Abstract
Background and aims
D-dimer is a marker of active fibrinolysis. Understanding how age-related factors affect D-dimer levels may help the interpretation of high D-dimer levels in older individuals.
Methods
776 Baltimore Longitudinal Study on Aging (BLSA) participants (mean age 68.4±13.9 yrs) were divided into three groups according to baseline D-dimer levels >200 ng/mL; 100–200 ng/mL and <100 ng/mL.
Results
D-dimer level increased with age (p<0.0001). Using polychotomous logistic regression models, we found that age, cholesterol, triglycerides, creatinine, erythrocyte sedimentation rate, hemoglobin and body mass index were independently associated with D-dimer level.
Conclusions
Rising levels of D-dimer with age can be explained in part by the high prevalence of pro-inflammatory conditions and increasing burden of lipid abnormalities, anemia and obesity. These factors compromise the specificity of D-dimer levels as a diagnostic aid to thrombosis in older individuals.
Keywords: D-dimer, inflammation, obesity
INTRODUCTION
D-dimer is one of the most commonly used clinical assays for the detection of active coagulation and in vivo fibrin formation and lysis. The D-dimer assay is widely used in clinical practice to rule out clinically suspected deep venous thrombosis and pulmonary embolism. In fact, a number of studies have demonstrated that normal D-dimer levels have a high negative predictive value for these medical conditions (1). The predictive value of D-dimer for thrombosis has been studied mostly in clinical populations or in patients who were at high risk of thrombosis based on clinical characteristics. The frequency of abnormally elevated D-dimer levels is higher in older individuals (2–5) even in the absence of detectable thrombosis; therefore, the specificity of elevated D-dimer in thrombosis declines with age. A better understanding of factors that affect D-dimer levels in old age may improve the clinical interpretation of elevated levels. In addition, understanding factors that affect D-dimer levels may be important to start developing age-specific values of D-dimer above which more in depth testing should be performed.
We hypothesize that elevated D-dimer levels in older people may be due to an increased rate of clot formation and fibrinolysis possibly activated by a systemic pro-inflammatory state, lipid abnormalities and obesity.
SUBJECTS AND METHODS
Study population
The Baltimore Longitudinal Study on Aging (BLSA) is a longitudinal rolling cohort study of the National Institute on Aging, initiated more than 49 years ago. A general description of the BLSA trial has been previously reported (6). Healthy volunteers aged 20 and older are enrolled in the study and participate in follow-up assessment visits approximately every 2 years; participants older than 80 years are evaluated yearly. Currently, the study population has about 1400 active participants. An independent institutional review board approved the BLSA study protocol, and participants provided informed consent. There were 776 participants, 388 female and 388 male, who had D-dimer values available for this analysis.
Methods
Blood samples were collected in the morning after the subjects had fasted for 12h. Laboratory assays performed at the time of participant visit at the Clinical Research Unit of the National Institute on Aging, included white blood count, MCV, LDH, reticulocyte, total cholesterol, total triglycerides, albumin, erythrocyte sedimentation rate (ESR), and other selected serum chemistries (creatinine, hemoglobin, iron, ferritin, folate levels, and homocysteine) which were all assayed by standard clinical methods. D-dimer was measured in our clinical laboratory using a fully automated, benchtop coagulation workstation with STA-Compact (Diagnostica Stago, Inc.) and levels are reported in nanograms per milliliter. Anthropometric measurements, including weight, height, and blood pressure (BP), were recorded at the time of the visit, body mass index was calculated as weight (kilograms)/(height [meters])2 using objectively assessed measures. Cigarette smoking history was assessed by self-report and coded as ever smoked vs never smoked. Physical activity was assessed by self-report of average time spent per week in 97 activities (7). BLSA participants completed a comprehensive health survey questionnaire at each visit, and all newly reported diagnoses were adjudicated by a nurse practitioner based on information from multiple sources including medical records and communication with the primary care physician.
