Abstract
Normal copper homeostasis is essential for human growth and development. Copper mutations, inadequate diet or surgical interventions, may lead to cardiac hypertrophy, poor neuronal myelination, blood vessel abnormalities and impaired immune response. Copper overload is associated with morphological and metabolic changes in tissues and, if untreated, eventual death. Recent reports also indicate that changes in the expression of copper transporters alter the sensitivity of cancer cells to major chemotherapeutic drugs, such as cisplatin, although the mechanism behind this important phenomenon remains unclear. This review summarizes current information on the molecular characteristics of copper transporters CTR1, CTR2, ATP7A and ATP7B, their roles in mammalian copper homeostasis and the physiological consequences of their inactivation. The mechanisms through which copper transporters may influence cell sensitivity to cisplatin are discussed. Regulation of human copper homeostasis has significant therapeutic potential and requires the detailed understanding of copper transport mechanisms.
Adequate copper levels are essential for normal metabolism
Copper is the cofactor of key metabolic enzymes
All cells in the human body require copper for their metabolic needs. Copper serves as an essential cofactor for the activity of cytochrome C oxidase in mitochondria, the enzyme that is central for respiration, and for Cu, Zn-dependent superoxide dismutase, which plays an important role in detoxification of oxygen radicals in the cytosol. In addition, many cells require copper in the secretory pathway, where enzymes including dopamine-β-hydroxylase, peptidyl-α-mono-oxygenase, ceruloplasmin, tyrosinase and others incorporate copper as a cofactor in their catalytic sites. These enzymes then critically contribute to a number of key physiological processes, such as iron export from the cells, the production of neuroendocrine peptides and neurotransmitters, pigmentation, blood clotting and others. Copper deficiency decreases the activity of these enzymes and thus adversely affects the corresponding physiological processes [1,2]. Heart function is particularly sensitive to inadequate copper supply [3]. In animals, experimental copper depletion is associated with cardiohypertrophy due to increased size and reduction in the number of cardiomyocytes [3]. During viral infection, copper-deficient hearts show increased inflammation and lower acquired immune response, which further add to the heart’s injury [4].
Copper deficiency can be inborn or induced
Severe dietary deficiency in humans is rare; however, marginal copper deficiency is not uncommon, particularly in pregnancy [5,6]. In addition, pathological states that cause an inadequate copper supply are becoming more recognized; Menkes disease (MND) is the best known disorder of copper deficiency. MND is an X-chromosome-linked disorder associated with impaired copper efflux from enterocytes into the blood and inadequate transport of copper to the brain. Patients with MND show delayed growth and development, poor temperature control, connective tissue abnormalities, seizures, mental retardation and die in their early childhood (for a review on diagnostic and treatment of MND, see elsewhere [7]).
In addition to genetic mutations, copper deficiency can be unintentionally induced when patients with disorders unrelated to copper metabolism undergo life-saving treatments. For example, bariatric surgery has emerged as an effective tool for decreasing body mass in morbidly obese patients. At the same time, recent follow-up studies indicate that, while such operations result in a significant improvement of body mass index, as well as glucose and cholesterol levels, the postoperative levels of essential micronutrients, including copper, are shown to continually decrease [8]. This deficiency may not be immediately detected and adverse consequences may become apparent only with time [9]. The copper and iron deficiency in these patients is associated with anemia and neutropenia [10,11], which can often be treated if adequate supplies of nutrients are provided. Recent reports also suggest that copper deficiency could be an underappreciated complication of celiac disease [12] or be acquired as a result of excessive zinc ingestion or general malabsorption [13].
Elevated copper is harmful
It is well established that excess copper is also deleterious for cell metabolism. Therefore, the level of copper in cells and tissue must be tightly regulated. The ATP-driven transporters, such as copper-transporting ATPases ATP7A and ATP7B, play a central role in this process. Inactivation of their transport activity is associated with diminished copper efflux from cells and, in some tissues, massive copper overload (e.g., in the intestines, upon ATP7A inactivation and in the liver when the function of ATP7B is lost). Harmful consequences of copper accumulation are particularly apparent in the case of Wilson’s disease (WND), an autosomal recessive disorder caused by genetic inactivation of ATP7B. WND patients have greatly elevated copper levels in the liver and in several other tissues and show a wide spectrum of hepatic abnormalities, including hepatitis, cirrhosis, fulminant liver failure and/or neurological and psychiatric disease [14]. Copper chelation is used successfully to alleviate many symptoms of WND; however, neurological symptoms can be difficult to treat and side-effects are common, necessitating the search for improved new treatments.
