Abstract
Both cultured neonatal rat hippocampal neurons and differentiated oligodendrocytes rapidly metabolized exogenous C2- and C6-ceramides to sphingosine (Sph) and sphingosine 1-phosphate (S1P) but only minimally to C16–24-ceramides. Dihydrosphinolipids were unaffected but were increased by exogenous C6-dihydroceramide. Conversely, quantitative liquid chromatography-tandem mass spectrometry technology showed that exogenous S1P (0.25–10 μm) was rapidly metabolized to both Sph (a >200-fold increase) and predominantly C18-ceramide (a >2-fold increase). Longer treatments with either C2-ceramide (>2.5 μm) or S1P (10 μm) led to apoptotic cell death. Thus, there is an active sphingolipid salvage pathway in both neurons and oligodendrocytes. Staurosporine-induced cell death was shown to be associated with decreased S1P and increased Sph and C16/18-ceramide levels. The physiological significance of this observation was confirmed by the analysis of affected white matter and plaques from brains of multiple sclerosis patients in which reduced S1P and increased Sph and C16/18-ceramides were observed.
Keywords: Apoptosis, HPLC, Mass Spectrometry, Neuron, Sphingolipid, Ceramide, HPLC-Mass Spectrometry, Neonatal Rat Oligodendrocytes, Sphingosine, Sphingosine 1-Phosphate
Introduction
Ceramides have been associated with cell growth arrest and death by apoptosis whereas their catabolite sphingosine 1-phosphate (S1P)3 enhances proliferation, differentiation, and cell survival in multiple cell types and tissues (1), including the brain (2). Ceramides have been implicated as effectors or enhancer molecules in both the death receptor (cytokine-extrinsic) and stress (BCL2 family/intrinsic) pathways which lead to cell death (2–5). This probably involves activation of protein phosphatase 2A by ceramides (6) or a direct action to increase mitochondrial membrane permeability and cause cytochrome c release (7). There is growing in vivo evidence to support the idea that regulation of ceramide levels is critical to normal cell function. Mutations that delete acid ceramidase activity lead to ceramide accumulation in lysosomes (Farber lipogranulomatosis) and subsequent neurodegeneration (8), and ceramidase inhibitors have potential use as anticancer agents (9). Although elevated lysosomal ceramides in Farber disease do not result in increased apoptosis (10), it is possible that failure to convert ceramide to the bioactive S1P could explain some of the pathology. Nonlysosomal ceramidases exist (5, 11) and must play a role in ceramide homeostasis, for example, in Drosophila the ceramidase controls presynaptic terminal sphingolipid composition to regulate vesicle fusion, trafficking, and synaptic function (12). Thus, regulation of ceramide catabolism must be critical for normal nervous system function in many species and phyla.
Sphingosine (Sph) is cytotoxic (13), but phosphorylation of Sph to S1P renders it bioprotective (1). Thus, the enzymes that regulate ceramide catabolism must themselves be highly regulated because they connect pathways with antagonistic properties. It is therefore important to understand the role of ceramide-metabolizing pathways in neurons and glia because different brain cell types may respond differently to drugs used to treat neurological disorders such as brain tumors, neurodegenerative diseases, and psychiatric disorders. This is especially important because of the current interest in treating lysosomal storage diseases in children and adults by restricting the synthesis of sphingolipids (14).
De novo biosynthesis of ceramides is initiated by serine palmitoyltransferase to produce 3-ketodihydrosphingosine, which is further converted to dihydrosphingosine (DHSph) dihydroceramides (DHCer), and ceramides (15). In contrast, S1P is not derived by the de novo biosynthesis but through ceramide degradation by ceramidases to Sph and Sph phosphorylation to S1P. Ceramides are also formed by catabolic degradation of sphingomyelin (SM) and glycosphingolipids in lysosomes (5) and extralysosomally (16, 17). Null mutations in lysosomal acid sphingomyelinase produce devastating neurovisceral storage of SM (Niemann Pick type A disease) but no depletion of ceramides in brain.4 Deletion of ceramide galactosyl- and glucosyl-transferases in mice did not lead to increased ceramide levels (19), suggesting active alternate pathways regulating cellular ceramide levels. In lysosomal acid sphingomyelinase (−/−) mice (20), the storage of lipids and the degeneration of Purkinje cells and other neurons occur very early, suggesting rapid turnover of SM, but there is only minor SM storage in oligodendrocytes (21), suggesting differences in sphingolipid metabolism in different brain cell types. This has been observed experimentally (22). Axonal dystrophy is pronounced in Niemann Pick disease type A (lysosomal acid sphingomyelinase-null mice), but there is little dysmyelination (20). In contrast, nonlysosomal neutral sphingomyelinase 2 (−/−) mice show specific brain pathology and developmental changes in brain and their skeletal systems (23), the latter resembling osteogenesis imperfecta (24). Many studies implicate this neutral pH active nonlysosomal neutral sphingomyelinase 2 as the main enzyme producing ceramides to induce apoptosis in response to stress (16, 25–29). Other studies have suggested that acid (lysosomal) sphingomyelinase or increased de novo synthesis of ceramides also plays key roles in elevating proapoptotic ceramides (5). Thus, the origin of increased ceramides may differ in different tissues and be dependent on the type of stress as well as the molecular species of ceramides generated.
Water-soluble ceramide analogs, N-acetylsphingosine (C2-ceramide) and C6-ceramide have been used in many thousands of studies to mimic ceramide-mediated stress-induced cell death pathways (16, 30, 31). Recently, it was shown that C2-ceramide occurs naturally in brain at low levels (32). Despite the ubiquitous use of C2- and C6-ceramides in stress-related studies, their metabolic fate has not been unequivocally established. To resolve this issue we analyzed neurons and oligodendrocytes to quantify ceramide metabolites after the addition of increasing concentrations of C2-ceramide or S1P. The use of quantitative LC-MS/MS technology (33, 34) allowed us to confirm a rapid interconversion between ceramides, Sph, and S1P. Importantly, we show for the first time the immediate conversion of exogenous C2-ceramide and C6-ceramide to Sph and S1P, which may help explain some of the contradictory actions of these water-soluble analogs in different biological systems including the brain.
EXPERIMENTAL PROCEDURES
Standards and Reagents
Sph, DHSph, a 17-carbon analog of Sph (C17-Sph), S1P, DHS1P, a 17-carbon analog of S1P (C17-S1P), N-myristoyl (14:0), N-palmitoyl (16:0), N-oleoyl (18:1), N-stearoyl (18:0), N-arachidoyl (20:0), N-nervonoyl (24:1), N-lignoceroyl (24:0) sphingosines (ceramides (Cer)), N-palmitoyl (16:0), N-oleoyl (18:1), N-stearoyl (18:0), N-arachidoyl (20:0), N-behenoyl (22:0), N-nervonoyl (24:1), N-lignoceroyl (24:0), DHSph (DHCer), N-heptadecanoyl sphingosine (Cer17:0), and the palmitoyl (16:0), stearoyl (18:0), arachidoyl (20:0), behenoyl (22:0), nervonoyl (24:1), and lignoceroyl (24:0) coenzyme A substrates were obtained from Avanti Polar Lipids (Alabaster, AL). 3-Keto-DHSph was purchased from Matreya (Pleasant Gap, PA). The nonphosphorylated lipid standards were dissolved in methanol, whereas the sphingoid base phosphates were dissolved in methanol containing a trace amount of concentrated HCl and were stored at −20 °C.
