Abstract
Preparative high-speed countercurrent chromatography (HSCCC) was successfully used for isolation and purification of caffeic acid from Eupatorium adenophorum Spreng with a solvent system composed of ethyl acetate-methanol-water at a volume ratio of 10:1:10, v/v. Using a preparative unit of the HSCCC centrifuge, about a 938 mg amount of the crude extract was separated, yielding 63.2 mg of caffeic acid at purity of 96.0%. Then, the anti-microbial and anti-virus drug caffeic acid (C9H8O4) was intercalated into layered double hydroxides for the first time by anion exchange under a nitrogen atmosphere. The product caffeic acid–LDH has been characterized by powder X-ray diffraction (XRD), Fourier transform-infrared (FT-IR), Scanning electron micrographs (SEM), indicating that the drug has been successfully intercalated into LDH.
Keywords: Isolation and purification, High-speed countercurrent chromatography, Layered double hydroxides, caffeic acid, Eupatorium adenophorum Spreng
Introduction
Eupatorium adenophorum Spreng is native to Central America, mainly Mexico. This weed is toxic to grazing animals, its strong allelopathy inhibits the growth of other plants and the strong ability to adapt to different environmental conditions results in the damage of many farmlands, pasture fields and forests [1]. Since the 1950s, this Crofton weed has spread rapidly across southwest China, damaging native ecosystems and causing great economic losses. Although much attention has been paid to management of this weed and several methods have been used to control it, including manual, chemical and biological control, there has been no significant progress [2, 3].
Compared to simple control processes, pharmaceutical utilization of the weed gives more benefits. As many other native plants, it has some bioactive components such as caffeic acid. The antioxidative properties of caffeic acid have previously been evaluated, and caffeic acid has been shown to exhibit both radical-scavenging and metal-chelating abilities[4].
High-speed countercurrent chromatography (HSCCC) is a support-free all liquid-liquid partition technique [5] which can eliminate irreversible adsorption of samples on solid support in conventional column chromatography [6]. Therefore, it is very suitable for separation of active compounds from traditional Chinese herbs and other natural products [7]. However, no report has been published on the use of HSCCC for the isolation and purification of caffeic acid from Eupatorium adenophorum Spreng. The present paper describes the successful preparative separation and purification of caffeic acid from Eupatorium adenophorum Spreng by HSCCC.
Layered double hydroxides (LDHs), or so-called anionic clays, consist of cationic brucite-like layers and exchangeable interlayer anions [8, 9]. Because of their biocompatibility, Mg-Al–LDHs can be used as host materials for drug-LDH host–guest supramolecular structures. The anti-microbial and anti-virus drug caffeic acid (C9H8O4) has been intercalated into layered double hydroxides for the first time by anion exchange under a nitrogen atmosphere. The product has been characterized by powder X-ray diffraction (XRD), Fourier transform-infrared spectra (FT-IR), and scanning electron micrographs (SEM).
Experimental
Apparatus
Preparative HSCCC was performed using a Model GS10A multilayer coil planet centrifuge (Beijing Institute of New Technology Application, Beijing, China) equipped with a PTFE(polytetrafluoroethylene) multilayer coil of 110 m × 1.6 mm I.D. with a total capacity of 230 mL. The β value of the preparative column ranged from 0.5 at the internal terminal to 0.8 at the external terminal of the multilayer coil. (The β value is given by dividing the distance between the coil and the holder shaft with the distance between the holder shaft and the central axis of the centrifuge. It is an important parameter to determine retention of the stationary phase in the coiled column.)
The solvent was pumped into the column with a Model NS-1007 constant-flow pump (Beijing Institute of New Technology Application, Beijing, China). Continuous monitoring of the effluent was achieved with a Model 8823A-UV Monitor (Beijing Institute of New Technology Application, Beijing, China) at 254 nm. A manual sample injection valve with a 10 mL loop (for the preparative HSCCC) was used to introduce the sample into the column. A portable recorder Yokogawa Model 3057 (Sichuan Instrument Factory, Chongqing, China) was used to plot the chromatogram. A rotary evaporator was also used.
