Abstract
Background
Alcohol and tobacco dependence are highly comorbid disorders, with preclinical evidence suggesting a role for nicotinic acetylcholine receptors (nAChRs) in alcohol consumption. Varenicline, a partial nicotinic agonist with high affinity for the α4β2 nAChR receptor, reduced ethanol intake in rodents. We aimed to test whether varenicline would reduce alcohol consumption and alcohol craving in humans.
Methods
This double-blind, placebo-controlled investigation examined the effect of varenicline (2 mg/day vs. placebo) on alcohol self-administration using an established laboratory paradigm in non-alcohol-dependent heavy drinkers (n = 20) who were daily smokers. Following 7 days of medication pretreatment, participants were first administered a priming dose of alcohol (.3 g/kg) and subjective, and physiologic responses were assessed. A 2-hour alcohol self-administration period followed during which participants could choose to consume up to 8 additional drinks (each .15 g/kg).
Results
Varenicline (.5 ± SE = .40) significantly reduced the number of drinks consumed compared to placebo (2.60 ± SE = .93) and increased the likelihood of abstaining from any drinking during the self-administration period. Following the priming drink, varenicline attenuated alcohol craving and reduced subjective reinforcing alcohol effects (high, like, rush, feel good, intoxicated). Adverse events associated with varenicline were minimal and, when combined with alcohol, produced no significant effects on physiologic reactivity, mood, or nausea.
Conclusions
This preliminary investigation demonstrated that varenicline significantly reduced alcohol self-administration and was well tolerated, alone and in combination with alcohol in heavy-drinking smokers. Varenicline should be investigated as a potential treatment for alcohol use disorders.
Keywords: Alcohol, craving, heavy drinkers, human laboratory, nicotinic acetylcholine receptors, self-administration, smokers, varenicline
Problems associated with alcohol use continue to be a major public health problem, and identifying effective medications for the treatment of alcohol use disorders remains a high priority (1). One molecular target that demonstrates promise for alcohol treatment is the nicotinic acetylcholine receptor (nAChR) system (2). It is known that alcohol and tobacco dependence are highly comorbid (3,4), and electrophysiologic, pharmacologic, genetic, and neurochemical studies suggest that nAChRs are involved in alcohol consumption and other alcohol-dependent behaviors (2,5–8).
Reinforcing effects of alcohol are thought to be mediated, in part, by dopamine release in the nucleus accumbens (NA) and the ventral tegmental area (VTA) (9–11). It has been hypothesized that alcohol may produce mesolimbic activation, at least in part by its effects on central nAChRs (8,12–14). Alcohol administration increases acetylcholine levels in the VTA and dopamine levels in the NA (15). Microdialysis studies have documented that mecamylamine (a noncompetitive and nonselective nAChR antagonist) administered directly into the VTA blocked alcohol-induced increases in dopamine release in the NA (16) and, when administered systemically, blocked alcohol-induced dopamine overflow in the NA (17). Additionally, alcohol preference and intake is attenuated when mecamylamine is either administered directly into the VTA (14) or administered systemically (13,18).
Although targeting the nAChR system to modify alcohol consumption shows promise, the lack of suitable and specific agents for investigation in humans has impeded progress. Mecamylamine, previously the only nAChR agent available for human administration, has limited efficacy as a smoking cessation medication (19) and has dose-limiting adverse effects (20). Human studies examining the effect of mecamylamine on subjective alcohol reactivity have been promising (21,22), but reduced alcohol consumption has yet to be demonstrated (23). The recent Food and Drug Administration approval of varenicline, a partial nicotinic agonist for smoking cessation, presents an opportunity to further our understanding of the role of nAChRs in human alcohol consumption and to determine whether this novel compound may have therapeutic potential for the treatment of alcohol use disorders.
Varenicline binds with higher affinity at α4β2 nAChRs than at other nAChR subtypes and stimulates dopamine release to 60% of the level produced by nicotine (24). The α4β2 subtype is known to be necessary for nicotine dependence (25,26), and varenicline is highly effective for smoking cessation (27,28). Given the role of nAChRs in alcohol effects (2,5–8), varenicline may be a promising candidate to modify alcohol reactivity and drinking behavior. Consistent with this hypothesis, varenicline was found to reduce ethanol seeking and consumption in rats, leading to the hypothesis that varenicline may reduce alcohol consumption through its partial agonist activity at α4β2 nAChRs by limiting the ability of alcohol to activate dopamine release in the NA (29).
