Abstract
Background
Multidimensional impairment of older patients may influence the clinical outcome of diseases. The aim of this study was to evaluate whether a Multidimensional Prognostic Index (MPI) based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure.
Methods and Results
In this prospective study with a 1-month follow-up, 376 patients aged 65 and older with a diagnosis of heart failure were enrolled. A standardized comprehensive geriatric assessment that included information on functional (activities of daily living and instrumental activities of daily living), cognitive (Short Portable Mental Status Questionnaire), and nutritional status (Mini Nutritional Assessment), as well as on risk of pressure sore (Exton-Smith Scale), comorbidities (Cumulative Illness Rating Scale Index), medications, and social support network, was used to calculate the MPI for mortality using a previously validated algorithm. The New York Heart Association, the Enhanced Feedback for Effective Cardiac Treatment, and the Acute Decompensated Heart Failure National Registry regression model scores were also calculated. Higher MPI values were significantly associated with higher 30-day mortality, both in men (MPI-1, 2.8%; MPI-2, 15.3%; MPI-3, 47.4%; P=0.000) and women (MPI-1, 0%; MPI-2, 6.5%; MPI-3, 14.6%; P=0.011). The discrimination of the MPI was also good, with areas under the receiver operating characteristic curves (men: 0.83; 95% CI, 0.75 to 0.90; women: 0.80; 95% CI, 0.71 to 0.89) greater than receiver operating characteristic areas of New York Heart Association (men: 0.63; 95% CI, 0.57 to 0.69; P=0.015; women: 0.65; 95% CI, 0.55 to 0.75; P=0.064), Enhanced Feedback for Effective Cardiac Treatment (men: 0.69; 95% CI, 0.58 to 0.79; P=0.045; women: 0.71; 95% CI, 0.55 to 0.87; P=0.443), and Acute Decompensated Heart Failure National Registry scores (men: 0.65; 95% CI, 0.52 to 0.78; P=0.023; women: 0.67; 95% CI, 0.49 to 0.83, P=0.171).
Conclusions
The MPI, calculated from information collected in a standardized comprehensive geriatric assessment, is useful to estimate the risk of 1-month mortality in older patients with heart failure.
Keywords: heart failure, mortality, risk factors, Multidimensional Prognostic Index (MPI), prognosis
The prevalence of heart failure (HF) increases with age, reaching figure as high as 10% to 12% in patients older than 80 years.1,2 HF is the leading medical cause of hospitalization among people aged 65 years or older in the United States and European countries,3,4 and imposes a substantial burden on individuals and society in terms of mortality, morbidity, and associated healthcare cost. Studies have shown that patients discharged from the hospital with a diagnosis of HF have high risk of mortality (11.3% at 30 days and 33.1% at 1 year)5,6 and rehospitalization (≈40% in the 6-month follow-up period after their index hospitalization).7
Assessing the effectiveness of intervention in older patients with HF is particularly challenging because the clinical evolution and mortality of these patients result from a combination of biological, functional, psychological, and environmental factors8 and is conditioned only in part by the cardiovascular signs and symptoms that are traditionally included in prognostic indexes.9 Predictive models of short-term10,11 and long-term12,13 mortality in patients with HF have been recently described. The Enhanced Feedback for Effective Cardiac Treatment (EFFECT)10 score is a clinical prognostic model based on physiological parameters, co-morbidities, and laboratory tests, which was derived and validated in 2 cohorts of patients with a mean age of >75 years. The Acute Decompensated Heart Failure National Registry (ADHERE) regression model11 combines information on blood urea nitrogen (BUN) levels, systolic blood pressure (SBP), heart rate, and age to predict in-hospital mortality in patients with HF. The ADHERE regression model was validated in 2 large cohorts of acutely decompensated patients with HF and mean age of 72.5 years.
More recently, another 4-item risk score based on laboratory tests, SBP, and peripheral arterial disease could identify older patients with HF at high risk for mortality at 6 months.12 Finally, in the Seattle Heart Failure Model,13 a 1-, 2-, and 3-year survival could be predicted using clinical, pharmacological, device, and laboratory characteristics in patients with HF.
Although these prognostic scores use information that is directly connected with the pathophysiology of HF, it is increasingly evident that the prognosis of older patients with chronic medical conditions is strongly affected by comorbidity, functional status, body composition, treatment, and other factors that are not directly related to the index disease.8 Unfortunately, a prognostic model that consider this comprehensive approach for the mortality risk assessment of older patients discharged from the hospital with a diagnosis of HF has never been developed.
Recently, we developed and validated in 2 different cohorts of elderly patients, a Multidimensional Prognostic Index (MPI) for 1-year mortality derived from a standardized comprehensive geriatric assessment (CGA) performed in older patients hospitalized for acute diseases or relapse of a chronic disease.14
The aim of this study was to evaluate the prognostic accuracy of MPI on posthospital 30-day mortality in older patients hospitalized for HF.
Methods
Subjects
All patients aged 65 years and older admitted with a diagnosis of HF from January 2005 to December 2007 to the Geriatrics Unit of the Casa Sollievo della Sofferenza Hospital, IRCCS, San Giovanni Rotondo, Italy, were screened for inclusion in the study.
