Abstract
Glioblastoma multiforme (GBM) is a devastating form of brain cancer for which there is no effective treatment. Here, we report a novel approach to brain tumor therapy through genetic modification of normal brain cells to block tumor growth and effect tumor regression. Previous studies have focused on the use of vector-based gene therapy for GBM by direct intratumoral injection with expression of therapeutic proteins by tumor cells themselves. However, as antitumor proteins are generally lethal to tumor cells, the therapeutic reservoir is rapidly depleted, allowing escape of residual tumor cells. Moreover, it has been difficult to achieve consistent transduction of these highly heterogeneous tumors. In our studies, we found that transduction of normal cells in the brain with an adeno-associated virus (AAV) vector encoding interferon-β (IFN-β) was sufficient to completely prevent tumor growth in orthotopic xenograft models of GBM, even in the contralateral hemisphere. In addition, complete eradication of established tumors was achieved through expression of IFN-β by neurons using a neuronal-restricted promoter. To our knowledge this is the first direct demonstration of the efficacy of targeting gene delivery exclusively to normal brain cells for brain tumor therapy.
INTRODUCTION
Glioblastoma multiforme (GBM) is the most malignant (World Health Organization Grade IV) astrocytoma and is the most frequent type of primary brain tumor.1 The Central Brain Tumor Registry of the United States (http://www.cbtrus.org) states that the 5-year survival rate from the time of diagnosis is only 3.3%. Standard-of-care treatment involves radiation, chemotherapy, and maximal surgical re-section of the tumor; however, as the poor survival rate indicates, these treatments have not been effective in preventing disease progression. One of the hallmarks of GBM is its invasiveness. Infiltrating cells extend from the tumor body into the surrounding brain parenchyma, making complete surgical removal of tumor nearly impossible and recurrent tumors often appear within 2 cm of the initial lesion.2
A number of viral vectors including herpes simplex virus, adenovirus, retrovirus, and adeno-associated virus (AAV) have been used to deliver therapeutic transgenes into experimental gliomas.3 These vectors are generally injected directly into the tumor and have shown promising results in animal models of GBM.4–7 The success of this approach relies on the ability of the vectors to transduce glioma cells efficiently based on data from a limited number of glioma cell lines. The main caveat being that GBMs are highly heterogeneous and the transduction efficiencies achieved in a given glioma cell line may not translate to those obtainable in human GBM tumors in vivo. Supporting this concern is data showing that transduction of primary spheroids prepared from different GBM patient biopsy samples was variable using the same AAV vector8 or herpes simplex virus vector. 9 Additionally, the high interstitial pressure within tumors has been shown to prevent homogeneous spread of macromolecules, including vectors, throughout the tumor.10 A promising alternative to the tumor-transduction approach would be to target gene delivery to normal brain tissue surrounding the tumor, as efficient transduction of normal brain parenchyma has been obtained with AAV vectors in several animal models of human central nervous system disease.11,12 Further, the efficiency of AAV-mediated gene transfer to the brain has been dramatically increased by the use of new AAV serotypes, which yield widespread transduction in many regions of the rodent brain.11,13–16 Expression of a therapeutic protein in the brain parenchyma may be used to accomplish two goals: (i) form a barrier against infiltrating GBM cells, and (ii) create a long-term reservoir of therapeutic proteins for extended exposure of tumor cells. This is in contrast to tumor-expressed therapeutic proteins, which ultimately kill the tumor cells themselves and thus limit the efficacy of tumor-directed gene delivery approaches.
In this study, we tested whether creating a “zone of resistance” in the brain by AAV-mediated expression of human interferon-β (hIFN-β) in normal brain tissue could prevent the growth of tumors in orthotopic xenograft models of GBM. First, in a proof-of-concept experiment, we found that pretreating normal brain with an AAV vector encoding hIFN-β could effectively prevent the establishment of intracranial tumors using two different human glioma cell lines, in contrast to mice pretreated with a control AAV vector. In fact, we observed a robust antitumor effect against tumors located distally to the vector injection site. Second, we demonstrated, via neuronal-restricted expression of IFN-β, that complete regression could be achieved without the need for transduction of tumor cells.
RESULTS
Transduction of normal brain with an AAV vector encoding hIFN-β before tumor implantation prevents tumor growth
We speculated that the ability of AAV vectors to mediate efficient gene transfer to the brain parenchyma could provide a robust source of an antitumor protein which could prevent brain tumor growth. We chose to use the pleotropic cytokine, hIFN-β, as a model therapeutic protein based on its potent antiangiogenic, antiproliferative, and proapoptotic effects on tumor cells.17–19 Two AAV vectors were initially constructed: one encoding hIFN-β (AAV-CBA-hIFN-β) and a control with no transgene [AAV-empty vector (AAV-ev)]. The ability of the AAV-CBA-hIFN-β vector to express hIFNβ was confirmed in vitro by enzyme-linked immunosorbent assay (ELISA) after transduction of mouse primary neuronal cultures and in vivo by immunoblot analysis after injection of vector into the striatum of nude mice (Supplementary Figure S1).
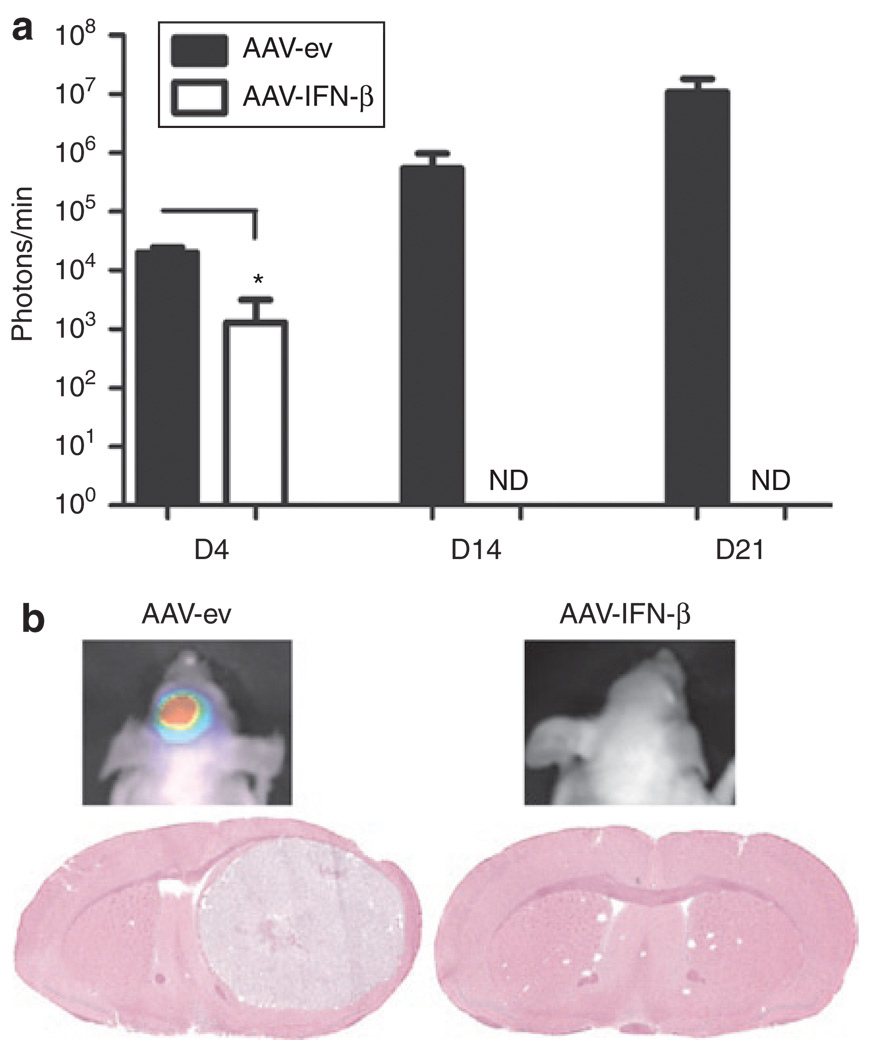
First we evaluated whether AAV-mediated expression of hIFN-β solely from brain parenchyma could prevent growth of glioma tumors in mice. Nude mice were pretreated by intrastriatal injection of either AAV-CBA-hIFN-β or AAV-ev [1011 genome copies (gc) each]. Two weeks later, we injected 105 U87fluc-mCherry-EGFRvIII cells, which stably express firefly luciferase (fluc), the red fluorescent protein, mCherry, and human epidermal growth factor receptor variant III (EGFRvIII), into the striatum in the same stereotaxic coordinates as the AAV vectors. Expression of fluc allows for continuous evaluation of tumor growth by sequential bioluminescence imaging of tumor-associated luciferase activity, and mCherry provides a means to distinguish tumors cells from normal cells in histological sections. Retrovirus-mediated EGFRvIII expression in U87 cells increases the rate of tumor growth in nude mice.20 Following tumor cell injection, we measured tumor-associated bioluminescence signal (TABS) in AAV-CBA-hIFN-β and AAV-ev-treated mice over time (Figure 1a). At day 4 post-tumor injection, TABS was significantly lower in AAV-CBA-hIFN-β-treated mice compared with AAV-ev-treated mice (Figure 1a; P < 0.0001). Strikingly, by day 14, no bioluminescence was detected in mice treated with AAV-CBA-hIFN-β, while the signal had increased by more than sevenfold between days 4 and 14 for mice treated with AAV-ev. At day 21, we still could not detect any TABS for mice treated with AAV-CBA-hIFN-β. Histological analysis of brain sections showed no evidence of tumor in the brain of AAV-CBA-hIFN-β-treated mice. In contrast, all AAV-ev-treated mice harbored large tumor masses in the brain (Figure 1b). Similar results were obtained using either U87fluc-mCherry or Gli36fluc+ glioma cells (105 cells were injected), albeit with different kinetics of tumor growth/regression (Supplementary Figures S2 and S3, respectively).