The high-performance liquid chromatography (HPLC) equipment used was a Shimadzu LC-20A system including two LC-20A solvent delivery units, an SPD-M20A UV-VIS photodiode array detector (DAD), a Model 7725 injection valve with a 20 μl loop, an SCL-20A system controller, and a Class-VP-LC workstation (Shimadzu, Kyoto, Japan).
Reagents
All organic solvents used for HSCCC were of analytical grade and purchased from Beijing Chemical Factory (Beijing, China). Methanol used for HPLC analysis was of chromatographic grade and purchased from Tianjin Huaxi Special Reagent Factory (Tianjin, China). Eupatorium adenophorum Spreng was obtained from Institute of Environment and Sustainable Development in Agriculture, The Chinese Academy of Agricultural Sciences. Caffeic acid standards were purchased from National Institute for the Control of Pharmaceutical & Biological Products (Beijing, China).
Preparation of Crude Extract of Eupatorium adenophorum Spreng
About 100 g of dried Eupatorium adenophorum Spreng was extracted (refluxed) for 1.5 h once with 1500 mL of ethanol solution, and then filtered by a six-layer pledget. The filtrate was concentrated to dryness under reduced pressure yielding 5.50 g of a crude sample, which contained caffeic acid.
Preparation of Two-Phase Solvent System and Sample Solutions
The solvent system utilized in the present study was prepared by mixing ethyl acetate-methanol-water (10:1:10, v/v), and thoroughly equilibrating the mixture in a separatory funnel at room temperature, two phases being separated shortly before use.
The sample solutions were prepared by dissolving the crude extract in the lower phase at suitable concentrations according to preparative purpose.
Separation Procedure
Preparative HSCCC was performed with a Model GS10A HSCCC instrument as follows: the multilayer coiled column was first entirely filled with the upper phase as stationary phase. Then the lower phase of the solvent system was pumped into the head end of the column at a flow-rate of 2 mL/min, while the apparatus was rotated at 800 rpm. After hydrodynamic equilibrium was reached, as indicated by a clear mobile phase eluting at the tail outlet, the sample solution (938 mg in 10 mL of lower phase) was injected through the injection valve. The effluent from the outlet of the column was continuously monitored with a UV detector at 254 nm. Each peak fraction was collected according to the chromatogram.
HPLC Analyses and Identification of HSCCC Peak Fractions
The crude extract of Eupatorium adenophorum Spreng and HSCCC peak fractions were each analyzed by HPLC. The analyses were performed with a Shimadzu ODS column (150 × 4.6 mm I.D.). The mobile phase composed of methanol: 0.05% phosphoric acid water (1:1, v/v) was isocratically eluted at a flow-rate of 0.5 mL/min and the effluent monitored at 254 nm by a DAD detector.
Identification of caffeic acid was based on MS, 1H-NMR; and 13C-NMR spectra.
Synthesis of NO3-Containing Mg–Al-LDHs
An aqueous solution (50 mL) containing Mg (NO3)2·6H2O (15.30 g, 0.06 mol) and Al (NO3)3·9H2O (11.40 g, 0.03 mol) (initial Mg/Al = 2.0) were added dropwise to a solution (75 mL) containing NaOH (7.20 g, 0.18 mol) under nitrogen atmosphere with vigorous stirring, then simultaneously adding 0.1 mol·L−1 NaOH solution to bring the final pH of ca. 10. The resulting slurry was aged at 80°C for 24 h. Then the resultant was filtered, washed with decarbonated distilled water until the pH of ca.7 and finally dried at 80°C for 24 h yielding the product NO3−LDHs [10-13].
Caffeic Acid–LDH Hybrid Synthesis
Under nitrogen atmosphere, 0.5 g of NO3--LDH was added to 100 mL of a 0.005 mol aqueous drug solution. The pH of mixture solution was held constant at 4.5 by simultaneous addition of 0.1 mol·L−1 NaOH solution [14, 15]. The exchange process was stirred vigorously for 24 h at 100°C. The precipitate was washed by decarbonated distilled water, and finally dried at 100°C for 24 h giving the product caffeic acid–LDH.