Statistical analysis
Statistical analyses were performed using the SAS statistical package 9.1 (SAS Institute, Inc, Cary, NC). D-dimer levels were divided into three groups: group 1= D-dimer >200 ng/mL, group 2= 100–200 ng/mL and group 3= <100 ng/mL. In our laboratory, values of D-dimer above 200 ng/mL are considered abnormal. The intermediate group was introduced in the analysis to test the hypothesis that inflammation and other age related factors may affect D-dimer levels even within the usual range. Different variables describing the D-dimer group were formally tested with analysis of variance for continuous variables and chi square test for categorical variables. Factors aside from age and sex that were independently correlated with D-dimer with a p-value <0.3 were entered into a polychotomous logistic regression model (CATMOD) predicting D-dimer group.
RESULTS
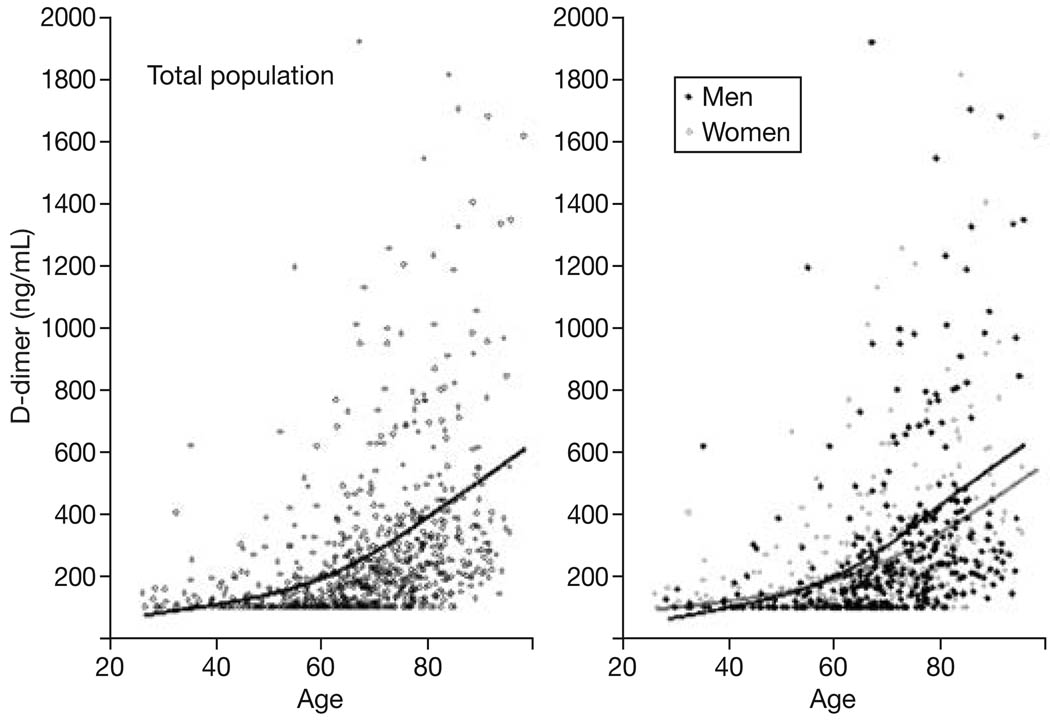
A total of 776 consecutive participants (mean age 68.4±13.9 yrs), 388 female and 388 male, with D-dimer measurements were used in these analyses. The characteristics of study participants according to D-dimer group are shown in Table 1. The proportion of women was similar in the three study groups (group 1= D-dimer >200 ng/mL, group 2= 100–200 ng/mL and group 3= <100 ng/mL) with 50.2%, 54.5% and 46.7% respectively (Table 1). D-dimer levels increased with age (p<0.0001) and were slightly higher in men than in women (Fig. 1). After adjusting for age and sex, hemoglobin, total cholesterol, BMI, total triglycerides, albumin, creatinine, homocysteine, LDH, MCV, iron, ferritin, folate, ESR and smoking were associated with D-dimer levels with a p-value <0.3 (Table 1). These variables were included as potential predictors of D-dimer group in a polychotomous logistic regression model. After removal of the variables that were not significantly predictive of D-dimer group, age, total cholesterol, total triglycerides, creatinine, ESR, hemoglobin and BMI were independently associated with D-dimer levels (Table 2). Interestingly, age and ESR discriminated the two groups with higher D-dimer from the “normal D-dimer group” while cholesterol, triglycerides, creatinine, hemoglobin and BMI only discriminated participants with D-dimer >200 ng/mL but not those with D-dimer 100–200 ng/mL from the reference group with D-dimer <100 ng/ml. None of the other variables included in the initial model were associated with D-dimer level.
