Abstract
Pair housing of laboratory macaques is widely considered to lead to positive changes in well-being, yet the process of introduction is viewed as potentially stressful and risk-prone. Behavioral and physiological data were collected on eight adult male rhesus macaques before, during, and after the process of introduction, in order to measure the initial stress of introduction as well as long-term changes in well-being. Socially experienced subjects, all implanted with biotelemetry devices, were studied in five successive phases: baseline (singly housed), 1 day each of protected contact and full contact introduction, post-introduction (1–3 weeks after introduction), and settled pairs (≥20 weeks after introduction). One hundred and seventy-six hours of behavioral data and 672 hr of heart rate data were analyzed. Fecal cortisol was also measured for the baseline, post-introduction, and settled pair phases. All introductions were successful and subjects showed no physiological or behavioral signs of stress, such as increased heart rate, abnormal behavior, or psychological indices of distress (depressive/anxiety-related behavior). Agonism was minimal throughout the introduction process and over the subsequent months; only one wound was incurred over the course of the study. Levels of abnormal behaviors, psychological indices of distress, locomotion, inactivity, and affiliation showed improvements within several weeks after introduction; these changes were still present 5–9 months later for the latter two categories. Heart rates during introduction fell significantly in the settled pair phase, and also varied predictably with time of day. Fecal cortisol levels were lower in settled pairs than in single housing. The fact that reductions in abnormal behavior did not persist over the long term may have been confounded by increasing duration of time spent caged. The results of this study may be of practical use for designing and monitoring social introductions and suggest that managers should not dismiss the feasibility of successful pairing of adult male rhesus macaques.
Keywords: Rhesus macaques, social introductions, biotelemetry, stress
Introduction
The most commonly used nonhuman primates in biomedical research are rhesus macaques [Fox et al., 2002]. Rhesus macaques are social animals by nature and for this reason their social needs must be addressed under federal regulation [U.S. Department of Agriculture, 1991]. The Guide for the Care and Use of Laboratory Animals recommends that social animals be housed with members of the same species whenever possible [National Research Council, 1996]. Studies have shown that social housing of compatible pairs leads to positive social interactions and the expression of species-appropriate social behavior not possible in single housing [Eaton et al., 1994; Lutz & Novak, 2005; Roberts & Platt, 2005]. It has been suggested that social housing is an effective form of environmental enrichment that can be used both to reduce the incidence of abnormal behaviors and enhance the psychological well-being of nonhuman primates that are caged for research purposes [Lutz & Novak, 2005; National Research Council, 1998; Reinhardt, 1989; Reinhardt et al., 1988]. Nonetheless, some investigators fear that their research will be hindered by social housing and may be reluctant to use it as a form of enrichment. However, in fact, compatible pairs show more species-representative behavior and deal with routine changes in their environment more efficiently than single-housed animals, which can make them better suited research models [Reinhardt, 2002; Roberts & Platt, 2005].
Although compatible animals seem to thrive in pair housing, some would argue that the process of social introduction is often a very stressful event [e.g., Clarke et al., 1995]. Even in the absence of serious wounding, stress indicators such as cortisol levels, behavior, and cellular immune responses support this idea [Clark et al., 1996; Gust et al., 1991; Line et al., 1996]. Another argument often made against social housing is that adult rhesus macaques may cause serious injury to one another during social introductions. Patterns of aggression and wounding during group formations [Bernstein et al., 1974a,b; Westergaard et al., 1999] suggest that introductions involving adult males may be of particular concern. However, many studies have demonstrated success in pairing both adult male and female rhesus macaques with a low incidence of serious wounding [Eaton et al., 1994; Reinhardt, 1994, 2002; Roberts & Platt, 2005]. Although the evidence suggests that pair housing is beneficial to rhesus macaques, these benefits must be balanced against the risk of stress and injury, a consideration that may be especially important in males. The aim of this study is to monitor both short- and long-term behavioral and physiological effects of introduction on male rhesus macaques. We hypothesize that the initial formation of pairs will be a stressful event, but that the long-term effects will be beneficial.
Materials and Methods
Subjects and Housing
Subjects included eight male rhesus macaques (Macaca mulatta) of Indian origin, ranging in age from 5.0 to 6.2 years, weighing 7–12kg at the start of the study. All individuals were obtained from the specific pathogen-free breeding colony at the Tulane National Primate Research Center (TNPRC), which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International. All aspects of housing, care, and research conformed to the Guide for the Care and Use of Laboratory Animals [National Research Council, 1996] and the USDA's Animal Welfare Regulations [U.S. Department of Agriculture, 1991], and adhered to the study's protocol as approved by the TNPRC Institutional Animal Care and Use Committee. Subjects had been mother-reared in social groups for at least the first 8 months of life, and remained in social housing for another 4 years or more before being placed in single housing. Time housed singly before the onset of the study ranged from 4 to 19 months. They were housed individually while baseline data were obtained and then pair housed for the remainder of the study, except for several 7–14 day periods following surgical accesses to address implant-related problems that occurred months after social introduction.
