Abstract
Thyroid hormone has profound and diverse effects on liver metabolism. Here we show that tri-iodothyronine (T3) treatment in mice acutely and specifically induces hepatic expression of the metabolic regulator fibroblast growth factor 21 (FGF21). Mice treated with T3 showed a dose-dependent increase in hepatic FGF21 expression with significant induction at doses as low as 100 μg/kg. Time course studies determined that induction is seen as early as 4 h after treatment with a further increase in expression at 6 h after injection. As FGF21 expression is downstream of the nuclear receptor peroxisome proliferator-activated receptor α (PPARα), we treated PPARα knock-out mice with T3 and found no increase in expression, indicating that hepatic regulation of FGF21 by T3 in liver is via a PPARα-dependent mechanism. In contrast, in white adipose tissue, FGF21 expression was suppressed by T3 treatment, with other T3 targets unaffected. In cell culture studies with an FGF21 reporter construct, we determined that three transcription factors are required for induction of FGF21 expression: thyroid hormone receptor β (TRβ), retinoid X receptor (RXR), and PPARα. These findings indicate a novel regulatory pathway whereby T3 positively regulates hepatic FGF21 expression, presenting a novel therapeutic target for diseases such as non-alcoholic fatty liver disease.
Keywords: Fatty Acid Oxidation, Gene Regulation, General Transcription Factors, Lipid Oxidation, Lipolysis, Liver, Liver Metabolism, Metabolic Diseases, PPAR, Thyroid
Introduction
The biochemical pathways mediating the metabolism of carbohydrates, lipids, and proteins are all regulated to some degree by thyroid hormone and the thyroid hormone receptors (α and β) (1, 2), which belong to the nuclear hormone receptor superfamily (1). In the liver, the β isoform of the thyroid hormone receptor (TRβ) is responsible for mediating the majority of the actions of tri-iodothyronine (T3),2 whereas in other tissues such as the heart and brown adipose tissue, the α isoform (TRα) is the main mediator of thyroid hormone effects (3, 4).
FGF21 is a member of the endocrine FGF subfamily, which also includes FGF19 and FGF23, all of which circulate and have hormone-like actions (5, 6). FGF21 is known to stimulate glucose uptake in mouse 3T3-L1 adipocytes and in primary cultures of human adipocytes and can improve glucose homeostasis when administered to obese mice (6) and non-human primates (7). Transgenic mice overexpressing FGF21 in liver display improved insulin sensitivity and glucose clearance, reduced plasma triglyceride concentrations, and are resistant to weight gain when fed a high fat diet (6).
Subsequent studies showed that administration of FGF21 to mice with high fat diet-induced obesity led to increased fat utilization and energy expenditure and reduced plasma glucose, insulin, serum lipid concentrations, and hepatic triglyceride concentrations (6, 8). In one study, it was shown that the decrease in hepatic triglyceride concentrations was accompanied by a decrease in lipogenic gene expression (8).
FGF21 expression is known to be downstream of the nuclear receptor PPARα, which itself plays a significant role in lipid oxidation. FGF21 is physiologically induced under conditions that activate PPARα including fasting and consumption of a ketogenic diet (KD) (9, 10). Fibrate treatment, which leads to pharmacological activation of PPARα, also causes increased FGF21 expression in rodents (10).
T3 can induce metabolic changes that are similar to those induced by FGF21 including weight loss and increased energy expenditure, and thus we speculated that FGF21 expression might be regulated by T3. We found that hepatic FGF21 gene expression responds rapidly and robustly to T3 administered peripherally in a dose-dependent manner. A substantial 3-fold induction is seen as soon as 4 h after treatment with a further increase in expression 6 h after injection. The induction of genes induced by T3 also included SPOT14 and glucose-6-phosphatse (G6Pase), but interestingly, induction of PPARα target genes is selective; thus T3 treatment did not increase expression of carnitine palmitoyltransferase 1A (CPT1a) or uncoupling protein 2 (UCP2) at these early time points. Remarkably, PPARα is required for T3-mediated effects on FGF21 expression as no induction was seen in PPARα KO mice after T3 treatment. However, other T3 targets including SPOT14 and G6Pase responded in PPARα KO mice in the expected direction following treatment with T3. Using cell culture studies, which introduced an FGF21 reporter construct into 293T cells, we also demonstrate that T3-mediated FGF21 induction requires both TRβ and PPARα as well as their common heterodimeric partner, the retinoid X receptor (RXR). These data suggest that T3 is able to rapidly and specifically regulate FGF21 expression in the liver through a PPARα-dependent pathway. Novel therapies based around TRβ agonists may permit specific hepatic up-regulation of FGF21 to treat conditions such as fatty liver and non-alcoholic hepatic steatosis.
