Abstract
Tumor cells, including SW480 carcinoma cells that carry a mutant p53, are addicted to the mutant for their survival and resistance to growth suppression by chemotherapeutic agents. Here, we investigated whether various classes of p53 mutants share a common property and functional domains necessary for mutant p53 gain of function. To test this, we generated SW480 cell lines in which endogenous mutant R273H/P309S can be inducibly or stably knocked down, whereas a small interfering RNA-resistant mutant p53 along with a mutated functional domain can be inducibly or stably expressed. We found that both contact-site (R248W and R273H) and conformation (G245S and R249S) mutants are able to maintain the transformed phenotypes of SW480 cells conferred by endogenous mutant p53. We also found that activation domains 1–2 and the proline-rich domain are required for mutant p53 gain of function. Interestingly, we showed that the C-terminal basic domain, which is required for wild-type p53 activity, is an inhibitory domain for mutant p53. Furthermore, we showed that deletion of the basic domain enhances, whereas a mutation in activation domains 1–2 and deletion of the proline-rich domain abolish mutant p53 to regulate Gro1 and Id2, both of which are regulated by and mediate endogenous mutant p53 gain of function. These results indicate that both conformation and contact-site mutants share a property for cell transformation, and the domains critical for wild-type p53 tumor suppression are also required for mutant p53 tumor promotion. Thus, the inhibitory basic domain and the common property for p53 mutants can be explored for targeting tumors with mutant p53.
Keywords: Mutant, Oncogene, p53, Transcription Target Genes, Tumor Suppressor, Gain of Function, Mutant p53
Introduction
The p53 tumor suppressor gene is frequently mutated in human cancer (1). The majority of tumor-derived p53 mutations is missense point mutation and clustered within the central DNA-binding domain. Mutant p53 is defective in sequence-specific DNA binding and growth inhibition (2, 3), which defines the classical loss of function mutation for a tumor suppressor. In addition, p53 mutants with an intact domain for tetramerization are dominant negative because the mutants can form a heterotetramer with wild-type p53 (4, 5). Interestingly, a growing number of studies have provided compelling evidence that p53 mutants have acquired additional functions independent of wild-type p53, called gain of function (2). For example, knock-in mice that carry one null allele and one mutant allele of the p53 gene (R172H or R270H) developed novel tumors when compared with p53-null mice (6, 7). Furthermore, mouse embryo fibroblasts from mutant mice homozygous in R172H displayed enhanced cell proliferation, DNA synthesis, and transformation potential (6). Accordingly, it appears that gain of function is dependent on the ability of mutant p53 to transactivate or repress specific target genes, such as c-Myc, Fas, and NF-κB2 genes (8–10). Indeed, the spectrum of genes regulated by mutant p53 is quite distinct from that regulated by wild-type p53 (2). In an effort to identify target genes in a physiologically relevant context, we found that inducible knockdown of mutant p53 in SW480 and MIA PaCa-2 cells leads to increased expression of Id2 (11) but decreased expression of Gro1 (12).
Structural and functional analyses have shown that wild-type p53 contains several functional domains (13). These are N-terminal activation domain 1 (AD1)2 within residues 1–42 and activation domain 2 (AD2) within residues 43–61, the proline-rich domain (PRD) within residues 62–91, the sequence-specific DNA-binding domain within residues 102–292, and the extreme C-terminal basic domain (BD) within residues 364–393. The functional domains in wild-type p53 have been subject to extensive analysis (13–15). p53 with a mutation in AD1 is deficient in transcriptional activity and subsequently unable to induce growth suppression and cell cycle arrest (16). Previously, we and others identified AD2, which is required for p53-dependent apoptosis (17, 18). In addition, we and others showed that the PRD is necessary for induction of apoptosis and contributes to growth suppression (19–21). The BD is found to be extensively modified and fine-tunes p53 transcriptional activity (15). For example, the DNA binding activity of p53 is increased by phosphorylation of multiple serine residues, acetylation of multiple lysine residues, and binding of a specific antibody or peptide to this domain (3, 14). Thus, the tumor suppression and transcriptional activity of p53 are tightly controlled by its functional domains.
Despite the wealth of information about the functional domains in wild-type p53, there are only a few reports on mutant p53 functional domains. Previous studies showed that the integrity of activation domain 1 is required for mutant p53 gain of function in p53-null cells (9, 16, 22). In addition, deletion of residues 360–393 impairs mutant p53(D281G) to regulate the c-Myc promoter (8). However, the underlying mechanism, and more importantly, the physiological significance of the functional domains are still unclear. Here, to determine whether various classes of p53 mutants differ in their capability to maintain the transformed phenotypes of tumor cells and functional domains necessary for mutant p53 gain of function, we generated a series of SW480 cell lines in which endogenous mutant p53 can be knocked down inducibly or stably by small interfering RNA (siRNA), whereas an siRNA-resistant mutant p53 along with a mutated functional domain can be stably or inducibly expressed. We found that both contact-site (R248W and R273H) and conformation (G245S and R249S) mutants are able to maintain the transformed phenotypes conferred by endogenous mutant p53 in SW480 cells. We found that AD1, AD2, and PRD are necessary for mutant p53 to maintain the transformed phenotypes and resistance toward chemotherapeutic drugs, whereas BD suppresses this effect. Thus, a strategy to target the inhibitory basic domain and the common property can be explored for tumors with mutant p53.
MATERIALS AND METHODS
Cell Culture
Colorectal adenocarcinoma cell line SW480, harboring mutant p53 R273H/P309S, was cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone). SW480-p53-KD cell line, in which siRNA targeting p53 can be inducibly expressed under the control of the tetracycline-regulated promoter, was used as described (23). The control cell line SW480-LacZ-KD, in which siRNA targeting bacterial β-galactosidase mRNA (LacZ) can be inducibly expressed, was generated as described (23).