Results and Discussion
HSCCC Separation of Caffeic Acid
As shown in Fig. 1, the HPLC analysis of the crude extract of Eupatorium adenophorum Spreng shows caffeic acid, the purity of which was 6.7% based on the HPLC external standard curve.
Fig.1.
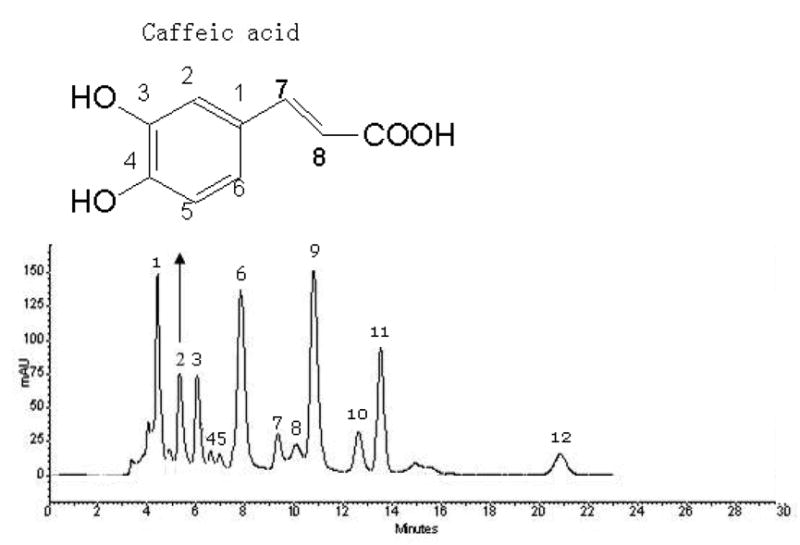
HPLC analyses of the crude extract of Eupatorium adenophorum Spreng.
HPLC conditions: a Shimadzu ODS column (150 × 4.6 mm I.D.). Mobile phase: methanol: 0.05% phosphoric acid water (1:1, v/v), flow-rate: 0.5 mL/min, monitored at 254 nm by a DAD detector. Peak 2 = caffeic acid
In order to achieve efficient resolution of the target compound, a two-phase solvent system composed of ethyl acetate-methanol-water was examined using preparative HSCCC by varying the mutual volume ratio, since this solvent system has been successfully applied to various samples with a moderate degree of polarity. Finally from 938 mg of the crude extract, preparative high-speed countercurrent chromatography at the solvent ratio (10:1:10, v/v) successfully isolated 63.2 mg of caffeic acid. at the purity of 96.0% based on HPLC peak area percentage. The chromatograms of HSCCC and the HPLC are illustrated in Fig. 2.
Fig. 2.
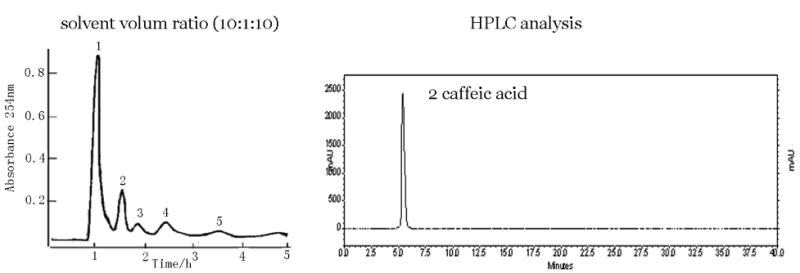
Preparative HSCCC separation of the crude extract of Eupatorium adenophorum Spreng with solvent system composed of ethyl acetate-methanol-water (10:1:10, v/v), stationary phase: upper organic phase of solvent system, mobile phase: lower aqueous phase of solvent system; flow-rate: 2.0 mL/min; revolution speed: 800 rpm; sample: 234.5 mg dissolved in 10 mL of lower phase of the solvent system. HPLC conditions: a Shimadzu ODS column (150 × 4.6 mm I.D.). Mobile phase: methanol: 0.05% phosphoric acid water (1:1, v/v), flow-rate: 0.5 mL/min, monitored at 254 nm by a DAD detector. Peak 2 = caffeic acid.