EXPERIMENTAL PROCEDURES
Animals
12-Week-old C57BL/6 male mice (The Jackson Laboratory) were fed a standard rodent chow (F6 Rodent Diet; Harlan Teklad), housed in a controlled environment under a 14/10-h light/dark cycle, and acclimatized to housing for 7 days prior to experimentation. For dose-response (five mice per group) and time course (eight mice per time point) studies, mice were housed in groups of four.
12-Week-old male PPARα KO (n = 4) mice and WT (n = 4) control littermates on a C57BL/6 background were purchased (Taconic Farms) and housed in groups of four under the same conditions as above. All procedures were approved by and performed in accordance with guidelines issued by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Dose Response
Mice were injected intraperitioneally with either PBS or increasing doses (0, 10, 25, 100, 250, and 500 μg/kg) of T3 diluted in PBS (T2877, Sigma-Aldrich) in a total volume of 150 μl. 6 h after injection, mice were sacrificed, and tissues were rapidly collected, flash-frozen in liquid nitrogen, and stored at −80 °C.
Time Course
Male mice were injected with either PBS or 500 μg/kg of T3. Mice were sacrificed at 2, 4, and 6 h after injection, and tissues were processed as noted above.
RNA Extraction and Real-time PCR
Total RNA was isolated from tissues with the RNeasy lipid tissue kit (Qiagen). 1 μg of RNA was used for generation of cDNA (Qiagen). Quantitative real-time PCR was performed on a MX3000P thermocycler (Stratagene) using SYBR Green master mix (Applied Biosystems). Primer pairs used are listed in supplemental Table S1. All samples were analyzed in duplicate, measuring both the gene of interest and cyclophilin as an internal control.
Serum Analysis
Non-esterified fatty acids (NEFAs) were measured in duplicate using an enzymatic colorimetric assay (Wako NEFA C, Wako Chemicals; Richmond, VA).
In Vitro Transfection
293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen). Transient transfections were performed in 6-well plates using Lipofectamine 2000 (Invitrogen). Each well received a total of 100 ng of a combination of an FGF21 reporter (FGF21 constructs were generously provided by D. Manglesdorf, University of Texas Southwestern medical school; see Inagaki et al. (9)) or DR4-TK luciferase reporter construct (11), pKCR2-TRβ1 (12), pCMV-PPARα (Origene, Rockville, MD), and pKCR2-hRXRα (11) along with 3 ng of a control pCMV-β-galactosidase plasmid (11) to account for transfection efficiency. 24 h after transfection, the medium was changed to Dulbecco's modified Eagle's media supplemented with 10% steroid-depleted serum (HyClone) either with no ligand or with 10 nm T3 (Sigma). Following another 24 h, the cells were lysed and assayed for luciferase and β-galactosidase activities. Experiments were performed three times in triplicate, and the luciferase activity was normalized to β-galactosidase.
To determine the requirement of the PPAR-response elements (PPREs) present in the FGF21 promoter, cells were transfected as above with plasmids expressing TRβ, PPARα, RXRα, β-galactosidase, along with one of three FGF21 luciferase reporter constructs with varying lengths of promoter −1497, −977, and −66, respectively (see Inagaki et al. (9) for details).
Statistical Analysis
All results are expressed as mean ± S.E. Statistical comparisons of groups were made using analysis of variance.