Figure 1. Pretreatment of brain with AAV-CBA-IFN-β prevents aggressive glioma growth in nude mice.
(a) Mice were infused with 1011 genome copies (gc) of either AAV-empty vector (AAV-ev) or AAV-hIFN-β by stereotaxic injection of the striatum (six mice per group). Two weeks after injection, 105 U87 glioma cells stably expressing luciferase and mCherry as well as expressing a constitutively active epidermal growth factor receptor (EGFR) variant III (U87fluc-mCherry-EGFRvIII) were implanted into the same site as vector and mice were imaged for tumor-associated bioluminescent signal at the indicated time points post-tumor injection. ND=, not detectable. (b) Representative bioluminescent images of mice injected with AAV-ev (left image) or AAV-CBA-hIFN-β (right image), both at day 21 post-tumor implantation. Corresponding hematoxylin and eosin–stained coronal brain sections from either AAV-ev-treated animals (left image, day 21 post-tumor) or AAV-CBA-hIFN-β-treated animals (right image, day 53 post-tumor).CBA, chicken β-actin; hIFN-β, human interferon-β.
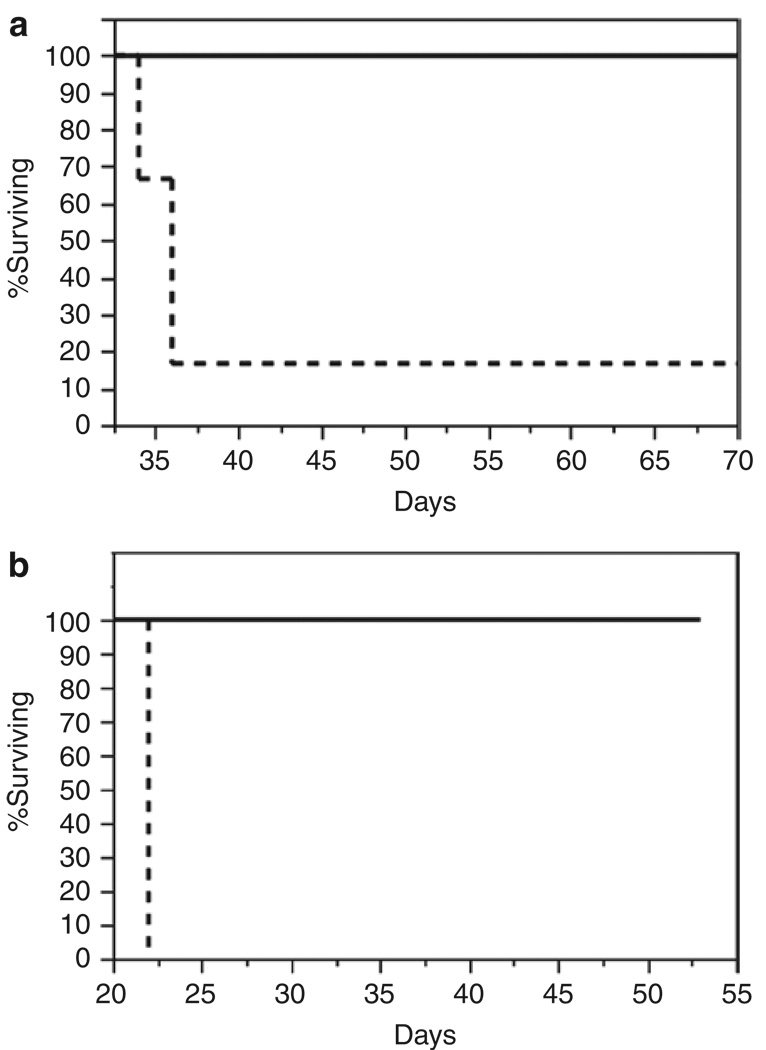
Next, we analyzed the effect of prior AAV-CBA-hIFN-β delivery to normal brain on animal survival after tumor injection. Groups of nude mice were pretreated with 1011 gc of either AAV-CBA-hIFN-β or AAV-ev vectors and 2 weeks later challenged with 5 × 105 U87fluc-mCherry cells or 105 U87fluc-mCherry-EGFRvIII cells. Mice were monitored for symptoms of tumor growth, and the end point was defined as >20% body weight loss. We observed 100% survival in both cohorts of mice pretreated with AAV-CBA-hIFN-β, while mice injected with AAV-ev followed by U87fluc-mCherry or U87fluc-mCherry-EGFRvIII cells had a median survival of 36 and 22 days, respectively (Figure 2a and b).
Figure 2. Pretreatment of brain with AAV-CBA-hIFN-b provides 100% survival against glioma challenge.
Kaplan–Meier survival analysis of mice pretreated as in (Figure 1a) and later implanted with (a) 5 × 105 U87fluc-mCherry cells, or (b) 105 U87fluc-mCherry-EGFRvIII cells. Solid line indicates AAV-CBA-IFN-β-treated mice, while dashed line indicates AAV-empty vector–treated mice (six mice per group). AAV, adeno-associated virus; CBA, chicken β-actin; EGFRvIII, epidermal growth factor receptor variant III; hIFN-β, human interferon-β.
