Abstract
Objective To assess the effectiveness of penicillin for three days and treatment for seven days compared with placebo in resolving symptoms in children with sore throat.
Design Randomised, double blind, placebo controlled trial.
Setting 43 family practices in the Netherlands.
Participants 156 children aged 4-15 who had a sore throat for less than seven days and at least two of the four Centor criteria (history of fever, absence of cough, swollen tender anterior cervical lymph nodes, and tonsillar exudate).
Interventions Patients were randomly assigned to penicillin for seven days, penicillin for three days followed by placebo for four days, or placebo for seven days.
Main outcome measures Duration of symptoms, mean consumption of analgesics, number of days of absence from school, occurrence of streptococcal sequelae, eradication of the initial pathogen, and recurrences of sore throat after six months.
Results Penicillin treatment was not more beneficial than placebo in resolving symptoms of sore throat, neither in the total group nor in the 96 children with group A streptococci. In the groups randomised to seven days of penicillin, three days of penicillin, or placebo, one, two, and eight children, respectively, experienced a streptococcal sequela.
Conclusion Penicillin treatment had no beneficial effect in children with sore throat on the average duration of symptoms. Penicillin may, however, reduce streptococcal sequelae.
Introduction
Acute sore throat is one of the most common complaints for which children visit doctors. Roughly 15-30% of all cases with pharyngitis presented to a doctor are caused by group A streptococci.1
We previously reported that penicillin treatment for seven days was superior to treatment for three days or placebo in resolving symptoms of sore throat in adult patients with group A streptococcal pharyngitis.2 Empirical evidence for children is, however, scarce.3
Simultaneously with our trial in adults we performed a double blind, randomised trial in children aged 4-15 presenting with sore throat, comparing the effectiveness of penicillin V for seven days, penicillin V for three days (followed by placebo for four days), and placebo for seven days.
Participants and methods
We described the methods in detail in our earlier report.2 Overall 308 children aged 4-15 contacted their general practitioner because of an acute sore throat. We excluded 45 children because of medical reasons, such as an imminent quinsy (n = 28), (suspected) scarlet fever (n = 9), an intercurrent disease requiring antibiotics (n = 6), and intolerance to penicillin (n = 2).4 The participating general practitioners were experienced users of these so called Centor criteria, which were developed to trace patients with streptococcal tonsillitis.5 Of the 262 eligible children 156 were randomly assigned to one of three treatment groups: penicillin V for seven days (n = 46), penicillin V for three days followed by placebo for four days (n = 54), or placebo for seven days (n = 56). The dosage was one 250 mg capsule three times daily for children aged 4-10 and two 250 mg capsules three times daily for children aged 10 and older.
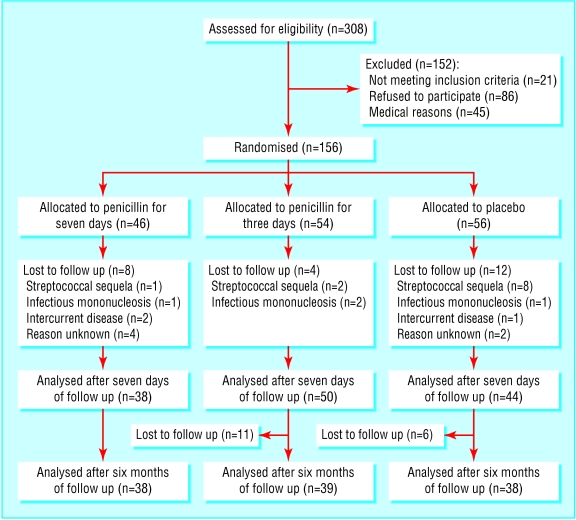
The patients were randomly assigned according to a computer generated list that was blinded to both patients and doctors. The figure shows the flow of patients through the trial. An independent pharmacist filled and numbered the medication trays. Each general practitioner received medication trays from the study coordinator, to be used in the numbered order. After six months of follow up the parents of the children were informed by telephone about the treatment received.
Figure 1.
Flow of participants through the trial of penicillin for acute sore throat in children
Throat swabs were taken after randomisation and again after two weeks, and a diary was given to the parents. During the study they recorded the children's attendance at school and possible side effects of penicillin.