C2- and C6-ceramides were purchased from Avanti and Matreya. MTT was from Sigma. The protein assay kit was from Bio-Rad.
Autopsy specimens from patients with active multiple sclerosis (bank codes 10103–10111), Alzheimer disease (AD; bank codes 10121–10128), and normal control brain samples (Human Brain and Spinal Fluid Resource 296, 635 and 712) were obtained from the Human Brain and Center, Los Angeles, CA. Multiple sclerosis patient samples typically showed plaques and sharply demarcated areas of demyelination in white matter (WM) of the cerebrum, cerebellar cortex, centrum ovale, and pons etc., neuropathological diagnosis confirmed those of chronic multiple sclerosis plaques of demyelination. Samples were dissected into plaques of demyelination in WM and adjacent normal-appearing white matter (NAWM) and quick-frozen. No attempt was made to distinguish possible clinical subtypes, and all multiple sclerosis samples were considered to be typical for multiple sclerosis. Age-matched AD brain samples were confirmed pathologically, dissected (NAWM), and quick-frozen. Control brain samples were from autopsy brains of auto accident, gunshot, and accidental hanging victims. Pathological examination of coronal sections of the left hemisphere (including lenticular nucleus and thalamus) showed no abnormalities.
Cell Culture: Neonatal (P3) Rat Hippocampal Neurons
Briefly, hippocampi were dissected from 10–12 anesthetized decapitated 3-day-old rats, sectioned (400-μm thickness), and incubated in oxygenated, HEPES-buffered saline. Tissue was treated with trypsin, proteases, and mechanically triturated to yield a cell suspension that was centrifuged through an iodixanol density gradient for 15 min. The pellet was treated with DNase, and cells were plated on polylysine-coated 6-well Co-star plates in 5% oxygen/5% CO2 in nitrogen in Neurobasal culture medium (Invitrogen) supplemented with B-27 (2 ml/100 ml; Invitrogen), 2-mercaptoethanol (0.14 mm), NaCl (35 mm), glutamine (0.5 mm), penicillin (1000 units/ml), and streptomycin (0.1 mg/ml) (35).
Neonatal (P3) Rat Oligodendrocytes
Neonatal rat oligodendrocytes were isolated from cerebral hemispheres of the same rats used for neonatal rat hippocampal neurons (NRH) culture by the Bottenstein (36) modification of the method of McCarthy and de Vellis (37). The first shake is carried out at 7 days and subsequently at 1-week intervals with shakes 2, 3, and 4 giving the highest yield of 98% pure astrocytes and microglia-free oligodendrocytes (NRO). All cultures were differentiated for 6 days prior to use (37, 38). Rat astrocytes were isolated by trypsinization of the bed layer (following oligodendrocyte removal by shaking) and culture in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. To assess cell viability, cells were plated in 24-well culture plates for MTT assay, which was carried out as described previously (26).
Lipid Extraction and Sample Preparation for LC-MS/MS
Cellular lipids were extracted by a modified Bligh and Dyer (39) procedure with the use of 0.1 n HCl for phase separation. C17-S1P (40 pmol), C17-Sph (30 pmol), and 17:0-Cer (30 pmol) were used as internal standards and were added during the initial step of lipid extraction. The extracted lipids were dissolved in methanol/chloroform (4:1, v/v), and aliquots were taken to determine the total phospholipid content as described previously (40). Samples were concentrated under a stream of nitrogen, redissolved in methanol, transferred to autosampler vials, and subjected to consecutive LC-MS/MS analysis of sphingoid bases, ceramides, and sphingoid base 1-phosphates.
Analysis of Sphingoid Bases, Sphingoid Base 1-Phosphates, and Ceramides
Analyses of the sphingolipids were performed by combined LC-MS/MS. The instrumentation used was an API4000 Q-trap hybrid triple quadrupole linear ion trap mass spectrometer (Applied Biosystems) equipped with a TurboIonSpray ionization source interfaced with an automated Agilent 1100 series liquid chromatograph and autosampler (Agilent Technologies, Wilmington, DE). The sphingolipids were ionized via electrospray ionization with detection via multiple reactions monitoring (MRM). Analysis of sphingoid bases and the molecular species of ceramides used electrospray ionization in positive ions with MRM analysis using a minor modification of published methods (41, 42). Briefly resolution of sphingoid bases was achieved with a Discovery C18 column (2.1 × 50 mm, 5-μm particle size; Supelco, Bellefonte, PA) and a gradient from methanol/water/formic acid (61:38:1, v/v) with 5 mm ammonium formate to methanol/acetonitrile/formic acid (39:60:1, v/v) with 5 mm ammonium formate at a flow rate of 0.5 ml/min. The MRM transitions used for detection of sphingoid bases were as follows: m/z 286 → 268 (C17-Sph, internal standard), m/z 300 → 282 (Sph), and m/z 302 → 284 (DHSph).
Ceramide molecular species were resolved using a 3- × 100-mm XTerra XDB-C8 column (3.5-μm particle size; Waters, Milford, MA) and a gradient from methanol/water/formic acid (61:39:0.5, v/v) with 5 mm ammonium formate to acetonitrile/chloroform/water/formic acid (90:10:0.5:0.5, v/v) with 5 mm ammonium formate at a flow rate of 0.5 ml/min. MRM transitions monitored for the elution of ceramide molecular species were as follows: m/z 510 → 264, 14:0-Cer; m/z 538 → 264, 16:0-Cer; m/z 540 → 284, 16:0-DHCer; m/z 552 → 264, 17:0-Cer (internal standard); m/z 564 → 264, 18:1-Cer; m/z 566 → 284, 18:1-DHCer; m/z 566 → 264, 18:0-Cer; m/z 568 → 284, 18:0-DHCer; m/z 594 → 264, 20:0-Cer; m/z 596 → 284, 20:0-DHCer; m/z 624 → 284, 22:0-DHCer; m/z 650 → 264, 24:1-Cer; m/z 652 → 284, 24:1-DHCer; m/z 652 → 264, 24:0-Cer; m/z 654 → 284, 24:0-DHCer; m/z 680 → 264, 26:1-Cer; m/z 682 → 264, 26:0-Cer; m/z 708 → 264, 28:1-Cer; and m/z 710 → 264, 28:0-Cer.
S1P and DHS1P were quantified as bisacetylated derivatives with C17-S1P as the internal standard using reverse-phase HPLC separation, negative ion electrospray ionization, and MRM analysis. Details of this approach were described previously (33).
Standard curves for each of the sphingoid bases, sphingoid base 1-phosphates, and ceramide molecular species were constructed via the addition of increasing concentrations of the individual analyte to 30 or 40 pmol of the corresponding structural analogs used as the internal standard. Linearity and the correlation coefficients of the standard curves were obtained via a linear regression analysis. The standard curves were linear over the range of 0.0–300 pmol of each of the sphingolipid analytes with correlation coefficients (R2) > 0.98.