Inclusion criteria were (1) age≥65 years; (2) diagnosis of HF; (3) ability to provide an informed consent or availability of a proxy for informed consent and willingness to participate in the study; and (4) complete CGA during hospitalization.
A structured interview, a clinical evaluation, and an extensive review of records from the patients’ general practitioners were performed at baseline. At baseline, serum samples were taken for the analysis of creatinine, BUN, hemoglobin, and serum sodium. An estimated glomerular filtration rate was calculated with the use of modified Modification of Diet in Renal Disease15 4-component equation incorporating age, gender, race, and serum creatinine level; GFR is expressed as milliliter per minute per 1.73 cm2, and estimated glomerular filtration rate <60 mL/min/1.73 cm2 was used to defined the presence of decreased renal function. Anemia was defined as hemoglobin level <12 g/dL and hyponatremia as sodium level <136 meq/mL.
In addition, all patients admitted to our unit received a standard CGA for clinical purposes. Vital status was assessed by directly contacting the participants or consulting the Registry Offices of the cities where the patients were residents at the time of hospital admission. Dates of death were identified from death certificates.
Diagnosis of HF
HF was diagnosed according to the diagnostic criteria of the European Society of Cardiology16 and the American Heart Association,17 ie, rapid onset of symptoms and signs secondary to abnormal cardiac function, with or without previous cardiac disease. Cardiac dysfunction could be related to systolic or diastolic dysfunction, to abnormalities in cardiac rhythm, or to preload and after load mismatch. Acute HF was defined as a new onset of decompensated HF or decompensation of chronic, established HF, with symptoms sufficient to warrant hospitalization. Clinical information pertinent to potential causes of HF was collected during hospitalization in all patients. Functional class of HF was rated according to the New York Heart Association (NYHA) classification, and ejection fraction (EF) was determinate by echocardiography. As recommended, an EF ≥50% was used to define HF with preserved EF.18
Two previously validated specific risk scores for short-term mortality in patients with HF, the EFFECT10 and ADHERE,11 were also computed. The EFFECT model10 is based on 11 variables including age, sex, physiological parameters (blood pressure and respiratory rate), the presence of 5 comorbid conditions (cerebrovascular disease, chronic obstructive pulmonary disease, dementia, hepatic cirrhosis, and cancer), and laboratory tests (BUN levels and serum sodium concentration). The linear regression ADHERE model11 is based on 4 variables: SBP, heart rate, BUN levels, and age in the following formula: log odds of mortality=0.0212×BUN–0.0192×SBP+0.0131×heart rate+0.0288× age–4.72. Both the EFFECT and ADHERE risk scores were considered as continuous variables.
The CGA
CGA is a widely used instrument in geriatric practice. Functional status was evaluated by activities of daily living (ADL) index,19 which defines the level of dependence or independence in a 6 daily personal care activities including bathing, toileting, feeding, dressing, urine and bowel continence, and transferring (in and out of bed or chair), and by instrumental ADL scale,20 which assesses independence in 8 activities that are more cognitively and physically demanding than ADL, including managing finances, taking medications, using telephone, shopping, using transportation, preparing meals, doing housework, and washing.
Cognitive status was assessed by the Short Portable Mental Status Questionnaire, a 10-item questionnaire that assesses orientation, memory, attention, calculation, and language.21
Comorbidity was examined using the Cumulative Illness Rating Scale.22 The Cumulative Illness Rating Scale uses a 5-point ordinal scale (scores 1 to 5) to estimate the severity of pathology in each of 13 systems, including cardiac, vascular, respiratory, eye-ear-nose-throat, upper and lower gastrointestinal, hepatic, renal, genitourinary, musculoskeletal, skin disorder, nervous system, endocrine-metabolic, and psychiatric behavioral disorders. Based on the ratings, the Comorbidity Index (Cumulative Illness Rating Scale-CI) score, which reflects the number of concomitant diseases, were derived from the total number of categories in which moderate or severe levels (grade 3 to 5) of disease were identified (range, 0 to 13).
Nutritional status was explored with the Mini Nutritional Assessment,23 which includes information on (1) anthropometric measures (body mass index in body weight/height2, midarm circumference in centimeter, calf circumference in centimeter, and weight loss); (2) lifestyle, medication, and mobility; (3) number of meals, food and fluid intake, and autonomy of feeding; and (4) self-perception of health and nutrition.
The Exton-Smith Scale was used to evaluate the risk of developing pressure sores. This 5-item questionnaire determines physical and mental conditions, activity, mobility, and incontinence. For each item, a score from 1 to 4 is assigned.24
Medication use was defined according to the Anatomic Therapeutics Chemical Classification code system, and the number of drugs used by patients at admission was recorded. Patients were defined as drug users if they took a medication included in the Anatomic Therapeutics Chemical Classification code system at the moment of admission.
Social aspects included household composition, home service, and institutionalization. The approximate time required for collecting data using CGA was 20 minutes (range, 15 to 25 minutes per person).
