Abstract
Despite the wealth of information on cannabinoid-induced peripheral antihyperalgesic and antinociceptive effects in many pain models, the molecular mechanism(s) for these actions remains unknown. Although metabotropic cannabinoid receptors have important roles in many pharmacological actions of cannabinoids, recent studies have led to the recognition of a family of at least five ionotropic cannabinoid receptors (ICRs). The known ICRs are members of the family of transient receptor potential (TRP) channels and include TRPV1, TRPV2, TRPV4, TRPM8 and TRPA1. Cannabinoid activation of ICRs can result in desensitization of the TRPA1 and TRPV1 channel activities, inhibition of nociceptors and antihyperalgesia and antinociception in certain pain models. Thus, cannabinoids activate both metabotropic and ionotropic mechanisms to produce peripheral analgesic effects. Here, we provide an overview of the pharmacology of TRP channels as ICRs.
Introduction
Although cannabinoids have been used for millennia for treating pain and other symptoms, their mechanisms of action remain obscure. With the heralded identification of multiple G-protein-coupled receptors (GPCRs) mediating cannabinoid effects nearly two decades ago, the mystery of cannabinoid pharmacology was thought to be solved [1,2]. However, continued studies demonstrate that many cannabinoid effects cannot be attributed solely to the CB1 and CB2 metabotropic GPCRs. For example, several important cannabinoid actions, such as peripherally mediated antihyperalgesia (see Glossary), persist in CB1- and CB2-gene knockout animals [3,4]. In addition, the dual generation of both neuroprotective and neurotoxic effects of cannabinoids [5,6] cannot be explained by the presence of known metabotropic cannabinoid receptors (MCRs). For example, the CB1 antagonist rimonabat is neuroprotective in a model of cerebral ischemia, and these effects are independent of actions on MCRs [6]. These and other findings have led to the suggestion of a ‘CBx’ – an unknown cannabinoid receptor [7]. Here, we focus on the role of transient receptor potential (TRP) channels serving as ionotropic cannabinoid receptors (ICRs) and contributing to the pharmacology of cannabinoids, with an emphasis on their key role in the pain system.
The detection of tissue injury is an essential function of somatosensation and is mediated primarily by a specialized class of peripheral nociceptive afferent neurons. Although tissue injury is detected via peripheral nociceptive neurons, the actual perception of pain occurs in the central nervous system (CNS) and is subject to both stimulatory and inhibitory central modulation [8]. Peripheral nociceptive neurons are classically organized by morphological attributes or conduction velocity properties, although contemporary research has focused on their expression of receptors and ion channels as a functional type of classification [9,10]. The growing recognition of receptors expressed on nociceptors has prompted research on peripheral mechanisms regulating their activity. For example, peripheral administration of cannabinoids into inflamed tissue inhibits nociceptor activity and produces a peripherally mediated antihyperalgesia [11]. Additional analgesic mechanisms are engaged after central administration of cannabinoids. Thus, drugs can modulate peripheral or central targets to produce peripherally or centrally mediated analgesia.
The classical cannabinoid receptors: metabotropic receptors: metabotropic receptors
The first family of cannabinoid receptors identified were the metabotropic GPCRs including CB1, CB2, GPR55 and possibly GPR119 and peroxisome-proliferator-activated receptors (PPARs) [1,2,7,12]. The expression pattern for CB1 (throughout the CNS and heart, gastrointestinal tract, kidney, spleen, liver, lung, testis, uterus and muscle), CB2 (primarily immune cells and some neurons) and GPR55 (adrenal tissue, ileum, jejunum, frontal cortex and striatum) is broad, implicating a complex modulation of multiple physiologic systems by cannabinoids [13,14]. These MCRs signal primarily via Gi/o-related pathways, although coupling to Gs and Gq signaling pathways have been reported under certain experimental conditions [15]. Thus, agonist-directed trafficking is observed with these receptors as with other GPCRs. Activation of the MCRs leads to generation of the cannabinoid tetrad of behavior in addition to many other of the classical effects attributed to cannabinoid pharmacology [16]. In general, two approaches have been used experimentally to implicate an observed effect with the MCRs. First, pretreatment with a cannabinoid antagonist is predicted to block the observed effect. However, many of these compounds have inverse agonist activities that complicate interpretation, and other compounds such as AM251 might have known antagonist actions against CB1 with only recently recognized actions against other receptors (i.e. antagonist or inverse agonist actions at GPR55) [7,14]. A second approach is the use of genetic knockout animals. Interestingly, the knockout studies implicate the existence of non-CB1 and/or non-CB2 cannabinoid receptors [17]. It is possible that these effects might be attributed to additional GPCRs activated by cannabinoids. However, the finding that certain cannabinoid actions persist after pretreatment with either pertussis toxin or GDPβS compounds capable of blocking GPCR functions provides strong evidence of a second family of non-MCRs [18,19]. In addition, parallel studies indicate that application of cannabinoids generates a slow inward current in the TRPV1 and TRPA1 ionotropic receptors [20,21]. Taken together, these studies have expanded the scope of cannabinoid research to focus on ionotropic receptor systems.