Statistical Analysis
The results of LC-MS/MS analyses are from duplicate experiments run in triplicate. The cell death assay results are from duplicate experiments run in triplicate. Statistical analyses were performed by Student's t test, and results were considered statistically significant when p < 0.05.
RESULTS
Exogenous C2-ceramide Dramatically Increases Both Sph and S1P Levels in Rat Primary Brain Cell Cultures
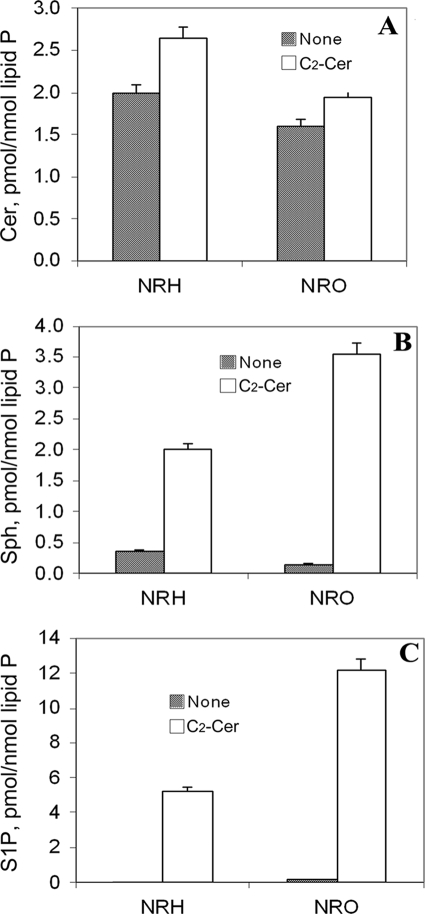
Basal ceramide levels were >80 times those of S1P in NRH and 10 times that of S1P in NRO (Figs. 1 and 2). Basal ceramide levels were 10 times that of Sph in both neurons and that of Sph in oligodendrocytes (Figs. 1, A and C, and 2). The significance of the higher basal S1P in oligodendrocytes compared with neurons is not known.
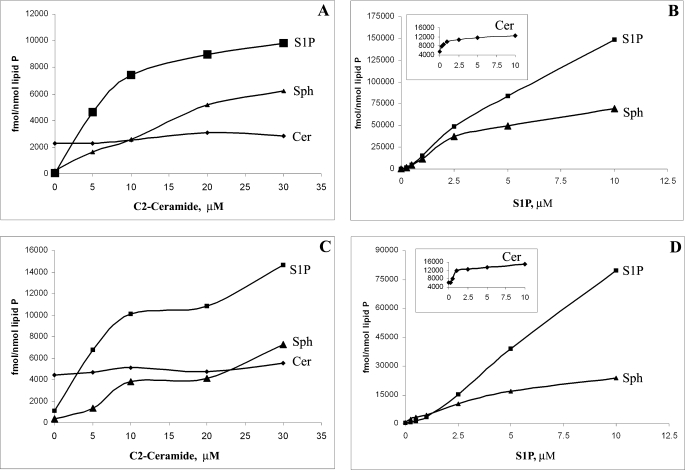
FIGURE 1.
Sphingolipid interconversions in rat hippocampal neurons and oligodendrocytes under exogenous C2-Cer and S1P. A, exogenous C2-ceramide dramatically increases the levels of Sph and S1P (but not total ceramides) in rat hippocampal neurons. B, exogenous S1P dramatically increases the levels of Sph and ceramides (inset) in rat hippocampal neurons. C, exogenous C2-ceramide dramatically increases the levels of Sph and S1P (but not total ceramides) in rat neonatal oligodendrocytes. D, exogenous S1P dramatically increases the levels of Sph and ceramides (inset) in rat neonatal oligodendrocytes. Sphingolipids were analyzed by HPLC-MS/MS as described under “Experimental Procedures.”
FIGURE 2.
Effect of C2-ceramide on the level of S1P, sphingosine, and ceramides in hippocampal neurons and oligodendrocytes. Exogenous C2-Cer has little effect on ceramide levels (A) but dramatically increases the levels of Sph (B) and S1P (C) in both NRH and NRO. Sph and S1P levels become comparable with that of ceramides in S1P-treated cells. Sphingolipids were analyzed by LC-MS/MS as described under “Experimental Procedures.”
Exogenous C2-ceramide rapidly (<30 min) increased both Sph and S1P in NRH (Fig. 1A) and NRO (Fig. 1C). S1P levels were not significantly reduced by changing culture medium and measuring S1P content after a further 2 h of culture. Interestingly, C16–24-ceramide levels were essentially unaffected by exogenous C2-ceramide (Fig. 1, A and C), confirming that Sph derived from C2-ceramide was not converted to long chain ceramides (31). The increase in exogenous C2-ceramide concentration proportionally increased intracellular levels of both Sph and S1P; time course studies with 20 μm C2-ceramide showed increases in S1P as early as 5 min after the treatment (see Fig. 4B). This suggests that C2-ceramide is rapidly deacylated by some type of ceramidase (probably neutral) initially to Sph and that neural cell sphingosine kinases SphK1 and/or SphK2 (2) are much more active than the S1P lyase and S1P phosphatases, which degrade S1P to hexadecenal and phosphorylethanolamine.
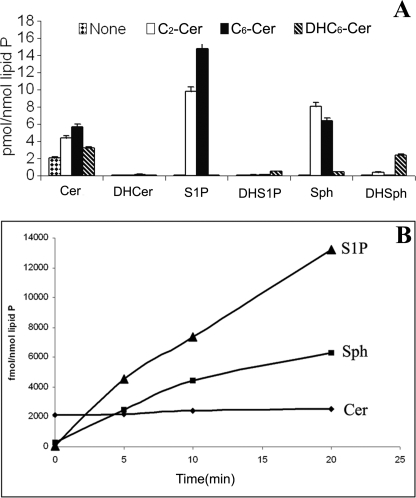
FIGURE 4.
Sphingolipid interconversions in rat hippocampal neurons. A, exogenous ceramide analogs (C2-Cer and C6-Cer, 20 μm for 2 h each) increase Sph and S1P, exogenous dihydroceramides (DHC6-Cer, 20 μm for 2 h) increase DHSph and DHS1P. B, time course of the conversion of exogenous C2-Cer (20 μm) to Sph and S1P by neonatal NRH. Sphingolipids were analyzed by LC-MS/MS as described under “Experimental Procedures.”
