Abstract
Increased nicotine deprivation and impulsivity have been associated with relapse but the degree to which they together influence cognitive processing has not been explored. We examined the effects of increasing levels of nicotine deprivation on of cognitive processing, and assessed the relationship of trait impulsivity with these effects in daily smokers (n = 30). Using a within-subject design with three deprivation conditions (nondeprived, 5-hr, 17-hr), volunteers completed the Conners’ Continuous Performance Task-II and the Cued Go/No-Go Task. Trait impulsivity was assessed at intake with the Barratt Impulsiveness Scale (Patton et al., 1995). Mixed-model regression analyses revealed deprivation slowed reaction time, increased errors, increased variability in responding, and increased failures of inhibitory control. Performance at 17 hours of deprivation was most likely to be affected. Significant deprivation and impulsivity interactions indicated impulsiveness was negatively correlated with deprivation-associated performance decrements. Less impulsive smokers were more affected by deprivation, demonstrating greater impairment. Research is needed to understand mechanisms by which impulsivity confers greater risk for relapse. Our results suggest deprivation may not increase relapse risk among impulsive smokers by increasing impairment of cognitive processing.
Keywords: nicotine deprivation, impulsivity, smoking cessation, cognitive processing
Self-control or impulsivity mechanisms have been identified in recent models of addiction, including nicotine addiction, as being at the core of the compulsive nature of addiction (Baker et al., 2004; Baumeister, 2003; Brady & Sinha, 2005; Everit & Robinson, 2005; Goldstein & Volkow, 2002; Jentsch & Taylor, 1999; Koob et al., 2004; Volkow, 2004). Historically conceptualized as a personality trait, self-control or impulsivity includes various facets such as impulse control, response inhibition, and behavioral regulation. When functioning properly, the exertion of self-control can serve to regulate immediate desires and delay gratification, with the goal of maximizing beneficial long-term outcomes (Hayes, Gifford, & Ruckstuhl, 1996). Along those lines, trait measures of self-control or impulsivity have been found to be associated with initiation (Wills & Stoolmiller, 2002), maintenance (Peluso et al., 1999; Piquero et al., 2002), and relapse to drug use (Doran, Spring, McChargue, Pergadia, & Richmond, 2004). For example, individuals who rated higher on trait impulsivity were likely to relapse to cigarette smoking faster than those individuals who rated lower (Doran et al., 2004).
Although addicted individuals are more likely to be impulsive, self-control problems are often exacerbated in high challenge states (Kelley, Schiltz, & Landry, 2005; Sinha, 2005; Volkow & Li, 2005). One example of a high challenge state is nicotine deprivation. Nicotine deprivation has been shown to produce decrements in self-control as measured by delay discounting, which is a measure of how much an individual discounts a delayed reward as a function of the length of delay to the reward (Field, Santacangelo, Sumnall, Goudie, & Cole, 2006; Mitchell, 2004). Mitchell observed that 24 hours of nicotine deprivation resulted in increased discounting for cigarette-related choices. Field and colleagues (2006) observed that periods of at least 13 hours of nicotine deprivation resulted in impulsive choices for monetary and cigarette-related rewards. Together these two studies indicate that nicotine deprivation can increase impulsive decision making. Observations that the challenge state produced by nicotine deprivation may produce additional decrements in self-control are consistent with what is seen clinically, as withdrawal is often associated with relapse. Typically, relapse episodes happen soon after the start of the quit attempt, while the smoker is experiencing acute withdrawal symptoms (for a review, see Brown, LeJuez, Kahler, Strong, & Zvolensky, 2005).