RESULTS
T3 Treatment Induces FGF21 Expression in the Liver in a Dose- and Time-dependent Manner
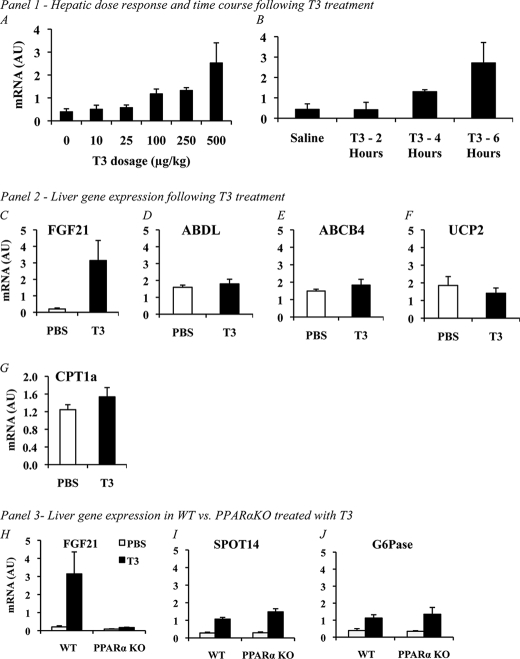
In euthyroid C57BL/6 mice injected with T3 intraperitoneally, we found a dose-dependent induction of FGF21 mRNA expression in the liver (Fig. 1A). At 6 h after injection, a greater than 2-fold increase in the expression of hepatic FGF21 was seen at a dose of 100 μg/kg of T3 (0.40 ± 0.13 versus 1.17 ± 0.21). We saw even greater levels of induction of FGF21 at dosages of 250 μg/kg (0.40 ± 0.13 versus 1.32 ± 0.13) and 500 μg/kg (0.40 ± 0.13 versus 2.52 ± 0.88). Using the highest dose of T3, we examined induction at different time points ranging from 2 to 6 h. At 2 h after injection, there was no increase in FGF21 expression following T3 treatment (0.44 ± 0.12 versus 0.42 ± 0.10; Fig. 1B); however, at 4 h, expression is significantly increased over baseline levels (0.44 ± 0.12 versus 1.30 ± 0.04, Fig. 1B) with a further rise in expression found at the 6-h time point (0.46 ± 0.119 versus 2.71 ± 0.22, Fig. 1B). The rapidity of induction of FGF21 suggests that the action of T3 on the liver is likely direct via TRβ activation rather than via a secondary effect such as increased circulating PPARα ligands.
FIGURE 1.
Panel 1, A, acute effects of T3 treatment in mice. T3 treatment of euthyroid male c57BL/6 mice via intraperitoneal injection causes a significant increase in FGF21 gene expression in the liver in a dose-dependent manner. AU, arbitrary units. B, increased FGF21 gene expression induced by T3 treatment in the liver is not significant at 2 h after injection (0.44 ± 0.12 versus 0.42 ± 0.10, n = 8 versus 8, NS); however, at 4 h after injection, expression is significantly elevated (0.44 ± 0.12 versus 1.30 ± 0.04, n = 8 versus 8, p = 0.021), and this increase in expression reaches maximal levels at 6 h (0.46 ± 0.119 versus 2.71 ± 0.22, n = 8 versus 8, p = <0.001). Panel 2, effects of T3 on hepatic PPARα targets in normal mice. To determine the extent to which PPARα target genes were induced by T3 treatment, we analyzed gene expression of several known hepatic PPARα target genes. Only expression of FGF21 (C) (PBS versus FGF21, 0.20 ± 0.06 versus 3.14 ± 1.22, 4 versus 4, p < 0.001) was significantly increased. No change between vehicle- and T3-treated mice was found for ABDL (D), ABCB4 (E), UCP2 (F), or CPT1a (G), indicating that in this paradigm, induction of FGF21 is unique among known PPARα targets. Panel 3, effects of T3 in PPARα-deficient mice. H, FGF21 is not induced in the liver of PPARα knock-out mice upon treatment with T3 using the maximal dose and optimum time point from our initial studies (WT versus PPARα KO, 3.14 ± 1.22 versus 0.17 ± 0.2, 4 versus 4, p < 0.001). Other T3-responsive genes such as SPOT14 (I) (WT versus PPARα KO, 1.07 ± 0.10 versus 1.48 ± 0.18,4 versus 4, p < 0.001) and G6Pase (J) (WT versus PPARα KO, 1.12 ± 0.20 versus 1.35 ± 0.40,4 versus 4, p < 0.001) show robust induction, indicating that PPARα is explicitly required for the induction of FGF21 expression by T3 in vivo. Error bars indicate mean ± S.E.