Exogenous S1P Increases S1P, Sph, and C16/18-ceramide Levels in Cells
To investigate the role of S1P in sphingolipid recycling in brain cells, we added exogenous S1P at increasing concentrations (0.25–10 μm) to both neonatal rat hippocampal neurons (NRH) and oligodendrocytes (NRO) under the same conditions as C2-ceramide (Fig. 1, B and D). This provided further evidence of an efficient recycling process because ceramide levels rapidly increased to up to 2-fold that of basal ceramide levels (Fig. 1, B and D, insets). However, the increase was not equal across all measured ceramide molecular species, and almost all of the increase was seen in ceramide C16:0 and C18:0 species (Fig. 3C) in NRO. This is most likely dependent on the differential expression of ceramide synthases (e.g. CerS1) (43). Consistent with this recycling, we had previously observed that [3H]Sph was rapidly acylated to [3H]ceramide by neonatal rat oligodendrocytes. Following the addition of exogenous S1P, Sph levels increased rapidly up to 40-fold (10 μm S1P for 2 h) (Fig. 1, B and D). The increase was greater in neurons than in oligodendrocytes and appeared linear up to 2 μm S1P. Thus, following addition of S1P, both intracellular S1P and Sph levels rapidly exceeded ceramide levels. This >200-fold increase over basal S1P and Sph levels is shown in Figs. 1 and 2 and suggests the rapid dephosphorylation of S1P to Sph and then reacylation to ceramides. Thus, the central nervous system must have a highly active S1P phosphatase 1 or 2 (44) rather than a S1P lyase.
FIGURE 3.
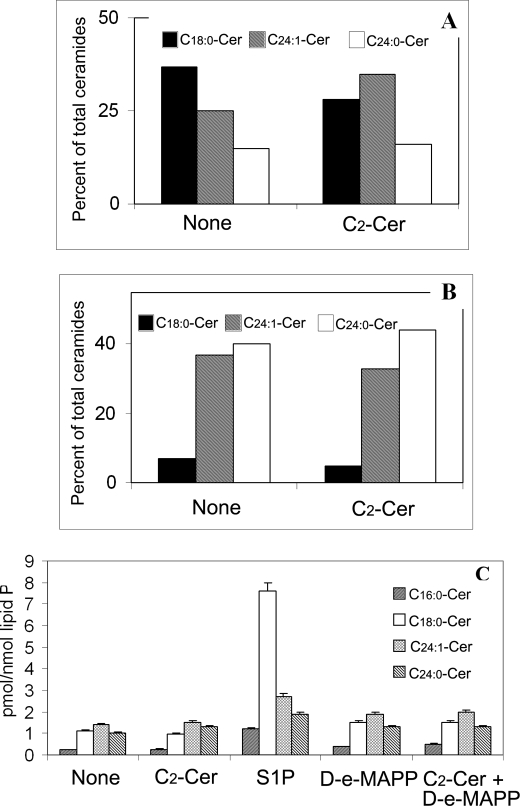
Neuronal ceramides contain more C18:0-ceramide and less C24-ceramides than oligodendrocytes, and their levels are unaffected by exogenous C2-ceramide. A and B, neonatal NRH (A) and NRO (B) have different relative proportions of C18:0-, C24:1-, and C24:0-ceramides. Culture in the presence of exogenous C2-ceramide minimally affects the relative proportion of C18:0-, C24:1-, and C24:0-ceramides in neurons (A) and oligodendrocytes (B). C, exogenous S1P (10 μm) increases the level of C18:0-ceramide in rat oligodendrocytes. Neither C2-Cer (10 μm), the ceramidase inhibitor d-e-MAPP (10 μm), nor a combination of C2-Cer (10 μm) and d-e-MAPP (10 μm) had any significant effect on relative proportion and the levels of ceramide molecular species. Ceramide levels were analyzed by LC-MS/MS as described under “Experimental Procedures.”
Different Brain Cell Types Have Distinctive Ceramide Composition
LC-MS/MS analysis showed that the major fatty acids in ceramides from rat hippocampal neurons were C18:0 (40%), C24:1 (25%), and C24:0 (15%) (Fig. 3A). The predominance of these shorter (C16 and C18) chain fatty acids in ceramides was observed previously in cultured embryonic chick neurons (employing a completely different shotgun MS method) (22), which revealed C16:0 (30%), C18:0 (50%), and C24:1 (10%). In contrast, LC-MS/MS analysis of oligodendrocytes cultured from the same rat brains (also in the absence of fetal calf serum) showed the major fatty acids in ceramides to be C24:1 (40%) and C24:0 (40%) with only 5% C18:0 (Fig. 3B). This difference was also confirmed by shotgun MS (22) of the same neonatal rat oligodendrocyte ceramides, which again showed C24:1 and C24:0 to be the major fatty acid species. Such differences in fatty acid chain length of ceramide could be due to different ceramide synthase expression levels and activity in neurons versus oligodendrocytes. Whole mouse brain expresses at least four ceramide synthases (1, 5, 43) although ceramide synthase 1 is the most specific for brain and produces mainly C16 and C18-ceramides. In all cells the predominant sphingosine base was assumed to be C18:0. However, despite the relatively low level of C18-ceramides in NRO, the exogenous S1P was preferentially incorporated into C18-ceramides in both neurons and oligodendrocytes (Fig. 3C). Neither C2-Cer (10 μm), the ceramidase inhibitor (d-e-MAPP, 10 μm) nor a combination of C2-Cer (10 μm) + MAPP (10 μm) had any significant effect on the fatty acid distribution in ceramides and did not significantly increase ceramide levels from oligodendrocytes (Fig. 3C) but did affect the C18/C24 + 24:1 ratio in neurons, decreasing it from 0.9 to 0.5 and did not significantly increase ceramide levels (Fig. 3A).
C2-ceramide and C6-ceramide Are Rapidly Converted to Sph and S1P but Have No Effect on Dihydrosphingosines
Exogenous C6-ceramide produced dramatic increases in Sph and S1P similar to those produced by C2-ceramide (Fig. 4A). The conversion of C2-ceramide to Sph and S1P was close to linear for the first 10 min (Fig. 4B) and continue to increase for at least 30 min. The increases were >10-fold for Sph and >100-fold for S1P in 5 min, whereas there was essentially no change in C16-C24 ceramides levels over the initial 30-min time period. Exogenous DHCer increase DHSph and DHS1P levels in cells. In hippocampal neurons, the basal DHCer levels were only 2% of ceramide levels and ∼2-fold higher than DHSph and DHS1P levels. Addition of C2-ceramide or C6-ceramide (20 μm) had little effect on DHSph and DHS1P levels (Fig. 5) for up to 2 h, and there was no increase in the level of DHCer (Fig. 5B). Similarly, in neonatal rat oligodendrocytes, the basal DHCer levels were only 3.5% of ceramide levels but 5-fold higher than DHS and DHS1P. Exogenous C6-DHCer (20 μm) did not increase Cer, Sph, or S1P levels, or even DHCer levels but increased DHSph levels 50-fold and DHS1P levels 10-fold in both neurons and oligodendrocytes (Fig. 4A). Exogenous S1P did not have any effect on DHSph or DHS1P levels. Thus, the Sph- and DHSph-based pathways appear to be regulated independently of each other.
FIGURE 5.