To generate SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas an siRNA-resistant mutant p53 is stably expressed, mutant p53 in pcDNA3 was transfected into p53-KD SW480 cells. The resulting cell lines were selected with G418 and then confirmed by Western blot analysis. To generate SW480 cell lines in which endogenous mutant p53 is inducibly knocked down, whereas an siRNA-resistant mutant p53 is inducibly expressed, mutant p53 in pcDNA4 was transfected into p53-KD SW480 cells. The resulting cell lines were selected with Zeocin and then confirmed by Western blot analysis. To generate SW480 cell lines in which endogenous mutant p53 is stably knocked down, whereas an siRNA-resistant mutant p53 is inducibly expressed, p53 siRNA-expressing pBabe-U6 and mutant p53 in pcDNA4 were co-transfected into SW480 cells, in which a tetracycline repressor is expressed by pcDNA6. The resulting cell lines were selected with puromycin and Zeocin and then confirmed by Western blot analysis.
Antibodies
Antibodies against p53 (FL-393 and PAb421), HA tag, and actin were purchased from Santa Cruz Biotechnology or Sigma.
Plasmids
The constructs that stably or inducibly express siRNA against p53 were used as described (24). To generate a construct that inducibly expresses siRNA against LacZ, one pair of oligonucleotides was synthesized and cloned into pBabe-H1 at HindIII and BglII sites, and the resulting construct was designated as pBabe-H1-LacZ siRNA. The siRNA oligonucleotides are sense, 5′-GATCCCCTTTAACCGCCAGTCAGGCTTTCAAGAGAAGCCTGACTGGCGGTTAAATTTTTGGAAA-3′, and antisense, 5′-AGCTTTTCCAAAAATTTAACCGCCAGTCAGGCTTCTCTTGAAAGCCTGACTGGCGGTTAAAGGG-3′, with the siRNA targeting region underlined.
To generate a construct expressing LacZ protein, an 1,163-bp DNA fragment encoding HA-tagged C-terminal 375-amino acid (GenBankTM NC_008563) was amplified with forward primer, 5′-AAGCTTACCATGTACCCATACGACGTACCAGATTACGCTGAGTTCCTGCACTGGATGG-3′, and reverse primer, 5′-CTCGAGTTATTTTTGACACCAGACCAAC-3′. The fragment was confirmed by sequencing and then cloned into pcDNA3 at HindIII and XhoI sites, and the resulting plasmid was designated as pcDNA3-HA-LacZ.
To generate a construct expressing siRNA-resistant mutant p53, an 1,182-bp DNA fragment containing the entire open reading frame of mutant p53, in which CAG (underlined) within siRNA targeting region (5′-GACTCCAGTGGTAATCTAC-3′) was replaced with ATC, was amplified with forward primer, 5′-AAGCTTACCATGGAGGAGCCGCAGTCAGATCC-3′, and reverse primer, 5′-CTCGAGTCAGTCTGAGTCAGGCCCTTC-3′. The fragment was confirmed by sequencing and then cloned into pcDNA3 or pcDNA4 at HindIII and XhoI sites, and the resulting plasmid was designated as pcDNA3- or pcDNA4-mutant p53.
To generate a construct expressing siRNA-resistant mutant p53 (ΔBD), an 1,104-bp DNA fragment encoding amino acids 1–363 was amplified with forward primer, 5′-AAGCTTACCATGGAGGAGCCGCAGTCAGATCC-3′, and reverse primer, 5′-CTCGAGTCACCTGCTCCCCCCTGGCTCCT-3′. The fragment was confirmed by sequencing and then cloned into pcDNA4.
To generate a construct expressing siRNA-resistant mutant p53(ΔPRD), which lacks residues 62–91, p53(Gln-22/Ser-23), or p53(Gln-53/Ser-54), a two-step PCR-based method was used. For p53(ΔPRD), fragment 1 was amplified with forward primer P1, 5′-AAGCTTACCATGGAGGAGCCGCAGTCAGATCC-3′, and reverse primer P2, 5′-GACAGAAGATGACAGGGGATCTGGACCTGGGTCTTC-3′; fragment 2 was amplified with forward primer P3, 5′-GAAGACCCAGGTCCAGATCCCCTGTCATCTTCTGTC-3′, and reverse primer P4, 5′-CTCGAGtCAGTCTGAGTCAGGCCCTTC-3′. For p53(Gln-22/Ser-23), fragment 1 was amplified with forward primer P1, and reverse primer, 5′-CGTTG TTTTCAGGAAGTAGTTTGGATTGGTCTGAAAATGTTTCCTGAC-3′; fragment 2 was amplified with forward primer, 5′-GTCAGGAAACATTTTCAGACCAATCCAAACTACTTCCTGAAAACAACG-3′, and reverse primer P4. For p53(Gln-53/Ser-54), fragment 1 was amplified with forward primer P1 and reverse primer, 5′-GGACCTGGGTCTTCAGTGGACTGTTGTTCAATATCGTCCG-3′; fragment 2 was amplified with forward primer, 5′-CGGACGATATTGAACAACAGTCCACTGAAGACCCAGGTCC-3′, and reverse primer P4. Then, fragments 1–2 were mixed together as a template and amplified with primers P1 and P4. The resulting fragments encoding p53(ΔPRD), p53(Gln-22/Ser-23), and p53(Gln-53/Ser-54) were confirmed by sequencing and cloned into pcDNA4.