Other PPARα Target Genes Are Not Induced by T3 Treatment
To determine the extent of the change in gene expression mediated by T3, we analyzed several other known hepatic PPARα target genes in WT mice treated with 500 μg/kg T3 at a 6-h time point. At this dosage and time frame, no change in the PPARα targets CPT1a (1.34 ± 0.07 versus 1.46 ± 0.10, Fig. 1G), UCP2 (1.85 ± 0.51 versus 1.41 ± 0.30, Fig. 1F), ABDL (1.59 ± 0.11 versus 1.83 ± 0.34, Fig. 1D), or ATP-binding cassette, subfamily B, member 4 (ABCB4) (0.11 ± 0.01 versus 0.12 ± 0.01 Fig. 1E) was noted. We also assayed expression of PPARα itself; however, no differences in expression were found in any of our studies (data not shown).
Induction of FGF21 by T3 in the Liver Is Mediated via PPARα
Following our initial observation that T3 induces FGF21 in the liver, we next treated both WT and PPARα KO mice with T3. As PPARα has been previously shown to be critical for the induction of FGF21 by both feeding of a KD and during the fasted state, we hypothesized that it may also be upstream of the FGF21 induction seen with T3 treatment. Using a dose of 500 μg/kg, which significantly increased FGF21 expression in our initial experiments, and at a time point of 6 h, we assessed the T3-mediated FGF21 response in WT and PPARα KO mice. WT mice responded as expected with a 16-fold induction of FGF21 (0.20 ± 0.06 versus 3.14 ± 1.22, Fig. 1H). In contrast, there was no induction of FGF21 above baseline levels in PPARα KO mice (0.09 ± 0.01 versus 0.17 ± 0.02, Fig. 1H), indicating that PPARα is required for the induction of FGF21 by T3.
To confirm that the T3 treatments were successful, we also measured induction of two known T3 liver response genes, SPOT14 and G6Pase, in PPARα KO mice. Expression of both SPOT14 and G6Pase was increased approximately 3–5-fold by T3 treatment in WT and PPARα KO mice (Fig. 1, I and J).
T3 Treatment Suppresses FGF21 Expression in White Adipose Tissue (WAT)
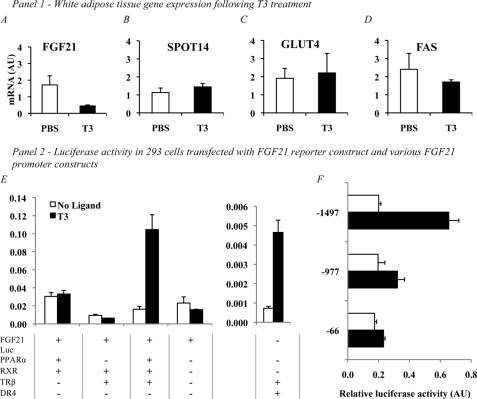
To examine the tissue-specific effects of T3, we evaluated expression of FGF21 in WAT 6 h after treatment intraperitoneally with 500 μg/kg of T3. In contrast to the induction seen in liver, we found reduced expression of FGF21 in WAT after T3 treatment (1.71 ± 0.54 versus 0.45 ± 0.06, Fig. 2A). Expression of known target genes including fatty acid synthase (FAS) (2.40 ± 0.88 versus 1.70 ± 0.12, Fig. 2D), SPOT14 (1.13 ± 0.25 versus 1.44 ± 0.19, Fig. 2B), and glucose transporter type 4 (GLUT4) (1.91 ± 0.55 versus 2.25 ± 1.10, Fig. 2C) was unaffected by T3 in this treatment paradigm. FGF21 expression was also suppressed in the WAT of PPARα KO mice under the same conditions, suggesting a PPARα-independent mechanism of action (data not shown).
FIGURE 2.