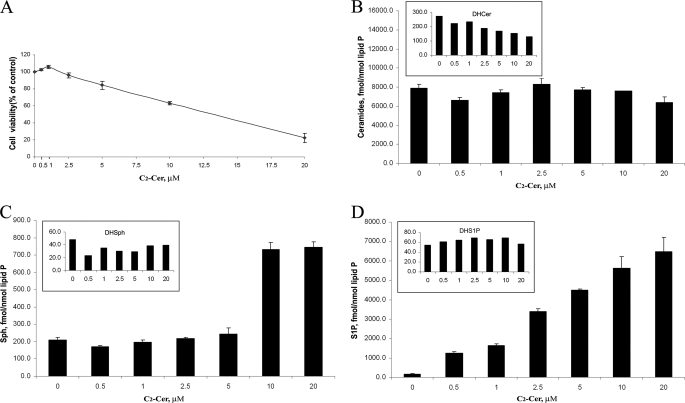
Concentration-dependent effect and sphingolipid interconversions of C2-ceramide in rat oligodendrocytes. A, dose response of treatment of rat neonatal cultured oligodendrocytes for 40 h with C2-Cer shows some protection below 2 μm concentration and cell death at higher concentrations (from 2.5 to 20 μm). Cell viability was determined by the MTT method as described under “Experimental Procedures.” B, short term (2-h) exposure of oligodendrocytes to 0.5–20 μm C2-Cer does not increase Cer but decreases DHCer levels in cells. C, short term (2-h) exposure of oligodendrocytes to 0.5–2.5 μm C2-Cer does not increase Sph, DHSph levels, but exposure of oligodendrocytes to higher concentrations of C2-Cer (10 and 20 μm) dramatically increases Sph in cells. D, short term (2-h) exposure of oligodendrocytes to 0.5–20 μm C2-Cer increases intracellular S1P level but does not affect DHS1P level in cells. Sphingolipids were analyzed by LC-MS/MS as described under “Experimental Procedures.”
Effect of C2-ceramide and S1P on Cell Death
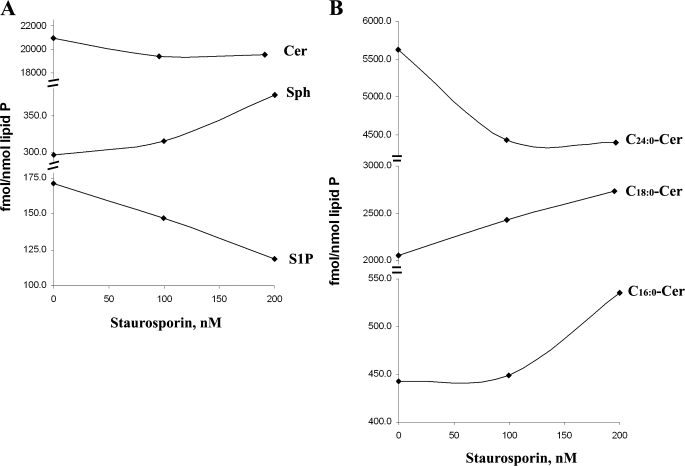
C2-ceramide (20 μm) induced >50% cell death in a dose-responsive manner in both neonatal rat oligodendrocytes and hippocampal neurons. However, the dose-response data (shown for oligodendrocytes in Fig. 5A) showed a protective role for C2-ceramide at low concentrations (0.5 and 1 μm). Higher concentrations of C2-ceramide (from 2.5 μm to 20 μm) started to induce cell death as expected. Further, we found that submicromolar concentrations of C2-ceramide increased S1P levels (Fig. 5D) but decreased ceramides and Sph to lower than the basal levels (Fig. 5, B and C). Treating cells with 2.5–20 μm C2-ceramide increased the levels of Sph and became cytotoxic. We also observed that oligodendrocytes were killed by higher concentrations of S1P (2–10 μm). From previous studies (Fig. 1, B and D) we know that S1P can be metabolized to Sph and ceramides very rapidly (<30 min), and our data suggested that changes in the Sph level of cells may decide their fate (life or death) even though the S1P level also increasing. To understand further how inducers of apoptosis affect ceramide, Sph, and S1P levels, we treated cells with 100 and 200 nm staurosporine for 3 h. The decrease in S1P occurred concomitantly with an increase in Sph. The increase in ceramides was more complex because C16/18 fatty acid species (believed to be proapoptotic) increased whereas C24-ceramides actually decreased (Fig. 6, A and B). The change in ceramide levels was observed previously with hypoxia/reoxygenation treatment in NT-2 cells, but on the other hand, Sph and S1P levels in these cells were unchanged (45).
FIGURE 6.
Staurosporine-induced cell death is associated with increased Sph, decreased S1P, and increased C16/18:0-ceramide levels. A, in oligodendrocytes with staurosporine treatment (100 and 200 nm) for 3 h, the level of ceramides is not increased, but Sph level is increased, and S1P level is decreased. B, further analysis shows that the levels of C16:0-Cer and C18:0-Cer species are increased, and the level of C24:0-Cer is decreased. Sphingolipids were analyzed by LC-MS/MS as described under “Experimental Procedures.”
Decreased S1P and Increased Sph and C16/18-ceramide Levels Are Observed in Multiple Sclerosis Lesions and Affected White Matter
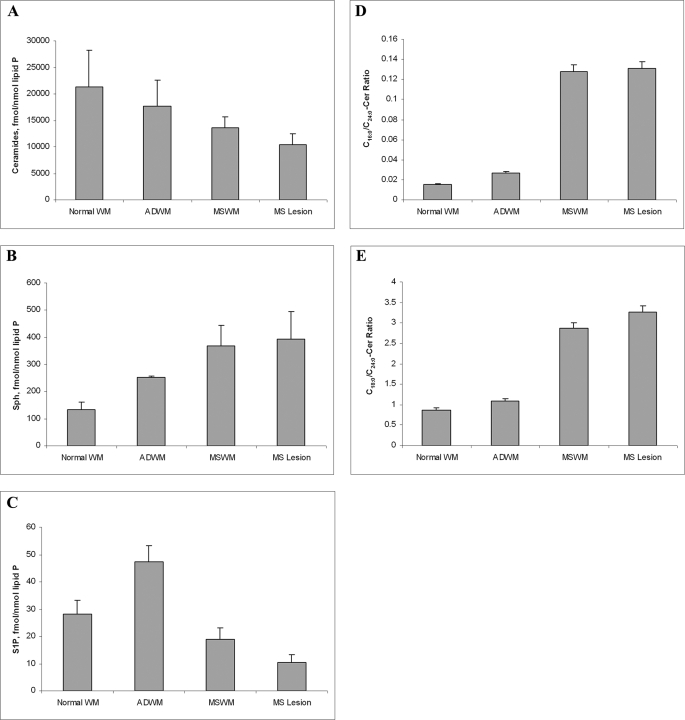
We analyzed postmortem brain samples from multiple sclerosis patients, dissected and identified as previously described (46), using accidental death brain WM samples as controls and AD WM as parallel compare samples. Total ceramides were decreased in multiple sclerosis brain WM and lesions compared with controls and AD WM (Fig. 7A). Sph was increased in the brain of a patient with multiple sclerosis WM and lesions compared with controls and AD WM (Fig. 7B) whereas S1P was decreased in multiple sclerosis brain WM and lesions compared with controls and AD WM (Fig. 7C). We then examined the ceramide data in more detail and observed that the C16/18- to C24-ceramide ratio was greatly increased in multiple sclerosis brain WM and lesions compared with controls and AD WM (Fig. 7, D and E).
