Abstract
Coupled with evaporative light scattering detection, high-speed countercurrent chromatography was successfully applied for the first time to separation and purification of four triterpene saponins including esculentoside A, B, C and D from roots of Radix Phytolaccae. The separation was performed with an optimized two-phase solvent system composed of chloroform-methanol-water (4:4:2, v/v) using the lower phase as the mobile phase at a flow rate of 1.5 ml/min,. From 150 mg of crude extract 46.3 mg of esculentoside A, 21.8 mg of esculentoside B, 7.3 mg of esculentoside C, and 13.6 mg of esculentoside D were obtained at purities of 96.7%, 99.2%, 96.5% and 97.8%, respectively, as determined by HPLC analysis. The structures of the four triterpene saponins were identified by ESI-MS,1H NMR and 13C NMR.
Keywords: High-speed countercurrent chromatography, Radix phytolaccae, Triterpene saponins
INTRODUCTION
Radix Phytollacae (Chinese name shanglu) is a famous Chinese herbal medicine derived from the root of Phytolacca acinosa Roxb. or P. americana L. which is widely distributed on the earth. It has long been used for treatment of edema, abdominal fullness, dysuria, abnormal defecation and external application for treatment of carbuncle and sore as a traditional Chinese medicine(TCM)[1]. The major biological activity of the compounds in R. Phytolaccae is attributed to its triterpene saponins, which have been reported to have a variety of activities such as induction of immune interferon (INF-γ), anti-inflammatory agent and tumor necrosis factor (TNF); enhancement of leukocyte phagocytosis and promotion of DNA transformation; antifertility; and molluscicidal effect, etc.[2]. In the past, the separation and purification of saponins from R. Phytolaccae were performed using conventional column methods. However, these methods are tedious and require several chromatographic steps on a silica gel or other columns resulting in low yields of target compounds [3–8].
HSCCC has been widely used for separation of bioactive compounds from a variety of kinds of Chinese herbal medicines in recent years[9,10,11], and several papers have reported the HSCCC separation coupled with an evaporative light scattering detector(ELSD)[12~14]. However, there are few reports that applied to the separation of saponins from R. Phytolaccae, probably due to the fact that triterpene saponins have low UV absorption. In this paper, we established a method for the purification of four saponins such as esculentosides A - D from a crude extract of R. Phytolaccae by HSCCC coupled with an evaporative light scattering detector.
EXPERIMENTAL
Apparatus
The HSCCC instrument employed in the present study was a TBE-300A HSCCC apparatus (Tauto Biotechnique, Shanghai, China) with three multilayer coil separation columns connected in series (i.d. of the tubing = 1.5 mm, total volume = 260 ml) equipped with a 20ml sample loop .The β values of the multilayer coil range from 0.5 at the internal termainl to 0.8 at the external terminal The revolution speed of the apparatus can be regulated with a speed controller in the range between 0 and 1000 rpm. The solvent was pumped into the column with a Model TBE5002 constant flow pump (Tauto Biotechnique, Shanghai, China). Continuous monitoring of the effluent was achieved with an Alltech 800 evaporative light scattering detector. The data were collected with a Model N2000 chromatography workstation (Zhejiang University, Hangzhou, China). The analytical HPLC equipment was a Hitachi system consisting of an Alltech 2000ES evaporative light scattering detector (Alltech, USA).
Reagents and plant materials
All solvents used for the preparation of crude sample and HSCCC separation were of analytical grade and purchased from Tianjin Chemical Factory (Tianjin, China). Methanol used for HPLC was of HPLC-grade which was also purchased from Tianjin Chemical Factory. Distilled water was used.
Roots of R. Phytolaccae were purchased from Shannxi Hongda drug manufactory (Shannxi, China) and identified by Professor Yazhou Wang, College of Life sciences, Northwest University, Xi’an, China.