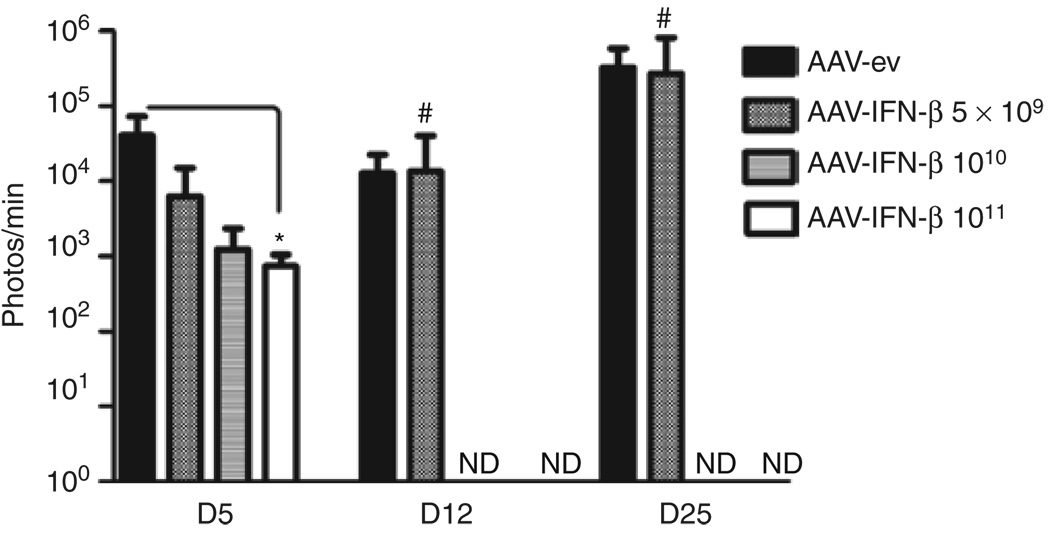
To analyze the therapeutic threshold of AAV-mediated hIFN-β expression, we performed a dose–response experiment in which mice were pretreated intracranially with 1011, 1010, or 5 × 109 gc of AAV-CBA-hIFN-β and 2 weeks later challenged with U87fluc-mCherry cells as described in Figure 1. Imaging at days 12 and 25 revealed that all AAV-CBA-hIFN-β-treated mice in the 1011 and 1010 gc groups, and three of four mice in the 5 × 109 gc group showed no detectable TABS in contrast to AAV-ev-treated mice, which all had detectable TABS at these time points (Figure 3).
Figure 3. Antitumor effect of different doses of AAV-CBA-hIFN-β.
AAV-CBA-hIFN-β was infused into the striatum of three groups of mice (n = 4) at the following doses: 1011 genome copies (gc), 1010 gc, 5 × 109 gc. Two weeks later, 5 × 105 U87fluc-mCherry cells were injected at the same coordinates used for vector injection and tumor growth was monitored by bioluminescent imaging. #Only one of four mice in the 5 × 109 gc AAV-CBA-hIFN-β group gave detectable signal at days 12 and 25. AAV-ev, adeno-associated virus–empty vector; CBA, chicken β-actin; EGFRvIII, epidermal growth factor receptor variant III; hIFN-β, human interferon-β; ND, not detected.*P < 0.05.
Intracranial injection of AAV-hIFN-β prevents growth of distal tumors in the brain
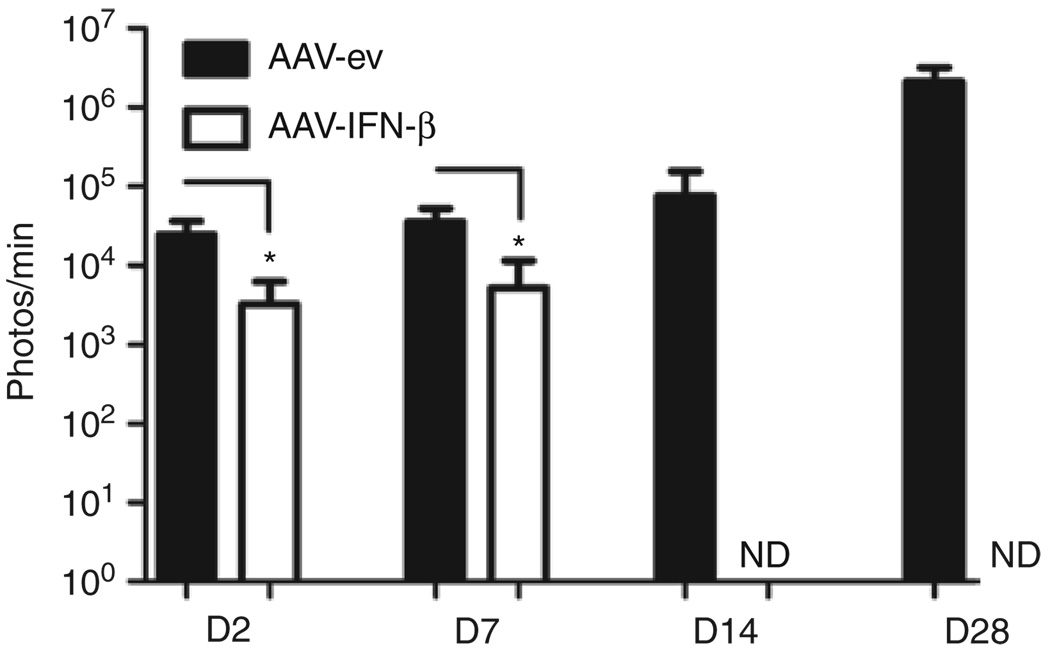
To model a tumor recurrence distant to the primary site, as seen in GBM patients, we injected either 5 × 1010 gc of AAV-CBA-hIFN-β or AAV-ev into the left striatum of nude mice and two weeks later injected 5 × 105 U87fluc-mCherry cells into the contralateral striatum. Remarkably, by day 2 and day 7 post-tumor injection there was a 7.6-fold (P = 0.0057) and 6.9-fold (P = 0.006) reduction, respectively, of TABS in mice injected with AAV-CBA-IFN-β compared to mice injected with AAV-ev (Figure 4). By day 14, the bioluminescent signal was undetectable in mice injected with AAV-CBA-hIFN-β and the entire group of mice (n = 5) survived symptom-free for 51 days post-tumor challenge, at which time the experiment was terminated. In contrast, the TABS increased in mice injected with control AAV vector, and all animals in this group reached the end point between days 31 and 42. These results show that delivery of AAV-CBA-hIFN-β vector to normal brain creates a far-reaching therapeutic zone. Also, we showed that brain-expressed IFN-β was superior to tumor-expressed IFN-β in preventing the growth of distal tumors (Supplementary Figure S4).
Figure 4. Intracranial injection of AAV-CBA-hIFN-β causes regression of distal tumors in the brain.
Mice were infused with 5 × 1010 genome copies of either AAV-empty vector (AAV-ev) or AAV-CBA-hIFN-β by stereotaxic injection of the striatum (five mice per group). Two weeks post-injection, 5 × 105 U87fluc-mCherry glioma cells were implanted into the striatum of the contralateral hemisphere and mice were imaged for tumor-associated bioluminescent signal at the indicated time points post-tumor injection. AAV, adeno-associated virus; CBA, chicken β-actin; hIFN-β, human interferon-β. *P < 0.01.