All subjects were housed in one room with specially equipped telemetry cages (Allentown, Inc., Allentown, NJ) and wall-mounted video cameras. Cages consisted of mesh ceilings, fronts, and floors with solid sides preventing visual or tactile contact between neighbors. Subjects were housed on one side of the room with visual access to others via wall- and/or cage-hung mirrors. Individuals to be introduced were placed immediately adjacent to their future pair mate before the onset of the study. Each individual stainless steel cage measured 36in in height with 8.6 ft2 of floor space. Each cage contained two perches, two foraging boards, one toy, and a small mirror hanging on the outside of the cage. The room was maintained on a 12:12-hr light:dark cycle. The ambient temperature remained between 64 and 72°F with a relative humidity of 30–70%. The subjects were fed commercial biscuits (Purina® Lab Fiber-Plus Monkey Diet, Richmond, Indiana) twice daily and had access to fresh, clean water ad libitum. They were also fed fresh produce and/or a foraging mix at least once daily five times per week.
Data Collection
Before the onset of this study, all eight subjects were surgically implanted with T29F-5B telemetry devices (Konigsberg Instruments, Pasedena, CA) to monitor their heart rate. The devices also measured body temperature, which would later be used as data in an infectious disease study. Each transmitter was placed in the flank region between the external and internal abdominal oblique muscles. The power coil was placed in the ventral abdominal region, the electrocardiogram (ECG) lead wires in the thoracic region, and the antenna in the dorsal region immediately lateral to the spine. The ECG lead wires were positioned according to vectors predetermined by an ECG monitor before surgery to produce the tallest and most consistent p and t waves on the ECG.
The telemetry devices were monitored and controlled using a base station and antenna matrix panel (Konigsberg Instruments, Pasedena, CA). Data were collected from the telemetry devices using CA recorder software and hardware (Data Integrated Scientific Systems, Pinckney, MI). Four video cameras were wall mounted across from the cages. The videotaped data were recorded on a computer equipped with Video Insight (Houston, TX). Videotaped recordings were analyzed using Observer XT® (Noldus Information Technology Inc., Leesburg, VA).
A temperament assessment was performed on each subject before the start of the study. The assessment score was based on degree of aggression, fear, receptivity to observer, and normality. Because the results of the assessment tests were similar between subjects, they were instead paired according to body weight. Ongoing studies by the second author suggest that compatible pairs show a larger weight disparity between the individuals (currently approximately 22%) than do pairs involved in unsuccessful introductions (approximately 15%). Therefore, pairs were determined in such a way as to maximize the weight differences within a pair (which resulted in a difference varying from 11 to 31%).
This study consisted of five phases (see Table I). Data collection began in April 2006 and was completed in January 2007. During baseline (BL), subjects were videotaped in either 12 30-min sessions or 18 20-min sessions over a 2-week period. A total of 6hr of videotape per animal was recorded for this phase. These sessions were evenly divided among four start times: 8:30, 10:30, 12:00, and 15:00. Videotaping was scheduled to avoid daily feedings, routine husbandry, and research procedures. Telemetry data were also collected on these days, over continuous 8-hr periods (8:00–16:00).
TABLE I. Study Phases.
| Phase | Abbreviation | Descriptions | Data collected |
|---|---|---|---|
| Baseline | BL | Individually housed animals before introduction | Behavioral data Telemetry data Fecal cortisol levels Physical examination Complete blood count (CBC) Chemistry panel (CP) |
| Protected contact | PC | Pairs introduced but remain separated by a panel consisting of bars spaced 2cm apart to allow social contact between subjects without allowing them to enter the other's cage | Behavioral data Telemetry data |
| Full contact | FC | Panel is pulled and animals are in full contact | Behavioral data Telemetry data |
| Post-introduction | PI | Pair housing beginning a week after introduction | Behavioral data Telemetry data Fecal cortisol levels Physical examination Complete blood count (CBC) Chemistry panel (CP) |
| Settled pairs | SP | Pair housing beginning at 20–39 weeks after initial introduction | Behavioral data Telemetry data Fecal cortisol levels Physical examination Complete blood count (CBC) Chemistry panel (CP) |
The subjects were anesthetized with 10mg/kg of ketamine hydrochloride on either Monday or Tuesday of the first week of BL for weighing, physical examination by clinical veterinarians, and blood collection to assess their general health. A fresh fecal sample was also collected from each subject's cage at the end of this first week to avoid the effects of the earlier anesthetic episode and to collect data reflective of normal weekday routines. Blood samples collected from each subject were sent to TNPRC clinical pathology laboratory for complete blood counts (Advia 120, Bayer, Tarrytown, NY) and chemistry panels (Olympus AU400, Center Valley, PA). Fecal samples (approximately 5g per subject) were placed in 50 ml polypropylene vials and mixed with 15 ml of methanol for 5min. The samples were then frozen at −20°C before shipment to Wisconsin National Primate Research Center (Madison, WI) for fecal cortisol analysis.
