Abstract
Homeostatic scaling of glutamatergic and GABAergic transmission is triggered by prolonged alterations in synaptic neuronal activity. We have previously described a presynaptic mechanism for synaptic homeostasis and plasticity that involves scaling the level of vesicular glutamate (VGLUT1) and γ-aminobutyric acid (GABA) (VGAT) transporter biosynthesis. These molecular determinants of vesicle filling and quantal size are regulated by neuronal activity in an opposite manner and bi-directionally. Here, we report that a striking induction of VGLUT2 mRNA and synaptic protein is triggered by a prolonged increase in glutamatergic synaptic activity in mature neocortical neuronal networks in vitro together with two determinants of inhibitory synaptic strength, the neuronal activity-regulated pentraxin (Narp), and glutamate decarboxylase (GAD65). Activity-dependent induction of VGLUT2 and Narp exhibits a similar intermediate-early gene response that is blocked by actinomycin D and tetrodotoxin, by inhibitors of ionotropic glutamate receptors and L-type voltage-gated calcium channels, and is dependent on downstream signaling via calmodulin, calcium/calmodulin-dependent protein kinase (CaMK) and extracellular signal-regulated kinase 1/2 (ERK1/2). The co-induction of VGLUT2 and Narp triggered by prolonged γ-aminobutyric acid type A receptor blockade is independent of brain-derived nerve growth factor and TrkB receptor signaling. VGLUT2 protein induction occurs on a subset of cortically derived synaptic vesicles in excitatory synapses on somata and dendritic processes of multipolar GABAergic interneurons, recognized sites for the clustering of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate glutamate receptors by Narp. We propose that VGLUT2 and Narp induction by excitation-transcription coupling leads to increased glutamatergic transmission at synapses on GABAergic inhibitory feedback neurons as part of a coordinated program of Ca2+-signal transcription involved in mechanisms of homeostatic plasticity after prolonged hyperactivity.
Keywords: Calcium/Calmodulin, Gene/Regulation, Neurotransmitters, Subcellular Organelles/Vesicles, Transport/Neurotransmitter, BDNF, Homeostatic Synaptic Plasticity, Inhibitory Feedback, Quantal Size, Vesicular Transporter
Introduction
Homeostatic plasticity is an adaptive response of neocortical and hippocampal neuronal networks after prolonged changes in synaptic activity that scales the strength of excitatory and inhibitory synapses to stabilize the firing rate of pyramidal neurons (1–3). Post-synaptic alterations in AMPA2 glutamate and GABAA receptor density is thought to account for homeostatic scaling of miniature excitatory and inhibitory postsynaptic current amplitude (4–10). However, recent evidence has established that in mature neurons, homeostatic plasticity also includes the presynaptic scaling of quantal size; i.e. the amount of glutamate and GABA released from individual synaptic vesicles (11–15). Variations in the quantal size of glutamate released at mammalian excitatory synapses in vivo is due to differences in the concentration of glutamate within vesicles (Ref. 16, but see Ref. 17). Indeed, activity-dependent scaling the level of gene transcription for the vesicular glutamate and GABA transporters (VGLUT1 and VGAT) is an endogenous mechanism used to regulate the number of these transporters on individual vesicles in synaptic terminals and, hence, the amount of glutamate and GABA that is available for exocytotic release (18).
Whereas decreased synaptic strength occurs at most excitatory synapses after prolonged neuronal hyperactivity (5), increased glutamatergic synaptic strength has been reported at GABAergic bipolar interneurons (19, 20) providing a mechanism for inhibitory feedback (21). Interestingly, the neuronal activity-regulated pentraxin (Narp) polypeptide appears to play a key role in regulating AMPA glutamate receptor clustering at excitatory synapses found on hippocampal inhibitory interneurons (22, 23). Sustained increases in Narp released from excitatory neurons may, therefore, contribute to increases in inhibitory feedback induced by chronic neuronal stimulation (24). Increased GABAergic transmission can also occur by increased vesicular filling with GABA by altering the biosynthesis, degradation, or re-uptake of GABA to control cytoplasmic GABA levels (25–27). A reduction in GAD65, GAD67, and GABA expression is observed by prolonged neuronal inactivity in vitro and in vivo (7, 13, 28, 29).
The molecular mechanisms responsible for scaling vesicular glutamate and GABA transporter biosynthesis during postnatal development and in mature cortical synapses by neuronal activity are not known. The functional implications for differential VGLUT gene regulation and trafficking of VGLUT isoforms to distinct axonal terminals distinguishes mammalian cortical excitatory synapses from glutamatergic synapses in simpler organisms like Drosophila, which only expresses a single VGLUT homologue (30–33). Although VGLUT1 is the predominant vesicular transporter expressed in neocortical pyramidal neurons in vivo and in vitro, VGLUT2 is co-expressed with VGLUT1 in most pyramidal neurons during development (12, 34–38). In addition, recent work indicates that intrinsic VGLUT2-encoded excitatory transmission is retained in the adult neocortex, and it appears to be involved in certain aspects of cognitive, emotional, and social behavior (39) and in seizure susceptibility (40).
Here, we describe an activity-dependent mechanism of VGLUT2 and Narp gene induction in mature neocortical neurons that involves excitation-transcription (E-T) coupling, a process initiated by Ca2+ signal transcription that results in early, intermediate-early, and long term changes in gene expression (41–45). E-T coupling in central cortical neurons can be initiated by Ca2+ influx through L-type voltage-gated Ca2+ channels (VGCCs) and includes calmodulin-signaling and phosphorylation via Ca2+/calmodulin-dependent protein kinases (CaMK) and mitogen-activated protein kinases (MAPKs) (45–48). Our results indicate that VGLUT2 and Narp co-induction is an intermediate-early gene response to prolonged hyperactivity that includes Ca2+ calmodulin, CaMK, and ERK1/2 signaling. Brain-derived neurotrophic factor (BDNF) and TrkB signaling also activates Narp expression alone. We conclude that E-T coupling triggered by a prolonged increase in glutamatergic synaptic activity in neocortical neurons in vitro is a mechanism used to activate Ca2+ signal transcription of VGLUT2 and Narp to scale the strength of glutamatergic synapses on inhibitory interneurons in long term activity-dependent synaptic plasticity.
EXPERIMENTAL PROCEDURES
Primary Neuronal Cultures and PC12 Cell Transfection
Primary neuronal cultures were prepared as described (12, 49) with minor modifications. The procedures utilized were approved by Louisiana State University Health Sciences Center Institutional Animal Care Committee and are consistent with recommendations by the Panel of Euthanasia of the American Veterinary Medical Association. Dissected cortical tissue from E18 embryos was placed in Neurobasal medium containing 0.02% bovine serum albumin, 0.1% papain, and 5 mm l-cysteine and incubated with gentle shaking at 37 °C for 20 min. The tissue pieces were then removed, rinsed three times with Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 2.5 μg/ml leupeptin and dissociated by gentle trituration using fire polished Pasteur pipettes in complete Neurobasal medium supplemented with B27 (Invitrogen) and 0.5 mm Glutamax (Invitrogen). Cell viability was determined using trypan blue exclusion to be >95%. Cells were plated at 7.5 × 105 cells per well in poly-d-lysine-coated (50 μg/ml; Sigma) 6-well clusters. Cells were initially plated in the presence of glutamate (25 μm), but thereafter complete medium without glutamate was used to refresh the cultures. For the first week the medium was changed twice (50% replacement), but thereafter the medium was changed 3 times per week (25% replacement).
PC12 cells (PC12A123.7) were cultured and transfected with cDNA plasmids containing VGLUT1 and/or VGLUT2 by Lipofectamine as described (50). After 18 h, cells were treated with sodium butyrate (5 mm) to increase transgene expression (51). Cells were harvested <48 h post-transfection.
Pharmacological Treatment of Neocortical Cultures in Vitro with Gabazine to Disinhibit Neuronal Network Activity
Blockade of ionotropic inhibitory transmission via pharmacological antagonism of the GABAA receptor using picrotoxin (52), bicuculline (5), or a newer, more specific antagonist, gabazine (53, 54) results in disinhibition. Disinhibition results in the increase in endogenous synaptic excitatory activity that includes the release of glutamate and other excitatory molecules. We generally pretreat cultures (15–30 min) with drugs before the addition of gabazine (20 μm). Tetrodotoxin was obtained from Alomone Labs (Jerusalem, Israel). 4′,6-Diamidino-2-phenylindole, recombinant TrkB/Fc chimera, and gabazine (SR-95531) were obtained from Sigma. KN-93 and K-252a were obtained from EMD Chemicals (Newark, NJ). All other drugs were obtained from Tocris (Ellisville, MO). DMSO concentrations, when required to dissolve compounds, did not exceed 0.05% and did not affect our results.