Expression of hIFN-β from nontumor tissue causes regression of established tumors
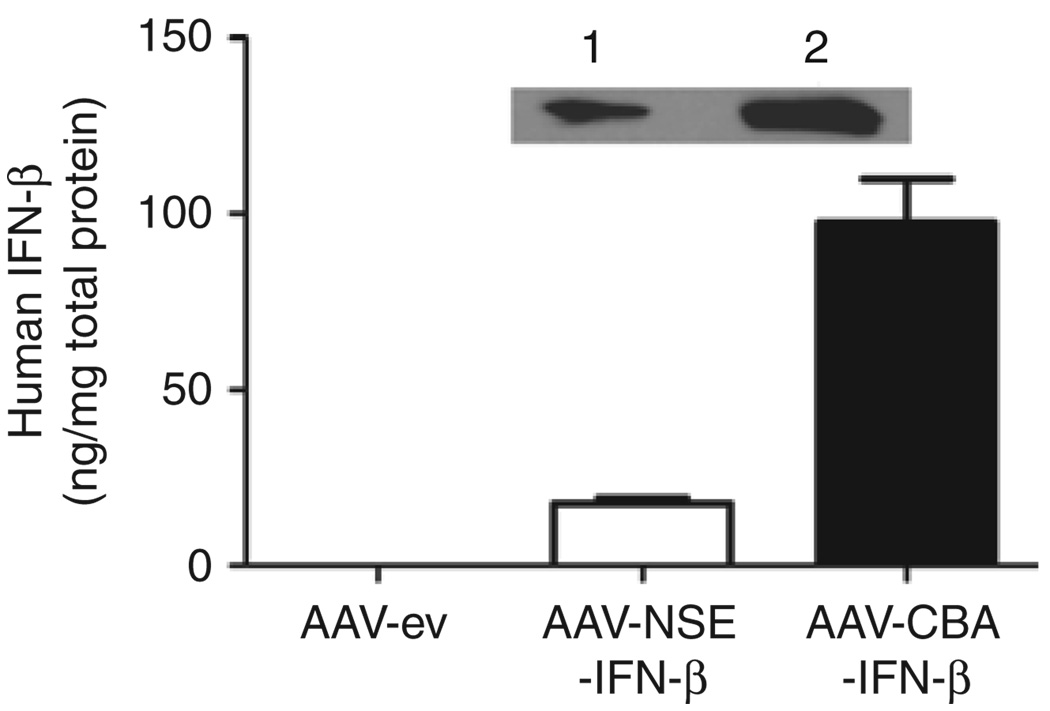
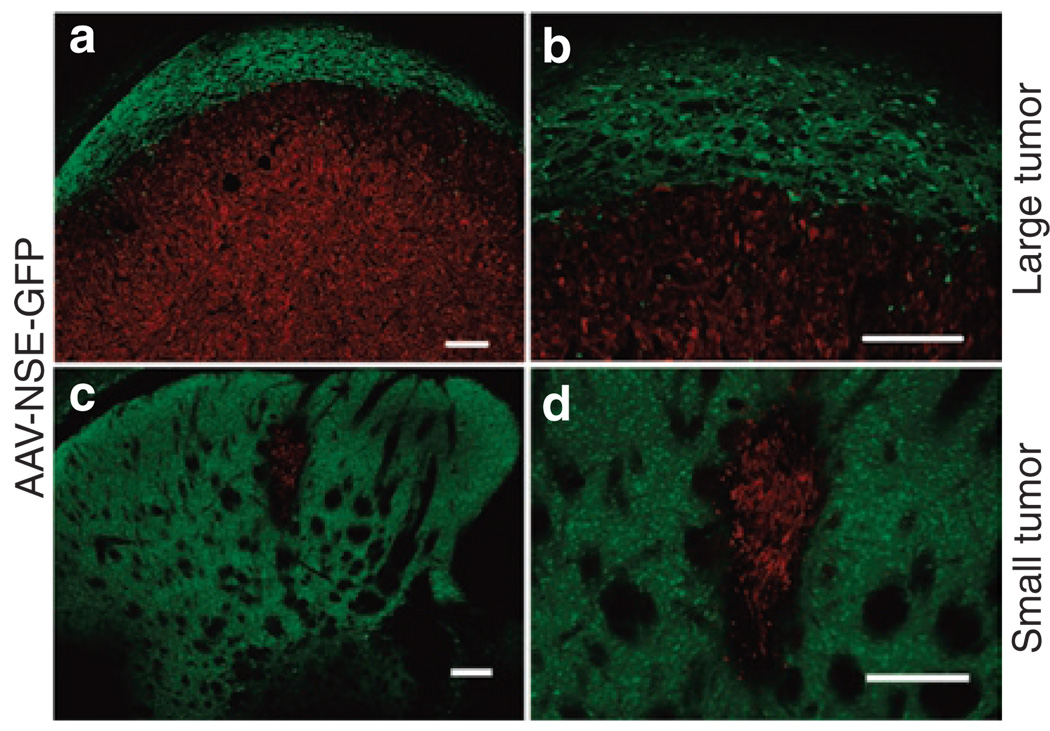
Infusion of AAV vector into the brain before tumor implantation is a useful paradigm to test the zone of resistance concept, but does not model the clinical situation where residual tumor cells exist beyond the re-section cavity. To test the zone of resistance principle for pre-existing tumors, we constructed an AAV vector encoding hIFN-β under the rat neuron–specific enolase (NSE) promoter. Given the neuronal specificity of NSE, we anticipated this AAV-NSE-hIFN-β vector to be inactive in U87 cells, which are of glial origin. This was confirmed by an in vitro transduction assay on U87 cells (Supplementary Figure S5). To confirm the functionality of the AAV-NSE-hIFN-β vector and compare it to AAV-CBA-hIFN-β, we injected 1011 gc of either construct into the striatum of nude mice. Two weeks later, brains were harvested and the striatum was analyzed for hIFN-β expression by immunoblot analysis and ELISA. IFN-β levels in the striatum of AAVNSE-hIFN-β-injected mice were 18 ng IFN-β/mg total protein as compared with 100 ng IFN-β/mg total protein in AAV-CBA-hIFN-β-injected mice (Figure 5). To demonstrate the neuronal specificity of transgene expression from AAV-NSE vectors in brain, we injected the AAV-NSE-GFP vector intratumorally in mice bearing U87fluc-mCherry-EGFRvIII tumors and analyzed green fluorescent protein (GFP) expression 7 days later (Figure 6a and b). As expected, the AAV-NSE-GFP vector–mediated robust expression in normal brain, but not in tumor cells (mCherry+) as there were no GFP+/mCherry+ cells in any of the sections analyzed. We also analyzed the transduction profile of AAV-NSE-GFP vectors in small tumors (Figure 6c and d). Small tumors allowed for better visualization of transduced cell distribution in normal brain before it was compressed by tumor growth. As before, GFP expression was found exclusively in nontumor tissue in the striatum.
Figure 5. AAV-NSE-hIFN-β vector function and in vivo levels.
Interferon-β (IFN-β) levels in striatum after injection with either AAV-CBA-hIFN-β or AAV-NSE-hIFN-β. Nude mice were infused into the striatum with 1011 genome copies of the indicated vectors, and 2 weeks later human IFN-β (hIFN-β) was detected by enzyme-linked immunosorbent assay and immunoblot analysis (inset). A band of the expected 25-kd MW was detected in lysate from brains transduced with both AAV-CBA-hIFN-β vector (lane 1) and AAV-NSE-hIFN-β vector (lane 2). AAV-ev, adeno-associated virus–empty vector; NSE, neuron-specific enolase.
Figure 6. AAV-NSE-GFP vector expresses exclusively in brain parenchyma.
(a, b; large tumor) 105 U87fluc-mCherry-EGFRvIII cells were implanted into the striatum of nude mice and 1 week later, 1010 genome copies AAV-NSE-GFP was infused intratumorally. Seven days later, brains were analyzed as above. Transduced cells are shown in green and tumor cells in red due to stable mCherry expression. Note the transduction of brain parenchyma on the perimeter of the tumor and also the few GFP+ cells within the tumor which did not co-localize with mCherry. Scale bar = 200 μm. (c, d; small tumor) Mice were injected with 104 U87fluc-mCherry cells and were intratumorally injected with AAV-NSE-GFP 7 days later. Ten days later, mice were killed and brains analyzed for transduction. Note the excellent transduction of brain which completely surrounds the tumor. Scale bars = 200 µm. AAV, adeno-associated virus; EGFRvIII, epidermal growth factor receptor variant III; GFP, green fluorescent protein; NSE, neuron-specific enolase.
