Abstract
Autism spectrum disorders are characterised by severe deficits in socialisation, communication, and repetitive or unusual behaviours. Increases over time in the frequency of these disorders (to present rates of about 60 cases per 10 000 children) might be attributable to factors such as new administrative classifications, policy and practice changes, and increased awareness. Surveillance and screening strategies for early identification could enable early treatment and improved outcomes. Autism spectrum disorders are highly genetic and multifactorial, with many risk factors acting together. Genes that affect synaptic maturation are implicated, resulting in neurobiological theories focusing on connectivity and neural effects of gene expression. Several treatments might address core and comorbid symptoms. However, not all treatments have been adequately studied. Improved strategies for early identification with phenotypic characteristics and biological markers (eg, electrophysiological changes) might hopefully improve effectiveness of treatment. Further knowledge about early identification, neurobiology of autism, effective treatments, and the effect of this disorder on families is needed.
Introduction
Autism is a neurodevelopmental disorder in the category of pervasive developmental disorders, and is characterised by severe and pervasive impairment in reciprocal socialisation, qualitative impairment in communication, and repetitive or unusual behaviour. The Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV)1 and the International Classification of Diseases, 10th edition (ICD-10),2 include autistic disorder, Asperger’s syndrome, pervasive developmental disorder-not otherwise specified (PDD-NOS), Rett’s syndrome, and childhood disintegrative disorder as pervasive developmental disorders. Clinicians and researchers use autism spectrum disorders to include autism, Asperger’s syndrome, and PDD-NOS, which we discuss in this Seminar. For children with Rett’s disorder or childhood disintegrative disorder, their clinical course, patho-physiology, and the diagnostic strategies used are different and are not addressed in this Seminar.
Epidemiology
We focus on prevalence of autism spectrum disorders and possible causes of changes in prevalence. Although estimates vary, prevalence seems to have increased greatly since the 1960s, when rates included only autistic disorder. In the 20 years since, in the USA and Europe prevalence rates ranged from five to 72 cases per 10 000 children.3,4 These estimates were affected by screening, case-confirmation strategies, and sample size, with small sample sizes resulting in high estimates. Prevalence of autistic disorder is between ten and 20 per 10 000 children.5 Estimates of autism spectrum disorders have been more consistent than have those for autistic disorder—perhaps because they are less sensitive to small differences in case definitions. These estimates are close to 60 per 10 000 children.6 However, in a prevalence study7 researchers reported a rate of 116 per 10 000 children for all autism spectrum disorders. They used a small sample of children in South Thames, UK, and relied on screening and case-confirmation methods, with a broad definition of these disorders. When the definition of autism was narrowed, they reported a prevalence of 25 per 10 000.
A rise in the number of children identified with autism spectrum disorders in educational systems has resulted in public health concern.8 Some of the reported increase is attributable to new administrative classifications in special-education settings and the subsequent re-classification of children from a different category to autism.8 Symptoms of these disorders might resemble or arise with intellectual disability, attention deficit-hyperactivity disorder, or obsessive- compulsive disorder.9 Policy and practice changes rather than true changes in community prevalence might be responsible for recorded increases. Substantial small-area variation in prevalence could be related to local health-care and education resources,10 and pressure to obtain intensive services might result in over-diagnosis.
Clinical characteristics and screening
Core symptoms of autism spectrum disorders affect domains of socialisation, communication, and behaviour (panel 1). Clinical signs are usually present by age 3 years, but typical language development might delay identification of symptoms. Results of prospective studies11 of infants at risk (ie, younger siblings of affected children) have shown that deficits in social responsiveness, communication, and play can be present in those as young as age 6–12 months. Diagnoses show heterogeneity of clinical phenotype, severity, and type and frequency of symptoms. Autism spectrum disorders have characteristic diagnostic criteria, ages of symptom recognition, associated medical and developmental features, standard effective treatments, and usual courses of development (table 1).
Panel 1: Core domains of autism1.
Socialisation
Impaired use of non-verbal behaviours to regulate interactions
Delayed peer interactions, few or no friendships, and little interaction
Absence of seeking to share enjoyment and interests
Delayed initiation of interactions
Little or no social reciprocity and absence of social judgment
Communication
Delay in verbal language without non-verbal compensation (eg, gestures)
Impairment in expressive language and conversation, and disturbance in pragmatic language use
Stereotyped, repetitive, or idiosyncratic language
Delayed imaginative and social imitative play
Restricted, stereotyped, and repetitive patterns of behaviour
Preoccupation with stereotyped or restricted interests or topics
Adherence to routines, rigidity, and perseverative behaviour
Stereotyped, repetitive motor mannerisms, and selfstimulatory behaviour
Preoccupation or fascination with parts of items and unusual visual exploration
Table 1.
Differential diagnostic features of autism spectrum disorders
| Autism | Asperger’s syndrome | Pervasive developmental disorder- not otherwise specified (PDD-NOS) |
|
|---|---|---|---|
| Age of recognition (diagnosis*) | 0–3 years (3–5 years) | >3 years (6–8 years) | Variable |
| Regression | About 25% (social or communication) | No | Variable |
| Sex ratio (male:female) | 2:1 | 4:1 | Male>female (variable) |
| Socialisation | Poor; >2 DSM-IV criteria | Poor | Variable |
| Communication | Delayed, deviant; might be non-verbal | No early delay; qualitative and pragmatic difficulties later |
Variable |
| Behaviour | More impaired than in Asperger’s syndrome or PDD-NOS (includes stereotypy) |
Variable (circumscribed interests) | Variable |
| Intellectual disability | >60% | Mild to none | Mild to severe |
| Cause | More likely to establish genetic or other cause than in Asperger’s syndrome or PDD-NOS |
Variable | Variable |
| Seizures | 25% over lifespan | Roughly 10% | Roughly 10% |
| Outcome | Poor to fair | Fair to good | Fair to good |
DSM-IV=Diagnostic and Statistical Manual of Mental Disorders, 4th edition.
Average age at diagnosis. Data adapted from Volkmar and Pauls.12
Early detection enables referral to intervention services and for family support, with the goal of an improved outcome.13 Two methods of identification have emerged. The strategy in the UK combines targeted or selective screening with recognition of alerting signals by clinicians and families.14 The practice endorsed in the USA is routine general developmental surveillance with disorder-specific screening for those who are identified to be at risk during routine screening, or universal autism-specific screening at high-risk ages (eg, 18 and 24 months or 30 months), or both.15 Few data are available to compare the effectiveness of these approaches. Universal whole-population screening with standardised tests has not been supported in the UK16 because of poor sensitivity and specificity of tests.14
Tebruegge and colleagues14 investigated the effect of targeted checks—more than a third of children with autism spectrum disorder were not identified at age 2 years. These researchers supported routine child health surveillance at age 2·0 years and 3·5 years by health professionals with awareness of typical development. The joint working party on child-health surveillance and the American Academy of Pediatrics (AAP)16 recommended education of clinicians and families to recognise red flags or alerting signals. Other countries have adopted similar strategies, combining routine child-health surveillance with a standardised method. In Japan, the young autism and other developmental disorders checkup tool (YACHT) is administered by public health nurses to children aged 18 and 24 months.17 Wong and colleagues18 have modified the checklist for autism in toddlers (CHAT) for use in China, with a two-stage screening strategy in CHAT-23, incorporating a questionnaire and a direct observation stage that has more sensitivity and specificity than does CHAT.
