Abstract
Objectives
Compare quality-of-life ratings of terminally ill cancer versus non-cancer patients over time.
Design
Secondary analysis of prospective data from a randomized clinical trial.
Setting
Trial conducted with terminally ill patients in Seattle, Washington, testing the efficacy of massage and guided meditation in improving patients' quality of life.
Participants
167 trial participants, of whom 127 provided follow-up data and died before data analysis.
Measurements
At enrollment, participants reported demographic characteristics, symptom distress, quality of life, and primary life-limiting diagnosis. At enrollment and at follow-up interviews after every two study-provided treatment sessions, participants rated their perceived quality of life on a scale from 0 (no quality of life) to 10 (perfect quality). At the end of the study the researchers added measures of patient's survival status, number of days between study enrollment and death, and receipt of hospice services.
Results
Multilevel models showed significantly steeper quality-of-life declines for cancer patients than for non-cancer patients after adjustment for time between study enrollment and death. Over a 4-month pre-death period, the average non-cancer patient was estimated to experience a quality-of-life decline of about 0.6 on a 0-10 scale, compared with a 1.2-point decline for cancer patients.
Conclusion
Cancer patients face more precipitous end-of-life challenges to quality of life than do other terminally ill persons. Therefore, clinicians must address quality-of-life issues – not just symptom burden and distress. By introducing and discussing expected quality-of-life declines at the end of life, clinicians may help to prepare, support, and reassure patients and their families.
Keywords: end of life, quality of life, cancer, multilevel modeling
Introduction
In the late 1960s Glaser and Strauss introduced the concept of trajectories of decline at the end of life, finding through qualitative research that hospital personnel used accumulated knowledge about varying trajectories to organize delivery of health care.1 Caregivers' expectations about the likely duration and shape of an individual patient's illness progression allowed them to anticipate and respond promptly to patients' changing needs. Almost 30 years later a report from the Institute of Medicine, aimed at improving end-of-life care, revisited the concept and provided schematics for three possible end-of-life trajectories, each having implications for optimal end-of-life care.2 Since then, researchers have focused on defining trajectories associated with leading causes of death, documenting their characteristics, and demonstrating the usefulness of this concept for end-of-life research.
Lunney and associates examined four distinct trajectories associated with specific causes of death: sudden death, death from cancer, organ failure deaths associated with congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD), and longer-term frailty and decline associated with stroke, dementia, and other diagnoses related to old age. The four groups exhibited significant differences in demographic characteristics, patterns of care, and healthcare expenditures.3 Other researchers have investigated the types of decline over time typically experienced by patients with specific terminal illnesses. Three studies4-6 examined symptom distress and quality of life, collecting data from patients/surrogates at multiple follow-up points over the last six months of life and from surrogates for the final three days of life. Each study focused on a single disease group (cancer,4 CHF,5 COPD6), presenting the pattern of decline typical for that disease, but none of them directly compared outcome trajectories of the three groups. All three studies substituted surrogate responses (albeit calibrated to those of patients) when patients became too ill to participate, thus potentially imputing some responses that did not perfectly match patients' own perceptions.
We are aware of only two studies that directly compared outcome trajectories of patients from different disease groups.7,8 Neither was based on data collected directly from patients, and neither considered quality of life. One used information regarding onset of functional impairments, gathered during a single retrospective interview with a decedent's family member or friend.7 The other used data collected quarterly during the year before patients' deaths from nurses in long-term care facilities.8 Both studies focused on dependence in activities of daily living – an area that could be reasonably reported by surrogate respondents. Both reported steeper functional declines over the last year of life among cancer patients than among patients who died from other causes.
To our knowledge, there have been no studies directly comparing disease groups on changes over time in perceived quality of life. A research group that found differences in functional decline between cancer and non-cancer patients noted as a study limitation the unavailability of data on patients' quality-of-life perceptions.7 Although health status indicators have sometimes been used as proxies for quality of life, they are conceptually distinct – the former representing more objectively observable aspects of health and function, and the latter representing patients' evaluations of their life circumstances, including but not limited to satisfaction with health status. One study of elderly patients found reasonably strong associations between global quality-of-life ratings and health-status outcomes but reported a substantial minority of patients whose perceptions of their quality of life differed markedly from what would have been predicted from their more objective health status.9
Patients' differential ability to adapt to declines in health suggests that studies directly comparing diagnosis groups on perceived quality of life might be useful. Such studies will be of particular value if they are based on repeated measures, reported over time by the same respondent. Studies that rely on a combination of patient and surrogate data, with surrogates providing data after patients become too impaired to respond for themselves, run the risk of inferring true declines in status from declines that might be more appropriately attributed to changes in respondent. A review of studies measuring agreement between patients and surrogates has suggested that family/friend respondents provide significantly more pessimistic reports of patient symptoms and function than patients report, themselves.10
Our primary goal in this study was to provide a direct test of whether, like functional status, declines in quality of life for cancer patients are disproportionately steep, when compared to those of patients dying from other causes. Results of this test could provide guidance to clinicians as they attempt to prepare and support patients and their families as patients approach death. As part of a recent clinical trial of complementary and alternative medicine therapies with patients at end of life, we collected quality-of-life assessments from patients throughout their study participation. We have reported the association of the therapeutic interventions with patient outcomes elsewhere.11 In this article we report the results of secondary analyses, examining differences in quality-of-life trajectories by disease group (cancer vs. other diseases).