An additional interest in copper transport and homeostasis was sparked by an intriguing observation from Komatsu and co-workers [15]. These investigators found that the transfection of human epidermoid KB carcinoma cells with the cDNA for the copper-transporting ATPase ATP7B and subsequent selection of cells using high copper concentrations increased cell survival in the presence of cisplatin, a widely used platinum-based chemotherapeutic drug. These authors also observed an upregulation of ATP7B expression in cisplatin-resistant cell lines originating from prostate carcinoma, epidermoind carcinoma and ovarian tumors. Altogether, these findings led to the suggestion that ATP7B mediates increased resistance of cancer cells to cisplatin. Over the last decade, this hypothesis has received significant support [16]. Although the mechanism of resistance remains controversial, the role of copper homeostasis in cells’ resistance to cisplatin appears solidly established.
Overall, clinical data provide compelling evidence for the need to better understand the cellular mechanisms regulating copper acquisition and subsequent distribution between tissues. In the next sections, we will briefly summarize current information on cellular machinery responsible for the entry and efflux of copper from the cell and then discuss the role of copper transporters in mediating resistance to cisplatin.
Copper uptake: the machinery & mechanism
CTR1 is required for high-affinity copper uptake into cells
Genetic and biochemical studies identified several proteins that play key roles in the uptake, distribution and export of copper from the cells. These include a high-affinity transporter CTR1, a low-affinity transporter CTR2, copper chaperones (CCS, Atox1, Cox17, SCO1 and SCO2) and the copper efflux transporters ATP7A and ATP7B. In recent years, it has also become apparent that, in addition to membrane transporters, cells contain a complex network of soluble regulator molecules that allow for the fine tuning of copper homeostasis and precise temporal and spatial allocation of copper in a cell. Interactions with dynactin, ADP-ribosylation factor (Arf)1 GTPase, phosphatidylinosytol-binding protein (COMMD1), ubiquitinating machinery and other proteins have been reported, illustrating the potential dependence of normal copper distribution on many cellular factors that have not been traditionally linked to copper metabolism [17–19].
It is thought that the major route of copper entry from the blood into cells is through the copper transporter CTR1 [20,21]. CTR1 was first discovered as a protein responsible for high-affinity copper uptake in Saccharomyces cerevisae [22]. In yeast, the inactivation of yCTR1 results in lower entry of copper into the cell and iron deficiency, due to a limited supply of copper to a copper-dependent ferroxidase (FET3) involved in iron uptake. The complementation of this phenotype was subsequently employed to clone the human CTR1 [23]. These studies also uncovered the presence in the human genome of another potential copper transporter, CTR2 [23]. CTR2 has sequence homology to CTR1, but does not complement the yeast phenotype and, as we discuss below in more detail, the precise role of CTR2 in copper transport in mammalian cells remains uncertain.
More recently, a processed pseudogene highly homologous to human CTR1, CTR1Ψ, was identified [24]. The pseudogene is transcribed and, if translated, would produce a truncated 95-amino acid residue product. However, the transfection of CTR1Ψ cDNA into cells does not lead to an increase in copper uptake. This result argues against the role of this product in copper translocation across the membrane, although the regulatory role of the CTRΨ product cannot be excluded [24].
CTR1 is ubiquitously expressed in tissues and is particularly abundant in the choroid plexus, renal tubules and in connective tissues of the eye, ovary and testis [25]. The levels of CTR1 in tissues may be influenced by a physiological state, such as pregnancy or lactation [26]. CTR1 mediates energy-independent copper transport with an apparent Km of 2–5 μM [27]. Silver is a potent inhibitor of CTR1-mediated transport, while divalent metal ions are ineffective [27], suggesting that CTR1 transports the reduced form of copper, Cu(I). Downregulation of CTR1 in cultured cell lines using siRNA technology eliminates the high-affinity component of copper uptake and, overall, decreases copper entry by 70% [28]. In vivo, the non-CTR1 component of copper uptake can sustain copper homeostasis if CTR1 is deleted in individual tissues, such as liver [29]; however, the whole-body genetic inactivation of CTR1 is embryonic lethal [25,30]. This severe phenotype is likely due to insufficient copper supply to the body, although an additional copper-independent role of CTR1 in early tissue morphogenesis has recently been proposed [31].