Social introduction began with subjects being placed in protected contact (PC) by substituting a panel consisting of bars spaced 2 cm apart for the solid panel that had previously separated the monkeys. Subjects were closely monitored for any fighting or repeated aggression that would have been cause for separation. Introductions were observed real time from an office equipped with monitors displaying the video feed from the animal holding room, and the first 2 hr of video feed were recorded for later analysis. Eight hours of telemetry data were also collected beginning with the social introduction, from 8:00 to 16:00. After 24 hr, as neither persistent aggression nor wounding was observed, each pair was placed into full contact (FC) by removing the barred panel so that individuals could enter one another's cages. Immediately after the subjects were introduced into full contact, videotaped recordings were collected for 2 continuous hours and telemetry data for 8 hr. The post-introduction (PI) period measured response to pair housing over the short term (1–3 weeks after introduction), whereas the settled pairs phase (SP) examined long-term response to pair formation, 20–21 weeks after introduction for four animals, and 39–40 weeks in the other four. The timing of SP data collection varied owing to implant-related problems (mechanical failures and/or thoracic erosions) which had to be resolved before SP telemetry data could be collected. Data collection procedures for PI and SP were identical to BL described above with respect to the amount of data and the times data were collected for videotaped recordings, telemetry data, physical examination, blood work, body weight measurement, and fecal collection. The only difference between these phases was that subjects were placed into protected contact overnight once in PI and once in SP for collection of feces attributable to individual subjects the following morning.
Scan sampling (with a 30sec intersample interval) was performed on the behavioral data collected during all five phases of the study. Data were coded using an ethogram containing 73 mutually exclusive behaviors, after the achievement of interobserver reliability (>85%) among the two coders. Telemetry data were collected every 30sec from 8:00 until 16:00 on the days that videotaped recordings were collected. The mean heart rate was calculated for each animal over four 1-hr sessions during this 8-hr period. These sessions started at the following times: 10:30, 11:45, 13:00, and 15:00. These times were chosen to represent periods of different human activity levels in the animal holding areas.
Statistical Analysis
Data were analyzed using repeated measures analysis of variance; significant results were then analyzed using post hoc Bonferroni tests to control for multiple comparisons. Heart rate data employed time points and phases as within-subject variables. Owing to technical problems experienced during telemetry data collection, data from one subject were excluded entirely, and among the remaining subjects, the following data points were not available for analysis, but were replaced with the respective variables' mean values: 11:45/PC (one subject), 13:00/PC (one subject), and 15:00/FC (four subjects). Fecal cortisol and behavioral measures used phases as within-subject variables, and behaviors were compiled in ten categories for analysis (see Table II). In some phases, particular behavioral categories were recorded for too few subjects to be included in the relevant repeated measures analysis of variance (owing to lack of variance); all of these instances are indicated in the results below.
TABLE II. Behavioral Categories.
Indices of Psychological Disturbance (i.e., Depressivea and Anxiety-relatedb)
|
| Abnormal: Locomotor stereotypes including bizarre posture, flip, floating limb, head toss, jump, pace, rock, spin, and stereotype; other abnormal behavior categories (e.g., overgrooming) occurred too rarely to be analyzed |
| Eat: Common usage, includes holding food |
| Forage: Manipulating foraging boards with hands or mouth; eating or attempting to remove seeds/foraging mix off board |
| Inactive: Passive or sleeping |
| Locomotion: Walk, climb, jump (two steps or more) |
Manipulate
|
| Non-contact affiliative: Attempt to touch, lip-smack, present, coo calls |
| Contact affiliative: Groom, affiliative contact, social overpluck, social play, cling, mount, and genital explore |
Agonisticc
|
Analysis performed on bolded behavioral categories.
After Shively et al. [1997, 2005].
Self-directed displacement behavior that has been demonstrated to be associated with stress [Baker & Aureli, 1997; Maestripieri et al., 1992; Schino et al., 1996].
Submissive behaviors (fear grimace, bob, rapid glances, cringe, and rump present) and receipt of submission were included in the ethogram but not analyzed because they were only observed in one animal in one phase.
Results
The social introductions for all four pairs went well with no complications, as do over 90% of social introductions of adult male Indian-rhesus macaques at the TNPRC [Baker, unpublished]. In PC, three of the four pairs were observed grooming. The pair that was not observed to groom in PC or FC established a clear rank relationship quickly and was recorded grooming at high levels by PI. Non-contact agonism was displayed by three pairs in the first 2hr of PC, but no agonism was displayed at all during the first 2hr of FC. Only two pairs showed agonism in PI; all did in SP, but it was always mild and infrequent. Over the entire study period (as well as to date), there was only one instance of wounding that required veterinary care. This event occurred 14 weeks after introduction. The pair involved appeared to have the most positive introduction, with frequent mounting and co-threatening of care staff, but also experienced rank reversal within the first week after introduction. Immediately following the injury, the individuals were moved from full to protected contact housing. One subject required veterinary care for a full thickness laceration on his hip. Sutures were used to repair the wound and the subject was given antibiotics to prevent infection. The pair was maintained in protected contact to allow the wounds to heal. The wounded subject also required a new implant during this time, so the pair remained in protected contact for a total of 4 weeks. After this time, the subjects were placed into full contact with no complications. At the time of writing, three subjects have been humanely sacrificed following inoculation of simian immunodeficiency virus and advancing disease; all pairs remained together without problems until the day of sacrifice. All other pairs remain together.