Methods
Study Sample
Data came from a randomized trial testing the effects of two active interventions (therapeutic massage and guided meditation) on quality of life at the end of life, when compared to the effects of an attention-control condition (friendly visits) that served as a proxy for volunteer services routinely provided as part of standard hospice care. Inasmuch as treatment group did not have a significant association with patients' self-reported quality of life,11 we combined the three treatment groups for the secondary analyses reported here.
Participants in the trial included hospice or palliative care patients from the Seattle metropolitan area, who spoke English, were at least 18 years old, were mentally capable of providing reliable responses during a 60-90 minute baseline interview, were expected to survive for at least 3 weeks after enrollment, and agreed to accept random assignment. Persons who met all other study requirements, but who were not receiving hospice or palliative care, could participate if they had been diagnosed with acquired immune deficiency syndrome or stage IV cancer. The University of Washington Human Subjects Division approved all study procedures.
The sample used for our primary analyses included 127 study participants who provided quality-of-life data at one or more follow-up interviews and who had died by the time of analysis. In preliminary analyses we compare the characteristics of this group with those of the full sample of 167 patients who were randomized to treatment.
Data Collection
Study interviewers conducted in-person structured baseline interviews at locations of the patients' choice, typically patients' residences. Computerized randomization to one of the three treatment groups occurred immediately following the baseline interview. After randomization, patients received up to two treatment visits per week of the assigned type, on a schedule and at a location of their choosing, with a brief follow-up interview after every two treatment visits. Follow-up interviews were either in person at patients' desired location or by telephone, depending upon patient ability and preference. Participation continued until the patient's death, voluntary withdrawal, or end of the study. Treatment visits were contingent upon regular provision of follow-up data until such time as study staff determined that the patient was too physically or cognitively impaired to complete interviews. Patients deemed incapable of further interviews continued to receive treatment until death, but we collected no data during this final treatment-only period.
Outcome
The outcome of interest was the patient's rating of global quality of life (QOL), a measure included as a validation item on an instrument constituting the Perceived Quality of Life (PQoL) Scale.12,13 At each interview we asked patients to rate their overall quality of life during the past 7 days, using a scale ranging from 0 (no quality of life) to 10 (perfect quality of life). Like the PQoL scale items, the global rating reflects respondents' perceptions, and the anchors on the single-item rating are somewhat similar in meaning to those used for the PQoL scale items (“extremely dissatisfied” to “extremely satisfied”). Quality of life is a complex and multidimensional construct often measured with multi-item scales, and at the baseline interview patients completed all 19 PQoL scale items. However, prior research has shown high correlations between overall quality-of-life ratings and multi-item scales,14,15 and in order to reduce respondent burden during frequent and numerous follow-up sessions, the study designers used an abbreviated version of the PQoL that included only five of the 19 scale items, along with the single-item rating. Because there has not been research evaluating the validity of scales based on subsets of the PQoL, we used the single-item global rating as the outcome for our analyses.
Primary Predictors
Predictors of interest occurred at two levels. We will refer to the predictor that was time specific (i.e., that could vary from one interview to the next for the same patient) as a “level-1” predictor, and to predictors that were constant for a patient over all interviews as “level-2” predictors. The level-2 predictor of interest was the patient's primary diagnosis, dichotomized into cancer versus other diseases. The level-1 predictor of interest measured how many days prior to the patient's death the interview occurred – a negative number, increasing with forward movement in time, with zero representing the day of death.
Other Variables
We used 14 level-2 variables to describe the sample or to test/adjust for confounding in regression models. Nine variables came from patients' self reports at baseline: gender, age at enrollment, race-ethnicity (white non-Hispanic vs. minority), education (six categories), average monthly income for 3 years before study enrollment (eight categories), disease group (five categories), baseline symptom distress (mean for 32 items from the Memorial Symptom Assessment Scale, each item ranging from 0 [symptom absent] through 4 [present and causing very great distress]),16 baseline PQoL12,13 scale score (mean for 19 PQoL items, each ranging from 0 [extremely dissatisfied] through 10 [extremely satisfied]), and baseline global quality of life (QOL; 0 [no quality of life] through 10 [perfect quality of life]). We computed five additional level-2 covariates after the study ended: receipt of hospice care during period of study participation, survival status at the time of data analysis, elapsed time from enrollment to final follow-up interview, and – for decedents – elapsed time from final follow-up to death and from study enrollment to death.