Next, we analyzed whether transduction of brain parenchyma with AAV-NSE-hIFN-β targeting expression to neurons could affect growth of established U87 tumors. Mice were first injected with 5 × 105 U87fluc-mCherry cells and 2 weeks later injected into the same site with 4 × 1011 gc of AAV-NSE-hIFN-β or AAV-ev. We chose to use more vector for this experiment due to the low expression levels of IFN-β obtained with the NSE-driven construct as compared with the chicken β-actin (CBA)-driven construct (Figure 5). Remarkably, by day 4 post-vector injection, TABS was significantly lower in AAV-hIFN-β-treated mice compared to AAV-ev-treated mice (Figure 7a; P = 0.0045). By day 14 post-vector infusion, no TABS could be detected in AAV-NSE-hIFN-β-treated mice (Figure 7a). In contrast, in AAV-ev-treated mice TABS increased between 4 and 14 days post-vector injection, and all mice reached the end point in the last imaging session (day 28 after tumor cell injection). Histological examination of the brain revealed massive tumors in all AAVev- treated animals (Figure 7b). AAV-NSE-hIFN-β-treated mice were imaged at day 35 post-vector infusion (day 49 post-tumor injection), and TABS remained undetectable. A subgroup of mice (n = 2) was killed after this imaging session and the brains were harvested for histological examination, which showed a glial scar with no definite tumor (Figure 7b, arrow; and Supplementary Figure S6). Another subgroup (n = 2) was continuously monitored and survived with no symptoms of tumor growth until the experiment was terminated at day 105 post-tumor injection.
Figure 7. Expression of human interferon-β (hIFN-β) from nontumor tissue causes regression of established tumors.
(a) 5 × 105 U87fluc-mCherry cells were implanted into nude mice (four mice per group) and 2 weeks later, mice were infused with 4 × 1011 genome copies of AAV-empty vector (AAV-ev) or AAV-NSE-hIFN-β. Mice were imaged as in Figure 1a. (b) Representative bioluminescent images of mice injected with AAV-ev (left image) or AAV-NSE-hIFN-β (right image), both at day 14 post-vector. Hematoxylin and eosin–stained coronal brain sections from either AAV-ev-treated animals (left image, day 38 post-tumor) or AAV-NSE-hIFN-B-treated animals (right image, day 49 post-tumor). Arrow points to glial scar.
DISCUSSION
This study demonstrates the principle of genetically engineering normal brain cells to prevent tumor growth and to cause regression of tumors by secretion of an antitumor agent. This new strategy provides a more predictable and stable means of antitumor therapy than direct targeting of tumor cells themselves. The majority of clinical trials for GBM using viral vectors focus on direct transduction of residual tumor cells following tumor re-section. However, we reasoned that normal cells would be a more desirable target for AAV transduction as the majority of tissue surrounding the re-section cavity is normal brain parenchyma infiltrated with tumor cells. AAV vectors have been shown to transduce normal brain with exceptionally high efficiency in a number of mammalian species including mice, rats, cats, dogs, and monkeys.12,21–24 Furthermore, transducing primarily or only tumor cells becomes a self-limiting approach because the cells producing the therapeutic molecule are eliminated by the therapy itself. To achieve a therapeutic effect, it would be necessary to transduce a very large percentage of infiltrating tumor cells, which is highly unlikely given the heterogeneous nature of primary GBM and their invasive properties. This spares nontransduced tumor cells from prolonged exposure to the antitumor therapy and may explain the results of one study where intratumoral injection of an AAV2 vector expressing thymidine kinase was unable to prevent tumor progression in a rat GBM model after ganciclovir administration even though a substantial percentage (39%) of tumor cells was initially transduced.25
The results described here demonstrate that efficient transduction of normal brain tissue with an AAV vector encoding a secreted antitumor protein can prevent the growth of human glioma cell tumors in an orthotopic xenograft mouse model. We injected AAV-hIFN-β vector into nude mouse brain followed 2 weeks later by intracranial injection of U87fluc-mCherry-EGFRvIII cells (Figure 1). In this model, AAV-transduced normal cells in the brain are the unequivocal source of therapeutic protein. Others have observed transduction of neurons bordering intracranial tumors as well as direct tumor cell transduction after AAV injection, but did not demonstrate whether one cell type or the other was responsible for the antitumor effect.4 In the present proof-of-concept model, we found that pretreatment of normal brain with AAV-hIFN-β vector was 100% effective in preventing brain tumor growth.
Next, we tested the validity of the zone of resistance concept in treatment of established U87 intracranial tumors. To confirm that a therapeutic effect could be obtained via AAV-transduced normal brain, we used an AAV vector encoding hIFN-β under the rat NSE promoter and we observed complete tumor regression of established U87 tumors (Figure 7). Moreover, we showed exclusive transduction of nontumor tissue around the tumor after intratumoral injection of AAV-NSE-GFP vectors confirming the neuronal specificity of the promoter (Figure 6). The remarkable antitumor effect reported here is likely the result of continued high-level expression of hIFN-β from AAV-transduced neurons that creates a three-dimensional therapeutic “cage” around the tumor. Two other studies using AAV vectors encoding antitumor proteins were able to cause complete tumor regression in mouse models of GBM, although an important difference exists between those studies and ours. Yoshida et al. found that intratumoral injection of an AAV2 vector encoding hIFN-β caused complete tumor regression after six injections of vector, and Mizuno et al. obtained tumor regression in the majority of animals after three intratumoral injections with an AAV vector carrying a transgene for herpes simplex virus thymidine kinase and subsequent ganciclovir treatment.26,27 In contrast, in our study a single injection of AAVrh.8-encoding hIFN-β was sufficient to cause tumor regression in all mice. AAV2 gives relatively limited transduction of tumor cells28 and brain parenchyma,29 which is confined to the needle track, whereas AAV vectors based on alternative AAV serotypes (such as the primate AAV serotype used in our study, AAVrh.8) yield a wider transduction pattern when injected into the murine striatum.29 Additionally, we found a robust antitumor effect when tumors were injected distally to the site of AAV-IFN-β injection (Figure 4). Our results suggest the importance of choosing the appropriate target cell (normal brain versus tumor cells), vector platform (AAV serotype), as well as the therapeutic molecule to achieve a maximal therapeutic effect.
The pluripotent effects of IFN-β on tumor growth have been described,30,31 although the exact mechanism of regression of intracranial U87 glioma tumors by AAV-mediated expression of hIFN-β in the nude mouse model in this study remains to be determined. As human IFN-β has relatively low activity on mouse cells,32 it is most likely that tumor regression resulted from direct effects on the human glioma cells (be it apoptosis, growth inhibition, or decreased secretion of proangiogenic proteins). However, it is possible that there was some species cross-reactivity due to the high levels of AAV-mediated IFN-β production. Even though nude mice lack cytotoxic T-cell activity they retain natural killer cells which can be activated by the immunomodulatory functions of IFN-β, apparently in some cases, across the species barrier.33
Clinical trials34,35 for GBM using recombinant IFN-β protein have reported only minimal to moderate efficacy, which could be due to short half-life and low intratumoral concentrations. Recently, a phase I clinical trial using an adenovirus serotype 5 (Ad5) vector encoding hIFN-β (Ad-hIFN-β) for GBM treatment has been completed. 36 Although a moderate increase in tumor cell apoptosis was reported at the higher doses of Ad-hIFN-β, all patients had disease progression after treatment. This may have been due to limited distribution and transduction of normal brain around the re-section cavity, and possibly short-term transgene expression resulting from immune responses to vector proteins.37 It is worth pointing out that because the Ad vector was injected into the peritumoral brain after tumor re-section, some transduction of brain parenchyma may have occurred, although based on the limitations of Ad vectors mentioned above, the expression of IFN-β in the peritumoral region may have been spatially and temporally limited. Currently, AAV vectors appear to be more suitable in achieving long-term, widespread gene expression in the brain after intraparenchymal delivery and have relatively low toxicity and inflammatory properties, with high transduction efficiency of neurons compared with standard Ad vectors.12,38–40 For example, long-term experiments in monkeys have shown that AAV-mediated gene expression in the brain can last at least 6 years.21
Translation of the zone of resistance concept into GBM patients is likely to require the use of convection-enhanced delivery to achieve widespread distribution of the AAV vectors around the tumor, and careful consideration of the best timing for vector delivery. The doses shown to be effective in this study (1010–1011 gc) are compatible with translation into the much larger human brain (~2,000-fold larger = 2 × 1013–2 × 1014 gc) in terms of existing AAV vector production capacity (1015–1016 gc). Additionally, it will be necessary to assess the toxicity of continuous IFN-β expression in the brain, and its correlation with AAV vector dose. The experiments presented here do not address the potential toxicity of longterm expression of human IFN-β in the brain because mouse cells are quite unresponsive to it. Thus, follow-up experiments using AAV vectors encoding mouse IFN-β infused into mouse brain will be performed. One possible way to address potential toxicity in humans would be to use drug-regulated AAV vectors expressing hIFN-β.
