Abstract
This study evaluated the application of positive reinforcement training (PRT) as an intervention for abnormal behaviors in singly housed laboratory rhesus macaques at 2 large primate facilities. Training involved basic control behaviors and body-part presentation. The study compared baseline behavioral data on 30 adult males and 33 adult females compared with 3 treatment phases presented in counterbalanced order: 6 min per week of PRT, 20 or 40 min per week of PRT, and 6 min per week of unstructured human interaction (HI). Within-subject parametric tests detected no main or interaction effects involving experimental phase. However, among a subset of subjects with levels of abnormal in the top quartile of the range (n = 15), abnormal behavior was reduced from 35% to 25% of samples with PRT but not with HI. These results suggest that short durations of PRT applied as enrichment for this species and in this context may not in itself be sufficient intervention for abnormal behavior because levels remained high. However, it may be appropriate as an adjunct to other interventions and may be best targeted to the most severely affected individuals.
Abnormal behaviors in captive nonhuman primates are common both in laboratories (Bayne, Dexter, & Suomi, 1992; Bellanca & Crockett, 2002; Erwin, Mitchell, & Maple, 1973; Lutz, Well, & Novak, 2003; Nash, Fritz, Alford, & Brent, 1999; Novak, Crockett, & Sackett, 2002) and in zoos (Bollen & Novak, 2000; Tarou, Bloomsmith, & Maple, 2005). Abnormal behaviors are typically a focus of behavioral management programs for captive nonhuman primates.
These behaviors vary in form and include species-inappropriate and/or apparently functionless locomotor and postural stereotypies, oral behaviors such as regurgitation or coprophagy, and self-directed behaviors (some of them self-injurious). Abnormal behaviors emerge from a number of conditions associated with captivity. Early social deprivation and later forms of social restriction (Berkson, 1968; Dienske & Griffin, 1978; Lutz et al., 2003; Meder, 1989), the duration of time living alone, and the proportion of a monkey's life spent individually housed (Lutz et al., 2003; Novak, 2003) relate positively to the incidence of some abnormal behavior patterns. Husbandry routines such as feeding schedules and restraint practices are implicated as causes of certain types of abnormal behavior (Bloomsmith & Lambeth, 1995; Lukas, 1999; Mason, 1991). Space limitations, suboptimal levels of stimulation, and lack of environmental control are also associated with abnormal behavior (Capitanio, 1986; Draper & Bernstein, 1963; Paulk, Dienske, & Ribbens, 1977).
A wide array of social and inanimate enrichment techniques have been tested as ameliorative strategies for abnormal behaviors. Given the myriad factors that can influence the development and maintenance of abnormal behaviors—Mason and Latham (2004) review animals in the laboratory and zoo—it is not surprising that different categories of abnormal behavior vary in their apparent resistance to treatment (Novak, 2003) and response to particular interventions. For example, some feeding enrichment techniques reduce appetitive abnormal behaviors such as coprophagy and regurgitation (Baker, 1997; Bloomsmith, Alford, & Maple, 1988; Lukas, 1999) but do not affect behaviors without an oral component such as locomotor stereotypies. As another example, paired rhesus macaques showed lower levels of hair pulling than singly housed controls, but differences were not detected in abnormal behaviors with oral components (Eaton, Kelley, & Axthelm, 1994). These differential responses have been found in a number of other studies as well (Baker, 2004; Bourgeois & Brent, 2005; Clarke, Juno, & Maple, 1982; Crockett, Shimoji, & Bowden, 2000) and suggest that studies of ameliorative strategies should distinguish between abnormal behavior categories.
Positive reinforcement training is one technique that may be applied with the intention of reducing abnormal behavior. However, this strategy has received relatively little objective evaluation as a tool to combat these behaviors. There are two general approaches to using training to address abnormal behavior. The first is targeted intervention applied to specific behavioral problems in individual animals. Published case studies have reported that individualized training intervention for specific behavioral problems (hereafter termed “targeted training intervention”), often in conjunction with other environmental changes or pharmacological interventions, has been associated with some reduction of the following:
Levels of repeated regurgitation in a chimpanzee (Morgan, Howell, & Fritz, 1993),
Self-wounding in a chimpanzee (Bourgeois, Vasquez, & Brasky, 2007),
Repeated self-slapping in an orangutan (Raper, Bloomsmith, Stone, & Mayo, 2002),
Coprophagy and self-biting in drills (Cox, 1987; Desmond, Laule, & McNary, 1987), and
A variety of appetitive and self-directed abnormal behaviors in a gorilla (Pizzutto, Nichi, Corrêa, Ades, & Guimarães, 2007).
In humans, this approach has been used as behavioral therapy for stereotypy and self-injurious behavior for more than 40 years and has successfully reduced these behaviors in more than 80% of the 700 patients studied (Kahng, Iwata, & Lewin, 2002). The value of targeted training intervention for addressing similar behavioral problems in nonhuman primates has not yet been evaluated outside of case studies; see Bloomsmith, Marr, and Maple (2007) for more information.
The second method is to apply positive reinforcement training in a manner more analogous to routine environmental enrichment techniques. In other words, it is “distributed” to a large number of animals regardless of the nature, frequency, or context of any behavior problems. Such "training as enrichment" has been evaluated in two primate species. Among singly housed baboons, several categories of abnormal behavior were reduced from baseline levels, both during and—to a lesser extent—outside of training sessions, which focused on basic husbandry commands (Bourgeois & Brent, 2005). Among socially housed chimpanzees, a similar regimen of training resulted in lower levels of overall abnormal behavior during the training sessions, but there was no generalization of this effect to periods of time other than the training sessions (Bloomsmith, Baker, Ross, & Lambeth, 1999).