Assessment
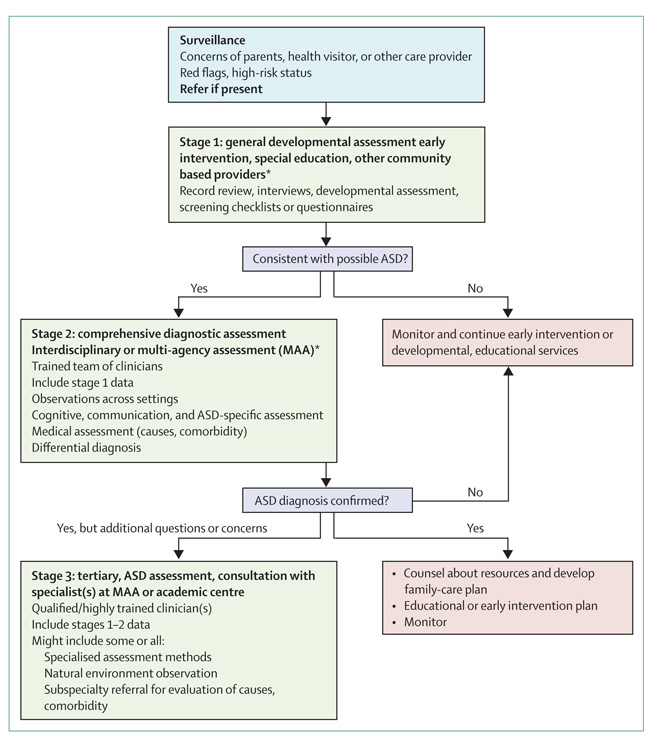
Children identified to be at risk should be referred for comprehensive developmental and diagnostic assessment for autism spectrum disorders. This assessment might be done through community resources (eg, early intervention staff, educators, psychologists, or speech pathologists), educational agencies, or local developmental clinicians. Reviews of early identification and screening are available.15,17,19 If concerns that a child has autism spectrum disorder are validated, comprehensive diagnostic assessment is needed (figure 1). These assessments should be multidisciplinary, addressing core symptoms, cognition, language, and adaptive, sensory, and motor skills. The multidisciplinary team should include clinicians skilled in speech and language therapy, occupational therapy, education, psychology, and social work. Ozonoff and colleagues19 reviewed domains of assessment, including neuropsychological, attention, executive function, and academic functioning.
Figure 1. Stages of identification and diagnosis of autism spectrum disorder.
ASD=autism spectrum disorder. Adapted from the National Austictic Society and 15 and the Pennsylvania Department of Public Welfare.20
Diagnostic assessment of autism spectrum disorders includes use of ICD or DSM diagnostic criteria,21 and standardised methods to assess core (panel 1) and comorbid symptoms (table 2). This multidisciplinary assessment includes a review of caregiver concerns, descriptions of behaviour, medical history, and questionnaires.21 Input from families about their observations and concerns are crucial. Although parents are often aware of developmental problems in their child from age 18 months, a diagnosis is often not made until 2 years after the initial expression of parental concern. In some cases diagnosis has not been confirmed until close to age 6 years,28 which is sometimes associated with delays attributable to access to services and regional variations in diagnosis.
Table 2.
Comorbid symptoms or disorders
| Frequency | Treatments | |
|---|---|---|
| Developmental5 | ||
| Cognitive; intellectual disability | 40–80% | Educational |
| Language deficits | 50–63% | Communication training, speech and language therapy |
| Attentional problems, impulsivity, or hyperactivity |
59% | Behavioural intervention, psychopharmacotherapy (eg, stimulants, atomoxetine, or alpha blockers) |
| Motor delay | 9–19% | Physical therapy |
| Hypotonia | 50% | Physical therapy |
| Psychiatric22 | ||
| Anxiety | 43–84% | Behavioural treatment such as relaxation, cognitive behavioural treatment, psychopharmacotherapy (eg, SSRIs or alpha-2 agonists) |
| Depression | 2–30% | Psychotherapy, antidepressants |
| Obsessive compulsive disorder or interfering repetitive behaviour |
37% | Behavioural treatment, SSRIs and other drugs such as atypical antipsychotics |
| Oppositional defiant disorder | 7% | Behavioural treatment |
| Behavioural problems | 3% | Behavioural treatment |
| Behavioural23 | ||
| Disruptive, irritable, or aggressive behaviour |
8–32% | Behavioural intervention, atypical antipsychotics (eg, risperidone or arapiprazole) |
| Self-injurious behaviour | 34% | Behavioural intervention; drugs (eg, risperidone, naltrexone, and others) |
| Sensory24 | ||
| Tactile | 80–90% | Occupational therapy, behavioural treatment, and desensitisation |
| Auditory sensitivity | 5–47% | Occupational therapy |
| Neurological25 | ||
| Seizures and epilepsy | 5–49% | Anticonvulsants |
| Tics | 8–10% | Alpha-2 agonists (clonidine and guanfacine) and others such as atomoxetine |
| Gastrointestinal26 | ||
| Food selectivity | 30–90% | Behavioural treatment, investigation as appropriate for gastrointestinal difficulties |
| Gastro-oesophageal reflux, constipation |
8–59% | Gastrointestinal investigations as appropriate (eg, barium swallow or milk scan for gastrooesophageal reflux; flat plate, clean out, stool softener, or cathartic for constipation as clinically indicated) |
| Sleep27 | ||
| Sleep disruption | 52–73% | Sleep diary, sleep hygiene, behavioural supports, investigation of possible medical comorbidities as cause, drugs (eg, melatonin and clonidine) |
SSRI=selective serotonin reuptake inhibitor.
Table 3 shows methods for diagnosis and categorisation of autism spectrum disorders.19 Standardised questionnaires such as the social responsiveness scale provide data about severity of core deficits of socialisation, and the revised repetitive behaviour scale provides information about stereotyped or repetitive behaviours (figure 1). Use of two research quality, gold-standard assessment methods based on DSM criteria, the autism diagnostic observation schedule (ADOS)58 and the revised autism diagnostic interview (ADI-R),59 have improved accuracy and reliability of diagnosis.19 The ADOS is a semistructured standardised assessment for social behaviour, communication, and imaginative play, and is used in research and clinical settings. To diagnose individuals with intellectual disability is difficult because behaviours might not be specific to autism spectrum disorders; the ADOS diagnostic algorithm was revised to address these issues. The time needed for administration of the ADI-R (1–3 h) precludes its use in many clinical settings.
Table 3.
Selected methods for autism diagnosis and categorisation
| Description | 0–3 years | 3–5 years | SA | Adult | Administration time (min) |
|
|---|---|---|---|---|---|---|
| Checklist or inventory | ||||||
| Screening method | ||||||
| Quantitative checklist for autism in toddlers29 | Parent questionnaire, 25 questions | X | ·· | ·· | ·· | 5–10 |
| Modified checklist for autism in toddlers30 | Questionnaire 23 questions | X | ·· | ·· | ·· | 10 |
| First year inventory31 | Parent report 60 questions | X | ·· | ·· | ·· | NS |
| Early childhood inventory-432 | Parent rating scale 108 items Teacher rating scale, 87 items |
·· | X | ·· | ·· | <20 |
| Child symptom inventory-433 | Parent rating scale 97 items, teacher rating scale 77 items | ·· | ·· | X | ·· | Variable |
| Social communication questionnaire34 | Parent interview or questionnaire 40 items | ·· | X | X | ·· | 10–15 |
| Asperger’s syndrome diagnostic scale35 | Parent or teacher rating scale 50 items | ·· | ·· | X | ·· | 10–15 |
| Krug Asperger diagnostic index36 | Parent, caregiver, or teacher questionnaire 32 items | ·· | ·· | X | ·· | 10–15 |
| Autism spectrum quotient37 | Parent questionnaire, for child, adolescent, and adult 50 questions |
·· | ·· | X | X | NS |
| Autism behaviour checklist38 | Parent or teacher questionnaire 57 items | ·· | X | X | ·· | 10–20 |
| Pervasive developmental disorder rating scale39 | Rating scale 51 items | X | X | ·· | ·· | NS |
| Pervasive developmental disorder in mental retardation scale40 | Clinician and teacher observation instrument 12 items | ·· | X | X | ·· | 10–20 |
| Dimensional assessment | ||||||
| Developmental behaviour checklist,41 and developmental behaviour checklist—early screen42 |
Questionnaire; 96 items and 17 items | X | X | ·· | ·· | 10–15 |
| Pervasive developmental disorder behaviour inventory43 | Parent, caregiver, or teacher rating scale 8–10 subscales (124–188 items) |
X | X | X | ·· | 20–45 |
| Aberrant behaviour checklist44 | Parent rating scale 58 items | ·· | X | X | ·· | 20–30 |
| Child communication checklist45 | Parent rating scale 70 items | ·· | X | X | ·· | 5–15 |
| Social responsiveness scale46 | Parent and caregiver questionnaire 65 items | ·· | X | X | X | 15–20 |
| Repetitive behaviour scale—revised47 | Parent and caregiver questionnaire 43 items | X | X | X | X | 15–20 |
| Social and communication disorders checklist48 | Parent and caregiver rating scale 12 items | ·· | ·· | X | X | 10–15 |
| Structured interview | ||||||
| Diagnostic method | ||||||
| Parent interview for autism49 | Parent interview 118 items | X | ·· | ·· | ·· | 20–30 |
| Diagnosis of social and communication disorder schedule50 | Semistructured caregiver interview schedule 2–4 h administration, more than 300 items |
·· | X | X | ·· | 180 |
| Autism diagnostic interview—revised51 | Semistructured, investigator-based interview for caregivers | X* | X | X | X | 90–180 |
| Diagnostic method, dimensional assessment | ||||||
| Developmental, dimensional, and diagnostic interview (3di)52 | Caregiver computerised interview 183 items (demographics), 266 items (ASD related), 291 items (current mental state) |
X | X | X | X | 45–90 |
| Observational measures | ||||||
| Screening method | ||||||
| Checklist for autism in toddlers53 | Caregiver rating scale plus observations | X | ·· | ·· | ·· | 10 |
| Screening tool for autism in children aged 2 years54 | Clinician observation 12 items | X | ·· | ·· | ·· | 20† |
| Diagnostic method, dimensional assessment | ||||||
| Autism observation schedule for infants55 | Clinician observation 18 item | X | ·· | ·· | ·· | 20† |
| Autism diagnostic observation schedule—modules 1–456 | Standardised observation method | X | X | X | X | 40–60† |
| Diagnostic method | ||||||
| Childhood autism rating scale57 | History and observation 15 items (Likert scale) | X | X | X | X | 20–30 |
SA=school-aged children. NS=not specified. ASD=austistic spectrum disorder. X=appropriate method
Mental age older than 2 years.