Statistical Analysis
In initial analyses we tested for differences between cancer and non-cancer groups (and between patients included versus excluded from the primary analyses), using Fisher's Exact Test for dichotomous variables and Mann-Whitney Z-approximations for ordinal variables. We compared estimated survival times for cancer and non-cancer patients, using Kaplan-Meier analysis with the Breslow generalized Wilcoxon test.
We based our primary analyses of quality-of-life trajectories on multilevel modeling,17-19 with interviews clustered within patients. At level 1 (interview level) we regressed patient j's QOL at interview i on the timing of interview i relative to patient j's death (measured in negative days). This procedure provided for each patient an outcome trajectory over time, represented by the slope of the time predictor. At level 2, we regressed patients' level-1 intercepts and slopes on their diagnoses (cancer/non-cancer), adjusted for confounders. The following is a simplified model with no covariates, predicting QOL from days before death (DBD) and the patient's diagnosis:
-
Level 1 model:
QOLij = β0j+β1jDBDij+eij
-
Level 2 model:
β0j = γ00 + γ01Cancerj + u0j
β1j = γ10 + γ11Cancerj + u1j
The level-2 model estimates the following four fixed effects, with the fourth being of particular interest in the current article:
γ00 The estimated average QOL for non-cancer patients on day of death
γ01 Value added to this average day-of-death QOL if the patient had cancer
γ10 Change in QOL for all patients for each day progressing toward death
γ11 Additional change for each day progressing toward death if the patient had cancer
A significant negative value on γ11 provided evidence that cancer patients experienced steeper declines in QOL over time than did non-cancer patients.
We anticipated that even in the absence of statistically significant curvilinear effects of time on QOL, patients might experience relatively slow QOL declines at times distant from death, with more rapid decline as death approached. As a result, patients who enrolled in the study nearer their deaths might display steeper average trajectories than those who enrolled earlier, simply because their trajectories did not include the slow-changing values distant from death. To adjust for this source of confounding, we included in all regression models a covariate representing the total number of days between the patient's study enrollment and death. We centered this covariate on the sample mean.
Unlike traditional repeated-measures analysis of variance, multilevel modeling does not require a constant number of follow-up points for each patient, or predetermined time points for data collection. Rather, it allows use of variable numbers of follow-up points, collected at varying intervals. Although the QOL rating showed statistically significant negative skew, platykurtosis, and departures from normality, histograms of the distribution conformed sufficiently to the normal distribution to justify modeling the outcome as normal, using a restricted maximum likelihood estimator that provided standard error estimates robust to non-normality.
For multilevel modeling, we used HLM 6.06,20 and for preliminary analyses SPSS 14.0 for Windows.21 We report 2-tailed p-values and consider those <.05 to be statistically significant.
Results
Sample Characteristics
A total of 167 patients enrolled in the study and were randomized to treatment. The majority were female (63.5%); few identified themselves as members of racial/ethnic minority groups (10.8%); median age at enrollment was 73 years (range 34-99); and more than three-quarters reported some post-high-school education. Median monthly income over the three years before study enrollment was $2000-$3000. Most enrollees (84.4%) had received hospice care by the time the study team ended regular visits to area hospice programs. At their baseline interviews, patients indicated low symptom distress (mean 1.05 on a 0-4 scale) and moderate PQoL scale scores (mean 5.94 on a 0-10 scale) and global QOL ratings (mean 6.08 on a 0-10 scale). Patients provided follow-up QOLs a median of 8 times (range 0-130). On average, the 153 patients who had died by the time of data analysis enrolled 4 months before death (median 123 days, range 6-1592 days), and the Kaplan-Meier estimate for median time from enrollment to death for all 167 patients was 144 days. We followed the 140 patients who provided follow-up data an average of 3.7 months after enrollment (median 112.5 days, range 9-1091).