Positive reinforcement training may influence captive primate well being through several routes. First, with targeted training intervention, desensitization to stimuli associated with the undesirable behavior or training of behaviors incompatible with the abnormal behavior is generally attempted. Alternatively, when training is applied as a more general enrichment technique, it may benefit primates by providing cognitive stimulation, increased physical activity, and opportunities for choice and control. Finally, it is also possible that it is the simple addition of human attention and the foods that may be associated with the training that impacts well being. Unstructured human interaction, consisting of treat feeding, grooming, or playing, has shown promise as an intervention for reducing stereotypic and self-directed abnormal behaviors among singly housed rhesus macaques (Bayne, Dexter, & Strange, 1993) and both stereotypic abnormal behavior and those with oral components among socially housed chimpanzees (Baker, 2004; Bloomsmith et al., 1999).
Rhesus macaques (Macaca mulatta) are the most prevalent species held in laboratories. According to a recent survey, approximately 60% of rhesus macaques caged in the laboratory are singly housed (Baker, Weed, Crockett, & Bloomsmith, 2007). The vast majority of individuals housed in this manner display at least one type of abnormal behavior, in many cases occupying significant proportions of the activity budget (Bayne et al., 1992; Lutz et al., 2003). Particularly resistant to treatment is self-injurious abnormal behavior, which typically refers to both self-wounding and behaviors with the potential to cause injury or pain, such as self-biting (Novak, 2003). Self-wounding has been observed in between 6% and 15% of singly housed rhesus macaques, a figure that varies between laboratories (Bayne et al., 1992; Bayne, Haines, Dexter, Woodman, & Evans, 1995; Lutz et al., 2003). For these reasons, identifying successful interventions for abnormal behavior among rhesus macaques has been a major focus of the literature of applied primatology.
This study aimed to evaluate the potential for positive reinforcement training, provided as environmental enrichment, to ameliorate a wide variety of abnormal behaviors among singly housed rhesus macaques. This study involved a large sample of male and female rhesus macaques, permitting an assessment of sex differences in response to training.
This study also explored the role of rearing history on the effects of training because rearing has long been known to affect development in a variety of respects relevant to training, such as sociality and cognition (Novak & Sackett, 2006). Because a preliminary study suggested that subjects who display self-injurious behavior react differently to human interaction (Baker, Bloomsmith, Griffis, & Gierhart, 2003), the effect of the presence of this behavioral pathology on subjects’ response to training was explored as well. To disentangle the effects of time interacting with a person or the food rewards being consumed from the effects of training, the study included a comparison with human interaction that did not include training.
METHODS
This experiment was designed as a collaborative research project between the Tulane National Primate Research Center (TNPRC) and Yerkes National Primate Research Center (YNPRC). The methodologies were identical except as noted here.
Study Animals
Subjects included 30 male and 33 female rhesus macaques between 3.3 and 16.7 years (mean ± SE = 6.7 ± 0.3 years) at the start of the study. Subjects were selected for inclusion in the study based on availability and compatibility between the study described here and biomedical studies to which the animals were assigned. However, approximately half of the subjects were included due to observations, made by behavioral management or veterinary staff, of relatively high levels of abnormal behaviors. Subjects derived from a variety of rearing backgrounds:
15 were reared by their mothers in a social group for at least the first 6 months of life,
19 were mother-reared by females in mother-infant pairs for at least the first 6 months,
18 were hand-reared with peer contact in a nursery for at least 2 months during the first year of life, and
11 were hand-reared in a nursery with no peer contact for at least the first 2 years of life.
Housing and Husbandry
The 63 subjects were housed at the TNPRC (n = 28) and the YNPRC (n = 35). Subjects were assigned to various research or animal holding protocols approved by the facilities’ Institutional Animal Care and Use Committee. All aspects of management and research use conformed to applicable U.S. federal regulations and the guidelines described in the National Research Council (1996).
All subjects were singly housed indoors in stainless steel cages with a height of 32–36 inches and floor space of 4.3–8.6 square feet, depending on body weight and in accordance with federal animal welfare regulations. Animal-care staff provided food biscuits twice daily, and water was available ad libitum. Feeding enrichment, consisting of fruits, vegetables, and other food treats, was distributed three to five times per week. Each cage included a perch and a manipulable object such as a toy, PVC piece, or hardwood segment. Some cages were also equipped with foraging or grooming devices, but the inanimate environmental enrichment conditions were held constant through the period of study for all subjects. Human interaction was estimated at 30 s/day in the course of routine husbandry and feeding enrichment implementation.
Experimental Procedures
This study used a within-subjects design with four phases, a baseline condition lasting 4 weeks, and three treatment conditions, each lasting 8 weeks. The baseline condition was compared with three experimental conditions in which subjects received one each of the following treatments: 6 min per week of positive reinforcement training, 20 (at the TNPRC) or 40 (at the YNPRC) min per week of positive reinforcement training, and 6 min per week of human interaction/treat feeding. Subjects were exposed to experimental conditions presented in a pseudorandomized balanced order. One person implemented all experimental phases for a particular animal. Types of treats and quantity given were held constant across all phases. Personnel safety was ensured through compliance with standard operating procedures for personal protective equipment as well as familiarity with macaque social behavior and individual subjects’ temperaments.
Positive reinforcement training sessions involved teaching the animals control behaviors (sitting, standing, and stationing) and presentation of body parts. A small, handheld clicker was employed as a secondary reinforcer or “bridge” with food, praise, or tactile contact used as primary reinforcers. During all phases involving training, sessions were conducted in two or three sessions per week. The human-interaction phase consisted of (a) treat feeding not contingent on the subject’s behavior; (b) play; and/or (c) grooming, if the animal presented for grooming against the cage bars. Interaction sessions were conducted two or three times per week with general durations from 2 to 20 min each; some individual sessions fell out of this range because of factors such as interruptions due to unexpected activity in the room. When subjects performed abnormal behavior during training or interaction sessions, the person, in an attempt to avoid inadvertent reinforcement of the abnormal behavior, ceased interacting with the animal and resumed when the behavior stopped. If bouts of abnormal behavior lasted longer than a few seconds or involved self-biting, the person faced away until the behavior ceased.