Although IFN-β proved very effective as a therapeutic protein in our study as well as in other preclinical models of cancer,26,41 it should not be considered the only protein with which to apply our strategy of genetically modifying normal brain with AAV to prevent tumor growth. A number of other therapeutic molecules could be used to achieve such an effect. In fact, it may be feasible to use our approach in a multimodal fashion whereby normal brain is transduced with a cocktail of AAV vectors encoding a number of therapeutic proteins each attacking a different mechanism responsible for GBM invasiveness and growth. Examples of potentially useful therapeutic proteins are bone morphogenetic proteins to induce differentiation of GBM stem cells, cytokines which stimulate an anti-tumor immune response or block immunosuppressive T-regulatory cells, antiangiogenic proteins, and proteins which inhibit extracellular matrix remodeling to block glioma cell invasion.
In conclusion, our results represent a new paradigm for GBM therapy, as well as other malignancies, based on AAV-mediated genetic engineering of normal tissue to manipulate the tumor microenvironment. Furthermore, the efficiency of tumor regression in the brain is currently unrivaled with existing preclinical GBM-therapy strategies using AAV vectors.4,26,27 This therapeutic approach could be translated into clinical trials by multiple injections of vector into the surrounding brain parenchyma during tumor re-section to create a zone of resistance to tumor recurrence.
MATERIALS AND METHODS
Cell culture
Human glioblastoma cell lines U87 and Gli36 were obtained from American Type Culture Collection (Manassas, VA) and kindly provided by Dr. Anthony Capanogni (University of California at Los Angeles, Los Angeles, CA),42 respectively. All cell lines were cultured in high glucose Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma, St Louis, MO) and 100 U/ml penicillin, 100 µg/ml streptomycin (Invitrogen) in a humidified atmosphere supplemented with 5% CO2 at 37°C. U87fluc-mCherry cells stably expressing both firefly luciferase (fluc) and mCherry (kindly provided by Dr. Roger Tsien) were generated by transduction with lentiviral vector CSCW2-Fluc-ImC, which carries a fluc-IRES-mCherry cassette under the immediate early cytomegalovirus promoter. This lentiviral backbone also carries a woodchuck hepatitis virus post-transcriptional regulatory element downstream of the expression cassette, and was obtained by deleting all human immunodeficiency virus–enhancer elements in the 3′-long-terminal repeat of CSCGW.43 U87fluc-mCherry-EGFRvIII cells were generated by transduction of U87fluc-mCherry cells with the retroviral vector Babe-EGFRvIII-Puro (EGFRvIII complementary DNA kindly provided by Dr. Webster Cavenee20 and cloned into pBabePuro44) and followed by selection in puromycin (1 µg/ml; Sigma). Gli36fluc+ cells have previously been described,45 and stably express fluc and enhanced GFP (EGFP). Lentivirus vectors were produced as previously described.43
AAV vector design and preparation
The AAV serotype used in this study is derived from the capsid sequence of AAVrh.8 (ref. 46). The AAV-CBA-hIFN-β vector was constructed by replacing EGFP in AAV-CBA-EGFP14 with the complementary DNA for hIFN-β (Invivogen, San Diego, CA). In this vector, gene expression is controlled by a hybrid cytomegalovirus enhancer/CBA promoter. The control AAV-ev is devoid of a transgene. The AAV-NSE-GFP vector encodes EGFP directly under the rat NSE promoter and was derived from pTR-UF4 ref. (47) (from Dr. Richard Snyder, University of Florida, FL). All vectors carry a woodchuck hepatitis virus post-trancriptional regulatory element downstream of the transgenes. This element was derived from the CSCGW plasmid.43 The AAV-NSE- hIFN-β vector was constructed by replacing GFP with the complementary DNA for human IFN-β. AAV vectors were prepared as described previously.14
Animals and stereotaxic injections of mice
Experiments using intracranial injections were performed in 6–8-week-old male athymic nude mice obtained from an in-house colony at the Massachusetts General Hospital. Tumor cells and AAV vectors for the data described in Figure 1–Figure 3 and Figure 5–Figure 7 were implanted/infused in the left midstriatum using the following coordinates from bregma in mm: anterior-posterior +0.5, medio-lateral +2.0, dorso-ventral −2.5. For the contralateral tumor experiment (Figure 4), vector was infused into the left midstriatum using the same coordinates as above and tumor was implanted using the following coordinates from bregma in mm: anterior-posterior +0.5, medio-lateral −2.0, dorso-ventral −2.5. All tumor cell injections were performed using a Micro 4 Microsyringe Pump Controller (World Precision Instruments, Sarasota, FL) attached to a Hamilton syringe with a 33-gauge needle (Hamilton, Rena, NV). Tumor cells in Opti-MEM (Invitrogen) were injected into the striatum in 1 µl (105 cells) or 2 µl (5 × 105 cells) at a rate of 0.2 µl/min. AAV vectors (2 µl; 5 × 1010 or 1011 gc for CBA vectors and 4 × 1011 gc for NSE vectors) were infused into the striatum at a rate of 0.2 µl/min using a Harvard 22 syringe pump (Harvard Apparatus, Holliston, MA) to drive a gas-tight Hamilton Syringe (Hamilton) attached to a 33-gauge steel needle (Hamilton) via PEEK tubing (Alltech, Deerfield, IL) and Luer adapters (Amersham Biosciences, Piscataway, NJ). In vector pretreatment experiments, mice were infused with AAV vectors 2 weeks before challenging with U87fluc-mCherry or Gli36fluc+ cells injected into the same stereotaxic coordinates as vectors (with the exception of Figure 4 in which tumor was injected contralaterally to vector). In the established tumor paradigm, mice were first injected with U87fluc-mCherry glioma cells followed by AAV vector injection into the tumor implantation coordinates 2 weeks later or 1 week later for U87fluc-mCherry-EGFRvIII cells. In survival experiments, mice were killed when they reached the humane end point defined by >20% body weight loss, or onset of symptoms associated with tumor growth. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the Massachusetts General Hospital and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Bioluminescence imaging
Bioluminescence imaging in live animals was carried out using a cryogenically cooled, high-efficiency CCD camera system (Roper Scientific, Duluth, GA) available to us through the Massachusetts General Hospital Small Animal Imaging Facility at the Center for Molecular Imaging Research. For fluc imaging, mice were injected intraperitoneally with D-luciferin (4.5 mg/animal in 150 µl saline) and imaged 5 minutes later. The spatial distribution and levels of luciferase activity in the brain were measured by recording total photon counts in the CCD with no illumination. Postprocessing and visualization were performed as previously described.48
Measurement of human IFN-β in brain lysates or cell culture media by ELISA
Quantitation of IFN-β in culture media and tissue lysates was performed using an ELISA kit for human IFN-β (PBL Biomedical Laboratories, Piscataway, NJ) according to the manufacturers’ instructions. The limit of detection at the utilized dilution is 125 pg IFN-β/ml. For detection in brain lysates, the striatum was dissected from a 2-mm coronal brain slice spanning the injection site, and placed in 400 µl MPER lysis buffer (Pierce, Rockford, IL) containing Complete Protease Inhibitor Cocktail (Roche, Indianapolis, IN). Lysates were diluted 1:1,000 in dilution buffer before ELISA. The limit of detection at this dilution is 25 ng IFN-β/ml. Total protein concentrations were determined using the DC protein assay (BioRad, Hercules, CA).