CTR1 makes copper bioavailable
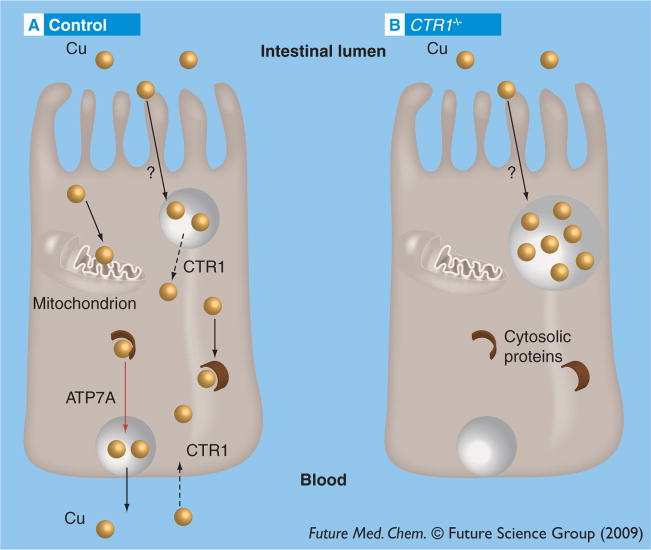
The consequences of CTR1 downregulation in individual tissues differ considerably. Targeted deletion of CTR1 in mouse liver leads to a 50% decrease in the liver copper content and a lower activity of copper-dependent enzymes, most likely due to diminished copper uptake from the blood [29]. By contrast, the targeted inactivation of CTR1 in intestine is associated with copper accumulation in enterocytes [32]. At the same time, copper that enters the CTR1−/− enterocytes is not bioavailable (i.e., it does not reach the copper-dependent enzymes in the cytosol and in mitochondria and is not available for efflux into the blood) (Figure 1) [32]. Thus, in both tissues, CTR1 is responsible for making copper available in the cytosol for utilization by various cellular proteins and further efflux (Figure 1). In other words, the functional role of CTR1 in two tissues is the same and the marked difference in the consequences of CTR1 inactivation is likely due to a considerable copper uptake via the apical membrane of enterocytes, which is absent in hepatocytes. This apical uptake does not depend on CTR1 (the CTR1−/− intestinal cells accumulate copper); it could be mediated by other copper transporters or may occur via vesicle-mediated endocytosis. This conclusion stems from the available functional data; however, the current literature disagreements on CTR1 localization in the intestine led to alternative suggestions about the CTR1 function. Consequently, we will discuss the available data on localization and function of CTR1 in enterocytes in more detail.
Figure 1. Currently available data on copper fluxes in normal (control) and CTR1-deficient (CTR1−/−) enterocytes.
(A) Copper (gold circles) enters enterocytes via two membranes: the apical membrane that faces the intestinal lumen and the basolateral membrane that faces the blood. The high-affinity copper uptake, inhibited by silver, takes place via the basolateral membrane and is likely to be mediated (as in other cells) by CTR1. The low-affinity copper uptake, which is not inhibited by silver, occurs via the apical membrane through an uncharacterized process (black arrow). Copper taken up via the apical pathway appears unavailable in the cytosol in the absence of CTR1 and an additional role of CTR1 could be to release dietary copper from subapical vesicles for further utilization. Once released, copper binds to cytosolic proteins (brown semicircles) and is also delivered to copper chaperones and cytochrome C oxidase in mitochondria, as well as to Cu-ATPases in the secretory pathway. Cu-ATPase ATP7A transports copper to vesicles, which then fuse with the basolateral membrane and copper is released into the blood. (B) Genetic inactivation of CTR1 in the intestine results in accumulation of copper in enterocytes; however, this copper is sequestered (presumably in vesicles) and does not reach cytosolic proteins, mitochondria or secretory pathways, resulting in low activity of copper-dependent enzymes and impaired copper efflux from the enterocytes.