ICRs regulate sensory neuron activities
Emerging data from several studies has led to the recognition of a second family of cannabinoid receptors, namely the ICRs. The ICRs are either activated or antagonized by cannabinoids in a variety of cell types by nM–μM concentrations of these compounds (Table 1). To date, the known ICRs belong to the TRP family of channels. The TRP channels are a broad family of ligand-gated ion channels that generate an inward flow of cations upon activation. Studies reported to date indicate that cannabinoids gate at least five distinct ICRs (Table 1).
Table 1.
Action of cannabinoids on ionotropic cannabinoid receptorsa
| Cannabinoid | Type | Action on ICR | MCR | Currentb |
|---|---|---|---|---|
| Anandamide | Endogenous | TRPV1 [21] | CB1; CB2 | 200–500 pA |
| >0.3 μMc | >10 nM | |||
| NADA | Endogenous | TRPV1 [61] | CB1; CB2 | 300–700 pA |
| >10 nM | >100 nM | |||
| 5′,6'-EA | Endogenous | TRPV4 [62] | NAd | 20–50 pA |
| >1 μM | ||||
| ACEA | Synthetic | TRPV1 [4,23] | CB1 | 300–700 pA |
| >5 μM | >1 nM | |||
| Δ9-THC | Plant | TRPV2 [63]; TRPA1 [30] | CB1; CB2 | 100–200 pA |
| >10 μM | >10 nM | |||
| Cannabinol | Plant | TRPV2 [63]; TRPA1 [30] | NAd | 50–100 pA |
| >10 μM | ||||
| Cannabidiol | Plant | TRPV2 [63]; TRPA1 [30] | NAd | 50–100 pA |
| >10 μM | ||||
| Cannabigerol | Plant | TRPM8 [46] | NAd | NA |
| NA | ||||
| AM404 | Synthetic | TRPV1 [64,65] | AEA-trans | 200–500 pA |
| >1 μM | NA | |||
| WIN55212 | Synthetic | TRPA1 [4] | CB1; CB2 | 200–300 pA |
| >5 μM | >10 nM | |||
| AM1241 | Synthetic | TRPA1-TRPV1e | CB2 | 100–200 pA |
| >25 μM | >1 nM |
Abbreviations: Δ9-THC, delta(9)-tetrahydrocannabinol; 5',6'-EA, 5',6'-epoxyeicosatrienoic acid; AEA-trans, antagonist for putative anandamide transporter; ICR, ionotropic cannabinoid receptor; MCR, metabotropic cannabinoid receptor; NA, non-applicable; NADA , N-arachidonoyl-dopamine.
Approximate value of current magnitudes in sensory neurons.
Approximate threshold concentrations of cannabinoids to activate receptors.
Cannabinoid-like compounds do not activate CB1 and CB2.
Activation of TRPA1 and TRPV1 co-expressing cells.