Preparation of cDNA and Real Time Reverse Transcription-PCR
Total RNA was isolated using Qiagen RNeasy MINI kit (Valencia, CA) according to the manufacturer's instructions. Cells in each well were harvested in a volume of 350 μl and then disrupted using three 1-s pulses with a probe sonicator. RNA (1 μg) was reverse-transcribed using Bio-Rad iScript cDNA synthesis kit (oligo(dT) + random hexamers) as described (12). Typical total RNA yields were ∼3–5 μg/well. Specific primers for rat VGLUT1, VGLUT2, GAD65, GAD67, BDNF, Narp, and β-actin were selected using Beacon Designer Software (Bio-Rad) or from the literature (55) and synthesized by Invitrogen. The forward and reverse primer sequences and amplicon length are listed as follows: VGLUT1, 5′-ggcagtttccaggacctccactc-3′, 5′-gcaagaggcagttgagaaggagagag-3′ (153 bp); VGLUT2, 5′-gggtatttggtctgtttggtgtcctg-3′, 5′-cgacacagcaagggttatggtcac-3′ (173 bp); GAD65, 5′-cgccagactagcagaacccatg-3′, 5′-tggcttctcagagtctccgtagag-3′ (172 bp); GAD67, 5′-gtgctgctccagtgttctgccatc-3′, 5′-aatcccacagtgccctttgctttcc-3′ (201 bp); Narp, 5′-ggcaagatcaagaagacgttg-3′, 5′-tccaggtgatgcagatatggt-3′ (238 bp); BDNF (exon III (55)), 5′-tgcgagtattacctccgccat-3′, 5′-aggatggtcatcactcttctc-3′ (220 bp); β-actin, 5′-taggcaccagggtgtgatggtggg-3′, 5′-cgcagctcattgtagaaggtgtggtg-3′ (171 bp). The reactions were setup in duplicate in a 25-μl total volume with 5 pmol of each primer, 12.5 μl of 2× SYBR Green Master Mix (Applied Biosystems, Foster City, CA), and 200 ng of template. The PCR cycle was 95 °C for 3 min, 42 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. A melt curve analysis performed for each primer pair was used to verify that a single product was amplified. Furthermore, the sizes of the amplified DNA fragments using the various primers sets were verified by gel electrophoresis and restriction analysis. The PCR amplification of each product is initially assessed using 10-fold dilution of a rat cDNA library prepared from dissociated neocortical neuronal cultures (DIV18) as a template and found to be linear over 5 orders of magnitude and greater than 95% efficient. The amplification and analysis were performed using an iCycler iQ Multicolor Real-Time PCR detection system (Bio-Rad). Samples were compared using the relative CT method as described (12). Data were normalized to the β-actin housekeeping gene. Group means are presented with their S.E. Statistical differences were determined by one-way analysis of variance followed by Bonferroni's multiple comparison test.
Immunocytochemistry
The culture medium was removed, and the cells were fixed with PBS containing 4% paraformaldehyde for 20 min on ice. Cells were rinsed 3 times for 10 min with PBS, post-fixed on ice with −20 °C methanol for 10 min, and followed by the same rinse in PBS. Cells were incubated with blocking buffer (0.2% Triton X-100 and 6% normal goat serum) in PBS for at least 1 h at room temperature or overnight at 4 °C. The antibodies against VGLUT1 and VGLUT2 were characterized previously (56, 57): rabbit anti-VGLUT1 (1:2000), guinea pig anti-VGLUT2 (1:4000). Other antibodies used were commercially available: mouse anti-synaptophysin (1:1000, SVP38; Sigma), mouse anti-GAD67 (1:100, Chemicon), rabbit anti-GABA (1:500, Sigma), mouse anti-MAP2 (1:250, Chemicon). Primary antibodies diluted in blocking buffer were incubated with cells overnight at 4 °C. After 3 washes for 10 min with PBS, species-specific and highly cross-adsorbed secondary antibodies coupled to Alexa 488, 594, or 647 (Molecular Probes, Eugene, OR) diluted 1:200 in blocking buffer were applied for 1 h at room temperature and followed by three PBS washes. In some samples nuclear staining was performed after the final wash by 10 min of incubation with 4′,6-diamidino-2-phenylindole (1 μg/ml) in PBS.
Cells were mounted with Prolong Gold antifade reagent (Invitrogen) and viewed. All images were obtained from a Leica DMRXA automated upright epifluorescent microscope (Nussloch, Germany), a Sensicam QE charge-coupled devise digital camera (Cooke Corp., Romulus, MI), and suitable filter sets (Chroma sets 41001, 31004, and 41008/41022; Chroma Technology, Brattleboro, VT). Some images were deconvolved using Slidebook 4.0 software (Intelligent Imaging Innovations, Denver, CO). For puncta quantification, control and gabazine images were acquired using equal exposure times in stacks of 15–25 planes at 0.2 μm depth intervals and deconvolved with a constrained iterative algorithm, which preserves the original image intensity scale as described (12). Analysis involved the creation of a digital binary mask by segmenting areas over threshold from each fluorescence channels within all captured planes and Slidebook-driven mathematical object statistics (size and mean intensity in red and green channels) calculations. Objects were pruned by size, and analysis with a pixel correlation algorithm identified colocalized objects as described (12).
Immunoisolation of Synaptic Vesicles and Western Blotting
Synaptic vesicles were prepared from differentiated neocortical cultures treated with gabazine and from transfected PC12 cells. Confluent cells (10 cm2) were rinsed with PBS, scraped in 1 ml of cold PBS containing 5 μg/ml pepstatin, 5 μg/ml aprotinin, and 5 μg/ml leupeptin, and “cracked” in the presence of 1 mm phenylmethylsulfonyl fluoride using 11 stokes in a ball-bearing device. Homogenates of PC12 cells were centrifuged at 27,000 × g for 20 min. Homogenates from neocortical neuronal cultures were centrifuged at 35,000 × g for 20 min. The supernatants containing vesicles were directly used for immunoprecipitation or immunoisolation experiments. For analysis of the time-course of VGLUT2 expression after gabazine treatment, vesicle membranes from post-35,000 × g supernatants were pelleted by high speed centrifugation (100,000 × g, 30 min) and subjected to SDS-PAGE electrophoresis and Western blotting.
Immobilized protein A-agarose beads (Roche Diagnostics) were washed three times with PBS. Then, 20 μg of affinity-purified rabbit antibody against VGLUT1, VGLUT2, or synaptophysin was coupled to 25 μl of beads by incubation for 2 h at room temperature. Coupled beads were washed 5 times in PBS and blocked for 10 min with PBS containing 2% glycine, 2% lysine (w/v) followed by a final PBS wash. For immunoisolation, the immunobeads were gently rotated with sample (∼400 μg of protein) overnight at 4 °C. For immunoprecipitation, vesicle suspensions were solubilized with nonionic detergent by the addition of Nonidet-P40 to 1% and EDTA/EGTA to 1 mm and incubated for 1 h at 4 °C. The solubilized samples were clarified by centrifugation at 150,000 × g for 30 min. Immunobeads were gently rotated with the sample overnight at 4 °C. Control beads were beads coupled with nonspecific rabbit IgGs. Beads were separated from unbound proteins in the supernatant by brief centrifugation (30 s) and washed 5 times with cold PBS with or without 1% Nonidet P-40.
Protein samples were dissolved in Laemmli SDS sample buffer, size-fractionated on 10% precise Tris-HCl polyacrylamide gels, and electrophoretically transferred to nitrocellulose membrane using standard protocols. VGLUT1, VGLUT2, and synaptophysin were detected using their respective primary antibodies (guinea pig anti-VGLUT1 or VGLUT2, 1/4000, and mouse anti-p38 from Sigma, 1/5000) and horseradish peroxidase-conjugated anti-guinea pig or mouse IgG secondary antibodies (Sigma, 1/8000) followed by enhanced chemiluminescence (West Pico, Pierce) and exposure to film (Hyperfilm ECL, Amersham Biosciences).
RESULTS
Coordinate Induction of VGLUT2 or Narp and GAD65 mRNA Expression by a Prolonged Increase in Glutamatergic Synaptic Activity in Primary Neocortical Neuronal Cultures
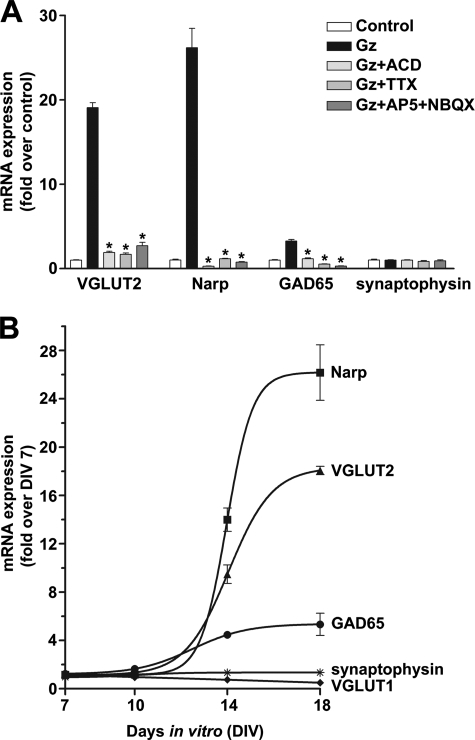
It has previously been established that blocking GABAA-mediated inhibition using picrotoxin, bicuculline, or gabazine eliminates the large inhibitory currents normally present in primary neuronal cultures (4, 52, 58), allowing the excitatory postsynaptic currents to more easily summate to produce postsynaptic depolarization and spiking (5). Thus, blocking GABAA-mediated inhibition initially raises firing rates of pyramidal neurons, but over a 48-h period firing rates return to close to control values (5). This pharmacological disinhibition is the standard protocol to induce homeostatic synaptic plasticity triggered by hyperactivity in neocortical and hippocampal neuronal networks (3). Here, we report that prolonged hyperactivity of dissociated neocortical neuronal cultures (DIV18), produced by 24 h GABAA receptor blockade (gabazine; 20 μm), results in the induction of VGLUT2, Narp, and GAD65 mRNA expression. The steady-state levels of mRNA for these genes increased by 17.6 ± 2.4 (VGLUT2), 28.6 ± 4.4 (Narp), and 5.31 ± 0.57 (GAD65)-fold over control values (Fig. 1A). GAD67 mRNA levels increased ∼2-fold (n = 3; 2.20 ± 0.10, p < 0.001) above control values after hyperactivity. The increase in mRNA levels is a result of de novo gene induction as they are completely blocked in the presence of actinomycin D, an inhibitor of mRNA transcription (Fig. 1A). The induction of these genes requires an increase in action potential-driven neural transmission as they are blocked in the presence of tetrodotoxin (TTX; 1 μm) (Fig. 1A). Although the N-methyl-d-aspartate glutamate receptor blocker dl-2-amino-5-phosphonopentanoic acid (50 μm) and the AMPA glutamate receptor blocker 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (20 μm) alone only partially attenuate the increase in gene expression (∼50%, data not shown), when present together they completely block VGLUT2, Narp, and GAD65 gene induction (Fig. 1A). Higher dl-2-amino-5-phosphonopentanoic acid concentrations (100 μm) alone inhibit to >80% (data not shown). Thus, we conclude that the increased synaptic glutamate receptor activation and the resulting depolarization of neurons after prolonged disinhibition by gabazine initiates a coordinated program of action-potential-dependent gene transcription for these three established molecular determinants of excitatory or inhibitory strength; VGLUT2 or Narp and GAD65.