CTR1 localization & function in intestine
Studies by Nose and colleagues provide convincing evidence that CTR1 in enterocytes is required for a complex process of transferring dietary copper from the lumen of the intestine into the blood. As described above, the targeted inactivation of intestinal CTR1 in mice disrupts this entire process, leaving animals copper deficient, but does not prevent copper entry into enterocytes [32]. A further understanding of specific functions of CTR1 in intestine has been hindered by a significant discrepancy between the results on the intracellular localization of the transporter (the apical, basolateral and intracellular localization were reported). We now summarize the strengths and limitations of different experimental approaches used to characterize CTR1 in enterocytes, largely to illustrate that more experiments in a physiologically relevant context are needed to better understand the functional role of intestinal CTR1.
Immunostaining has been the most frequently used and the least reliable approach to determine the localization of CTR1. This is because CTR1 protein has poor antigenicity and high-quality specific antibodies are difficult to obtain. Typically, western blot analysis of cells and tissue extracts shows multiple bands, some of which are related to CTR1, while others (often many) are not. Pre-absorption with specific peptides, which is sometimes used as a control, is insufficient to assure the antibody specificity, because this procedure does not deal with the issue of antibody cross-reactivity. The siRNA or other targeted inactivation of CTR1 is probably the best way to identify CTR1-related staining. However, in the majority of experiments such control was not (or could not have been) performed. To date, the immunostaining of CTR1 in cultured intestinal cells and tissue has shown intracellular staining [26,48], apical membrane staining [26], diffuse vesicular staining in proximity to apical membrane [32] and basolateral staining [33]. Clearly, these observations are difficult to reconcile without additional studies.
It should be noted that the utilization of epitope-tagged recombinant CTR1 is a tempting approach to investigate its localization. However, it is worth considering that the overexpression of membrane proteins is often associated with their mislocalization due to trapping in the endoplasmic reticulum (ER) and/or ‘spill-over’ to other compartments. Thus, given the current significant discrepancy of observations and conclusions, the firm identification of CTR1 localization must first be completed for the endogenous protein.
Direct measurements of a high-affinity copper flux that is dependent on the presence of the transporter would provide an ultimate proof for the CTR1 function at a particular membrane. In vivo, such experiments are difficult. However, indirect evidence using an intestinal knockout [32] suggests that CTR1 is not critical for apical copper uptake. Direct-uptake measurements using radioactive copper have been carried out in polarized Caco-2 cells (a model of intestinal epithelial cells [33]). These experiments yielded convincing evidence that, under basal conditions, the high-affinity copper uptake in intestinal cells is indeed mediated via the basolateral and not the apical membrane [33]. Silver, an inhibitor of CTR1, was used to demonstrate that this basolateral copper uptake was silver-inhibited (and by implication CTR1-related), while the low-affinity apical copper uptake was not inactivated by silver [33]. It is interesting that copper depletion increases absorptive capacity of Caco-2 cells [34]; the molecular basis for this phenomenon has yet to be established. Further confirmation of the involvement of CTR1 in high-affinity copper uptake at the basolateral membrane may include the downregulation of CTR1 using specific siRNA and a parallel loss of protein and high-affinity copper uptake across this membrane.
If CTR1 does not mediate a high-affinity copper uptake via the apical membrane, then what is the function of CTR1 in its apical location, which was observed by several investigators? It should be noted that all available immunostaining data are of insufficient resolution to determine with certainty whether CTR1 is targeted to the plasma membrane or is located in vesicles in the vicinity of the membrane. This is an important distinction, because, in the plasma membrane, CTR1 would physically translocate copper from the outside of the cell to the cytosol. In vesicles, the CTR1 function could be to release copper that was absorbed through other mechanisms and trapped in the vesicular lumen (Figure 1). The lack of bioavailable copper (despite overall copper accumulation) in the intestinal CTR1 knockout supports this second model [32]. An additional support for the vesicular (rather than plasma membrane) apical location of CTR1 is provided by surface biotinylation experiments in polarized Caco-2 cells [33]. In these studies, the labeling of cells with the impermeable biotinylating reagent followed by immunoprecipitation demonstrated the absence of CTR1 at the apical membrane. Plasma membrane staining of CTR1 in these experiments was only observed at the basolateral surface, consistent with high-affinity copper transport observed in this location [33].