Reverse Transcription-PCR Assay
Total RNA was isolated from cells using TRIzol reagent (Invitrogen). cDNA was synthesized using an IscriptTM cDNA synthesis kit (Bio-Rad). To measure Id2 mRNA, RT-PCR was done with forward primer 5′-ATGAAAGCCTTCAGTCCCGTG-3′ and reverse primer 5′-TCAGCCACACAGTGCTTTGC-3′. To measure Gro1 mRNA, RT-PCR was done with forward primer 5′-ATGGCCCGCGCTGCTCTCTCCG-3′ and reverse primer 5′-TCAGTTGGATTTGTCACTGTTCAG-3′. Glyceraldehyde-3-phosphate dehydrogenase was amplified with forward primer 5′-CATCTTCTTTTGCGTCGCCAG-3′ and reverse primer 5′-CTTGATTTTGGAGGGATCTCGC-3′.
Colony Formation Assay
SW480 cells (1,000 per well) in a six-well plate were cultured in the absence or presence of tetracycline (1.0 μg/ml) for 72 h and then untreated or treated with 50 nm camptothecin for 4 h followed by one wash with Dulbecco's modified Eagle's medium to remove camptothecin. The cells were maintained in fresh medium for the next 15–17 days and then fixed with methanol/glacial acetic acid (7:1) and stained with 0.1% of crystal violet.
Statistics
Two-tailed Student's t test was used to determine the statistical significance. p < 0.05 was considered significant.
RESULTS
The Proliferation Defect of SW480 Cells Induced by Knockdown of Endogenous Mutant p53 Can Be Rescued by Both Contact-site and Conformation Mutants
We and others showed that tumor cell lines, including SW480 cells, which carry a mutant p53, are addicted to the mutant for cell survival and proliferation (11, 25, 26). However, it is not clear whether in the same genetic background both contact-site and conformation mutants are capable of maintaining the transformed phenotypes of tumor cells. Thus, the SW480 cell line, which carries mutant R273H/P309S, was chosen to address this. Previously, we generated multiple inducible p53 knockdown SW480 cell lines and showed that mutant p53 is required for the colony-forming ability of SW480 cells (11). Similar to our previous observation (11), here we showed that upon induction of siRNA against p53, the level of mutant p53 protein was reduced (Fig. 1A), which inhibited SW480 cells to form colonies (Fig. 1B). In addition, knockdown of mutant p53 sensitized SW480 cells to treatment with camptothecin, an inhibitor of DNA topoisomerase I and a chemotherapeutic drug (Fig. 1B). We also generated SW480 cell lines in which siRNA against LacZ transcript can be inducibly expressed under the control of the tetracycline-regulated promoter. We showed that upon induction of LacZ siRNA, the level of LacZ protein was decreased, whereas the level of mutant p53 protein was not affected in SW480 cells transiently transfected with LacZ-expressing vector (Fig. 1C). In addition, inducible expression of LacZ siRNA in SW480 cells had little if any effect on cell proliferation regardless of treatment with camptothecin (Fig. 1D).
FIGURE 1.
Mutant p53 is required for maintaining the transformed phenotypes of SW480 cells. A, Western blots were prepared with extracts from SW480 cells uninduced (−) or induced (+) to knock down mutant p53 for 3 days and then probed with antibodies against p53 and actin, respectively. B, left, colony formation assay was performed with SW480 cells uninduced or induced to knock down endogenous mutant p53 along with mock treatment or treatment with camptothecin (CPT). Right, quantification of the number of colonies with a diameter of >1 mm. *, p < 0.05. Error bars indicate S.E. C, generation of SW480 cell lines in which siRNA against bacterial LacZ can be inducibly expressed. Western blots were prepared with extracts from SW480 cells, which were transiently transfected with HA-tagged LacZ for 1 day and then uninduced (−) or induced (+) to express siRNA against LacZ mRNA for 3 days. The blots were then probed with antibodies against HA tag, p53, and actin, respectively. D, left, colony formation assay was performed with SW480 cells uninduced or induced to express siRNA against LacZ along with mock treatment or treatment with CPT. Right, quantification of the number of colonies with a diameter of >1 mm. Error bars indicate S.E.
Next, we generated a series of SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas an siRNA-resistant tumor-derived hot spot mutant, including contact-site mutants (R248W and R273H) and conformation mutants (G245S and R249S), was stably expressed. We would like to note that codon 72 in endogenous mutant p53 encodes an arginine, whereas that in exogenous mutant p53 encodes a proline. As a result, exogenous mutant p53 can be separated from endogenous mutant p53 in an SDS-PAGE gel. Upon induction of siRNA against p53, endogenous mutant p53 was knocked down (Fig. 2, A, C, E, and G, compare lanes 1 and 3 with lanes 2 and 4, respectively). In addition, exogenous mutant p53 was stably expressed (Fig. 2, A, C, E, and G). Except for G245S-producing clone 9 and R249S-producing clone 2 in which exogenous mutant was expressed at an extremely low level, all other clones expressed mutant p53 at a level equivalent to that for endogenous mutant p53. To test whether these mutants are capable of substituting endogenous R273H/P309S for colony formation, one clone was chosen for further analysis. We showed that in the presence of an exogenous mutant p53, knockdown of endogenous mutant p53 had no effect on the number of colonies formed by SW480 cells regardless of treatment with camptothecin (Fig. 2, B, D, F, and H). This suggests that stable replacement with G245S, R248W, R249S, and R273H was capable of rescuing the proliferative defect in SW480 cells induced by knockdown of endogenous mutant p53 and conferring resistance to growth suppression by treatment with camptothecin.
FIGURE 2.