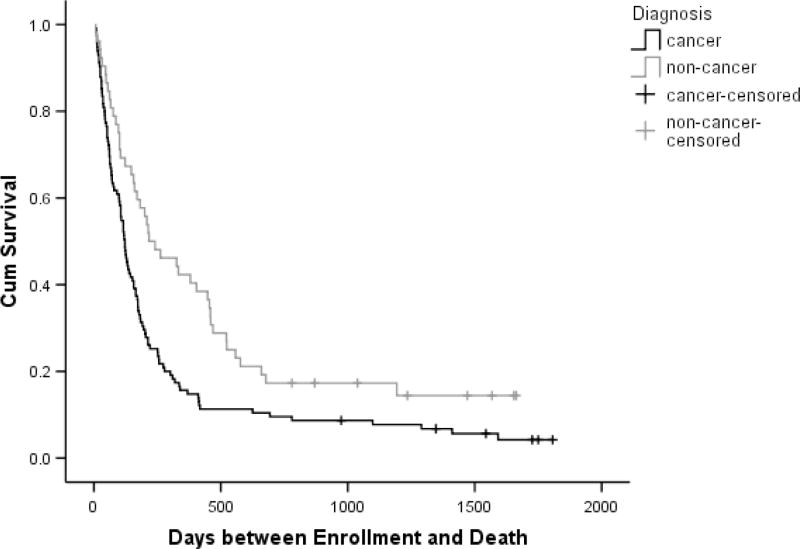
Cancer was the most common terminal diagnosis (68.9%), followed by cardiac disease (15.0%), pulmonary disease (7.2%), neurologic conditions (5.4%), and other diagnoses (3.6%). Cancer patients were significantly younger at enrollment than were patients with other primary conditions (P < .001) and had significantly higher income (P = .014) and education (P = .033), but the two groups did not differ on gender, race, hospice services, or baseline symptom distress or quality of life. Cancer patients had lower levels of involvement in the study, completing significantly fewer follow-up interviews (P = .001) and being followed for significantly shorter time periods (P = .002). Kaplan-Meier estimates for total time between enrollment and death were significantly shorter for cancer patients than for those with other conditions (P = .002). (Table 1, Figure 1)
Table 1. Patient Characteristics.
| Total Sample n = 167 | Analysis Sample n = 127* | |||
|---|---|---|---|---|
| Cancer (115) | Other (52) | Cancer (87) | Other (40) | |
| % in Analysis Sample | 75.7 | 76.9 | 100.0 | 100.0 |
| % with Follow-up QOL† Data | 80.9 | 90.4 | 100.0 | 100.0 |
| % Deceased by Time of Analysis | 94.8 | 84.6 | 100.0 | 100.0 |
| % Female | 66.1 | 57.7 | 71.3 | 57.5 |
| Age at Enrollment: Md (range) | 68 (34,99) | 81 (36,98) | 69 (37,98) | 81 (42,98) |
| % Minority Race/Ethnicity | 11.3 | 9.6 | 8.0 | 7.5 |
| Formal Education | ||||
| % 8th Grade or Less | 0.0 | 5.7 | 0.0 | 5.0 |
| % Some High School | 6.1 | 7.7 | 5.7 | 7.5 |
| % High School Diploma / GED | 13.0 | 19.2 | 12.6 | 17.5 |
| % Trade School or Some College | 34.8 | 34.6 | 40.2 | 37.5 |
| % 4-Year College Degree | 24.3 | 19.2 | 20.7 | 17.5 |
| % Graduate/Professional School | 21.7 | 13.5 | 20.7 | 15.0 |
| Average Monthly Income, Past 3 Yrs | ||||
| % $0 | 0.0 | 1.9 | 0.0 | 2.5 |
| % $1-$500 | 3.5 | 5.8 | 4.6 | 5.0 |
| % $501-$1000 | 7.0 | 7.7 | 5.7 | 10.0 |
| % $1001-$1500 | 13.9 | 17.3 | 13.8 | 10.0 |
| % $1501-$2000 | 6.1 | 17.3 | 4.6 | 17.5 |
| % $2001-$3000 | 21.7 | 13.5 | 20.7 | 15.0 |
| % $3001-$4000 | 13.0 | 9.6 | 14.9 | 10.0 |
| % $4001 and Over | 27.8 | 13.5 | 27.6 | 17.5 |
| % Unknown | 7.0 | 13.5 | 8.0 | 12.5 |
| Primary Diagnosis | ||||
| % Cancer | 100.0 | 0.0 | 100.0 | 0.0 |
| % Cardiac Disease | 0.0 | 48.1 | 0.0 | 47.5 |
| % Pulmonary Disease | 0.0 | 23.1 | 0.0 | 25.5 |
| % Neurologic Disease | 0.0 | 17.3 | 0.0 | 17.5 |
| % Other Diseases | 0.0 | 11.5 | 0.0 | 10.0 |
| Baseline Symptoms / Quality-of-Life | ||||
| Symptom Distress Score‡: Mean | 1.1 (±0.6) | 1.0 (±0.4) | 1.1 (±0.5) | 1.0 (±0.4) |
| PQoL Score§: Mean | 6.0 (±1.8) | 5.8 (±1.7) | 6.0 (±1.7) | 5.9 (±1.7) |
| QOL Rating†: Mean | 6.3 (±2.6) | 5.7 (±2.3) | 6.2 (±2.6) | 5.8 (±2.3) |
| Last QOL† before Death: Mean | 5.2 (±2.8) | 5.4 (±2.7) | 4.7 (±2.8) | 5.2 (±2.8) |
| % Received Hospice Care¶ | 81.7 | 90.4 | 83.9 | 90.0 |
| # Follow-up Points: Md (Range) | 6 (0,109) | 15.5 (0,130) | 8 (1,109) | 15.5 (1,130) |
| Elapsed Time (Days) | ||||
| Enrollment to Death: | ||||
| Actual: Md (Range), Decedents# | 116 (6,1592) | 191 (7,1193) | 123 (20,1592) | 211.5 (24,1193) |
| Estimated: Md, Total Sample** | 122 | 217 | -- | -- |
| Time Followed: Md (Range) †† | 94 (11,1091) | 162 (9,966) | 91 (11,1067) | 147 (9,966) |
| Last Follow-up to Death: Md (Range) | -- | -- | 22 (3,946) | 13 (2,558) |
Abbreviations: Md (median)
Analysis sample included all patients who provided at least one follow-up assessment of quality of life, and who had died by the time of analysis.