For this investigation, we used an established laboratory model developed by our group (30) that evaluates medication effects on reactivity to a priming drink and subsequent alcohol self-administration behavior (31–34). On the basis of the preclinical findings (29), we predicted as our primary hypothesis that varenicline, compared with placebo, would reduce the number of drinks consumed. Additionally, we predicted that varenicline would attenuate alcohol craving and that craving responses would be predictive of subsequent drinking behavior (29). Finally, we evaluated the safety and tolerability of varenicline, alone and in combination with alcohol in heavy-drinking smokers. This initial study excluded nonsmokers because the safety of administering varenicline to steady-state levels in this population was unknown.
Methods and Materials
Participants
Participants were eligible if they were ≥21 years and smoked ≥10 cigarettes per day. Women and men had to consume >7 or 14 drinks per week, and >3 or 4 drinks per episode at least once per week, respectively (35), in the previous 30 days. Exclusion criteria included alcohol dependence, illicit drug use (except for occasional cannabis use), past 30-day use of psychoactive drugs, treatment-seeking for alcohol or smoking, current Axis I disorders (except for nicotine dependence or alcohol abuse) (36), current suicidal or homicidal ideation, pregnant or nursing, or medical conditions contraindicating alcohol use (e.g., liver enzymes ≥3× normal) or varenicline administration (e.g., known allergy to varenicline).
Procedures
Eligibility Screening
The Human Investigation Committee of Yale University approved this study. Written informed consent was obtained at the start of the intake session. Physical examination included an electrocardiogram, urine toxicology, pregnancy test, and basic blood chemistries. Of the 55 potential participants who were screened, 6 declined to participate and 26 did not meet eligibility criteria related to illicit drug use (14) or smoking or drinking criteria (8), had medical contraindications (4), or were treatment seeking (1). Twenty-two individuals met eligibility criteria; one lost interest in the study before randomization, one lost interest after randomization. Twenty participants completed the study.
Medication
The medication condition was double-blind and placebo-controlled. Randomization to varenicline (2 mg/day) or a matching placebo (0 mg/day) was stratified by sex. Varenicline was titrated to steady-state levels over 7 days (.5 mg daily for Days 1 and 2, .5 mg twice daily for Days 3–5, and 1.0 mg twice daily on Days 6 and 7) (18). Medication compliance (which was 100%) was monitored with pill counts and riboflavin marker on Days 5 and 8 (37).
Laboratory Session
Each subject completed a 14-hour laboratory session conducted at the Yale Center for Clinical Investigation, New Haven, Connecticut. The laboratory procedures were similar to those used in our previous alcohol self-administration studies (30,33,34), which conform to guidelines for alcohol administration (38). Laboratory sessions started at 8:00 AM, and baseline assessments of breath alcohol, plasma cotinine and nicotine levels, urine drug screen, and urine pregnancy screen were obtained. The final dose of medication was provided at 9:00 AM. To ensure that participants were not nicotine deprived during the session, 15-min smoke breaks were provided at 10:00 AM, 12:00 PM, and 2:00 PM.
Priming Dose
The alcohol priming drink (.3 g/kg) (39) was administered from 3:00–3:05 PM and consisted of 1 part 80-proof liquor of the subject’s choosing to 3 parts mixer chosen from a selection of equicaloric, noncaffeinated, noncarbonated drinks.
Alcohol Self-Administration
Starting at 50 and 120 min following the priming drink, participants were exposed to two, 1-hour ad libitum drinking periods during which they were permitted to drink up to four alcoholic drinks (each .15 g/kg), or to receive monetary reinforcement ($3 per drink) for each drink not consumed. Participants were discharged at 10:00 PM at which time their breath alcohol levels had fallen below .02 g%.