The proliferation defect of SW480 cells induced by knockdown of endogenous mutant p53 can be rescued by stable expression of an siRNA-resistant mutant p53. A, C, E, and G, generation of SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas an siRNA-resistant mutant G245S (A), R248W (C), R249S (E), or R273H (G) was stably expressed. Western blots were prepared with extracts from SW480 cells uninduced (−) or induced (+) to knock down endogenous mutant p53 for 3 days and then probed with antibodies against p53 and actin, respectively. B, D, F, and H, left, SW480 cells, in which siRNA-resistant mutant G245S (B), R248W (D), R249S (F), or R273H (H) was stably expressed, were uninduced or induced to knock down endogenous mutant p53 along with mock treatment or treatment with CPT and then cultured for 14 days. Right, quantification of the number of colonies with a diameter of >1 mm. Error bars indicate S.E.
To further expand the above observations, we generated SW480 cell lines in which endogenous mutant p53 was stably knocked down by siRNA, whereas an siRNA-resistant mutant, including G245S, R248W, R249S, and R273H, can be inducibly expressed. As shown in Fig. 3, mutant p53 was expressed upon induction, whereas endogenous mutant p53 was nearly undetectable (Fig. 3, A, E, and G) or expressed at a low level (Fig. 3C). Interestingly, we showed that inducible expression of an exogenous mutant p53 was able to promote the colony-forming ability of SW480 cells along with reduced sensitivity to treatment with camptothecin when endogenous mutant p53 was stably knocked down (Fig. 3, B, D, F, and H). Here, we would like note that colony quantification and statistical analyses were not performed for R273H-producing SW480 cells in the absence of camptothecin treatment due to the large number of colonies (Fig. 3H, right panel).
FIGURE 3.
Inducible expression of an siRNA-resistant mutant p53 promotes the colony-forming ability of SW480 cells in which endogenous mutant p53 was stably knocked down. A, C, E, and G, generation of SW480 cell lines in which endogenous mutant p53 was stably knocked down, whereas an siRNA-resistant mutant G245S (A), R248W (C), R249S (E), or R273H (G) can be inducibly expressed. Western blots were prepared with extracts from SW480 cells uninduced (−) or induced (+) to express an exogenous mutant p53 for 1 day and then probed with antibodies against p53 and actin, respectively. B, D, F, and H, left, SW480 cells, in which endogenous mutant p53 was stably knocked down, were uninduced or induced to express mutant G245S (B), R248W (D), R249S (F), or R273H (H) along with mock treatment or treatment with CPT and then cultured for 14 days. Right, quantification of the number of colonies with a diameter of >1 mm. *, p < 0.05. Error bars indicate S.E.
The gain of function in colony-forming activity for each of the four p53 mutants appears to be stronger in SW480 cells in which endogenous mutant p53 was stably knocked down than that in which endogenous mutant p53 was inducibly knocked down. This may be due to multiple factors, including the extent of knockdown for endogenous mutant p53 by stable and inducible approaches and the length of time for which SW480 cells were cultured without endogenous mutant p53. To address this, we generated SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down and at the same time an siRNA-resistant mutant p53 can be inducibly expressed. We showed that upon induction, endogenous mutant p53 was markedly knocked down, whereas exogenous p53 mutants, G245S and R248W, were expressed (Fig. 4, A and C). We would like to note that only a minute amount of exogenous mutant p53 was expressed in SW480-producing line 11 (Fig. 4C). Next, a colony formation assay was performed and showed that G245S and R248W were able to rescue the colony-forming defect caused by inducible knockdown of endogenous mutant p53 and to confer resistance to growth suppression by treatment with camptothecin (Fig. 4, B and D). We would also like to note that the colony-forming activity of SW480 cells was slightly enhanced by R248W, which may be due to a slightly higher expression level of the mutant. Nevertheless, three separate approaches led to the similar conclusion that the loss of the transformed phenotypes (cell proliferation and survival) upon knockdown of endogenous mutant R273H/P309S can be restored by an exogenous mutant p53 in SW480 cells.
FIGURE 4.
The proliferation defect of SW480 cells induced by knockdown of endogenous mutant p53 can be rescued by inducible expression of an siRNA-resistant mutant p53. A and C, generation of SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas siRNA-resistant mutant G245S (A) or R248W (C) can be inducibly expressed. Western blots were prepared with extracts from SW480 cells uninduced (−) or induced (+) to knock down endogenous mutant p53 and simultaneously express an exogenous mutant p53 for 1 day and then probed with antibodies against p53 and actin, respectively. B and D, left, SW480 cells were uninduced or induced to knock down endogenous mutant p53 and simultaneously express mutant G245S (B) or R248W (D) along with mock treatment or treatment with CPT and then cultured for 14 days. Right, quantification of the number of colonies with a diameter of >1 mm. *, p < 0.05. Error bars indicate S.E.
AD1, AD2, and PRD Are Required for Mutant p53 Gain of Function, whereas BD Is an Inhibitory Domain
In addition to the DNA-binding domain, AD1, AD2, PRD, and BD are known to regulate wild-type p53 activity for tumor suppression (13, 15), but the requirement of these domains for mutant p53 in a physiologically relevant setting is still not certain. Thus, we generated siRNA-resistant G245S and R248W mutants along with a mutation in AD1 that carries a double-point mutation (Gln-22/Ser-23), in AD2 that carries a double-point mutation (Gln-53/Ser-54), in PRD that lacks residues 62–91, or in BD that lacks residues 364–393.
To determine the requirement of AD1 for mutant p53 gain of function, we generated multiple SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas AD1-deficient G245S or R248W can be inducibly expressed (Fig. 5, A and C). Next, a colony formation assay was performed and showed that some colonies were formed upon expression of G245S(Gln-22/Ser-23) and R248W(Gln-22/Ser-23), even with treatment of camptothecin (Fig. 5, B and D). However, the extent of the rescue was much less by G245S(Gln-22/Ser-23) and R248W(Gln-22/Ser-23) than that by G245S and R248W (Fig. 4, B and D).
FIGURE 5.