Table 1.
Characteristics of study participants according to D-dimer group.
| >200 ng/mL | 100–200 ng/mL | <100 ng/mL | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Mean | n | Mean | n | Mean | n | p1 | p2 |
| Female, % | 46.70% | (359) | 54.50% | (244) | 50.20% | (173) | 0.18 | |
| Age | 75.2 ± 0.59 | (359) | 64.9 ± 0.85 | (244) | 59.2 ± 0.92 | (173) | <0.0001 | |
| Hemoglobin | 13.3 ± 0.08 | (356) | 13.8 ± 0.09 | (243) | 14.1 ± 0.1 | (171) | <0.0001 | <0.0001 |
| Cholesterol | 191.23 ± 2.13 | (359) | 190.9 ± 2.24 | (244) | 189.9 ± 2.76 | (173) | 0.92 | 0.16 |
| BMI | 27.1 ± 0.26 | (354) | 27.7 ± 0.32 | (241) | 26.8 ± 0.35 | (170) | 0.10 | <0.01 |
| Triglycerides | 98.5 ± 2.64 | (359) | 113.3 ± 4.18 | (244) | 108.9 ± 5.07 | (173) | <0.007 | <0.02 |
| Albumin | 4±0.02 | (359) | 4.1 ± 0.02 | (244) | 4.2 ± 0.02 | (173) | <0.0001 | <0.004 |
| Creatinine | 1.1 ± 0.02 | (359) | 0.98 ± 0.01 | (244) | 0.96 ± 0.015 | (173) | <0.0001 | <0.04 |
| Homocysteine | 11.5 ± 0.34 | (355) | 9.4 ± 0.16 | (243) | 9.5 ± 0.32 | (172) | <0.0001 | <0.01 |
| LDH | 498.9 ± 6.87 | (359) | 479 ± 7.13 | (244) | 456.5 ± 8.18 | (173) | <0.0006 | 0.28 |
| MCV | 90.9 ± 0.29 | (356) | 89.4 ± 0.36 | (243) | 89.8 ± 0.39 | (171) | <0.001 | 0.24 |
| Iron | 86.3 ± 1.72 | (359) | 89.7 ± 2.09 | (244) | 96.4 ± 2.41 | (173) | <0.004 | 0.21 |
| Ferritin | 106.7 ± 4.92 | (359) | 115.9 ± 7.63 | (243) | 106.8 ± 7.45 | (173) | 0.51 | 0.30 |
| Folate | 24.3 ± 0.76 | (355) | 23.7 ± 0.81 | (240) | 23.5 ± 1.1 | (172) | 0.78 | 0.24 |
| ESR | 16.1 ± 0.76 | (350) | 12 ± 0.72 | (239) | 8.1 ± 0.57 | (172) | <0.0001 | <0.0001 |
| Smoking | <0.03 | |||||||
| Current | 2.10% | (359) | 1.70% | (244) | 1.20% | (173) | ||
| Former | 22% | 13% | 7% | |||||
| Never | 22.20% | 16.50% | 13.80% | |||||
Values represent % (n) or mean ± SD (n), where n is the total number of subjects in the respective categories.
p-values from basic model;
p-values from analysis of covariance adjusted for age and sex.
Note that the distribution of physical activity, creatine kinase, white blood cell, reticulocytes, myocardial infarction, diabetes, cancer, angina, stroke, transitory ischemic attack, atrial fibrilation, congestive heart failure, chronic renal insufficiency, hypertension, rheumatic arthritis and osteoarthritis, were substantially similar between groups (adjusted p-value >0.3) and therefore they are not represented in this table.
Fig. 1.
Relationship between age and D-dimer levels in the whole study population and separately in men and women (Baltimore Longitudinal Study on Aging, n = 776).
Table 2.