Preparation of crude sample
The roots (400 g) of R. Phytolaccae were percolated with 70% ethanol (3.5 L), and the extract was dried under reduced pressure yielding 100 g of crude extract. This extract was dissolved in water (800 ml).and extracted with ethyl acetate (800 ml, 6 times). The ethyl acetate phase was pooled and evaporated to yield 15.7 g of the crude saponin-rich extract that was subjected to HSCCC separation.
Measurement of partition coefficient (K)
The two-phase solvent systems were selected according to the partition coefficient (K) of the target components that is the most important step in HSCCC[15] First, a mixture of chloroform-methanol-water and chloroform-methanol-n-butanol-water at different volume ratios were equilibrated in a separatory funnel by repeating vigorous shaking at room temperature. Then, 1.5 ml of each phase of the equilibrated two-phase solvent system was delivered into a 10-ml test tube to which approximately 1.5 mg of the sample was added. Then the tube was capped and shaken vigorously to equilibrate the contents. After two phases are separated, an aliquot sampled from each phase was analyzed by the HPLC coupled with ELSD to determine the partition coefficient (K) values. The K value was calculated by dividing the peak area of the upper phase with that of the lower phase from each pair of corresponding peaks in the chromatogram.
Preparation of two-phase solvent system and sample solution
The two-phase solvent system composed of chloroform-methanol-water (4:4:2, v/v/v) was used for HSCCC separation,. The solvent mixture was thoroughly equilibrated in a separatory funnel at room temperature. Then, the two phases were separated and degassed by sonication at least for 15 min before use. The sample solution was prepared by dissolving 120mg of the crude extract in 4 ml of equal volumes of each phase (total 8 ml) of the solvent system used for HSCCC separation.
HSCCC separation procedure
The preparative HSCCC was performed with a Model TBE300A HSCCC instrument as follows: the multilayer coiled column was first entirely filled with the upper phase as stationary phase. The lower phase was then pumped into the head end of the column at a flow-rate of 1.5 ml/min, while the apparatus was run at a revolution speed of 800 rpm. After hydrodynamic equilibrium was reached (about 60 min later), the sample solution (120 mg) was injected into the separation column through the injection valve. The effluent from the tail end of the separation column was continuously monitored with an Alltech 800 ELSD. The tube temperature of ELSD was adjusted at 40°C and the gas flow rate set at 2.8 L/min. Peak fractions were collected into test tubes at 3 min intervals (4.5 ml/tube). Each purified compound was stored at 0°C before ESI-MS and NMR analyses.
HPLC analysis and identification of HSCCC peak fractions
The HPLC analysis of every HSCCC peak fraction was performed with a Kromasil C18 (250 mm×4.6 mm, I.D.) column at room temperature as follows: The mobile phase was methanol-0.4% glacial acetic acid (70:30, v/v) and the flow rate was set at 1.0 mL/min throughout. Tube temperature of ELSD was kept at 76.5°C, and the gas flow rate was set at 2.1 L/min.
The identification of HSCCC peak fractions was carried out by electron spray ionization mass spectrometry (ESI-MS) and NMR. ESI-MS analyses were carried out using a Thermo Scientific LTQ XL ion trap mass spectrometer (ThermoFnnigan, San Jose, CA, USA) equipped with an electrospray ionization (ESI) source. NMR spectra were obtained with a Bruker Avance 400 NMR.
RESULTS AND DISCUSSION
Optimization of suitable two-phase solvent system and other conditions of HSCCC
In order to achieve an ideal separation result, the selection of a two-phase solvent system is the most significant step which may require many days of trial and error experiments[15]‥ . Firstly, the target compounds should be stable and soluble in the system, the volume ratios of the system should also be acceptable to avoid the waste, then the solvent system should provide a suitable partition coefficient (K) for the target compounds. The suitable K value for HSCCC is between 0.5 and 1, if the K value is smaller, the compounds would be eluted near the solvent front without sufficient peak resolution, while a larger K value requires a long separation time and yields dilute peak fractions[15]. Table 1 lists the K values of two target compounds in 7 different two-phase solvent systems. Among them, ethyl acetate-n-butanol-water gives too large K values for both compounds while chloroform-methanol-n-butanol-water at a volume ratio of 5:6:1:4 gives too small K values. When the volume ratio of chloroform-methanol-n-butanol-water was adjusted at 5:6:1:4, the K values of compounds 1 and 4 became similar Finally, it can be seen from the table that compounds 1 and 4 have an ideal K values when the chloroform-methanol-water (4:4:2, v/v/v) was used. Since the polarities of compounds 2 and 3 were between those of compounds 1 and 4, we chose this two-phase solvent system for HSCCC separation.