Mutant G245S or R248W with a double-point mutation (Gln-22/Ser-23) in activation domain 1 is deficient in rescuing the proliferation defect of SW480 cells induced by knockdown of endogenous mutant p53. A and C, generation of SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas siRNA-resistant mutant G245S(Gln-22/Ser-23) (A) or R248W(Gln-22/Ser-23) (C) can be inducibly expressed. The experiment was performed as in Fig. 4A. B and D, left, SW480 cells were uninduced or induced to knock down endogenous mutant p53 and simultaneously express mutant G245S(Gln-22/Ser-23) (B) or R248W(Gln-22/Ser-23) (D) along with mock treatment or treatment with CPT and then cultured for 14 days. Right, quantification of the number of colonies with a diameter of >1 mm. *, p < 0.05. Error bars indicate S.E.
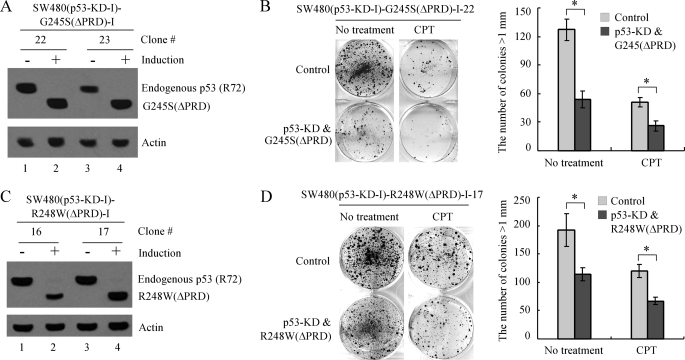
Similarly, several SW480 cell lines were generated in which endogenous mutant p53 can be inducibly knocked down, whereas G245S or R248W along with a mutation in AD2 (Gln-53/Ser-54) or a deletion of PRD (ΔPRD) can be inducibly expressed (Fig. 6, A and C; Fig. 7, A and C). We showed that AD2 mutation and PRD deletion made G245S and R248W less efficient in rescuing the colony-forming ability and in conferring resistance to growth suppression by treatment of camptothecin in SW480 cells upon knockdown of endogenous mutant p53 (Fig. 6, B and D; Fig. 7, B and D).
FIGURE 6.
Mutant G245S or R248W with a double-point mutation (Gln-53/Ser-54) in activation domain 2 is deficient in rescuing the proliferation defect of SW480 cells induced by knockdown of endogenous mutant p53. A and C, generation of SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas siRNA-resistant mutant G245S(Gln-53/Ser-54) (A) or R248W(Gln-53/Ser-54) (C) can be inducibly expressed. The experiment was performed as in Fig. 4A. B and D, left, SW480 cells were uninduced or induced to knock down endogenous mutant p53 and simultaneously express mutant G245S(Gln-53/Ser-54) (B) or R248W(Gln-53/Ser-54) (D) along with mock treatment or treatment with CPT and then cultured for 14 days. Right, quantification of the number of colonies with a diameter of >1 mm. *, p < 0.05. Error bars indicate S.E.
FIGURE 7.
Mutant G245S or R248W without the proline-rich domain (Δ62–91) is deficient in rescuing the proliferation defect of SW480 cells induced by knockdown of endogenous mutant p53. A and C, generation of SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas siRNA-resistant mutant G245S(ΔPRD) (A) or R248W(ΔPRD) (C) can be inducibly expressed. The experiment was performed as in Fig. 4A. B and D, left, SW480 cells were uninduced or induced to knock down endogenous mutant p53 and simultaneously express mutant G245S(ΔPRD) (B) or R248W(ΔPRD) (D) along with mock treatment or treatment with CPT and then cultured for 14 days. Right, quantification of the number of colonies with a diameter of >1 mm. *, p < 0.05. Error bars indicate S.E.
The C-terminal BD is known to regulate the DNA binding and transcriptional activity of wild-type p53 via modifications, such as phosphorylation, acetylation, and methylation (15). In addition, deletion of the BD inhibits p53 transcriptional activity in vivo (27), although the in vitro DNA binding activity for wild-type p53 can be enhanced by such deletion (3). Similarly, deletion of the BD impairs mutant p53 D281G to activate the c-Myc promoter (8). To further define the activity of BD for mutant p53 gain of function, we generated multiple SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas siRNA-resistant G245S(ΔBD) or R248W(ΔBD) can be inducibly expressed. Western blot analysis showed that upon induction, endogenous mutant p53 was knocked down, whereas exogenous G245S(ΔBD) or R248W(ΔBD) was expressed (Fig. 8, A and C, top panel). We would like to note that due to an abnormal migration pattern, G245S(ΔBD) and R248W(ΔBD), migrated at the same position as endogenous full-length mutant p53 in SDS-PAGE gels. This phenomenon was also observed previously (28). To address this, Pab421 antibody, which recognizes an epitope within residues 371–380 and thus would not recognize BD-deleted p53, was used for Western blot analysis. We showed that upon induction, endogenous full-length mutant p53 was inducibly knocked down, whereas exogenous G245S(ΔBD) and R248W(ΔBD) were not detected (Fig. 8, A and C, middle panel). Next, colony formation assay was performed and showed that upon induction of G245S(ΔBD) or R248W(ΔBD), the colony-forming ability of SW480 cells was markedly enhanced at both the mock-treated and the camptothecin-treated conditions (Fig. 8, B and D). We would like to note that colony quantification and statistical analyses were not performed for R248W(ΔBD)-producing SW480 cells in the absence of camptothecin treatment due to the large number of colonies (Fig. 8D, right panel). To further confirm this, we generated SW480 cell lines in which endogenous mutant p53 was stably knocked down, whereas siRNA-resistant G245S(ΔBD) or R248W(ΔBD) can be inducibly expressed (Fig. 8, E and G). Similarly, we showed that G245S(ΔBD) and R248W(ΔBD) promoted the colony-forming activity of SW480 cells at both the mock-treated and the camptothecin-treated conditions (Fig. 8, F and H).
