, no differences were found that could account for the significantly lower hepatic TG in ACAT2 knockdown livers. In data not shown, homogenates were made from the frozen liver samples of ASO-treated hyperlipidemic mice, as described (12). 25 μg of total protein were loaded per well, and immunoblots were performed to compare the protein expression of SCD1, ACC1, and p-AMPK. There was a trend toward lower expression of SCD1 from livers that had ACAT2 knocked down, but there was no change in protein expression between ASO control and ACAT2 ASO-treated livers for ACC1 or p-AMPK. Taken together, these data provide little convincing evidence that there is a general decrease in the expression of genes involved in the de novo synthesis of hepatic TG that could lead to decreased hepatic triglyceride concentrations of the ACAT2-depleted mouse livers.
In summary, the current studies have implicated a novel mechanism by which the accumulation of hepatic cholesteryl ester can lead to hepatic triglyceride accumulation. The mechanism by which this occurs is apparently not through an enhancement in lipogenesis and de novo triglyceride synthesis coupled to insulin resistance and hyperglycemia as is often reported in mice fed high fat diets (34, 35). Instead, the current data indicate that the presence of cholesteryl ester is able to limit the mobilization of triglycerides from the liver, perhaps through the relative substrate flexibility previously reported in some neutral lipid-esterifying enzymes (32, 33), such that the presence of CE interferes with TG hydrolysis and mobilization from cytoplasmic lipid droplets independent of lipogenesis. This study provides novel insight into dietary cholesterol-driven NAFLD and provides additional evidence that ACAT2-specific inhibitors hold promise to not only protect against atherosclerosis but also NAFLD.
Supplementary Material
Acknowledgments
We thank Dr. Robert Farese, Jr. (Gladstone Institute of University of California, San Francisco) for the gift of ACAT2−/− breeder mice, Dr. Stephen Young (UCLA) for the gift of apoB100/100,LDLr−/− breeder mice, and Dr. Rosanne Crook and Mark Graham from Isis Pharmaceuticals, Carlsbad, CA, for the generous gift of antisense oligonucleotides that were used in these studies.
This work was supported, in whole or in part, by National Institutes of Health Grant HL-49373 (to L. L. R.). L. L. R. has ongoing collaborations with Amgen and Glaxo-Smith Kline. He participates in a speaker's bureau for Merck-Schering Plough.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
R. Temel and L. L. Rudel, unpublished findings.
- NAFLD
- non-alcoholic fatty liver disease
- ACAT2
- acyl-CoA:cholesterol O-acyl transferase 2
- apoB
- apolipoprotein B
- CE
- cholesteryl ester
- TG
- triglyceride
- VLDL
- very low density lipoprotein
- LDLr
- low density lipoprotein receptor
- ASO
- antisense oligonucleotide
- TGH
- triacylglycerol hydrolase.
REFERENCES
- 1.Ludwig J., Viggiano T. R., McGill D. B., Oh B. J. (1980) Mayo Clin. Proc. 55, 434–438 [PubMed] [Google Scholar]
- 2.Schreuder T. C., Verwer B. J., van Nieuwkerk C. M., Mulder C. J. (2008) World J. Gastroenterol. 14, 2474–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varela-Rey M., Embade N., Ariz U., Lu S. C., Mato J. M., Martínez-Chantar M. L. (2009) Int. J. Biochem. Cell Biol. 41, 969–976 [DOI] [PubMed] [Google Scholar]
- 4.Bradbury M. W. (2006) Am. J. Physiol. Gastrointest. Liver Physiol. 290, G194–G198 [DOI] [PubMed] [Google Scholar]
- 5.Adams L. A., Angulo P. (2006) Postgrad. Med. J. 82, 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marra F., Gastaldelli A., Svegliati Baroni G., Tell G., Tiribelli C. (2008) Trends. Mol. Med. 14, 72–81 [DOI] [PubMed] [Google Scholar]
- 7.Wieckowska A., McCullough A. J., Feldstein A. E. (2007) Hepatology 46, 582–589 [DOI] [PubMed] [Google Scholar]
- 8.Fan J. G. (2008) J. Dig. Dis. 9, 63–67 [DOI] [PubMed] [Google Scholar]
- 9.Adams L. A., Lymp J. F., St Sauver J., Sanderson S. O., Lindor K. D., Feldstein A., Angulo P. (2005) Gastroenterology 129, 113–121 [DOI] [PubMed] [Google Scholar]