Table 1.
The K (partition coefficient) values of compounds 1 and 4 in 7 different two-phase solvent systems
| solvent system | ratio | K1 of compound 1 | K2 of compound 4 |
|---|---|---|---|
| Ethyl acetate-n-butanol-water | 5:3:5 | 923.11 | 212.37 |
| 5:2:5 | - | 523.67 | |
| Chloroform-methanol-n-butanol-water | 5:6:1:4 | 0.46 | 0.26 |
| 5:6:0.5:4 | 0.53 | 0.55 | |
| Chloroform-methanol-water | 4:3:2 | 1.39 | 2.07 |
| 4:5:3 | 4.32 | 1.88 | |
| 4:4:2 | 0.62 | 0.88 |
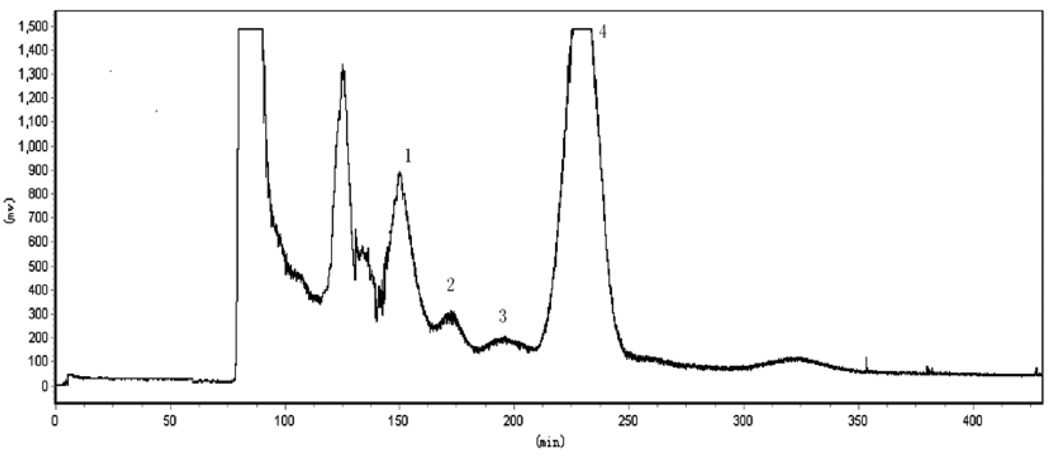
We have also investigated the effects of the revolution speed and the flow rate of the mobile phase on HSCCC separation. The results indicated that high flow rates of the mobile phase reduced both peak resolution and retention of the stationary phase though the separation time was shortened. ‥ According to the results obtained from a series of preliminary runs, the revolution speed was set at 800 rpm and the flow rate at 1.5 mL/min which yielded an ideal retention level of the stationary phase at 61% with good peak resolution between the target compounds (see Figure 2 and Figure 3).
Figure 2.
HSCCC chromatogram of the crude extract of R. Phytolaccae. Peak 1, compound 1; peak 2, compound 2; peak 3, compound 3; peak 4, compound 4. Experimental conditions: two-phase solvent system: chloroform-methanol-water (4:4:2, v/v/v); stationary phase: upper phase; mobile phase: lower phase; flow-rate:1.5 mL/min; revolution speed: 800 rpm; ELSD conditions: drift tube temperature, 40°C; gas flow, 2.8 L/min; impactor, off; sample size, 120 mg of crude extract dissolved in 8 ml of a mixture of upper and lower phases (1:1, v/v) of the solvent system used for HSCCC.