The majority of ICRs are expressed in nociceptive sensory neurons, which can detect and respond to noxious mechanical, thermal and chemical stimuli [22]. Therefore, it could be predicted that activation of sensory neurons, by cannabinoid gating of inward currents generated by these ICRs, could result in nociception and, ultimately, pain perception [23–25]. However, a vast majority of behavioral studies indicate that cannabinoids do not produce nociception [19,23,26] but instead induce a peripherally mediated and efficacious antihyperalgesia and antinociception [27–29]. The interpretation of these data is complex because cannabinoids activate ionotropic receptors and generate inward currents but still produce a profound antihyperalgesia. One possible hypothesis addressing this issue is that partial activation of ICRs does not necessarily generate excitation (i.e. action potential) of nociceptors. From this perspective, it is interesting to note that cannabinoids are not full agonists for TRP channels [4,23,30]. Indeed, cannabinoids typically evoke a slow generation of small inward currents and Ca2+ accumulation [4,19,20,30] (Table 1). As a result, cannabinoid-gated responses might not reach the threshold levels required to excite nociceptors. Moreover, slow depolarization of nociceptor membrane potentials might lead to inactivation of voltage-gated channels that, in turn, inhibits the generation of action potentials [31].
Because cannabinoids can trigger peripherally mediated antihyperalgesia and antinociception, it is important to address whether their modulation of ICRs regulates peripheral nociceptor activity. Application of cannabinoids elevates internal Ca2+ ([Ca2+]i) levels in nociceptors because known ICRs (TRP channels) are permeable to Ca2+ (Table 1). An elevation of [Ca2+]i can ignite numerous cellular cascades, including induction of Ca2+-dependent kinases, phosphatases and phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) [32–35]. It is well established that these enzymes and lipids can be effective modulators of activities of the TRPV1 and TRPA1 channels [33,34,36–39], which have crucial roles in regulating nociception [40–43]. Thus, one hypothesis is that certain cannabinoids regulate nociceptors by activating Ca2+-permeable channels. As detailed later, the remarkable outcome of this activation leads to a desensitization of nociceptor activities. Recent studies have indeed demonstrated that cannabinoids that selectively activate TRPV1 (e.g. arachidonoylchloro-ethanolamide [ACEA]) or TRPA1 (e.g. WIN55212 or AM1241) induce a homologous and cross-desensitization (i.e. the desensitization of one channel after activation of another channel) of the activities of both of these TRP channels [4,19,20], ultimately resulting in peripherally mediated antihyperalgesia [4,19]. The simultaneous desensitization of multiple TRP channels might lead to a broader inhibition of nociceptor responsiveness than that observed by inhibition of only one channel and, thus, represents a potentially novel approach for the generation of analgesic compounds. Studies conducted in cell expression systems indicate that TRPV1-selective cannabinoids can only desensitize TRPA1 when TRPV1 is co-expressed. Similarly, TRPA1-selective cannabinoids desensitize TRPV1 only under conditions in which TRPA1 is present [4]. Thus, the cross-desensitization produced by these TRP-selective cannabinoids requires the co-expression of both TRPV1 and TRPA1.
To understand how activation of ICRs leads to inhibition of nociceptors, molecular mechanisms of desensitization of TRP channels by ICR-activating cannabinoids were investigated. The results indicate that cross-desensitization between the TRPA1 and TRPV1 channels in sensory neurons seems to involve multiple separate mechanisms.
First, TRPV1 is desensitized by TRPV1- and TRPA1-activating cannabinoids via Ca2+-dependent calcineurin (phosphatase 2B)-induced dephosphorylation of the channel [19,20] (Figure 1). A similar mechanism is employed in the desensitization of TRPV1 by the TRPA1-specific agonist mustard oil (MO) [33,44]. Interestingly, Ca2+-induced PtdIns(4,5)P2 biosynthesis, a mechanism involved in tachyphylaxis of capsaicin responses in heterologous expression systems [34,38], does not play a part in desensitization of TRPA1 by activation of TRPV1 or in cross-desensitization of TRPV1 by activation of the TRPA1 channel in sensory neurons [33]. Furthermore, Ca2+-dependent activation of kinases can be responsible for recovery from desensitization [37]. Overall, Ca2+-dependent desensitization prevails over sensitization because calcineurin is more effectively induced by low concentrations of Ca2+ than kinases [45]. Thus, calcineurin-mediated dephosphorylation is an important mechanism for cannabinoid desensitization of TRPV1 and compounds that block calcineurin produce a significant inhibition of peripheral cannabinoid antihyperalgesia [18] (Figure 1).
Figure 1.