Data Collection and Statistical Analysis
Six observers collected data after establishment of interobserver reliability among a minimum of two observers with a minimum of 85% agreement between observers. After the collection of baseline data, each experimental phase was implemented for 4 weeks before the onset of data collection. Behavioral data consisted of 60-min focal observations collected via videotape. Observation time was held steady for study conditions in recognition of the effect of time of day on behavior. The schedule of training/interaction sessions relative to videotaped observations was variable. In each phase, 4 to 8 hr of data were collected per animal, for a total of 1494 hr. All data analyzed were collected outside of the times during which the person was working with the subject. Data were coded with an exhaustive and mutually exclusive ethogram of 62 behaviors, using instantaneous sampling with a 15-s intersample interval. Predetermined decision rules were applied for priority of data entry for samples in which more than 1 behavior occurred. Only the 19 behaviors that were categorized as “abnormal” were used in this analysis.
All point samples for individual subjects were pooled across observation periods, and statistical analyses were performed using percentage of samples for each behavior in each study phase. Data were collapsed into a single category of abnormal behavior (all 19 behaviors) for some analyses. Abnormal behaviors were also grouped into the following subcategories for further analysis:
Appetitive disorders (coprophagy, deposit food and reingest, feces paint, regurgitate and reingest, urophagy),
Self-directed abnormal behavior (eye poke, overgroom, self-clasp, self-mouth),
Self-injurious behavior (self-bite, self-mutilate, self-slap), and
Stereotyped behavior (bizarre posture, flip, floating limb, head toss, pace, rock, and other stereotypies).
See Table 1 for operational definitions. All subjects spent at least 1% of samples performing abnormal behavior in the baseline condition.
TABLE 1.
Operational Definitions of Abnormal Behavior
| Appetitive Disorders |
|---|
| Coprophagy: Ingest feces |
| Deposit food: Expel chewed food/water on a substrate and reingest |
| Feces paint: Smear and rub fecal material onto a surface; may be associated with picking food out of feces |
| Regurgitate and reingest: Deliberately cause stomach contents to flow into mouth and then consume them |
| Urophagy: Lick or suck pooled urine from a surface or directly from penis |
| Self-Directed Abnormal Behavior |
| Eye poke: Digit in eye socket |
| Overgroom: Deliberately pluck hair from self, using hands or teeth |
| Self-clasp: Clutch of one’s own body with hand(s) and/or feet |
| Self-mouth: Placement of a part of the subject's body into its mouth |
| Self-Injurious Behavior |
| Self-bite: Any vigorous biting of one’s own body |
| Self-mutilate: Cause wounding to self |
| Self-slap: Contact the body in a way that is presumably painful to the monkey without causing obvious wounding |
| Stereotyped Behavior |
| Bizarre posture: Hold seemingly uncomfortable or unnatural posture |
| Flip: Repetitive somersaulting movements |
| Floating limb: Limb raised in air, subject appearing as if not in control or aware of it |
| Head toss: Throw head back and forth and move it to the side in a circular manner when sitting or standing |
| Other stereotypy: Any repetitive ritualized behavior pattern that is idiosyncratic, serves no obvious function (i.e., is not a part of play, sex, or grooming), and meets no other definitions in the category of stereotyped behavior |
| Pace: Repetitive locomotion involving at least two complete repetitions of the same path |
| Rock: Any rhythmic motions of the body from a stationary position; animal remains sitting or standing while the upper torso sways back and forth |
Subjects were classified according to their sex, facility in which they were housed, early rearing history (grouped for analysis as either mother-reared in a social setting or other setting), and on a documentation pertaining to self-injurious behavior. In addition, subjects were ranked by the percentage of time devoted to abnormal behavior during the baseline period; a quartile division was performed and subjects categorized accordingly.
These factors were used as grouping variables in a series of separate statistical analyses. Analyses of variance (ANOVA) for repeated measures were used for the comparisons of the single category of abnormal behavior across the baseline and three test conditions.
Multivariate analyses of variance (MANOVA) for repeated measures were used for the analyses of the four subcategories of abnormal behavior, again with sex, facility, rearing background, history of self-injurious behavior, and level of baseline abnormal behavior used as grouping factors. Alpha was set at 0.05 for these analyses. Significant ANOVAs were followed by t tests with alpha adjusted to 0.008 to correct for multiple comparisons, with a trend defined with alpha set between 0.008 and 0.02. Because the main intention of the study was to determine whether the various interventions differentially affected levels of abnormal behavior, interactions between study phase and the dependent measures were the statistical measure of interest.
RESULTS
An ANOVA for the single category of all abnormal behavior showed no differences across the four study phases in the time devoted to abnormal behavior (F = 0.10, p = .96, df = 3; baseline: 19.6%; 6 min/week training: 19.1%; 20–40 min/week training: 19.9%; human interaction: 18.6%). The series of between-subject ANOVAs used to test for effects with sex, facility, rearing history, and history of self-injurious behavior as grouping factors found no statistically significant interactions between any of these grouping factors and study phase (sex: F = 0.11, p = .75, df = 3; facility: F = 0.64, p = .59, df = 3; rearing history: F = 0.99, p = .40, df = 3; history of self-injurious behavior: F = 0.88, p = .45, df = 3).