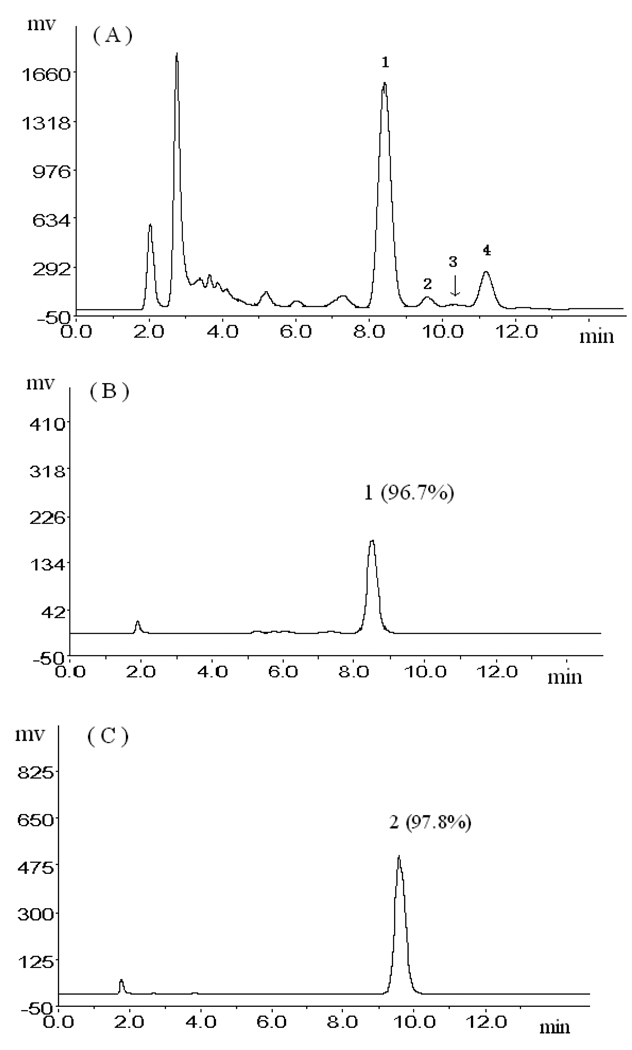
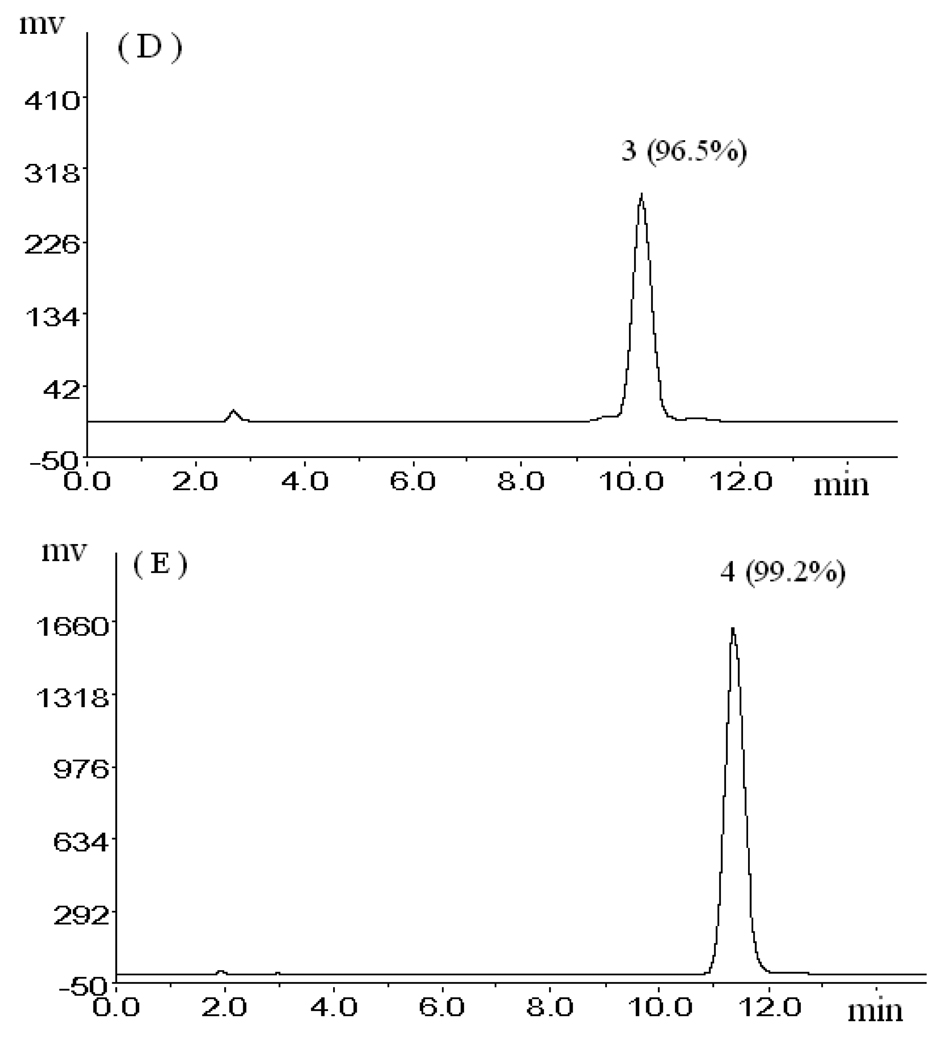
Figure 3.
(A):HPLC analysis of pooled ethyl acetate crude extract from the roots of R. Phytolaccae; (B), (C), (D) and (E): The HPLC analyses of compounds 4 to 1. Experimental conditions.: column: Kromasil C18 (5 µm, 250 mm × 4.6 mm I.D.); mobile phase: 0.4% glacial acetic acid and methanol (30:70, v/v); flow rate: 1.0 ml/min.
Confirmation of chemical structures
The structure identification of peak fractions was performed by IR, ESI-MS, 1H NMR and 13C NMR.
Compound 1:white powder, with a positive reaction when treated with the Liebermann-Buchard and Molish reagents. ESI-MS(m/z): 687[M+Na]+, 1H NMR(400MHz, DMSO, ppm): 0.69(3H, s, CH3), 0.80(3H, s, CH3), 