Single-item quality-of-life rating: potential range = 0 (no quality of life) through 10 (perfect quality of life)
Total scale score from the 32-item Memorial Symptom Assessment Scale (mean value for valid responses to the 32 items): potential range = 0 (no symptoms) through 4 (all 32 symptoms present and causing very great distress).
Total scale score from the 19-item Perceived Quality-of-Life Questionnaire (mean value for valid responses to the 19 items): potential range = 0 (extremely dissatisfied with all 19 areas assessed) through 10 (extremely satisfied with all 19 areas assessed).
Information on hospice care was available only through August 31, 2007 (the point at which the study team ended regular visits to hospice programs)
Based on patients who had died by the time of data analysis
Based on Kaplan-Meier analysis, with 14 non-decedents censored at the point of data analysis.
Time from study enrollment to last follow-up interview; for the total sample, this included times for only the 140 patients who completed at least one follow-up interview.
Figure 1. Cumulative Survival by Primary Diagnosis*.
* Cumulative survival estimates are based on Kaplan-Meier analysis of 167 study enrollees, with 153 who had died by the time of analysis, and the remaining 14 censored.
More than three-quarters of those randomized (76.0%) remained in the primary analysis sample. The 40 enrollees excluded from analysis of quality-of-life trajectories either failed to provide follow-up data (26 total; evenly split between patients who withdrew from the study before follow-up and patients who died before a follow-up interview could be completed), were still alive at the point of data analysis (13), or both (1). The cancer and non-cancer groups did not differ significantly with regard to inclusion in the analysis sample or provision of follow-up data, but non-cancer patients were significantly more likely to have been excluded because they had not died (P = .037). (Table 1)
Characteristics of the analysis sample were similar to those of the total sample. The 127 patients included in the analysis differed significantly from the 40 who were excluded on only one baseline characteristic: racial/ethnic minority status (7.9% vs. 20.0%, P = .041). As in the full sample of enrollees, cancer patients in the analysis sample were significantly younger at enrollment than non-cancer patients (P < .001). However, the two disease groups did not differ significantly on other baseline characteristics or on receipt of hospice services. On average, patients in the analysis sample enrolled about 4.7 months before death (median = 144 days, range 20-1592), were followed for about 3.4 months (median = 102 days, range 9-1067), completed 10 follow-up interviews (range = 1-130), and had their final follow-up interview about 20 days before death (range = 2-946). Cancer patients in the analysis sample completed fewer follow-up interviews (P = .003), enrolled nearer death (P = .014), and were followed for fewer days (P = .002), but did not differ significantly from other patients regarding elapsed time between their final interview and death. (Table 1)
Estimated Change in Quality of Life of Decedents over Time. (Table 2 and Figure 2)
Table 2. Predictors of Quality-of-Life Trajectories for 127 Decedents.
| Estimate | p | |
|---|---|---|
| Estimated Fixed Effects | ||
| Estimated QOL* on Day of Death | ||
| QOL if enrolled the mean number of days before death† | 4.967674 | <0.001 |
| Addition to Death-Day QOL if patient had cancer | -0.126999 | 0.791 |
| Alteration in Death-Day QOL for each 1-day increase in length of patient's enrollment-to-death period | 0.001048 | 0.309 |
| Change in QOL Each Day Leading Up to Death | ||
| Per-day change in QOL if enrolled mean days before death | -0.003593 | 0.001 |
| Additional per-day change in QOL if patient had cancer | -0.005306 | 0.003 |
| Alteration in per-day change in QOL (slope) for each 1-day increase in length of patient's enrollment-to-death period | 0.000009 | <0.001 |
| Estimated Random Effects | Std Dev | p |
| QOL on Day of Death | 2.25933 | <0.001 |
| Per-day Change | 0.00600 | <0.001 |
| Within Patients | 1.49854 | --- |
QOL = single-item global quality-of-life rating (range = 0 [no quality of life] to 10 [perfect quality of life])
The variable measuring days between enrollment and death was centered on its grand mean for the sample (238.04).