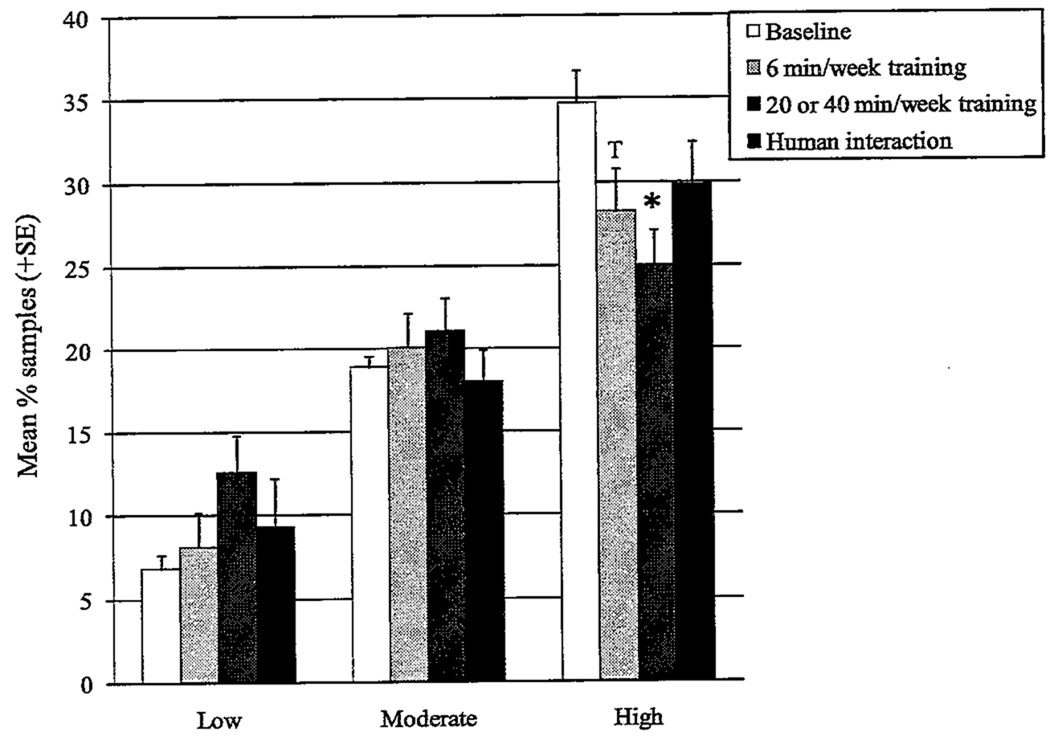
However, there was a significant interaction between quartiie grouping and study phase (F = 2.73, p = .02, df = 6). Subjects in the lowest quartiie showed no change with study phase (n = 16, F = 0.92, p = .44, df = 3), nor did subjects in the middle quartile (n = 32, F = 0.93, p = .43, df = 3). The top quartiie showed a significant change across study phases (n = 15, F = 3.45, p = .03, df = 3); pairwise comparisons with baseline found a significant reduction during 20–40 min/week training (t = 3.56, p = .003), with a trend toward a reduction 6 min/week training (t = 2.70, p = .02) but none during human interaction (t = 2.23, p = .05; Figure 1).
FIGURE 1.
Levels of abnormal behavior across study conditions; contrasts between subjects with low, moderate, or high levels of abnormal behavior in baseline conditions (* = p < .008 for pairwise comparisons with baseline; t = 0.008 < p < .02 in comparison with baseline). T = value shows trend toward significant difference from another value.
In the baseline condition, stereotypic behavior was observed in all but one subject. Fifty-three showed self-directed behavior, 43 showed self-injurious, and 14 showed appetitive-abnormal behaviors. A MANOVA applied to these four subcategories of abnormal behavior showed a significant interaction between phase of the study and these subcategories of abnormal behavior (F = 17.08, p = .00, df = 12). ANOVAs showed no effect on individual behavioral categories (stereotypic behavior: F = 0.20, p = .90, df = 3; appetitive behavior (F = 0.80, p = .50, df = 3); self-directed behavior (F = 0.89, p = .45, df = 3); self-injurious behavior (F = 0.25, p = .86, df = 3).
As with the analysis of all abnormal behaviors, in the MANOVA applied to the subcategories of abnormal behavior, there was a significant interaction between quartiie grouping and study phase (F = 5.67, p = .00, df = 24). Subjects in the lowest quartile showed no change with study phase (F = 61.50, p = .10, df = 12). Subjects in the middle quartile did (F = 36.64, p = .00, df = 12), but ANOVAs showed no effect on individual behavioral categories: appetitive behavior (F = 0.45, p = .72, df = 3); self-directed behavior (F = 2.48, p = .07, df = 3); self-injurious behavior (F = 0.06, p = .98, df = 3); stereotypic behavior (F = 0.46, p = .71, df = 3).
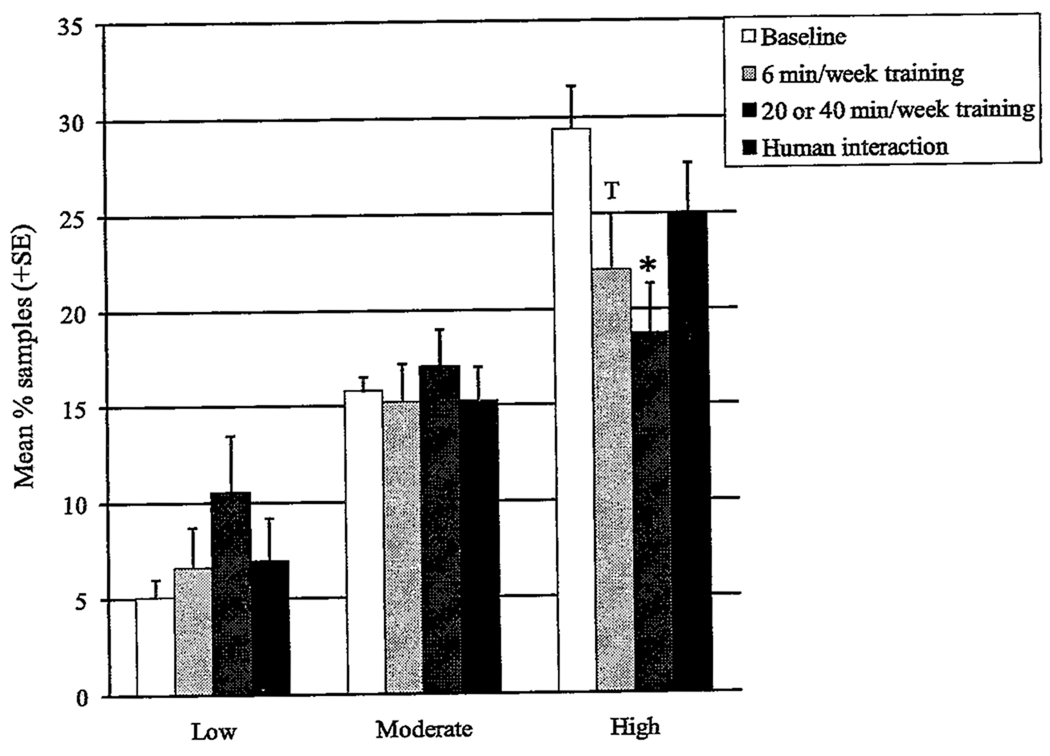
The top quartile showed a significant change (F = 42.18, p = .03, df = 12). ANOVAs showed significant reductions in stereotypic behavior (F = 3.55, p = .02, df = 3), with pairwise comparisons revealing a drop during 20–40 min/week training (t = 3.44, p = .004) and a trend in the same direction for 6 min/week training (t = 2.63, p = .02); no difference was seen with human interaction (t = 1.97, p = .07). Differences were not seen for appetitive behavior (F = 0.37, df = 3, p = .78), self-directed behavior (F = 0.12, df = 3, p = .95), or self-injurious behavior (F = 0.89, df = 3, p = .45) (Figure 2).