FIGURE 8.
Mutant G245S or R248W without the C-terminal basic domain (Δ364–393) enhances the colony-forming ability of SW480 cells upon knockdown of endogenous mutant p53. A and C, generation of SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas siRNA-resistant mutant G245S(ΔBD) (A) or R248W(ΔBD) (C) can be inducibly expressed. The experiment was performed as in Fig. 4A. B and D, left, SW480 cells were uninduced or induced to knock down endogenous mutant p53 and simultaneously express mutant G245S(ΔBD) (B) or R248W(ΔBD) (D) along with mock treatment or treatment with CPT and then cultured for 14 days. Right, quantification of the number of colonies with a diameter of >1 mm. *, p < 0.05. Error bars indicate S.E. E and G, generation of SW480 cell lines in which endogenous mutant p53 was stably knocked down, whereas siRNA-resistant mutant G245S(ΔBD) (E) or R248W(ΔBD) (G) can be inducibly expressed. The experiment was performed as in Fig. 3A. F and H, left, SW480 cells, in which endogenous mutant p53 was stably knocked down, were uninduced or induced to express mutant G245S(ΔBD) (F) or R248W(ΔBD) (H) along with mock treatment or treatment with CPT and then cultured for 14 days. Right, quantification of the number of colonies with a diameter of >1 mm. *, p < 0.05. Error bars indicate S.E.
To determine the underlying mechanism by which mutations in AD1, AD2, or PRD inhibit, whereas deletion of BD enhances, the gain of function for mutant p53, we examined the expression pattern of Id2 and Gro1 in SW480 cells. Previously, we showed that upon knockdown of endogenous mutant p53 in SW480 and MIA PaCa-2 cells, Id2 expression is induced, whereas Gro1 expression is inhibited (11, 12). In addition, we showed that knockdown of Id2 and ectopic expression of Gro1 are able to rescue the proliferative defect in SW480 and MIA PaCa-2 cells induced by knockdown of mutant p53 (11, 12). Thus, Id2 and Gro1 expression was examined by RT-PCR in SW480 cell lines in which endogenous mutant p53 can be inducibly knocked down, whereas an siRNA-resistant mutant p53 can be inducibly expressed. We showed that upon knockdown of endogenous mutant p53 but not expression of siRNA against LacZ, Gro1 expression was inhibited, whereas Id2 expression was increased (Fig. 9A), consistent with our previous reports (11, 12). We also showed that inducible replacement with G245S or R248W in SW480 cells upon inducible knockdown of endogenous mutant p53 restored the expression pattern of Gro1 to a level similar to that at the control condition (Fig. 9B, Gro1 panel, compare lanes 1 and 3 with lanes 2 and 4, respectively). Furthermore, the expression of Id2 was repressed by G245S and R248W to a level slightly lower than that at the control condition (Fig. 9B, Id2 panel, compare lanes 1 and 3 with lanes 2 and 4, respectively). However, the altered expression pattern of Gro1 and Id2 in SW480 cells due to knockdown of endogenous mutant p53 was not fully restored to the basal level by mutant p53 with a double-point mutation in AD1 (Fig. 9C) and in AD2 (Fig. 9D) or with a deletion of PRD (Fig. 9E). In contrast, we showed that deletion of the BD made mutant G245S and R248W efficiently restore the normal expression pattern of Gro1 and Id2 in SW480 cells altered by knockdown of endogenous mutant p53 (Fig. 9F). In fact, the level of Gro1 was slightly increased upon inducible expression of R248W(ΔBD) in p53 knockdown SW480 cells (Fig. 9F), which may be responsible for the enhanced colony-forming ability of SW480 cells with knockdown of endogenous mutant p53 (Fig. 8).
FIGURE 9.
AD1, AD2, and PRD are necessary for mutant p53 to regulate Gro1 and Id2, whereas BD is an inhibitory domain. A, semiquantitative RT-PCR was performed to measure the level of Gro1 and Id2 in SW480 cells uninduced or induced to express siRNA against LacZ or knock down endogenous mutant p53 for 3 days. The level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was measured as loading control. B–F, the experiment was performed as in A except that SW480 cells were uninduced or induced to knock down endogenous mutant p53 and simultaneously express siRNA-resistant mutant G245S or R248W (B), G245S(Gln-22/Ser-23) or R248W(Gln-22/Ser-23) (C), G245S(Gln-53/Ser-54) or R248W(Gln-53/Ser-54) (D), G245S(ΔPRD) or R248W(ΔPRD) (E), or G245S(ΔBD) or R248W(ΔBD) (F).
DISCUSSION
Molecular oncogenic properties for mutant p53 have been extensively investigated, mostly via ectopic expression in p53-null cells to examine gain of function. Although many properties for mutant p53 have been identified, such as mutant p53 having a transcriptional activity distinct from wild-type p53, these approaches have limitations (2). The most challenging one is that most tumor cells carrying no p53, such as the H1299 cell line, may have already been transformed and do not require mutant p53 for maintaining their transformed phenotypes (11). Thus, the ideal approach to investigate mutant p53 is to use tumor cells in which endogenous mutant p53 is required for keeping their transformed phenotypes. Previously, we and others showed that endogenous mutant R273H/P309S is required for SW480 cell proliferation and survival (11, 12, 25). With such a system, we demonstrated that both conformation (G245S and R249S) and contact-site (R248W and R273H) mutants share a common property in rescuing the colony-forming defect in SW480 cells induced by knockdown of endogenous mutant p53. In addition, we found that activation domains 1–2 and the proline-rich domain are necessary for mutant p53 transcriptional activity, whereas the C-terminal basic domain is an inhibitory domain. Therefore, the inhibitory basic domain and the common property for both classes of mutants can be explored for cancer therapeutic development.