Factors associated with D-dimer group levels, results are from a polychotomous logistic regression model. Parsimonious set of predicting variables was selected by backward selection.
| <100 vs 100–200 ng/mL | <100 vs >200 ng/mL | |||
|---|---|---|---|---|
| Effect | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age (yrs) | 1.03 (1.01–1.05) | <0.0001 | 1.11 (1.09–1.13) | <0.0001 |
| Cholesterol (mg/dL) | 1.00 (1.00–1.01) | 0.53 | 1.01 (1.00–1.02) | <0.01 |
| Triglyceride (mg/dL) | 1.00 (1.00–1.00) | 0.78 | 0.99 (0.99–1.00) | <0.01 |
| Creatinine (mg/dL) | 1.33 (0.47–3.71) | 0.59 | 3.29 (1.17–9.27) | <0.05 |
| ESR | 1.04 (1.01–1.07) | <0.05 | 1.05 (1.02–1.08) | <0.001 |
| Hemoglobin (mg/dL) | 0.90 (0.75–1.07) | 0.22 | 0.82 (0.68–0.98) | <0.05 |
| BMI (kg/m2) | 1.04 (0.99–1.09) | 0.11 | 1.06 (1.01–1.11) | <0.05 |
From polychotomous logistic regression model. Parsimonious model based on backward selection method, after removal of variables not associated with D-dimer levels, such as physical activity, albumin, creatine kinase, white blood cell, homocysteine, lactate dehydrogenase (LDH), mean corpuscular volume (MCV), iron, ferritin, folate, reticulocytes, myocardial infarction, diabetes, cancer, angina, stroke, transitory ischemic attack, atrial fibrillation, congestive heart failure, chronic renal insufficiency, hypertension, rheumatic arthritis, osteoarthritis and smoking.
DISCUSSION
Using data from 776 subjects in the BLSA, we found that age and ESR were statistically different between individuals with D-dimer <100 ng/mL and those with D-dimer 100–200 ng/mL, and the difference was even greater between participants with D-dimer <100 ng/mL and those with D-dimer >200 ng/mL. Total cholesterol, total triglycerides, creatinine, hemoglobin and BMI only discriminated participants with D-dimer >200 ng/mL but not those with D-dimer 100–200 ng/mL from the reference group with D-dimer <100 ng/mL. These findings suggest that the current threshold of 200 ng/mL established in our laboratory as a cut-off that divides normal from abnormal, is too high to capture the effect of inflammation on D-dimer levels. In fact, some individuals in whom inflammation is already causing hypercoagulability, as expressed by high D-dimer levels, will have values below 200 ng/mL. Since other studies have used cut-off thresholds that are even higher than the threshold used in our study, the amount of misclassification is probably even higher (2, 8–10). Consistent with results from previous studies, we found that the level of D-dimer as well as the prevalence of elevated D-dimer increases with age (2, 4, 10, 11). The explanation for the increasing levels of D-dimer in the elderly is uncertain. An intriguing hypothesis is that the rising levels of D-dimer with aging is due to a mild pro-inflammatory state and increasing levels of co-morbidities. Accordingly, ESR, a marker of systemic inflammation, was independently associated with elevated D-dimer levels (10). An elevated ESR is a nonspecific but reliable marker of infection and inflammation (12, 13). D-dimer increment could be viewed as an acute phase reactant that responds to all underlying inflammatory processes. However, unlike other acute phase reactants, D-dimer is only produced after clotting has taken place. It is not synthesized by tissues as a consequence of inflammation. Thus, it may be hypothesized that chronic inflammation is associated with an upregulation of coagulation and reactive compensatory fibrinolysis which leads to excess production of D-dimer. Regardless of mechanism, the age-associated increase in D-dimer levels makes D-dimer assay virtually useless as a screening test for venous thromboembolic disease in older persons (2, 14–16). Interestingly, elevated D-dimer was independently associated with total cholesterol (p<0.01), total triglyceride (p<0.01) and BMI (p<0.01) (Table 2), which are components of the metabolic syndrome. This is consistent with the notion that D-dimer is a cardiovascular disease risk factor (17, 18). Surprisingly, low hemoglobin was also independently associated with elevated D-dimer levels. The meaning of this finding is uncertain and will require further investigation.
Our study has limitations. Additional important markers of inflammation such as IL-6 and C-reactive protein levels were not available at the time of this analysis. Ideally, future analyses should include a broad panel of inflammatory markers to verify the hypothesis that the age-associated pro-inflammatory state is also associated with up-regulation of coagulation and fibrinolysis. It is possible that increased fibrinolysis is a component of certain types of inflammation but not others.
ACKNOWLEDGEMENTS
This research was supported entirely by the Intramural Research Program, National Institute on Aging (NIA), National Institutes of Health, USA.