Timing of Assessments
Blood alcohol level (BAL), alcohol craving (40), tobacco craving (41), and physiologic measures (systolic and diastolic blood pressure, heart rate, skin temperature) were assessed 15 min before and 10, 20, 30, 40, 80, 110, 150, and 180 min following the consumption of the priming drink. Subjective effects of alcohol (mean of high, like, rush, feel-good, intoxicated) (42), mood (43), nicotine withdrawal (44), and potential adverse effects (nauseous, dizzy, jittery) were assessed at baseline, 20, 40, 110, and 180 min following priming drink consumption.
Adverse Events
Adverse effects were assessed in person on Days 1, 5, and 8 and by phone on Day 2 (45). Common varenicline side effects (>5% and twice the rate seen in placebo-treated patients for smoking cessation) include nausea, abnormal dreams, insomnia, constipation, flatulence, and vomiting (46).
Statistical Analysis
Baseline characteristics and frequency counts of total side effects were compared across medication (2 mg varenicline vs. 0 mg placebo) with either t tests or chi-square tests. Separate repeated-measures analyses of variance (ANOVAs) were conducted for the priming drink and self-administration periods to examine BALs, subjective measures, and physiologic measures across medication and within time. For the self-administration period, an ANOVA was conducted to examine the number of drinks consumed by medication based on our a priori hypothesis.
Results
Baseline Characteristics
Varenicline and placebo groups were well matched for baseline demographic variables, and smoking and drinking behavior with the exception of race/ethnicity (Table 1).
Table 1.
Baseline Characteristics by Medication Condition, Mean (SD) or n (%)
| Varenicline (2 mg/day) (n = 10) | Placebo (0 mg/day) (n = 10) | |
|---|---|---|
| Age | 34.20 (12.08) | 35.30 (12.71) |
| Sex (% male) | 80% | 80% |
| Race/Ethnicitya | ||
| Non-Hispanic White | 4 (40%) | 9 (90%) |
| Non-Hispanic Black | 4 (40%) | 0 (0%) |
| Hispanic | 2 (20%) | 1 (10%) |
| Education | ||
| ≤ High school | 4 (40%) | 5 (50%) |
| ≥ College | 6 (60%) | 5 (50%) |
| Marital Status | ||
| Not married | 7 (70%) | 9 (90%) |
| Married | 3 (30%) | 1 (10%) |
| Tobacco Use | ||
| Cigarettes per day | 21.23 (8.02) | 21.28 (8.73) |
| CO levels (ppm) | 27.70 (15.05) | 35.20 (12.44) |
| Urine cotinine (ng/mL) | 1629.10 (988.54) | 1648.10 (921.40) |
| FTND scoresb | 5.10 (1.60) | 5.30 (2.21) |
| Alcohol Use | ||
| Weekly frequency (days)c | 4.34 (1.63) | 4.01 (1.42) |
| Drinks per episodec | 8.04 (4.69) | 6.23 (3.42) |
| AUDIT scoresd | 10.30 (4.19) | 9.70 (4.37) |
| Current DSM-IV alcohol abuse | 5 (50%) | 4 (40%) |
| Lifetime DSM-IV alcohol abuse | 7 (70%) | 8 (80%) |
| Lifetime DSM-IV alcohol | ||
| Dependence | 4 (40%) | 2 (20%) |
AUDIT, Alcohol Use Disorders Identification Test; CO, breath carbon monoxide; FTND, Fagerström Nicotine Dependence.
p < .05 chi-square. Participants in the placebo group were more likely to be non-Hispanic White (χ2 = 6.26, p < .05).
Range: scores ≥ 4 for nicotine dependence.
Means calculated over 30 days before intake.
Scores ≥ 8 for alcohol misuse.
Pretreatment Period
During the medication titration period, rates of adverse events did not differ by medication (Table 2). All severity ratings were minimal or mild. No participants discontinued the study because of adverse events. There were no significant changes from baseline in alcohol or cigarette use during the 1-week drug titration period; however, a trend for reduced alcohol frequency was demonstrated (varenicline mean = −1.54 ± SE = .73 vs. placebo mean = −.01 ± SE = .28, p = .07).
Table 2.
