Abstract
The expansion of a trinucleotide repeat sequence, such as CAG/CTG, has been pinpointed as the molecular basis for a number of neurodegenerative disorders. It has been proposed that as part of the expansion process, these repetitive sequences adopt non-B conformations such as hairpins. However, the prevalence of these hairpins and their contributions to the DNA expansion have not been well defined. In this work, we utilized a molecular beacon strategy to examine the stability of the (CAG)10 hairpin and also its behavior in the presence of the complementary (CTG)10 hairpin. We find that the two hairpins represent kinetically trapped species that can coexist but irreversibly convert to duplex upon thermal induction. Furthermore, as monitored by fluorescence and optical analysis, modifications to the base composition of either the loop or stem region have a profound effect on the ability of the trinucleotide repeat hairpins to convert to duplex. Additionally, the rate of duplex formation is also reduced with these loop and stem-modified hairpins. These results demonstrate that the trinucleotide repeat hairpins can convert to duplex via two independent mechanisms as follows: the loop-loop interactions found in kissing hairpins or the stem-stem interactions of a cruciform.
Keywords: DNA, DNA/Damage, DNA/Repair, DNA/Structure, Nucleic Acid/Chemistry, Nucleic Acid/Structure
Introduction
The expansion of a DNA trinucleotide repeat (TNR)3 sequence has been identified as the molecular basis for a number of neurodegenerative disorders (1–5). For example, Huntington disease is caused by the expansion of a CAG/CTG sequence. These TNR sequences have been shown previously to fold into discrete conformations in vitro, notably hairpins (6–9). It has been proposed that formation of these hairpins may contribute to the expansion process by facilitating polymerase slippage during replication and/or repair (9–13). Recent studies using a mouse model of Huntington disease have implicated oxidative damage and the DNA repair enzyme 8-oxoguanine glycosylase (OGG1) in TNR expansion (14). Furthermore, we have shown that when present in the hairpin conformation, TNR sequences are more susceptible to oxidative damage yet less efficiently repaired by OGG1 (15). Given the proposed roles for hairpins in TNR expansion, the stability of these structures and their ability to persist warrant consideration. Toward that end, in this work we examine the behavior of the (CAG)10 hairpin in the presence of the complementary (CTG)10 hairpin.
It has been reported that complementary CAG and CTG sequences can form a duplex. These previous studies have relied predominantly on UV-visible spectroscopy and calorimetry (16–19). These methodologies, although powerful, are inherently limited because they provide a collective observation of both DNA strands. Therefore, in this work we utilized a molecular beacon strategy to selectively monitor the behavior of a single hairpin in the presence of the complementary sequence. This strand-specific strategy has allowed us to identify molecular recognition events that are important in the conversion from individual hairpins to the CAG/CTG duplex.
Extensive research on the secondary structure of RNA has shown that complementary nucleic acid hairpins can convert to duplex via the formation of kissing hairpins and/or a cruciform intermediate (20–28). In a kissing complex, the two hairpins interact via the loop regions; in a cruciform, the interaction is via the terminal region of the stem. Given the precedent for these molecular recognition events in RNA hairpin to duplex conversion, we examined their potential role in TNR DNA hairpin to duplex conversion. To accomplish this, we designed a series of TNR hairpins that are modified in either the loop or stem region. Using our molecular beacon strategy, along with other chemical, biochemical, and biophysical methods, we have found that both kissing hairpins and cruciform intermediates provide viable pathways for the hairpin to duplex conversion.
EXPERIMENTAL PROCEDURES
Oligonucleotide Synthesis and Purification
Oligonucleotides were synthesized using standard phosphoramidite chemistry (29). All phosphoramidites, including the 5′-fluorescein phosphoramidite (6-(3′,6′-dipivaloylfluoresceinyl-6-carboxamido)-hexyl-1-O-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite), and the 3′-dabcyl CPG supports (1-dimethoxytrityloxy-3-[O-(N-4′-carboxy-4-(dimethylamino)-azobenzene)-3-aminopropyl)]-propyl-2-O-succinoyl-alkylamino-controlled pore glass) were purchased from Glen Research. The (CAG)10 sequence containing the pendant 5′-fluorescein and 3′-dabcyl groups was purified using a Dynamax Microsorb C18 reverse phase HPLC column (10 × 250 mm). The mobile phases were 30 mm ammonium acetate and acetonitrile with a gradient of 5–25% acetonitrile over 25 min (3.5 ml/min). Product identity was confirmed by electrospray ionization-mass spectrometry (supplemental Fig. 1). During the synthesis of all other oligonucleotides (i.e. those lacking the pendant fluorophore and quencher), the 5′-dimethoxytrityl group was retained to aid in purification. The crude products were purified using a Dynamax Microsorb C18 reverse phase HPLC column (10 × 250 mm). The mobile phases were 30 mm ammonium acetate and acetonitrile with a gradient of 5–25% acetonitrile over 25 min (3.5 ml/min). The 5′-dimethoxytrityl group was removed by incubation in 80% glacial acetic acid for 12 min followed by ethanol precipitation. A second HPLC purification was performed using the same column and mobile phases as above with a gradient of 0–15% acetonitrile over 35 min (3.5 ml/min). Quantification of oligonucleotides was performed at 90 °C using the ϵ260 values estimated for single-stranded DNA (30) and a Beckman Coulter DU800 UV-visible spectrophotometer equipped with a Peltier thermoelectric device.
Fluorescence Measurements
Fluorescence profiles were obtained using an MX4000 real time PCR instrument (Stratagene). Hairpins (3 μm) were obtained by incubating each oligonucleotide at 90 °C for 5 min followed by slow cooling to room temperature in a buffer of 20 mm sodium phosphate, 10 mm NaCl, pH 7.0. Each hairpin was chilled on ice for ∼30 min prior to mixing with another hairpin. Two hairpins were then combined to yield a 25-μl sample containing 1.5 μm of each hairpin in 20 mm sodium phosphate, 10 mm NaCl, pH 7.0. The temperature cycle was as follows: 10 °C for 10 min, 10–90 °C at 1 °C/min, 90 °C for 5 min, and 90 to 10 °C at 1 °C/min. The excitation wavelength was 492 nm, and fluorescence was monitored at 520 nm. Melting temperatures (Tm) were obtained by taking the maximum in the first derivative of the fluorescence profile. The sequence of the noncomplementary control oligonucleotide is 5′-TGTGATAGCACTGATATATACCTTAGACCCATGA-3′.
Optical Analysis
Melting temperatures for each oligonucleotide were obtained with a Beckman Coulter DU800 UV-visible spectrophotometer equipped with a Peltier thermoelectric device. Samples contained varying concentrations of an oligonucleotide in 20 mm sodium phosphate, 10 mm NaCl, pH 7.0. Prior to optical analysis, the samples were incubated at 90 °C for 5 min followed by slow cooling to room temperature. The samples were then heated from 25 to 90 °C at a rate of 1 °C/min while monitoring absorbance at 260 nm; for the reverse experiment, the samples were cooled from 90 to 25 °C at a rate of 1 °C/min. Melting temperatures were obtained by taking the maximum in the first derivative of the absorbance profile generated while heating from 25 to 90 °C.