FIGURE 1.
The coordinate induction of VGLUT2, Narp, and GAD65 gene expression triggered by prolonged network hyperactivity. A, shown is stimulation of VGLUT2, Narp, and GAD65 mRNA levels by prolonged (24 h) treatment with gabazine (Gz; 20 μm) that produces hyperactivity by disinhibition. Hyperactivity increased VGLUT2, Narp, and GAD65 mRNA levels to ∼18×, 28×, and 5×, respectively, over control values. Pretreatment of differentiated neocortical neuronal cultures (DIV18) by actinomycin, an inhibitor of mRNA transcription (ACD; 7.5 μg/ml), tetrodotoxin (TTX; 1 μm), an inhibitor of action-potential driven transmitter release, or collective inhibition of AMPA (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX); 10 μm) and N-methyl-d-aspartate (dl-2-amino-5-phosphonopentanoic acid; 50 μm) glutamate receptors significantly antagonized the induction of VGLUT2, Narp, and GAD65 gene expression by hyperactivity. Data are the mean ± S.E. (bars) values (n = 3). The asterisk denotes significantly different from gabazine-treated samples (p < 0.001). B, shown is the dependence of VGLUT2, Narp, and GAD65 mRNA induction on maturation of the neocortical neuronal network. Cultures were plated at 7.5 × 104 cells/cm2 and treated with gabazine (20 μm) for 24 h after culturing for various days in vitro (DIV 7, 10, 14, and 18). Data are the mean ± S.E. (bars) values (n = 3) relative to DIV7, which was not different from control values. Note VGLUT2, Narp, and GAD65 mRNA induction by hyperactivity does not occur at DIV10 and is maximal at DIV18.
Activity-dependent VGLUT2, Narp, and GAD65 Gene Induction Is Developmentally Regulated
We next examined the dependence of VGLUT2, Narp, and GAD65 gene induction on the presynaptic maturation of our dissociated neuron-rich culture preparation in vitro. We can define presynaptic maturation of functional excitatory and inhibitory transmitter release by the levels of expression of VGLUT1 and VGAT mRNA and synaptic protein that coincides with the maturation of vesicle cycling in synapses (11, 12). The endogenous coordinated postnatal induction of VGLUT1 and VGAT mRNA and protein expression is the same in the neocortex and hippocampus in vivo (59, 60). Here, we find that in young cultures (DIV7 and DIV10), prolonged hyperactivity fails to induce expression of VGLUT2, Narp, and GAD65 altogether (Fig. 1B). At this time, endogenous VGLUT1 and VGAT mRNA and synaptic protein expression is quite low compared with DIV18 (12); quantal size is also reduced in immature cultures (11). By DIV14, VGLUT2, Narp, and GAD65 mRNA induction can be observed, but the maximal induction of these genes by increased glutamatergic synaptic activity occurs at DIV > 18 (Fig. 1B). At the same time, a decrease in VGLUT1 mRNA levels results from hyperactivity with maximal reduction (50%) seen by DIV18 (12). Together, our data indicate that the ability of neocortical neurons to induce expression of VGLUT2, Narp, and GAD65 after a 24-h period of increased glutamatergic synaptic activity develops during the “critical” period of functional maturation of presynaptic excitatory/inhibitory strength and network stability (corresponding to the second and third postnatal weeks in rodents). Because activity-dependent induction of VGLUT2, Narp, and GAD65 is maximal in synapse-differentiated neurons, we conclude that VGLUT2, Narp, and GAD65 gene induction after prolonged hyperactivity is a result of an excitatory/inhibitory imbalance produced by an endogenous increase in exocytotic-released glutamate.
VGLUT2 and Narp Induction Is a Coordinate Intermediate-Early Gene Response to Pharmacological Disinhibition
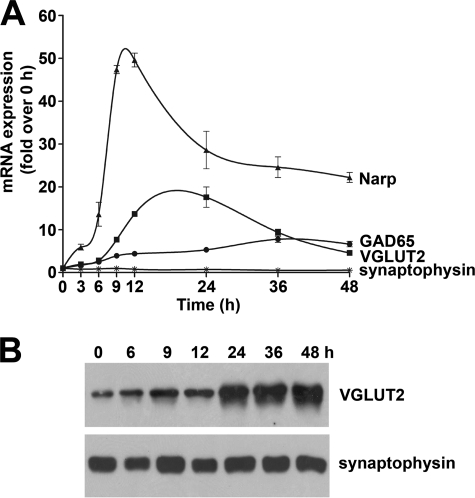
Homeostatic synaptic plasticity is generally studied after 48 h of inactivity or hyperactivity (4, 5). Here, we have assessed the time course of VGLUT2 gene induction after gabazine (20 μm; DIV18) and compared this to the induction of Narp, an established intermediate-early gene (22). In accordance, we find that Narp mRNA induction is biphasic with maximal levels observed by 12 h (50×), and elevated levels persist (>20×) at 48 h. Likewise, VGLUT2 induction displays a similar intermediate-early-like and biphasic gene response to prolonged glutamatergic synaptic hyperactivity with maximal stimulation (>15×) between 12–24 h, with elevated levels persisting (>5×) at 48 h (Fig. 2A). To confirm that changes in VGLUT2 mRNA expression result in corresponding changes in protein, we assessed the time-course of VGLUT2 protein expression by Western analysis. Western blots of enriched synaptic vesicle preparations (post 35,000 × g membranes) were prepared from differentiated neocortical cultures treated with gabazine (Fig. 2B). VGLUT2 protein levels are markedly increased by 24 h of disinhibition whereas synaptophysin protein levels do not vary. The time-course of GAD65 mRNA induction displays a later response than both Narp and VGLUT2 and is maximal at 48 h. Synaptophysin mRNA and protein levels are not affected by prolonged gabazine treatment of mature neuronal networks as previously reported (12). Taken together, our data suggest that VGLUT2 and Narp gene induction are coordinately linked (with GAD65 following a later time course) after an endogenous increase of glutamatergic synaptic activity in mature neocortical neuronal cultures.
FIGURE 2.
Time course of changes in VGLUT2, Narp, GAD65 expression triggered by hyperactivity. Cultures (DIV18) were incubated for 0, 3, 6, 9, 12, 24, 36, and 48 h with gabazine (20 μm) before harvesting for RNA (left) and protein (right) and analyzed by reverse transcription-PCR and Western blot. Data for reverse transcription-PCR are the mean ± S.E. (bars) values (n = 3). Note by 12 h (RNA) or 24 h (protein) VGLUT2 levels increase, whereas synaptophysin levels are not affected. Also note biphasic induction of intermediate-early gene transcription of Narp and VGLUT2 mRNA and delayed increase in GAD65 levels.
E-T Coupling of VGLUT2 and Narp by Hyperactivity Involves Ca2+ Signaling through L-type VGCCs
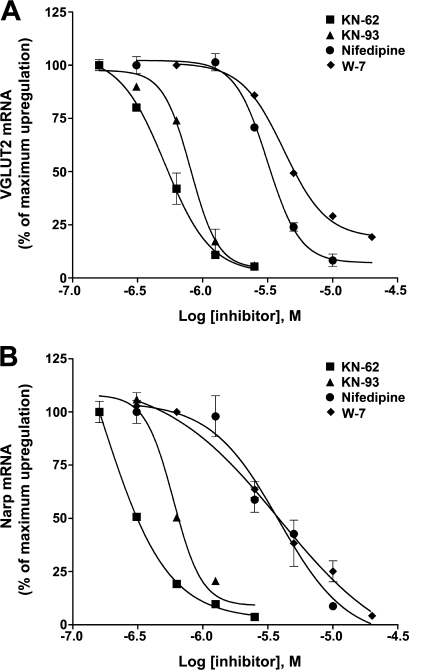
E-T coupling of gene regulation via Ca2+ signaling represents a key component to long term regulation of synaptic plasticity (46–48, 61). Here, we examined if the mechanism of VGLUT2 and Narp gene induction involves E-T coupling via L-type VGCCs. Cells were pretreated with selective L-type VGCC blockers, nifedipine or verapamil, at various concentrations for 30 min before gabazine was added. Nifedipine blocked the increase of VGLUT2 (n = 3; IC50 = 3.2 ± 0.3 μm) and Narp (n = 3; IC50 = 3.8 ± 0.3 μm) mRNA levels (Fig. 3). Nifedipine (10 μm) also completely blocks GAD65 induction (n = 3; 5.0× ± 0.2 versus 0.8× ± 0.04). When present without gabazine, nifedipine had minor effects (X-fold change) on VGLUT2 (2.5X ± 0.3), Narp (0.8X ± 0.1), and GAD65 (0.8X ± 0.05) mRNA levels. A different L-type channel blocker, verapamil (20 μm), also blocked increases in VGLUT2, Narp, and GAD65 mRNA after hyperactivity (data not shown). Our results indicate that neuronal Ca2+ influx through L-type VGCCs after hyperactivity initiates E-T coupling of VGLUT2, Narp, and GAD65 via Ca2+ signal transcription.
FIGURE 3.