Training needed to administer this method.
Comorbid disorders are common in children60 and families of children with autism spectrum disorders, and might have a greater effect on function and outcome than do core symptoms (table 2). Parents of affected children have increased rates of stress and mental health comorbidity (eg, anxiety and depression), which might be associated with their child’s behavioural problems.61 Comorbid behavioural or developmental disorders include intellectual delays, inattention or other symptoms of attention deficit-hyperactivity disorder, externalising behaviours (such as aggression and disruption), affective difficulties (such as depression or anxiety), sleep disruption, and sensory differences.22 Medical comorbidities, such as gastrooesophageal reflux, food selectivity, and neurological disorders (eg, tics and seizures) also have a substantial effect on management and on the family. Some behavioural or affective comorbidities might be targets for pharmacotherapy.
A comprehensive diagnostic assessment should include medical investigation for causes and associated diagnoses.62 Results will inform families about related genetic, neurological, or medical problems, and risk of recurrence in future siblings. An appropriate medical investigation for causes includes a detailed history and physical examination (with careful examination for dysmorphology). Clinical genetic assessment might include laboratory studies ordered by the primary care practitioner or referral to a clinical geneticist. Genetic laboratory studies can include routine karyotype and molecular DNA testing for fragile X, or comparative genomic hybridisation, or both.63 Associated medical problems such as seizures show a need for electroencephalogram (EEG), substantial regression a need for metabolic investigation, and abnormal head size a need for neuroimaging in some. Routine brain imaging or EEG is not recommended unless specific clinical features are indicative of an active neurological process needing clinical diagnosis.
Neurobiology
Attempts to identify unified theories explaining core and comorbid deficits have been unsuccessful, which is not surprising in view of the heterogeneous expression of autism spectrum disorders. In studies64 of this disorder as a neurodevelopmental disorder of prenatal and postnatal brain development, researchers have attempted to elucidate these theories by examination of brain growth, functional neural networks, neuropathology, electrophysiology, and neurochemistry. Neurocognitive theories include pragmatic language impairment and difficulties in intersubjectivity (theory of mind), executive function and problem-solving mindset, weak central coherence and difficulty with integration of information into meaningful wholes,12 and deficits in connectivity and processing demands.65
Neurobiological findings support different theories. Macrocephaly is noted by age 2–3 years in 20% of children with autism spectrum disorder. Brain growth accelerates at 12 months.65 These changes arise in parallel with onset of core symptoms during the first 2 years of life. Results of neuroimaging studies66 have shown overgrowth in cortical white matter and abnormal patterns of growth in the frontal lobe, temporal lobes, and limbic structures such as the amygdala. These brain regions are implicated in development of social, communication, and motor abilities that are impaired in autism spectrum disorder. In post-mortem brain studies,67 researchers have also noted cytoarchitectural abnormal findings, including reduced number and size of purkinje cells, and abnormal findings in the cortical minicolumn.
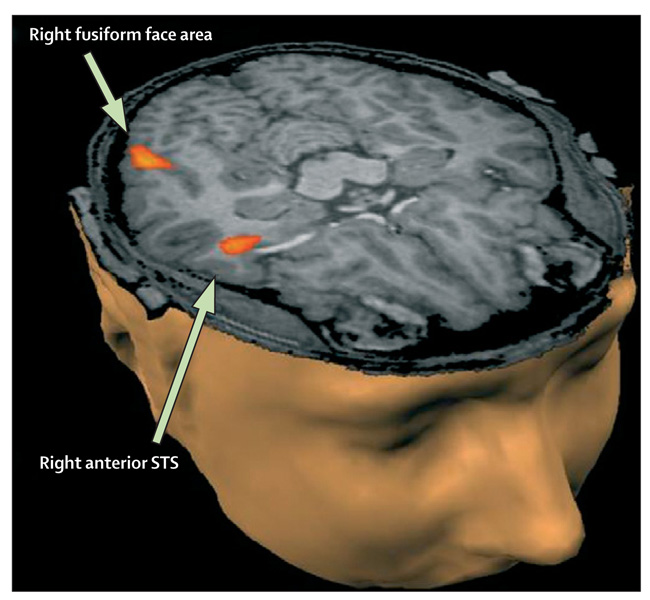
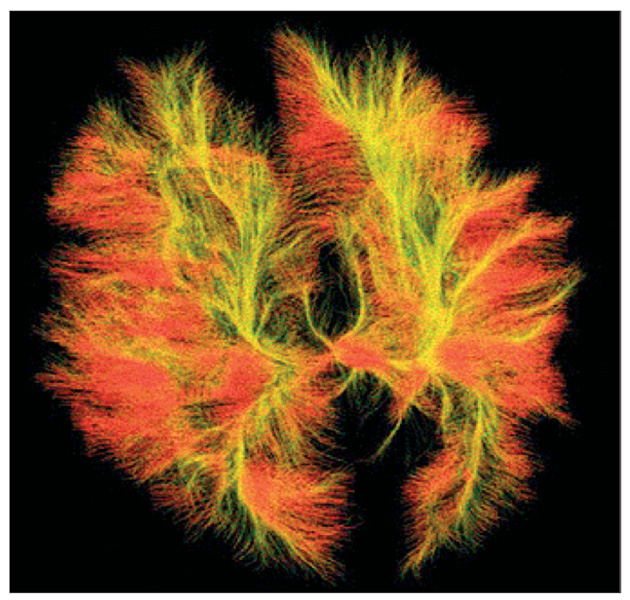
Functional MRI has shown differences in patterns of activation and timing of synchronisation across cortical networks, with lowered functional connectivity relating to language, working memory, social cognition or perception, and problem solving. The most reliably replicated functional MRI abnormal finding is hypoactivation of the fusiform face area, associated with deficits in perception of people compared with objects (figure 2).64,67 Results of other functional MRI studies68 done during imitation tasks have suggested impaired mirror neuron activity in the inferior frontal gyrus (pars opercularis). With diffusion tensor imaging (figure 3), researchers65 have shown disruption of white matter in brain regions associated with social functioning.
Figure 2. Functional MRI.
Functional MRI is used to study activity of the relation of the amygdala to the fusiform face area and superior temporal sulcus (STS), which are responsible for face perception.
Figure 3. Diffusion tensor imaging.
Used for measurement of axonal pathways, providing visualisation of connectivity of brain regions.