For the hairpin to duplex conversion experiments, each hairpin was first formed by incubating the appropriate oligonucleotide (3 μm) at 90 °C for 5 min followed by slow cooling to room temperature in a buffer of 20 mm sodium phosphate, 10 mm NaCl, pH 7.0. Prior to mixing, each hairpin was incubated at 10 °C. The hairpins were combined to yield a 325-μl sample containing 1.5 μm of each hairpin in 20 mm sodium phosphate, 10 mm NaCl, pH 7.0. The temperature cycle was as follows: 10–90 °C at 1 °C/min followed by the reverse experiment of 90 to 10 °C at 1 °C/min. Absorbance was monitored at 260 nm.
Characterization of Hairpins Using the Chemical Probe DEPC
Oligonucleotides were 5′-32P-end-labeled using T4 polynucleotide kinase according to the manufacturer's protocol. Hairpins were obtained by incubating each oligonucleotide at 90 °C for 5 min followed by slow cooling to room temperature in a buffer of 20 mm sodium phosphate, 10 mm NaCl, pH 7.0. Samples for chemical probe experiments contained a single hairpin (5 μm) and 13.3–267 μm DEPC in 20 mm sodium phosphate, 10 mm NaCl, pH 7.0, and were incubated at 37 °C for 30 min. Following incubation with DEPC, samples were dried under vacuum, treated with 10% piperidine (v/v) at 90 °C for 30 min, and again dried under vacuum. Samples were resuspended in denaturing loading buffer (80% formamide, 0.1% xylene cyanol, and 0.1% bromphenol blue) and electrophoresed through an 18% denaturing polyacrylamide gel. Products were visualized using phosphorimagery.
Determination of Rate Constants for Hairpin to Duplex Conversion
The (CAG)10 sequence was 5′-32P-end-labeled. Individual hairpins were obtained by incubation of the appropriate oligonucleotide at 90 °C for 5 min followed by slow cooling to room temperature. Experiments were performed under pseudo first-order conditions using 0.25 μm (CAG)10 and 2.5 μm of the complementary hairpin in 20 mm sodium phosphate, 10 mm NaCl, pH 7.0. Prior to mixing, each hairpin was incubated at 37 °C for ∼30 min; the hairpins were then combined and incubated at 37 °C. The longest time point was mixed first, and subsequent samples were timed and loaded onto the same gel with a 15-min delay between the loading of each sample. This procedure was followed for the determination of all rate constants with the exception of an experiment performed at 4 °C, in which there was no delay between the loading of each sample and an experiment performed in the presence of 80 mm NaCl, which had a 10-min delay between the loading of each sample. Samples were electrophoresed through a 12% native polyacrylamide gel. Electrophoresis was performed at 25–50 V for 7–8 h at 4 °C. Products were visualized using phosphorimagery. The amount of duplex product was quantified and plotted as a function of time. The data were fitted in Origin to a monoexponential curve using the following equation: D = H0 (1 − e−k′t), where D is percentage of duplex at time t (in seconds), H0 is percentage of hairpin at t = 0, which was set to 100% during data fitting (31), and k′ is the observed rate constant. The reported second-order rate constant was obtained by dividing k′ by the concentration of the sequence that is in excess (2.5 μm). For the (CAG)10 and (CTG)10 hairpin to duplex conversion experiments, a significant amount of duplex product appeared at t = 0; therefore, in this case, as described previously, we included a dead time parameter to account for this time lapse (32). The dead time was 19 s on average.
RESULTS
Design and Characterization of the FL-(CAG)10-DCL Molecular Beacon
To probe the behavior of the (CAG)10 hairpin in the presence of the complementary sequence, we utilized a molecular beacon strategy. Our molecular beacon consists of a (CAG)10 sequence with a fluorophore (fluorescein) and quencher (dabcyl) covalently attached at the 5′- and 3′-ends, respectively (Fig. 1A). By design, when the molecular beacon is in the hairpin conformation, the fluorophore and quencher are in close proximity; this results in fluorescence quenching (Fig. 1B). In contrast, when the molecular beacon is present in an unfolded conformation or is base-paired to the complementary sequence to form a double-stranded duplex, the fluorophore and quencher are spatially separated, and fluorescence will be observed (Fig. 1C).
FIGURE 1.
(CAG)10 molecular beacon. A, chemical structure of the FL-(CAG)10-DCL molecular beacon. B, schematic illustration of the non-fluorescent conformation of the molecular beacon. C, schematic illustration of the fluorescent conformations of the molecular beacon.
We first obtained the fluorescence profile of the FL-(CAG)10-DCL molecular beacon by monitoring fluorescence intensity at 520 nm as a function of temperature (Fig. 2). At temperatures below 40 °C, only low levels of fluorescence were observed. However, as the temperature was increased above 40 °C, there was an increase in fluorescence intensity, which plateaus at 60 °C, and remains constant at higher temperatures. The temperature at the mid-point of this transition, 49.0 ± 1.0 °C, corresponds to the Tm of the FL-(CAG)10-DCL hairpin. These fluorescence data indicate that, consistent with our design strategy, the molecular beacon adopts a hairpin conformation. At temperatures above the Tm of the hairpin, however, the hairpin melted, and the molecular beacon was present in an unfolded conformation.
FIGURE 2.
Fluorescence profile for the (CAG)10 molecular beacon. The fluorescence intensity at 520 nm was monitored as a function of temperature for the FL-(CAG)10-DCL molecular beacon. Conditions used were 1.5 μm FL-(CAG)10-DCL in 20 mm sodium phosphate, 10 mm NaCl, pH 7.0.
To determine the contribution of the pendant fluorophore and quencher to thermal stability of the molecular beacon hairpin, a (CAG)10 sequence lacking these pendant moieties was also prepared. The thermal stability of this hairpin was obtained using optical melting analysis; a Tm of 53.5 ± 0.1 °C was observed (supplemental Fig. 2A). Furthermore, the Tm does not vary over a 5-fold range in concentration. This concentration-independent behavior is consistent with an intramolecular fold (33), as opposed to a multistranded conformation. When analyzed by optical melting, the FL-(CAG)10-DCL molecular beacon displays a Tm of 53.4 ± 0.1 °C (supplemental Fig. 2B). Based on the similar Tm obtained by optical melting for (CAG)10 and FL-(CAG)10-DCL, we concluded that the pendant fluorophore and quencher do not significantly modulate the thermal stability of the molecular beacon hairpin.