Altogether, the current data are consistent with the following preliminary model (Figure 1) [33]. Copper crosses the apical membrane of enterocytes via a process that is largely independent of CTR1. This step may involve more than one transport mechanism (i.e., it could be a process mediated by transporters such as DMT1 and/or be driven by endocytosis/pinocytosis), resulting in a sequestration of copper in the subapical vesicles. CTR1 is targeted to these vesicles either as a result of normal cycling between the basolateral membrane and intracellular compartment (see below for current evidence on CTR1 trafficking) or, possibly, as a result of post-translational modification/interaction with a specific cellular machinery. In the vesicles, CTR1 would facilitate the release of copper from the lumen into the cytosol, where it is picked up by copper chaperones and delivered to various intracellular targets (Figure 1). In its basolateral location, CTR1 retrieves copper from the blood and transfers copper to the cytosol, as it does in other tissues. One wonders whether the copper-induced endocytosis of CTR1 [35, 36] could be a part of the uptake mechanism at the basolateral membrane (i.e., following copper binding, CTR1 is first endocytosed and then it releases copper into the cytosol from the vesicles). Experiments in insect cells showing stable levels of CTR1 at the plasma membrane seem to argue against this possibility [37]. However, it remains to be established how closely the in vitro uptake experiments recapitulate the endogenous processes.
The structure of CTR1 suggests a ‘pore with two-gates’ mechanism for copper transport
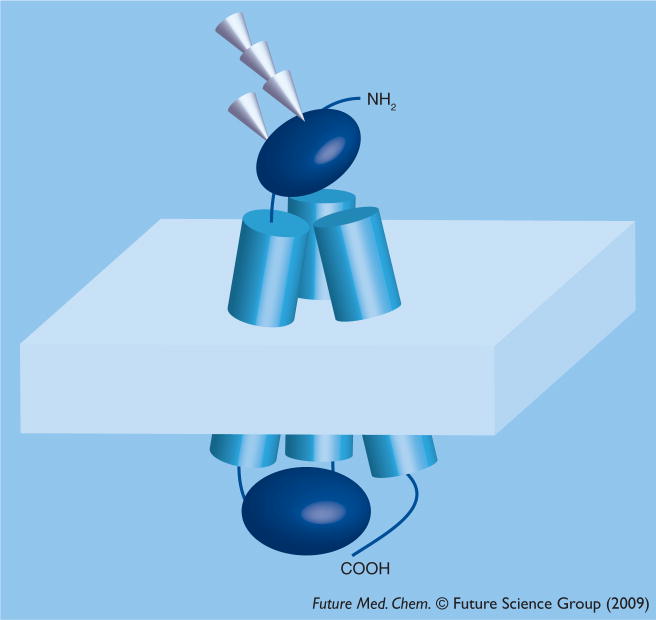
The best characterized yeast and human CTRs differ in size and show only moderate sequence similarity. Nevertheless, they share an overall homotrimer architecture, in which each monomer has four key domains (Figure 2):
Figure 2. Overall architecture of the CTR1 monomer.
CTR1 has a glycosylated N-terminal domain exposed to the outside milieu; three transmembrane (TM) segments, a cytosolic loop connecting TM1 and TM2 and the cytosolic C-terminus. In the fully assembled functional CTR1, the three monomers associate into a stable complex. For the actual structure of the oligomer see Jiang et al. [40].
An extracellular N-terminus
Three transmembrane (TM) helices
An intracellular loop of variable length, connecting the first and second TM helices
An intracellular C-terminal tail
Of these four domains, the extracellular N-terminus, which is rich in Met and His residues, has been implicated in copper binding [38]. In this review, we will focus on the properties of human CTR1 (hCTR1), but will refer to the yeast orthologue in cases where important insights were obtained using this model system.
Human CTR1 monomer consists of 190 amino acid residues. HCTR1 is N- and O-glycosylated [39] with the glycosylation sites located at the extracellular N-terminal domain (residues Asn-15 and Thr-27, respectively). Glycosylation increases the mass of the protein and in polyacrylamide gels the monomeric CTR1 migrates as an approximately 35-kDa protein. The ability of CTR1 to retain dimeric and trimeric forms during electrophoresis under denaturing conditions has been a common experimental complication that sometimes hinders unambiguous detection of endogenous CTR1 on Western blots.