Counts and Severity Ratings of Treatment Emergent Symptomsa During 1-Week Medication Lead-in Period (Days 1–8)
| Varenicline 2 mg/day (n = 10) | Placebo 0 mg/day (n = 10) | |
|---|---|---|
| Any Adverse Event | 3 | 4 |
| Nausea | 1 (minimal) | 0 |
| Sleep Difficulties | 1 (minimal) | 4 (minimal) |
| Abnormal Dreams | 1 (minimal) | 1 (minimal) |
| Constipation | 0 | 2 (minimal) |
| Flatulence | 0 | 1 (mild) |
| Vomiting | 0 | 1 (minimal) |
More than 5% and twice the rate seen in placebo-treated patients for smoking cessation; frequency counts do not differ by medication condition.
Priming Drink Period
Plasma nicotine, cotinine, and breath CO did not differ by medication.
Smoking Behavior
Over the course of the three smoking breaks, varenicline (mean = 3.78 ± SE = .38) significantly reduced the number of cigarettes smoked (p < .05) compared with placebo (mean = 5.39 ± SE = .59).
Blood Alcohol Levels
BALs demonstrated a significant quadratic effect of time (F = 35.04, p < .0005) but did not differ by medication. Maximum mean BALs achieved during the priming drink period did not differ across varenicline (.021 g/dL ± SE = .003) or placebo conditions (.021 g/dL ± SE = .002).
Subjective Measures
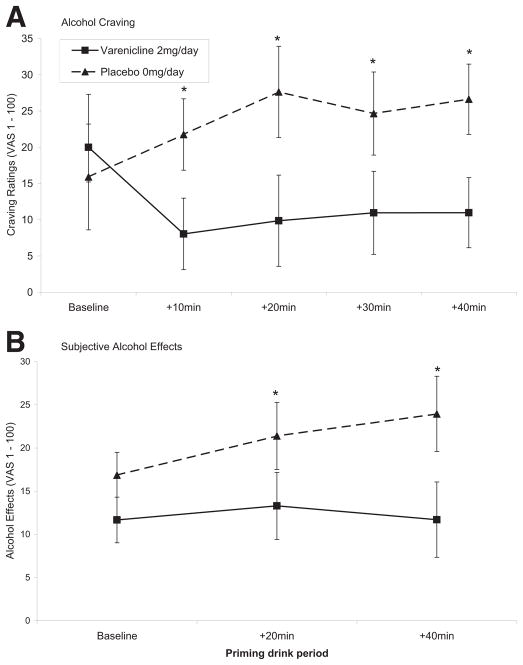
Alcohol craving increased following the priming drink in the placebo group but decreased in the varenicline group (F = 2.57, p < .05; Figure 1A). An effect of medication was demonstrated for subjective effects of alcohol (F = 4.53, p < .05; Figure 1B). Mood ratings and tobacco craving did not differ by medication. Nicotine withdrawal scores (overall mean = 2.18 out of possible 32, SE = .37) and adverse effects (nausea, dizzy, jittery) were minimal (all mean values <4 out of possible 100) and did not differ by medication.
Figure 1.
Mean subjective measures of alcohol following consumption of the .3 g/kg priming dose by medication condition and time. (A) Alcohol craving (time × medication, F = 2.57, p < .05). (B) Subjective alcohol effects (mean of high, like, rush, feel-good, intoxicated) (medication, F = 4.53, p < .05]. VAS, visual analogue scale (1–100). Time points +10, 20, 30, and 40 are adjusted for baseline time point. *p < .05 for paired comparisons of varenicline versus placebo.