FIGURE 2.
Levels of stereotypic abnormal behavior categories in the four study phases (* = p < .008 for pairwise comparisons with baseline; t = 0.008 < p < .02 in comparison with baseline). T = value shows trend toward significant difference from another value.
DISCUSSION
This study found evidence that positive reinforcement training as enrichment (involving control and body-part presentation behaviors and provided regardless of individual behavior) reduced levels of abnormal behavior in singly housed rhesus macaques. However, this improvement was seen only among the most seriously affected individuals, was restricted to one type of abnormal behavior, and abnormal behavior remained elevated well above levels documented with other forms of enrichment intervention for caged laboratory macaques (Bayne et al., 1992; Bayne et al., 1991; Bellanca & Crockett, 2002; Bryant, Rupniak, & Iversen, 1988; Crockett, Bellanca, Heffernan, Ronan, & Bonn, 2001; Crockett et al., 1995; Crockett et al., 2000; Eaton et al., 1994; Kessel & Brent, 1998; Lam, Rupniak, & Iverson, 1991; Schapiro, Bloomsmith, Porter, & Suarez, 1996).
The behavioral effects of training as enrichment have been poorly studied and, to our knowledge, have shown carry-over effects on abnormal behavior in only one study, involving baboons (Bourgeois & Brent, 2005); however, for several reasons, one still might have expected this approach to be more fruitful. In a prior study of rhesus macaques (Bayne et al., 1993) and in several studies of chimpanzees (Baker, 2004; Bloomsmith et al., 1999), unstructured human interaction as enrichment has been associated with improvements in abnormal behavior. Because training provides not only human interaction but also cognitive stimulation and opportunities for choice and control, it would be surprising that its benefits would not include those associated with human interaction. It is important to note, however, that this study found no effects of unstructured human interaction on abnormal behavior.
This result contrasts with the findings of Bayne et al. (1993) in terms of statistical significance, and it is striking to note that the mean decrease in abnormal behavior in the current study (5%) is considerably smaller than that found in the 1993 study (approximately 13%). It was important to attempt replicating that study with a larger sample size of subjects. Although rearing backgrounds of subjects in the Bayne et al. (1993) study were not indicated, the current study did not detect any role of rearing in the effects of either unstructured interaction or positive reinforcement training; therefore, differences in rearing background between subjects in the two studies are unlikely to explain the difference in their findings.
Several factors may account for the contrast between the current study and previous research. First, species’ differences may play a role in the overall response to humans. Several factors, such as the degree of similarity between human and chimpanzee social signals or body size in chimpanzees and baboons, may reduce their fear of humans and make them more receptive to interaction of any type. All previous studies of training as enrichment involved only large-bodied primate species; the same is true of cases of successful targeted-training intervention. Also, in the chimpanzee studies, subjects were socially housed. Although one might anticipate that response to a particular behavioral management technique might increase in magnitude with lower baseline level of environmental complexity, it may be that the effects of long-term single housing are severe enough to overwhelm the effect of the periodic social stimulation provided by people.
Was training a positive experience for the subjects of the current study? Subjects chose to interact with the trainer during an average of 96% of the sessions. This suggests that the subjects were not avoiding training interactions. Because they came to reliably perform a mean of three different commands over the course of the study, the monkeys clearly learned commands.
Because the training protocol involved brief pauses in training if abnormal behavior was performed, it is unlikely that intermittent reinforcement of abnormal behavior influenced results. Analyses of other data associated with this project (training and interaction records) found that subjects showed no differences in level of abnormal behavior between the different treatment phases. However, fearful behavior was shown during a larger proportion of training sessions than human interaction sessions of equal length; in addition, the frequency of fearful behavior increased over the training phase but not over the human interaction phase (Maloney et al., 2007).
Although the training techniques of desensitization can be applied to reduce rhesus macaques’ fear of humans (Clay, Bloomsmith, Marr, & Maple, 2009), they were not used in this study. It is possible, therefore, that, for some captive rhesus monkeys, training interactions with humans may have an aversive component that worked against the benefits of cognitive stimulation and control. More work should be done to understand the role of fear; perhaps the routine application of desensitization before initiating other training sessions would be beneficial with rhesus macaques (Clay et al., 2009).
It is notable that the majority of studies demonstrating a positive effect of training on abnormal behavior involved targeted training intervention for particular abnormal behaviors in individual animals (see introduction) rather than the more generalized application not tailored to particular animals or problems (training as enrichment). This difference suggests that behavioral management programs may produce more of an effect on abnormal behavior by implementing individualized training programs to combat particular abnormal behavior problems in a relatively small subset of animals rather than attempt to apply generalized training as enrichment on a broad scale. That this study only observed benefits to those animals with extreme levels of abnormal behavior supports the provision of targeting training for a subset of the population. The need to identify subsets of animals who might benefit from training is also suggested by the fact that only stereotypic behaviors, but not other abnormal behavior categories, were reduced in any treatment condition.