Although these fluorescence and optically based methods have allowed us to confirm that the (CAG)10 sequence does, in fact, adopt a hairpin conformation, they do not provide a picture of the hairpin at the molecular level. For instance, they do not reveal how many bases are in the loop of the hairpin. This structural information was obtained based on modification of the hairpin by DEPC, which is a chemical probe of nucleobase accessibility. DEPC selectively modifies unpaired purines (A ≫ G) such as those in base bulges or loops (34). Hyper-reactivity toward DEPC of two adenines and one guanine in the (CAG)10 hairpin suggests that the loop is defined as the four nucleobases shown in Fig. 3. The reactivity toward DEPC at G18 is above background, but not as intense as that observed at G15, and it has led us to assign this nucleobase as being part of the loop-closing base pair. However, it is possible that the G-C base pair that we have assigned as the loop-closing base pair is also part of the loop that would then describe a six-base loop. Although the structure shown in Fig. 3 positions both A14 and A17 in the loop of the hairpin, we find that these two adenines are not modified by DEPC to the same extent. This may be due to different stacking interactions with the adjacent bases. Furthermore, we find that the adenines in the stem of the hairpin are also modified by DEPC, although to a lesser extent than those in the loop; this reactivity is consistent with increased dynamics at A·A mismatch sites.
FIGURE 3.
Characterization of the (CAG)10 hairpin using the chemical probe DEPC. A, autoradiogram revealing strand cleavage of (CAG)10 hairpin assembly. Conditions are 5 μm (CAG)10 in 20 mm sodium phosphate, 10 mm NaCl, pH 7.0. Lanes 1 and 2 contain DNA alone and piperidine-treated DNA, respectively. Lane 3 contains DNA incubated for 30 min at 37 °C in the presence of 13.3 μm DEPC followed by piperidine treatment. A/G and C/T are Maxam/Gilbert sequencing reactions. Schematic representation of the location of damage induced by DEPC. The asterisk represents the location of the 32P label. B, histogram representing strand cleavage in lane 3, revealing hyper-reactivity of two adenines and one guanine located in the loop of the (CAG)10 hairpin.
Behavior of the (CAG)10 Hairpin in the Presence of the Complementary (CTG)10 Sequence, Equilibrium Studies
We next used the FL-(CAG)10-DCL molecular beacon to examine the behavior of the (CAG)10 hairpin in the presence of the complementary (CTG)10 sequence. As shown here by optical melting analysis and modification by DEPC, the (CTG)10 sequence also adopts a hairpin conformation (supplemental Fig. 3). Furthermore, similar to the (CAG)10 hairpin, the loop of the (CTG)10 hairpin contains either four or six nucleobases.
The FL-(CAG)10-DCL and (CTG)10 hairpins were combined in a 1:1 ratio, and the fluorescence intensity was monitored as a function of temperature (Fig. 4). At temperatures below 25 °C, fluorescence quenching was observed; therefore, at these temperatures the FL-(CAG)10-DCL hairpin persisted in the presence of the complementary sequence. However, as the temperature is increased, an increase in fluorescence intensity was observed. Please note that the behavior of the FL-(CAG)10-DCL hairpin was monitored selectively in this assay because the (CTG)10 hairpin was not fluorescently labeled. Therefore, in the presence of the (CTG)10 hairpin, the increase in fluorescence centered at 38.8 ± 0.8 °C reflected melting of the FL-(CAG)10-DCL hairpin. Thus, the FL-(CAG)10-DCL hairpin had a Tm of 49.0 ± 1.0 °C in the absence of (CTG)10 and 38.8 ± 0.8 °C in the presence of (CTG)10. The complementary sequence lowered the thermal stability of the (CAG)10 hairpin ∼10 °C. Indeed, this effect on the thermal stability of the (CAG)10 hairpin was specific to the complementary (CTG)10 sequence. In the presence of a noncomplementary oligonucleotide, no change in the thermal stability of the FL-(CAG)10-DCL hairpin is observed (Fig. 4).
FIGURE 4.
Fluorescence profile for the (CAG)10 molecular beacon in the presence of complementary and noncomplementary DNA. The fluorescence intensity at 520 nm was monitored as a function of temperature for the FL-(CAG)10-DCL molecular beacon in the absence of other DNAs (open circles) or in the presence of equimolar amounts of (CTG)10 (closed circles) or a noncomplementary oligonucleotide (crosses). Conditions were 1.5 μm FL-(CAG)10-DCL and 1.5 μm (CTG)10 or noncomplementary sequence in 20 mm sodium phosphate, 10 mm NaCl, pH 7.0.
Although our fluorescence-based assay was particularly well suited for examining the thermal stability of the FL-(CAG)10-DCL hairpin, it did not allow us to determine whether the hairpin merely melted in the presence of (CTG)10 or if the complementary sequences have converted to duplex; fluorescence would be observed in both cases. To determine whether destabilization of the FL-(CAG)10-DCL hairpin occurred as part of the conversion to FL-(CAG)10-DCL/(CTG)10 duplex, optical analysis was performed. For these experiments, an equimolar amount of the FL-(CAG)10-DCL and (CTG)10 hairpins was combined, and absorbance at 260 nm was monitored as a function of temperature (Fig. 5). Two prominent transitions were observed in the absorbance profile. The first was a decrease in absorbance centered at 37.3 ± 0.6 °C; this decrease reflected an increase in order and base stacking that was consistent with duplex formation. Thus, although the individual hairpins persist at low temperatures, upon thermal induction the two complementary hairpins convert to duplex. Further confirmation that the decrease in absorbance corresponds to duplex formation was provided by the observation of a second transition, an increase in absorbance, at 75.7 ± 1.2 °C. This increase in absorbance reflects melting of the FL-(CAG)10-DCL/(CTG)10 duplex. As expected, no duplex melting transition was observed by fluorescence because the unstructured and duplex conformations of the FL-(CAG)10-DCL molecular beacon were indistinguishable.
FIGURE 5.
Absorbance profile of (CAG)10 and (CTG)10 hairpins. Absorbance at 260 nm was monitored as a function of temperature for a sample containing 1.5 μm (CAG)10 and (CTG)10 in 20 mm sodium phosphate, 10 mm NaCl, pH 7.0.
In addition to demonstrating that the FL-(CAG)10-DCL and (CTG)10 hairpins convert to duplex via a thermally induced process, we have also examined the reversibility of the conversion. Reversibility can be established readily by observing the fluorescence intensity as the temperature is decreased from 90 to 10 °C. If the process is reversible, meaning the FL-(CAG)10-DCL and (CTG)10 hairpins reform upon cooling, a decrease in fluorescence would be observed as the temperature is lowered. However, if the process is irreversible, meaning FL-(CAG)10-DCL/(CTG)10 duplex is formed upon cooling, the fluorescence intensity would remain high. Indeed, upon cooling from 90 to 10 °C, the fluorescence intensity remains high (supplemental Fig. 4A). The individual hairpins do not reform but rather the complementary sequences form duplex. In addition to the fluorescence data, upon cooling, optical analysis is also indicative of duplex and not hairpin formation (supplemental Fig. 4B).