The MPI
We used the MPI, an algorithm developed and previously validated in 2 independent cohorts of elderly hospitalized patients and elsewhere reported.14 This MPI was developed by the inclusion of information from the above reported 8 domains of the CGA, ie, ADL, instrumental ADL, Short Portable Mental Status Questionnaire, Cumulative Illness Rating Scale-CI, Mini Nutritional Assessment, Exton-Smith Scale, medication use, and social aspects. For each domain, a tripartite hierarchy was used, ie, 0=no problems, 0.5=minor problems, and 1=major problems, based on conventional cutoff points derived from the literature for the Short Portable Mental Status Questionnaire,21 Mini Nutritional Assessment,23 Exton-Smith Scale,24 and ADL or instrumental ADL25 or observing the frequency of distribution of patients in the previous validation study14 for comorbities and number of medication (Table 1). The sum of the calculated scores from the 8 domains was divided by 8 to obtain a final MPI risk score between 0=no risk and 1=higher risk of mortality. Also, the MPI was expressed as 3 grades of risk: MPI-1=low risk (MPI value ≤0.33), MPI-2=moderate risk (MPI value between 0.34 and 0.66), and MPI-3=severe risk (MPI value >0.66).14
Table 1.
MPI Score Assigned to Each Domain Based on the Severity of the Problems
| Problems |
|||
|---|---|---|---|
| No | Minor | Severe | |
| Assessment | (Value=0) | (Value=0.5) | (Value=1) |
| ADL* | 6–5 | 4–3 | 2–0 |
| Instrumental ADL* | 8–6 | 5–4 | 3–0 |
| Short portable mental status questionnaire† | 0–3 | 4–7 | 8–10 |
| Comorbidity index (cumulative illness rating scale-CI)‡ | 0 | 1–2 | ≥3 |
| Mini nutritional assessment§ | ≥24 | 17–23.5 | <17 |
| Exton-smith scale¶ | 16–20 | 10–15 | 5–9 |
| No. of medications | 0–3 | 4–6 | ≥7 |
| Social support network | Living with family | Institutionalized | Living alone |
No. of active functional activities.
No. of errors.
No. of diseases.
Mini Nutritional Assessment score: ≥24, satisfactory nutritional status; 17–23.5, at risk of malnutrition; <17, malnutrition.
Exton-Smith Scale score: 16–20, minimum risk; 10–15, moderate risk; 5–9 high risk of developing scores.
Statistical Analysis
All analyses were performed using SPSS version 13 for Windows (SPSS Inc, Chicago, Ill). Continuous variables were presented as means and standard deviations and categorical variables as frequencies and percentages. Comparisons between men and women were made using the χ2 test for categorical variables (ie, gender, anemia, diabetes mellitus, hypertension, atrial fibrillation, coronary heart disease, medication, and mortality) and the t test for continuous variables (ie, age, educational level, SBP, and EF).
The discrimination of the model for 1-month mortality was assessed by calculating area under the receiver operating characteristic (ROC) curves for the MPI, EFFECT, and ADHERE risk scores considered as continuous variables. Note that for NYHA, the score was based on the standard 4 classes and was considered as an approximately continuous risk score.
To compare the areas under the ROC curves of the MPI with the NYHA, EFFECT, and ADHERE models, we used the DeLong’s approach that accounts for correlation.26 For sake of completeness, we also run a bootstrapped version of the same approach that yielded overlapping results (data not shown). The Spearman pairwise correlations were made to test the correlations between MPI and each of the other risk scores. A P value <0.05 was considered for statistical significance.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Overall Study Population
During the enrolment period, 416 patients were consecutively admitted to the geriatric unit with a diagnosis of HF. Five patients were excluded because they were younger than 65 years, 10 patients were excluded because the CGA was not completed, and 25 patients were excluded because they did not consent to participate in the study. Thus, the final study population included 376 patients, 163 men (43.4% of the total population) and 213 women (56.6%). The mean age was 80.5±7.3 years with a range from 65 to 100 years.
As shown in Table 2, women had significantly more ADL (P=0.000) and instrumental ADL disabilities (P=0.020), lower Mini Nutritional Assessment scores (P=0.000), lower educational level (P=0.000), lower Exton-Smith scores (P=0.001), and higher Short Portable Mental Status Questionnaire score (P=0.001) than men. The prevalence of coronary artery disease was significantly higher in men than women (P=0.000), whereas no differences between men and women were observed in the prevalence of nonischemic cardiomyopathy, atrial fibrillation, hypertension or diabetes mellitus, mean EF values, percentage of HF with preserved EF, as well as in hyponatremia, anemia, mean SBP, the percentage of decreased renal function, and in the use of β-blockers, angiotensin converting enzyme inhibitors, diuretics, and low-dose aspirin.
Table 2.