FIGURE 7.
Changes in Cer, Sph, and S1P levels in oligodendrocytes undergoing cell death correlate with Cer, Sph, and S1P levels in multiple sclerosis (MS) WM. A, ceramide level is moderately decreased in multiple sclerosis brain WM and lesions compared with controls and AD WM. B, Sph is increased in multiple sclerosis brain WM and lesions compared with controls and AD WM. C, S1P is decreased in multiple sclerosis brain WM and lesions compared with controls and AD WM. D, C16-Cer/C24-Cer ratio is greatly increased in multiple sclerosis brain WM and lesions compared with controls and AD WM. E, C18-Cer/C24-Cer ratio is greatly increased in multiple sclerosis brain WM and lesions compared with controls and AD WM. Results are the average of patients/controls run in triplicate.
DISCUSSION
The relative levels of ceramides, Sph, and S1P are believed to determine cell viability in the central nervous system and are strongly influenced by the activity of hydrolases (sphingomyelinases and ceramidases), SphKs, S1P phosphatases, and S1P lysase (Fig. 8). Many bioactivities have been ascribed to ceramides, and these can generally be mimicked by water-soluble analogs (C2- and C6-ceramides) but not by DHCer. C2-ceramide maybe a natural brain component because Van Overloop et al. (32) detected small amounts of C2-ceramide in brain (10 pmol/g) and liver (25 pmol/g). However, the physiological origins or metabolic fate of C2-ceramide have not yet been clearly determined. In terms of synthesis, Van Overloop et al. (32) negated the idea that C2-ceramide was formed by acyl transfer from platelet-activating factor to Sph, as suggested by Lee et al. (47) because there was no reduction in C2-ceramide in platelet-activating factor-deficient Pex5 (−/−) mice. Although the amounts of C2-ceramide in brain are small (C2-ceramide is present at concentrations 5000-fold less than the typical C16–24 ceramides), there is sufficient C2-ceramide (10 fmol/mg of protein) to be the precursor of S1P (2 fmol/mg of protein or 50 fmol/nmol of lipid phosphorus), We now report that C2- and C6-ceramides can be metabolized to Sph and S1P in brain cells at levels vastly higher than previously suspected and that S1P can be efficiently recycled to Sph and C16/18:0 ceramides in cells of central nervous system origin.
FIGURE 8.
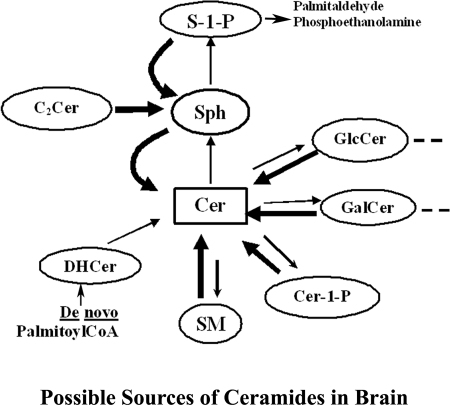
Sphingolipid metabolic interconversions in the brain. The de novo pathway of ceramide formation consists of consequential generation of 3-keto-DHSph, DHSph, DHCer, and Cer. Cer are also produced via catabolic degradation of SM and glycosphingolipids by sphingomyelinases and β-galactosidase. Cer is further hydrolyzed by ceramidases to Sph, which is consequently converted to S1P by kinases SphK1 and SphK2. S1P can be degraded to ethanolamine phosphate and hexadecanal by a S1P lyase or recycled (via phosphatases) to Sph and then reacylated to ceramides. Other sources of ceramide in brain cells include Cer1P (via a Cer 1P-phosphatase), and C2-ceramide (see the Introduction and Ref. 32) S1P appears to be protective and Sph and C16/18:0-Cer are proapoptotic, as revealed by experimental manipulation of primary cultures on neurons and oligodendrocytes and analyses of multiple sclerosis brain tissue.
Our results differ from previous reports (e.g. 31), which showed that exogenous C6-Cer was metabolized to Sph by A549 adenocarcinoma cells and then reacylated to ceramides (32). Using our improved LC-MS/MS analytical approach, we confirmed that exogenous C2- and C6-ceramides were rapidly deacylated to Sph but then converted to S1P rather than to long chain ceramides. Intriguingly, exogenous S1P is an important precursor for both Sph and ceramides in neural cells because rather than being degraded by lyase activity as observed in other tissues such as lung (48), it is quickly metabolized to Sph and C16/18:0 ceramides. The low “resting” levels of Sph and S1P observed in cells suggest that they may in fact be rapidly metabolized intermediates.
The rapid conversion of C2-ceramide to roughly equal amounts of Sph and S1P suggests that SphKs are very active in both neurons and oligodendrocytes. Two kinases have been described, SphK1 and SphK2, and a combined knock out causes defects in neurogenesis and is embryologically lethal (49). However, the actual substrate for these kinases is not clear because it has been shown (in two different cell lines, one neural and one nonneural), that overexpression of SphK1 generated DHS1P rather than S1P (34). In these studies, after SphK1 overexpression, the DHS1P/S1P ratio increased about 100-fold (from a ratio of 0.04:1 to 3:1). DHS1P might also be an important second messenger, and this is supported by the fact that DHS1P has affinity to S1P receptors similar to that of S1P (1). In this current study, exogenous C2-ceramide did not increase DHS1P levels, but exogenous addition of DHCer led to a large increase in DHS1P levels in the cells. Addition of exogenous C6-DHCer gave qualitatively comparable results to C6-Cer (increased DHSph and DHS1P) but quantitatively much less of an effect, suggesting two noninteractive parallel pathways. Regarding the recycling of S1P, two phosphohydrolases, S1P phosphatase 1 and 2, have been shown to convert S1P to Sph and are bulk-expressed in brain (44). The S1P lyase appears important in some model systems, e.g. Caenorhabditis elegans, because inhibition by small interfering RNA led to accumulation of both phosphorylated and unphosphorylated sphingosines, poor feeding, delayed growth, reproductive abnormalities, and intestinal damage (50). However, in mammalian central nervous system, the role of S1P lyase in dealing with the constant production of S1P may be replaced by sphingolipid recycling.
S1P has many important actions through a family of high affinity S1P receptors (S1PR1–5), which can couple differentially via Gi, Gq, G12/13, and Rho to multiple effector systems, including adenylate cyclase, phospholipases C and D, extracellular signal-regulated kinase 1, c-Jun N-terminal kinase, p38 mitogen-activated protein kinase, and nonreceptor tyrosine kinases (2). The receptors are usually described as “high affinity,” although many studies have used high micromolar S1P concentrations (10–100 μm) to show biological effects. S1P (1 μm) has been claimed to promote cell proliferation and prevent programmed cell death initiated by staurosporine in mesoangioblasts (51); our study showed that concentrations as low as 0.25 μΜ S1P produced a rapid 50% increase in C16/18 ceramides. Our data explain the duality of S1P, protection at low concentrations and death (through Sph or ceramide) at high concentrations which was also recently reported by Hagen et al. (52). Ceramides may have a similar dual role (protection at low concentrations and killing at high concentrations), and this was reported previously in rat spinal motor neurons by Irie and Hirabayashi (53).