There were no significant findings on the complete blood counts or chemistry panels for any subject during any phase of data collection. No individuals lost weight, but rather gained an average of 1.5kg (range: 0.1–3.1 kg) or a mean of 18% of their baseline weight [mean annual increase in weight among comparably aged singly housed male rhesus macaques at the TNPRC range from 16 to 19% annually; Falkenstein, unpublished].
Telemetry Data (Heart Rate)
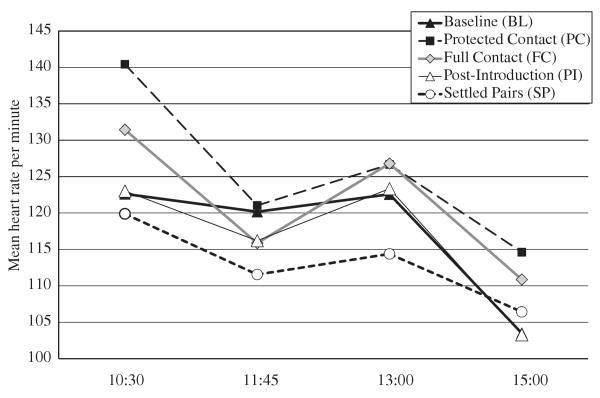
There was a significant effect of phase (F4,24 =2.88, P<0.05); post hoc Bonferroni test showed significant decreases in heart rate between PC and SP (P<0.05); see Figure 1. Heart rate also varied with time of day (F3,18 =17.57, P<0.00001), with significant contrasts between 10:30 and 11:45 (P<0.01), 10:30 and 15:00 (P<0.00005), and all pairwise contrasts among 11:45, 13:00, and 15:00 (P<0.005–0.05). No interaction effect was detected (F12,72 =1.08, NS).
Fig. 1.
Mean heart rate at predetermined times of day throughout the study (significant contrasts include the following [see text]: PC vs. SP, 10:30 vs. 11:45, 10:30 vs. 15:00, 11:45 vs. 15:00, 13:00 vs. 15:00). PC, protected contact; SP, settled pairs.
Fecal Cortisol Levels
Fecal cortisol levels ranged from 3.6 to 83.8 ng/g over the course of the three phases sampled, except for one baseline sample measuring 395.8 ng/g. No significant difference in subjects' fecal cortisol levels was detected across BL (82.5±45.6 ng/g), PI (10.4± 4.6), and SP (8.9±1.8; F2,14±2.46, NS). To test whether these significant results were biased by the outlier, the analysis was rerun without data from the relevant individual. That analysis detected a significant difference in levels across BL (37.7±9.9 ng/g), PI (10.8±5.3), and SP (9.3±2.0; F2,12=4.87, P<0.05), owing to a trend toward reduction between BL and PI (P<0.07) and significant reduction between BL and SP (P<0.05).
Behavior
The following behaviors differed significantly across phases of study: indices of psychological disturbance (F4,28=5.18, P<0.005), foraging (F4,28= 4.36, P<0.01), inactivity (F4,28=7.77, P<0.0005), locomotion (F4,28=6.79, P<0.0001), and contact affiliation (F3,21=24.76, P<0.00001), which was not recorded during the BL. A trend toward difference across phases was detected for abnormal behavior (F2,14=3.52, P<0.06), which could only be compared across BL, PI, and SP as it was recorded in too few subjects in PC and FC for statistical analysis. Post hoc pairwise tests showed that indices of psychological disturbance were seen at higher levels during BL than PC (P<0.01), FC (P<0.05), and PI (P<0.01) (see Fig. 2). Levels of abnormal behavior tended to be higher during BL than PI (P<0.06) but not than SP (see Fig. 2). Subjects spent more time foraging during BL than during PC (P<0.05) and than during SP (P<0.05) (see Fig. 2). Subjects locomoted less during BL than during FC (P<0.0005), and less during PC than FC (P<0.005; see Fig. 3). There was also more inactivity recorded during PC than both PI (P<0.01) and SP (P<0.005; see Fig. 3). Contact affiliation, which was of course absent in BL, was higher in PI and SP than in PC (P<0.005 and 0.0005, respectively) and than in FC (P<0.00001 and 0.000001, respectively; see Fig. 3).
Fig. 2.