Magnetoencephalography is a non-invasive measure of magnetic fields generated by neuronal activity, providing spatial and temporal localisation of activity within the brain. This technique has been used to investigate auditory processing deficits with the hope to identify an electro physiological signature that might enable early detection or monitoring of progress.69 Neurochemical investigations with animal models and empirical drug studies remain inconclusive. Serotonin and genetic differences in serotonin transport seem to have the most empirical evidence for a role in autism spectrum disorder,70 whereas data lending support to the roles of dopaminergic and glutaminergic systems are presently less robust, but are evolving. Study of the role of the dopaminergic and cholinergic system, oxytocin, and aminoacid neurotransmitters shows promise.70 Together, results of clinical, neuroimaging, neuropathological, and neurochemical studies66 show that autism spectrum disorders are disorders of neuronal-cortical organisation that cause deficits in information processing in the nervous system, ranging from synaptic and dendritic organisation to connectivity and brain structure. These changes probably alter developmental trajectory of social communication and seem to be affected by genetic and environmental factors.
Causes
Autism spectrum disorder is highly genetic. The relative risk of a second child having this diagnosis is 20–50 times higher than the population base rate,71 and thus families should consider genetic counselling. Parents and siblings often show mild, subsyndromal manifestations of autism, the broad autism phenotype,72 including delayed language, difficulties with social aspects of language (pragmatics), delayed social development, absence of close friendships, and a perfectionistic or rigid personality style.73 Heritability estimates from family and twin studies71 suggest that about 90% of variance is attributable to genetic factors, making this disorder the neuropsychiatric disorder most affected by genetic factors. Dependent on the definition used, 60–90% of monozygotic twins are concordant for autism spectrum disorder, compared with about 10% for dizygotic twins.74
Autism spectrum disorder is multifactorial, with many risk factors acting together to produce the phenotype. The difference between monozygotic and dizygotic concordance rates suggests some risk factors interact (ie, gene–gene or gene–environmental interactions). These effects could be a result of toxic environmental factors or epigenetic factors that alter gene functions, in turn altering neural tissue. Epigenetic factors can be specific aspects of the physical environment (eg, biochemically active compounds) or specific types of psychological experiences (eg, stress) that alter brain chemistry, turn genes off or on at specific times during development, or regulate gene expression in other ways. The possible role of environmental and epigenetic factors is an area being studied.
Autism spectrum disorder is associated with known genetic causes in 10–15% of cases. The most common causes include fragile X syndrome (about 3%), tuberous sclerosis (about 2%), and various cytogenetic abnormal findings such as maternal duplication of 15q1-q13 (roughly 2%), and deletions and duplications of 16p11 (about 1%).75 None of these causes are specific to the disorder, but rather are specific to a range of phenotypes, including intellectual disability.
Genetics
Since 2003,12 fundamental changes in our understanding of the genetics of autism have taken place. Previously, this specialty was guided almost exclusively by the common disorder–common gene model,76 proposing that many genes frequently identified in the general population each confer small-to-moderate effects on the phenotype. Only a few common variants have been identified as possible candidate genes in linkage and association studies,77 and many of these have not been verified in subsequent independent sample replication studies, pointing to the difficulty of finding common causes in a heterogeneous disorder. Difficulty in finding robust common variants is not unique to autism spectrum disorder. Encouragingly, the largest genome-wide association study78 has identified a common variant of statistical signficance—an intergenic region between cadherin 9 and 10. This finding is exciting because cadherins are important for neuronal connectivity, and thus represents a possible biological mechanism to explain under-connectivity.
Another promising development in understanding the genetics of autism spectrum disorder is the discovery of variations in the gene copy number as a risk factor.79 Copy-number variation is a structural variation in the genome in which material is either duplicated or deleted. Copy-number variations can be de novo or inherited. Almost all these variations are deletions, with many fragments containing several genes.76 De-novo copy-number variations seem to be strongly associated with intellectual impairment and dysmorphology.79 Most seem to be individually unique, although we do not know the full implications of them because their relation to phenotype is not established,76 and affected siblings do not always share specific variations.80 Furthermore, to know whether a given de-novo variant is abnormal is difficult, because the population distribution of specific copy-number variations is unknown.
Insights into underlying biological mechanisms for autism spectrum disorder have been gained from study of syndromes with increased rates of this disorder. For example, functions of the genes underlying fragile X (FMR1) and Rett’s syndrome (MECP2) implicate synaptic dysfunction in cause and pathogenesis.81 Further evidence82 for synaptic dysfunction as a unifying cause has come from findings of rare mutations in neural cell adhesion and synaptic molecules such as X-linked neuroligin 4 (NLGN4X) and neuroligin 3 (NLGN3). Convergence of genetic findings with implications for synaptic maturation is especially notable because findings from neuroimaging research also suggest that structural and functional brain connectivity is aberrant in autism spectrum disorders.65 Thus, genetic and neurobiological evidence point to a good causal model of this disorder—namely, genetically mediated abnormalities of synaptic maturation and connectivity.
Researchers need to explain how genes that affect maturation of the synapse can account for specific behaviours and brain functions that are altered, while other processes are simultaneously spared. Possibly, problems of synaptic maturation are not ubiquitous in the brain and genes that affect brain function are regionally expressed, affecting only some systems. Alternatively, all circuits and synapses throughout the brain could be affected, but those mediating social and communicative skills and behavioural flexibility might be vulnerable to a common underlying synaptic defect. An important implication of this model is that an opportunity to intervene prophylactically during the first months of life might be available. With Drosophila models of fragile X syndrome and mouse models of Rett’s syndrome, investigators have already shown that phenotype can be altered through administration of metabotropic glutamate antagonists (in the fragile X model),83 or reinstatement of the MeCP2 gene after birth (in Rett’s syndrome).84
Treatment
New developments
Advances in cognitive and affective developmental neuroscience, neurobiology, and the genetics of autism spectrum disorder have resulted in potentially novel methods for early detection and improved targeting and effectiveness of treatments.85 For example, neuroimaging strategies such as functional MRI and magneto encephalo-graphy might provide biomarkers to monitor physiological changes before and after treatment. We still do not know which treatments or combinations of types of treatments will be most effective and for whom they will be effective.86 Many interventions address core deficits (panel 2) and associated conditions (table 2). Core symptoms might be more malleable when treatment is initiated in early childhood, making early screening and diagnosis important.93 Behavioural or developmental manifestations of core symptoms are most obvious, and thus are the main focus of treatment.
Panel 2: Examples of treatments for core symptoms of autism spectrum disorder.
Socialisation
Educational curricula87
Treatment and education of autistic and related communication handicapped children (TEACCH), strategies for teaching based on autism research (STAR), parent training, and inclusion with trained shadow
Communication and language88
Didactic and intensive training, and Milieu teaching
Social-skills training89
Social skills training and social stories
Behavioural treatment86
Discrete trial instruction, pivotal response training, and relationship development intervention
Communication
Communication intervention88
Within a comprehensive programme (eg, pivotal response training, or other centre), social pragmatics approach, and parent training
Augmentative and assistive communication90
Picture exchange communication system, sign language, and assistive technology (eg, vocal output devices)
Behavioural (eg, play, reciprocal communication)91
Floor time/developmental, individual differences, relationship-based approach, applied verbal behaviour
Educational87
TEACCH, STAR
Behaviour
Behavioural intervention91
Discrete trial instruction, and other comprehensive programmes using applied behaviour analysis
Psychopharmacology92
Selective serotonin reuptake inhibitors, anticonvulsants, atypical antipsychotics, α-2 agonists
For most children, the main source of intervention is their family or educational system.71 Comprehensive treatment programmes include combinations of specialised educational curricula, developmental therapies, behaviourally based treatments, and intensive parent training in the home, community, or school setting (panel 2).86,94,95 Parental stress might impede the effectiveness of early interventions;96 hence, support for families must be integrated into treatment. Goals of treatment are to improve functional status of the individual through acquisition of skills in core deficit areas, and decrease effects of comorbid conditions. Medical treatments might be effective for addressing behavioural or medical comorbidities. No biological treatment to ameliorate all symptoms of autism spectrum disorder is presently available.