Mechanisms for cannabinoid cross-desensitization of TRPV1 and TRPA1. Cannabinoids desensitize TRPV1 via activation of calcineurin and dephosphorylation of the ion channel. Homologous desensitization of TRPV1 can occur by application of TRPV1-selective cannabinoids (e.g. ACEA), and heterologous desensitization of TRPV1 can occur by administration of TRPA1-selective cannabinoids (e.g. WIN55212). Cannabinoids desensitize TRPA1 via activation of a calcium-independent pathway. Abbreviations: DAG, diacylglycerol; IP3, inositol (1,4,5)-trisphosphate; PHD, pleckstrin homology domain; PIP2, phosphatidylinositol (4,5)-bisphosphate; PLC, phospholipase C.
A second potential mechanism for TRPA1 desensitization is via a Ca2+-dependent depletion of PtdIns(4,5)P2 [33]. However, unlike capsaicin [33,34], tested cannabinoids cannot induce Ca2+-dependent depletion of PtdIns(4,5)P2, possibly owing to their slow or reduced accumulation of intracellular Ca2+ levels [20]. Thus, this mechanism does not seem to be utilized for cannabinoid desensitization of TRP channels.
A third mechanism for desensitization of TRPA1 is via activation of a Ca2+-independent pathway [33,44]. This pathway is evident for TRPA1 desensitization by low concentrations of MO [46] and possibly by a partial agonist for the TRPA1 channel, notably the cannabinoid WIN55212 [4,33]. Altogether, certain cannabinoids are able to inhibit nociception by activating ICRs – TRP channels.
Roles of ICRs in peripheral antihyperalgesia
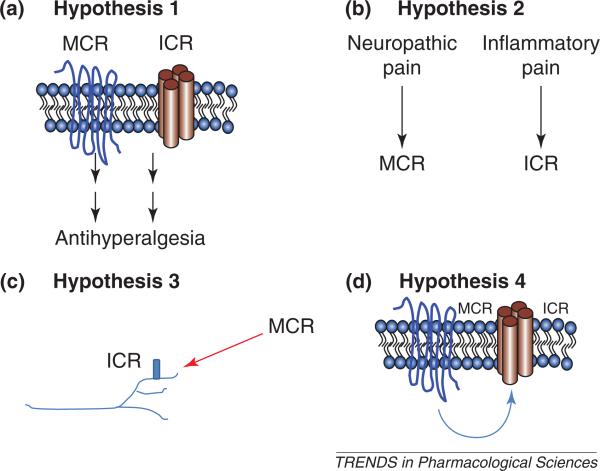
There is broad agreement that cannabinoids can produce peripherally mediated antihyperalgesic and antinociceptive effects by any of several proposed mechanisms [4,19,47–49]. Experimental findings support at least four distinct hypotheses (Figure 2). The first hypothesis is that cannabinoids mediate their actions via different metabotropic or ionotropic receptors. Thus, peripherally restricted doses of WIN55212 significantly inhibit capsaicin-induced nocifensive behavior in wild-type animals, and this effect is lost in either TRPV1 or CB1-gene knockouts but not in mice with genetic deletion of the CB2 receptor [4]. Thus, cannabinoids might activate multiple receptor mechanisms to produce peripherally mediated antihyperalgesia. The second hypothesis is based upon the observation that cannabinoid receptor systems are differentially activated in various pain models (e.g. inflammatory versus neuropathic versus basal pain thresholds) or by distinct experimental approaches (e.g. local versus systemic versus intrathecal injection). For example, certain immune cells expressing CB2 might have greater contributions to nociceptor activation in inflammatory pain models compared with neuropathic pain models. In addition, inflammation has been reported to trigger upregulation of the CB1 receptor in sensory neurons [50]. The third hypothesis is based upon the finding that cannabinoids can modulate sensory neurons in addition to non-neuronal peripheral cells. Thus, the overall cannabinoid effect might be an integration of activity across several peripheral cell types (e.g. nociceptor terminals or keratinocytes [32]). Fourth, it is possible that ICRs and MCRs can functionally cooperate under certain conditions [5,51] because ionotropic and metabotropic receptors are co-expressed in many cells [52].
Figure 2.