Baseline Characteristics of Patients Divided According to Gender
| All (n=376) | Men (n=163) | Women (n=213) | P Value | |
|---|---|---|---|---|
| Patients, % | 100 | 43.4 | 56.6 | … |
| Age, y | 80.5±7.3 | 80.1±7.8 | 80.8±6.9 | 0.335 |
| MPI value | 0.44±0.2 | 0.39±0.16 | 0.48±0.19 | 0.000 |
| Educational level, y | 4.1±3.1 | 5.0±3.7 | 3.4±2.5 | 0.000 |
| ADL score | 4.0±2.1 | 4.5±1.9 | 3.4±2.5 | 0.000 |
| IADL score | 3.4±2.7 | 3.8±2.5 | 3.1±2.8 | 0.020 |
| SPMSQ score | 2.1±2.1 | 1.67±2.0 | 2.44±2.1 | 0.001 |
| Exton-Smith score | 15.6±3.0 | 16.2±2.8 | 15.1±3.1 | 0.001 |
| CIRS-CI score | 3.6±1.7 | 3.5±1.8 | 3.6±1.6 | 0.433 |
| MNA score | 21.5±4.5 | 22.6±4.1 | 20.7±4.6 | 0.000 |
| No. of drugs | 5.7±2.6 | 5.8±2.7 | 5.6±2.6 | 0.314 |
| Coronary heart disease | 60 (15.9) | 39 (23.9) | 21 (9.8) | 0.000 |
| Nonischemic cardiomyopathy | 183 (48.6) | 79 (48.4) | 104 (48.8) | 0.928 |
| Atrial fibrillation | 226 (60.1) | 105 (64.4) | 121 (56.8) | 0.205 |
| Hypertension | 214 (56.9) | 84 (51.3) | 130 (61.0) | 0.066 |
| Diabetes | 106 (28.1) | 47 (28.8) | 59 (27.6) | 0.992 |
| EF | 47.9±13 | 45.6±12.6 | 49.7±13.2 | 0.083 |
| Preserved EF | 115 (30.5) | 48 (29.4) | 67 (31.4) | 0.760 |
| Hyponatremia* | 43 (11.4) | 18 (11.0) | 25 (11.7) | 0.890 |
| Anemia† | 174 (46.3) | 66 (40.5) | 108 (50.7) | 0.062 |
| Decrease renal function‡ | 221 (58.7) | 87 (53.4) | 134 (62.9) | 0.083 |
| Systolic blood pressure | 134.8±21.3 | 133.6±19.7 | 135.7±22.4 | 0.334 |
| Angiotensin-converting enzyme inhibitors | 162 (43.2) | 78 (47.8) | 85 (39.9) | 0.153 |
| β-blockers | 46 (12.2) | 23 (14.1) | 23 (10.8) | 0.418 |
| Diuretics | 263 (69.9) | 110 (67.5) | 153 (71.8) | 0.430 |
| Low-dose aspirin | 126 (33.5) | 58 (35.5) | 68 (31.9) | 0.520 |
| Mortality at 30 d | 36 (9.6) | 22 (13.5) | 14 (6.6) | 0.038 |
Data are presented as mean±SD or n (%).
Sodium level <136 meq/mL.
Hemoglobin level <12 g/dL.
eGFR <60 mL/min/1.73 m2.
After 30 days of follow-up, the overall mortality rate was 9.6% with significant differences between men and women (13.5% versus 6.6%, P=0.038).
MPI
Tables 3 and 4 report the characteristics of patients divided according to their MPI grade; 130 patients (34.6%) were included in MPI-1 group, 179 patients (47.6%) in MPI-2 group, and 67 patients (17.8%) in MPI-3 group. Both men and women patients with higher MPI values were more likely to be older (P=0.000) and less likely to have preserved EF (P=0.001). Moreover, women with increasing MPI values had a significant higher prevalence of decreased renal function (P=0.009) No differences between the 3 MPI groups were observed in the prevalence of cardiac pathologies, anemia, hyponatremia, hypertension, or diabetes mellitus, the EF mean values, and percentages of drug use.
Table 3.
Baseline Characteristics of Men Divided According to MPI Grade
| Characteristics | MPI |
|||
|---|---|---|---|---|
| Low Risk | Moderate Risk | Severe Risk | P Value | |
| Patients | 72 (44.2) | 72 (44.2) | 19 (11.7) | |
| MPI value | 0.22±0.07 | 0.46±0.08 | 0.75±0.08 | |
| Age, y | ||||
| Range | 65–98 | 65–97 | 70–98 | |
| Mean±SD | 78.2±7.8 | 80.2±7.4 | 84.6±7.3 | 0.003 |
| Educational level years | 4.93±3.8 | 4.71±2.9 | 7.33±5.8 | 0.145 |
| Mortality at 30 d | 2 (2.8) | 11 (15.3) | 9 (47.4) | 0.000 |
| Coronary heart disease | 17 (23.6) | 17 (23.6) | 5 (26.3) | 0.954 |
| Nonischemic cardiomyopathy | 37 (51.3) | 36 (50) | 6 (31.5) | 0.237 |
| Atrial fibrillation | 45 (62.5) | 49 (68.5) | 11 (57.8) | 0.545 |
| Hypertension | 35 (48.6) | 39 (54.1) | 10 (52.6) | 0.621 |
| Diabetes | 17 (23.6) | 23 (31.9) | 7 (36.8) | 0.384 |
| EF | 46.3±12.3 | 44.2±12.1 | 50.0±19.7 | 0.690 |
| Preserved EF | 30 (41.7) | 15 (20.39) | 3 (15.7) | 0.009 |
| Anemia† | 27 (37.5) | 30 (41.6) | 9 (47.4) | 0.676 |
| Hyponatremia* | 5 (7.0) | 12 (16.6) | 1 (5.3) | 0.104 |
| Decrease renal function‡ | 36 (50) | 43 (59.7) | 8 (42.1) | 0.292 |
| Angiotensin-converting enzyme inhibitors | 36 (50) | 33 (45.8) | 9 (47.3) | 0.596 |
| β-blockers | 12 (16.6) | 9 (12.5) | 2 (10.5) | 0.765 |
| Diuretics | 49 (68) | 51 (70.8) | 10 (52.6) | 0.624 |
| Low-dose aspirin | 27 (37.5) | 23 (31.9) | 8 (41.2) | 0.220 |
Data are presented as n (%) or mean±SD.