Physiologic Measures
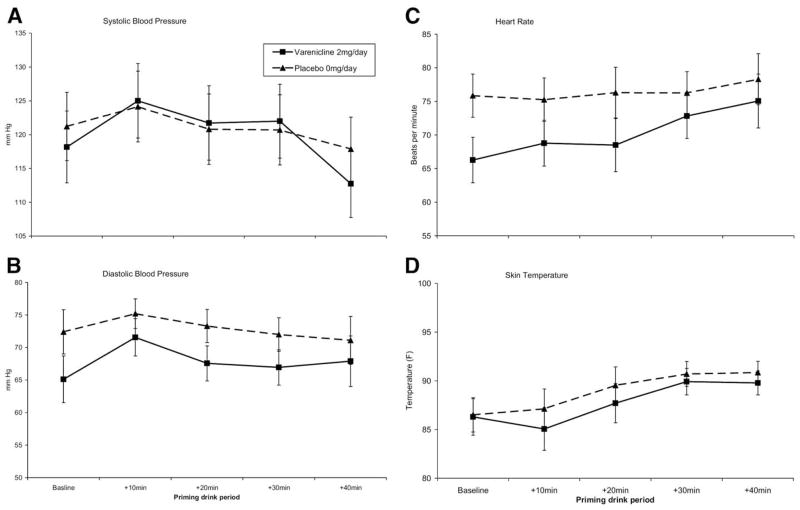
Systolic and diastolic blood pressure, heart rate, and skin temperature demonstrated significant effects of time (ps < .05). Effects of medication or medication by time were not significant (Figure 2).
Figure 2.
Mean physiologic reactivity following consumption of the .3 g/kg priming dose by medication condition and time. No significant effects of medication or of time by medication were demonstrated. (A) Systolic blood pressure (time, F = 6.64, p < .05). (B) Diastolic blood pressure (time, F = 3.47, p < .05). (C) Heart rate (time, F = 6.54, p < .05). (D) Skin temperature (time, F = 8.40, p < .05).
Ad-Libitum Drinking Period
Drinking Behavior
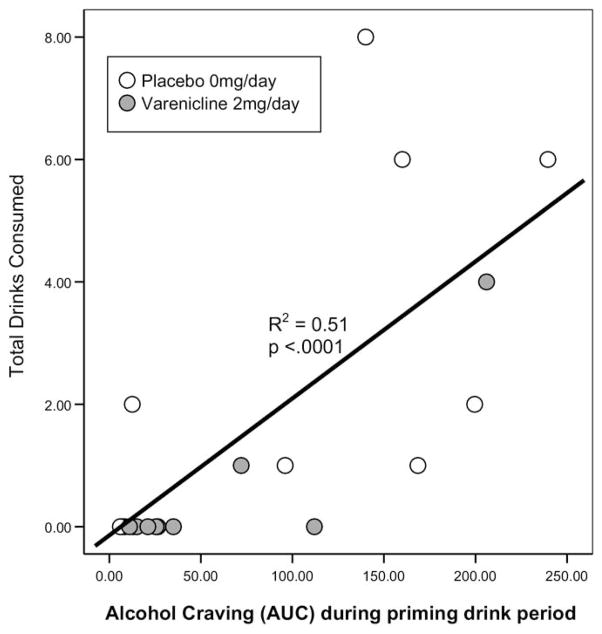
Varenicline (mean = .5± SE = .40) compared with placebo (mean = 2.60 ± SE = .9), significantly reduced the number of drinks consumed during the 2-hour self-administration period (F = 4.27, p < .05). The effect of medication on drinking was large (d = .97). Varenicline significantly increased the likelihood of remaining completely abstinent (χ2 = 5.05, p < .03) with 8 of 10 varenicline versus 3 of 10 placebo participants declining all drinks. As predicted, alcohol craving was significantly associated with drinks consumed (Figure 3).
Figure 3.
Scatterplot with line of best fit for alcohol craving during the priming drink (assessed as area under the curve [AUC]) by total drinks consumed during the 2-hour self-administration period.
Blood Alcohol Levels
BALs decreased in the varenicline condition but continued to increase in the placebo condition mirroring drinking behavior (time × medication F = 2.31, p = .07). At the end of the 2-hour period, varenicline blood alcohol levels were .010 g/dL ± SE = .001 and placebo levels were .031 g/dL ± SE = .011.
Subjective Measures
Mean subjective alcohol effects were greater in the placebo (21.11 ± SE = 3.44) versus varenicline condition (10.61 ± SE = 3.44; F = 4.64, p < .05). Nicotine withdrawal scores, although remaining minimal, increased slightly in the placebo condition (F = 6.48, p < .05) but were not significantly associated with medication effects on drinking behavior. Alcohol and tobacco craving and mood did not differ by medication.