It is still uncertain as to which molecules deliver copper to CTR1 from the serum. In vitro, free copper can be a substrate for CTR1 and the large portion of the CTR1 N-terminus (exposed to the blood site or to the lumen of vesicles) is not required for the transport activity. Whether this is true in vivo is unknown. The N-terminus of hCTR1 contains two so-called Mets motifs, M1 (M7GMSYM12) and M2 (M40MMMPM45) and two His-rich sequences, H1 (H3SHH6) and H2 (H22HH24). Met and His residues are both excellent ligands for copper and the ability of the Mets motif to bind copper was directly demonstrated in vitro [40]. Therefore, it is tempting to think that, in vivo, the N-terminus may serve as a docking site for copper carriers in the blood, which facilitates the retrieval and delivery of copper to the membrane portion of CTR1.
Pioneering studies from Aller, De Feo and Unger provided an initial glimpse into the molecular architecture of hCTR1 [41]. CTR1 oligomer has a symmetrical channel-like structure. The protein has a visible pore surrounded by monomers, where copper is likely to enter the transporter. The conserved Met residues of the MxxxM motif in TM2 may provide binding sites for copper and facilitate copper entry into the pore [41]. The precise coordination environment is not essential for copper translocation; replacing the Met residues in the MxxxM motif by Cys or His residues [42] or one of the Met with Ile does not disrupt transport activity [37].
The biochemical mechanism of copper movement through CTR1 remains unclear. Mutational studies of the TM domains suggest that, after copper enters the intramembrane portion of hCTR1, there are few, if any, sites of tight binding [37]. Thus, hCTR1 provides a channel-like pathway for, apparently, free diffusion of copper in a low dielectric environment.
In the hCTR1 structure, the presumed entry to the pore is covered by an electron-dense material (most likely the N-terminal domain, Figure 2), which may act as a ‘gate’ or a ‘concentrator’ for copper ions. At the opposite end, the exit from CTR1 is also closed by the protein mass, which could be formed by the cytosolic loop and/or the CTR1 C-terminus (Figure 2). How do the ‘gates’ open and close? It has been shown (by proteolysis of hCTR1 [43] and using fluorescence resonance energy transfer [FRET] measurements for yCTR1 [44]) that copper binding at the outer surface of the transporter causes a conformational change in its cytoplasmic portion. This structural rearrangement is probably important for copper release. Replacement of His-139 at the cytosolic side of TM2 with Arg facilitates copper transport [37], presumably by decreasing the tight packing and mimicking an open state.
Whether copper is released from the transporter in a free form or is retrieved by interacting proteins (copper chaperones) is still unknown. Recent experimental evidence in yeast illustrates that the C-terminal tail may play an important role in regulating the rate of transport by yCTR1 [45]; whether this regulatory mechanism is shared by the human transporter is still uncertain. Deletion of the entire C-terminal tail at Lys178 reduces copper uptake in hCTR1 by 70% [37]; while in yeast, similar deletion appears to increase copper transport [45]. Mutational studies indicate that, in the human protein, the C-terminal Cys-161 and Cys-189 contribute to hCTR1 oligomerization and stability, but are not critically involved in copper transport [45]. By contrast, the C-terminus of yCTR1 has been implicated in direct transfer of Cu(I) to the yeast copper binding chaperone ATX1 [46]. The transfer function of yCTR1 C-terminus could be due to its larger size and the presence of additional Cys residues in this region. Further structural comparison of the yCTR1 and hCTR1 is likely to yield useful insights into general and specific features of TM copper transporters in different eukaryotes and help to identify structural features that regulate rates of copper transport.
CTR1 levels at the plasma membrane can be modulated
Current data suggest that the uptake of copper via CTR1 is regulated. In cultured cells, CTR1 is typically observed in two locations: at the plasma membrane and intracellularly in vesicles. The intracellular localization in subapical vesicles is particularly noticeable in the intestinal cells (Figure 1), where CTR1 apparently functions to release copper and make it bioavailable in the cytosol (see above). In animal studies, a change in diet (from regular to copper-deficient food) was reported to induce the relocalization of intestinal CTR1 towards the apical membrane [26]. Accumulation of CTR1 in the vicinity of the apical membrane under conditions of copper deficiency may be required to facilitate the release of dietary copper into the cytosol and thus be beneficial. It would be interesting to determine whether the increase in copper absorption in copper-deficient Caco-2 cells [34] involves CTR1 moving towards the apical membrane/vesicles or whether other mechanisms are involved.