S1P presumably exerts most of its physiological action at the submicromolar level. Drug analogs that appear to mimic Sph, such as FTY720, have recently been shown to function as inhibitors of ceramide synthase (48), resulting in decreased ceramide and S1P in lung cells. However, recent work by Lahiri et al. (54) reported elevated levels of ceramides, SM, and hexosylceramides after incubation with FTY720. FTY720 has been shown to benefit multiple sclerosis patients, possibly by preventing release of lymphocytes into the central nervous system. To investigate a possible connection between S1P levels and multiple sclerosis, we first showed that cell death induced by staurosporine decreased S1P level but elevated the levels of proapoptotic Sph and C16/18:0-ceramides. We then looked at multiple sclerosis plaques and WM and found S1P to be decreased and Sph to be increased. Total ceramides were actually decreased, as reported by Wheeler et al. (18) for unaffected MS WM. However, detailed analysis revealed that proapoptotic C16/18:0-ceramides were actually elevated in MS WM compared with matched controls or pathological autopsy specimens from disease such as AD. This could reflect the loss of oligodendrocytes in MS WM because we also showed in this study that oligodendrocytes contain very little C18:0-ceramide compared with neurons.
We (46) previously reported increased OxPC in MS brain, and Wheeler et al. (18) reported increased 4-hydroxynonenal (the other aldehyde product of unsaturated PC oxidation) (46) in multiple sclerosis brain, both being the hallmark of inflammatory degenerative disease. Normal WM from MS patients was found to be enriched in sterols and phosphoglycerides at the expense of sphingolipids (18). They speculated that S1P might be reduced in MS brain and that FTY720 could be acting by restoring S1P signaling. In this study, we show for the first time that S1P is indeed greatly reduced in multiple sclerosis brain and that Sph and proapoptotic ceramide species are increased. At present, we have no evidence to comment on their notion that hyperactive metabolism of S1P in multiple sclerosis may lead to increased formation of phosphoethanolamine to phosphatidylethanolamine, although our observation of low de facto lyase activity (to generate phosphatidylethanolamine) would seem to argue against this idea.
Current therapies for neurodegenerative disease involve the use of UCGlcT inhibitors such as N-butyldeoxynojirimycin to lower glycolipid substrate levels in the neurological forms of Gaucher disease and juvenile Tay-Sachs disease (14). However, such inhibitors also elevate ceramides (and therefore potentially S1P) in neurons and oligodendrocytes. The possible toxic effects of Sph and S1P from chronically used drugs at high doses, by mechanisms revealed in this study, should be taken into account of when attempting therapy of brain diseases.
Acknowledgments
All mass spectrometric analyses were performed by the University of Chicago, Department of Medicine Lipidomic mass spectrometry facility. We thank Sylvia Dawson for excellent technical assistance in preparing the neonatal rat oligodendrocyte cultures and Janice Wang in the Neural Cell Culture Core of P30 HD054275 J. P. Kennedy Mental Retardation and Developmental Disabilities Center for the preparation of rat hippocampal neurons. We thank the members of the Human Brain and Spinal Fluid Resource Center, Los Angeles, CA, for providing brain tissues for this study.
This work was supported by U. S. Public Health Service Grant NS36866-33 and Pilot Grant PP1319 from the National Multiple Sclerosis Society (to G. D.). Clinical and Translational Science Award Grant UL1RR024999 (to J. Q.), and U.S. Public Health Service Grant HL079853 (to V. N.) from USPHS.
J. Qin, S. Dawson, and G. Dawson, in preparation.
- S1P
- sphingosine 1-phosphate
- Sph
- sphingosine
- SphK
- sphingosine kinase
- DHSph
- dihydrosphingosine
- DHS1P
- dihydrosphingosine 1-phosphate
- SM
- sphingomyelin
- Cer
- ceramide
- DHCer
- dihydroceramide
- C2-Cer
- ceramide (N-acetylsphingosine)
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- AD
- Alzheimer disease
- WM
- white matter
- NAWM
- normal-appearing WM
- NRH
- neonatal rat hippocampal neurons
- NRO
- neonatal rat oligodendrocytes
- HPLC
- high pressure liquid chromatography
- MRM
- multiple reactions monitoring
- d-e-MAPP
- (1S,2R)-d-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol.
REFERENCES
- 1.Chalfant C. E., Spiegel S. (2005) J. Cell Sci. 118, 4605–4612 [DOI] [PubMed] [Google Scholar]
- 2.Bryan L., Kordula T., Spiegel S., Milstien S. (2008) Biochim. Biophys. Acta 1781, 459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goswami R., Dawson G. (2000) J. Neurosci. Res. 60, 141–149 [DOI] [PubMed] [Google Scholar]
- 4.Chalfant C. E., Rathman K., Pinkerman R. L., Wood R. E., Obeid L. M., Ogretmen B., Hannun Y. A. (2002) J. Biol. Chem. 277, 12587–12595 [DOI] [PubMed] [Google Scholar]
- 5.Kitatani K., Idkowiak-Baldys J., Hannun Y. A. (2008) Cell. Signal. 20, 1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhyay A., Saddoughi S. A., Song P., Sultan I., Ponnusamy S., Senkal C. E., Snook C. F., Arnold H. K., Sears R. C., Hannun Y. A., Ogretmen B. (2009) FASEB J. 23, 751–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siskind L. J., Kolesnick R. N., Colombini M. (2006) Mitochondrion 6, 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Futerman A. H., Van Meer G. (2004) Nat. Rev. 5, 554–565 [DOI] [PubMed] [Google Scholar]
- 9.Dagan A., Wang C., Fibach E., Gatt S. (2003) Biochim. Biophys. Acta 1633, 161–169 [DOI] [PubMed] [Google Scholar]
- 10.Tohyama J., Oya Y., Ezoe T., Vanier M. T., Nakayasu H., Fujita N., Suzuki K. (1999) J. Inherit. Metab. Dis. 22, 649–662 [DOI] [PubMed] [Google Scholar]
- 11.Kono M., Dreier J. L., Ellis J. M., Allende M. L., Kalkofen D. N., Sanders K. M., Bielawski J., Bielawska A., Hannun Y. A., Proia R. L. (2006) J. Biol. Chem. 281, 7324–7331 [DOI] [PubMed] [Google Scholar]