The primary outcome variable was the duration of symptoms, defined as the number of days of symptoms after randomisation until the pain had resolved permanently. Secondary outcome variables included mean consumption of analgesics (in days), absence from school, development of streptococcal sequelae such as an (imminent) quinsy, eradication of the initial pathogen after two weeks, and recurrent episodes of sore throat during the six month follow up period. Imminent quinsy was defined as a unilateral, peritonsillar or tonsillar swelling with oedema and exudate, pushing aside the uvula. Informed consent was obtained from the child's parent or carer.
With at least 52 children in each of the three groups a difference of one day of duration of symptoms could be detected at a 5% level of significance with 90% power. For subgroup analysis a total of 20 children per group would be needed to detect a difference of 1.5 days of duration of symptoms. We performed all analyses on an intention to treat basis.
Results
The mean age of the included children was 10, half (78) were boys, and the mean duration of complaints before inclusion in the study was three days. We observed no relevant baseline differences between the three treatment groups (table 1).
Table 1.
Baseline characteristics of patients in the three treatment groups. Values are numbers (percentages) of children unless otherwise indicated
|
Duration of penicillin treatment per group
|
|||
|---|---|---|---|
| Seven days (n=46) | Three days (n=54) | Placebo group (n=56) | |
| Mean age in years (SD) | 9.9 (3.8) | 10.5 (3.8) | 10.1 (3.9) |
| Male | 20 (44) | 20 (37) | 30 (54) |
| Healthcare insurance | 32 (70) | 34 (63) | 36 (64) |
| Resident in cities >30 000 inhabitants | 21 (46) | 22 (41) | 28 (50) |
| Presented to general practitioner in October-March | 28 (61) | 42 (78) | 37 (66) |
| Sore throat >3 days | 30 (65) | 29 (54) | 33 (59) |
| Fever (reported) | 43 (94) | 49 (91) | 47 (84) |
| Absence of cough | 34 (74) | 37 (69) | 40 (71) |
| Absence from school | 28 (72) | 37 (69) | 40 (74) |
| Tonsillar exudate | 35 (76) | 43 (79) | 42 (75) |
| Cervical lymph nodes | 43 (94) | 53 (97) | 54 (96) |
| All four Centor criteria present | 19 (41) | 24 (44) | 25 (45) |
| History of tonsillectomy | 2 (4) | 3 (6) | 2 (4) |
| Throat culture: | |||
| Positive for group A streptococci | 28 (70) | 26 (48) | 43 (77) |
The mean duration of sore throat was the same in children taking penicillin for seven days (mean 3.8 days, 95% confidence interval 3.2 to 4.4) and in children taking placebo (3.8 days, 3.3 to 4.3); the difference was 0 days (-0.9 to 0.9). The number of days with a sore throat did not differ between children who took penicillin for three days (mean 4.6 days, 4.0 to 5.2) and children taking placebo (difference 0.8 days, -0.1 to 1.7; table 2). The number of days missed at school and the incidence of recurrent episodes of sore throat were also similar in all treatment groups (tables 3 and 4).
Table 2.
Mean duration of sore throat (in days, with 95% confidence intervals) in the three treatment groups
|
No of patients
|
Duration of penicillin treatment per group
|
|||
|---|---|---|---|---|
| Culture | Seven days | Three days | Placebo group | |
| All | 156 | 3.8 (3.2 to 4.4) | 4.6 (4.0 to 5.2) | 3.8 (3.3 to 4.3) |
| Positive for group A streptococci | 96 | 3.0 (2.4 to 3.6) | 4.8 (4.0 to 5.6) | 3.5 (2.9 to 4.1) |
| Other or negative | 60 | 4.9 (4.1 to 5.7) | 4.4 (3.6 to 5.4) | 4.7 (3.5 to 5.9) |
Table 3.
Mean duration of absence from school in days (with 95% confidence intervals) in the three treatment groups
|
Duration of penicillin treatment
|
|||
|---|---|---|---|
| Treatment group | 0 days | 3 days | 7 days |
| All (n=156) | 2.4 (1.8 to 3.0) | 2.3 (1.7 to 2.9) | 2.8 (2.2 to 3.5) |
| Children with group A streptococci (n=96) | 2.2 (1.6 to 2.8) | 2.2 (1.3 to 3.0) | 2.5 (1.8 to 3.1) |
| Children without group A streptococci (n=60) | 3.2 (1.6 to 4.7) | 2.4 (1.6 to 3.3) | 3.3 (2.0 to 4.6) |
Table 4.