Psychosocial treatments
Increased numbers of children identified to have autism spectrum disorder and restricted availability of resources means that implementation of psychosocial interventions needs to include several approaches. Educational settings for interventions range from full time special education classes (in mainstream or special schools), part time or resource room special education support (eg, dual placement) in which the child is included in a typical education class, or typical class placement (mainstream) with supports provided to the child. In a US survey97 of special education directors and autism consultants in Georgia, USA, researchers reported use of several strategies addressing socialisation or interpersonal relationships, acquisition of language, play, and other skills, and comorbid conditions such as cognitive deficits, physiological issues, and maladaptive behaviours.
Socialisation deficits can be addressed individually or in small group settings. Behavioural strategies and skills training can teach social skills, enhance peer interaction, and promote play skills. Because communication deficits are central to autism spectrum disorders, speech and language therapy is very important.88 In young non-verbal children, strategies include use of principles of positive reinforcement to promote attention and imitation. For children with verbal apraxia, augmentative strategies such as the picture exchange communication system might improve communication and ameliorate behavioural difficulties. Assessment of the effectiveness of complex and technologically sophisticated augmentative systems is needed.88
The most well researched treatment programmes are based on principles of applied behaviour analysis.79 Treatments based on such principles represent a wide range of early intervention strategies for children with autism—from highly structured programmes run in one-on-one settings to behaviourally based inclusion programmes that include children with typical development. The first types of behavioural treatment programmes developed and examined were very structured, intensive, one-on-one programmes called discrete trial training, which were highly effective for up to half of children enrolled in about ten randomised clinical trials98 done in the past 20 years.
These intensive programmes are expensive, and children have difficulty generalising the information from a very structured session to group and community settings. Less structured, more naturalistic behavioural programmes have been developed, such as pivotal response training99 and incidental teaching.100 In individual and non-randomised group studies,101 researchers noted that about half of children have good outcomes in these types of programmes. Presently, even structured sessions typically include naturalistic methods for increasing generalisation and maintenance. A combination of these behavioural methods is more effective than is usual care for improvement of outcomes for children with autism.102
Parent-mediated interventions have been shown in controlled studies to be an important aspect of intervention. Investigators identified that generalisation and maintenance of behaviour changes were improved when parents were trained in highly structured behavioural methods.103 As behavioural programming for children with autism evolved from teaching one behaviour at a time to a broadened focus of increasing general motivation and responsiveness,104 parent education also began to change. Parents were taught naturalistic strategies that were easier to use in the home, needed fewer hours of training, increased both leisure and teaching time, and improved parent satisfaction and enjoyment of the treatment. Parents are now thought to be important collaborators at all stages—from assessment through to goal development and treatment delivery.105
Developmental models such as developmental individual-difference, relationship-based floortime model,106 the social com munication, emotional regulation and trans actional support model,107 and the Denver model108 have shown some promising results. These models derive from research showing an association between social relation ships and communicative development. Although the theory underlying these models differs from learning theory, many techniques used in naturalistic behavioural interventions are common to developmental approaches.109
Pharmacological and medical approaches
Although existing pharmacotherapeutic agents are not effective for treatment of core symptoms of autistism spectrum disorders, research has provided the impetus to study potential drug effects on core social and language impairment.110 In a double-blind, placebo–control crossover study111 of children with autistic spectrum disorders and comorbid attention-deficit-hyperactivity disorder, methylphenidate treatment was shown to have a positive effect on joint attention. Drugs might be helpful to address comorbid symptoms and as an adjunct to appropriate educational, behavioural, and develop mental treatments (table 2).
The most common comorbid symptoms addressed by pharmacotherapy are attentional difficulties, hyperactivity, affective difficulties (eg, anxiety and depression), interfering repetitive activity, irritability, aggression, self-injurious behaviour, and sleep disruption (table 2). Until recently, drugs were selected on the basis of extrapolation of their use in other disorders such as attention deficit-hyperactivity disorder and anxiety.92 Data from the Research Units on Pediatric Psychopharmacology112 autism network provided support for use of atypical antipsychotics (such as risperidone) for treatment of irritability in children with autism spectrum disorders. Because evidence exists for abnormal serotonin function in individuals with this disorder, selective serotonin reuptake inhibitors have been used to treat anxiety, or rigid or repetitive behaviours. Results from clinical trials have been mixed. Unlike in adults, the side-effect profile (irritability and activation) might restrict use of these drugs in children with autism spectrum disorders.113 A multicentre trial114 of citalopram for repetitive behaviours showed that this drug did not improve these behaviours. Effectiveness of other widely used agents needs to be explored. King and Botsic92 reviewed pharmacological treatments for autism spectrum disorders.
Treatments should be prioritised by risks, dysfunction, and effect on the family of autism spectrum disorder.115 For example, a child with maladaptive behaviour in one situation might have an improved response to a behavioural plan after a functional behavioural analysis. Such an analysis would identify triggering events and consequences that might perpetuate undesired behaviour. Behaviours that are severe or arise in many settings, or both, and are not adequately treated with behavioural strategies alone might be helped by a combination of behavioural and drug treatment.115 Medical problems such as seizures and tics are more frequent in individuals with autism spectrum disorders than in those without the disorders, and should be treated appropriately.116
Complementary and alternative medical treatments are often used by families. Their popularity is in part attributable to the chronicity of symptoms of autism spectrum disorder and the absence of effective medical treatments. Popular biologically based treatments (panel 3) include supplements, specialised diets, immune therapies, gastrointestinal treatments, chelation, and withholding immunisations. Other non-biological treatments include manipulative and body-based treatments (eg, craniosacral manipulation, and auditory integration), mind-based and body-based therapies (eg, yoga), and energy medicine. Levy and Hyman117 reviewed studies of effectiveness of complementary and alternative medicine. So far, few studies have addressed safety and effectiveness of most of these treatments. Practitioners should support families as they assess the effectiveness, risks, and cost of treatments and assist in monitoring potential side-effects.
Panel 3: Examples of complementary and alternative medical treatments97.
Biological
Supplements—eg, vitamin B6 or magnesium ion, dimethyl glycine, and cod-liver oil)
Anti-infectives—eg, antibiotics, antifungals, and antivirals
Immunoglobulins
Off-label drugs—eg, secretin
Chelation medications
Gastrointestinal medications
Elimination or special diets—eg, gluten free or casein free
Hyperbaric oxygen administration
Non-biological
Auditory integration training
Chiropractic therapy
Craniosacral manipulation
Facilitated communication
Massage and qigong
Interactive metronome
Reiki
Transcranial stimulation
Yoga
Although treatments might be effective for alleviation of symptoms, improvement of functional skills, and lessening of stress in families, no cure for autism spectrum disorder is yet available. Outcomes are improved with early detection and intensive treatment.12 Data for long-term prognosis are scarce. Factors associated with poor outcomes are intellectual function in childhood (intelligence quotient<70), early com munication deficits, and continued ritualistic and stereotyped behaviour.118 Some adults with autism gain employment, live independently, and establish relation ships; however, most adults remain dependent on family or others for support.118
Future directions
In the past 10 years,119 much progress has been made with diagnosis and management of autism spectrum disorder. Hopefully, early detection and diagnosis of infants and children at risk will enable treatments to be designed and implemented to alter the course of early behaviour and brain development.85 Amaral and colleagues120 suggested that the heterogeneity of factors affecting brain development predicts a heterogeneous pattern of neuropathology. Through neuroimaging approaches such as diffusion tensor imaging, functional MRI, and magnetoencephalography, abnormal findings have been identified in neuronal patterning, cortical connectivity, synaptic organisation,66 and electrophysiology. Improved early identification with phenotypic characteristics and possible biological markers should allow for increasingly individualised and effective treatment. Promotion of early identification, improved understanding of brain mechanisms, development of effective treatments, and strategies to moderate the effect of autism spectrum disorder on families is needed.
Search strategy and selection criteria
We searched Medline, Psychinfo, and Cochrane Library databases from January, 1998, to December, 2008, with the search terms “autism”, “autistic disorder”, “pervasive developmental disorder”, “autism spectrum disorder”, and “Asperger syndrome” in combination with “evaluation”, “diagnosis”, “treatment”, “therapy”, “medication”, “pharmacotherapy”, “epidemiology”, “genetics”, “neuroimaging”, “behavior therapy”, “early identification”, “outcome”, and “complementary and alternative therapy.” We largely selected reports published within the past 5 years, but did not exclude commonly referenced and highly regarded older publications. We also searched references from recent reviews and other reports identified by this search strategy, and selected those we judged relevant. Review articles and book chapters are cited to provide more details and references than are provided in this Seminar. Our reference list was modified on the basis of comments from peer reviewers.