The high challenge state of nicotine deprivation has well-known detrimental effects on cognitive performance. Nicotine deprivation attributed to smoking abstinence has been associated with impaired performance on a wide variety of tasks, including those assessing attention (e.g., Bell, Taylor, Singleton, Henningfield, & Heishman, 1999; Harakas & Foulds, 2002; Myers, Taylor, Moolchan, & Heishman, 2008;), vigilance (e.g., Parrott, Garnham, Wesnes, & Pincock, 1996), working memory (e.g., Myers et al., 2008; Xu et al., 2006), cognitive processing and response accuracy (e.g., Snyder, Davis, & Henningfield, 1989) and overall cognitive performance (e.g., al’Absi, Amunrud, & Wittmers, 2002). Although the effects of nicotine deprivation on cognition are well characterized, the relationship between deprivation-induced cognitive impairment and trait impulsivity is largely unknown. As nicotine deprivation increases, individuals with varying levels of trait impulsivity could be differentially affected. Moreover, various facets of impulsivity could have different relationships with nicotine deprivation-induced cognitive deficits. These cognitive deficits, in turn, could have a role as a motivating influence in smoking relapse and could in part explain why more impulsive individuals relapse faster than those who are less impulsive (e.g., Doran et al., 2004).
The purpose of the present investigation was to examine the influences of increasing levels of nicotine deprivation and of trait impulsivity on cognitive processing in daily smokers. To examine these effects, trait impulsivity was measured at study intake with the Barratt Impulsiveness Scale (BSI) (Patton, Stanford, & Barratt, 1995), a well known measure of trait impulsivity. The study used a within-subject design in daily smokers who were nondeprived, or nicotine deprived for 5 or 17 hours. The 5-hr nicotine deprivation condition represented acute deprivation, targeting increases in tobacco craving (Drobes & Tiffany, 1997). The 17-hr deprivation condition represented a period of more prolonged deprivation, targeting increases in craving, as well as additional tobacco withdrawal symptoms (Hatsukami, Hughes, Pickens, & Svikis, 1984). Main effects and interactions of nicotine deprivation and trait impulsivity were assessed on measures of cognitive performance, which included the Conners’ Continuous Performance Task (CPT-II; Conners, 2000) and the Cued Go/No-Go Task (CGNG; Marczinski & Fillmore, 2003). Cognitive testing occurred within each level of nicotine deprivation. The CPT-II task is designed to assess sustained attention, concentration, response inhibition, and impulsivity. Nicotine deprivation has been found to produce performance decrements in CPT-II outcomes, which were ameliorated with smoking resumption (e.g., Sacco et al., 2005). The CGNG task is sensitive to drug effects and assesses behavioral activation and inhibition. Prior research has indicated that overnight nicotine deprivation resulted in impaired performance on the task (e.g., McClernon et al., 2008).
Given past research indicating the general finding that nicotine deprivation impairs cognitive processing (e.g., Bell et al., 1999; Synder et al., 1989), nicotine deprivation-induced changes in task performance were predicted. Associations between trait impulsivity and performance at each level of nicotine deprivation were examined using mixed model regression analyses. We explored the degree to which nicotine deprivation-related changes in cognitive processing were associated with trait impulsivity. More impulsive individuals might experience differential changes after nicotine deprivation. An understanding of the relationship between trait impulsivity and changes in cognitive performance after nicotine deprivation could in part explain differences observed in rates of smoking cessation among individuals of varying levels of impulsivity.
Method
Participants
Participants were eligible to enroll in the study if they were 18 to 55 years of age and smoked between 10 and 30 cigarettes per day for at least the past year. Participants were excluded if they were using illicit drugs (cocaine, opiate, benzodiazepine, barbiturate, or amphetamine), currently seeking treatment for smoking behavior, presented with current severe psychiatric disorders, or had medical conditions that would contraindicate smoking. Thirty-five participants were scheduled to start the laboratory sessions. Three were dismissed before the first laboratory session, for illicit drug use. Two were dismissed before the second laboratory session, for illicit drug use. A total of 30 participants completed the study (15 females, 15 males). The average age was 31.93 (SD = 10.29), and 47% of participants were African American, 33% were White, and none were Hispanic. Fifty-three percent of participants had completed up to a high-school diploma, 47% had at least some college education, and 90% were single. Participants smoked on average 16.67 (SD = 6.38) cigarettes per day, had baseline carbon monoxide readings of 25.03 ppm (SD = 13.32), and average Fagerström Nicotine Dependence Scores (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) of 5.13 (SD = 1.70; possible range 1–10) indicating moderate nicotine dependence.