The structural identification of target compound was carried out by MS, 1H-NMR and 13C-NMR Spectra as follows: EI-MS m/z: 180, 163, 136, 89, 77, 44. 13C-NMR (DMSO- d6) δ: 168.7 (C-9), 148.7 (C-4), 146.1 (C-3), 145.3 (C-7), 126.5 (C-1), 121.9 (C-6), 116.5 (C-5), 115.9 (C-8), 115.3 (C-2). 1H-NMR DMSO–d6δ : 12.130 (1H, COOH), 7.702 (1H, H-8), 7.519 (1H, H-7), 6.895 (1H, H-6), 6.881 (1H, H-5), 6.915 (1H, H-2), 5.011 (1H, 3C - OH) 4.998 (1H, 4C - OH), compared with the data given in [16], it was identified as caffeic acid.
Characterization of Caffeic Acid–LDH
The X-ray diffraction patterns of NO3-form of LDH and caffeic acid–LDH were shown in Fig.3. In fact, the thickness of basic brucite layer does not change very much during the anion exchange process. The basal d spacing of the [003] plane was dependent on the size of the guest anion in interlayer of NO3-form of hydrotalcite clay where the d spacing of the [003] plane of NO3--, form of LDH is × Å. In the present study, the X-ray diffraction patterns of NO3- form of LDH and caffeic acid–LDH were compared. The basal d spacing value of LDH layer was 7.8 Å that was expanded to 22.54 Å, representing the intercalation of caffeic acid into interlayer of Mg–Al LDH. This reveals that caffeic acid–LDH hybrid has been synthesized.
Fig. 3.

XRD patterns of NO3-form of LDH (a) and synthesized caffeic acid–LDH hybrid (b).
FT-IR was used to distinguish the materials with very similar structures and it is possible to find a trace amount of materials, which are not detectable by XRD analysis. In Fig. 4, the spectrum of NO3- form of LDH showed a broad peak from 3000 to 3600 cm-1 that was due to the water molecules in interlayer and to the hydroxyl groups in the brucite-like layers, with a shoulder at 2927 cm-1 due to the H-bonded OH-stretching vibration. The strong peak at 1485 cm-1 was attributed the NO3- anion in a symmetric environment. The bands in the range from 760 to 550 cm-1 were attributed to oxygen–metal–oxygen stretching. The spectrum of caffeic acid contained peaks at 1690 cm-1 (C=O), 1639 cm-1(C=C), 1617, 1521 and 1447 cm-1 (aromatic ring, C=C), 1247 and 1294 cm-1 (carboxylic C–O), 1189 cm-1 (carboxylic, O–H). The caffeic acid–LDH exhibited characteristic IR peaks of both the LDH and pure caffeic acid. Some of the peaks of caffeic acid–LDH hybrid occurred at the same frequency as of pure caffeic acid, while others were slightly shifted and some new peaks appeared. The results indicated that caffeic acid was intercalated into interlayer of Mg–Al LDH.
Fig.4.
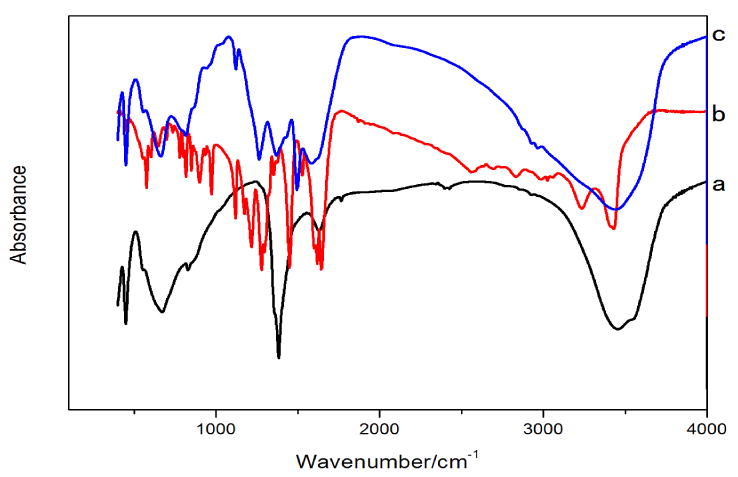
Fourier transform-infrared spectra of NO3- form of LDH (a), caffeic acid (b) and caffeic acid–LDH (c)
The comparison between scanning electron micrographs (Fig.5) of NO3 form of LDH and caffeic acid–LDH show that NO3- form of LDH has well-formed and regular sized microcrystals, while the caffeic acid–LDH powder appears to be constituted of non-uniform irregular aggregates. Very likely, the presence of drug molecules taken up on the microcrystal surface can produce a variation of the superficial interactions that influences the aggregation behavior of the microcrystals. As a consequence of the enlargement of the interlayer region, the layering of caffeic acid–LDH is also evident and fringes are occasionally observed.