Acknowledgments
This work was supported in part by grants from: the Center for Disease Control and Prevention (grant number 1-U10-DD-000182-03), the National Institutes of Health (NIH; 1-RO1-MH-073807, 1-RO1-DC008871-01, and RO1 ES016443-01) for SEL; from the National Science Foundation (sbe-0542013), NIH/NIMS (5RO1MH073084), NIH (1 P50 HD055726-01, 1 RO1 HD055741-01, and 5R01mh076189-03), and Pennsylvania Department of Health (SAP#410042728) for RTS; the National Institute of Mental Health (1K01MH067628-1 and 1 R01 MH077000-01) and Department of Defense (W81XWH-07-ASDRP-CA) for DSM; and NIMH (1 RO1 MH083717-01A1) and Department of Education (IES-NCSER-2008-1) for DSM and SEL.0
Footnotes
Contributors
All authors contributed to the design, search strategy, synthesis of information identified in the search, writing and editing, and integration of editing as suggested by co-authors. No medical writer was involved in creation of this Seminar.
Conflicts of interest
We declare that we have no conflicts of interest.
Contributor Information
Susan E Levy, Children’s Hospital of Philadelphia, University of Pennsylvania, School of Medicine, Center for Autism Research, Philadelphia, PA, USA; Department of Psychiarty, University of Pennsylvania, School of Medicine, Center for Autism Research, Philadelphia, PA, USA.
David S Mandell, Department of Psychiarty, University of Pennsylvania, School of Medicine, Center for Autism Research, Philadelphia, PA, USA.
Robert T Schultz, Children’s Hospital of Philadelphia, University of Pennsylvania, School of Medicine, Center for Autism Research, Philadelphia, PA, USA; Department of Psychiarty, University of Pennsylvania, School of Medicine, Center for Autism Research, Philadelphia, PA, USA.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.WHO. The ICD-10 classification of mental and behavioral disorders: diagnostic criteria for research. Geneva: World Health Organization; 1993. [Google Scholar]
- 3.Sponheim E, Skjeldal O. Autism and related disorders: epidemiological findings in a Norwegian study using ICD-10 diagnostic criteria. J Autism Dev Disord. 1998;28:217–227. doi: 10.1023/a:1026017405150. [DOI] [PubMed] [Google Scholar]
- 4.Kadesjo B, Gillberg C, Hagberg B. Brief report: autism and Asperger syndrome in seven-year-old children: a total population study. J Autism Dev Disord. 1999;29:327–331. doi: 10.1023/a:1022115520317. [DOI] [PubMed] [Google Scholar]
- 5.Newschaffer CJ, Croen LA, Daniels J, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Surveillance Summaries. MMWR Morbid Mortal Wkly Rep. 2007;56:1–28. [Google Scholar]
- 7.Baird G, Simonoff E, Pickles A, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP) Lancet. 2006;368:210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- 8.Shattuck P. The contribution of diagnostic substitution to the growing administrative prevalence of autism in US special education. Pediatrics. 2006;117:1028–1037. doi: 10.1542/peds.2005-1516. [DOI] [PubMed] [Google Scholar]
- 9.Gillberg C, Billstedt E. Autism and asperger syndrome: coexistence with other clinical disorders. Acta Psychiatr Scand. 2000;102:321–333. doi: 10.1034/j.1600-0447.2000.102005321.x. [DOI] [PubMed] [Google Scholar]
- 10.Mandell D, Morales K, Marcus S, Stahmer A, Doshi J, Polsky D. Psychotropic medication use among children with autism spectrum disorders. Pediatrics. 2008;121:e441–e448. doi: 10.1542/peds.2007-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkmar FR, Chawarska K. Autism in infants: an update. World Psychiatry. 2008;7:19–21. doi: 10.1002/j.2051-5545.2008.tb00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkmar FR, Pauls D. Autism. Lancet. 2003;362:1133–1141. doi: 10.1016/S0140-6736(03)14471-6. [DOI] [PubMed] [Google Scholar]
- 13.Howlin P, Magiati I, Charman T. Systematic review of early intensive behavioral interventions for children with autism. Am J Intellect Dev Disabil. 2009;114:23–41. doi: 10.1352/2009.114:23;nd41. [DOI] [PubMed] [Google Scholar]
- 14.Tebruegge M, Nandini V, Ritchie J. Does routine child health surveillance contribute to the early detection of children with pervasive developmental disorders? An epidemiological study in Kent, UK. BMC Pediatr. 2004;4:4–11. doi: 10.1186/1471-2431-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 16.National Initiative for Autism. [accessed June 8, 2009];The National Autism Plan for Children. Screening and assessment. 2003 http://www.nas.org.uk/nas/jsp/polopoly.jsp?d=368&a=2178.
- 17.Honda H, Shimizu Y, Nitto Y, et al. Extraction and refinement strategy for detection of autism in 18-month-olds: a guarantee of higher sensitivity and specificity in the process of mass screening. J Child Psychol Psychiatry. 2009;50:972–981. doi: 10.1111/j.1469-7610.2009.02055.x. [DOI] [PubMed] [Google Scholar]
- 18.Wong V, Hui LH, Lee WC, et al. A modified screening tool for autism (checklist for autism in toddlers [CHAT-23]) for Chinese children. Pediatrics. 2004;114:166–176. doi: 10.1542/peds.114.2.e166. [DOI] [PubMed] [Google Scholar]
- 19.Ozonoff S, Goodlin-Jones BL, Solomon M. Evidence-based assessment of autism spectrum disorders in children and adolescents. J Clin Child Adolesc Psychol. 2005;34:523–540. doi: 10.1207/s15374424jccp3403_8. [DOI] [PubMed] [Google Scholar]
- 20.Pennsylvania Department of Public Welfare. Pennsylvania autism assessment and diagnosis expert work group: supporting quality diagnostic practices for persons with suspected autism spectrum disorder. Philadelphia, PA: 2007. [accessed June 15, 2009]. http://www.dpw.state.pa.us/Resources/Documents/Pdf/Publications/PA_ASD_DiagnosisExpertWorkgroup.pdf. [Google Scholar]
- 21.Charman T, Baird G. Practitioner review: diagnosis of autism spectrum disorder in 2- and 3-year-old children. J Child Psychol Psychiatry. 2002;43:289–305. doi: 10.1111/1469-7610.00022. [DOI] [PubMed] [Google Scholar]
- 22.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 23.Hartley SL, Sikora DM, McCoy R. Prevalence and risk factors of maladaptive behaviour in young children with autistic disorder. J Intellect Disabil Res. 2008;52:819–829. doi: 10.1111/j.1365-2788.2008.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 2003;33:631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- 25.Rapin I, Tuchman R. Autism: definition, neurobiology, screening, diagnosis. Pediatr Clin North Am. 2008;55:1129–1146. doi: 10.1016/j.pcl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Nikolov RN, Bearss KE, Lettinga J, et al. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J Autism Dev Disord. 2008;39:405–413. doi: 10.1007/s10803-008-0637-8. [DOI] [PubMed] [Google Scholar]
- 27.Limoges E, Mottron L, Bolduc C, Berthiaume C, Godbout R. Atypical sleep architecture and the autism phenotype. Brain. 2005;128:1049–1061. doi: 10.1093/brain/awh425. [DOI] [PubMed] [Google Scholar]
- 28.Howlin P, Asgharian A. The diagnosis of autism and Asperger syndrome: findings from a survey of 770 families. Dev Med Child Neurol. 1999;41:834–839. doi: 10.1017/s0012162299001656. [DOI] [PubMed] [Google Scholar]
- 29.Allison C, Baron-Cohen S, Wheelwright S, et al. The Q-CHAT (Quantitative CHecklist for Autism in Toddlers): a normally distributed quantitative measure of autistic traits at 18–24 months of age: preliminary report. J Autism Dev Disord. 2008;38:1414–1425. doi: 10.1007/s10803-007-0509-7. [DOI] [PubMed] [Google Scholar]
- 30.Robins DL, Fein D, Barton M, et al. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders.”. J Autism Dev Disord. 2001;31:131–144. doi: 10.1023/a:1010738829569. [DOI] [PubMed] [Google Scholar]
- 31.Reznick J, Baranek G, Reavis S, Watson L, Crais E. A parent-report instrument for identifying one-year-olds at risk for an eventual diagnosis of autism: the first year inventory. J Autism Dev Disord. 2007;37:1691–1710. doi: 10.1007/s10803-006-0303-y. [DOI] [PubMed] [Google Scholar]
- 32.DeVincent C, Gadow KD, Strong G, et al. Screening for autism spectrum disorder with the Early Childhood Inventory-4. J Dev Behav Pediatr. 2008;29:1–10. doi: 10.1097/DBP.0b013e3181468c32. [DOI] [PubMed] [Google Scholar]