Panel 1, effects of T3 on WAT gene expression of FGF21 and previously described T3 target genes. To examine the tissue specificity of the effects of T3, we examined WAT, which has previously been described as a key tissue for FGF21 action. A, FGF21 expression was significantly suppressed following treatment with 500 μg/kg of T3 at the 6-h time point (1.71 ± 0.54 versus 0.45 ± 0.06, n = 4 versus 4, p = 0.0041). AU, arbitrary units. However, expression of known T3 targets SPOT14 (B) (1.13 ± 0.25 versus 1.44 ± 0.19, n = 4 versus 4, NS), GLUT4 (C) (1.91 ± 0.55 versus 2.25 ± 1.10, n = 4 versus 4, NS), and FAS (D) was unaffected by the treatment. Suppression of FGF21 expression by T3 also occurred in a similar manner in WAT of PPARα KO mice (data not shown), suggesting a PPARα-independent mechanism of action. Panel 2, E, reconstitution of the T3 signaling pathway in 293T cells indicates that alongside the FGF21 reporter, only a combination of PPARα, TRβ, and RXR is sufficient for the induction of FGF21 by T3. Transfection with FGF21 reporter alone led to luciferase (Luc) accumulation; however, this effect was not altered by T3 treatment of the cells. The addition of PPARα and RXR increased the accumulation seen with reporter alone; however, as with the reporter, this effect was not changed by T3 treatment. Transfection with TRβ and RXR led to a slight reduction in expression when compared with reporter alone; however, this change was not significant and was not affected by T3 treatment. When cells were transfected with TRβ, RXR, PPARα, and the reporter construct, we did see a significant increase in luciferase accumulation. DR4, a T3-responsive element, is included as a positive control and was induced by T3 in an appropriate manner similar to that seen in previous experiments. To determine the requirement of the two known PPAR-response elements in the FGF21 promoter, 293T cells were transfected with PPARα, TRβ, and RXR along with FGF21 reporter constructs of varying lengths (F) (−1497, full length containing both PPREs, −977, containing only the proximal PPRE, and −66, which had neither PPRE present). Induction occurred as in our previous experiments using the full-length (−1497) promoter construct; however, when the distal PPRE was removed in the −977 construct, luciferase induction dropped significantly. All data are corrected for β-Gal expression to account for differences in transfection efficiency. Error bars indicate mean ± S.E.
Reconstitution of a T3-responsive Signaling Pathway in 293T Cells
To establish the regulatory elements required for induction of FGF21 by T3, we transfected 293T cells with an FGF21 luciferase reporter plasmid accompanied by a combination of RXR, TRβ, and PPARα expression vectors. Transfection with the FGF21 luciferase construct alone did not allow induction of FGF21 after the cells were treated with T3 (0.02 ± 0.07 versus 0.02 ± 0.01). We then added PPARα and RXR without TRβ but still did not see significant induction upon treatment with T3 (0.03 ± 0.01 versus 0.03 ± 0.01). We did, however, see a small but significant reduction in FGF21 expression with a combination of RXR and TRβ without PPARα (0.0094 ± 0.011 versus 0.0064 ± 0.001), suggesting that PPARα is required even for basal expression of FGF21. Finally, cells were transfected with a combination of PPARα, RXR, and TRβ, and under these conditions, a robust and significant induction of FGF21 expression was seen following treatment with T3 (0.02 ± 0.01 versus 0.11 ± 0.02). As a control, a known T3-responsive DR4 element was also transfected into cells and showed robust induction following treatment with T3 and in the presence of TRβ (Fig. 2E).
To determine the requirement of the two known PPREs present in the FGF21 promoter, we used three FGF21 luciferase reporter constructs comprising various lengths of the FGF21 promoter (−1497, which spans both PPREs, −977, which lacks the distal PPRE, and −66, which lacks both). As in our earlier assays, transfection with the full-length −1497 reporter construct led to a robust induction of FGF21 (0.20 ± 0.01 versus 0.65 ± 0.07; Fig. 2F). When cells were transfected with the −977 and −66 reporter constructs, the induction of FGF21 was dramatically reduced (Fig. 2F), indicating that only the distal PPRE is required for induction, whereas the proximal PPRE does not seem to affect induction in our model.
DISCUSSION
FGF21 has emerged as a novel hepatic regulator of metabolism that plays a role in both glucose homeostasis and lipid oxidation. FGF21 expression in the liver is regulated by both fasting and consumption of a KD (9, 10, 13). Induction of FGF21 with fasting explicitly requires PPARα. However, partial induction can be seen with feeding of a ketogenic diet even in PPARα-deficient mice, suggesting that alternate pathways may also exist. Pharmacologically FGF21 expression is increased by treatment with fibrates, which are known PPARα agonists (14). Here we show that hepatic FGF21 is also regulated by thyroid hormone.