Sodium level <136 meq/mL.
Hemoglobin level <12 g/dL.
eGFR <60 mL/min/1.73 m2.
Table 4.
Baseline Characteristics of Women Divided According to MPI Grade
| Characteristics | MPI |
|||
|---|---|---|---|---|
| Low Risk | Moderate Risk | Severe Risk | P Value | |
| Patients | 58 (27.2) | 107 (50.2) | 48 (21.5) | |
| MPI value | 0.23±0.07 | 0.49±0.09 | 0.75±0.06 | |
| Age, y | ||||
| Range | 65–91 | 65–97 | 66–100 | |
| Mean±SD | 77.5±6.6 | 81.9±6.6 | 82.5±7.0 | 0.000 |
| Educational level years | 4.03±2.3 | 3.3±2.6 | 3.04±2.5 | 0.288 |
| Mortality at 30 d | 0 | 7 (6.5) | 7 (14.6) | 0.000 |
| Coronary heart disease | 5 (8.6) | 11 (10.2) | 5 (10.4) | 0.985 |
| Nonischemic cardiomyopathy | 28 (48.2) | 54 (50.4) | 22 (45.3) | 0.679 |
| Atrial fibrillation | 29 (50) | 63 (58.8) | 29 (60.4) | 0.491 |
| Hypertension | 42 (72.4) | 63 (58.8) | 25 (52.8) | 0.059 |
| Diabetes | 17 (29.3) | 25 (23.3) | 17 (35.4) | 0.169 |
| EF | 52.9±12.9 | 47.8±11.9 | 45.6±16.2 | 0.183 |
| Preserved EF | 28 (48.2) | 28 (26.2) | 11 (22.9) | 0.019 |
| Anemia† | 25 (43.1) | 58 (54.2) | 25 (52.0) | 0.413 |
| Hyponatremia* | 3 (5.1) | 15 (14.0) | 7 (14.5) | 0.209 |
| Decrease renal function‡ | 29 (50) | 67 (62.6) | 38 (79.1) | 0.009 |
| Angiotensin-converting enzyme inhibitors | 24 (41.3) | 46 (42.9) | 15 (31.5) | 0.421 |
| β-blockers | 7 (12.0) | 11 (10.2) | 5 (10.4) | 0.533 |
| Diuretics | 41 (70.6) | 80 (74.7) | 32 (66.6) | 0.816 |
| Low-dose aspirin | 21 (36.2) | 30 (28.0) | 17 (35.0) | 0.361 |
Data are presented as n (%) or mean±SD.
Sodium level <136 meq/mL.
Hemoglobin level <12 g/dL.
eGFR <60 mL/min/1.73 m2.
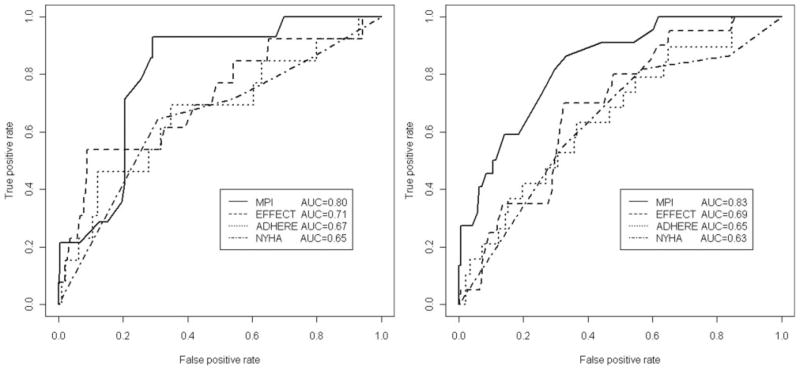
With the increasing of MPI grade, 30-day mortality rates were progressively higher both in men (MPI-1=2.8%, MPI-2=15.3%; MPI-3=47.4%; P=0.000) and in women (MPI-1=0%, MPI-2=6.5%; MPI-3=14.6%; P=0.011). The discrimination of the MPI was also good, with an ROC area for mortality of 0.83 (95% CI, 0.76 to 0.90) in men and of 0.80 (95% CI, 0.71 to 0.89) in women (Figure).
Figure.
ROC curves for the MPI, NYHA, EFFECT, and ADHERE risk scores at 30 days of follow-up in men (right) and women (left).