Physiologic Measures
Physiologic measures did not demonstrate any effects of medication during the self-administration period (data not shown).
Discussion
Using an established alcohol self-administration paradigm (30–34), we found that varenicline compared with placebo significantly reduced the number of drinks consumed by heavy-drinking smokers and increased the likelihood of remaining completely abstinent during the 2-hour self-administration period. This result is consistent with preclinical findings (29) examining ethanol seeking and choice and further supports a role for nAChR effects in alcohol consumption. Varenicline also attenuated alcohol craving and subjective reinforcing alcohol effects (e.g., intoxicated) following consumption of the priming drink. Moreover, alcohol craving in response to the priming drink was significantly associated with subsequent alcohol consumption, accounting for half of the variance in drinks consumed. The magnitude of the effect of varenicline on drinks consumed and craving responses is similar to what is demonstrated with naltrexone using an identical laboratory paradigm (30). Given that naltrexone has demonstrated efficacy for the treatment of alcohol use disorders (47), this suggests that the observed effects with varenicline have clinical relevance.
On the basis of these findings, we speculate that varenicline may reduce drinking in a similar manner to how it works for smoking: 1) by acting as a partial agonist at α4β2 receptors stimulating adequate levels of dopamine release to prevent alcohol craving and 2) by acting as a competitive antagonist blocking the effect of alcohol at nAChRs to augment dopamine levels further, thus inhibiting alcohol-related reinforcement, craving, and self-administration behavior. A recent microdialysis study (48) lends support to this hypothesized mechanism. Following 5 days of varenicline pretreatment, an acute injection of varenicline completely abolished the extracellular NA dopamine response to alcohol and nicotine coadministration. Additionally, it is possible that varenicline shifted the pharmacodynamic effect of the priming drink to the right. In contrast to the placebo condition, varenicline attenuated the ability of the priming drink to prompt further ad libitum consumption. Future work examining the mechanisms underlying these findings would further clarify nAChR effects on alcohol consumption.
Overall, varenicline was well tolerated in heavy-drinking smokers during this short-term laboratory study. Side effects experienced during the pretreatment week were minimal, consistent with those observed in smokers undergoing smoking cessation treatment, and rates did not differ between varenicline and placebo groups. When varenicline was combined with alcohol during the laboratory session, we observed no effect of medication on physiologic reactivity or mood ratings in response to the low-dose priming drink. Additionally, adverse effects (nausea, dizzy, jittery) assessed throughout the laboratory session were minimal and did not differ by medication. Although these effects must be replicated in larger samples and studied at higher alcohol doses, our findings indicate that combining varenicline with low doses of alcohol appears safe and well tolerated. This finding is of immediate clinical relevance for the approved use of varenicline in smoking cessation given that the majority of smokers drink (3,4). Although there has been concern that varenicline is associated with neuropsychiatric side effects (49), a recent report examining varenicline for smoking cessation in smokers with psychiatric conditions (including alcohol problems) found that varenicline was safe, well tolerated, and did not exacerbate mental illness (50).
Our results, in combination with preclinical findings (29,47), suggest that varenicline warrants further development as a treatment for alcohol use disorders. However, our results were obtained in heavy-drinking smokers (with 45% meeting criteria for current alcohol abuse), and it remains to be determined whether these findings will extend to those with alcohol dependence. Among smokers, the potential of varenicline as a dual treatment for alcohol and tobacco disorders should be investigated. Diseases related to tobacco use are the leading cause of morbidity and mortality in alcoholics (51), and the relative risk of mortality increases with the combined versus singular abuse of alcohol and tobacco (52–54). Medications such as varenicline, which may target shared neurobiological substrates of alcohol and nicotine use, hold promise in this regard (2).