The PPARs are ligand-activated receptors that heterodimerize with RXR and bind to response elements in target genes. Available ligand alters co-activator/co-repressor dynamics to induce transcription of downstream target genes. Fatty acids are a preferred PPARα ligand with a wide array of other lipids also implicated in PPAR activation (15, 16). PPARα is expressed at high levels in liver, where its activation promotes fatty acid oxidation, ketogenesis, lipid transport, and gluconeogenesis (17). Systemic levels of free fatty acids change with nutritional status, making PPARα an attractive candidate sensor of energy balance that might respond to fatty acids by accelerating their metabolism. In our studies, we did not see any significant changes in circulating free fatty acids, indicating that production of PPARα ligand is not the mechanism via which T3 induces FGF21 expression (supplemental Fig. 1), suggesting a direct interaction between TRβ and PPARα. Furthermore, our gene expression data from WAT suggest that the effects of T3 on FGF21 expression are tissue-specific, possibly due to mediation by different TR or PPAR subtypes as PPARα is expressed at high levels in the liver and to a much lesser extent in WAT with the same being true of TRβ. It is possible that under different treatment conditions such as a longer period of T3 treatment, we could see induction of FGF21 expression in WAT. Due to the systemic effects that long term T3 treatment has on physiology, it would be difficult to ascertain whether these effects are direct effects on gene expression by T3 or are mediated by indirect mechanisms. Direct effects of T3 on gene expression are supported by our cell culture studies, which demonstrate that a specific subset of nuclear receptors are required for the FGF21 induction to occur in a rapid manner.
Thyroid hormone influences many metabolic pathways, particularly pathways that mediate lipolysis, and promote fatty acid oxidation in the liver. T3 treatment in rats stimulates thermogenesis from fatty acid β oxidation as a result of lipolysis and increased caloric intake (18). Lipogenesis is also stimulated by T3. However, this effect occurs to a much lesser extent and is mainly seen in the context of restoration of depleted fat stores after a period of energy deficit (19). Knock-out mouse models with deletion of either TRα or TRβ display a range of defects in lipogenes, lipolysis, cholesterol metabolism, and fatty acid oxidation (20, 21).
Previous studies have shown that treatment with T3 itself or with selective agonists of TRβ can improve the metabolic status of diet-induced obese rodents (22–24). Activation of TRβ with a selective thyromimetic (GC-1) in rats results in the induction of UCP1 gene expression, whereas only minimally mediating synergism between thyroid hormone and the sympathetic nervous system (20). The use of GC-1 or other TRβ-selective agonists in rodents and primates has recently been shown to increase energy expenditure and decrease fat mass and plasma levels of cholesterol (23), while sparing the heart (25) and the skeletal system (26).
Thyroid hormone action is mediated by a complex interaction between TRs and other nuclear receptors including the PPARs and the liver X receptor, which respond to circulating metabolite levels (27, 28). Cross-talk between thyroid hormone signaling and these nutrient-responsive factors occurs through a variety of mechanisms, including but not limited to competition for RXR, transcriptional co-factors, or DNA-binding sites and transcriptional cofactors. In our animal experiments, we show that PPARα is required for induction of FGF21 expression by T3, which occurs over a very rapid time frame. Our in vitro experiments demonstrate that induction requires PPARα as well as TRβ and RXR, suggesting that there is a unique interaction between the TR and PPARα to mediate FGF21 induction. Furthermore, we show that of the two known PPREs present in the FGF21 promoter, the distal site is required for the induction of FGF21 by thyroid hormone, whereas the proximal site does not seem to play a role.
Hepatic steatosis is observed in several animal models with inactivation of nuclear receptors involved in metabolic control, including the PPARα KO mouse. In both humans and animal models, obesity is associated with lipid deposition in the liver, which can lead to fibrosis and even cirrhosis (29, 30). In both human and murine microarray studies, the greatest -fold change in liver gene expression as a consequence of hepatic lipid accumulation is the down-regulation of a set of T3-responsive genes including genes involved in energy metabolism (4, 31).