Comparison Between the MPI and the NYHA, EFFECT, and ADHERE Models
Figure shows the ROC areas for models with MPI, NYHA, EFFECT, and ADHERE, evaluated as continuous variables, both in men (right) and women (left). In men, the MPI risk score demonstrated a significantly higher prognostic value for 30-day mortality than the NYHA (0.83; 95% CI, 0.76 to 0.90 versus 0.63; 95% CI, 0.57 to 0.69; P=0.015), the EFFECT (0.83; 95% CI, 0.76 to 0.90 versus 0.68; 95% CI, 0.58 to 0.79; P=0.045), and the ADHERE risk scores (0.83; 95% CI, 0.76 to 0.90 versus 0.65; 95% CI, 0.52 to 0.78; P=0.023). In women, also the MPI demonstrated an higher discrimination value than the NYHA (0.80; 95% CI, 0.71 to 0.89 versus 0.65; 95% CI, 0.55 to 0.75; P=0.06), the EFFECT (0.80; 95% CI, 0.71 to 0.89 versus 0.71; 95% CI, 0.55 to 0.88; P=0.443), and the ADHERE (0.80; 95% CI, 0.71 to 0.89 versus 0.66; 95% CI, 0.49 to 0.83, P=0.171) risk scores, but the differences were not significant. For sake of completeness, we also run a bootstrapped version of the same approach that yielded overlapping results (data not shown). Pairwise Spearman correlation coefficients between MPI and the other risk scores were in men: MPI versus NYHA=0.23, P=0.003; MPI versus EFFECT=0.36, P<0.0001; and MPI versus ADHERE=0.36, P<0.0001 and in women: MPI versus NYHA=0.15, P=0.03; MPI versus EFFECT=0.36, P<0.0001; and MPI versus ADHERE=0.32, P<0.0001. The results confirmed that weaker correlations were associated with higher under the ROC areas differences according to the tests obtained for the ROC comparisons.
Discussion
In this study, we demonstrated that MPI derived from a CGA can be used to stratify hospitalized older patients with HF into categories that experienced very different short-term mortality. The MPI was derived from standard measures of CGA that are routinely collected in geriatric patients affected by acute and chronic morbidities. Our findings demonstrate that CGA is a powerful tool to estimate 30-day mortality in older patients discharged after an episode of hospitalization for HF. Moreover, the prognostic value of mortality risk, as estimated by the multidimensional integrated information, seems to be more accurate than the prognostic value based only on information derived from disease-specific parameters. Indeed, no differences in the prevalences of cardiac pathologies, hypertension, or cardiological drug use were observed in patients with different MPI values, and no significant differences were also found in the EF mean values, whereas a significant reduction in the percentage of patients with preserved EF was observed both in men and in women with higher MPI values (P=0.001). In this study, ≈30% of total population with HF had preserved EF. This finding was consistent with those of a previous study.27 Interestingly, no other specific concomitant diseases, such as anemia, diabetes mellitus, and hyponatremia were associated with higher MPI values, whereas with the increasing of the MPI values, a significant decreased renal function was observed in women.
As shown by the areas under the ROC curves, in this population, the predictive value of the MPI was higher than the predictive value of the NYHA, EFFECT, and ADHERE models both in men and in women. However, the differences were significant in men and not significant in women. A lower 1-month mortality observed in women compared with men may explain this apparent discrepancy in results. Interestingly, in this population, the ADHERE model demonstrated an area under the ROC curve quite similar to the value observed in the original validation study (0.69),11 whereas the EFFECT model demonstrated an area slightly lower than the original study (0.80).10
Of note, in this study, the overall 30-day mortality was 9.6%, quite similar to the rates of 6% to 13% reported in other studies.28–30 As expected, mortality rates were higher in men than women (13.5% versus 6.6%, P=0.035), whereas the MPI mean values were significantly lower in men than women (0.39±0.16 versus 0.48±0.19, P=0.0000). These findings are in agreement with recent studies in older people,8,14,31 reporting that the sex difference in life expectancy was not entirely due to sex difference in impairment, because men with the same chronological age and biological impairments (ie, frailty index) had a higher risk of death compared with women.
Predicting the outcome in elderly patients with HF is very important in clinical practice for decision-making process. Previous epidemiological studies suggested that a wide variety of factors was associated with an increased risk of death, including demographic factors (age and male sex),32 clinical characteristics such us cardiopulmonary functions or NYHA class,33 renal function,34 and other comorbidities (ie, depression, anemia, and diabetes mellitus).35–37 Two previous studies have explored the role of the functional assessment in older patients with HF. The first study9 carried out in 120 patients with chronic HF reported that frailty, assessed by the Frailty Staging System,38 was an independent variable for predicting long-term mortality in elderly subject with chronic HF. The second study demonstrated that the functional status, evaluated by means of the ADL Barthel Index in 88 elderly patients hospitalized for HF, was an independent predictor of mortality.29
To the best of our knowledge, this is the first description of a short-term prognostic index based on data available from a standard CGA for older patients hospitalized with HF in an acute care setting. These data are in agreement with studies carried out in older patients hospitalized for an acute disorder or relapse of a chronic disease independently from the diagnosis14 as well as in elderly patients hospitalized for an acute upper gastrointestinal bleeding,39 pneumonia,40 and dementia.41 Overall, these findings support the concept that considering multidimensional aggregate information is very important for predicting short-term mortality in older patients with acute diseases or relapse of chronic diseases, including HF.