Episodes of upper respiratory tract infection and sore throat reported by the parent of carer during the 6 month follow up period. Values are numbers (percentages) of patients with at least one episode per given period per treatment group
|
Duration of penicillin treatment
|
P value (χ2 test)
|
|||
|---|---|---|---|---|
| 7 days | 3 days | 0 days | ||
| Upper respiratory tract infection:
|
|
|
|
|
| Day 8-15 | 8/39 (20) | 8/39 (20) | 7/40 (18) | 0.9 |
| Day 16-180 | 32/39 (82) | 31/39 (80) | 30/40 (75) | 0.6 |
| Sore throat: | ||||
| Day 8-15 | 4/39 (10) | 6/39 (15) | 4/40 (10) | 0.8 |
| Day 16-180 | 24/39 (62) | 18/39 (46) | 18/40 (45) | 0.2 |
In children with group A streptococci (n = 96) who were treated with penicillin for seven days the sore throat resolved permanently 0.5 (-0.6 to 1.5) days sooner than in children who took placebo, whereas in those who took penicillin for three days the sore throat resolved permanently 1.3 (0.2 to 2.4) days later than in the placebo group (table 5). We found no differences in mean consumption of analgesics (table 6).
Table 5.
Difference in mean duration of sore throat (in days, with 95% confidence intervals) of seven days of penicillin treatment versus placebo, and three days of penicillin treatment versus placebo
|
Penicillin versus placebo
|
|||
|---|---|---|---|
| Throat cultures | No of patients | Seven days | Three days |
| All | 156 | 0.0 (−0.9 to 0.9) | 0.8 (−0.1 to 1.7) |
| Positive for group A streptococci | 96 | −0.5 (−1.5 to 0.6) | 1.3 (0.2 to 2.4) |
| Other or negative | 60 | −0.2 (−2.0 to 1.6) | 0.3 (−1.4 to 1.9) |
Table 6.
Mean consumption of analgesics (in days, with 95% confidence intervals) in the three randomised groups
|
Duration of penicillin treatment per group
|
||||
|---|---|---|---|---|
| Culture | No of patients | Seven days | Three days | Placebo group |
| All | 156 | 1.1 (0.7 to 1.6) | 1.4 (1.0 to 1.9) | 1.4 (1.0 to 1.8) |
| Positive for group A streptococci | 96 | 0.8 (0.3 to 1.3) | 1.3 (0.7 to 1.9) | 1.2 (0.8 to 1.6) |
| Other or negative | 60 | 1.6 (0.7 to 2.5) | 1.6 (0.9 to 2.3) | 2.0 (1.1 to 2.9) |
Penicillin treatment for seven days was more effective than treatment for three days and placebo in eradicating group A streptococci. The eradication rates were 68%, 35%, and 28% in children who took penicillin for seven days, three days, or placebo, respectively (P = 0.003).
Eleven children developed a streptococcal sequela: nine had an imminent quinsy, one scarlet fever, and one impetigo. In the group taking penicillin for seven days one child (0.02%) experienced a streptococcal sequela, compared with two (0.04%) in the group taking penicillin for three days, and eight (0.1%) in the placebo group. The incidence rate ratio of seven days of penicillin versus placebo was 0.15 (95% confidence interval 0.02 to 1.2); the incidence rate ratio of three days of penicillin versus placebo was 0.26 (0.06 to 1.2). After breaking the treatment code all 11 children received new, prolonged, or alternative antibiotic treatment. They recovered uneventfully, without referral to a hospital. The occurrence of possible side effects, such as abdominal pain (38%), diarrhoea (26%), and vomiting (30%) did not differ between the three treatment groups.