- 12.Rohrbough J., Rushton E., Palanker L., Woodruff E., Matthies H. J., Acharya U., Acharya J. K., Broadie K. (2004) J. Neurosci. 24, 7789–7803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vartanian T., Dawson G., Soliven B., Nelson D. J., Szuchet S. (1989) Glia 2, 370–379 [DOI] [PubMed] [Google Scholar]
- 14.Jeyakumar M., Dwek R., Butters T. D., Platt F. M. (2005) Nat. Rev. 6, 1–12 [DOI] [PubMed] [Google Scholar]
- 15.Perry D. K. (2002) Biochim. Biophys. Acta 1585, 146–152 [DOI] [PubMed] [Google Scholar]
- 16.Kolesnick R., Hannun Y. A. (1999) Trends Biochem. Sci. 24, 224–225; author reply, 227 [DOI] [PubMed] [Google Scholar]
- 17.Goswami R., Ahmed M., Kilkus J., Han T., Dawson S. A., Dawson G. (2005) J. Neurosci. Res. 81, 208–217 [DOI] [PubMed] [Google Scholar]
- 18.Wheeler D., Bandaru V. V., Calabresi P. A., Nath A., Haughey N. J. (2008) Brain 131, 3092–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saadat L., Dupree J. L., Kilkus J., Han X., Trakal M., Proia R. L., Dawson G., Popko B. (2010) Glia 58, 391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodge J. C., Clarke J., Treleaven C. M., Taksir T. V., Griffiths D. A., Yang W., Fidler J. A., Passini M. A., Karey K. P., Schuchman E. H., Cheng S. H., Shihabuddin L. S. (2009) Exp. Neurol. 215, 349–357 [DOI] [PubMed] [Google Scholar]
- 21.Kuemmel T. A., Schroeder R., Stoffel W. (1997) J. Neuropathol. Exp. Neurol. 56, 171–179 [DOI] [PubMed] [Google Scholar]
- 22.Kilkus J. P., Goswami R., Dawson S. A., Testai F. D., Berdyshev E. V., Han X., Dawson G. (2008) J. Neurochem. 106, 1745–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoffel W., Jenke B., Blöck B., Zumbansen M., Koebke J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 4554–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aubin I., Adams C. P., Opsahl S., Septier D., Bishop C. E., Auge N., Salvayre R., Negre-Salvayre A., Goldberg M., Guénet J. L., Poirier C. (2005) Nat. Genet. 37, 803–805 [DOI] [PubMed] [Google Scholar]
- 25.Wiesner D. A., Kilkus J. P., Gottschalk A. R., Quintáns J., Dawson G. (1997) J. Biol. Chem. 272, 9868–9876 [DOI] [PubMed] [Google Scholar]
- 26.Testai F. D., Landek M. A., Dawson G. (2004) J. Neurosci. Res. 75, 66–74 [DOI] [PubMed] [Google Scholar]
- 27.Marchesini N., Luberto C., Hannun Y. A. (2003) J. Biol. Chem. 278, 13775–13783 [DOI] [PubMed] [Google Scholar]
- 28.Lee J. T., Xu J., Lee J. M., Ku G., Han X., Yang D. I., Chen S., Hsu C. Y. (2004) J. Cell Biol. 164, 123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S., Lee J.-M., Zeng C., Chen H., Hsu C. Y., Xu J. (2006) J. Neurochem. 97, 631–640 [DOI] [PubMed] [Google Scholar]
- 30.Wiesner D. A., Dawson G. (1996) J. Neurochem. 66, 1418–1425 [DOI] [PubMed] [Google Scholar]
- 31.Ogretmen B., Pettus B. J., Rossi M. J., Wood R., Usta J., Szulc Z., Bielawska A., Obeid L. M., Hannun Y. A. (2002) J. Biol. Chem. 277, 12960–12969 [DOI] [PubMed] [Google Scholar]
- 32.Van Overloop H., Denizot Y., Baes M., Van Veldhoven P. P. (2007) Biol. Chem. 388, 315–324 [DOI] [PubMed] [Google Scholar]
- 33.Berdyshev E. V., Gorshkova I. A., Garcia J. G. N., Natarajan V., Hubbard W. C. (2005) Anal. Biochem. 339, 129–136 [DOI] [PubMed] [Google Scholar]
- 34.Berdyshev E. V., Gorshkova I. A., Usatyuk P., Zhao Y., Saatian B., Hubbard W., Natarajan V. (2006) Cell. Signal. 18, 1779–1792 [DOI] [PubMed] [Google Scholar]
- 35.Marks J. D., Bindokas V. P., Zhang X. M. (2000) Brain Res. Dev. Brain Res. 124, 101–116 [DOI] [PubMed] [Google Scholar]
- 36.Bottenstein J. E. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 1955–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy K., de Vellis J. (1980) J. Cell Biol. 85, 890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scurlock B., Dawson G. (1999) J. Neurosci. Res. 55, 514–522 [DOI] [PubMed] [Google Scholar]
- 39.Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 40.Vaskovsky V. E., Kostetsky E. Y., Vasendin I. M. (1975) J. Chromatogr. 114, 129–141 [DOI] [PubMed] [Google Scholar]
- 41.Murphy R. C., Fiedler J., Hevko J. (2001) Chem. Rev. 101, 479–526 [DOI] [PubMed] [Google Scholar]
- 42.Merrill A. H., Caligan T. B., Wang E., Peters K., Ou J. (2000) Methods Enzymol. 312, 3–9 [DOI] [PubMed] [Google Scholar]
- 43.Laviad E. L., Albee L., Pankova-Kholmyansky I., Epstein S., Park H., Merrill A. H., Jr., Futerman A. H. (2008) J. Biol. Chem. 283, 5677–5684 [DOI] [PubMed] [Google Scholar]
- 44.Maceyka M., Milstien S., Spiegel S. (2007) Methods Enzymol. 434, 243–256 [DOI] [PubMed] [Google Scholar]
- 45.Jin J., Hou Q., Mullen T. D., Zeidan Y. H., Bielawski J., Kraveka J. M., Bielawska A., Obeid L. M., Hannun Y. A., Hsu Y. T. (2008) J. Biol. Chem. 283, 26509–26517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin J., Goswami R., Balabanov R., Dawson G. (2007) J. Neurosci. Res. 85, 977–984 [DOI] [PubMed] [Google Scholar]
- 47.Lee T. C., Ou M. C., Shinozaki K., Malone B., Snyder F. (1996) J. Biol. Chem. 271, 209–217 [DOI] [PubMed] [Google Scholar]
- 48.Berdyshev E. V., Gorshkova I., Skobeleva A., Bittman R., Lu X., Dudek S. M., Mirzapoiazova T., Garcia J. G., Natarajan V. (2009) J. Biol. Chem. 284, 5467–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizugishi K., Yamashita T., Olivera A., Miller G. F., Spiegel S., Proia R. L. (2005) Mol. Cell. Biol. 25, 11113–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendel J., Heinecke K., Fyrst H., Saba J. D. (2003) J. Biol. Chem. 278, 22341–22349 [DOI] [PubMed] [Google Scholar]
- 51.Donati C., Cencetti F., Nincheri P., Bernacchioni C., Brunelli S., Clementi E., Cossu G., Bruni P. (2007) Stem Cells 25, 1713–1719 [DOI] [PubMed] [Google Scholar]
- 52.Hagen N. H., Van Veldhoven P. P., Proia R. L., Park H., Merrill A. H., Jr., van Echten-Deckert G. (2009) J. Biol. Chem. 284, 11346–11353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irie F., Hirabayashi Y. (1998) J. Neurosci. Res. 54, 475–485 [DOI] [PubMed] [Google Scholar]
- 54.Lahiri S., Park H., Laviad E. L., Lu X., Bittman R., Futerman A. H. (2009) J. Biol. Chem. 284, 16090–16098 [DOI] [PMC free article] [PubMed] [Google Scholar]