REFERENCES
- 1.Michiels JJ, Palareti G, de Moerloose P, editors. Semin Vasc Med. Vol. 5. 2005. Fibrin D-dimer testing for venous and arterial thrombotic disease; pp. 311–334. [DOI] [PubMed] [Google Scholar]
- 2.Harper PL, Theakston E, Ahmed J, Ockelford P. D-dimer concentration increases with age reducing the clinical value of the D-dimer assay in the elderly. Intern Med J. 2007;37:607–613. doi: 10.1111/j.1445-5994.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee AJ, Fowkes FG, Lowe GD, Rumley A. Determinants of fibrin D-dimer in the Edinburgh Artery Study. Arterioscler Thromb Vasc Biol. 1995;15:1094–1097. doi: 10.1161/01.atv.15.8.1094. [DOI] [PubMed] [Google Scholar]
- 4.Al-Zahrani H, Lowe GD, Fowkes FG, Douglas JT, et al. Increased fibrin turnover in peripheral arterial disease: comparison with a population study. Clin Hemorheol. 1992;12:867–872. [Google Scholar]
- 5.Giansante C, Fiotti N, Cattin L, Da Col PG, Calabrese S. Fibrinogen. D-dimer and thrombin-antithrombin complexes in a random population sample: relationships with other cardiovascular risk factors. Thromb Haemost. 1994;71:581–586. [PubMed] [Google Scholar]
- 6.Shock NW, Gruelich RC, Andres RA, et al. The Baltimore Longitudinal Study of Aging. Washington, DC: US Government Printing Office; 1984. [Google Scholar]
- 7.Talbot LA, Metter EJ, Fleg JL. Leisure-time physical activities and their relationship to cardiorespiratory fitness in healthy men and women 18–95 years old. Med Sci Sports Exerc. 2000;32:417–425. doi: 10.1097/00005768-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Mavromatis BH, Kessler CM. D-Dimer testing: the role of the clinical laboratory in the diagnosis of pulmonary embolism. J Clin Pathol. 2001;54:664–668. doi: 10.1136/jcp.54.9.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takefumi M, Hiroko K, Kazuomi K, Shunji S. Fibrin D-Dimer in thrombogenic disorders. Semin Thromb Hemost. 2000;26(1) doi: 10.1055/s-2000-9811. [DOI] [PubMed] [Google Scholar]
- 10.Swartz JE, Jacobson BF, Connor MD, Bernstein PL, Fritz VU. Erythrocyte sedimentation rate as a marker of inflammation and ongoing coagulation in stroke and transient ischaemic attack. S Afr Med J. 2005;95:607–612. [PubMed] [Google Scholar]
- 11.Reid D. MD Thesis. University of Glasgow; 1991. The clinical role of fibrinogen and fibrin in peripheral arterial disease. [Google Scholar]
- 12.Dacie JV, Lewis SM. Miscellaneous tests. In: Dacie JV, Lewis SM, editors. Practical Haematology. London Churchill Livingstone; 1995. pp. 559–574. [Google Scholar]
- 13.Saadeh C. The erythrocytes sedimentation rate: old and new clinical applications. South Med J. 1998;91:220–225. [PubMed] [Google Scholar]
- 14.Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med. 2000;109:357–361. doi: 10.1016/s0002-9343(00)00493-9. [DOI] [PubMed] [Google Scholar]
- 15.Righini M, Le Gal G, Perrier A, Bounameaux H. The challenge of diagnosing pulmonary embolism in elderly patients: influence of age on commonly used diagnostic tests strategies. J Am Geriatr Soc. 2005;53:1039–1045. doi: 10.1111/j.1532-5415.2005.53309.x. [DOI] [PubMed] [Google Scholar]
- 16.Shutgens RE, Haas FJ, Biesma DH. Reduced efficacy of clinical probability score and D-dimer assay in elderly subjects suspected of having deep vein thrombosis. Br J Haematol. 2005;129:653–657. doi: 10.1111/j.1365-2141.2005.05515.x. [DOI] [PubMed] [Google Scholar]
- 17.Lowe GD. A cardiovascular risk factor. 2nd ed. Mannheim: Boehringer Mannheim; 1997. Fibrinogen. [Google Scholar]
- 18.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993;118:956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]