Varenicline should also be investigated as a primary treatment for alcohol use disorders. Although our study was conducted in smokers, they were not deprived of nicotine, suggesting that varenicline affects alcohol drinking independent of its effects on nicotine withdrawal. Moreover, the preclinical studies of varenicline demonstrating reductions in ethanol seeking and consumption were conducted in nicotine-naïve animals (29). Future studies in heavy drinkers and alcohol-dependent drinkers who are nonsmokers are indicated. Although we selected the recommended dose for smoking cessation for this study, the dose may not be the optimal one to target drinking behavior. Preclinical investigations of the effect of varenicline on alcohol consumption (29) and dopamine response (48) have not demonstrated linear dose effects, supporting the need for a dose-ranging study.
In summary, our results suggest that the nAChR system holds promise as a medication target for alcohol use disorders. In heavy-drinking smokers, we found that varenicline significantly reduced drinking and increased the likelihood of remaining completely abstinent during the self-administration session. Reductions in drinking behavior were associated with attenuated craving responses following the consumption of the priming drink. Given that varenicline was found to be well tolerated, alone and in combination with alcohol, clinical trials examining varenicline as a primary treatment for alcohol use disorders and as a potential dual treatment for alcohol and tobacco use disorders should be pursued.
Acknowledgments
This work was supported by Grant Nos. 15596, 15496, 15632, 14715 from the National Institute for Alcohol Abuse and Alcoholism; National Institutes of Health Grant Nos. CTSA-UL1RR024139 DA00436; Department of Mental Health and Addiction Services, State of Connecticut.
Drs. McKee, Harrison, Tetrault, and Shi and Ms. Estevez and Balchunas report no biomedical financial interests or potential conflicts of interest. Dr. O’Malley and Krishnan-Sarin are inventors on patents held by Yale University for the use of naltrexone for smoking cessation, since abandoned. Dr. O’Malley received travel reimbursement for an invited talk at the Meeting of the Controlled Release Society; an honorarium from the American College of Neuropsychopharmacology; was a consultant to Ortho-McNeil (1 day); and was a speaker at the Medical Education Speakers Network (1 day). Dr. Picciotto received a small grant from Pfizer in 2007 to study varenicline and other compounds in preclinical models of antidepressant efficacy. Dr. Petrakis was a principal investigator on an investigator-initiated study sponsored by Forest.
References
- 1.Li TK. FY 2006 President’s budget request for NIAAA Director’s statement before the House and Senate appropriations subcommittees. [Accessed February 10, 2008.];NIAAA. 2006 Available at: http://www.niaaa.nih.gov/AboutNIAAA/CongressionalInformation/Testimony/statement3_05.
- 2.Li TK, Volkow ND, Baler RD, Egli M. The biological bases of nicotine and alcohol co-addiction. Biol Psychiatry. 2007;61:1–3. doi: 10.1016/j.biopsych.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 3.McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG. Smoking status as a clinical indicator for alcohol misuse in US adults. Arch Int Med. 2007;167:716–721. doi: 10.1001/archinte.167.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant BF. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. J Subst Abuse. 1998;10:59–73. doi: 10.1016/s0899-3289(99)80141-2. [DOI] [PubMed] [Google Scholar]
- 5.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 6.Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci Biobehav Rev. 2004;27:713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Söderpalm B, Reicson M, Olausson P, Blomqvist O, Engel JA. Nicotine mechanisms involved in dopaminergic activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- 9.Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res. 1999;23:1751–1760. [PubMed] [Google Scholar]
- 11.Middaugh LD, Szumlinski KK, van Patten Y, Marlow A-L, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice alters the behavioral and neurochemical effects of ethanol: blockade by naltrexone. Alcohol Clin Exp Res. 2003;27:1892–1900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- 12.Blomqvist O, Engel JA, Nissbrandt H, Söderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- 13.Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: Effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- 14.Ericson M, Blomqvist O, Engel JA, Söderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- 15.Larsson A, Edstrom L, Svensson L, Söderpalm B, Engel JA. Voluntary ethanol intake increases extracellular acetylcholine levels in the ventral tegmental area in the rat. Alcohol Alcohol. 2005;40:349–358. doi: 10.1093/alcalc/agh180. [DOI] [PubMed] [Google Scholar]
- 16.Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- 17.Larsson A, Svensson L, Soderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neuro-chemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- 18.Lê AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotine receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- 19.Frishman WH, Mitta H, Coopersmith A, Ky T. Nicotine and non-nicotine smoking cessation pharmacotherapies. Cardiol Rev. 2006;14:57–73. doi: 10.1097/01.crd.0000172309.06270.25. [DOI] [PubMed] [Google Scholar]
- 20.Targacept, Inc. [Accessed September 17, 2008.];Inversine. 2002 Available at: http://www.targacept.com/pdf/inversine_pi.pdf.