Behavior of the (CAG)10 Hairpin in the Presence of the Complementary (CTG)10 Sequence, Kinetic Studies
Having demonstrated that the FL-(CAG)10-DCL and (CTG)10 hairpins irreversibly convert to FL-(CAG)10-DCL/(CTG)10 duplex via a thermally induced process, we next sought to determine the rate of duplex formation by the complementary sequences. To accomplish this, 5′-32P-labeled (CAG)10 hairpin was mixed with the (CTG)10 hairpin under pseudo first-order conditions. A 10-fold excess of the (CTG)10 hairpin was utilized; importantly, under these conditions, no homoduplex side reaction was observed for the (CTG)10 sequence (supplemental Fig. 5). The samples were incubated at 37 °C; the slower migrating duplex was then resolved from the single-stranded hairpin by native PAGE. Notably, when the (CAG)10 hairpin alone is analyzed by native PAGE, a single band is observed indicating that (CAG)10 adopts a single conformation (supplemental Fig. 5). At t = 3 min, approximately half of the (CAG)10 hairpin has converted to (CAG)10/(CTG)10 duplex, and by 15 min the conversion is nearly complete (Fig. 6A). The amount of duplex product was quantitated and plotted as a function of time, and the data were fit to a monoexponential curve to obtain the rate constant for duplex formation (Fig. 6B). For the pair of hairpins (CAG)10 and (CTG)10, the rate for duplex formation was 1721 ± 98 m−1 s−1.
FIGURE 6.
Kinetics of hairpin to duplex conversion for (CAG)10 and (CTG)10, LP1, LP2, ST1, or LP1/ST1. A, autoradiogram revealing conversion of (CAG)10 hairpin to (CAG)10/(CTG)10 duplex after incubation at 37 °C. B, graphical representation of percent duplex product as a function of time for (CAG)10 and (CTG)10 (closed circles), (CAG)10 and LP1 (open circles), (CAG)10 and LP2 (closed triangles), (CAG)10 and ST1 (open diamonds), or (CAG)10 and LP1/ST1 (open squares).
As expected for a thermally induced process, kinetic experiments performed at 4 °C, instead of 37 °C, reveal that duplex conversion is significantly slower at lower temperature (supplemental Fig. 6). In fact, at the longest time point assayed, t = 25.5 h, only 23% of the (CAG)10 hairpin has converted to (CAG)10/(CTG)10 duplex. Because such a limited amount of hairpin to duplex conversion was observed, quantitative kinetic data could not be obtained.
Both Loop-Loop and Stem-Stem Interactions Can Mediate Hairpin to Duplex Conversion
Having demonstrated that, in a thermally induced and irreversible process, the (CAG)10 and (CTG)10 hairpins convert to duplex, we next sought to determine the mechanism by which the two hairpins interact. To accomplish this, we prepared a series of modified (CTG)10 sequences (Table 1). To probe the role of the unpaired bases in the loop of the hairpin in modulating the conversion to duplex, two loop-modified (CTG)10 sequences were prepared, LP1 and LP2. In LP1, the two bases 5′-TT-3′ are substituted for the apical loop bases of the (CTG)10 hairpin. In LP2, four bases in the loop are changed such that they are no longer capable of canonical Watson-Crick base pairing with the loop of the (CAG)10 hairpin. To examine the potential role of the terminal bases of the stem of the hairpin in modulating the conversion to duplex, the stem-modified sequence ST1 was prepared. In this sequence, the three terminal bases of the stem, at both the 5′- and 3′-ends, are no longer complementary to the terminal bases of the (CAG)10 hairpin; A-T base pairs have been exchanged for G-C base pairs and vice versa. Optical melting analysis and chemical probe experiments with DEPC show that these loop and stem-modified sequences form hairpins (supplemental Figs. 7–9).
TABLE 1.
DNA sequences used in this study
Underlined bases represent those changed with respect to (CTG)10 or (CAG)10.
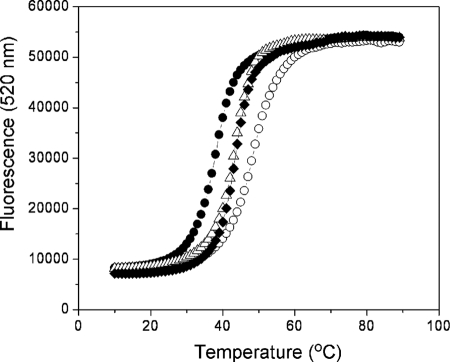
The behavior of the FL-(CAG)10-DCL molecular beacon in the presence of these modified (CTG)10 hairpins was examined first. The modified hairpins were individually combined with the FL-(CAG)10-DCL molecular beacon, and fluorescence intensity was monitored as a function of temperature (Fig. 7). Table 2 shows the Tm of the FL-(CAG)10-DCL hairpin in presence of the modified (CTG)10 hairpins. Although the FL-(CAG)10-DCL hairpin has a Tm of 49.0 °C in the absence of other DNA and 38.8 °C in the presence of (CTG)10, in the presence of the loop-modified (CTG)10 hairpins, intermediate melting temperatures were observed. Therefore, although the loop-modified hairpins destabilize the FL-(CAG)10-DCL hairpin, they do so to a lesser extent than the fully complementary (CTG)10 hairpin. Interestingly, changing only the two apical bases of the (CTG)10 loop (LP1) has the same effect on the thermal stability of the FL-(CAG)10-DCL hairpin as changing four loop bases (LP2). Consistent with the extent of destabilization of the FL-(CAG)10-DCL hairpin by the loop-modified (CTG)10 sequences, by optical analysis the temperature at which duplex forms between (CAG)10 and the loop-modified (CTG)10 sequences is higher than that observed with (CTG)10 (Table 2 and supplemental Figs. 10 and 11). Taken together, the results of these experiments with the loop-modified (CTG)10 sequences LP1 and LP2 suggest that, relative to the fully complementary (CTG)10 sequence, the conversion to duplex is disfavored with these sequences; higher temperatures are needed to melt the FL-(CAG)10-DCL hairpin and also to induce the hairpin to duplex conversion.
FIGURE 7.
Fluorescence profile for the (CAG)10 molecular beacon in the presence of the loop-modified (CTG)10 hairpins. The fluorescence intensity at 520 nm was monitored as a function of temperature for the FL-(CAG)10-DCL molecular beacon in the absence of other DNAs (open circles) or the presence of (CTG)10 (closed circles), LP1 (closed diamonds), or LP2 (open triangles). Conditions were 1.5 μm FL-(CAG)10-DCL and 1.5 μm loop-modified or (CTG)10 hairpin in 20 mm sodium phosphate, 10 mm NaCl, pH 7.0.
TABLE 2.