- 33.Gadow KD, Schwartz J, DeVincent C, et al. Clinical utility of autism spectrum disorder scoring algorithms for the child symptom inventory-4. J Autism Dev Disord. 2008;38:419–427. doi: 10.1007/s10803-007-0408-y. [DOI] [PubMed] [Google Scholar]
- 34.Chandler S, Charman T, Baird G, et al. Validation of the social communication questionnaire in a population cohort of children with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1324–1332. doi: 10.1097/chi.0b013e31812f7d8d. [DOI] [PubMed] [Google Scholar]
- 35.Myles BS, Bock S, Simpson R. ASDS Asperger syndrome diagnostic scale. San Antonio, TX: Pearson Education; 2000. [Google Scholar]
- 36.Krug DA, Arick JR. Krug Asperger’s Disorder Index. Austin, TX: Pro-Ed; 2003. [Google Scholar]
- 37.Auyeung B, Baron-Cohen S, Wheelwright S, Allison C. The autism spectrum quotient: children’s version (AQ-Child) J Autism Dev Disord. 2008;38:1230–1240. doi: 10.1007/s10803-007-0504-z. [DOI] [PubMed] [Google Scholar]
- 38.Rellini E, Tortolani D, Trillo S, et al. Childhood autism rating scale (CARS) and autism behavior checklist (ABC) correspondence and conflicts with DSM-IV criteria in diagnosis of autism.”. J Autism Dev Disord. 2004;34:703–708. doi: 10.1007/s10803-004-5290-2. [DOI] [PubMed] [Google Scholar]
- 39.Eaves RC, Campbell HA, Chambers D. Criterion-related and construct validity of the pervasive developmental disorders rating scale and the autism behavior checklist. Psychol Sch. 2000;37:311–321. [Google Scholar]
- 40.Kraijer D, de Bildt A. The PDD-MRS: an instrument for identification of autism spectrum disorders in persons with mental retardation. J Autism Dev Disord. 2005;35:499–513. doi: 10.1007/s10803-005-5040-0. [DOI] [PubMed] [Google Scholar]
- 41.Taffe JR, Gray KM, Einfeld SL, et al. Short form of the developmental behaviour checklist. Am J Ment Retard. 2007;112:31–39. doi: 10.1352/0895-8017(2007)112[31:SFOTDB]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray KM, Tonge BJ, Sweeney DJ, Einfeld SL. Screening for autism in young children with developmental delay: an evaluation of the developmental behaviour checklist: early screen. J Autism Dev Disord. 2008;38:1003–1010. doi: 10.1007/s10803-007-0473-2. [DOI] [PubMed] [Google Scholar]
- 43.Cohen IL. Criterion-related validity of the PDD behavior inventory. J Autism Dev Disord. 2003;33:47–53. doi: 10.1023/a:1022278420716. [DOI] [PubMed] [Google Scholar]
- 44.Karabekiroglu K, Aman M. Validity of the aberrant behavior checklist in a clinical sample of toddlers.”. Child Psychiatry Hum Dev. 2008;40:99–110. doi: 10.1007/s10578-008-0108-7. [DOI] [PubMed] [Google Scholar]
- 45.Geurts HM, Verte S, Oosterlaan J, et al. Can the children’s communication checklist differentiate between children with autism, children with ADHD, and normal controls?”. J Child Psychol Psychiatry. 2004;45:1437–1453. doi: 10.1111/j.1469-7610.2004.00850.x. [DOI] [PubMed] [Google Scholar]
- 46.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 47.Lam KSL, Aman MG. The repetitive behavior scale-revised: independent validation in individuals with autism spectrum disorders. J Autism Dev Disord. 2007;37:855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- 48.Skuse DH, Mandy WP, Scourfield J. Measuring autistic traits: heritability, reliability and validity of the social and communication disorders checklist. Br J Psychiatry. 2005;187:568–572. doi: 10.1192/bjp.187.6.568. [DOI] [PubMed] [Google Scholar]
- 49.Stone WL, Coonrod EE, Pozdol SL, Turner LM. The parent interview for autism-clinical version (PIA-CV): a measure of behavioral change for young children with autism. Autism. 2003;7:9–30. doi: 10.1177/1362361303007001003. [DOI] [PubMed] [Google Scholar]
- 50.Wing L, Leekam S, Libby S, et al. The diagnostic interview for social and communication disorders: background, inter-rater reliability and clinical use. J Child Psychol Psychiatry. 2002;43:307–325. doi: 10.1111/1469-7610.00023. [DOI] [PubMed] [Google Scholar]
- 51.Lecavalier L, Aman MG, Scahill L, et al. Validity of the autism diagnostic interview-revised. Am J Ment Retard. 2006;111:199–215. doi: 10.1352/0895-8017(2006)111[199:VOTADI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.Skuse D, Warrington R, Bishop D, et al. The developmental, dimensional and diagnostic interview (3di): a novel computerized assessment for autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2004;43:548–558. doi: 10.1097/00004583-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Baron-Cohen S, Wheelwright S, Cox A, et al. Early identification of autism by Checklist for Autism in Toddlers (CHAT) J R Soc Med. 2000;93:521–525. doi: 10.1177/014107680009301007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone WL, Coonrod EE, Turner LM, et al. Psychometric properties of the STAT for early autism screening. J Autism Dev Disord. 2004;34:691–701. doi: 10.1007/s10803-004-5289-8. [DOI] [PubMed] [Google Scholar]
- 55.Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The autism observation scale for infants: scale development and reliability data. J Autism Dev Disord. 2008;38:731–738. doi: 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- 56.Lord C, Risi S, Lambrecht L, et al. The ADOS-G (Autism Diagnostic Observation Schedule-Generic): a standard measure of social-communication deficits associated with autism spectrum disorders. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 57.Magyar C, Pandolfi V. Factor structure evaluation of the childhood autism rating scale. J Autism Dev Disord. 2007;37:1787–1794. doi: 10.1007/s10803-006-0313-9. [DOI] [PubMed] [Google Scholar]
- 58.Lord C, Risi S, Lambrecht L, et al. The ADOS-G (Autism Diagnostic Observation Schedule-Generic): a standard measure of social-communication deficits associated with autism spectrum disorders. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 59.Le Couteur A, Rutter M, Lord C, et al. Autism diagnostic interview: a standardized investigator-based instrument. J Autism Dev Disord. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- 60.Matson JL, Nebel-Schwalm MS. Comorbid psychopathology with autism spectrum disorder in children: an overview. Res Dev Disabil. 2006;28:341–352. doi: 10.1016/j.ridd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Herring S, Gray K, Taffe J, Torge B, Seeney D, Einfeld S. Behavioral and emotional problems in toddlers with pervasive developmental disorders and developmental delay and assocation with parental mental health. J Intellect Disabil Res. 2006;50:874–882. doi: 10.1111/j.1365-2788.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- 62.Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- 63.Schaefer GB, Mendelsohn NJ. Genetics evaluation for the etiologic diagnosis of autism spectrum disorders. Genet Med. 2008;10:4–12. doi: 10.1097/GIM.0b013e31815efdd7. [DOI] [PubMed] [Google Scholar]
- 64.DiCicco-Bloom E, Lord C, Zwaigenbaum L, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17:434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts TP, Schmidt GL, Egeth M, et al. Electrophysiological signatures: magnetoencephalographic studies of the neural correlates of language impairment in autism spectrum disorders. Int J Psychophysiol. 2008;68:149–160. doi: 10.1016/j.ijpsycho.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lam KSL, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: A review of the literature. Res Dev Disabi. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 71.O’Roak BJ, State MW. Autism genetics: strategies, challenges, and opportunities. Autism Res. 2008;1:4–17. doi: 10.1002/aur.3. [DOI] [PubMed] [Google Scholar]
- 72.Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry. 1997;154:185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- 73.Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: a comparison across parents of multiple-and single-incidence autism families. Am J Med Genet B Neuropsychiatr Genet. 2008;147:424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bailey A, Le Couteur A, Gottesman I, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 75.Kumar R, Christian S. Genetics of autism spectrum disorders. Curr Neurol Neurosci Rep. 2009;9:188–197. doi: 10.1007/s11910-009-0029-2. [DOI] [PubMed] [Google Scholar]