Wild-type p53 deficient in activation domain 1 (Gln-22/Ser-23) is defective in inducing cell cycle arrest but retains limited activity in inducing apoptosis (28–30). This is likely due to compromised transcriptional activity for p53(Gln-22/Ser-23), which has a limited activity in regulating a subset of p53 target genes (18, 30, 31). As a result, thymus-specific knock-in of mouse p53(Gln-25/Ser-26) makes mice prone to thymus lymphomas, suggesting that transcriptional activity conferred by activation domain 1 is required for p53-dependent tumor suppression (32). For mutant p53, several studies showed that Gln-22/Ser-23 mutation inactivates mutant p53 to regulate the promoters of putative mutant p53 target genes, such as MDR1 and c-Myc (8, 16, 22). In addition, knock-in mice with mutant p53(A135V) deficient in activation domain 1 are prone to tumors in a manner similar to p53-null mice (33) instead of being similar to that of mutant R172H and R270H knock-in mice, which are prone to metastatic tumors (6, 7). Consistent with the above studies, here we showed that inducible expression of G245S(Gln-22/Ser-23) and R248W(Gln-22/Ser-23) is not able to rescue the colony-forming defect and proper expression of Gro1 and Id2 in SW480 cells induced by knockdown of endogenous mutant p53 (Figs. 5 and 9).
Wild-type p53 deficient in activation domain 2 (Gln-53/Ser-54) is defective in inducing apoptosis but retains an activity in inducing cell cycle arrest (18, 20). This is probably due to compromised activity for p53(Gln-53/Ser-54) to regulate proapoptotic p53 target genes, such as Bax and PIG3 (18, 20, 34). However, the role of activation domain 2 in mutant p53 has not been examined. Here, we showed that inducible expression of G245S(Gln-53/Ser-54) and R248W(Gln-53/Ser-54) is not able to rescue the colony-forming defect and proper expression of Gro1 and Id2 in SW480 cells induced by knockdown of endogenous mutant p53 (Figs. 6 and 9). Thus, like activation domain 1, activation domain 2 is also necessary for mutant p53 gain of function.
PRD is required for wild-type p53 to suppress colony formation (21), and lack of PRD makes wild-type p53 deficient in inducing proapoptotic target genes, and to a lesser extent, growth-arrest genes (35). p53(ΔPRD) knock-in mice are prone to B-cell lymphoma instead of T cell lymphoma in p53-null mouse (36). In addition, mice with a mutation in PRD are able to rescue lethality by loss of Mdm4 but not Mdm2 (37). This suggests that PRD is required for proper function of wild-type p53. Here, we showed that inducible expression of G245S(ΔPRD) and R248W(ΔPRD) is not able to rescue the colony-forming defect and proper expression of Gro1 and Id2 in SW480 cells induced by knockdown of endogenous mutant p53 (Figs. 7 and 9). Thus, like wild-type p53, mutant p53 is also dependent on PRD for its gain of function.
The BD and its modifications fine-tune p53-dependent transcriptional activity and tumor suppression in vitro and in vivo (14, 15, 38). The BD is also found to be an inhibitory domain for p53 to activate IGFBP3, an effector of apoptosis (27). For mutant p53, the BD is necessary for mutant D281G to regulate the c-Myc promoter (8), and Ser-392 nonphosphorylatable p53 mutants, His-175/Ala-392 and Trp-248/Ala-392, are more potent in transforming rat embryo fibroblasts in cooperation with the ras oncogene than His-175/Ser-392 and Trp-248/Ser-392 (39). Here, we found that the colony-forming ability for SW480 cells is markedly enhanced at both the mock-treated and the camptothecin-treated conditions by G245S(ΔBD) and R248W(ΔBD) (Fig. 8). Furthermore, we showed that in SW480 cells upon knockdown of endogenous mutant p53, G245S(ΔBD) is capable of restoring normal expression pattern of Gro1 and Id2, whereas R248W(ΔBD) efficiently represses Id2 expression and further enhances Gro1 expression (Fig. 9). Because ectopic expression of Gro1 is able to rescue the proliferative defect in SW480 cells induced by knockdown of endogenous mutant p53 (12), the enhanced expression of Gro1 by R248W(ΔBD) is at least in part responsible for the enhanced colony-forming activity for the mutant. Nevertheless, it is also possible that other novel pro-growth target genes may be highly induced or that other antigrowth target genes may be highly repressed by mutant p53 without the inhibitory basic domain. Thus, future studies are warranted to identify such mutant p53 target genes and the mechanism by which the BD regulates mutant p53 transcriptional activity.
Supplementary Material
This work was supported in part by National Institutes of Health Grant CA121137 (to X. C.).
This article was selected as a Paper of the Week.
- AD
- activation domain 1
- BD
- basic domain
- PRD
- proline-rich domain
- KD
- knockdown
- HA
- hemagglutinin
- siRNA
- small interfering RNA
- RT-PCR
- reverse transcription-PCR
- CPT
- camptothecin.