L-type Ca2+ channels, CaM, and CaM-dependent kinases are involved in the induction of VGLUT2 and Narp mRNA levels triggered by prolonged hyperactivity. Pretreatment of cultures (DIV18) for 30 min with a selective L-type Ca2+ channel blocker nifedipine (10 μm), selective inhibitors of CaM kinases (KN-62, KN-93; 5 μm), or by a CaM antagonist (W7; 20 μm) significantly antagonized (>90%) the induction of VGLUT2 and Narp gene expression by hyperactivity after 24 h of treatment with gabazine (20 μm). Dose response curves of these compounds for VGLUT2 (left) and Narp (right) from a typical experiment were performed in triplicate.
E-T Coupling of VGLUT2 and Narp by Hyperactivity Involves Ca2+/Calmodulin
Ca2+/calmodulin (CaM) is a ubiquitous small Ca2+-binding protein that acts as a Ca2+ sensor in neurons and binds to several enzymes including CaMK, modulating their activity (62). To determine whether Ca2+ binding to CaM is involved in E-T coupling of VGLUT2 and Narp, we used the CaM inhibitor W7 (20 μm). W7 significantly reduced (>90% inhibition) the induction of VGLUT2, Narp, and GAD65 mRNA levels. The relative IC50 value of W7 to inhibit VGLUT2 and Narp induction was 4.23 ± 0.51 and 2.88 ± 0.33 μm, respectively (Fig. 3).
E-T Coupling of VGLUT2 and Narp by Hyperactivity Involves CaMK and ERK Signaling
Sustained increases in the phosphorylation of the cyclic AMP response element-binding protein (CREB) and ERK1/2 in vivo are involved in long term Ca2+ signal transcription-dependent synaptic plasticity (41, 46, 62–64). Because Ca2+-signal transcription by prolonged hyperactivity leads to VGLUT2 and Narp induction, we sought to determine whether CaMK/ERK signaling are also involved.
CaM and the CaM kinase isoforms II and IV have been implicated in Ca2+ signal transcription (65, 66). Interestingly, CaMKII is induced in neuronal somata after pharmacologic disinhibition by prolonged GABAA receptor blockade in vitro (67). To determine whether CaMKs are involved in E-T coupling of VGLUT2 and Narp, we used two antagonists of CaMKs, KN-62 and KN-93, which inactivate all CaMK isoforms (Fig. 3). At 5 μm concentration, KN-62 or KN-93 completely blocked induction of VGLUT2, Narp, and GAD65. The relative IC50 values of KN-62 and KN-93 to inhibit VGLUT2 induction were 0.51 ± 0.06 and 0.81 ± 0.08 μm and for Narp were 0.26 ± 0.04 and 0.59 ± 0.05 μm, respectively (Fig. 3). When present without gabazine, KN-62 had minor effects (X-fold change) on VGLUT2 (2.0X ± 0.2), Narp (0.5X ± 0.1), and GAD65 (0.7X ± 0.1) mRNA levels.
Both CREB and ERK1/2 have emerged as critical points of convergence in the signaling pathways regulating the expression of genes in Ca2+-signal transcription-dependent synaptic plasticity (68–71). To determine whether ERK1/2 signaling is also involved in E-T coupling of VGLUT2 and Narp, we used two antagonists of MAPK (also called ERK1/2 kinases), PD98059 and U0126. At 50 μm concentration, PD98059 significantly reduced both VGLUT2 and Narp gene induction after gabazine treatment (Fig. 4, C and D). U0126 (10 μm) was similarly effective (>80% inhibition). Taken together, our results indicate that CaM, CaMK, and ERK1/2 are important components in Ca2+ signal transcription involved in the coordinate induction of VGLUT2 and Narp triggered by prolonged endogenous increase in glutamatergic synaptic activity.
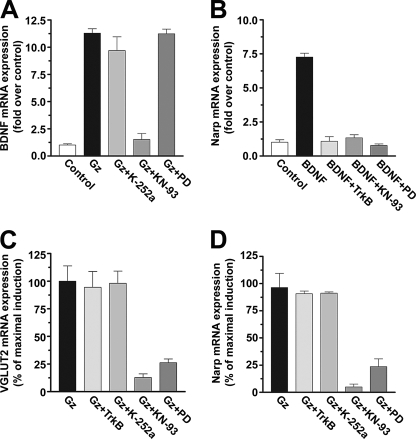
FIGURE 4.
BDNF and TrkB receptor signaling does not mediate co-induction of VGLUT2 and Narp triggered by prolonged hyperactivity. A, neuronal cultures (DIV18) were pretreated with various drugs (200 nm K-252a, 10 μm KN-93, 50 μm PD98059 (PD), or TrykB/Fc (TrkB) at 2 μg/ml (B) or 10 μg/ml (C and D) 15 min before the addition of 20 μm gabazine (Gz). BDNF mRNA induction by 12 h hyperactivity is shown. B, BDNF alone induces Narp expression. C and D, VGLUT2 and Narp induction is not BDNF/TrkB-dependent. Experiments shown are averages of two independent experiments performed in duplicate. Error bars indicate the S.D.
BDNF and TrkB Receptor Signaling Do Not Mediate VGLUT2 and Narp Induction Triggered by Hyperactivity
Activation of the L-type VGCC or Ca2+-permeable glutamate receptors specifically increases the expression of a group of Ca2+-regulated genes including BDNF, which is important for learning, neuronal survival, and other adaptive responses in the nervous system (63, 72–75). Activity-dependent scaling of postsynaptic strength (after relief from tetrodotoxin-induced inactivity) has been shown to be dependent on BDNF (8, 20). Furthermore, BDNF expression is induced by neuronal activity (76–78), and both BDNF and Narp are induced by seizure activity in the neocortex and hippocampus (24, 79–81). We, therefore, tested if E-T coupling of VGLUT2 and Narp involves BDNF-mediated signaling. First, we measured the induction of BDNF mRNA containing the activity-dependent exon III (55, 82) after prolonged gabazine treatment (Fig. 4A). BDNF mRNA induction by gabazine treatment (∼10X at 12 h) is selectively blocked by KN-93 (10 μm) but not by PD98059 (50 μm) or U0126 (10 μm). This indicates that E-T coupling of BDNF transcription arises via CaMK signaling directly to CREB, as expected (63, 72–74, 83).
To determine whether VGLUT2 and Narp induction is a result of BNDF-induced gene transcription, we exposed cultures to recombinant BDNF (25 ng/ml) in the presence and absence of various inhibitors. Interestingly, BDNF stimulates the expression of Narp (8x) but does not stimulate VGLUT2 (Fig. 4B). Higher concentrations of BDNF (100 ng/ml) also did not induce VGLUT2 transcription (data not shown). Recent work has shown that Narp is up-regulated during BDNF-induced long term potentiation in adult rat dentate gyrus in vivo (55). To determine whether BDNF-induced Narp induction in primary cortical cultures relies on signaling via the tyrosine kinase receptor, we pretreated cultures with K-252a (200 nm) to inhibit tyrosine kinase activity or with a recombinant TrkB/Fc fragment (2 μg/ml) that binds BDNF to prevent activation of the endogenous membrane-bound TrkB receptor. Both K-252a and the TrkB/Fc fragment completely block BDNF-triggered Narp induction (Fig. 4B). We also examined whether BDNF-induced Narp expression is dependent on CaMK and ERK signaling, similar to that described for Arc in vivo (71). Both KN-93 (10 μm) and U0126 (10 μm) completely block BDNF-triggered Narp induction (Fig. 4B).
BDNF increases only Narp expression, yet E-T coupling triggered by gabazine treatment increases both Narp and VGLUT2 transcription. The magnitude of Narp induction by prolonged hyperactivity is greater (3×) than that produced by BDNF alone. To determine whether Narp and VGLUT2 induction after prolonged hyperactivity relies on TrkB signaling, we blocked TrkB receptor kinase activity using K-252a (200 nm) or by adsorbing secreted BDNF using the TrkB/Fc fragment (10 μg/ml) in cultures and treated with gabazine (24 h). We report that VGLUT2 and Narp mRNA induction by hyperactivity is not affected by the blockade of BDNF actions or by TrkB receptor signaling. We conclude that E-T coupling that leads to the coordinate induction of VGLUT2 and Narp triggered by a prolonged increase in endogenous glutamatergic activity does not rely on CREB-mediated BDNF expression.
Immunodetection of VGLUT2 Synapses on GABAergic Interneurons
Narp clusters AMPA glutamate receptors on hippocampal GABAergic interneurons (22, 23). Hence, if cortical excitatory synapses encoded by VGLUT2 release glutamate at sites where Narp may also cluster AMPA glutamate receptors, then VGLUT2 terminals should be identifiable on GABAergic inhibitory neurons. GABAergic neurons can be identified by immunocytochemistry due to their expression of glutamic acid decarboxylase isoforms (GAD65 and GAD67) and by the presence of GABA itself (84). GAD65 and GABA are associated with synaptic vesicles that traffic through axons and are concentrated in nerve endings. Dendrites and neuronal somata also are immunoreactive for GABA due to the presence of GAD67. GAD67 mRNA expression is induced after prolonged hyperactivity (albeit to a lesser degree than GAD65), and we can visualize a modest increase in GABA levels in the processes of some neurons following gabazine treatment (Fig. 5, A and B) using an antibody against GABA itself. By double-label immunocytochemistry using antibodies against the dendritic marker MAP2 and GABA, processes that contain GABA also contain MAP2 (Fig. 5C), as expected. Thus, we can identify neuronal somata and dendritic processes of inhibitory neurons using anti-GABA or anti-GAD67 antibodies.
FIGURE 5.