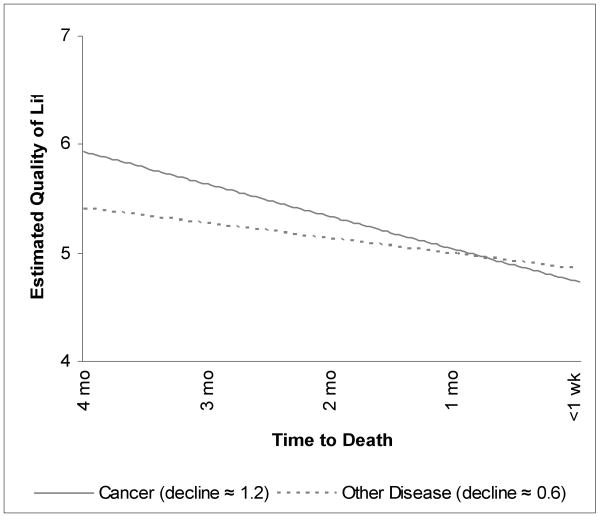
Figure 2. Estimated Quality of Life Trajectories*.
* Trajectories were estimated with multilevel modeling, using responses of 127 respondents over time, in a model including diagnosis and time from enrollment to death as predictors of the slope. The estimated trajectories depicted in the graph are for patients who enrolled in the study 123 days before death (the median for patients with cancer). Quality of life ratings could range from 0 (no quality of life) through 10 (perfect quality of life).
Because cancer patients survived for significantly shorter periods after study enrollment than did patients with other diseases, we adjusted regression models for the patient's total days between enrollment and death (subsequently called the “length of the pre-death period”), with this variable centered on the sample mean (238.04 days, which we label the “typical pre-death period”). Addition of other covariates (gender, age, racial/ethnic minority status, education, receipt of hospice services, and baseline measures of symptom distress and quality of life) had little impact on the association between cancer and the QOL trajectory, nor were any of these variables significantly associated with either the day-of-death QOL or its trajectory over time. Thus, our model includes only the primary predictor (cancer vs. non-cancer diagnosis) and the single covariate (length of the pre-death period). We will illustrate model implications with two examples: patients with the typical 238.04-day (7.8-month) pre-death period and patients with a shorter 123-day (4-month) pre-death period (the median for cancer patients in the study and 115.04 days shorter than the typical period).
A model based on 127 patients who had died by the time of data analysis showed moderately high estimated QOL even on the day of death: almost 5 on a 0-10 scale. For patients with typical pre-death periods, model-based day-of-death estimates were 4.968 for non-cancer patients and 4.841 for cancer patients. For patients with 123-day pre-death periods, the estimates were 4.847 for non-cancer patients and 4.720 for cancer patients. Day-of-death differences for the four groups were too small to suggest any generalizable effect of diagnosis or length of pre-death period on the day-of-death QOL.
However, estimates from the model suggest that most patients experienced significant average daily QOL declines during the period leading up to death and that these declines were significantly affected by both diagnosis and the length of the pre-death period. Irrespective of diagnosis, a patient with the typical pre-death period experienced, on average, at least a 0.004-point decrease in QOL every day as death approached. Patients with cancer experienced an additional 0.005-point daily decrease (thus totaling 0.009 points per day – or more than twice that of non-cancer patients). However, these slopes changed for patients with other than the typical pre-death period. Daily declines leveled off by 0.000009 for each day the pre-death period exceeded the typical period and steepened by the same amount for each day the pre-death period under-represented the typical.
Thus, among patients with the typical 238.04-day pre-death period, non-cancer patients experienced a total QOL change of -0.855 (i.e., [-0.003593] × 238.04) during study participation, whereas cancer patients experienced change of -2.118 (i.e., [-0.008899] × 238.04). For these patients, cancer patients' declines were almost 2.5 times those of non-cancer patients.
Among patients with the shorter 123-day pre-death period, the total QOL change for non-cancer patients was -0.569 (i.e., [-0.0046283] × 123), and the change for cancer patients was -1.222 (i.e., [-0. 0099343] × 123). For these patients, cancer patients' total declines over the course of their participation were over 2.1 times those of non-cancer patients.
Figure 2 shows estimated QOL trajectories for the last 4 months of life for cancer and non-cancer patients, using the parameter estimates from Table 2, with time from enrollment to death fixed at 4 months. At 4 months before death the cancer patients' average estimated QOL ratings were substantially higher than those of patients with other diseases. By the time of death, cancer patients viewed their quality of life as slightly (although not significantly) lower than did other patients.
Finally, although both diagnosis and the length of the pre-death period accounted for significant differences in QOL trajectories between patients, there remained substantial variance that was unexplained by these two factors (slope SD = 0.006). This leaves open the possibility of considerable overlap in individual slopes between cancer and non-cancer patients.