This study evaluated up to 40 min per week of positive reinforcement training. Although it is of course possible that training with a more intensive schedule than the range investigated in this study could result in more significant behavioral improvements, even the amount of training studied here, if applied colonywide, would realistically require a full-time trainer for as few as 50 animals. Because few laboratory facilities in the United States staff enrichment programs with this low technician-to-animal ratio—facilities average one enrichment technician for about 360 caged nonhuman primates (Baker et al., 2007)—greater amounts of training for any significant number of animals is clearly outside the bounds of practicality. If the goal is to reduce abnormal behavior, the time that could be invested in positive reinforcement training might be more effective were it devoted instead to increasing use of social housing or various inanimate enrichment strategies of demonstrated benefit.
Whereas broad implementation of generalized training as enrichment is unlikely to reduce abnormal behavior in most of a facility’s singly housed rhesus macaque population, the approach used to treat abnormal behaviors in institutionalized humans may provide a productive model for using training to moderate abnormal behavior in nonhuman primates. The literature on “functional assessment” techniques, which involve investigating the causes and contexts of the undesirable behavior and the tailoring of intervention to the specific cause, has been used to address other unwanted behaviors in captive primates (Clay, et al., 2009; Martin et al., 2007) and may provide more effective models for treating abnormal behavior with training (Bloomsmith et al., 2007).
Training benefits captive animal well being through a variety of routes other than the amelioration of abnormal behavior. For example, training can be used to boost levels of desirable social behaviors in rhesus macaques (Cox, 1987; Desmond et al., 1987; Schapiro, Perlman, & Boudreau, 2001) as well as to reduce agonism in chimpanzees (Bloomsmith et al., 1999; Bloomsmith, Laule, Alford, & Thurston, 1994). It has also been found to reduce stress as measured with cortisol, hematological, and cardiovascular measures in a variety of species (Bassett, Buchanan-Smith, McKinley, & Smith, 2003; Koban, Miyamoto, Donmoyer, & Hammar, 2005; Lambeth, Hau, Perlman, Martino, & Schapiro, 2006; Moseley & Davis, 1989; Reinhardt, Cowley, Scheffler, Vertein, & Wegner, 1990).
These findings highlight the need for behavioral management programs to increase emphasis on the prevention of abnormal behavior rather than aiming only for intervention once behavioral problems are established. This study may also be significant in light of the U.S. Department of Agriculture (USDA) Draft Policy on Environmental Enhancement (USDA, 1999), which stated the following:
Housing without the animal having the opportunity for continuous visual, auditory, olfactory, and tactile contact with a compatible primate [requires] daily positive interaction with compatible human care givers. The human contact should be of sufficient type and duration to compensate for restricted social housing. (p. 38147)
CONCLUSION
Given the correspondence between social housing and abnormal behavior found in surveys (Bayne et al., 1992; Bellanca & Crockett, 2002; Lutz et al., 2003) and in experimental studies (Baker, 1996; Bourgeois & Brent, 2005; Chamove, Anderson, & Nash, 1984; Eaton et al., 1994; Kessel & Brent, 2001; Schapiro, 2002) and the results of the current study, it is unlikely that a practical application of human interaction or training will be effective in replacing conspecific social housing with regard to its benefit on abnormal behavior. The findings of this study may be useful for guiding the most effective allocation of human and financial resources toward improving the well being of captive primates.
ACKNOWLEDGMENTS
This study was supported by the Base Grants to the Tulane National Primate Research Center (NCRR/NIH P51 RR00164) and Yerkes National Primate Research Center (NCRR/NIH P51 RR00165). Both facilities are fully accredited by the Association for the Assessment and Accreditation of Laboratory Care International.
REFERENCES
- Baker KC. Chimpanzees in single cages and small social groups: Effects of housing on behavior. Contemporary Topics in Laboratory Animal Science. 1996;35:71–74. [Google Scholar]
- Baker KC. Straw and forage material ameliorate abnormal behaviors in adult chimpanzees. Zoo Biology. 1997;16:225–236. [Google Scholar]
- Baker KC. Benefits of positive human interaction for socially-housed chimpanzees. Animal Welfare. 2004;13:239–245. [PMC free article] [PubMed] [Google Scholar]
- Baker K, Bloomsmith M, Griffis C, Gierhart M. Self-injurious behavior and response to human interaction as enrichment in rhesus macaques. American Journal of Primatology. 2003;60:94–95. [Google Scholar]
- Baker KC, Weed JL, Crockett CC, Bloomsith MA. Survey of behavioral management programs for laboratory primates. American Journal of Primatology. 2007;69:377–394. doi: 10.1002/ajp.20347. [DOI] [PubMed] [Google Scholar]
- Bassett L, Buchanan-Smith HM, McKinley J, Smith TE. Effects of training on stress-related behavior of the common marmoset (Callithrix jacchus) in relation to coping with routine husbandry procedures. Journal of Applied Animal Welfare Science. 2003;6:221–233. doi: 10.1207/S15327604JAWS0603_07. [DOI] [PubMed] [Google Scholar]
- Bayne KAL, Dexter SL, Strange MS. The effects of food treat provisioning and human interaction on the behavioral well-being of rhesus monkeys (Macaca mulatta) Contemporary Topics in Laboratory Animal Science. 1993;32:6–9. [PubMed] [Google Scholar]