Procedure
Intake session
The study was approved by the Yale University Human Investigation Committee and written informed consent was obtained at the start of the intake session. The Structured Clinical Interview for DSM–IV (First, Spitzer, Gibbon, & Williams, 1997) was used to exclude individuals who met diagnostic criteria for substance abuse or dependence (other than nicotine dependence or alcohol abuse) or other Axis I disorders. The Timeline Followback (Sobell & Sobell, 1993) was used to assess past 30-day smoking behavior. Participants completed the BIS (Patton et al., 1995). Additionally, participants provided demographic and smoking history information. Breath carbon monoxide levels were assessed using a CO-meter (MCO2 Monitor, MicroDirect, Auburn, ME) and negative breath alcohol levels were confirmed (Alco-Sensor III, Intoximeter, St. Louis, MO). The absence of recent cocaine, opiate, benzodiazepine, barbiturate, or amphetamine was determined by urine drug test (JANT Pharmaceuticals, Encino, CA).
Laboratory sessions
Each participant completed three 6.5 hour laboratory sessions (nondeprived, 5 hr, 17 hr) that took place at the Yale Center for Clinical Investigation. Laboratory sessions started at 9:00 a.m. Before the cognitive testing, participants relaxed in a comfortable room equipped with TV, movies, and reading materials. Lunch was served at 12:15 p.m.
Nicotine deprivation conditions
For the 17-hr deprivation condition, participants were instructed to have their last cigarette at 10:00 p.m. the prior evening. Compliance was initially biochemically confirmed with carbon monoxide readings (less than 50% of their CO level at intake) and later with serum nicotine levels (less than 2 ng/ml). Volunteers who failed to comply with instructions were rescheduled. For the 5-hr deprivation condition, participants were able to smoke as they normally would before the laboratory session and had their last cigarette at 10:00 a.m. Participants in the nondeprived condition were able to smoke whenever they wished during the laboratory session, and all smoked a final cigarette at 3:00 p.m. The order of deprivation sessions was counterbalanced.
Task performance
During the morning of the first laboratory session, participants completed practice sessions on the CPT-II and CGNG tasks. For each of the three laboratory sessions, at 3:05 p.m. participants completed the CPT-II and CGNG tasks. The order of administration did not vary across sessions.
Instruments
BIS-11
This 30-item questionnaire was designed to assess different aspects of trait impulsivity such as attentional, motor, and nonplanning impulsiveness (Patton, Stanford, & Barratt, 1995). Participants indicated the degree to which they agreed with provided statements on a 4-point scale (1 = Rarely/Never; 4 = Almost Always). Scores range from 30 (low impulsivity) to 120 (high impulsivity). A sample statement is, “I do things without thinking.” Total scores and second order subscale scores (Attentional Impulsiveness, Motor Impulsiveness, and Nonplanning Impulsiveness) were calculated.
CPT-II
The CPT-II is a computerized assessment tool originally designed to aid in the identification of attention problems (Conners, 2000). The CPT-II has been used in other research to measure the effects of cigarette smoking, alcohol, and other drugs (e.g., Sacco et al., 2005). Over the course of a 14-min assessment, the participant is instructed to respond as quickly as possible to target stimuli (all letters but “X”) and to refrain from responding to a more rarely occurring nontarget stimulus (“X”). The CPT-II provides the following outcome measures of interest in the present study; rates of omission (failures to respond to target) and commission errors (responses to nontarget), hit reaction time (reaction time in milliseconds to target), and hit reaction time standard error (standard error of reaction time to target; a measure of variability in performance). It has been used for repeated testing with virtually no practice effects (Conners, 2000).