Discussion
No rationale exists for treatment with antibiotics in most children with sore throat, irrespective of the presence of streptococci. This finding is in agreement with the Dutch and Scottish guidelines on the management of sore throat.5,6
Resolution of symptoms and group A streptococci
In the total group of children and the children who were positive for group A streptococci treatment with penicillin for seven days failed to shorten the duration of sore throat, reduce non-attendance at school, or reduce recurrence of sore throat in the following six months. In our previous study, however, adult patients experienced an accelerated recovery of two days when treated with penicillin, most notably patients from whom group A streptococci were cultured.2 This discrepancy between children and adults is probably attributable to the high carrier rate of group A streptococci in asymptomatic children in our region (30% compared with 7% of adults).7 The 30% yield of group A streptococci in asymptomatic children is relatively high. Previous population based studies reported prevalences between 6% and 16%.8,9 As serotyping and genotyping of the group A streptococci did not show a predominant strain the existence of a local epidemic during the time of our study is unlikely.7,10
The preparatory training of the general practitioners to take throat swabs for culture, the use of two swabs instead of one per patient, and updated laboratory techniques11 are likely to have contributed to the high yield of streptococci in this study. In this way group A streptococci, possibly those in cultures with a low colony count, were detected that would have been missed by the techniques applied in previous studies. Cultures with a low colony count have been assumed to be more prevalent among carriers of group A streptococci who do not have an infection than among those having true streptococcal disease.7,9,12 Therefore we believe that the prevalence of streptococcal infections in our study is unlikely to differ from clinical practice or earlier studies. We probably included many carriers of group A streptococci in our group A positive children, which may have diluted the effect of penicillin.
Effects of penicillin for three days
The finding that the three day penicillin group had a slower recovery than the placebo group is in accordance with our finding in adult patients.2 The increased recurrence rate of sore throat at the end of the first week is probably due to the three days' exposure to penicillin, reducing the natural immune response without eradicating the pathogenic streptococci.
Generalisability
To our knowledge our study is the first large, randomised, double blind, placebo controlled trial in children. A recent systematic review on the management of sore throat reported important methodological limitations in all of the few available studies in children.3
As we expected, not all children with acute sore throat were included by the participating doctors. The inclusion rate, however, is in line with other randomised trials on the effects of antibiotics in upper respiratory tract infections in primary care. Possible reasons for a lower inclusion rate are that, firstly, doctors did not include all eligible patients, notably during busy office hours and during holiday periods, and secondly, patients may have visited other doctors who were not participating in the study—for example, because their own general practitioner was on leave or because they did not present during office hours. Since this selection is expected to be independent of patients' characteristics, it will not influence the generalisability.
We decided to use only two out of the four Centor criteria as an inclusion criterion in children because we considered them to be less useful than in adults. Nevertheless, we were concerned to include too many children with an unspecified upper respiratory tract infection if we did not use any criterion apart from “acute sore throat.”
We excluded severely ill children. In clinical practice this small subgroup might benefit more from penicillin than the children included in our trial. In the future such a subgroup might be identifiable by new diagnostic tools, such as genetic techniques, which help doctors to detect harmless colonisers as well as virulent invaders among the group A streptococci.13
Risk of streptococcal sequelae
The results of our study showed that streptococcal sequelae seem to occur more often in the placebo group than in the penicillin groups, but the power of our study, like all other individual trials, was too limited to draw firm conclusions. On the other hand, the observed trend is in concordance with a Cochrane review of trials in adults and children with sore throat.3 In this review the incidence of suppurative sequelae, such as acute otitis media and sinusitis in patients treated with antibiotics, was 50-75% lower than in the placebo group. Furthermore, these sequelae can safely be treated at the moment of occurrence, and their prevention is not a specific indication for antibiotic treatment in sore throat.
What is already known on this topic
Complications of sore throat that presented a serious problem in the past, such as acute rheumatic fever and post-streptococcal glomerulonephritis, have become extremely rare in affluent Western communities
Evidence is lacking whether penicillin treatment is needed for children with an acute sore throat who test positive for streptococci, and the advice given in national guidelines diverges
What this study adds
Penicillin V does not reduce the duration of symptoms or the use of analgesics, nor does it affect school attendance or recurrences of sore throat, irrespective the presence of group A streptococci
Penicillin V may reduce the development of streptococcal sequelae, such as quinsy, scarlet fever, or impetigo
Once a sequela is diagnosed, sufficient time is left to start antibiotic treatment
Nearly all children with a sore throat in Western communities can be treated safely without penicillin
Conclusion
In view of the extremely low incidence of potentially severe post-streptococcal sequelae such as rheumatic fever in affluent Western communities, rising antibiotic resistance rates, and the high carrier rate of group A streptococci in children, we advocate prudent prescription behaviour with respect to penicillin. General practitioners are recommended to treat children having an acute sore throat only when they are severely ill (unable to drink, an imminent quinsy) or at high risk (history of rheumatic fever, having an anatomical or immunological disorder, high incidence of streptococcal infections in the community).
bmj.com 2003;327:1324
Contributors: SZ initiated the research, designed the protocol, and collected the data. MMR analysed the data. RAdM was involved in the study design and the interpretation of data. AWH was involved in the study design and data analyses. All authors participated in writing the report. SZ will act as guarantor for the paper.