- Bayne K, Dexter S, Suomi S. A preliminary survey of the incidence of abnormal behavior in rhesus monkeys (Macaca mulatta) relative to housing condition. Lab Animal. 1992;21:38–46. [Google Scholar]
- Bayne K, Haines M, Dexter S, Woodman D, Evans C. Nonhuman primate wounding prevalence: a retrospective analysis. Lab Animal. 1995;24:40–44. [Google Scholar]
- Bayne K, Mainzer H, Dexter SL, Campbell G, Yamada R, Suomi S. The reduction of abnormal behaviors in individually housed rhesus monkeys (Macaca mulatta) with a foraging/grooming board. American Journal of Primatology. 1991;23:23–35. doi: 10.1002/ajp.1350230104. [DOI] [PubMed] [Google Scholar]
- Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. American Journal of Primatology. 2002;58:57–69. doi: 10.1002/ajp.10052. [DOI] [PubMed] [Google Scholar]
- Berkson G. Development of abnormal stereotyped behaviors. Developmental Biology. 1968;1:118–132. [Google Scholar]
- Bloomsmith MA, Alford PL, Maple TL. Successful feeding enrichment for captive chimpanzees. American Journal of Primatology. 1988;16:155–164. doi: 10.1002/ajp.1350160206. [DOI] [PubMed] [Google Scholar]
- Bloomsmith MA, Baker KC, Ross SK, Lambeth SP. Comparing animal training to non-training human interaction as environmental enrichment for chimpanzees. American Journal of Primatology. 1999;49:35–36. [Google Scholar]
- Bloomsmith MA, Lambeth SP. Effects of predictable versus unpredictable feeding schedules on chimpanzee behavior. Applied Animal Behaviour Science. 1995;44:65–74. [Google Scholar]
- Bloomsmith MA, Laule GE, Alford PL, Thurston RH. Using training to moderate chimpanzee aggression during feeding. Zoo Biology. 1994;13:557–566. [Google Scholar]
- Bloomsmith MA, Marr MJ, Maple TL. Addressing nonhuman primate behavioral problems through the application of operant conditioning: Is the human treatment approach a useful model? Applied Animal Behaviour Science. 2007;102:205–222. [Google Scholar]
- Bollen KS, Novak MA. A survey of abnormal behavior in captive zoo primates. American Journal of Primatology. 2000;51:47. [Google Scholar]
- Bourgeois SR, Brent L. Modifying the behaviour of singly caged baboons: Evaluating the effectiveness of four enrichment techniques. Animal Welfare. 2005;14:71–81. [Google Scholar]
- Bourgeois SR, Vazquez M, Brasky KM. Combination therapy reduces self-injurious behavior in a chimpanzee (Pan troglodytes troglodytes): A case report. Journal of Applied Animal Welfare Science. 2007;10:123–140. doi: 10.1080/10888700701313454. [DOI] [PubMed] [Google Scholar]
- Bryant CE, Rupniak NMJ, Iversen SD. Effects of different environmental enrichment devices on cage stereotypies and autoaggression in captive cynomolgus monkeys. Journal of Medical Primatology. 1988;17:257–269. [PubMed] [Google Scholar]
- Capitanio JP. Behavioral pathology. In: Mitchell G, Erwin J, editors. Comparative primate biology: Vol. 2, Pt. A. Behavior, conservation, and ecology. New York: Alan R. Liss; 1986. pp. 411–454. [Google Scholar]
- Chamove AS, Anderson JR, Nash VJ. Social and environmental influences on self-aggression in monkeys. Primates. 1984;25:319–325. [Google Scholar]
- Clarke AS, Juno CJ, Maple TL. Behavioral effects of a change in the physical environment: A pilot study of captive chimpanzees. Zoo Biology. 1982;1:371–380. [Google Scholar]
- Clay AW, Bloomsmith MA, Marr MJ, Maple TL. Comparing training methods to reduce fearful behavior in singly housed rhesus macaques. American Journal of Primatology. 2009;71:30–39. doi: 10.1002/ajp.20622. [DOI] [PubMed] [Google Scholar]
- Cox C. Increase in the frequency of social interactions and the likelihood of reproduction among drills. Proceedings of the American Association of Zoological Parks and Aquariums Annual Conference; Portland, OR. 1987. [Google Scholar]
- Crockett CM, Bellanca RU, Heffernan KS, Ronan DA, Bonn WF. Puzzle Ball foraging device for laboratory monkeys. Laboratory Primate Newsletter. 2001;40:4–7. [Google Scholar]
- Crockett CM, Bowers CL, Shimoji M, Leu M, Bowden DM, Sackett GP. Behavioral responses of longtailed macaques to different cage sizes and common laboratory experiences. Journal of Comparative Psychology. 1995;109:368–383. doi: 10.1037/0735-7036.109.4.368. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Shimoji M, Bowden DM. Behavior, appetite, and urinary cortisol responses by adult female pigtailed macaques to cage size, cage level, room change, and ketamine sedation. American Journal of Primatology. 2000;52:63–80. doi: 10.1002/1098-2345(200010)52:2<63::AID-AJP1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Desmond T, Laule G, McNary J. Training to enhance socialization and reproduction in drills; Proceedings of the American Association of Zoological Parks and Aquariums Annual Conference; Portland, OR. 1987. [Google Scholar]
- Dienske H, Griffin R. Abnormal behavior patterns developing in chimpanzee infants during nursery care: A note. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1978;19:387–391. doi: 10.1111/j.1469-7610.1978.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Draper WA, Bernstein IS. Stereotyped behavior and cage size. Perceptual and Motor Skills. 1963;16:231–234. doi: 10.2466/pms.1963.17.2.368. [DOI] [PubMed] [Google Scholar]
- Eaton GE, Kelley ST, Axthelm MK. Psychological well-being in paired adult female rhesus (Macaca mulatta) American Journal of Primatology. 1994;33:89–99. doi: 10.1002/ajp.1350330204. [DOI] [PubMed] [Google Scholar]
- Erwin J, Mitchell G, Maple T. Abnormal behavior in non-isolate-reared rhesus monkeys. Psychological Reports. 1973;33:515–523. doi: 10.2466/pr0.1973.33.2.515. [DOI] [PubMed] [Google Scholar]