CGNG test
The CGNG is a computerized task that assesses behavioral activation and inhibitory control by measuring participants’ ability to inhibit prepotent responses (Marczinski & Fillmore, 2003). It has been used in past research to measure to effects of nicotine deprivation, alcohol, cocaine, and d-amphetamine (e.g., Fill-more, Marczinski, & Bowman, 2005; Fillmore, Rush, & Hays, 2006; Fillmore, Rush, & Marczinski, 2003; Marczinski & Fillmore, 2003; McClernon et al., 2008). Participants completed 250 trials, which took approximately 15 minutes. A trial involved the following sequence of events: presentation of a fixation point (+) for 800 milliseconds; a blank, white screen for 500 milliseconds; a cue (a white rectangle) displayed for one of five. stimulus onset asynchronies (SOAs: 100, 200, 300, 400, and 500 milliseconds); a go or no-go target (green or blue rectangle) that remained visible until the participant made a response or 1,000 milliseconds had elapsed; and an intertrial interval of 700 milliseconds. Participants were instructed to press a key (/) for green rectangles (go target) and to inhibit pressing a key for blue rectangles (no-go target). The cue was a white rectangle outlined in black. Horizontal rectangles cued an upcoming go target and vertical rectangles cued an upcoming no-go target. Volunteers were correctly cued about the upcoming target (go) or no-go target (no-go) on 80% of trials. Outcome measures of interest were reaction time to go targets after go and no-go cues and accuracy in responding to go targets following go and no-go cues.
Statistical Analysis
Performance outcome measures on the CPT-II and CGNG served as markers of cognitive functioning. The primary measures of interest on the CPT-II were reaction time, reaction time standard error, omission errors, and commission errors. The primary measures of interest on the CGNG were reaction time to go targets following the go and no go cues, and accuracy in responding to the go target following the go and no go cues. Mixed model regression analyses, using a compound symmetry covariance structure, were used to examine the main and interactive effects of impulsivity and deprivation for each dependent variable. Deprivation was a within-subject variable with three levels (nondeprived, 5 hours, 17 hours). The three deprivation conditions were treated as a nominal level variable with the nondeprived condition as the reference group.
Results
Parameter estimates from the mixed model regression analyses using the BIS total score are presented in Table 1. These fixed effects parameters can be interpreted as regression coefficients. Summary statements regarding those analyses are reported below.
Table 1.
Parameter Estimates From Mixed Model Regression Analyses of Nicotine Deprivation at 5 and 17 Hours and Trait Impulsivity (Total Score From Barratt Impulsiveness Scale) on Performance of the Cued Go/No-Go Task (CGNG) and the Conners’ Continuous Performance Task-II (CPT-II)
| 5 hours nicotine deprivation vs. nondeprived referent group |
17 hours nicotine deprivation vs. nondeprived referent group |
|||
|---|---|---|---|---|
| DEP Estimate, t (df)*, p | DEP × IMP Estimate, t (df), p | DEP Estimate, t (df), p | DEP × IMP Estimate, t (df), p | |
| CGNG | ||||
| Reaction time go cue | 95.32, 3.78 (53.51), <.001 | −1.51, −3.66 (53.47), .001 | 109.58, 4.34 (53.51), <.001 | −1.74, −4.22 (53.46), <.001 |
| Reaction time no-go cue | 98.29, 3.20 (53.54), .002 | −1.55, −3.08 (53.51), .029 | 73.55, 2.40 (53.54), .02 | −1.13, −2.24 (53.49), .031 |
| Accuracy go cue | n.s. | n.s. | n.s. | n.s. |
| Accuracy no-go cue | n.s. | n.s. | n.s. | n.s. |
| CPT-II | ||||
| Omissions | n.s. | n.s. | 13.63, 3.82 (51.39), <.001 | −0.20, −3.47 (51.39), .001 |
| Commissions | n.s. | n.s. | n.s. | n.s. |
| Reaction time | n.s. | n.s. | 111.94, 2.57 (51.07), .013 | n.s. |
| Reaction time standard error | n.s. | n.s. | 10.49, 3.14 (51.19), .003 | −0.14, −2.60 (51.19), .012 |
Note. DEP is main effect of deprivation; DEP × IMP is interaction of deprivation and impulsivity; no main effects of impulsivity were demonstrated.