1.08(3H, s, CH3), 1.09(3H, s, CH3), 1.19(3H, s, CH3), 3.61(3H, s, OCH3), 4.25(IH, d, J=7.0 Hz, H in the end group), 5.15(IH, m, C12-H).13CNMR(400MHz, DMSO, ppm): 43.7(C-1), 69.7(C-2), 80.3(C-3), 43.3(C-4), 47.4(C-5), 16.9(C-6), 32.0(C-7), 41.4(C-8), 47.4(C-9), 35.9(C-10), 23.0(C-11), 122.1(C-12),143.7(C-13), 41.7(C-14), 27.2(C-15), 22.8(C-16), 46.2(C-17), 42.2(C-18), 42.2(C-19), 45.0(C-20), 29.8(C-21), 33.3(C-22), 63.2(C-23), 14.2(C-24), 16.6(C-25), 16.9(C-26), 25.6(C-27), 178.4(C-28), 27.9(C-29), 176.4(C-30), 51.7(C-31), 105.3(C-1 of Xyl), 73.7(C-2 of Xyl), 76.7(C-3 of Xyl),69.7(C-4 of Xyl), 65.7(C-5 of Xyl). According to the references[3], the compound 1 was identified as 3-O-β-D-xylopyranosy phytolaccagenin (esculentoside B).