- 10.Lee R. G., Willingham M. C., Davis M. A., Skinner K. A., Rudel L. L. (2000) J. Lipid Res. 41, 1991–2001 [PubMed] [Google Scholar]
- 11.Parini P., Davis M., Lada A. T., Erickson S. K., Wright T. L., Gustafsson U., Sahlin S., Einarsson C., Eriksson M., Angelin B., Tomoda H., Omura S., Willingham M. C., Rudel L. L. (2004) Circulation 110, 2017–2023 [DOI] [PubMed] [Google Scholar]
- 12.Lee R. G., Kelley K. L., Sawyer J. K., Farese R. V., Jr., Parks J. S., Rudel L. L. (2004) Circ. Res. 95, 998–1004 [DOI] [PubMed] [Google Scholar]
- 13.Bell T. A., 3rd, Brown J. M., Graham M. J., Lemonidis K. M., Crooke R. M., Rudel L. L. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1814–1820 [DOI] [PubMed] [Google Scholar]
- 14.Bell T. A., 3rd, Kelley K., Wilson M. D., Sawyer J. K., Rudel L. L. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 1396–1402 [DOI] [PubMed] [Google Scholar]
- 15.Willner E. L., Tow B., Buhman K. K., Wilson M., Sanan D. A., Rudel L. L., Farese R. V., Jr. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1262–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishina P. M., Wang J., Toyofuku W., Kuypers F. A., Ishida B. Y., Paigen B. (1993) Lipids 28, 599–605 [DOI] [PubMed] [Google Scholar]
- 17.Brown J. M., Bell T. A., 3rd, Alger H. M., Sawyer J. K., Smith T. L., Kelley K., Shah R., Wilson M. D., Davis M. A., Lee R. G., Graham M. J., Crooke R. M., Rudel L. L. (2008) J. Biol. Chem. 283, 10522–10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown J. M., Chung S., Sawyer J. K., Degirolamo C., Alger H. M., Nguyen T., Zhu X., Duong M. N., Wibley A. L., Shah R., Davis M. A., Kelley K., Wilson M. D., Kent C., Parks J. S., Rudel L. L. (2008) Circulation 118, 1467–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Temel R. E., Lee R. G., Kelley K. L., Davis M. A., Shah R., Sawyer J. K., Wilson M. D., Rudel L. L. (2005) J. Lipid Res. 46, 2423–2431 [DOI] [PubMed] [Google Scholar]
- 20.Carr T. P., Parks J. S., Rudel L. L. (1992) Arterioscler. Thromb. 12, 1274–1283 [DOI] [PubMed] [Google Scholar]
- 21.Rudel L. L., Davis M., Sawyer J., Shah R., Wallace J. (2002) J. Biol. Chem. 277, 31401–31406 [DOI] [PubMed] [Google Scholar]
- 22.Lee R. G., Shah R., Sawyer J. K., Hamilton R. L., Parks J. S., Rudel L. L. (2005) J. Lipid Res. 46, 1205–1212 [DOI] [PubMed] [Google Scholar]
- 23.Van Harken D. R., Dixon C. W., Heimberg M. (1969) J. Biol. Chem. 244, 2278–2285 [PubMed] [Google Scholar]
- 24.Demel R. A., Jackson R. L. (1985) J. Biol. Chem. 260, 9589–9592 [PubMed] [Google Scholar]
- 25.McGill H. C., Jr. (1979) Am. J. Clin. Nutr. 32, 2664–2702 [DOI] [PubMed] [Google Scholar]
- 26.Wilson M. D., Rudel L. L. (1994) J. Lipid Res. 35, 943–955 [PubMed] [Google Scholar]
- 27.Ioannou G. N., Morrow O. B., Connole M. L., Lee S. P. (2009) Hepatology 50, 175–184 [DOI] [PubMed] [Google Scholar]
- 28.Davis R. A., McNeal M. M., Moses R. L. (1982) J. Biol. Chem. 257, 2634–2640 [PubMed] [Google Scholar]
- 29.Anderson R. A., Joyce C., Davis M., Reagan J. W., Clark M., Shelness G. S., Rudel L. L. (1998) J. Biol. Chem. 273, 26747–26754 [DOI] [PubMed] [Google Scholar]
- 30.Cases S., Novak S., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Welch C. B., Lusis A. J., Spencer T. A., Krause B. R., Erickson S. K., Farese R. V., Jr. (1998) J. Biol. Chem. 273, 26755–26764 [DOI] [PubMed] [Google Scholar]
- 31.Alam M., Gilham D., Vance D. E., Lehner R. (2006) J. Lipid Res. 47, 375–383 [DOI] [PubMed] [Google Scholar]
- 32.Dolinsky V. W., Gilham D., Alam M., Vance D. E., Lehner R. (2004) Cell Mol. Life Sci. 61, 1633–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh S., Mallonee D. H., Hylemon P. B., Grogan W. M. (1995) Biochim. Biophys. Acta 1259, 305–312 [DOI] [PubMed] [Google Scholar]
- 34.Deevska G. M., Rozenova K. A., Giltiay N. V., Chambers M. A., White J., Boyanovsky B. B., Wei J., Daugherty A., Smart E. J., Reid M. B., Merrill A. H., Jr, Nikolova-Karakashian M. (2009) J. Biol. Chem. 284, 8359–8368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Browning J. D., Horton J. D. (2004) J. Clin. Invest. 114, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.