Human IFN-β immunoblot
Brain lysates were obtained using the same procedure as for ELISA. Fifty microgram of total protein for each sample was separated in 10% sodium dodecyl sulfate/polyacrylamide Tris–HCl Ready gels (BioRad). Immunoblot detection was performed with 1:1,000 dilution of rabbit anti-human IFN-β antibody (Abcam, Cambridge, MA) followed by 1:10,000 dilution of horseradish peroxidase–conjugated donkey anti-rabbit (GE Healthcare, Little Chalfont, UK), Supersignal chemiluminescent detection reagent (Pierce), and exposure to Biomax film (Kodak, Rochester, NY).
In vivo tumor-transduction analysis
To analyze the transduction profile of the AAV-NSE-GFP vector, the vector was infused intratumorally in tumor-bearing nude mice. For the small tumor model, we infused 1011 gc/animal at day 7 after implantation of 104 U87fluc-mCherry cells. Ten days later, mice were perfused with 4% paraformaldehyde. The brain was cryoprotected in 30% sucrose in phosphate-buffered saline, pH 7.4, before preparing 10-µm coronal sections in a cryostat. For the large tumor model, we infused 2 × 1010 gc/animal at day 7 after implantation of 105 U87flucmCherry- EGFRvIII cells. Seven days later, mice were perfused and the brain was treated as before. Vector transduction (GFP+ cells) and tumor cells (mCherry+) were visualized using a Nikon Eclipse TE2000-U fluorescence microscope. Images were captured with a Retiga EXi CCD digital camera (Qimaging, Surrey, Canada) and viewed with Metavue imaging software (version 6.2r4; Molecular Devices, Downingtown, PA).
Statistical analysis
Data presented provides the mean ± SD. For some figures, group comparisons were computed by an unpaired Student’s t- test using GraphPad PRISM software (version 5.0; San Diego, CA). For statistical analysis of group comparisons in Kaplan–Meier survival curves, the Wilcoxon Log-Rank test was performed using JMP version 7.0 software (SAS, Cary, NC).
Supplementary Material
Figure S1. IFN-β is expressed in primary neurons and in brain after transduction with AAV-CBA-hIFN-β vector.
Figure S2. Pretreatment of brain with AAV-CBA-IFN-β prevents U87 glioma growth in nude mice.
Figure S3. Pretreatment of brain with AAV-CBA-IFN-β prevents Gli36 glioma growth in nude mice.
Figure S4. Superior anti-tumor effect of brain-expressed IFN-β over tumor-expressed IFN-β.
Figure S5. The neuron-specific enolase (NSE) promoter is not efficiently utilized in U87 cells.
Figure S6. Histological analysis of brains from mice which had established tumors treated with AAV-NSE-hIFN-β.
ACKNOWLEDGMENTS
This work was supported by a Young Investigator Award from the Alliance for Cancer Gene Therapy (to M.S.E.), National Institutes of Health (NIH) CA69246 (to X.O.B.), and NIH 5 T32 CA073479-09 (to C.M.). We acknowledge Rakesh Jain and the Edwin L. Steele Laboratory at Massachusetts General Hospital (MGH) for support during this study. Also, we acknowledge the MGH Neuroscience Center Microscopy and Image Analysis Core (NIH grant P30NS045776) for the use of the Nikon scanner, and Ralph Weissleder, Director of the Center for Molecular Imaging Research, for the use of the bioluminescence imager, Katy Bercury and Bradley Hyman for kindly providing the primary murine neuronal cultures, and Jeffrey Hewett for technical advice on the immunoblot experiments. Finally, we thank Johan Skog, Bakhos Tannous, and Okay Saydam for critical reading of the manuscript.
REFERENCES
- 1.Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther. 2006;6:1087–1104. doi: 10.1586/14737140.6.7.1087. [DOI] [PubMed] [Google Scholar]
- 2.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 3.Lam PY, Breakefield XO. Potential of gene therapy for brain tumors. Hum Mol Genet. 2001;10:777–787. doi: 10.1093/hmg/10.7.777. [DOI] [PubMed] [Google Scholar]
- 4.Harding TC, Lalani AS, Roberts BN, Yendluri S, Luan B, Koprivnikar KE, et al. AAV serotype 8-mediated gene delivery of a soluble VEGF receptor to the CNS for the treatment of glioblastoma. Mol Ther. 2006;13:956–966. doi: 10.1016/j.ymthe.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Samoto K, Ehtesham M, Perng GC, Hashizume K, Wechsler SL, Nesburn AB, et al. A herpes simplex virus type 1 mutant with gamma 34.5 and LAT deletions effectively oncolyses human U87 glioblastomas in nude mice. Neurosurgery. 2002;50:599–605. doi: 10.1097/00006123-200203000-00031. discussion 605–606. [DOI] [PubMed] [Google Scholar]
- 6.Wang WJ, Tai CK, Kasahara N, Chen TC. Highly efficient and tumor-restricted gene transfer to malignant gliomas by replication-competent retroviral vectors. Hum Gene Ther. 2003;14:117–127. doi: 10.1089/104303403321070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowers G, He J, Schulz K, Olivarria G, Maneval D, Olson JJ. Efficacy of adenoviral p53 delivery with SCH58500 in the intracranial 9l and RG2 models. Front Biosci. 2003;8:a54–a61. doi: 10.2741/946. [DOI] [PubMed] [Google Scholar]
- 8.Thorsen F, Afione S, Huszthy PC, Tysnes BB, Svendsen A, Bjerkvig R, et al. Adeno-associated virus (AAV) serotypes 2, 4 and 5 display similar transduction profiles and penetrate solid tumor tissue in models of human glioma. J Gene Med. 2006;8:1131–1140. doi: 10.1002/jgm.939. [DOI] [PubMed] [Google Scholar]
- 9.Rueger MA, Winkeler A, Miletic H, Kaestle C, Richter R, Schneider G, et al. Variability in infectivity of primary cell cultures of human brain tumors with HSV-1 amplicon vectors. Gene Ther. 2005;12:588–596. doi: 10.1038/sj.gt.3302462. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK, Baxter LT. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988;48:7022–7032. [PubMed] [Google Scholar]
- 11.Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18:195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- 12.Mandel RJ, Manfredsson FP, Foust KD, Rising A, Reimsnider S, Nash K, et al. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Mol Ther. 2006;13:463–483. doi: 10.1016/j.ymthe.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther. 2006;13:517–527. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138:501–510. doi: 10.1016/j.neuroscience.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 15.Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther. 2008;16:89–96. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao G, Su J, Lu W, Zhang F, Zhao G, Marteralli D, et al. Adenovirus-mediated interferon-beta gene therapy suppresses growth and metastasis of human prostate cancer in nude mice. Cancer Gene Ther. 2001;8:497–505. doi: 10.1038/sj.cgt.7700333. [DOI] [PubMed] [Google Scholar]
- 18.Olson MV, Lee J, Zhang F, Wang A, Dong Z. Inducible nitric oxide synthase activity is essential for inhibition of prostatic tumor growth by interferon-beta gene therapy. Cancer Gene Ther. 2006;13:676–685. doi: 10.1038/sj.cgt.7700941. [DOI] [PubMed] [Google Scholar]