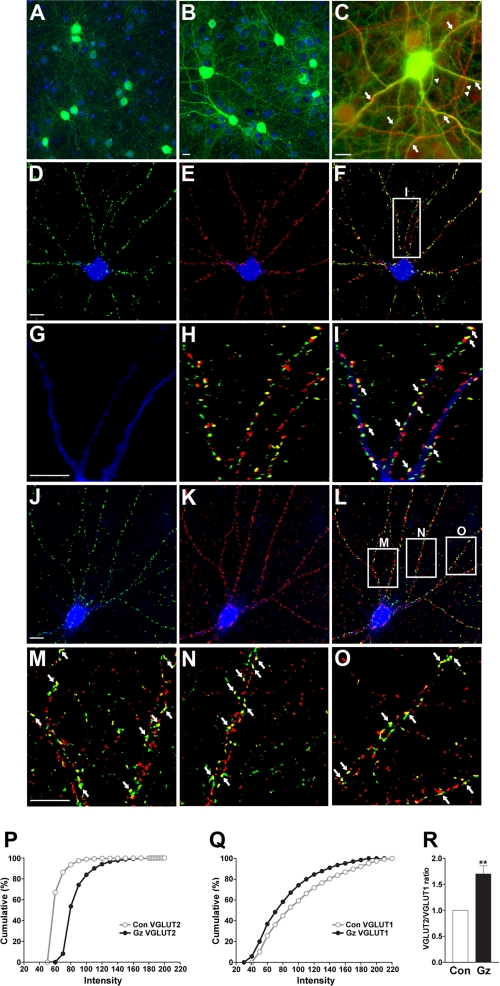
Immunodetection of VGLUT2 synapses on GABAergic interneurons. A, untreated control neuronal culture is shown. Inhibitory interneurons (green) can be identified using antibody against GABA itself. Unlabeled cells are visualized using 4′,6-diamidino-2-phenylindole (blue) nuclear staining. B, using the same fluorescence exposure time as A, treatment with gabazine (20 μm, 24 h) results in an apparent increase in GABA immunoreactivity (green) in cell bodies and in processes in inhibitory neurons. C, double staining with antibodies against GABA (green) and MAP2 (red), a marker for neuronal soma/dendrites, reveals co-localization predominantly in dendritic processes (arrows). Note green-only processes (arrowhead) that are likely inhibitory axonal projections and red-only processes (double arrowhead) that are likely excitatory dendritic processes. d–F, triple labeling with antibodies against GABA (blue), VGLUT2 (green), and synaptophysin (red) reveal abundant VGLUT2-positive synapses along GABA-containing interneuron processes and somata (40×). G–I show high power images (63×) of the boxed area in F revealing GABA-positive processes decorated with red, green, and yellow puncta. J–L, triple labeling with antibodies against GAD67 (blue), VGLUT2 (green), and VGLUT1 (red) reveals abundant VGLUT2-positive synapses along GAD67-containing interneuron processes and somata (40×). M–O, high power images (100×) of boxed areas in (L) reveal red, green, and yellow puncta on processes that correspond to synapses containing VGLUT1, VGLUT2, or both VGLUT1 and VGLUT2. Scale bar, 10 μm. P–R, the intensity profile for VGLUT2 (P) is right-shifted, whereas the VGLUT1 (Q) profile is left-shifted in gabazine-treated cultures on the cumulative percentage plot. Quantitation of VGLUT2 and VGLUT1 intensities in individual co-expressing puncta reveals an increase in VGLUT2/VGLUT1 ratio in gabazine (Gz)-treated cultures (R). Results shown are of a representative experiment that includes 1000 synapses. **, p < 0.01. Con, control.
VGLUT1 and VGLUT2 expression in differentiated cultures is confined to nerve terminals (11, 12). After 48 h of hyperactivity, VGLUT2 induction is also localized in excitatory synapses (12). Here, we find that VGLUT2-containing boutons are readily identifiable and abundantly expressed on cell somata and dendrites of GABAergic inhibitory neurons using double labeling with antibodies against VGLUT2 and with anti-GABA or anti-GAD67 antibodies (Fig. 5, d--O). VGLUT1-containing synapses are also present along GAD67-positive processes (Fig. 5, K–O). VGLUT2-encoded synapses are prominent on GABAergic neurons with varied morphology in vitro. Multipolar inhibitory neurons that express GAD67 or GABA are the most abundant cell type decorated with VGLUT2-containing axon terminals on cell somata and all along dendritic processes. Numerous VGLUT2 puncta that do not contain VGLUT1 are also found in the same processes of inhibitory neurons (Fig. 5, l–O).
We have previously reported that 48 h of treatment of mature cortical cultures by GABAA receptor blockade results in a marked increase (150%) in the immunofluorescence intensity of all VGLUT2 puncta, VGLUT1 expression in puncta is decreased ∼15–30%, and synaptophysin levels remain unchanged (11, 12). Synaptic VGLUT1 levels are bi-directionally regulated in hippocampal synapses (in an opposite manner to VGLUT2), whereas synaptotagmin and synapsin levels are not regulated by neural activity (11). Here, we show that many VGLUT2 synapses visualized on GABAergic processes co-express synaptophysin (Fig. 5, D–I) and VGLUT1 (Fig. 5, J–O). Previous work indicates that most VGLUT1/VGLUT2 co-expressing sites contain synaptophysin and oppose PSD95-containing post-synaptic elements (12). In the present study VGLUT2/VGLUT1 co-expressing synapses were most commonly found on inhibitory interneurons identified by GABA or GAD67 expression. VGLUT2 induction is also robust in puncta that do not contain VGLUT1 or synaptophysin (12), which are also present in the same dendritic processes of inhibitory interneurons (Fig. 5, L–O). Of 15,111 excitatory synapses present on dendrites and neuronal somata of GABAergic interneurons (n = 20 cells), ∼80% of them contain VGLUT1 (12,005/15,111), and half of these also contain VGLUT2 (5,850/15,111). We measured the fluorescence intensity of VGLUT1 and VGLUT2 in all co-expressing synapses and find opposite changes in the intensity of VGLUT2 and VGLUT1 (Fig. 5, P–R). The cumulative plots illustrate this phenomenon; the intensity of VGLUT2 puncta shifts to the right, and VGLUT1 shifts to the left (Fig. 5, P and Q). At VGLUT2/VGLUT1 co-expressing synapses, VGLUT2/VGLUT1 ratios show an increase of 1.79 ± 0.21 (p < 0.01; 1000 puncta analyzed). Together, our results indicate that although VGLUT1-encoded neocortical and hippocampal excitatory neurons down-regulate this transporter at all (or most) of their synapses after prolonged hyperactivity (11, 12), a subset of VGLUT1 synapses on GABAergic interneurons increase expression of VGLUT2 (Fig. 5, J–O). BecauseVGLUT2 is also a critical determinant for vesicle glutamate filling (57, 85–88), increased expression of VGLUT2 in a subset of VGLUT1 terminals may prevent a reduction in vesicle filling and release that would be expected at only VGLUT1-encoded excitatory synapses after prolonged hyperactivity (11).
Immunoisolation of Synaptic Vesicles from Intrinsic Neocortical Neuronal Culture Reveals Subpopulations of Glutamatergic Vesicles That Contain VGLUT1 or VGLUT2, and Only a Minority of Vesicles Co-express Both VGLUT1 and VGLUT2
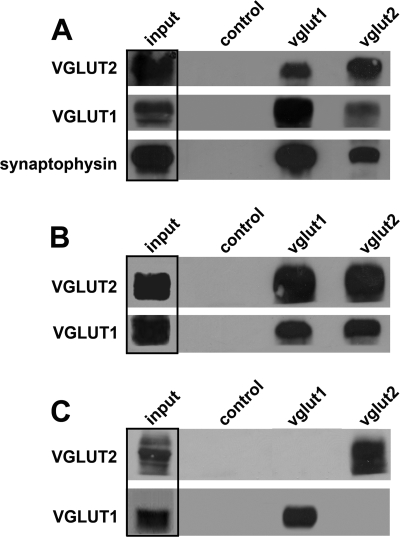
Because many VGLUT2-positive synapses also contain VGLUT1 in our cultures, we determined whether VGLUT1 and VGLUT2 are co-expressed on a subset of vesicles or if intracortically derived vesicles that only express VGLUT2 exist. We immunoisolated either VGLUT1- or VGLUT2-positive vesicle populations from differentiated cultures and tested for co-purification of the two transporters. Immunoisolation of synaptic vesicles was performed with beads coated with anti-VGLUT1 or anti-VGLUT2 antibodies. Nonspecific IgG-coated beads were used as a control. To increase expression of VGLUT2 protein, neuronal cultures were treated with gabazine (48 h). The immunobeads were incubated with enriched synaptic vesicle preparations (post 35,000 × g supernatants) in the absence of detergent. Material that remained bound to the immunobeads after several washing steps was analyzed by standard Western blotting (Fig. 6). Both anti-VGLUT1 and anti-VGLUT2 beads immunoisolated VGLUT1 and VGLUT2, respectively. In addition, both antibody-coupled beads could immunoisolate the respective other VGLUT isoform, albeit in lesser quantities, indicating that VGLUT1 and VGLUT2 can be present on the same vesicles in differentiated neocortical neurons in primary culture (Fig. 6A). If detergent was included in the vesicle preparation to disrupt lipid membranes, only the protein recognized by the respective bead-coupled antibody was detected in the bead fraction (data not shown). No association of VGLUT1 and VGLUT2 was detected in solubilized extracts of neocortical vesicles from DIV18 cultures, as previously reported for P14 hippocampus (37). To confirm that the co-isolation of VGLUT1 and VGLUT2 from neocortical neuronal cultures is the result of the presence of both isoforms on the same vesicles, we also isolated VGLUT1- and VGLUT2-containing vesicles (post 27,000 × g supernatants) from homogenates prepared from PC12 cells transfected with either VGLUT1 or VGLUT2 separately as well as from PC12 cells co-transfected with both VGLUT1 and VGLUT2 (Fig. 6, B and C). Using vesicles isolated from PC12 cells co-transfected with VGLUT1 and VGLUT2 cDNAs, we find that both anti-VGLUT1 and anti-VGLUT2 coupled beads immunoisolate both VGLUT1 and VGLUT2, indicating that both isoforms target the same vesicles when present in the same PC12 cell (Fig. 6B). On the other hand, when PC12 cells are separately transfected with VGLUT1 or with VGLUT2 and then the enriched vesicle preparations are mixed before immunoisolation, we find anti-VGLUT1 beads are only able to immunoisolate VGLUT1-containing vesicles, and anti-VGLUT2 beads are only able to immunoisolate VGLUT2-containing vesicles (Fig. 6C). These results, therefore, indicate that our antibody-vesicle and antibody-transporter reactions are specific and that nonspecific vesicle attachment to the beads and vesicle aggregations do not occur. Taken together, our results indicate that a minority subpopulation of vesicles in DIV18 neocortical neuronal cultures contains both VGLUT1 and VGLUT2. The majority of excitatory vesicles present in gabazine-treated differentiated neocortical primary neuronal cultures contain either VGLUT1 or VGLUT2 alone. Thus, in at least half of the glutamatergic synapses present on multipolar GABAergic interneurons in primary neocortical–derived neuronal cultures, multiple glutamatergic vesicle populations defined by expression of VGLUT1, VGLUT2, or VGLUT1/VGLUT2 are present.