- 76.Cook EH, Jr, Sherer MR. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455:919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- 77.Veenstra-Vanderweele J, Christian SL, Cook EH., Jr Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. [DOI] [PubMed] [Google Scholar]
- 78.Wang K, Zhang H, Ma D, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–533. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marshall CR, Noor A, Vincent JB, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramocki MD, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tabuchi K, Blundell J, Etherton M, et al. A neuroligin-3 mutation implicated in autism increases inhibatory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 84.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurologic defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- 86.Rogers SJ, Vismara LA. Evidence-based comprehensive treatments for early autism. J Clin Child Adolesc Psychol. 2008;37:8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Myers SM. Management of autism spectrum disorders in primary care. Pediatr Ann. 2009;38:42–49. doi: 10.3928/00904481-20090101-08. [DOI] [PubMed] [Google Scholar]
- 88.Paul R. Interventions to improve communication in autism. Child Adolesc Psychiatr Clin N Am. 2008;17:835–856. doi: 10.1016/j.chc.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seida JK, Ospina MB, Karkhaneh M, Hartling L, Smith V, Clark B. Systematic reviews of psychosocial interventions for autism: an umbrella review. Dev Med Child Neurol. 2009;51:95–104. doi: 10.1111/j.1469-8749.2008.03211.x. [DOI] [PubMed] [Google Scholar]
- 90.Schlosser RW, Wendt O. Effects of augmentative and alternative communication intervention on speech production in children with autism: a systematic review. Am J Speech Lang Pathol. 2008;17:212–230. doi: 10.1044/1058-0360(2008/021). [DOI] [PubMed] [Google Scholar]
- 91.Ospina MB, Krebs Seida J, Clark B, et al. Behavioural and developmental interventions for autism spectrum disorder: a clinical systematic review. PLoS ONE. 2008;3:e3755. doi: 10.1371/journal.pone.0003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.King BH, Bostic JQ. An update on pharmacologic treatments for autism spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2006;15:161–175. doi: 10.1016/j.chc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 93.Lord C, Wagner A, Rogers S, et al. Challenges in evaluating psychosocial interventions for Autistic Spectrum Disorders. J Autism Dev Disord. 2005;35:695–708. doi: 10.1007/s10803-005-0017-6. discussion 709–611. [DOI] [PubMed] [Google Scholar]
- 94.National Research Council. Educating children with autism. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 95.Parr J. Autism. BMJ Clin Evid. 2007;12:322. [Google Scholar]
- 96.Osborne L, McHugh L, Saunders J, Reed P. Parenting stress reduces the effectiveness of early teaching interventions for autistic spectrum disorders. J Autism Dev Disord. 2008;38:1092–1103. doi: 10.1007/s10803-007-0497-7. [DOI] [PubMed] [Google Scholar]
- 97.Hess K, Morrier M, Heflin L, Ivey M. Autism treatment survey: services received by children with autism spectrum disorders in public school classrooms. J Autism Dev Disord. 2008;38:961–971. doi: 10.1007/s10803-007-0470-5. [DOI] [PubMed] [Google Scholar]
- 98.Rogers S, Vismara L. Evidence-based comprehensive treatments for early autism. J Clin Child Adolesc Psychol. 2008;37:8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koegel R, Koegel L, McNerney E. Pivotal areas in intervention for autism. J Clin Child Psychol. 2001;30:19–32. doi: 10.1207/S15374424JCCP3001_4. [DOI] [PubMed] [Google Scholar]
- 100.McGee GG, Morrier MJ, Daly T. An incidental teaching approach to early intervention for toddlers with autism. J Assoc Pers Sev Handicaps. 1999;24:133–146. [Google Scholar]
- 101.Schreibman L. The science and fiction of autism. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 102.Howard J, Sparkman C, Cohen H, Green G, Stanislaw H. A comparison of intensive behavior analytic and eclectic treatments for young children with autism. Res Dev Disabil. 2005;26:359–383. doi: 10.1016/j.ridd.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 103.Ingersoll B. The effect of a parent-mediated imitation intervention on spontaneous imitation skills in young children with autism. Res Dev Disabil. 2007;28:163–175. doi: 10.1016/j.ridd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 104.Koegel R, O’Dell M, Koegel L. A natural language teaching paradigm for nonverbal autistic children. J Autism Dev Disord. 1987;17:187–200. doi: 10.1007/BF01495055. [DOI] [PubMed] [Google Scholar]
- 105.Albin R, Lucyshyn J, Horner R, Flannery K. Contextual fit for behavioral support plan: a model for “goodness of fit”. In: Koegel L, Koegel R, Dunlap G, editors. Positive behavioral support: including people with difficult behavior in the community. Baltimore, MD: Paul H. Brookes Publishing; 1996. [Google Scholar]
- 106.Wieder S, Greenspan SI. Climbing the symbolic ladder in the DIR model through floortime interactive play. Autism. 2003;7:425–435. doi: 10.1177/1362361303007004008. [DOI] [PubMed] [Google Scholar]
- 107.Prizant B, Wetherby A, Rubin E, Laurent A. The SCERTS Model: a transactional, family-centered approach to enhancing communication and socioemotional abilities of children with autism spectrum disorder. Infants Young Child. 2003;16:296–316. [Google Scholar]
- 108.Rogers S, Dilalla D. A comparative study of the effects of a developmentally based instructional model on young children with autism and young children with other disorders of behavior and development. Topics Early Child Special Edu. 1991;11:29–47. [Google Scholar]
- 109.Ingersoll B, Dvortcsak A, Whalen C, Sikora D. The effects of a developmental, social-pragmatic language intervention on rate of expressive language production in young children with autistic spectrum disorders. Focus Autism Other Dev Disabi. 2005;20:213–222. [Google Scholar]
- 110.Posey DJ, Erickson CA, McDougle CJ. Developing drugs for core social and communication impairment in autism. Child Adolesc Psychiatr Clin N Am. 2008;17:787–801. doi: 10.1016/j.chc.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jahromi L, Kasari C, McCracken J, et al. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord. 2009;39:395–404. doi: 10.1007/s10803-008-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McDougle CJ, Scahill L, McCracken JT, et al. Research units on pediatric psychopharmacology (RUPP) autism network. Background and rationale for an initial controlled study of risperidone. Child Adolesc Psychiatr Clin N Am. 2000;9:201–224. [PubMed] [Google Scholar]
- 113.Kolevzon A, Mathewson KA, Hollander E. Selective serotonin reuptake inhibitors in autism: a review of efficacy and tolerability. J Clin Psychiatry. 2006;67:407–414. doi: 10.4088/jcp.v67n0311. [DOI] [PubMed] [Google Scholar]
- 114.King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66:583–590. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hollander E, Phillips AT, Chin-Chin Y. Targeted treatments for symptom domains in child and adolescent autism. Lancet. 2003;362:732–734. doi: 10.1016/S0140-6736(03)14236-5. [DOI] [PubMed] [Google Scholar]
- 116.Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–358. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 117.Levy SE, Hyman SL. Complementary and alternative medicine treatments for children with autism spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2008;17:803–820. doi: 10.1016/j.chc.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry. 2004;45:212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 119.Wing L. The autistic spectrum. Lancet. 1997;350:1761–1766. doi: 10.1016/S0140-6736(97)09218-0. [DOI] [PubMed] [Google Scholar]
- 120.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:37–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]