- 19.Kaynor C, Xin M, Wakefield J, Barsoum J, Qin XQ. Direct evidence that IFN-beta functions as a tumor-suppressor protein. J Interferon Cytokine Res. 2002;22:1089–1098. doi: 10.1089/10799900260442511. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Ciron C, Desmaris N, Colle MA, Raoul S, Joussemet B, Verot L, et al. Gene therapy of the brain in the dog model of Hurler’s syndrome. Ann Neurol. 2006;60:204–213. doi: 10.1002/ana.20870. [DOI] [PubMed] [Google Scholar]
- 23.Hadaczek P, Kohutnicka M, Krauze MT, Bringas J, Pivirotto P, Cunningham J, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 24.Vite CH, Passini MA, Haskins ME, Wolfe JH. Adeno-associated virus vector-mediated transduction in the cat brain. Gene Ther. 2003;10:1874–1881. doi: 10.1038/sj.gt.3302087. [DOI] [PubMed] [Google Scholar]
- 25.Hadaczek P, Mirek H, Berger MS, Bankiewicz K. Limited efficacy of gene transfer in herpes simplex virus-thymidine kinase/ganciclovir gene therapy for brain tumors. J Neurosurg. 2005;102:328–335. doi: 10.3171/jns.2005.102.2.0328. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida J, Mizuno M, Nakahara N, Colosi P. Antitumor effect of an adeno-associated virus vector containing the human interferon-beta gene on experimental intracranial human glioma. Jpn J Cancer Res. 2002;93:223–228. doi: 10.1111/j.1349-7006.2002.tb01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuno M, Yoshida J, Colosi P, Kurtzman G. Adeno-associated virus vector containing the herpes simplex virus thymidine kinase gene causes complete regression of intracerebrally implanted human gliomas in mice, in conjunction with ganciclovir administration. Jpn J Cancer Res. 1998;89:76–80. doi: 10.1111/j.1349-7006.1998.tb00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada H, Miyamura K, Itoh T, Hagiwara M, Wakabayashi T, Mizuno M, et al. Gene therapy against an experimental glioma using adeno-associated virus vectors. Gene Ther. 1996;3:957–964. [PubMed] [Google Scholar]
- 29.Harding TC, Dickinson PJ, Roberts BN, Yendluri S, Gonzalez-Edick M, Lecouteur RA, et al. Enhanced gene transfer efficiency in the murine striatum and an orthotopic glioblastoma tumor model, using AAV-7- and AAV-8-pseudotyped vectors. Hum Gene Ther. 2006;17:807–820. doi: 10.1089/hum.2006.17.807. [DOI] [PubMed] [Google Scholar]
- 30.Izawa JI, Sweeney P, Perrotte P, Kedar D, Dong Z, Slaton JW, et al. Inhibition of tumorigenicity and metastasis of human bladder cancer growing in athymic mice by interferon-beta gene therapy results partially from various antiangiogenic effects including endothelial cell apoptosis. Clin Cancer Res. 2002;8:1258–1270. [PubMed] [Google Scholar]
- 31.Indraccolo S, Pfeffer U, Minuzzo S, Esposito G, Roni V, Mandruzzato S, et al. Identification of genes selectively regulated by IFNs in endothelial cells. J Immunol. 2007;178:1122–1135. doi: 10.4049/jimmunol.178.2.1122. [DOI] [PubMed] [Google Scholar]
- 32.Derynck R, Remaut E, Saman E, Stanssens P, De Clercq E, Content J, et al. Expression of human fibroblast interferon gene in Escherichia coli. Nature. 1980;287:193–197. doi: 10.1038/287193a0. [DOI] [PubMed] [Google Scholar]
- 33.Mizuno M, Yoshida J. Effect of human interferon beta gene transfer upon human glioma, transplanted into nude mouse brain, involves induced natural killer cells. Cancer Immunol Immunother. 1998;47:227–232. doi: 10.1007/s002620050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida J, Mizuno M, Wakabayashi T. Interferon-beta gene therapy for cancer: basic research to clinical application. Cancer Sci. 2004;95:858–865. doi: 10.1111/j.1349-7006.2004.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yung WK, Prados M, Levin VA, Fetell MR, Bennett J, Mahaley MS, et al. Intravenous recombinant interferon beta in patients with recurrent malignant gliomas: a phase I/II study. J Clin Oncol. 1991;9:1945–1949. doi: 10.1200/JCO.1991.9.11.1945. [DOI] [PubMed] [Google Scholar]
- 36.Chiocca EA, Smith KM, McKinney B, Palmer CA, Rosenfeld S, Lillehei K, et al. A phase I trial of Ad.hIFN-beta gene therapy for glioma. Mol Ther. 2008;16:618–626. doi: 10.1038/sj.mt.6300396. [DOI] [PubMed] [Google Scholar]
- 37.Byrnes AP, MacLaren RE, Charlton HM. Immunological instability of persistent adenovirus vectors in the brain: peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination. J Neurosci. 1996;16:3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCown TJ. Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther. 2005;5:333–338. doi: 10.2174/1566523054064995. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham J, Oiwa Y, Nagy D, Podsakoff G, Colosi P, Bankiewicz KS. Distribution of AAV-TK following intracranial convection-enhanced delivery into rats. Cell Transplant. 2000;9:585–594. doi: 10.1177/096368970000900504. [DOI] [PubMed] [Google Scholar]
- 40.Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Streck CJ, Ng CY, Zhang Y, Zhou J, Nathwani AC, Davidoff AM. Interferon-mediated anti-angiogenic therapy for neuroblastoma. Cancer Lett. 2005;228:163–170. doi: 10.1016/j.canlet.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 42.Kashima T, Vinters HV, Campagnoni AT. Unexpected expression of intermediate filament protein genes in human oligodendroglioma cell lines. J Neuropathol Exp Neurol. 1995;54:23–31. doi: 10.1097/00005072-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods. 2004;122:131–139. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 44.Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah K, Tung CH, Breakefield XO, Weissleder R. In vivo imaging of S-TRAIL-mediated tumor regression and apoptosis. Mol Ther. 2005;11:926–931. doi: 10.1016/j.ymthe.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein RL, Meyer EM, Peel AL, Zolotukhin S, Meyers C, Muzyczka N, et al. Neuron-specific transduction in the rat septohippocampal or nigrostriatal pathway by recombinant adeno-associated virus vectors. Exp Neurol. 1998;150:183–194. doi: 10.1006/exnr.1997.6736. [DOI] [PubMed] [Google Scholar]
- 48.Shah K, Tang Y, Breakefield X, Weissleder R. Real-time imaging of TRAIL-induced apoptosis of glioma tumors in vivo. Oncogene. 2003;22:6865–6872. doi: 10.1038/sj.onc.1206748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. IFN-β is expressed in primary neurons and in brain after transduction with AAV-CBA-hIFN-β vector.
Figure S2. Pretreatment of brain with AAV-CBA-IFN-β prevents U87 glioma growth in nude mice.
Figure S3. Pretreatment of brain with AAV-CBA-IFN-β prevents Gli36 glioma growth in nude mice.
Figure S4. Superior anti-tumor effect of brain-expressed IFN-β over tumor-expressed IFN-β.
Figure S5. The neuron-specific enolase (NSE) promoter is not efficiently utilized in U87 cells.
Figure S6. Histological analysis of brains from mice which had established tumors treated with AAV-NSE-hIFN-β.