We propose that the improvements in lipid profiles and fat accumulation after treatment with T3- and TRβ-specific agonists is due at least in part to induction of hepatic FGF21 and possibly suppression of WAT FGF21. Previous studies from our laboratory and others have demonstrated that modulation of FGF21 levels either via pharmacology or using molecular interventions lead to a phenotype similar to that seen with TRβ agonist treatment i.e. a significant improvement in serum lipid profile (10, 22), increased rates of lipolysis, and an increase in liver fatty acid metabolism (4, 32). These findings suggest that stimulation of lipolysis and hepatic fatty acid oxidation via FGF21 induction using TRβ-specific agonists has significant therapeutic potential.
Supplementary Material
Acknowledgments
We thank Dr. D. Manglesdorf and Dr. T. Inagaki (University of Texas Southwestern Medical Center) for the generous provision of FGF21 luciferase constructs.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1-DK56123 (to A. N. H.), RO1-DK069983 (to E. M.-F.), and R37-DK28082 (to J. S. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1 and Table S1.
- T3
- tri-iodothyronine
- FGF
- fibroblast growth factor
- PPAR
- proliferator-activated receptor
- PPRE
- PPAR-response element
- TR
- thyroid hormone receptor
- RXR
- retinoid X receptor
- KD
- ketogenic diet
- WAT
- white adipose tissue
- KO
- knock-out
- PBS
- phosphate-buffered saline
- WT
- wild type.
REFERENCES
- 1.Hulbert A. J. (2000) Biol. Rev. Camb. Philos. Soc. 75, 519–631 [DOI] [PubMed] [Google Scholar]
- 2.Klieverik L. P., Coomans C. P., Endert E., Sauerwein H. P., Havekes L. M., Voshol P. J., Rensen P. C., Romijn J. A., Kalsbeek A., Fliers E. (2009) Endocrinology 150, 5639–5648 [DOI] [PubMed] [Google Scholar]
- 3.Erion M. D., Cable E. E., Ito B. R., Jiang H., Fujitaki J. M., Finn P. D., Zhang B. H., Hou J., Boyer S. H., van Poelje P. D., Linemeyer D. L. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 15490–15495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perra A., Simbula G., Simbula M., Pibiri M., Kowalik M. A., Sulas P., Cocco M. T., Ledda-Columbano G. M., Columbano A. (2008) FASEB J. 22, 2981–2989 [DOI] [PubMed] [Google Scholar]
- 5.Kharitonenkov A., Shanafelt A. B. (2009) Curr. Opin. Investig. Drugs 10, 359–364 [PubMed] [Google Scholar]
- 6.Kharitonenkov A., Shiyanova T. L., Koester A., Ford A. M., Micanovic R., Galbreath E. J., Sandusky G. E., Hammond L. J., Moyers J. S., Owens R. A., Gromada J., Brozinick J. T., Hawkins E. D., Wroblewski V. J., Li D. S., Mehrbod F., Jaskunas S. R., Shanafelt A. B. (2005) J. Clin. Invest. 115, 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharitonenkov A., Wroblewski V. J., Koester A., Chen Y. F., Clutinger C. K., Tigno X. T., Hansen B. C., Shanafelt A. B., Etgen G. J. (2007) Endocrinology 148, 774–781 [DOI] [PubMed] [Google Scholar]
- 8.Xu J., Lloyd D. J., Hale C., Stanislaus S., Chen M., Sivits G., Vonderfecht S., Hecht R., Li Y. S., Lindberg R. A., Chen J. L., Jung D. Y., Zhang Z., Ko H. J., Kim J. K., Véniant M. M. (2009) Diabetes 58, 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V., Li Y., Goetz R., Mohammadi M., Esser V., Elmquist J. K., Gerard R. D., Burgess S. C., Hammer R. E., Mangelsdorf D. J., Kliewer S. A. (2007) Cell Metab. 5, 415–425 [DOI] [PubMed] [Google Scholar]
- 10.Badman M. K., Pissios P., Kennedy A. R., Koukos G., Flier J. S., Maratos-Flier E. (2007) Cell Metab. 5, 426–437 [DOI] [PubMed] [Google Scholar]
- 11.Hollenberg A. N., Monden T., Madura J. P., Lee K., Wondisford F. E. (1996) J. Biol. Chem. 271, 28516–28520 [DOI] [PubMed] [Google Scholar]