FIGURE 6.
Immunoisolation of synaptic vesicles from mature neocortical neurons in vitro reveals three populations of glutamatergic vesicles that contain VGLUT1, VGLUT2, or both VGLUT1 and VGLUT2. A, shown are synaptic vesicle fractions from DIV18 neocortical neuronal cultures treated with gabazine (20 μm) for 24 h. Antibodies conjugated to beads are listed above the lanes; antibodies used for Western blotting are listed on the left. Note that less VGLUT2 is immunoisolated with anti-VGLUT1 beads than with anti-VGLUT2 beads, and the opposite is true for VGLUT1. B, shown is immunoisolation of VGLUT1 and VGLUT2 from homogenates prepared from PC12 cells co-transfected with both VGLUT1 and VGLUT2. Both antibody-coupled beads are able to immunoisolate vesicles that contain VGLUT1 and VGLUT2. C, shown is immunoisolation of VGLUT1 and VGLUT2 from PC12 cells that were transfected with either VGLUT1 or VGLUT2 and the homogenates mixed. No co-isolation of VGLUT1 and VGLUT2 was seen when the immunobeads were incubated with a mixture of vesicles that contained either only VGLUT1 or VGLUT2.
DISCUSSION
E-T coupling via Ca2+ signal transcription is triggered by the prolonged endogenous increase in glutamatergic synaptic activity resulting from chronic blockade of GABAA receptor-mediated inhibitory transmission. The homeostatic adaptation to this prolonged hyperactivity, or disinhibition, at the level of gene transcription includes early responses and intermediate-early and -late effects that are likely more long-lived. In cortical neurons, the induction of early gene responses by hyperactivity such as junB, c-fos, zif268, and fos via L-type VGCC has been described (89). These transcription factors are involved in the regulation of transcription of a set of genes that affect immediate early and late responses involved in the maintenance of increased synaptic strength. Co-activation of L-type VGCCs and synaptic N-methyl-d-aspartate/AMPA glutamate receptors results in the phosphorylation of both CREB and ERK1/2 (46, 64, 90–92). Downstream signaling involved in E-T coupling via Ca2+ signal transcription may include BDNF/TrkB signaling, CaMK, and/or ERK1/2 signaling to regulate expression of intermediate-early and -late gene responses (63, 64, 72, 93).
E-T coupling of gene regulation by L-type VGCCs represents a key component to long term regulation of synaptic plasticity (46–48, 61). The results of our current study indicate that the induction of VGLUT2 mRNA and transporter level on small synaptic vesicles by prolonged synaptic ionotropic glutamatergic activity is a consequence of increased gene transcription after a signaling cascade initiated by Ca2+ influx into neurons mediated by L-type Ca2+ channels. L-type VGCCs contribute a relatively minor component of synaptic Ca2+ transients and to neurotransmitter release, but they play a key role in coupling synaptic excitation to activation of transcriptional events thought to contribute to neuronal plasticity (61, 94). A second important component of E-T coupling includes CaMKs (62). CaMKs are important mediators of many forms of Ca2+-dependent plasticity (95, 96), and the two major isoforms of CaMKII in the brain, αCaMKII and βCaMKII, are expressed in neurons (67). We show that the blockade of all members of the CaMK family (CaMKI, CaMKII, and CaMKIV), using specific inhibitors KN-62 or KN-93, completely prevents the induction of VGLUT2, Narp, and GAD65 after hyperactivity.
It has previously been shown that αCaMKII and βCaMKII are bidirectionally regulated by neural activity at the protein level both in the cell somata and synaptic processes of neurons (67). Indeed, αCaMKII immunoreactive levels are increased in excitatory neuronal somata after hyperactivity produced by GABAA receptor blockade, whereas βCaMKII levels are decreased (67). CaMKIV has a predominant somatic/nuclear localization (95, 97) and is a critical component in the signaling pathway that regulates BDNF mRNA expression (95). A recent study by Ibata et al. (98) suggests that CaMKIV rather than CaMKII is the Ca2+ sensor for postsynaptic scaling of glutamate receptors. We confirm that BDNF stimulates Narp expression via TrkB-dependent signaling (55), but BDNF does not stimulate VGLUT2 expression. Furthermore, co-induction of VGLUT2 and Narp after prolonged hyperactivity is not sensitive to inhibition by TrkB-Fc nor is it blocked by inhibition of tyrosine receptor kinase activity with K-252a. Together, our data support the model of E-T coupling that can utilize discrete temporal and spatial requirements for Ca2+ signal transcription to support enhanced gene transcription (41, 42, 44, 45).
Narp is a recognized intermediate-early gene product induced by hyperactivity in vivo (24, 55, 99). Here, we show that Narp mRNA expression is dramatically induced together with VGLUT2 after a prolonged increase in endogenous glutamatergic synaptic activation of neocortical neuronal networks in vitro. The increase in Narp gene transcription observed likely leads to increased Narp protein biosynthesis, vesicular packaging, and release. We postulate that action-potential-driven Narp transcription may, therefore, be an important component of homeostatic synaptic plasticity after prolonged hyperactivity. Importantly, VGLUT2 gene induction shares a similar time-course of expression at the level of mRNA, as does Narp. Although BDNF mRNA induction after prolonged hyperactivity is not affected by inhibition of ERK1/2 signaling, the downstream transcription of Narp via TrkB signaling is ERK1/2-dependent. Hence, E-T coupling after prolonged hyperactivity bestows greater up-regulation of Narp mRNA after hyperactivity (∼3×) than does BDNF treatment alone. Thus, our results suggest that at least two discrete Ca2+ signal transcription pathways exist for activity-dependent Narp expression: 1) BDNF-driven and 2) another driven by CaMK/ERK signaling together with VGLUT2.
E-T coupling that triggers VGLUT2 induction after a prolonged increase in glutamatergic synaptic activity relies on CaMK/ERK signaling. This distinct E-T coupled Ca2+ signal transcription pathway does not involve BDNF and TrkB signaling. Because VGLUT2 and Narp induction by hyperactivity does not occur in young neurons (DIV < 10) and the magnitude of VGLUT2 and Narp induction increases during the maturation period of presynaptic vesicular glutamate and GABA storage and release and synaptic vesicle cycling (11, 12, 18), we postulate that the co-induction of VGLUT2 and Narp gene expression triggered by hyperactivity is linked to a prolonged excess in the synaptic activation by glutamate. In this event, glutamate signaling via VGLUT2-encoded synapses and Narp are important downstream consequences of E-T coupling after prolonged hyperactivity that could serve to restore excitatory/inhibitory network balance.
Cortical pyramidal neurons make synapses onto the dendrites of other pyramidal neurons to mediate excitatory feed-forward transmission. Pyramidal neurons are also able to regulate inhibitory feedback via distinct connections onto bipolar and multipolar GABAergic inhibitory neurons (21). Opposite changes in the strength of glutamatergic synapses onto excitatory and inhibitory interneurons has been recorded after prolonged changes in neuronal activity, suggesting that synapse-specific mechanisms govern synaptic scaling (19, 20). After prolonged hyperactivity, glutamatergic strength at pyramidal neuron-pyramidal neuron synapses is diminished (21), and there VGLUT1 levels in synapses and glutamate levels in individual small synaptic vesicles for release are decreased (11, 12). On the other hand, prolonged hyperactivity also increases glutamatergic strength at pyramidal neuron-bipolar inhibitory interneuron synapses (21), and here we show that there are increased VGLUT2 levels on subsets of small synaptic vesicles in synapses that target multipolar GABAergic inhibitory neurons (see also Ref. 12). The presence of VGLUT2 in VGLUT1-encoded cortical excitatory neurons offers a second population of vesicles that could affect glutamate release properties. For instance, VGLUT1 and VGLUT2 have been considered to be markers for neurons that exhibit low and high release probability, respectively (57, 85). Narp is known to be released from excitatory synapses and cluster post-synaptic AMPA-type glutamate receptors found on GABAergic interneurons (22, 23, 100, 101). Because Narp and VGLUT2 are co-regulated by prolonged hyperactivity, we postulate that Narp and glutamate are co-released from synapses.