- 21.Blomqvist O, Hernadez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin Exp Res. 2002;26:326–331. [PubMed] [Google Scholar]
- 22.Chi H, de Wit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- 23.Young EM, Mahler S, Chi H, de Wit H. Mecamylamine and ethanol preference in healthy volunteers. Alcohol Clin Exp Res. 2005;29:58–65. doi: 10.1097/01.alc.0000150007.34702.16. [DOI] [PubMed] [Google Scholar]
- 24.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline, an α4β2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 25.Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio L, Merlo-Pich E, et al. Acetylcholine receptors containing the α2-subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 26.Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of alpha 4 receptors: Sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- 27.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billings CB, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion in placebo for smoking cessation. A randomized clinical trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline an α4β2 nicotinic acetylcholine receptor partial agonist vs placebo or sustained-release bupropion for smoking cessation. A randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectivity decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek J. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 31.Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: Temporal effects of drinking. Psychopharmacology. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- 32.Drobes DJ, Anton RF, Thomas SE, Voronin K. A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: Naltrexone and nalmefene. Neuropsychopharmacology. 2003;28:755–764. doi: 10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry. 2007;62:694–697. doi: 10.1016/j.biopsych.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 34.McKee SA, O’Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacol. 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobell LC, Sobell MB. Timeline Followback: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Techniques to Assess Alcohol Consumption. Totowa, NJ: Humana Press; 1993. pp. 41–72. [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 37.DelBoca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 38.National Advisory Council on Alcohol Abuse and Alcoholism. [Accessed August 25, 2008.];NIAAA guidelines (National Advisory Council on Alcohol Abuse and Alcoholism's Recommended guidelines on ethyl alcohol administration) 2005 Available at: http://www.niaaa.nih.gov/Resources/ResearchResources/job22.htm#dependent.
- 39.Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow KE, Batt RD, editors. Human Metabolism of Alcohol. I. Boca Raton, FL: CRC Press; 1989. pp. 41–56. [Google Scholar]
- 40.Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 41.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 42.Schuckit M. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psych. 1984;41:879–885. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- 43.Russell JA. A circumplex model of affect. J Personality Social Psychol. 1980;39:1161–1178. [Google Scholar]
- 44.Hughes JF, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psych. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 45.Levine J, Schooler NR. SAFTEE: A technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- 46.Pfizer Inc. [Accessed February 10, 2008.];Chantix. 2008 Available at: http://www.pfizer.com/pfizer/download/uspi_chantix.pdf.
- 47.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. for the COMBINE Study Research Group. Combined pharma-cotherapies and behavioral interventions for alcohol dependence. The COMBINE study: A randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 48.Ericson M, Löf E, Stomberg R, Söderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system [published online ahead of print January 6] J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- 49.Food and Drug Administration. [Accessed January 17, 2009.];Varenicline (marketed as Chantix) Information. 2009 Available at: http://www.fda.gov/CDER/Drug/infopage/varenicline/default.htm.
- 50.Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, Sutherland G. Varenicline in the routine treatment of tobacco dependence: A pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103:146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- 51.Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, et al. Mortality following inpatient addictions treatment: role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 52.Grucza RA, Beirut LJ. Co-occurring risk factors for alcohol dependence and habitual smoking: Update on findings from the Collaborative Study on the Genetics of Alcoholism. Alcohol Res Health. 2006;29:172–177. [PMC free article] [PubMed] [Google Scholar]
- 53.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–224. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Cancer risk associated with alcohol and tobacco use: Focus on upper aero-digestive tract and liver. Alcohol Res Health. 2007;3:193–198. [PMC free article] [PubMed] [Google Scholar]