REFERENCES
- 1.Levine A. J., Oren M. (2009) Nat. Rev. Cancer 9, 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brosh R., Rotter V. (2009) Nat. Rev. Cancer 9, 701–713 [DOI] [PubMed] [Google Scholar]
- 3.Ko L. J., Prives C. (1996) Genes Dev. 10, 1054–1072 [DOI] [PubMed] [Google Scholar]
- 4.Milner J., Medcalf E. A. (1991) Cell 65, 765–774 [DOI] [PubMed] [Google Scholar]
- 5.Willis A., Jung E. J., Wakefield T., Chen X. (2004) Oncogene 23, 2330–2338 [DOI] [PubMed] [Google Scholar]
- 6.Lang G. A., Iwakuma T., Suh Y. A., Liu G., Rao V. A., Parant J. M., Valentin-Vega Y. A., Terzian T., Caldwell L. C., Strong L. C., El-Naggar A. K., Lozano G. (2004) Cell 119, 861–872 [DOI] [PubMed] [Google Scholar]
- 7.Olive K. P., Tuveson D. A., Ruhe Z. C., Yin B., Willis N. A., Bronson R. T., Crowley D., Jacks T. (2004) Cell 119, 847–860 [DOI] [PubMed] [Google Scholar]
- 8.Frazier M. W., He X., Wang J., Gu Z., Cleveland J. L., Zambetti G. P. (1998) Mol. Cell Biol. 18, 3735–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scian M. J., Stagliano K. E., Anderson M. A., Hassan S., Bowman M., Miles M. F., Deb S. P., Deb S. (2005) Mol. Cell Biol. 25, 10097–10110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalcenstein A., Stambolsky P., Weisz L., Müller M., Wallach D., Goncharov T. M., Krammer P. H., Rotter V., Oren M. (2003) Oncogene 22, 5667–5676 [DOI] [PubMed] [Google Scholar]
- 11.Yan W., Liu G., Scoumanne A., Chen X. (2008) Cancer Res. 68, 6789–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan W., Chen X. (2009) J. Biol. Chem. 284, 12178–12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harms K. L., Chen X. (2006) Cell Death Differ. 13, 890–897 [DOI] [PubMed] [Google Scholar]
- 14.Brooks C. L., Gu W. (2006) Mol. Cell 21, 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vousden K. H., Prives C. (2009) Cell 137, 413–431 [DOI] [PubMed] [Google Scholar]
- 16.Lin J., Teresky A. K., Levine A. J. (1995) Oncogene 10, 2387–2390 [PubMed] [Google Scholar]
- 17.Candau R., Scolnick D. M., Darpino P., Ying C. Y., Halazonetis T. D., Berger S. L. (1997) Oncogene 15, 807–816 [DOI] [PubMed] [Google Scholar]
- 18.Zhu J., Zhou W., Jiang J., Chen X. (1998) J. Biol. Chem. 273, 13030–13036 [DOI] [PubMed] [Google Scholar]
- 19.Venot C., Maratrat M., Dureuil C., Conseiller E., Bracco L., Debussche L. (1998) EMBO J. 17, 4668–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J., Zhang S., Jiang J., Chen X. (2000) J. Biol. Chem. 275, 39927–39934 [DOI] [PubMed] [Google Scholar]
- 21.Walker K. K., Levine A. J. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 15335–15340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matas D., Sigal A., Stambolsky P., Milyavsky M., Weisz L., Schwartz D., Goldfinger N., Rotter V. (2001) EMBO J. 20, 4163–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan W., Chen X. (2007) Cancer Res. 67, 9117–9124 [DOI] [PubMed] [Google Scholar]
- 24.Liu G., Chen X. (2006) Mol. Cell Biol. 26, 1398–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin M. S., Kondo K., Marin M. C., Cheng L. S., Hahn W. C., Kaelin W. G., Jr. (2003) Cancer Cell 3, 403–410 [DOI] [PubMed] [Google Scholar]
- 26.Bossi G., Lapi E., Strano S., Rinaldo C., Blandino G., Sacchi A. (2006) Oncogene 25, 304–309 [DOI] [PubMed] [Google Scholar]
- 27.Harms K. L., Chen X. (2005) Mol. Cell Biol. 25, 2014–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Ko L. J., Jayaraman L., Prives C. (1996) Genes Dev. 10, 2438–2451 [DOI] [PubMed] [Google Scholar]
- 29.Haupt Y., Rowan S., Shaulian E., Vousden K. H., Oren M. (1995) Genes Dev. 9, 2170–2183 [DOI] [PubMed] [Google Scholar]
- 30.Johnson T. M., Attardi L. D. (2006) Cell Death Differ. 13, 902–908 [DOI] [PubMed] [Google Scholar]
- 31.Baptiste-Okoh N., Barsotti A. M., Prives C. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1937–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaidarenko O., Xu Y. (2009) Oncogene 28, 4397–4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nistér M., Tang M., Zhang X. Q., Yin C., Beeche M., Hu X., Enblad G., van Dyke T., Wahl G. M. (2005) Oncogene 24, 3563–3573 [DOI] [PubMed] [Google Scholar]
- 34.Hammond E. M., Mandell D. J., Salim A., Krieg A. J., Johnson T. M., Shirazi H. A., Attardi L. D., Giaccia A. J. (2006) Mol. Cell Biol. 26, 3492–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J., Jiang J., Zhou W., Zhu K., Chen X. (1999) Oncogene 18, 2149–2155 [DOI] [PubMed] [Google Scholar]
- 36.Slatter T. L., Ganesan P., Holzhauer C., Mehta R., Rubio C., Williams G., Wilson M., Royds J. A., Baird M. A., Braithwaite A. W. (2010) Cell Death Differ. 17, 540–550 [DOI] [PubMed] [Google Scholar]
- 37.Toledo F., Krummel K. A., Lee C. J., Liu C. W., Rodewald L. W., Tang M., Wahl G. M. (2006) Cancer Cell 9, 273–285 [DOI] [PubMed] [Google Scholar]
- 38.Donehower L. A., Lozano G. (2009) Nat. Rev. Cancer 9, 831–841 [DOI] [PubMed] [Google Scholar]
- 39.Yap D. B., Hsieh J. K., Zhong S., Heath V., Gusterson B., Crook T., Lu X. (2004) Cancer Res. 64, 4749–4754 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.