Thermal stability of (CAG)10-containing structures
Melting temperature of FL-(CAG)10-DCL in the absence or presence of complementary hairpins was monitored by fluorescence, and duplex formation and melting were determined by optical analysis. Error represents standard deviation resulting from a minimum of three experiments.
| Complementary hairpin | Tm (°C) of FL-(CAG)10-DCL hairpin | Formation (°C) of (CAG)10-containing duplex | Tm (°C) of (CAG)10-containing duplex |
|---|---|---|---|
| None | 49.0 ± 1.0 | ||
| (CTG)10 | 38.8 ± 0.8 | 37.3 ± 0.6 | 75.7 ± 1.2 |
| LP1 | 43.7 ± 0.9 | 46.5 ± 0.1 | 65.2 ± 1.9 |
| LP2 | 41.8 ± 1.1 | 46.4 ± 1.3 | 63.7 ± 1.1 |
| ST1 | 39.9 ± 0.3 | 43.8 ± 0.1 | 69.2 ± 1.2 |
| LP1/ST1 | 45.0 ± 0.1 | 45.1 ± 0.1 | 56.5 ± 0.1 |
In contrast to the results obtained with LP1 and LP2, no change in the Tm of the FL-(CAG)10-DCL hairpin was observed in the presence of the stem-modified sequence ST1 (supplemental Fig. 12A). The melting temperature of the molecular beacon is the same within error in the presence of (CTG)10 and ST1 (Table 2). This result suggests that the terminal bases of the stem of the hairpin are not involved in the hairpin to duplex conversion. However, by optical melting analysis, the temperature at which duplex forms between (CAG)10 and the stem-modified (CTG)10 sequence is significantly higher than that observed for (CAG)10 and (CTG)10 (compare supplemental Fig. 12B with supplemental Fig. 4B). These seemingly contradictory results suggested that our molecular beacon hairpin might not be amenable to studies designed to probe stem-stem interactions. Indeed, the placement of the fluorophore and quencher at the termini of the (CAG)10 hairpin stem may complicate analysis of the results obtained by fluorescence.
Nevertheless, these complications due to the pendant fluorophore and quencher are avoided in our kinetics assay that uses a 32P-labeled (CAG)10 hairpin. Therefore, the rate constants for duplex formation were determined for the (CAG)10 hairpin and LP1, LP2, and ST1. Consistent with the results obtained by fluorescence, in which the loop-modified (CTG)10 hairpins disrupt duplex formation, we find that the rate of conversion to duplex is significantly slower with the loop-modified sequences relative to (CTG)10 (Table 3). Furthermore, relative to (CTG)10, we find that the rate of duplex formation is also slower with the stem-modified sequence. In fact, for the loop and the stem-modified sequences, the rate of duplex formation is 4–6 times slower than that observed for the fully complementary (CAG)10 and (CTG)10 hairpins. These results indicate that changing either the bases in the loop or terminal region of the stem can modulate the hairpin to duplex conversion.
TABLE 3.
Rate constants for hairpin to duplex conversion at 37 °C
Error represents standard deviation resulting from a minimum of three experiments.
| Hairpin pair | k |
|---|---|
| m−1s−1 | |
| (CAG)10 and (CTG)10 | 1721 ± 98 |
| (CAG)10 and LP1 | 271 ± 29 |
| (CAG)10 and LP2 | 464 ± 54 |
| (CAG)10 and ST1 | 447 ± 36 |
| (CAG)10 and LP1/ST1 | 92 ± 11 |
| comp-LP1 and LP1 | 417 ± 182 |
| comp-LP2 and LP2 | 419 ± 18 |
| comp-ST1 and ST1 | 1380 ± 261 |
| comp-LP1/ST1 and LP1/ST1 | 649 ± 52 |
Rate of Duplex Formation by the (CAG)10 Hairpin and an LP1/ST1 Doubly Modified Hairpin
Our experiments with singly modified hairpins, (CTG)10 hairpins modified either in the loop or stem region, revealed that either modification decreases the rate of duplex formation relative to that observed for the fully complementary (CTG)10 hairpin. It is noteworthy that although the rate is reduced, duplex formation does still occur with these modified hairpins. To address the possibility that loop-loop and stem-stem interactions provide two independent modes of interaction by which the (CAG)10 and (CTG)10 hairpins can convert to duplex, we prepared a doubly modified hairpin, LP1/ST1. This sequence contains both the loop modification of LP1 and the stem modification of ST1. Optical melting analysis and characterization by DEPC indicate that the LP1/ST1 sequence adopts a hairpin conformation (supplemental Fig. 13). Using this doubly modified hairpin, the rate of duplex formation was 19-fold slower than that observed with (CTG)10.
Compensatory Modifications to (CAG)10 Hairpin Restore Rate of Duplex Formation
Relative to (CTG)10, we find that the rate of duplex formation is 4–6 times slower for the loop and stem-modified sequences LP1, LP2, and ST1. If this decrease in rate is due to the noncomplementarity introduced by the loop and stem modifications, then compensatory modifications to the (CAG)10 hairpin should restore the rate. Indeed, we find that a compensatory modification to the (CAG)10 hairpin (comp-ST1), which makes the hairpin complementary to ST1, restores the rate of duplex conversion (Table 3 and supplemental Fig. 15). Interestingly, however, this restoration in rate is not observed when compensatory loop modifications are made to (CAG)10. Partial restoration was observed for the doubly compensatory hairpin comp-LP1/ST1.
DISCUSSION
Previous work using CAG and CTG sequences containing 10 repeats has shown that these sequences form stable hairpins; moreover, it is known that these complementary sequences can also form duplex (11–13). However, the mechanism by which the complementary hairpins interact to convert to duplex has not been established. In this work we have used a multifaceted approach, including chemical, biochemical, and biophysical techniques to characterize the mechanism of hairpin to duplex conversion for these complementary TNR sequences.
As a starting point for characterizing the interactions between complementary TNR sequences, we utilized a molecular beacon strategy. Similar methodology has been employed effectively to characterize the folded structures adopted by nucleic acids, including guanine quadruplexes (35), duplex and triplex DNA (36), and hairpin DNA (37) and RNA (25). In this work, we designed the FL-(CAG)10-DCL molecular beacon to selectively monitor the behavior of the (CAG)10 hairpin in the presence of the complementary sequence.
It is noteworthy that fluorescence measurements and optical melting analysis yield melting temperatures that differ by ∼4 °C for the FL-(CAG)10-DCL molecular beacon hairpin. However, these two methods are relying on distinct phenomena to measure Tm values. The melting temperature obtained by fluorescence reflects the spatial separation of the fluorophore and quencher that is induced upon hairpin melting. In contrast, optical melting analysis relies on the unstacking of the nucleobase chromophores that occurs during hairpin melting. In fact, the Tm obtained by fluorescence is lower than that observed by optical analysis, which is consistent with the terminal region of the stem being more dynamic due to end fraying. Nevertheless, despite the different Tm values obtained by fluorescence and optical analysis, the melting temperature obtained by fluorescence always serves as the benchmark for comparison in our molecular beacon studies.