In conclusion, our study demonstrates that VGLUT2 and Narp gene expression is highly regulated by a prolonged increase in glutamatergic synaptic activity in vitro. It has recently been established that alterations in the level of either VGLUT1 or VGLUT2 mRNA expression directly affect the levels of these transporter proteins in synapses on small synaptic vesicles and the quantal size of glutamate release (11, 88, 102). Thus, the induction of VGLUT2 mRNA and VGLUT2 protein at synaptic sites that are most prominent on neuronal processes containing GABA provides molecular and morphologic evidence of increased VGLUT2-encoded transmission at excitatory-inhibitory connections. The coordinate induction of VGLUT2 with Narp and GAD65 via E-T coupling described here provides a novel link to Ca2+ signal transcription in the interplay between excitatory and inhibitory neurotransmission and homeostatic synaptic scaling by GABAergic inhibitory feedback.
This work was supported, in whole or in part, by National Institutes of Health Grant NS36936. This work was also supported by a National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award (NARSAD Southwest Florida Investigator) and a Research Enhancement Fund by Louisiana State University Health Sciences Center (to J. D. E.).
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- BDNF
- brain-derived nerve growth factor
- CaM
- Ca2+/calmodulin
- CaMK
- CaM-dependent protein kinase
- CREB
- cyclic AMP response element-binding protein
- DIV
- days in vitro
- E-T
- excitation-transcription
- ERK1/2
- extracellular signal-regulated kinase 1/2
- GAD
- glutamic acid decarboxylase
- KN-62
- 4-[2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenl-1-piperazinyl)propyl]phenylisoquinoline sulfonic acid ester
- KN-93
- 2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine
- VGCC
- voltage-gated calcium channel
- MAPK
- mitogen-activated protein kinase
- Narp
- neuronal activity-regulated pentraxin
- PD98059
- 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one
- PBS
- phosphate-buffered saline
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis[2-aminohenylthio] butadiene
- VGLUT1
- vesicular glutamate transporter-1
- VGLUT2
- vesicular glutamate transporter-2
- W7
- N-(6-aminohexyl)-5-chloro-1-napthalenesulfonamide
- GABAergic
- γ-aminobutyric acid-ergic
- GABA
- γ-aminobutyric acid
- GABAA
- γ-aminobutyric acid, type A
- VGAT
- vesicular GABA/glycine amino acid transporter.
REFERENCES
- 1.Davis G. W. (2006) Annu. Rev. Neurosci. 29, 307–323 [DOI] [PubMed] [Google Scholar]
- 2.Thiagarajan T. C., Lindskog M., Malgaroli A., Tsien R. W. (2007) Neuropharmacology 52, 156–175 [DOI] [PubMed] [Google Scholar]
- 3.Turrigiano G. (2007) Curr. Opin. Neurobiol. 17, 318–324 [DOI] [PubMed] [Google Scholar]
- 4.O'Brien R. J., Kamboj S., Ehlers M. D., Rosen K. R., Fischbach G. D., Huganir R. L. (1998) Neuron 21, 1067–1078 [DOI] [PubMed] [Google Scholar]
- 5.Turrigiano G. G., Leslie K. R., Desai N. S., Rutherford L. C., Nelson S. B. (1998) Nature 391, 892–896 [DOI] [PubMed] [Google Scholar]
- 6.Wierenga C. J., Ibata K., Turrigiano G. G. (2005) J. Neurosci. 25, 2895–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilman V., van Rossum M. C., Turrigiano G. G. (2002) J. Neurosci. 22, 1328–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swanwick C. C., Murthy N. R., Kapur J. (2006) Mol. Cell. Neurosci. 31, 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saliba R. S., Michels G., Jacob T. C., Pangalos M. N., Moss S. J. (2007) J. Neurosci. 27, 13341–13351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saliba R. S., Gu Z., Yan Z., Moss S. J. (2009) J. Biol. Chem. 284, 32544–32550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson N. R., Kang J., Hueske E. V., Leung T., Varoqui H., Murnick J. G., Erickson J. D., Liu G. (2005) J. Neurosci. 25, 6221–6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Gois S., Schäfer M. K., Defamie N., Chen C., Ricci A., Weihe E., Varoqui H., Erickson J. D. (2005) J. Neurosci. 25, 7121–7133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman K. N., Pal S. K., Burrone J., Murthy V. N. (2006) Nat. Neurosci. 9, 642–649 [DOI] [PubMed] [Google Scholar]
- 14.Wierenga C. J., Walsh M. F., Turrigiano G. G. (2006) J. Neurophysiol. 96, 2127–2133 [DOI] [PubMed] [Google Scholar]
- 15.Han E. B., Stevens C. F. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 10817–10822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X. S., Xue L., Mohan R., Paradiso K., Gillis K. D., Wu L. G. (2007) J. Neurosci. 27, 3046–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulzer D., Edwards R. (2000) Neuron 28, 5–7 [DOI] [PubMed] [Google Scholar]
- 18.Erickson J. D., De Gois S., Varoqui H., Schafer M. K., Weihe E. (2006) Neurochem. Int. 48, 643–649 [DOI] [PubMed] [Google Scholar]
- 19.Rutherford L. C., DeWan A., Lauer H. M., Turrigiano G. G. (1997) J. Neurosci. 17, 4527–4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutherford L. C., Nelson S. B., Turrigiano G. G. (1998) Neuron 21, 521–530 [DOI] [PubMed] [Google Scholar]
- 21.Turrigiano G. G., Nelson S. B. (2004) Nat. Rev. Neurosci. 5, 97–107 [DOI] [PubMed] [Google Scholar]
- 22.O'Brien R. J., Xu D., Petralia R. S., Steward O., Huganir R. L., Worley P. (1999) Neuron 23, 309–323 [DOI] [PubMed] [Google Scholar]
- 23.Mi R., Tang X., Sutter R., Xu D., Worley P., O'Brien R. J. (2002) J. Neurosci. 22, 7606–7616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reti I. M., Baraban J. M. (2000) Neuropsychopharmacology 23, 439–443 [DOI] [PubMed] [Google Scholar]
- 25.Engel D., Pahner I., Schulze K., Frahm C., Jarry H., Ahnert-Hilger G., Draguhn A. (2001) J. Physiol. 535, 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartmann K., Bruehl C., Golovko T., Draguhn A. (2008) PLoS One 3, e2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirmse K., Dvorzhak A., Kirischuk S., Grantyn R. (2008) J. Physiol. 586, 5665–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendry S. H., Jones E. G. (1988) Neuron 1, 701–712 [DOI] [PubMed] [Google Scholar]
- 29.Kinney J. W., Davis C. N., Tabarean I., Conti B., Bartfai T., Behrens M. M. (2006) J. Neurosci. 26, 1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniels R. W., Collins C. A., Gelfand M. V., Dant J., Brooks E. S., Krantz D. E., DiAntonio A. (2004) J. Neurosci. 24, 10466–10474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniels R. W., Collins C. A., Chen K., Gelfand M. V., Featherstone D. E., DiAntonio A. (2006) Neuron 49, 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank C. A., Kennedy M. J., Goold C. P., Marek K. W., Davis G. W. (2006) Neuron 52, 663–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frank C. A., Pielage J., Davis G. W. (2009) Neuron 61, 556–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakata-Haga H., Kanemoto M., Maruyama D., Hoshi K., Mogi K., Narita M., Okado N., Ikeda Y., Nogami H., Fukui Y., Kojima I., Takeda J., Hisano S. (2001) Brain Res. 902, 143–155 [DOI] [PubMed] [Google Scholar]
- 35.Boulland J. L., Qureshi T., Seal R. P., Rafiki A., Gundersen V., Bergersen L. H., Fremeau R. T., Jr., Edwards R. H., Storm-Mathisen J., Chaudhry F. A. (2004) J. Comp. Neurol. 480, 264–280 [DOI] [PubMed] [Google Scholar]
- 36.Gras C., Vinatier J., Amilhon B., Guerci A., Christov C., Ravassard P., Giros B., El Mestikawy S. (2005) Neuropharmacology 49, 901–911 [DOI] [PubMed] [Google Scholar]
- 37.Herzog E., Takamori S., Jahn R., Brose N., Wojcik S. M. (2006) J. Neurochem. 99, 1011–1018 [DOI] [PubMed] [Google Scholar]
- 38.Liguz-Lecznar M., Skangiel-Kramska J. (2007) Int. J. Dev. Neurosci. 25, 107–114 [DOI] [PubMed] [Google Scholar]
- 39.Wallén-Mackenzie A., Nordenankar K., Fejgin K., Lagerström M. C., Emilsson L., Fredriksson R., Wass C., Andersson D., Egecioglu E., Andersson M., Strandberg J., Lindhe O., Schiöth H. B., Chergui K., Hanse E., Långström B., Fredriksson A., Svensson L., Roman E., Kullander K. (2009) J. Neurosci. 29, 2238–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schallier A., Massie A., Loyens E., Moechars D., Drinkenburg W., Michotte Y., Smolders I. (2009) Neurochem. Int. 55, 41–44 [DOI] [PubMed] [Google Scholar]
- 41.Tsuda M. (1996) Neurochem. Int. 29, 443–451 [DOI] [PubMed] [Google Scholar]