Compound 2:white powder. ESI- MS(m/z): 717[M+Na]+, 1HMR(400MHz, DMSO, ppm): 0.70(3H, CH3), 0.82 (3H, s, CH3), 1.05 (3H, s, CH3), 1.09 (3H, s, CH3), 1.20 (3H, s, CH3), 3.63 (3H, s, OCH3), 4.27 (IH, d, J=8.0Hz, H of the end group), 5.19 (IH, m, C12-H).13C NMR (400MHz, DMSO, ppm): 43.5(C-1), 69.2(C-2), 80.7 (C-3), 43.3 (C-4), 47.4(C-5),16.9 (C-6), 32.0(C-7), 41.4(C-8), 48.7(C-9), 35.9(C-10), 23.0(C-11), 122.1 (C-12),143.6(C-13), 41.6(C-14), 27.2(C-15), 23.0(C-16), 46.2(C-17), 42.1(C-18), 42.1(C-19), 45.0(C-20), 29.7(C-21), 33.3(C-22), 63.5(C-23), 14.3(C-24), 16.5(C-25), 16.9(C-26), 25.6(C-27), 178.3(C-28), 27.8(C-29), 176.4(C-30), 51.7(C-31),104.2(C-1 of Glc), 73.8(C-2 of Glc), 76.6(C-3 of Glc), 70.0(C-4 of Glc), 76.9(C-5 of Glc), 61.1(C-6 of Glc). Compound 2 was identified as 3-O-β-D-glucopyranosyl phytolaccagenin(esculentoside D).
Compound 3:white powder, positively reacts in the Liebermann-Buchard and Molish reagents. ESI-MS(m/z): 833[M+Na]+, 13C NMR(400MHz, C5D5 ppm) 44.1(C-1), 70.9 (C-2), 82.9(C-3), 42.8(C-4), 47.7(C-5), 17.9(C-6), 32.8(C-7), 40.0(C-8), 48.4(C-9), 36.9(C-10 , 23.9(C-11),123.4(C-12), 144.2(C-13), 42.2(C-14), 28.2(C-15), 23.9(C-16), 46.1(C-17), 43.2(C-18), 42.9(C-19), 45.0(C-20), 30.5(C-21), 34.3(C-22), 64.2(C-23), 14.8(C-24), 17.6(C-25), 17.2(C-26), 26.2(C-27), 179.4(C-28), 28.2(C-29), 177.4(C-30), 51.7(C-31), 106.4(C-1 of Xyl), 75.3(C-2 of Xyl), 76.5(C-3 of Xyl), 78.0(C-4 of Xyl), 64.7(C-5 of Xyl), 103.7(C-1 of Glc), 74.3(C-2 of Glc), 78.1(C-3 of Glc), 71.7(C-4 of Glc), 78.8(C-5 of Glc), 62.6(C-6 of Glc). According to the references[16], compound 3 was identified as 3-O-[β-D-glucopyranosyl-(1→4)-β-D-xylopyranosyl] sylphytolaccagenin(esculentoside C).
Compound 4:white powder, with a positive reaction when were dealt with the Liebermann-Buchard and Molish reagents. ESI-MS(m/z):849[M+Na]+,1H NMR(400MHz, DMSO, ppm): 0.70(3H, s, CH3), 0.82(3H, s, CH3), 1.09(3H, s, CH3),1.10(3H, s, CH3), 1.20(3H, s, CH3), 3.63(3H, s, OCH3), 4.26(IH, d, J=8.0Hz, H of end group), 4.30(IH, d, J = 7.6Hz, H of end group) 5.17(1H, m, C12-H).13C NMR (400MHz, DMSO, ppm):43.7(C-1), 69.9(C-2), 80.3(C-3), 43.4(C-4), 47.5(C-5), 17.0(C-6), 32.1(C-7), 41.5(C-8), 48.7(C-9), 36.0(C-10), 23.1(C-11), 122.2(C-12), 143.7(C-13), 41.7(C-14), 27.3(C-15), 2.8(C-16), 46.2(C-17), 42.2(C-18), 41.8(C-19), 45.0(C-20), 29.8(C-21), 33.4(C-22), 63.3(C-23), 14.3(C-24), 16.6(C-25), 17.0(C-26), 25.6(C-27), 178.4(C-28), 27.9(C-29), 176.5(C-30), 51.7(C-31), 105.1(C-1 of Xyl), 73.5(C-2 of Xyl), 74.8(C-3 of Xyl), 77.1(C-4 of Xyl), 63.3(C-5 of Xyl), 101.7(C-1 of Glc), 72.8(C-2 of Glc), 76.4(C-3 of Glc), 70.2(C-4 of Glc), 76.5(C-5 of Glc), 61.2(C-6 of Glc). Compound 4 was identified as 3-O-[β-D-glucopyranosyl-(1→4)-β-D-xylopyranosyl] phytolaccagenin(esculentoside A).