Estimated degrees of freedom are reported.
Nicotine Deprivation
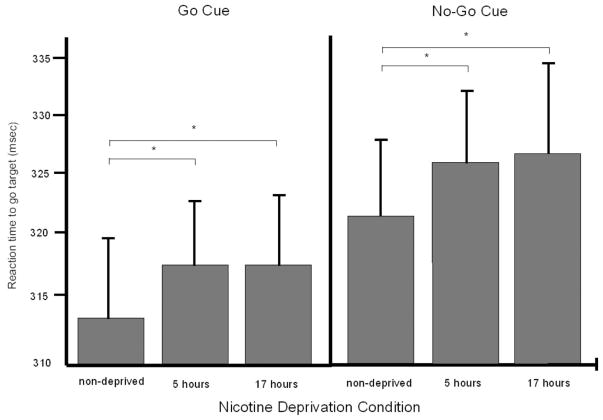
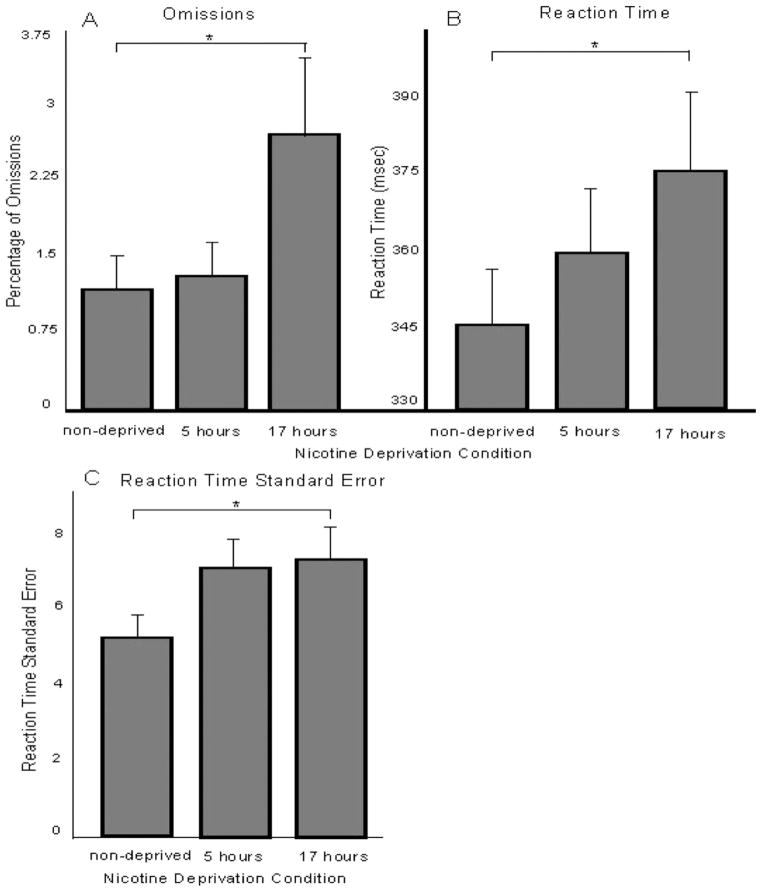
Figure 1 presents mean reaction time values to go targets following go cues (left panel) and no-go cues (right panel) in each deprivation condition. Figure 2 presents mean omission errors (Panel A), reaction time (Panel B), and reaction time standard error (Panel C) in each deprivation condition. Significant main effects of deprivation, as listed in Table 1, are depicted in the figures. Five hours of nicotine deprivation, compared to the nondeprived condition, significantly increased reaction time to the go and no-go cues in the CGNG (see Table 1).
Figure 1.
Mean task performance (+SE) outcome measures of the Cued Co/No-go Task (CGNG) across nicotine deprivation conditions. The left panel presents mean reaction times to the go target following the go cue and the right panel presents mean reaction times to the go target following the no-go cue. Asterisks denote ps < .05, from the mixed model findings (listed in Table 1), for within-subject comparisons.