Proposed mechanisms for the peripherally mediated antihyperalgesic effects of cannabinoids. (a) Hypothesis 1 proposes that cannabinoids produce peripheral antihyperalgesia by activation of both MCRs and ICRs. (b) Hypothesis 2 proposes that different pain models (or routes of drug injection) selectively activate either MCR or ICR pathways. (c) Hypothesis 3 proposes that the net effect of cannabinoids on modulating nociception is due to activation of MCRs and/or ICRs located on multiple cell types that are capable of interacting. (d) Hypothesis 4 proposes that MCRs and ICRs interact in the same cell leading to desensitization of activity. Note that the selective involvement of MCR and/or ICR in each hypothesis is for illustrative purposes only.
How important are ionotropic cannabinoid mechanisms in such a diversity of analgesic cannabinoid hypotheses? Interestingly, evidence from knockout animals indicates that both pathways are equally important in at least some pain models because knockout of either ICR or MCR genes reduces peripherally mediating antihyperalgesic effects of cannabinoids [4]. Several hypotheses could explain these findings. First, ICRs and MCRs operate independently and mediate the actions of cannabinoids on different types of cells. For example, CB2-mediated antinociception occurs via activation of CB2 on keratinocytes [53], whereas TRPA1-mediated effects of cannabinoids take place on nociceptors [4]. Thus, the relative role of specific cell types in various pain models could alter the contribution of distinct cannabinoid receptor systems. Second, ICRs and MCRs effectively mediate antihyperalgesia or antinociception in distinct pain models. Third, MCRs could cooperate with ionotropic receptors on cells where both are co-expressed. This could result in either direct inhibition of nociceptors or promotion of Ca2+-dependent release of nociception inhibitory factors (i.e. opioids, endocannabinoids etc) by non-neuronal peripheral cells. When co-expressed, ICRs and MCRs can cooperate in a variety of ways. For example, the activation of CB1 on mesencephalic dopaminergic neurons can produce intracellular 12(S)-hydroxyeicosatetraenoic acid, which is a TRPV1 agonist [5]. Cannabinoids can also be coupled to Gq/11 proteins [54,55], and this could lead to activation of the phospholipase C pathway which, in turn, could result in the activation of several TRP channels including TRPA1 [56]. In addition, fatty acid amine hydrolase (FAAH) is involved in anandamide metabolism and is co-localized with TRPV1 in several areas, indicating an important regulatory interaction [57]. Indeed, inhibition of FAAH rapidly leads to accumulation of fatty acid amides, some of which are efficacious TRP agonists [58]. Thus, URB597, a potent and systemically active inhibitor of FAAH, activates TRPA1 channels in addition to peroxisome proliferator-activated receptor-α [58,59].
Conclusion and future perspectives
Despite the wealth of information on cannabinoid-induced peripheral antihyperalgesic and antinociceptive actions in many pain models, the molecular mechanism(s) for these effects remains unknown. Recent investigation in these mechanisms has yielded the hypothesis that activation of ICRs can result in desensitization of the TRPA1 and TRPV1 channel activities, inhibition of nociceptors and antihyperalgesia and antinociception in certain pain models. One important conclusion is that, although cannabinoids differ in their activations of various receptors, they could exert inhibitory effects by acting through ICRs. In addition, the control of intracellular activities by cannabinoids could occur via two very distinct pathways (i.e. metabotropic versus ionotropic), providing multiple mechanisms for triggering antihyperalgesia and antinociception. Thus, it is conceivable that partial TRP-channel-specific agonists could constitute a novel class of peripherally selective analgesics without the typical side effects of conventional cannabinoids.
Nevertheless, many challenges still remain. Among these is the detailed characterization of molecular mechanisms responsible for desensitization of nociceptor-specific TRP channels by ICR-activating cannabinoids, the investigation of the function of ICR in peripheral cells (such as blood and skin cells) that might have roles in the process of nociception, and the evaluation of possible co-operation between MCRs and ICRs in nociceptive neurons in addition to other peripheral cells contributing to nociception.
Acknowledgements
Research was supported by grants DE014928 (A.N.A.), DA19585 and UL1 RR025767 (K.M.H.) and DE016500 (N.A.J.).
Glossary
- Activation*
increased functional activity of neurons (e.g. action potential or exocytosis).
- Allodynia
occurrence of nociception in response to a non-noxious stimulus.
- Antiallodynia
reduction of allodynia.
- Antihyperalgesia
reduction of hyperalgesia.
- Antinociception
increase in nociceptive thresholds above basal nociceptive levels.