- 12.Monden T., Yamada M., Ishii S., Hosoya T., Satoh T., Wondisford F. E., Hollenberg A. N., Mori M. (2003) Thyroid 13, 427–435 [DOI] [PubMed] [Google Scholar]
- 13.Kennedy A. R., Pissios P., Otu H., Roberson R., Xue B., Asakura K., Furukawa N., Marino F. E., Liu F. F., Kahn B. B., Libermann T. A., Maratos-Flier E. (2007) Am. J. Physiol. Endocrinol. Metab. 292, E1724–E1739 [DOI] [PubMed] [Google Scholar]
- 14.Kliewer S. A., Mangelsdorf D. J. (2010) Am. J. Clin. Nutr. 91, 254S–257S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hostetler H. A., Kier A. B., Schroeder F. (2006) Biochemistry 45, 7669–7681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakravarthy M. V., Lodhi I. J., Yin L., Malapaka R. R., Xu H. E., Turk J., Semenkovich C. F. (2009) Cell 138, 476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernal-Mizrachi C., Weng S., Feng C., Finck B. N., Knutsen R. H., Leone T. C., Coleman T., Mecham R. P., Kelly D. P., Semenkovich C. F. (2003) Nat. Med. 9, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 18.Oppenheimer J. H., Schwartz H. L., Lane J. T., Thompson M. P. (1991) J. Clin. Invest. 87, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holness M. J., French T. J., Schofield P. S., Sugden M. C. (1987) Biochem. J. 247, 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribeiro M. O., Bianco S. D., Kaneshige M., Schultz J. J., Cheng S. Y., Bianco A. C., Brent G. A. (2010) Endocrinology 151, 432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ying H., Araki O., Furuya F., Kato Y., Cheng S. Y. (2007) Mol. Cell. Biol. 27, 2359–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryzgalova G., Effendic S., Khan A., Rehnmark S., Barbounis P., Boulet J., Dong G., Singh R., Shapses S., Malm J., Webb P., Baxter J. D., Grover G. J. (2008) J. Steroid Biochem. Mol. Biol. 111, 262–267 [DOI] [PubMed] [Google Scholar]
- 23.Grover G. J., Mellström K., Malm J. (2007) Curr. Vasc. Pharmacol. 5, 141–154 [DOI] [PubMed] [Google Scholar]
- 24.Villicev C. M., Freitas F. R., Aoki M. S., Taffarel C., Scanlan T. S., Moriscot A. S., Ribeiro M. O., Bianco A. C., Gouveia C. H. (2007) J. Endocrinol. 193, 21–29 [DOI] [PubMed] [Google Scholar]
- 25.Trost S. U., Swanson E., Gloss B., Wang-Iverson D. B., Zhang H., Volodarsky T., Grover G. J., Baxter J. D., Chiellini G., Scanlan T. S., Dillmann W. H. (2000) Endocrinology 141, 3057–3064 [DOI] [PubMed] [Google Scholar]
- 26.Freitas F. R., Capelo L. P., O'Shea P. J., Jorgetti V., Moriscot A. S., Scanlan T. S., Williams G. R., Zorn T. M., Gouveia C. H. (2005) J. Bone Miner. Res. 20, 294–304 [DOI] [PubMed] [Google Scholar]
- 27.Buroker N. E., Young M. E., Wei C., Serikawa K., Ge M., Ning X. H., Portman M. A. (2007) Am. J. Physiol. Endocrinol. Metab. 292, E453–460 [DOI] [PubMed] [Google Scholar]
- 28.Araki O., Ying H., Furuya F., Zhu X., Cheng S. Y. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 16251–16256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silveira M. G., Mendes F. D., Diehl N. N., Enders F. T., Lindor K. D. (2009) Liver Int. 29, 1094–1100 [DOI] [PubMed] [Google Scholar]
- 30.Cable E. E., Finn P. D., Stebbins J. W., Hou J., Ito B. R., van Poelje P. D., Linemeyer D. L., Erion M. D. (2009) Hepatology 49, 407–417 [DOI] [PubMed] [Google Scholar]
- 31.Pihlajamäki J., Boes T., Kim E. Y., Dearie F., Kim B. W., Schroeder J., Mun E., Nasser I., Park P. J., Bianco A. C., Goldfine A. B., Patti M. E. (2009) J. Clin. Endocrinol. Metab. 94, 3521–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badman M. K., Koester A., Flier J. S., Kharitonenkov A., Maratos-Flier E. (2009) Endocrinology 150, 4931–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.