Interestingly, we find that the presence of the complementary (CTG)10 hairpin lowers the thermal stability of the FL-(CAG)10-DCL hairpin by ∼10 °C. This destabilization is specific to the complementary sequence; no change in the thermal stability of the FL-(CAG)10-DCL hairpin is observed in the presence of a noncomplementary oligonucleotide. Using optical analysis, we have confirmed that this destabilization of the molecular beacon occurs as a result of conversion to duplex. A similar destabilization has been reported for replication protein A. For single strands of DNA capable of adopting a folded conformation, replication protein A altered the equilibrium between melting and formation of duplex (38). Furthermore, our fluorescence measurements established that the FL-(CAG)10-DCL molecular beacon hairpin melts at 38.8 °C; optical analysis reveals that duplex formation occurs at 37.3 °C. Although the similarities in temperature for hairpin melting and duplex formation suggest that these two processes may occur concurrently, it is important to recall that fluorescence-based and optical methods are measuring related but unique processes.
Nevertheless, both fluorescence and optical analysis demonstrate that the (CAG)10 and (CTG)10 hairpins coexist at low temperature but upon thermal induction convert to duplex in an irreversible manner. Völker et al. (17, 19) also reported an irreversible conversion to duplex by Ω-DNA structures that contain a (CAG)6 or (CTG)6 single-stranded hairpin embedded within duplex. Because the hairpin to duplex conversion process is irreversible, we conclude that the FL-(CAG)10-DCL/(CTG)10 duplex is the thermodynamically more stable state at low temperature. Therefore, the FL-(CAG)10-DCL and (CTG)10 hairpins represent kinetically trapped states that coexist in solution below a threshold temperature. Interestingly, for sequences containing 10 repeats, the threshold temperature is similar to physiological temperature.
Using native PAGE, we next examined the kinetics of the hairpin to duplex conversion and obtained a rate constant of ∼1,700 m−1 s−1 for (CAG)10 and (CTG)10. Gacy and McMurray (16) previously reported a rate constant of 40,000 m−1 s−1 for duplex formation by (CAG)10 and (CTG)10. However, this previous study was performed at a higher ionic strength that has been shown to modulate duplex formation by repetitive DNA sequences (39). Indeed, for (CAG)10 and (CTG)10, we find that ionic strength affects the rate constant describing duplex formation; in the presence of an 8-fold greater concentration of NaCl, the rate of duplex formation increases 3-fold (supplemental Fig. 14).
Having shown that the complementary TNR sequences do convert to duplex above a threshold temperature, we used modified (CTG)10 hairpins to examine the mechanism. To determine whether a kissing complex is relevant for the (CAG)10 and (CTG)10 hairpin to duplex conversion, we used the FL-(CAG)10-DCL molecular beacon in conjunction with loop-modified (CTG)10 sequences. In these loop-modified sequences, the bases in the loop are no longer complementary to the loop bases of (CAG)10. In designing these modified sequences, it was important that the Tm of each hairpin was not significantly altered relative to (CTG)10 to avoid introducing the variable of thermal stability; indeed, the LP1 and LP2 hairpins have melting temperatures within 1 °C of (CTG)10. Thus, as expected, changing the unpaired bases in the loop has very little effect on the thermal stability of the hairpin.
If the conversion to duplex involves kissing hairpins, the loop-modified (CTG)10 sequences are expected to disrupt the process because G-C and A-T base pairs can no longer form between the apical loop bases of the two hairpins. Furthermore, having shown that FL-(CAG)10-DCL and (CTG)10 do, in fact, convert to duplex at ∼37 °C, we can use the Tm of FL-(CAG)10-DCL as a way to qualitatively gauge how readily the process occurs. For example, if the Tm of FL-(CAG)10-DCL is higher in the presence of LP1 than in the presence of (CTG)10, we would infer that the conversion to duplex is impeded by LP1. Likewise, if the Tm of FL-(CAG)10-DCL is lower in the presence of a given oligonucleotide, then the conversion to duplex is facilitated by that DNA.
We find that the Tm of FL-(CAG)10-DCL increases from 38.8 ± 0.8 °C with (CTG)10 to 42.7 ± 0.2 °C and 42.6 ± 0.2 °C, with LP1 and LP2, respectively. These results are supportive of a kissing hairpin intermediate. The 4–6-fold decrease in the rate with LP1 and LP2 relative to (CTG)10 also supports a role for loop-loop interactions in the hairpin to duplex conversion. Previous studies of the kinetics of duplex formation by the transactivation response element RNA and DNA hairpins showed a comparable decrease in the rate when loop-modified sequences were employed (40).
In addition to examining the role of kissing hairpins in the conversion of (CAG)10 and (CTG)10 hairpins to duplex, similar experiments were performed with the stem-modified sequence ST1 to determine whether a cruciform intermediate contributes to duplex formation. Importantly, whereas modifying the unpaired bases in the loop of the (CTG)10 hairpin did not have a significant effect on the Tm of the hairpin, ST1 has a melting temperature that is 5 °C lower than the unmodified (CTG)10 hairpin.
In contrast to the changes seen with the loop-modified sequences by fluorescence, no change in the Tm of FL-(CAG)10-DCL was observed with ST1. Because changing the terminal bases in (CTG)10 has no effect on the thermal stability of FL-(CAG)10-DCL relative to FL-(CAG)10-DCL in the presence of the unmodified (CTG)10 hairpin, one might conclude that a cruciform intermediate does not play a role in hairpin to duplex conversion. However, because the fluorophore and quencher are covalently attached at the 5′- and 3′-ends of (CAG)10, they may influence cruciform formation. Indeed, in this system we may have modulated cruciform formation with these pendant groups.
Cruciform formation is more adequately addressed using sequences without pendant groups, as is the case for our native PAGE assay. Indeed, relative to the (CAG)10 and (CTG)10 hairpins, we observe a 4-fold decrease in the rate constant for duplex formation by (CAG)10 and ST1. Because modifications to the terminal region of the stem of (CTG)10 slow the conversion process, this result suggests that a cruciform intermediate does contribute to duplex formation. Studies of duplex formation by the + and − copies of the primer-binding site of human immunodeficiency virus type 1 RNA also showed that alterations in terminal stem overhangs decreased the rate of duplex formation by 2 orders of magnitude (32). It is of note that our results are not simply an artifact of the lower thermal stability of the ST1 hairpin. At a given temperature, lower thermal stability would be manifested as a more dynamic or unfolded structure; if the hairpin were less structured, the rate of duplex formation would be faster. Indeed, Kushon et al. (41) have reported that formation of folded structures such as hairpins imposes a kinetic blockage upon peptide nucleic acid/DNA duplex formation. As expected for persisting secondary structures, we find that the rate of duplex formation is slowed with ST1.