Funding: This study was funded by Groene Land Achmea Health Insurances and the Stichting Gezondheidszorgonderzoek Ysselmond in Zwolle.
Competing interests: None declared.
Ethical approval: The study protocol was approved by the medical ethics committee of the Isala Clinics, Zwolle.
References
- 1.Bisno AL, Gerber MA, Gwaltney JM Jr, Kaplan EL, Schwartz RH, Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 2002;35: 113-25. [DOI] [PubMed] [Google Scholar]
- 2.Zwart S, Sachs, APE, Ruijs GJHM, Gubbels JW, Hoes AW, de Melker RA. Penicillin for acute sore throat: randomised double blind trial of seven days versus three days treatment or placebo in adults. BMJ 2000;320: 150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev 2000;(4): CD000023. [DOI] [PubMed]
- 4.Centor RM, Whitherspoon JM, Dalton HP, Brody ChE, Link K. The diagnosis of strep throat in adults in the emergency room. Med Decis Making 1981;1: 239-46. [DOI] [PubMed] [Google Scholar]
- 5.Dagnelie CF, Zwart S, Balder FA, Romeijnders ACM, Geijer RMM. NHG Standard “Acute sore throat.” Utrecht: Dutch College of General Practitioners, 2002. http://nhg.artsennet.nl/guidelines2/E11.htm (accessed 21 Nov 2003).
- 6.Scottish Intercollegiate Guidelines Network. Guideline 34: management of sore throat and indications for tonsillectomy. Edinburgh: Royal College of Physicians, 2001. www.sign.ac.uk/guidelines/fulltext/34/summary.html (accessed 17 Nov 2003).
- 7.Zwart S, Ruijs GJHM, Sachs APE, van Leeuwen WJ, Gubbels JW, de Melker RA. Beta-haemolytic streptococci isolated from acute sore-throat patients: cause or coincidence? A case-control study in general practice. Scand J Infect Dis 2000;32: 377-84. [DOI] [PubMed] [Google Scholar]
- 8.Begovac J, Bobinac E, Benic B, Desnica BB, Maretic T, Basnec A, et al. Asymptomatic pharyngeal carriage of beta-hemolytic streptococci and streptococcal pharyngitis among patients at an urban hospital in Croatia. Eur J Epidemiol 1993;9: 405-10. [DOI] [PubMed] [Google Scholar]
- 9.Gunnarsson RK, Holm SE, Söderström M. The prevalence of beta-hemolytic streptococci in throat specimens from healthy children and adults. Implications for the clinical value of throat cultures. Scand J Prim Health Care 1997;15: 149-55. [DOI] [PubMed] [Google Scholar]
- 10.Zwart S, Ruijs GJHM, Sachs APE, Schellekens JFP, de Melker RA. Potentially virulent strains and high colony counts of group A beta-haemolytic streptococci in pharyngitis patients having a delayed recovery or a complication. J Antimicrob Chemother 2001:47: 689-91. [DOI] [PubMed] [Google Scholar]
- 11.Ruoff KL, Whiley RA, Beighton D. Streptococcus. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, eds. Manual of clinical microbiology. 7th ed. Washington: ASM Press, 1999: 283-96.
- 12.Kaplan EL, Couser R, Ballard Huwe B, McKay C, Wannamaker LW. Significance of quantitative salivary cultures for group A and non-group A beta-hemolytic streptococci in patients with pharyngitis and in their family contacts. Pediatrics 1979;64: 904-12. [PubMed] [Google Scholar]
- 13.Musumeci R, Bue CL, Milazzo I, Nicoletti G, Serra A, Speciale A, Blandino G. Internalization-associated proteins among Streptococcus pyogenes isolated from asymptomatic carriers and children with pharyngitis. Clin Infect Dis 2003;37: 173-9. [DOI] [PubMed] [Google Scholar]