Discussion
In this study of patients who participated in a randomized trial of comfort care at end of life, we found significantly steeper declines over time in perceived quality of life among patients with cancer than among patients with other terminal illnesses. Although cancer patients died more quickly than other patients after study enrollment, thus potentially accounting for the diagnosis effect on QOL trajectories, the difference between the two groups remained after adjusting regression models for the total time between study enrollment and death. This finding is consistent with results of studies by Teno and Chen and their colleagues, who lacked data on patients' perceived quality of life, but found cancer patients to have significantly steeper declines in functional status than other patients.7,8 Reports of analyses based on the SUPPORT data were also suggestive of a difference between cancer patients and others, showing a significant decline in perceived QOL over the last 6 months of life for cancer patients but not for CHF and COPD patients; however, these studies did not directly compare the QOL trajectories of the diagnosis groups.4-6
Our study has a number of strengths – among them, the use of quality-of-life assessments collected exclusively from terminally ill patients and over multiple time points. Some studies of end-of-life trajectories have lacked quality-of-life assessments altogether, basing their results solely on surrogate reports of functional decline, sometimes made during a single assessment covering a lengthy retrospective period. Others have included quality-of-life ratings, but have relied on a combination of patient and surrogate reports, with increases in use of surrogates as death approached leaving open the possibility that status declines near death were in part a function of pessimistic reports provided by respondents other than the patients themselves. In addition, the latter studies have restricted their attention to patients with one life-limiting condition, rather than directly comparing trajectories of patients with different primary diagnoses. Our analysis also benefited from use of multilevel modeling techniques, based on use of a large number of data points collected frequently and over an extended time period. This methodology allowed flexibility in both the number and timing of interviews with declining patients, greater power and precision than would have been possible in analyses based on mean values for patients, and less recall error than would have been possible with data collected only once, but covering a lengthy retrospective period. With 127 level-2 clusters available for analysis, and with median of 11 observations per cluster (mean = 17), both the number of clusters and the cluster sizes were large enough to allow reliable estimation of the parameters of interest and their standard errors.24
The study is limited primarily by its dependence on a restricted patient sample: all drawn from a narrow geographic area, all participating in a randomized trial of comfort care at the end of life, and the large majority receiving hospice care. Patients in the study were perhaps atypical, given their willingness to contribute substantial time and energy to a clinical trial at the end of their lives and to discuss issues related to health status and quality of life on a regular basis. Moreover, quality-of-life trajectories for patients in hospice care may be considerably different from those for patients not receiving this type of end-of-life care, and may exhibit different patterns of decline. Attempts to generalize to wider groups should be made with caution.
Conclusions
Our study provides results that are consonant with those of Teno and Chen and their associates7,8 that show cancer patients facing end-of-life challenges that are often more precipitous than those of other terminally ill persons. The combined results suggest that, in addition to whatever functional declines cancer patients experience that exceed those of others, their perceived quality of life declines more steeply, as well. The findings of the three studies provide evidence that support differing end-of-life trajectories, dependent to some extent on the primary terminal diagnosis. Our findings may provide guidance to clinicians who, by introducing and discussing expected quality-of-life declines at the end of life, may be able to prepare, support, and reassure patients and families.25
Acknowledgments
The authors gratefully acknowledge the contributions of Dr. William E. Lafferty, who was principal investigator of the clinical trial from which data for this article were drawn. It was his vision that provided the impetus for the study and his efforts that resulted in funding and implementation. We are also appreciative of Yuki Durham's assistance in locating background materials for the article, and of the efforts by the study's research and clinical teams, who collected the research data and provided direct services to patient participants. Finally, we appreciate the time, energy, and good will provided by the participating patients, their family and friends, and their hospice and palliative care providers.
This study was funded by the National Institutes of Health / National Cancer Institute (grant #5R01-CA106204) and the Lotte & John Hecht Memorial Foundation
Sponsor's Role: The funding agencies had no role in the study design, methods, participant recruitment, data collection, analysis, or preparation of this article.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: As part of the research team for the clinical trial, Lois Downey contributed to development of study recruitment and data collection protocols, developed the research database, worked as a liaison with recruitment sites, and actively recruited and interviewed study participants. Her specific contributions to the current manuscript were literature review, statistical analyses, and drafting of the original manuscript.
Ruth A. Engelberg served as an Investigator on the original clinical trial and during development of the study's design provided consultation for the selection of survey measures, based on experiences with these measures by the University of Washington End-of-Life Care Research Program, of which she is Associate Director. Her role on this manuscript include contributions to the reference list, revision and refinement of the article's intellectual content, and suggestions for clarification of the description of statistical methods.