The experiments performed with (CAG)10 hairpins containing compensatory stem modifications also suggest that molecular recognition between hairpins influences hairpin-duplex conversion. The rate of duplex formation was restored when comp-ST1 was combined with ST1. This result suggests that the stem modifications made to the (CTG)10 hairpin can be thought of as changes to the base composition.
In contrast, comp-LP1 and comp-LP2 do not restore the rate of duplex formation. Therefore, in the case of loop modifications, changing the base composition may also have introduced subtle structural changes. For example, the extent of stacking with adjacent loop bases may be altered. Notably, however, these changes are not detected by DEPC suggesting that they are not gross structural changes. A more comprehensive structural analysis by NMR or x-ray crystallography would likely be necessary to discern changes in the extent of base stacking in the loop. Nevertheless, these alterations are sufficient to disrupt conversion to duplex via a kissing complex. Importantly, the observation of a decreased rate of duplex formation with LP1 and LP2, relative to (CTG)10, underscores the fact that the loop region can mediate conversion to duplex. Therefore, kissing hairpins are viable mechanistic precursors that allow for conversion to duplex.
Interestingly, by native PAGE we observed only hairpin and duplex species; there were no detectable intermediates. Van Melckebeke et al. (28) reported that the kissing interaction between the transactivation response RNA hairpin and a hairpin aptamer forms a quasi-continuous helix that would likely result in similar electrophoretic mobilities for a kissing hairpin intermediate and the final duplex product. Thus, in our experiments a kissing hairpin intermediate may co-migrate with the duplex product. In contrast to our results, when examining hairpin to duplex conversion of the human immunodeficiency virus, type 1, dimerization initiation site RNA hairpins, Bernacchi et al. (25) observe a third species by native PAGE in addition to hairpin and duplex, which migrate slower than the duplex product, that they attribute to a cruciform intermediate. However, consistent with our results by native PAGE, Gacy and McMurray (16) did not observe an intermediate in the conversion of CAG and CTG triplet repeat hairpins to duplex. Finally, we expect that kissing or cruciform hairpins would be transient in nature and once formed would rapidly convert to the observed duplex.
Taken together, the results obtained by fluorescence, optical analysis, and native PAGE provide evidence that both loop-loop and stem-stem interactions can modulate the conversion of the (CAG)10 and (CTG)10 hairpins to the (CAG)10/(CTG)10 duplex. Therefore, we considered that these two modes of interaction represent two independent mechanisms by which the complementary hairpins can convert to duplex. If this is the case, although LP1 and LP2 would disrupt duplex formation via kissing hairpins, these loop-modified sequences can still exploit a cruciform intermediate. Similarly, the ability to form kissing hairpins between (CAG)10 and the stem-modified sequence ST1 is not altered relative to (CTG)10. One would anticipate that the rate of duplex formation would be further decreased if the ability to form kissing hairpins and a cruciform intermediate were both disrupted.
To address this issue, we designed the LP1/ST1 sequence, which cannot form canonical base pairs with the (CAG)10 hairpin via the stem or loop regions. Whereas the rate of duplex formation decreased 4–6-fold with the sequences modified in either the loop or the stem region, a 19-fold decrease was observed with LP1/ST1. Thus, the results from the loop-modified and the stem-modified hairpins as well as for the doubly modified LP1/ST1 hairpin suggest two distinct mechanisms for duplex formation by (CAG)10 and (CTG)10 hairpins, i.e. kissing hairpins or cruciform intermediate. Using single-molecule fluorescence resonance energy transfer studies, these two mechanisms have also been proposed to be operational for duplex formation by the complementary transactivation response RNA and DNA hairpins (42).
It is noteworthy that although the rate of duplex formation is reduced 19-fold relative to the (CAG)10 and (CTG)10 pair, the (CAG)10 and LP1/ST1 hairpins are able to convert to duplex. It is possible that there may be another mechanism, other than kissing hairpins or cruciform, by which the hairpins interact. Alternatively, duplex formation may still occur via loop-loop and stem-stem interactions, but instead of exploiting A-T and G-C base pairs, these interactions would involve noncanonical base pairing. Although not as favorable energetically as the formation of Watson-Crick base pairs, C·T, C·A, and G·T pairs (43–44) can form between the (CAG)10 and modified (CTG)10 hairpins. As a third possibility, others have suggested that hairpin melting may occur prior to duplex conversion (25); in this scenario, the unstructured sequences would then anneal to form duplex. However, as monitored by optical methods, for all the hairpin pairs utilized in our experiments the temperature at which duplex formation occurs is well below the Tm of the individual hairpins. Therefore hairpin melting cannot be a requirement for duplex formation. Rather, our data support a mechanism that includes a molecular recognition event between two complementary hairpins.
In conclusion, the use of a FL-(CAG)10-DCL molecular beacon has revealed that the complementary sequence destabilizes the (CAG)10 hairpin. Furthermore, although the (CAG)10 and (CTG)10 hairpins represent kinetically trapped conformations, thermal induction mediates conversion to duplex via loop-loop or stem-stem interactions. Therefore, these results have implications with respect to the persistence of these secondary structures during TNR expansion and potential hindrance of recognition between CAG and CTG hairpins during the proposed strand slippage step. In vivo DNA topology might impose restrictions on hairpin-hairpin interactions; thus, kissing or cruciform intermediates may not be available to mediate conversion to the thermodynamically more stable duplex. Thus, the absence of these proposed kissing and cruciform pathways could allow for the prolonged existence of hairpin species resulting in slippage-dependent expansion. The findings presented in this work contribute to our understanding of TNR sequences and require consideration with respect to the mechanism of expansion by these repetitive sequences. Because the threshold temperature for duplex formation by sequences containing 10 repeats is near physiological, it remains to be seen if such sequences could persist as hairpins in vivo or, rather, would be present as duplex.
Supplementary Material
Acknowledgments
We thank Professor David Cane for use of phosphorimaging equipment, Craig Yennie for assistance with acquisition of mass spectra, and Daniel Jarem, Douglas Cattie, and Nicole Wilson for helpful discussions. The real time PCR instrument used in this work is located in the Molecular Pathology Core of the COBRE Center for Cancer Research Development and is supported by National Institutes of Health Grant P20 RR17695, awarded by the NCRR, Institutional Development Award Program.
This work was supported by Brown University in the form of start-up funds and a Richard B. Salomon faculty research award (to S. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–15.
- TNR
- trinucleotide repeat
- FL-(CAG)10-DCL
- fluorescein-5′-(CAG)10-3′-(E)-N-(3-(dihydroxymethoxy)propyl)-4-((4-(dimethylamino)phenyl)diazenyl) benzamide
- DEPC
- diethyl pyrocarbonate
- HPLC
- high pressure liquid chromatography
- comp
- compensatory.