Figure 2.
Mean task performance (+SE) outcome measures of the Conners’ Continuous Performance Task (CPT-II) across nicotine deprivation conditions. Panel A presents mean omissions, panel B presents mean reaction time, and panel C presents mean reaction time standard error. Asterisks denote ps < .05, from the mixed model findings (listed in Table 1), for within-subject comparisons.
Seventeen hours of nicotine deprivation, compared to the nondeprived condition, negatively affected performance on each task (see Table 1). On the CGNG, reaction time to the go cues and to the no-go cues increased significantly. On the CPT-II, omissions, reaction time, and reaction time standard error increased significantly.
Trait Impulsivity
No significant main effects of trait impulsivity were observed on performance of either task.
Interactions of Nicotine Deprivation and Trait Impulsivity
A significant interaction of nicotine deprivation at 5 hours and trait impulsivity was observed on reaction time to the go and no-cues on the CGNG task (see Table 1). The relationship between deprivation and impulsivity was negative in relation to the nondeprived referent group. No interactions were observed at this deprivation window for performance on the CPT-II.
Significant interactions of nicotine deprivation at 17 hours and trait impulsivity were observed on dependent measures of both tasks (see Table 1). On the CGNG, significant interactions was observed on reaction time to the go and no-go cues. On the CPT-II, significant interactions were observed on omissions and reaction time standard error. On both tasks, the relationship between deprivation and impulsivity were negative relative to the nondeprived referent group, suggesting that more impulsive individuals were less affected by nicotine deprivation. Analyses were repeated with the BIS second-order subscales, with each of the subscales replicating the same findings produced by the total score.
Second, to understand the nature of the interaction, we explored change scores in significant outcomes. Change scores were calculated as performance at 17 hours of deprivation minus performance at the nondeprived session. Scores were calculated for those measures with which significant interactions of impulsivity and deprivation at 17 hours were observed. We examined possible associations of these change scores with impulsivity. Pearson correlation values for CGNG go cue reaction time, CGNG no-go cue reaction time, CPT-II omission errors, and CPT-II reaction time standard error were −0.64 (p < .001), −0.40 (p = .036), −0.50 (p = .007), and −0.42 (p = .025), respectively. As rates of impulsivity increased, change scores decreased, indicating that more impulsive individuals exhibited less change in performance following deprivation.
Discussion
The present study examined the effects of increasing levels of nicotine deprivation on measures of cognitive processing. Cognitive processing was assessed by performance on the CPT-II and on the CGNG under three levels of nicotine deprivation (nondeprived, 5 hours, 17 hours). Across both tasks, nicotine deprivation was observed to negatively affect task performance. Nicotine deprived individuals were slower to respond and exhibited more variability in their response times. Decrements in performance were observed on both tasks at 17 hours of nicotine deprivation but only on the CGNG at 5 hours.
This study explored the relationship between nicotine deprivation-induced changes in cognitive processing, and trait impulsivity. Individual differences in impulsivity have been associated with a variety of substance use and relapse outcomes, including differences in smoking relapse rates (e.g., Doran et al., 2004) and differential changes in cognitive functioning because of nicotine deprivation could be implicated. Trait impulsivity was assessed in this study by self-report on the BIS (Patton et al., 1995). At 17 hours of nicotine deprivation with the CPT-II, and at both 5 hours and 17 hours with the CGNG, some performance measures and trait impulsivity were negatively correlated compared with the nondeprived referent comparison. These negative correlations indicated that more impulsive smokers in this study experienced less cognitive decline under nicotine deprivation. By contrast, less impulsive smokers were more likely to exhibit changes in performance under nicotine deprivation.