Fig.5.

Scanning electron micrographs of NO3- form of LDH (a) and caffeic acid–LDH hybrid (b).
Conclusions
High purity of caffeic acid was obtained by HSCCC with a two-phase solvent system composed of ethyl acetate-methanol-water at volume a ratio of 10:1:10. The purity was assessed by HPLC and the confirmation of chemical structure was performed by ESIMS, NMR spectroscopy. The present method is suitable for preparing preparative quantities of pure caffeic acid.
Hydrotalcite-like anionic clays can be used as agents able to intercalate drugs, and this study was conducted to synthesize a new caffeic acid–LDH and to elucidate the characteristics of the new drug. In this experiment, caffeic acid was intercalated into NO3- form of LDH. Conclusively, LDH could be an excellent biocompatible inorganic host for drug reservoirs and delivery carriers. Caffeic acid–LDH would be a functional and effective drug.
Acknowledgments
Financial support from Ministry of Agriculture of the people's republic of China (Project 200803022) and State Key Laboratory of Chemical Resource Engineering (Project CRE-B-2008104) are gratefully acknowledged.
References
- 1.Zhao XB, Zhang LH, Liu DH. Bioresource Technol. 2008;99:3729–3736. doi: 10.1016/j.biortech.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Gu WB, Sang WG, Ling HB, Axmacher JC. Agriculture, Ecosystems and Environment. 2008;124:173–178. [Google Scholar]
- 3.Wang L, Qin RHJ. Southwest forestry college. 2004;24:72–75. [Google Scholar]
- 4.Horn AF, Nielsen NS, Jacobsen C. Food Chem. 2009;112:412–420. [Google Scholar]
- 5.Gutzeit D, Winterhalter P, Jerz GJ. Chromatogr A. 2007;1172:40–46. doi: 10.1016/j.chroma.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 6.Ito YJ. Chromatogr. 1991;538:3–25. doi: 10.1016/s0021-9673(01)91617-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhou TT, Fan GR. J Chin Pharm Univ. 2007;38:391–395. [Google Scholar]
- 8.Li BX, He J, Evans DG, Duan X. Applied Clay Science. 2004;27:199–207. [Google Scholar]
- 9.Lu Z, Duan X. Chinese Journal of Catalysis. 2008;29(9):839–856. [Google Scholar]
- 10.Meng JH, Zhang H, Evans DG. Chem J Chinese Universities. 2003;24(7):1315–1319. [Google Scholar]
- 11.Khan MA, Choi CL, Lee DH, Park M, Lim BK, Lee JY, Choi J. Journal of Physics and Chemistry of Solids. 2007;68:1591–1597. [Google Scholar]
- 12.Hussein MZB, Zainal Z, Yahaya AH, Foo DWVJ. Controlled Release. 2002;82:417–427. doi: 10.1016/s0168-3659(02)00172-4. [DOI] [PubMed] [Google Scholar]
- 13.Inacio J, Taviot-Guého C, Forano C, Besse JP. Applied Clay Science. 2001;18:255–264. [Google Scholar]
- 14.Ren LL, He J, Evans DG, Duan X. Chinese Universities. 2001;24(1):169–173. [Google Scholar]
- 15.Zhang WF, Li DQ, Sun Q, Evans DG, Duan X. Chinese Univ. 2004;25(10):1799–1803. [Google Scholar]
- 16.Li YM, Wang TZ, Wang ZX. China Journal of Chinese Materia Medica. 2001;26:45–47. [PubMed] [Google Scholar]