REFERENCES
- 1.Imarisio S., Carmichael J., Korolchuk V., Chen C. W., Saiki S., Rose C., Krishna G., Davies J. E., Ttofi E., Underwood B. R., Rubinsztein D. C. (2008) Biochem. J. 412, 191–209 [DOI] [PubMed] [Google Scholar]
- 2.Kovtun I. V., McMurray C. T. (2008) Cell Res. 18, 198–213 [DOI] [PubMed] [Google Scholar]
- 3.Pearson C. E., Nichol, Edamura K., Cleary J. D. (2005) Nat. Rev. Genet. 6, 729–742 [DOI] [PubMed] [Google Scholar]
- 4.Gatchel J. R., Zoghbi H. Y. (2005) Nat. Rev. Genet. 6, 743–755 [DOI] [PubMed] [Google Scholar]
- 5.Wells R. D., Dere R., Hebert M. L., Napierala M., Son L. S. (2005) Nucleic Acids Res. 33, 3785–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gacy A. M., Goellner G., Juranić N., Macura S., McMurray C. T. (1995) Cell 81, 533–540 [DOI] [PubMed] [Google Scholar]
- 7.Mitas M. (1997) Nucleic Acids Res. 25, 2245–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paiva A. M., Sheardy R. D. (2004) Biochemistry 43, 14218–14227 [DOI] [PubMed] [Google Scholar]
- 9.Mirkin S. M. (2006) Curr. Opin. Struct. Biol. 16, 351–358 [DOI] [PubMed] [Google Scholar]
- 10.Petruska J., Hartenstine M. J., Goodman M. F. (1998) J. Biol. Chem. 273, 5204–5210 [DOI] [PubMed] [Google Scholar]
- 11.Owen B. A., Yang Z., Lai M., Gajec M., Gajek M., Badger J. D., 2nd, Hayes J. J., Edelmann W., Kucherlapati R., Wilson T. M., McMurray C. T. (2005) Nat. Struct. Mol. Biol. 12, 663–670 [DOI] [PubMed] [Google Scholar]
- 12.Wells R. D. (2007) Trends Biochem. Sci. 32, 271–278 [DOI] [PubMed] [Google Scholar]
- 13.Delagoutte E., Goellner G. M., Guo J., Baldacci G., McMurray C. T. (2008) J. Biol. Chem. 283, 13341–13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovtun I. V., Liu Y., Bjoras M., Klungland A., Wilson S. H., McMurray C. T. (2007) Nature 447, 447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarem D. A., Wilson N. R., Delaney S. (2009) Biochemistry 48, 6655–6663 [DOI] [PubMed] [Google Scholar]
- 16.Gacy A. M., McMurray C. T. (1998) Biochemistry 37, 9426–9434 [DOI] [PubMed] [Google Scholar]
- 17.Völker J., Makube N., Plum G. E., Klump H. H., Breslauer K. J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14700–14705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paiva A. M., Sheardy R. D. (2005) J. Am. Chem. Soc. 127, 5581–5585 [DOI] [PubMed] [Google Scholar]
- 19.Völker J., Klump H. H., Breslauer K. J. (2007) J. Am. Chem. Soc. 129, 5272–5280 [DOI] [PubMed] [Google Scholar]
- 20.Chang K. Y., Tinoco I., Jr. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 8705–8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paillart J. C., Marquet R., Skripkin E., Ehresmann B., Ehresmann C. (1994) J. Biol. Chem. 269, 27486–27493 [PubMed] [Google Scholar]
- 22.Chang K. Y, Tinoco I., Jr. (1997) J. Mol. Biol. 269, 52–66 [DOI] [PubMed] [Google Scholar]
- 23.Ennifar E., Yusupov M., Walter P., Marquet R., Ehresmann B., Ehresmann C., Dumas P. (1999) Structure 7, 1439–1449 [DOI] [PubMed] [Google Scholar]
- 24.Kim C. H., Tinoco I., Jr. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 9396–9401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernacchi S., Ennifar E., Tóth K., Walter P., Langowski J., Dumas P. (2005) J. Biol. Chem. 280, 40112–40121 [DOI] [PubMed] [Google Scholar]
- 26.Ulyanov N. B., Mujeeb A., Du Z., Tonelli M., Parslow T. G., James T. L. (2006) J. Biol. Chem. 281, 16168–16177 [DOI] [PubMed] [Google Scholar]
- 27.Ennifar E., Dumas P. (2006) J. Mol. Biol. 356, 771–782 [DOI] [PubMed] [Google Scholar]
- 28.Van Melckebeke H., Devany M., Di Primo C., Beaurain F., Toulmé J. J., Bryce D. L., Boisbouvier J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9210–9215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaucage S. L., Caruther M. H. (2000) Curr. Protoc. Nucleic Acid Chem. 3.3.1–3.3.20 [Google Scholar]
- 30.Warshaw M. M., Tinoco I., Jr. (1966) J. Mol. Biol. 20, 29–38 [DOI] [PubMed] [Google Scholar]
- 31.Vo M. N., Barany G., Rouzina I., Musier-Forsyth K. (2006) J. Mol. Biol. 363, 244–261 [DOI] [PubMed] [Google Scholar]
- 32.Ramalanjaona N., de Rocquigny H., Millet A., Ficheux D., Darlix J. L., Mély Y. (2007) J. Mol. Biol. 374, 1041–1053 [DOI] [PubMed] [Google Scholar]
- 33.Marky L. A., Breslauer K. J. (1987) Biopolymers 26, 1601–1620 [DOI] [PubMed] [Google Scholar]
- 34.Herr W. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 8009–8013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risitano A., Fox K. R. (2003) Biochemistry 42, 6507–6513 [DOI] [PubMed] [Google Scholar]
- 36.Darby R. A., Sollogoub M., McKeen C., Brown L., Risitano A., Brown N., Barton C., Brown T., Fox K. R. (2002) Nucleic Acids Res. 30, e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonnet G., Krichevsky O., Libchaber A. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 8602–8606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartos J. D., Willmott L. J., Binz S. K., Wold M. S., Bambara R. A. (2008) J. Biol. Chem. 283, 21758–21768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gacy A. M., McMurray C. T. (1994) Biochemistry 33, 11951–11959 [DOI] [PubMed] [Google Scholar]
- 40.Vo M. N., Barany G., Rouzina I., Musier-Forsyth K. (2009) J. Mol. Biol. 386, 789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kushon S. A., Jordan J. P., Seifert J. L., Nielsen H., Nielsen P. E., Armitage B. A. (2001) J. Am. Chem. Soc. 123, 10805–10813 [DOI] [PubMed] [Google Scholar]
- 42.Liu H. W., Cosa G., Landes C. F., Zeng Y., Kovaleski B. J., Mullen D. G., Barany G., Musier-Forsyth K., Barbara P. F. (2005) Biophys. J. 89, 3470–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allawi H. T., SantaLucia J. (1997) Biochemistry 36, 10581–10594 [DOI] [PubMed] [Google Scholar]
- 44.Leontis N. B., Stombaugh J., Westhof E. (2002) Nucleic Acids Res. 30, 3497–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.