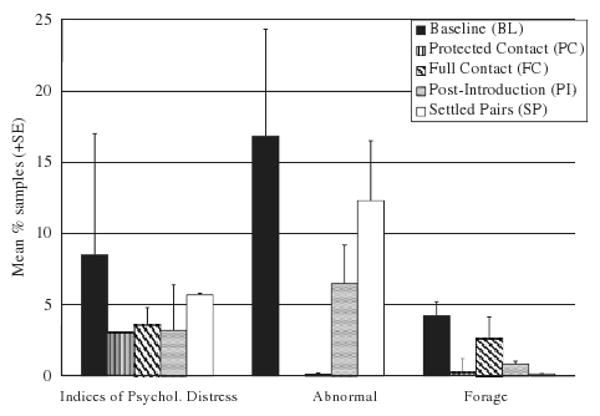
Mean percent of samples for indices of psychological disturbance (i.e., behaviors associated with depression or anxiety), abnormal behaviors, and foraging observed over the different phases of the study (indices of psychological disturbance varied between BL and PC, BL and FC, and BL and PI; abnormal behavior tended to vary between BL and PI; foraging varied between BL and PC, and BL and FC). BL, baseline; PC, protected contact; FC, full contact; PI, post-introduction; SP, settled pairs.
Fig. 3.
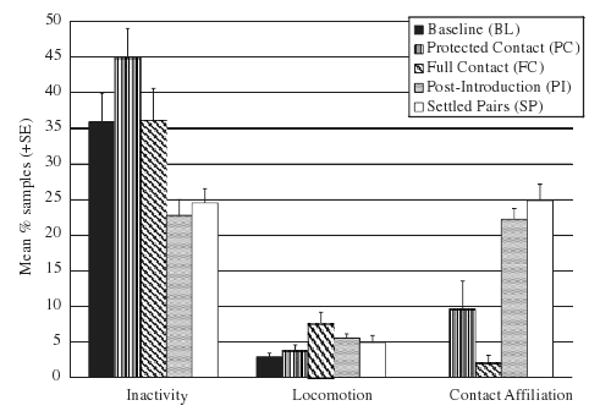
Mean percent of samples for inactivity, locomotion, and contact affiliation observed over the different phases of the study (inactivity varied between PC and PI, PC and SP; locomotion between BL and FC and between PC and FC; and contact affiliation between PC and PI, PC and FC, and PC and SP). BL, baseline; PC, protected contact; FC, full contact; PI, post-introduction; SP, settled pairs.
There was no significant difference in object manipulation (F4,28=1.01), eating (F4,28=1.70), or non-contact affiliation (F4,28=2.01) across the five phases of this study, nor agonistic behavior in the three phases in which it was recorded (PC, PI, and SP; F2,14=0.25).
Discussion
Our hypothesis that the initial formation of adult male pairs of rhesus macaques would be a stressful event was not supported by our data. There was no increase in heart rate over the course of the introduction or in the immediate post-introduction period. Significant stress would be expected to manifest in elevated heart rate on the basis of studies of social introduction in baboons [Coelho et al., 1991], as well as studies of the response of various macaque species to laboratory procedures and novelty [Bowers et al., 1998; Clarke et al., 1994; Hassimoto & Harada, 2003; Line et al., 1989]. Fecal cortisol values also showed no elevation at 1–2 weeks after introduction, as might be expected in previous studies of short-term response to social disruption [e.g., Gust et al., 1996] and stressful laboratory procedures in macaques [Clarke et al., 1988; Reinhardt et al., 1990], but rather tended to fall in the post-introductory period. Last, there were no increases in indices of psychological distress or abnormal behaviors throughout the introduction process. In fact, levels fell. A reduction in abnormal behavior in the immediate post-introduction period has also been seen in female cynomolgus monkeys [Line et al., 1990] and adolescent male baboons [Bourgeois & Brent, 2005]. Increased locomotion and decreased inactivity were also among the positive changes seen.
One factor that may have moderated the potentially stressful nature of initial relationship formation was the rapidity with which social partners began grooming (75% within the first 2hr of physical access) and the high levels of grooming observed (13% [range: 5–27%] of samples over the first 2hr, among the pairs that groomed during PC). All pairs were observed grooming by 24hr after being placed in full contact. Receipt of grooming in pigtailed macaques is associated with reduced heart rate [Boccia et al., 1989] and the receipt of high levels of grooming and low levels of aggression in rhesus macaques has been found to correspond to low levels of cortisol [Gust et al., 1993]. Very little agonism was observed over the course of the study. There was a complete lack of contact aggression during introductions, and non-contact agonism was only observed in two of the pairs (0.7% [range: 0.4–0.9%] of the samples over the first 2hr among the pairs that showed any aggression during PC). It was of considerable practical interest to observe that levels of contact affiliation during PC were significantly higher than that during FC and that agonism was seen during the PC but not FC introductions; these males interacted at high levels early in the introduction process despite the presence of the barrier that constrained physical access between them. This pattern suggests that protected contact may function as behavioral managers intend; to permit initial relationship formation in a context that allows control over proximity and possible reduction in risk of injury.