- Kahng S, Iwata BA, Lewin AB. Behavioral treatment of self-injury, 1964 to 2000. American Journal of Mental Retardation. 2002;107:212–221. doi: 10.1352/0895-8017(2002)107<0212:BTOSIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kessel AL, Brent L. Cage toys reduce abnormal behavior in individually housed pigtail macaques. Journal of Applied Animal Welfare Science. 1998;1:227–234. doi: 10.1207/s15327604jaws0103_3. [DOI] [PubMed] [Google Scholar]
- Kessel AL, Brent L. The rehabilitation of captive baboons. Journal of Medical Primatology. 2001;30:71–80. doi: 10.1034/j.1600-0684.2001.300201.x. [DOI] [PubMed] [Google Scholar]
- Koban TL, Miyamoto M, Donmoyer G, Hammar A. Effects of positive reinforcement training on cortisol, hematology and cardiovascular parameters in cynomolgus macaques (Macaca fascicularis) American Journal of Primatology. 2005;66:148. [Google Scholar]
- Lam K, Rupniak NMJ, Iversen SD. Use of a grooming and foraging substrate to reduce cage stereotypies in macaques. Journal of Medical Primatology. 1991;20:104–109. [PubMed] [Google Scholar]
- Lambeth SP, Hau J, Perlman JE, Martino M, Schapiro SJ. Positive reinforcement training affects hematologic and serum chemistry values in captive chimpanzees (Pan troglodytes) American Journal of Primatology. 2006;68:245–256. doi: 10.1002/ajp.20148. [DOI] [PubMed] [Google Scholar]
- Lukas KE. A review of nutritional and motivational factors contributing to the performance of regurgitation and reingestation in captive lowland gorillas (Gorilla gorilla gorilla) Applied Animal Behaviour Science. 1999;63:237–249. [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. American Journal of Primatology. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Maloney MA, Baker KC, Griffis C, Neu K, Bloomsmith M, Martinez M. Behavioral responses by singly housed adult rhesus macaques (Macaca mulatta) during positive reinforcement training and human interaction. American Journal of Primatology. 2007;69:53. [Google Scholar]
- Martin AL, Bloomsmith MA, Clay AW, Kelley ME, Marr MJ, Maple TL. The role of behavior analysis in the behavioral management of nonhuman primates. American Journal of Primatology. 2007;69:119–120. [Google Scholar]
- Mason GJ. Stereotypies: A critical review. Animal Behavior. 1991;41:1015–1037. [Google Scholar]
- Mason GJ, Latham N. Can#x02019;t stop, won’t stop: Is stereotypy a reliable animal welfare indicator? Animal Welfare. 2004;13:S57–S69. [Google Scholar]
- Meder A. Effects of hand-rearing on the behavioral development of infant and juvenile gorillas (Gorilla g. gorilla) Developmental Psychobiology. 1989;22:357–376. doi: 10.1002/dev.420220404. [DOI] [PubMed] [Google Scholar]
- Morgan L, Howell SM, Fritz J. Regurgitation and reingestion in a captive chimpanzee (Pan troglodytes) Lab Animal. 1993;22:42–45. [Google Scholar]
- Moseley JR, Davis JA. Psychological enrichment techniques and New World monkey restraint device reduce colony management time. Lab Animal. 1989;18:31–33. [Google Scholar]
- Nash LT, Fritz J, Alford PA, Brent L. Variables influencing the origins of diverse abnormal behaviors in a large sample of captive chimpanzees (Pan troglodytes) American Journal of Primatology. 1999;48:15–29. doi: 10.1002/(SICI)1098-2345(1999)48:1<15::AID-AJP2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Novak MA. Self-injurious behavior in rhesus monkeys: New insights into its etiology, physiology, and treatment. American Journal of Primatology. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Novak MA, Crockett CM, Sackett GP. Self-injurious behavior in captive macaque monkeys. In: Schroeder S, Oster-Granite ML, Thompson T, editors. Self-injurious behavior: Gene-brain-behavior relationships. Washington, DC: APA Books; 2002. pp. 151–161. [Google Scholar]
- Novak MA, Sackett GP. The effects of rearing experiences: The early years. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery rearing of nonhuman primates in the 21st century. New York: Springer; 2006. pp. 5–19. [Google Scholar]
- Paulk HH, Dienske H, Ribbens LG. Abnormal behavior in relation to cage size in rhesus monkeys. Journal of Abnormal Psychology. 1977;86:87–92. doi: 10.1037//0021-843x.86.1.87. [DOI] [PubMed] [Google Scholar]
- Pizzutto CS, Nichi M, Corrêa SHR, Ades C, Guimarães MADBV. Reduction of abnormal behavior in a gorilla (Gorilla gorilla gorilla) through social interaction with human beings. Laboratory Primate Newsletter. 2007;46:6–10. [Google Scholar]
- Raper JR, Bloomsmith MA, Stone A, Mayo L. Use of positive reinforcement training to decrease stereotypic behaviors in a pair of orang-utans (Pongo pygmaeus) American Journal of Primatology. 2002;57:70–71. [Google Scholar]
- Reinhardt V, Cowley D, Scheffler J, Vertein R, Wegner F. Cortisol response of female rhesus monkeys to venipuncture in homecage versus venipuncture in restraint apparatus. Journal of Medical Primatology. 1990;19:601–606. [PubMed] [Google Scholar]
- Schapiro SJ. Effects of social manipulations and environmental enrichment on behavior and cell-mediated immune responses in rhesus macaques. Pharmacology, Biochemistry, and Behavior. 2002;73:271–278. doi: 10.1016/s0091-3057(02)00779-7. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Porter LM, Suarez SA. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Applied Animal Behaviour Science. 1996;48:159–172. [Google Scholar]
- Schapiro SJ, Perlman JE, Boudreau BA. Manipulating the affiliative interactions of group-housed rhesus macaque using positive reinforcement training techniques. American Journal of Primatology. 2001;55:137–149. doi: 10.1002/ajp.1047. [DOI] [PubMed] [Google Scholar]
- Tarou LR, Bloomsmith MA, Maple TL. Survey of stereotypic behavior in prosimians. American Journal of Primatology. 2005;65:181–196. doi: 10.1002/ajp.20107. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. Animal welfare: Draft policy on environmental enhancement for nonhuman primates. Federal Register. 1999;64:38145–38150. [Google Scholar]