- 42.Bading H. (2000) Eur. J. Biochem. 267, 5280–5283 [DOI] [PubMed] [Google Scholar]
- 43.Dolmetsch R. (2003) Sci. STKE 2003, PE4. [DOI] [PubMed] [Google Scholar]
- 44.Cohen S., Greenberg M. E. (2008) Annu. Rev. Cell Dev. Biol. 24, 183–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wheeler D. G., Barrett C. F., Groth R. D., Safa P., Tsien R. W. (2008) J. Cell Biol. 183, 849–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolmetsch R. E., Pajvani U., Fife K., Spotts J. M., Greenberg M. E. (2001) Science 294, 333–339 [DOI] [PubMed] [Google Scholar]
- 47.Burgoyne R. D. (2007) Nat. Rev. Neurosci. 8, 182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbado M., Fablet K., Ronjat M., De Waard M. (2009) Biochim. Biophys. Acta 1793, 1096–1104 [DOI] [PubMed] [Google Scholar]
- 49.Brewer G. J., Torricelli J. R., Evege E. K., Price P. J. (1993) J. Neurosci. Res. 35, 567–576 [DOI] [PubMed] [Google Scholar]
- 50.Varoqui H., Erickson J. D. (1996) J. Biol. Chem. 271, 27229–27232 [DOI] [PubMed] [Google Scholar]
- 51.Zhu H., Duerr J. S., Varoqui H., McManus J. R., Rand J. B., Erickson J. D. (2001) J. Biol. Chem. 276, 41580–41587 [DOI] [PubMed] [Google Scholar]
- 52.Seeburg D. P., Feliu-Mojer M., Gaiottino J., Pak D. T., Sheng M. (2008) Neuron 58, 571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heaulme M., Chambon J. P., Leyris R., Molimard J. C., Wermuth C. G., Biziere K. (1986) Brain Res. 384, 224–231 [DOI] [PubMed] [Google Scholar]
- 54.Kurt S., Crook J. M., Ohl F. W., Scheich H., Schulze H. (2006) Hear. Res. 212, 224–235 [DOI] [PubMed] [Google Scholar]
- 55.Wibrand K., Messaoudi E., Håvik B., Steenslid V., Løvlie R., Steen V. M., Bramham C. R. (2006) Eur J. Neurosci. 23, 1501–1511 [DOI] [PubMed] [Google Scholar]
- 56.Schäfer M. K., Varoqui H., Defamie N., Weihe E., Erickson J. D. (2002) J. Biol. Chem. 277, 50734–50748 [DOI] [PubMed] [Google Scholar]
- 57.Varoqui H., Schäfer M. K., Zhu H., Weihe E., Erickson J. D. (2002) J. Neurosci. 22, 142–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueno S., Bracamontes J., Zorumski C., Weiss D. S., Steinbach J. H. (1997) J. Neurosci. 17, 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minelli A., Edwards R. H., Manzoni T., Conti F. (2003) Brain Res. Dev. Brain Res. 140, 309–314 [DOI] [PubMed] [Google Scholar]
- 60.Minelli A., Alonso-Nanclares L., Edwards R. H., DeFelipe J., Conti F. (2003) Neuroscience 117, 337–346 [DOI] [PubMed] [Google Scholar]
- 61.Murphy T. H., Worley P. F., Baraban J. M. (1991) Neuron 7, 625–635 [DOI] [PubMed] [Google Scholar]
- 62.Wayman G. A., Lee Y. S., Tokumitsu H., Silva A. J., Soderling T. R. (2008) Neuron 59, 914–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.West A. E., Chen W. G., Dalva M. B., Dolmetsch R. E., Kornhauser J. M., Shaywitz A. J., Takasu M. A., Tao X., Greenberg M. E. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 11024–11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas G. M., Huganir R. L. (2004) Nat. Rev. Neurosci. 5, 173–183 [DOI] [PubMed] [Google Scholar]
- 65.Matthews R. P., Guthrie C. R., Wailes L. M., Zhao X., Means A. R., McKnight G. S. (1994) Mol. Cell. Biol. 14, 6107–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Limbäck-Stokin K., Korzus E., Nagaoka-Yasuda R., Mayford M. (2004) J. Neurosci. 24, 10858–10867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thiagarajan T. C., Piedras-Renteria E. S., Tsien R. W. (2002) Neuron 36, 1103–1114 [DOI] [PubMed] [Google Scholar]
- 68.Atkins C. M., Selcher J. C., Petraitis J. J., Trzaskos J. M., Sweatt J. D. (1998) Nat. Neurosci. 1, 602–609 [DOI] [PubMed] [Google Scholar]
- 69.Impey S., Obrietan K., Wong S. T., Poser S., Yano S., Wayman G., Deloulme J. C., Chan G., Storm D. R. (1998) Neuron 21, 869–883 [DOI] [PubMed] [Google Scholar]
- 70.Davis S., Vanhoutte P., Pages C., Caboche J., Laroche S. (2000) J. Neurosci. 20, 4563–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ying S. W., Futter M., Rosenblum K., Webber M. J., Hunt S. P., Bliss T. V., Bramham C. R. (2002) J. Neurosci. 22, 1532–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finkbeiner S., Tavazoie S. F., Maloratsky A., Jacobs K. M., Harris K. M., Greenberg M. E. (1997) Neuron 19, 1031–1047 [DOI] [PubMed] [Google Scholar]
- 73.Tao X., Finkbeiner S., Arnold D. B., Shaywitz A. J., Greenberg M. E. (1998) Neuron 20, 709–726 [DOI] [PubMed] [Google Scholar]
- 74.Shieh P. B., Hu S. C., Bobb K., Timmusk T., Ghosh A. (1998) Neuron 20, 727–740 [DOI] [PubMed] [Google Scholar]
- 75.Tabuchi A., Nakaoka R., Amano K., Yukimine M., Andoh T., Kuraishi Y., Tsuda M. (2000) J. Biol. Chem. 275, 17269–17275 [DOI] [PubMed] [Google Scholar]
- 76.Zafra F., Castrén E., Thoenen H., Lindholm D. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lindholm D., Castrén E., Berzaghi M., Blöchl A., Thoenen H. (1994) J. Neurobiol. 25, 1362–1372 [DOI] [PubMed] [Google Scholar]
- 78.Wetmore C., Olson L., Bean A. J. (1994) J. Neurosci. 14, 1688–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Isackson P. J., Huntsman M. M., Murray K. D., Gall C. M. (1991) Neuron 6, 937–948 [DOI] [PubMed] [Google Scholar]
- 80.Nibuya M., Morinobu S., Duman R. S. (1995) J. Neurosci. 15, 7539–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ploski J. E., Newton S. S., Duman R. S. (2006) J. Neurochem. 99, 1122–1132 [DOI] [PubMed] [Google Scholar]
- 82.Shieh P. B., Ghosh A. (1999) J. Neurobiol. 41, 127–134 [PubMed] [Google Scholar]
- 83.Zafra F., Hengerer B., Leibrock J., Thoenen H., Lindholm D. (1990) EMBO J. 9, 3545–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soghomonian J. J., Martin D. L. (1998) Trends Pharmacol. Sci. 19, 500–505 [DOI] [PubMed] [Google Scholar]
- 85.Fremeau R. T., Jr., Troyer M. D., Pahner I., Nygaard G. O., Tran C. H., Reimer R. J., Bellocchio E. E., Fortin D., Storm-Mathisen J., Edwards R. H. (2001) Neuron 31, 247–260 [DOI] [PubMed] [Google Scholar]
- 86.Herzog E., Bellenchi G. C., Gras C., Bernard V., Ravassard P., Bedet C., Gasnier B., Giros B., El Mestikawy S. (2001) J. Neurosci. 21, RC181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takamori S., Rhee J. S., Rosenmund C., Jahn R. (2001) J. Neurosci. 21, RC182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moechars D., Weston M. C., Leo S., Callaerts-Vegh Z., Goris I., Daneels G., Buist A., Cik M., van der Spek P., Kass S., Meert T., D'Hooge R., Rosenmund C., Hampson R. M. (2006) J. Neurosci. 26, 12055–12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murphy T. H., Worley P. F., Nakabeppu Y., Christy B., Gastel J., Baraban J. M. (1991) J. Neurochem. 57, 1862–1872 [DOI] [PubMed] [Google Scholar]
- 90.Xia Z., Dudek H., Miranti C. K., Greenberg M. E. (1996) J. Neurosci. 16, 5425–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mao X., Bravo I. G., Cheng H., Alonso A. (2004) Exp. Cell Res. 292, 304–311 [DOI] [PubMed] [Google Scholar]
- 92.Tauskela J. S., Fang H., Hewitt M., Brunette E., Ahuja T., Thivierge J. P., Comas T., Mealing G. A. (2008) J. Biol. Chem. 283, 34667–34676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choe E. S., Wang J. Q. (2002) Neuroscience 114, 557–565 [DOI] [PubMed] [Google Scholar]
- 94.Agoston D. V., Eiden L. E., Brenneman D. E. (1991) J. Neurosci. Res 28, 140–148 [DOI] [PubMed] [Google Scholar]
- 95.Soderling T. R. (1999) Trends Biochem. Sci 24, 232–236 [DOI] [PubMed] [Google Scholar]
- 96.Lisman J., Schulman H., Cline H. (2002) Nat. Rev. Neurosci. 3, 175–190 [DOI] [PubMed] [Google Scholar]
- 97.Nakamura Y., Okuno S., Sato F., Fujisawa H. (1995) Neuroscience 68, 181–194 [DOI] [PubMed] [Google Scholar]
- 98.Ibata K., Sun Q., Turrigiano G. G. (2008) Neuron 57, 819–826 [DOI] [PubMed] [Google Scholar]
- 99.Reti I. M., Miskimon M., Dickson M., Petralia R. S., Takamiya K., Bland R., Saini J., During M. J., Huganir R. L., Baraban J. M. (2008) Neuroscience 151, 352–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Brien R., Xu D., Mi R., Tang X., Hopf C., Worley P. (2002) J. Neurosci. 22, 4487–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reti I. M., Reddy R., Worley P. F., Baraban J. M. (2002) J. Neurochem. 82, 935–944 [DOI] [PubMed] [Google Scholar]
- 102.Wojcik S. M., Rhee J. S., Herzog E., Sigler A., Jahn R., Takamori S., Brose N., Rosenmund C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 7158–7163 [DOI] [PMC free article] [PubMed] [Google Scholar]