CONCLUSIONS
Four triterpene saponins, 46.3 mg of esculentoside A, 21.8 mg of esculentoside B, 7.3 mg of esculentoside C, and 13.6 mg of esculentoside D were successfully isolated and separated from 120 mg of the roots of R. Phytolaccae by high-speed countercurrent chromatography with a two-phase solvent system composed of chloroform-methanol- water (4:4:2, v/v/v) Our study demonstrated that HSCCC coupled with an ELSD is a powerful method in separation and isolation of bioactive compounds with low ultraviolet absorbance from natural products. The method is simple and gives a high sample recovery rate.
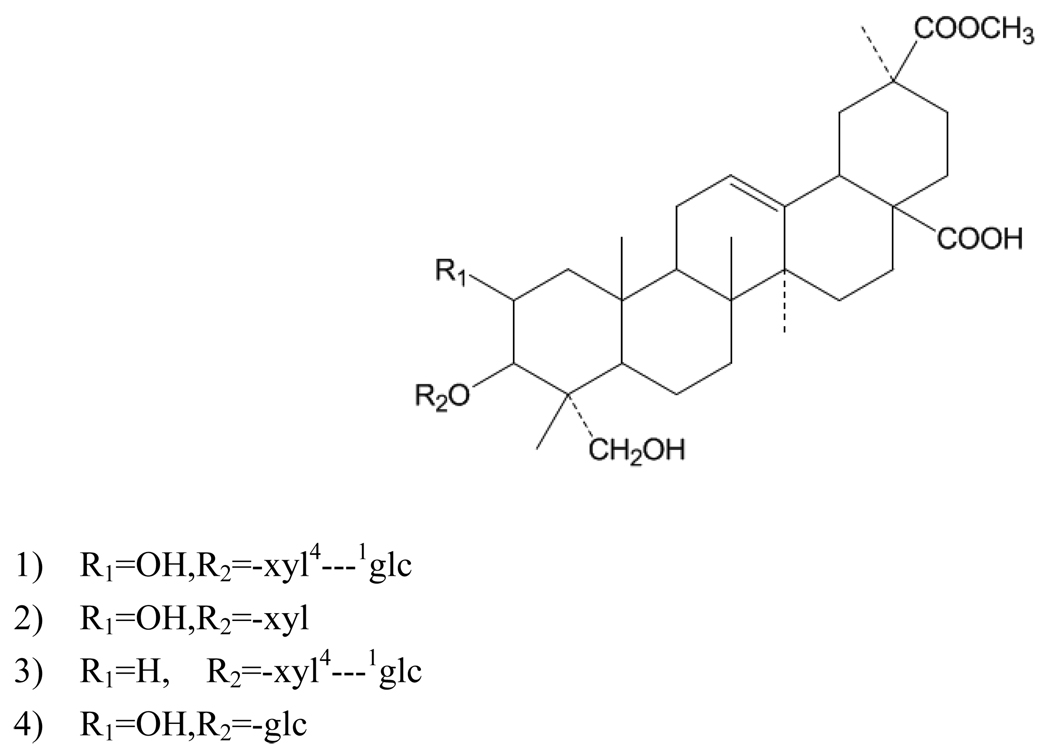
Figure 1.
Chemical structures of four triterpene saponins: (1) esculentoside A; (2) esculentoside B; (3) esculentoside C; (4) esculentoside D.
ACKNOWLEDGEMENTS
The authors thank Ying Liu, Xi Xu and Wancui Fan of the Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, Northwest University, Xi’an, China for their assistance in ESI-MS experiments.