These results add to our understanding of the negative influence of nicotine deprivation on cognitive processing. Although much research attention has been focused on this relationship (e.g., Bell et al., 1999; Myers et al., 2008; Snyder et al., 1989), one novel aspect of the present findings is the consideration of how a trait personality factor, such as impulsivity, can differentially affect the relationship between nicotine deprivation and cognitive processing. These findings differ from those of a recent study comparing the influence of nicotine deprivation on the cognitive performance of smokers with and without Attention Deficit Hyperactivity Disorder (ADHD) (McClernon et al., 2008). ADHD is a disorder in part characterized by impairments in impulsiveness and inattention (American Psychological Association, 2000). In the study, individuals with and without ADHD completed the CGNG and CPT-II after overnight nicotine deprivation or no deprivation. Under nicotine deprivation, individuals with ADHD exhibited poorer performance on some measures from the CPT-II. Interestingly, no group differences in nicotine deprivation-induced impairment were observed on the CGNG. Differences between these studies include that the present study involved multiple levels of nicotine deprivation and a larger sample of community daily smokers.
Together the findings from the present study and a recent one (McClernon et al., 2008) indicate that more research attention toward understanding the relationship between nicotine deprivation-induced changes in cognition, and trait impulsivity, is warranted. Possible mechanisms underlying the relationship between trait impulsivity and cognitive performance after nicotine deprivation are unclear. Individual differences in trait impulsivity can be pronounced. Nicotine deprivation is a high challenge state that can exacerbate these differences (Kelley et al., 2005; Sinha, 2005; Volkow & Li, 2005). In the present study, individuals who rated highly in impulsivity exhibited fewer changes after deprivation, possibly resulting in the observation of a floor effect. Alternatively, individuals who were less impulsive might have been more motivated to perform well, but were unable to maintain this level of motivation under nicotine deprivation. Future research exploring possible mechanisms underlying the associations observed in this study would be of benefit.
Possible limitations of the study include the relatively small sample size, which could have limited our options for statistical analyses. However, the current sample size is similar to studies examining the relationship between nicotine deprivation and impulsivity (e.g., Field et al., 2006; Mitchell, 2004) and by using a within-subject design we were able to demonstrate robust effects of nicotine deprivation and of the interaction between nicotine deprivation and trait impulsivity. This study should be considered an initial exploration of the how multiple levels of nicotine deprivation are associated with various facets of trait impulsivity and cognitive processing. An additional potential limitation is the generalizability of the study. The relationships between nicotine deprivation, trait impulsivity, and cognitive processing in a community sample of regular daily smokers only extend up to 17 hours of deprivation. More work across extended periods of nicotine deprivation could determine whether trait impulsivity continues to factor into nicotine deprivation-associated changes in cognitive processing across longer periods of nicotine deprivation. Other possible limitations include that nicotine deprivation was confounded with cigarette abstinence, that subjective effects were not assessed that could differentiate between the 5 hours and 17 hours nicotine deprivation conditions, and that no measures of cotinine, the primary nicotine metabolite, were assessed immediately before task performance.
Along with past research, the present findings highlight the importance of considering how nicotine deprivation might interact with personality factors such as impulsivity to differentially affect cognitive functioning in smokers attempting to quit smoking. This study demonstrated that multiple levels of nicotine deprivation slowed cognitive processing and that these changes were related to the smokers’ levels of trait impulsivity. As higher rates of impulsivity have been associated with less successful smoking cessation attempts (e.g., Doran et al., 2004), understanding how nicotine deprivation interacts with impulsivity continues to be an area of future examination. Moreover, a greater under standing of how trait personality factors, such as impulsivity, influence smoking cessation rates could aid in the development of individualized treatments.