References
- 1.Glaser BG, Strauss AL. Time for Dying. Chicago: Aldine Publishing Co.; 1968. [Google Scholar]
- 2.Institute of Medicine. Approaching death: improving care at the end of life. Washington, DC: National Academies Press; 1997. [PubMed] [Google Scholar]
- 3.Lunney JR, Lynn J, Hogan C. Profiles of older Medicare decedents. J Am Geriatr Soc. 2002;50:1108–1112. doi: 10.1046/j.1532-5415.2002.50268.x. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy EP, Phillips RS, Zhong Z, et al. Dying with cancer: Patients' function, symptoms, and care preferences as death approaches. J Am Geriatr Soc. 2000;48:S110–21. doi: 10.1111/j.1532-5415.2000.tb03120.x. [DOI] [PubMed] [Google Scholar]
- 5.Levenson JW, McCarthy EP, Lynn J, et al. The last six months of life for patients with congestive heart failure. J Am Geriatr Soc. 2000;48:S101–9. doi: 10.1111/j.1532-5415.2000.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 6.Lynn J, Ely EW, Zhong Z, et al. Living and dying with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2000;48:S91–100. doi: 10.1111/j.1532-5415.2000.tb03147.x. [DOI] [PubMed] [Google Scholar]
- 7.Teno JM, Weitzen S, Fennell ML, et al. Dying trajectory in the last year of life: Does cancer trajectory fit other diseases? J Palliat Med. 2001;4:457–464. doi: 10.1089/109662101753381593. [DOI] [PubMed] [Google Scholar]
- 8.Chen JH, Chan DC, Kiely DK, et al. Terminal trajectories of functional decline in long-term care setting. J Gerontol A Biol Sci Med Sci. 2007;62:531–536. doi: 10.1093/gerona/62.5.531. [DOI] [PubMed] [Google Scholar]
- 9.Covinsky KE, Wu AW, Landefeld CS, et al. Health status versus quality of life in older patients: does the distinction matter? Am J Med. 1999;106:435–440. doi: 10.1016/s0002-9343(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 10.McPherson CJ, Addington-Hall JM. Judging the quality of care at the end of life: Can proxies provide reliable information? Soc Sci Med. 2003;56:95–109. doi: 10.1016/s0277-9536(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 11.Downey L, Diehr P, Standish LJ, et al. Might massage or guided meditation provide “means to a better end”? primary outcomes from an efficacy trial with patients at the end of life. J Palliat Care. 2009;25:100–108. [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick DL, Danis M, Southerland LI, et al. Quality of life following intensive care. J Gen Intern Med. 1988;3:218–223. doi: 10.1007/BF02596335. [DOI] [PubMed] [Google Scholar]
- 13.Patrick DL, Kinne S, Engelberg RA, et al. Functional status and perceived quality of life in adults with and without chronic conditions. J Clin Epidemiol. 2000;53:779–785. doi: 10.1016/s0895-4356(00)00205-5. [DOI] [PubMed] [Google Scholar]
- 14.de Boer AG, van Lanschot JJ, Stalmeier PF, et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13:311–320. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- 15.Locke DE, Decker PA, Sloan JA, et al. Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients. J Pain Symptom Manage. 2007;34:628–638. doi: 10.1016/j.jpainsymman.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang VT, Hwang SS, Feuerman M, et al. The Memorial Symptom Assessment Scale Short Form (MSAS-SF) Cancer. 2000;89:1162–1171. doi: 10.1002/1097-0142(20000901)89:5<1162::aid-cncr26>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Snijders TAB, Bosker RJ. Multilevel analysis: An Introduction to Basic and Advanced Multilevel Modeling. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- 18.Hox JJ. Multilevel Analysis: Techniques and Applications. Mahwah, NJ: Erlbaum; 2002. [Google Scholar]
- 19.Raudenbush SW, Bryk AS. Hierarchical Linear Models. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 20.Raudenbush S, Bryk A, Congdon R. [April 30, 2009];HLM 6.06 for Windows. Available from http://www.ssicentral.com/hlm/index.html.
- 21.SPSS, Inc. SPSS for Windows, Release 14.0.0. Chicago: SPSS Inc.; 2005. [Google Scholar]
- 22.Wolinsky FD, Stump TE, et al. Consistency and change in functional status among older adults over time. J Aging Health. 1996;8:155–182. doi: 10.1177/089826439600800201. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, LaCroix AZ, Branch LG, et al. Morbidity and disability in older persons in the years prior to death. Am J Public Health. 1991;81:443–447. doi: 10.2105/ajph.81.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maas CJM, Hox JJ. Sufficient sample sizes for multilevel modeling. Methodology. 2005;1:86–92. [Google Scholar]
- 25.Reinke LF, Engelberg RA, Shannon SE, et al. Transitions regarding palliative and end-of-life care in severe chronic obstructive pulmonary disease or advanced cancer: Themes identified by patients, families, and clinicians. J Palliat Med. 2008;11:601–609. doi: 10.1089/jpm.2007.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]