REFERENCES
- 1.Xiao PG. Modern Chinese Materia Medica. Vol 1. Beijing: Chemical Industry Press; 2001. pp. 926–930. [Google Scholar]
- 2.Chinese Materia Medica. Vol Shanghai: Shanghai Science and Technology Publishers; 1999. State Administration of Traditional Chinese Medicine of People’s Republic of China,≪Chinese Materia Medica≫Editorial Department; pp. 737–745. [Google Scholar]
- 3.Yi YH, Wang ZL. Studies on the active principles of the Chinese drug “Shang l u”(Phytolacca esculenta Van Houtte.) The isolation and structure of triterpene saponins. Chinese Traditional and herbal drugs. 1984;15(2):7–11. [PubMed] [Google Scholar]
- 4.Wang ZL, Yi YH. Studies on the active principles of the Chinese drug “Shang lu”(Phytolacca esculenta Van Houtte.) The isolation and structure of esculentoside E and F.Acta Phamaceutica Sinca. 1984;19(11):825–829. [PubMed] [Google Scholar]
- 5.Yi YH, Wang ZL. A new active saponin from Phytolacca esculenta. Planta Med. 1989;55(6):551–552. [PubMed] [Google Scholar]
- 6.Yi YH, Esculentoside L, K Two new saponins from Phytolacca esculenta. Planta Med. 1990;56(3):301–303. [PubMed] [Google Scholar]
- 7.Yi YH, Huang X. Isolation and structure of three new saponins from Phytolacca esculenta. Acta Phamaceutica Sinca. 1990;25(10):745–749. [PubMed] [Google Scholar]
- 8.Yi YH, Dai FT. A new triterpenoid and its glycoside from Phytolacca esculenta. Planta Med. 1991;57(2):162–164. doi: 10.1055/s-2006-960056. [DOI] [PubMed] [Google Scholar]
- 9.Du QZ, Jerz G, Waibel R, Winterhalter P. Isolation of dammarane saponins from Panax notoginseng by high-speed countercurrent chromatography. J. Choromatogr. A. 2003;1008:173–180. doi: 10.1016/s0021-9673(03)00988-9. [DOI] [PubMed] [Google Scholar]
- 10.Xin XL, Yang Y, Zhong J, Aisa HA, Wang HQ. Preparative isolation and purification of isobenzofuranone derivatives and saponins from seeds of Nigella glandulifera Freyn by high-speed countercurrent chromatography combined with gel filtration. J. Choromatogr. A. 2009;1216:4258–4262. doi: 10.1016/j.chroma.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 11.Cao XL. Isolation technology and utilization of high-speed countercurrent chromatography. Beijing: Chemical industry press; 2005. p. 264. [Google Scholar]
- 12.Shi SP, Jiang D, Zhao MB, Tu PF. Preparative isolation and purification of triterpene saponins from Clematis mandshurica by high-speed countercurrent chromatography coupled with evaporative light scattering detection. J.Choromatogr.B. 2007;852:679–683. doi: 10.1016/j.jchromb.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 13.Yao S, Liu RM, Huang XF, Kong LY. Preparative isolation and purification of chemical constituents from the root of Adenophora tetraphlla by high-speed countercurrent chromatography with evaporative light scattering detection. 2007;1139:254–262. doi: 10.1016/j.chroma.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 14.Liu ZL, Jin Y, Shen PN, Wang J, Shen YJ. Seperation and purification of verticine and verticinone from Bulbus Fritillariae Thunbergii by high-speed countercurrent chromatography coupled with evaporative light scattering detection. Talanta. 2007;71:1873–1876. doi: 10.1016/j.talanta.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y. Golden rules and pitfalls in selecting optimum conditions for high-speed countercurrent chromatography. J. Choromatogr. A. 2005;1065:145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 16.Wang LY, Bai LM, et al. Bioactive triterpene saponins from the roots of Phytolacca americana. J. Nat. Prod. 2008;71:35–40. doi: 10.1021/np078012m. [DOI] [PubMed] [Google Scholar]