In clinical practice, this MPI may be useful in identifying both high- and low-risk patients, so that specific interventions can be eventually targeted to each group. This could be important particularly for identifying those elderly low-risk patients who can benefit from screening and/or prevention programs and who are actually excluded because of merely the old age. Conversely, the MPI is useful in identifying those high-risk patients for whom advanced care assistance programs could be appropriate and cost effective.
This study has some limitations. Because the study population comprised hospitalized older patients, it is possible that our findings may not be applicable to other settings. Second, the patients were recruited within a single hospital. Larger prospective multicenter studies are therefore needed to confirm our findings and to explore the variability of performance across different institutions. Finally, the CGA needs to be administered by expert geriatricians and requires time.
In conclusion, we described a MPI, based on data derived from CGA, in hospitalized older patients with HF. This index is a sensitive measure of the multidimensional impairment and might be useful in identifying elderly patients with HF who are at different risk of mortality. Further studies are needed to evaluate the potential usefulness of this prognostic tool in clinical practice.
CLINICAL PERSPECTIVE
The clinical evolution of older patients with acute diseases is conditioned only in part by the disease-specific signs and symptoms that are traditionally included in prognostic indices, whereas multidimensional integrated information seems to be more accurate to predict the risk of mortality. In this study, we demonstrated that a Multidimensional Prognostic Index (MPI) based on a comprehensive geriatric assessment can be used to stratify hospitalized older patients with heart failure into different categories of risk of mortality. In this population, higher MPI was associated with significantly higher mortality, although no differences in the prevalence of cardiac pathologies, hypertension, or cardiologic drug use were observed in patients with different MPI values, and no significant differences were also found in the ejection fraction mean values. These findings support the concept that considering multidimensional aggregate information is very important for predicting short-term mortality in older patients with heart failure. In clinical practice, this MPI may be useful in identifying both high- and low-risk patients, so that specific interventions can be eventually targeted to each group. This could be important particularly for identifying those low-risk elderly patients who can benefit from screening and/or prevention programs and who are actually excluded because of merely the old age. Conversely, the MPI may be useful in identifying those high-risk patients for whom appropriate advance care assistance programs could be appropriate and cost effective.
Acknowledgments
Sources of Funding
This work was supported by grants from Ministero della Salute (Italy), IRCCS Research Program 2006–2008, Line 2: “Malattie di rilevanza sociale,” and by the Intramural Research Program of the National Institute of Aging (Baltimore, Md).
Footnotes
Disclosures
None.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Thomas TS, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Clin Geriatr Med. 2007;23:1–10. doi: 10.1016/j.cger.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Koelling TM, Chen RS, Lubwama RN, L’Italien GJ, Eagle KA. The expanding national burding of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004;147:74–78. doi: 10.1016/j.ahj.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Fang J, Mensah JA, Croft JB, Keenan NL. Heart Failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 5.Zannad F, Braicon S, Juilliere Y, Mertes PM, Villemot JP, Alla F, Virion JM. Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: the EPICAL study. J Am Coll Cardiol. 1999;33:734–742. doi: 10.1016/s0735-1097(98)00634-2. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Zannad F, Sopko G, Klein L, Piña IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L International Working Group on Acute Heart Failure Syndromes. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 7.Westert GP, Lagoe RJ, Keskimäki I, Leyland A, Murphy M. An international study of hospital readmission and related utilization in Europe and the USA. Health Policy. 2002;61:269–278. doi: 10.1016/s0168-8510(01)00236-6. [DOI] [PubMed] [Google Scholar]
- 8.Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D’Ambrosio D, Gargiulo G, Testa G, Santis D, Galizia G, Ferrara N, Rengo F. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure. Derivation and validation of a clinical model. J Am Med Assoc. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. J Am Med Assoc. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 12.Huynh BC, Rovner A, Rich MW. Identification of older patients with heart failure who may be candidates for hospice care: development of a simple four-item risk score. J Am Geriatr Soc. 2008;56:1111–1115. doi: 10.1111/j.1532-5415.2008.01756.x. [DOI] [PubMed] [Google Scholar]