Whereas we found no confirmation of the hypothesis that the initial formation of pairs would be a stressful event, we did find support for our hypothesis that the long-term effects of pair housing would be positive. Partners familiar in the long term showed lower heart rates than during their initial introduction. The trend toward reduced cortisol in the PI condition continued and, in fact, magnified over the subsequent months of pair housing, a finding that differs from a previous study, which found no long-term change in cortisol among paired male rhesus macaques [Reinhardt et al., 1991]. Several of the positive changes in behavior observed during and in the first weeks following introductions persisted long term, including decreased inactivity and the considerable proportion of the monkeys' activity budgets engaged in affiliative behaviors. This latter finding is in line with the pattern previously observed among female rhesus macaques [Eaton et al., 1994], but is at odds with the suggestion that social partners may experience a decline in social interaction over time [Novak & Suomi, 1988].
Based on previous research of the distribution of abnormal behavior in different housing settings for macaques [Bayne et al., 1992; Bellanca & Crockett, 2002; Eaton et al., 1994; Lutz et al., 2003; Schapiro et al., 1996] and previous studies of the long-term effects of pairing macaques [Reinhardt et al., 1988] and forming groups of two or three in baboons [Kessel & Brent, 2001], the fact that the post-introduction reduction of abnormal behavior did not persist at 20–21 or 39–40 weeks was surprising. Although it may relate to our small sample size, the finding that pairing singly housed macaques may not be associated with long-term reductions in abnormal behavior is reminiscent of a previous study of single-vs. pair/trio-housed chimpanzees, which found that the presence of one or two companions during long-term indoor-housed chimpanzees did not result in levels of abnormal behaviors different from indoor single housing [Baker, 1996]. It may be that a more enriched social or physical environment is required to maintain low levels of abnormal behavior over long durations of indoor pair housing. However, an alternative hypothesis rests on the fact that the subjects of this study were removed from outdoor social housing as little as 4 months before the onset of baseline data collection. Levels of stereotypic locomotion, the form of abnormal behavior that predominated in our study population, are highly correlated with duration singly caged [Baker, unpublished data], which may relate not only to duration housed without social contact but also to duration housed in the restricted space provided by laboratory caging. As subjects had been recently moved into the caged environment, it is possible that increased time in a restricted area, despite changes in the social environment, confounded the effects of long-term pairing. We do not currently have data sufficient to test this hypothesis, but the fact that this abnormal behavior can represent the normal impulse to move away from a stimulus in an abnormally small space makes this suggestion plausible and may need to be controlled for in future study.
Although it was not surprising that levels of foraging were lower in settled pairs than in single housing, it was interesting to note that high levels of foraging were observed when the monkeys were placed in full contact. This level began to decrease as the subjects became acquainted with one another. We speculate that foraging (an activity that was not monopolizable owing to the presence of several boards per pair) may have functioned as a distraction or a way to relieve tension and avoid conflict until a relationship was established between the pairs.
The results of this study have several practical implications. First, it has been recommended [e.g., Reinhardt, 1989] that the formation of macaque pairs be performed in a space that is novel for both partners to avoid territorial reactions. This suggestion is frequently followed [e.g., Eaton et al., 1994; Kurth & Bryant, 1998; Lynch, 1998; Watson, 2002] but has not to the authors' knowledge been tested. Moving individuals immediately before introduction could also be argued to promote undesirable levels of stress and adversely affect introduction outcome. Furthermore, it is frequently impractical to take this step in laboratories that maintain populations close to the capacity of their facilities. Our findings indicate that pairing in a neutral space is not necessarily required to ensure safety and lack of distress during pair formation. It must be noted, however, that visual access to other individuals in the room was more restricted (i.e., via mirrors only) than would be the case in rooms housing monkeys on both sides of the room. Thereby, the potential for other monkeys in the room to trigger aggression was somewhat reduced. Second, this study supports the idea that initial introduction via a short period of restricted contact (i.e., through bars) may be an effective preliminary step in the formation of pairs of adult male rhesus macaques, permitting the establishment of rank and the exchange of affiliation while maintaining the ability of partners to withdraw from one another. Last, this study suggests that low levels of affiliation in the first few hours of unrestricted contact may not be predictive of a poor introduction outcome. This period of time may be most intensively monitored during social introductions; hence, behavioral staff should be aware that monkeys that exchange little affiliation at first may not be experiencing distress or rejecting their partners, and may in fact come in the long run to spend a considerable portion of their activity budget grooming.
The fact that cortisol levels were higher during single housing than in settled pairs, let alone soon after introductions, suggests that macaques can be significantly distressed when caged alone. Our results also suggest that the amount of stress experienced by laboratory primates relates to levels of human activity, as heart rates were highest during the hourly intervals covering the busiest times in animal holding areas (10:30 and 13:00). A late morning and early afternoon peak in heart rate has also been observed in other studies of caged Old World primates [Coelho et al., 1991; Line et al., 1989]. Descriptively, the difference in heart rate between the period with the highest level of human activity and the lowest level was smaller among settled pairs (11%) than in single housing (15%). Continued research on additional subjects, as well as studies investigating short-term responses to laboratory stressors, will permit us to explore the role of pair housing in supplying a social buffer [e.g., Gust et al., 1993, 1994, 1996; Winslow et al., 2003] against specific stressors associated with life in the laboratory.