- Basal nociceptive thresholds
stimulus intensity capable of evoking an escape response under basal (un-injured) conditions. Usually a thermal or mechanical stimulus.
- Hyperalgesia
increased magnitude of nociception in response to a noxious stimulus.
- Inflammatory pain models
animal models in which nociception occurs owing to injury of a peripheral tissue.
- Inverse agonist
a compound that binds to the same site as an agonist but reverses the constitutive activity of the receptor.
- Neuropathic pain models
animal models in which nociception occurs owing to injury to a peripheral nerve.
- Nociception
the neural encoding and modulation of noxious stimuli.
- Pain
an unpleasant sensory and emotional experience associated with actual or potential tissue damage.
- Peripheral nociceptive neurons
specialized class of peripheral afferent neurons that detects and encodes stimuli that induce tissue injury or chemical factors released during tissue injury.
- Sensitization
increased responsiveness of neurons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Some terms are modified from Ref. [60].
References
- 1.Matsuda LA, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda LA. Molecular aspects of cannabinoid receptors. Crit. Rev. Neurobiol. 1997;11:143–166. doi: 10.1615/critrevneurobiol.v11.i2-3.30. [DOI] [PubMed] [Google Scholar]
- 3.Zimmer A, et al. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akopian AN, et al. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J. Neurosci. 2008;28:1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SR, et al. Roles of transient receptor potential vanilloid subtype 1 and cannabinoid type 1 receptors in the brain: neuroprotection versus neurotoxicity. Mol. Neurobiol. 2007;35:245–254. doi: 10.1007/s12035-007-0030-1. [DOI] [PubMed] [Google Scholar]
- 6.Pegorini S, et al. Vanilloid VR1 receptor is involved in rimonabant-induced neuroprotection. Br. J. Pharmacol. 2006;147:552–559. doi: 10.1038/sj.bjp.0706656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryberg E, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br. J. Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 9.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Hagenacker T, et al. Feedback mechanisms in the regulation of intracellular calcium ([Ca2+]i) in the peripheral nociceptive system: role of TRPV-1 and pain related receptors. Cell Calcium. 2008;43:215–227. doi: 10.1016/j.ceca.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Richardson JD, et al. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Bennett A. Cannabinoids: a new group of agonists of PPARs. PPAR Res. 2007;2007:23513. doi: 10.1155/2007/23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackie K. Understanding cannabinoid psychoactivity with mouse genetic models. PLoS Biol. 2007;5:e280. doi: 10.1371/journal.pbio.0050280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackie K. Signaling via CNS cannabinoid receptors. Mol. Cell. Endocrinol. 2008;286:S60–S65. doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiley CR, Kaup SS. GPR55 and the vascular receptors for cannabinoids. Br. J. Pharmacol. 2007;152:559–561. doi: 10.1038/sj.bjp.0707421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pertwee RG. Pharmacological actions of cannabinoids. Handb. Exp. Pharmacol. 2005;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- 17.Brown AJ. Novel cannabinoid receptors. Br. J. Pharmacol. 2007;152:567–575. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans RM, et al. Modulation of sensory neuron potassium conductances by anandamide indicates roles for metabolites. Br. J. Pharmacol. 2008;154:480–492. doi: 10.1038/bjp.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patwardhan AM, et al. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11393–11398. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeske NA, et al. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. J. Biol. Chem. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zygmunt PM, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 22.Tominaga M, Caterina MJ. Thermosensation and pain. J. Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- 23.Price TJ, et al. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br. J. Pharmacol. 2004;141:1118–1130. doi: 10.1038/sj.bjp.0705711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischbach T, et al. Effects of anandamide and noxious heat on intracellular calcium concentration in nociceptive drg neurons of rats. J. Neurophysiol. 2007;98:929–938. doi: 10.1152/jn.01096.2006. [DOI] [PubMed] [Google Scholar]
- 25.Lee MG, et al. Effect of olvanil and anandamide on vagal C-fiber subtypes in guinea pig lung. Br. J. Pharmacol. 2005;146:596–603. doi: 10.1038/sj.bjp.0706339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price TJ, et al. Cannabinoid receptor-independent actions of the aminoalkylindole WIN 55,212-2 on trigeminal sensory neurons. Br. J. Pharmacol. 2004;142:257–266. doi: 10.1038/sj.bjp.0705778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarberg BH, Barkin RL. The future of cannabinoids as analgesic agents: a pharmacologic, pharmacokinetic, and pharmacodynamic overview. Am. J. Ther. 2007;14:475–483. doi: 10.1097/MJT.0b013e3180a5e581. [DOI] [PubMed] [Google Scholar]