- 13.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model. Prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 14.Pilotto A, Ferrucci L, Franceschi M, D’Ambrosio LP, Scarcelli C, Cascavilla L, Paris F, Placentino G, Seripa D, Dallapiccola B, Leandro G. Development and validation of a multidimensional prognostic index for 1-year mortality from the comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11:151–161. doi: 10.1089/rej.2007.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Nieminen MS, Böhm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, Hasin Y, Lopez-Sendon J, Mebazaa A, Metra M, Rhodes A, Swedberg K, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie MR, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Garcia MA, Dickstein K, Albuquerque A, Conthe P, Crespo-Leiro M, Ferrari R, Follath F, Gavazzi A, Janssens U, Komajda M, Morais J, Moreno R, Singer M, Singh S, Tendera M, Thygesen K ESC Committee for Practice Guideline (CPG) Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 17.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Chest Physician and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 18.Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. 2000;101:2118–2121. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 19.Katz S, Downs TD, Cash HR, Grotz RC. Progress in the development of an index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 21.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 22.Linn B, Linn M, Gurel L. The cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 23.Guigoz Y, Vellas B. The mini nutritional assessment (MNA) for grading the malnutrition states of elderly patients: presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme. 1999;1:3–11. doi: 10.1159/000062967. [DOI] [PubMed] [Google Scholar]
- 24.Bliss MR, McLaren R, Exton-Smith AN. Mattresses for preventing pressure sores in geriatric patients. Mon Bull Minis Health Public Health Lab Serv. 1966;25:238–268. [PubMed] [Google Scholar]
- 25.Thomas VS, Rockwood K, McDowell I. Multidimensionality in instrumental and basic activities of daily living. J Clin Epidemiol. 1998;51:315–321. doi: 10.1016/s0895-4356(97)00292-8. [DOI] [PubMed] [Google Scholar]
- 26.DeLong ER, DeLong DM, Clarke Pearson DL. Comparing the areas under two or more correlated receiver-operating characteristic curves; a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 27.Bathia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 28.Fonarow GC. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008;155:200–207. doi: 10.1016/j.ahj.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Formiga F, Chivite D, Solè A, Manito N, Ramon JM, Pujol R. Functional outcomes of elderly patients after the first hospital admission for decompensated heart failure. A prospective study. Arch Gerontol Geriatr. 2006;43:175–185. doi: 10.1016/j.archger.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Velavan P, Khan NK, Goode K, Rigby AS, Loh PH, Komajada M, Follath F, Swedberg K, Madeira H, Cleland JG. Predictors of short term mortality in heart failure—insights from the Euro Heart Failure survey. Int J Cardiol. 2008;138:63–69. doi: 10.1016/j.ijcard.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a chinese population. J Gerontol A Biol Sci Med Sci. 2005;60:1046–1051. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- 32.Ghali JK, Krause-Steinrauf HJ, Adams KF, Khan SS, Rosenberg YD, Yancy CW, Young JB, Goldman S, Peberdy MA, Lindenfeld J. Gender differences in advanced heart failure: insights from the BEST study. J Am Coll Cardiol. 2003;42:2128–2134. doi: 10.1016/j.jacc.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Scrutinio D, Lagioia R, Ricci A, Clemente M, Boni L, Rizzon P. Prediction of mortality in mild to moderately symptomatic patients with left ventricular dysfunction. The role of the New York Heart Association classification, cardiopulmonary exercise testing, two-dimensional echo-cardiography and Holter monitoring. Eur Heart J. 1994;15:1089–1095. doi: 10.1093/oxfordjournals.eurheartj.a060633. [DOI] [PubMed] [Google Scholar]
- 34.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 35.Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, Blazing MA, Davenport C, Califf RM, Krishnan RR, O’Connor CM. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161:1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 36.Maggioni AP, Opasich C, Anand I, Barlera S, Carbonieri E, Gonzini L, Tavazzi L, Latini R, Cohn J. Anemia in patients with heart failure: prevalence and prognostic role in a controlled trial and in clinical practice. J Card Fail. 2005;11:91–98. doi: 10.1016/j.cardfail.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Tribouilloy C, Rusinaru D, Mahjoub H, Tartière JM, Kesri-Tartière L, Godard S, Peltier M. Prognostic impact of diabetes mellitus in patients with heart failure and preserved ejection fraction. A prospective 5-years study. Heart. 2008;94:1450–1455. doi: 10.1136/hrt.2007.128769. [DOI] [PubMed] [Google Scholar]
- 38.Lachs MS, Feinstein AR, Cooney LM, Jr, Drickamer MA, Marottoli RA, Pannill FC, Tinetti ME. A simple procedure for general screening for functional disability in elderly patients. Ann Intern Med. 1990;112:699–706. doi: 10.7326/0003-4819-112-9-699. [DOI] [PubMed] [Google Scholar]
- 39.Pilotto A, Ferrucci L, Scarcelli C, Niro V, Di Mario F, Seripa D, Andriulli A, Leandro G, Franceschi M. Usefulness of comprehensive geriatric assessment in older patients with upper gastrointestinal bleeding: a two year follow-up study. Dig Dis. 2007;25:124–128. doi: 10.1159/000099476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilotto A, Addante F, Ferrucci L, Leandro G, D’Onofrio G, Corritore M, Niro V, Scarcelli C, Dallapiccola B, Franceschi M. The multidimensional prognostic index (MPI) predicts short- and long-term mortality in hospitalized geriatric patients with pneumonia. J Gerontol A Med Sci. 2009;64:880–889. doi: 10.1093/gerona/glp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilotto A, Sancarlo D, Panza F, Paris F, D’Onofrio G, Cascavilla L, Addante F, Seripa D, Solfrizzi V, Dallapiccola B, Franceschi M, Ferrucci L. The Multidimensional Prognostic Index (MPI) based on a comprehensive geriatric assessment predicts short- and long-term mortality in hospitalized older patients with dementia. J Alzheimers Dis. 2009;18:191–199. doi: 10.3233/JAD-2009-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]