Whereas a study of eight subjects cannot be used to predict the frequency of introduction success, this study does suggest that social introductions with adult male rhesus macaques can be performed successfully and without measurable distress. This conclusion is significant given the hesitance to pair adult male rhesus macaques at many facilities. In addition, in a biomedical setting, the duration of pair housing may be restricted by changing protocol requirements, subject reassignment, or planned euthanasia. As social introduction is widely viewed to be somewhat stressful, behavioral managers may factor in this perception of stress when considering whether pairing would be in the best interests of monkeys that cannot remain socially housed for a long period of time. This study hints that for adult male rhesus macaques, improvements to quality of life even over a few months would not be outweighed by the potential for a short-term decrement in well-being over the introduction process. Although the subjects of this study have been successfully pair housed for 18 months at the time of writing, such a duration of pair housing is not always necessary to confer net benefits of well-being to laboratory macaques.
Acknowledgments
This project was supported by NIH Grant P01-MH076388 to Steven D. Douglas, The Joseph Stokes, Jr. Research Institute at the Children's Hospital of Philadelphia and RR000167, RR00164, RR019628, RR012112, and RR05169 to the Tulane National Primate Research Center. All research involving subjects of this study complied with the Institutional Animal Care and Use Committee Regulations at the Tulane National Primate Research Center. We thank the husbandry staff for their assistance with this study and excellent care of the monkeys. We also thank the following veterinarians who assisted with the telemetry surgeries: Skip Bohm, Tara Ooms, Erin Ribka, and Morgan Singletary. Thanks also go to Mike Aertker for working on the telemetry system, Wayne Buck for developing software used to organize the telemetry data, Dan Wittwer for analyzing the fecal cortisol levels for this study, and Shelley Falkenstein for providing statistical support.
Contract grant sponsor: NIH; Contract grant numbers: P01-MH076388, RR000167, RR00164, RR019628, RR012112, RR05169.
References
- Baker K. Chimpanzees in single cages and small social groups: effects on behavior and wellbeing. Contemp Top Lab Anim Sci. 1996;35:61–64. [Google Scholar]
- Baker K, Aureli F. Behavioural indicators of anxiety: an empirical test in chimpanzees. Behaviour. 1997;134:1031–1050. [Google Scholar]
- Bayne K, Dexter S, Suomi S. A preliminary survey of the incidence of abnormal behavior in rhesus monkeys (Macaca mulatta) relative to housing condition. Lab Anim. 1992;21:38–46. [Google Scholar]
- Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. Am J Primatol. 2002;58:57–69. doi: 10.1002/ajp.10052. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkeys (Macaca mulatta) groups revealed in group formation studies. Folia Primatol. 1974a;21:81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Factors influencing the expression of aggression during introductions to rhesus monkey groups Primate aggression, territoriality, and xenophobia. New York: Academic Press; 1974b. pp. 211–240. [Google Scholar]
- Boccia ML, Reite M, Laudenslager M. On the physiology of grooming in a pigtail macaque. Physiol Behav. 1989;45:667–670. doi: 10.1016/0031-9384(89)90089-9. [DOI] [PubMed] [Google Scholar]
- Bourgeois SR, Brent L. Modifying the behaviour of singly caged baboons: evaluating the effectiveness of four enrichment techniques. Anim Welfare. 2005;14:71–81. [Google Scholar]
- Bowers CL, Crockett CM, Bowden DM. Differences in stress reactivity of laboratory macaques measured by heart period and respiratory sinus arrhythmia. Am J Primatol. 1998;45:245–261. doi: 10.1002/(SICI)1098-2345(1998)45:3<245::AID-AJP2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Clark MR, Harrison RM, Didier ES. Behavioral, immunological, and hormonal responses associated with social change in rhesus monkeys (Macaca mulatta) Am J Primatol. 1996;39:223–233. doi: 10.1002/(SICI)1098-2345(1996)39:4<223::AID-AJP3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Mason WA, Moberg GP. Differential behavior and adrenocortical responses to stress among three macaque species. Am J Primatol. 1988;14:37–52. doi: 10.1002/ajp.1350140104. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Mason WA, Mendoza SP. Heart rate patterns under stress in three species of macaques. Am J Primatol. 1994;33:133–148. doi: 10.1002/ajp.1350330207. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Czekala MN, Lindburg DG. Behavioral and adrenocortical responses of male cynomolgus and lion-tailed macaques to social stimulation and group formation. Primates. 1995;36:41–56. [Google Scholar]
- Coelho AM, Carey KD, Shade RE. Assessing the effects of social environment on blood pressure and heart rate of baboons. Am J Primatol. 1991;23:257–267. doi: 10.1002/ajp.1350230406. [DOI] [PubMed] [Google Scholar]
- Eaton GG, Kelley ST, Axthelm MK, Iliff-Sizemore SA, Shiigi SM. Psychological well-being in paired adult female rhesus (Macaca mulatta) Am J Primatol. 1994;33:89–99. doi: 10.1002/ajp.1350330204. [DOI] [PubMed] [Google Scholar]
- Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine. San Diego: Academic Press; 2002. [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav Immun. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK, Wilson ME. Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta) Horm Behav. 1993;27:318–331. doi: 10.1006/hbeh.1993.1024. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Brodie AR, McClure HM. Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiol Behav. 1994;55:681–684. doi: 10.1016/0031-9384(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Brodie AR, McClure HM. Effect of companions in modulating stress associated with new group formation in juvenile rhesus macaques. Physiol Behav. 1996;59:941–945. doi: 10.1016/0031-9384(95)02164-7. [DOI] [PubMed] [Google Scholar]
- Hassimoto M, Harada T. Use of a telemetry system to examine recovery of the cardiovascular system after excitement induced by handling stress in a conscious cynomolgus monkey (Macaca fascicularis) J Med Primatol. 2003;32:346–352. doi: 10.1046/j.1600-0684.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- Kessel AL, Brent L. The rehabilitation of captive baboons. J Med Primatol. 2001;30:71–80. doi: 10.1034/j.1600-0684.2001.300201.x. [DOI] [PubMed] [Google Scholar]
- Kurth B, Bryant D. Pairing female Macaca fascicularis. Lab Primate Newsl. 1998;37:3. [Google Scholar]
- Line SW, Morgan KN, Markowitz H. Heart rate and activity of rhesus monkeys in response to routine events. Lab Primate Newsl. 1989;28:9–12. [Google Scholar]
- Line SW, Morgan KN, Markowitz H, Roberts JA, Riddell M. Behavioral responses of female long-tailed macaques (Macaca fascicularis) to pair formation. Lab Primate Newsl. 1990;29:1–5. [Google Scholar]
- Line SW, Kaplan JR, Heise ER, Hilliard JK, Cohen S, Rabin BS, Manuck SB. Effects of social reorganization on cellular immunity in male cynomolgus monkeys. Am J Primatol. 1996;39:235–249. doi: 10.1002/(SICI)1098-2345(1996)39:4<235::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Novak MA. Environmental enrichment for nonhuman primates: theory and application. ILAR J. 2005;46:178–191. doi: 10.1093/ilar.46.2.178. [DOI] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Lynch R. Successful pair-housing of male macaques (Macaca fascicularis) Lab Primate Newsl. 1998;37:4–5. [Google Scholar]
- Maestripieri D, Schino G, Aureli F, Troisi A. A modest proposal: displacement activities as an indicator of emotions in primates. Anim Behav. 1992;44:967–979. [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- The psychological well-being of nonhuman primates. National Research Council; Washington, DC: National Academy Press; 1998. [Google Scholar]
- Novak MA, Suomi SJ. Psychological well-being of primates in captivity. Am Psychol. 1988;43:765–773. doi: 10.1037//0003-066x.43.10.765. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Behavioral responses of unrelated adult male rhesus monkeys familiarized and paired for the purpose of environmental enrichment. Am J Primatol. 1989;17:243–248. doi: 10.1002/ajp.1350170305. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Pair-housing rather than single-housing for laboratory rhesus macaques. J Med Primatol. 1994;23:426–431. doi: 10.1111/j.1600-0684.1994.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Addressing the social needs of macaques used for research. Lab Primate Newsl. 2002;41:7–10. [Google Scholar]
- Reinhardt V, Houser D, Eisele S, Cowley D, Vertein R. Behavioral responses of unrelated rhesus monkey females paired for the purpose of environmental enrichment. Am J Primatol. 1988;14:135–140. doi: 10.1002/ajp.1350140204. [DOI] [PubMed] [Google Scholar]
- Reinhardt V, Cowley D, Scheffler J. Cortisol response of female rhesus monkeys to venipuncture in homecage versus venipuncture in restraint apparatus. J Med Primatol. 1990;19:601–606. [PubMed] [Google Scholar]
- Reinhardt V, Cowley D, Eisele S. Serum cortisol concentrations of single-housed and isosexually pair-housed adult rhesus macaques. J Exp Anim Sci. 1991;34:73–76. [PubMed] [Google Scholar]
- Roberts SJ, Platt ML. Effects of isosexual pair-housing on biomedical implants and study participation in male macaques. Contemp Top Lab Anim Sci. 2005;44:13–18. [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Porter LM, Suarez SA. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Appl Anim Behav Sci. 1996;48:159–171. [Google Scholar]
- Schino G, Perretta G, Taglioni AM, Monaco V, Troisi A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety. 1996;2:186–191. doi: 10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychiatry. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. Final rules: code of federal regulations, title 9, part 3. Fed Regist. 1991;55:6426–6505. [Google Scholar]
- Watson LM. A successful program for same- and cross-age pair-housing adult and subadult male Macaca fascicularis. Lab Primate Newsl. 2002;41:6–9. [Google Scholar]
- Westergaard GC, Izard MK, Drake JD. Rhesus macaque (Macaca mulatta) group formation and housing: wounding and reproduction in a specific pathogen free (SPF) colony. Am J Primatol. 1999;49:339–347. doi: 10.1002/(SICI)1098-2345(199912)49:4<339::AID-AJP4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects of cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]