References
- al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacology, Biochemistry, and Behavior. 2002;72:707–716. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders DSM–IV–TR. 4 2000. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baumeister RF. Ego depletion and self-regulation failure: A resource model of self-control. Alcoholism: Clinical and Experimental Research. 2003;27:281–284. doi: 10.1097/01.ALC.0000060879.61384.A4. [DOI] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine & Tobacco Research. 1999;1:45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and sub stance use disorders. American Journal of Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ. Distress tolerance and early smoking lapse. Clinical Psychology Review. 2005;25:713–733. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Conners’ Continuous Performance Test user’s manual. Toronto, Canada: Multi-Health Systems; 2000. [Google Scholar]
- Doran N, Spring B, McChargue D, Pergadia M, Richmond M. Impulsivity and smoking relapse. Nicotine & Tobacco Research. 2004;6:641–647. doi: 10.1080/14622200410001727939. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urges through imaginal and in vivo procedures: Physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Everitt B, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Field M, Santacangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: The effects of nicotine deprivation. Psychopharmacology. 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. Journal of Studies on Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Hays L. Acute effects of cocaine in two models of inhibitory control: Implications of non-linear dose effects. Addiction. 2006;101:1323–1332. doi: 10.1111/j.1360-0443.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Marczinski CA. Effects of d-amphetamine on behavioral control in stimulant abusers: The role of pre-potent response tendencies. Drug and Alcohol Dependence. 2003;71:143–152. doi: 10.1016/s0376-8716(03)00089-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM–IV Axis I disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakas P, Foulds J. Acute effects of glucose tablets on craving, withdrawal symptoms, and sustained attention in 12-h abstinent tobacco smokers. Psychopharmacology. 2002;161:271–277. doi: 10.1007/s00213-002-1035-0. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Hurghes JF, Pickens RW, Svikis D. Tobacco withdrawal symptoms: An experimental analysis. Psychopharmacology. 1984;84:231–236. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Gifford EV, Ruckstuhl LEJ. Relational frame theory and executive function: A behavioral analysis. In: Lyon GR, Krasnegor NA, editors. Attention, memory, and executive function. Baltimore: Brookes; 1996. pp. 279–306. [Google Scholar]
- Heatherton TF, Kozlowski LK, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: Studies of gene activation in corticolimbic regions. Physiology & Behavior. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen S, Kenny PJ, Markou A, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Experimental and Clinical Psychopharmacology. 2003;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH, Lutz AM, Fitzgerald DP, Murray DW, Redman C, et al. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: Results of a preliminary study. Psychopharmacology. 2008;197:95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Effects of short-term nicotine deprivation on decision-making: Delay uncertainty, and effort discounting. Nicotine & Tobacco Research. 2004;6:819–828. doi: 10.1080/14622200412331296002. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Garnham MJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: Comparative effects upon cognitive task performance and mood state over 24 hours. Human Psychopharmacology. 1996;11:391–400. [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peluso T, Ricciardelli LA, Williams RJ. Self-control in relation to problem drinking and symptoms of disordered eating. Addictive Behaviors. 1999;24:439–442. doi: 10.1016/s0306-4603(98)00056-2. [DOI] [PubMed] [Google Scholar]
- Piquero AR, Gibson CL, Tibbetts SG. Does self-control account for the relationship between binge drinking and alcohol-related behaviours? Criminal Behaviour and Mental Health. 2002;12:135–154. doi: 10.1002/cbm.492. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia. Archives of General Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Sinha R. Lofexidine attenuates stress induced drug craving and arousal to improve stress-induced relapse outcomes. Neuropsychopharmacology. 2005;30(Suppl 1):S55. [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: Performance decrements assessed on a computerized test battery. Drug and Alcohol Dependence. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Techniques to assess alcohol consumption. New Jersey: Humana Press; 1993. [Google Scholar]
- Volkow N. Imaging the addicted brain: From molecules to behavior. Journal of Nuclear Medicine. 2004;45:13N–24N. [PubMed] [Google Scholar]
- Volkow N, Li T. Drug addiction: The neurobiology of behaviour gone awry. Nature Review Neuroscience. 2005;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Wills TA, Stoolmiller M. The role of self-control in early escalation of substance use: A time-varying analysis. Journal of Consulting and Clinical Psychology. 2002;70:986–997. doi: 10.1037//0022-006x.70.4.986. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Brody AL, et al. Effects of acute smoking on brain activity vary with abstinence in smokers performing the N-Back Task: A preliminary study. Psychiatry Research. 2006;148:103–109. doi: 10.1016/j.pscychresns.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]