- 28.Hohmann AG, Suplita RL., 2nd Endocannabinoid mechanisms of pain modulation. AAPS J. 2006;8:E693–E708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mbvundula EC, et al. Cannabinoids in pain and inflammation. Inflammopharmacology. 2004;12:99–114. doi: 10.1163/1568560041352275. [DOI] [PubMed] [Google Scholar]
- 30.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, et al. The responses of rat trigeminal ganglion neurons to capsaicin and two nonpungent vanilloid receptor agonists, olvanil and glyceryl nonamide. J. Neurosci. 1997;17:4101–4111. doi: 10.1523/JNEUROSCI.17-11-04101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- 33.Akopian AN, et al. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J. Physiol. 2007;583:175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukacs V, et al. Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 2007;27:7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Docherty RJ, et al. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 36.Koplas PA, et al. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J. Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhave G, et al. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 38.Liu B, et al. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karashima Y, et al. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch. 2008;457:77–89. doi: 10.1007/s00424-008-0493-6. [DOI] [PubMed] [Google Scholar]
- 40.Vennekens R, et al. Vanilloid transient receptor potential cation channels: an overview. Curr. Pharm. Des. 2008;14:18–31. doi: 10.2174/138161208783330763. [DOI] [PubMed] [Google Scholar]
- 41.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 42.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 43.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 44.Ruparel NB, et al. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135:271–279. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung J, et al. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J. Biol. Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 46.De Petrocellis L, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 2008;325:1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- 47.Rice AS, et al. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- 48.Malan TP, Jr, et al. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr. Opin. Pharmacol. 2003;3:62–67. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 49.Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb. Exp. Pharmacol. 2005;168:509–554. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- 50.Amaya F, et al. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain. 2006;124:175–183. doi: 10.1016/j.pain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Evans RM, et al. Chronic exposure of sensory neurones to increased levels of nerve growth factor modulates CB1/TRPV1 receptor crosstalk. Br. J. Pharmacol. 2007;152:404–413. doi: 10.1038/sj.bjp.0707411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cristino L, et al. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 53.Ibrahim MM, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Petrocellis L, et al. Mechanisms for the coupling of cannabinoid receptors to intracellular calcium mobilization in rat insulinoma beta-cells. Exp. Cell Res. 2007;313:2993–3004. doi: 10.1016/j.yexcr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 55.McIntosh BT, et al. Agonist-dependent cannabinoid receptor signalling in human trabecular meshwork cells. Br. J. Pharmacol. 2007;152:1111–1120. doi: 10.1038/sj.bjp.0707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 57.Zimov S, Yazulla S. Vanilloid receptor 1 (TRPV1/VR1) co-localizes with fatty acid amide hydrolase (FAAH) in retinal amacrine cells. Vis. Neurosci. 2007;24:581–591. doi: 10.1017/S095252380707054X. [DOI] [PubMed] [Google Scholar]
- 58.Niforatos W, et al. Activation of TRPA1 channels by the fatty acid amide hydrolase inhibitor 3′-carbamoylbiphenyl-3-yl cyclohexylcarbamate (URB597). Mol. Pharmacol. 2007;71:1209–1216. doi: 10.1124/mol.106.033621. [DOI] [PubMed] [Google Scholar]
- 59.Sagar DR, et al. Inhibition of fatty acid amide hydrolase produces PPAR-α-mediated analgesia in a rat model of inflammatory pain. Br. J. Pharmacol. doi: 10.1038/bjp.2008.335. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loeser JD, Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137:473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 61.Huang SM, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe H, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 63.Qin N, et al. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zygmunt PM, et al. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur. J. Pharmacol. 2000;396:39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]
- 65.Roberts LA, et al. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br. J. Pharmacol. 2002;137:421–428. doi: 10.1038/sj.bjp.0704904. [DOI] [PMC free article] [PubMed] [Google Scholar]