The effects of high copper concentrations on CTR1 localization have also been explored. In cultured HEK293 cells, high levels of copper were reported to induce CTR1 endocytosis and degradation [35], although, in other reports, degradation was not observed [37]. Copper-induced endocytosis is consistent with the model, where the removal of CTR1 from the membrane serves to prevent excess copper from entering cells.
New modes of regulating copper uptake via CTR1 have recently been discovered. O-glycosylation at Thr-27 was shown to protect CTR1 against proteolytic cleavage [39]. Interestingly, the subsequent studies have also revealed an important role for the first 30 residues (and perhaps glycosylation) in the regulation of the rate of copper uptake, which was not previously apparent [39]. At the cellular level, it has also been shown that the changes in the expression levels of Glu-Cys ligase (GCL), a rate-limiting enzyme in glutathione biosynthesis, increases the levels of CTR1 expression and copper uptake [47]. Whether this regulation is due to diminishing levels of bioavailable copper when glutathione is present in excess or due to some other mechanisms remains to be determined. The copper sensitivity of CTR1 levels has also been recently demonstrated [48].
CTR2 is a predominantly intracellular low-affinity copper transporter
CTR2 was originally cloned by Zhou and Gitschier, who noticed the sequence homology between CTR2 and CTR1 and suggested that CTR2 could be another copper transporter in human cells [23]. Interestingly, CTR2 lacks the extended N-terminal domain characteristic of CTR1. However, the residues in the TM portion that are known to be essential for the transport function of CTR1 are conserved in CTR2 [49]. Assuming that the N-terminus in CTR1 is required for the initial recognition of copper (or for concentrating copper in the proximity of the pore), one would hypothesize that either these two transporters detect different forms of copper and/or they function in distinct environments where the local concentrations of copper could be markedly different.
Recent studies on localization of CTR2 give some credence to the latter hypothesis. Specifically, the analysis of either the endogenous or recombinant CTR2 demonstrated that the vast majority of this protein was located to intracellular compartments [50,51]. Biotinylation experiments revealed that less than 5% of CTR2 is located at the plasma membrane [51]. Given its predominantly intracellular location, what could be the function of mammalian CTR2 in copper homeostasis? Although CTR2 does not complement the loss of CTR1 function in yeast, in mammalian cells, the recombinant CTR2 facilitates selective silver-inhibitable copper transport [52]. Based on this observation and a relatively low sensitivity for copper [51], the role of CTR2 in low-affinity copper uptake at the plasma membrane was proposed. This is a logical suggestion and it could be that the predominantly intracellular localization of CTR2 simply reflects a pool of inactive transporters that could be recruited to the plasma membrane under certain physiological conditions.
Alternatively, or in addition, the intracellular CTR2 may facilitate copper release from intra-cellular copper stores, as it does in yeast [51]. It is interesting that the CTR2-containing vesicles show overlap with lysosomal markers [48]. Could it be that CTR2 releases copper from the lysosomes, thus retrieving copper from degraded cuproenzymes and returning it back into the cytosol for further utilization? More experiments are needed to clarify the role of CTR2 in different cellular locations.
ATP-driven export & homeostatic control of copper in human cells
Copper-transporting ATPases play a central role in the maintenance of cellular copper levels
Human cells express two homologous Cu-ATPases: ATP7A and ATP7B. These transporters use the energy of ATP hydrolysis to transport copper (in the reduced Cu(I) form) from the cytosol across cellular membranes, thus decreasing cytosolic copper concentration. The transported copper is either released into the bloodstream for further distribution to tissues (in the case of ATP7A) or it is exported into the bile for eventual removal from the body (ATP7B). In both cases, copper export across the plasma membrane is indirect because the Cu-ATPases are not constitutively present at the plasma membrane [53,54]. Instead, they are thought to transport copper into the intracellular vesicles; the vesicles then fuse with the plasma membrane and copper is released (Figure 1). Whether vesicle exocytosis is a constitutive or regulated process is currently unknown. Recent data suggest that copper export can be stimulated in response to Ca2+-channel activation [55], which presumably stimulates vesicle fusion.
In addition to their copper export function, human Cu-ATPases are required for the delivery of copper cofactors to various copper-containing proteins. Secreted cuproproteins and the copper-containing proteins at the plasma membrane are thought to acquire their metal within the secretory pathway from the Cu-ATPases. The process of copper transfer from the Cu-ATPase to the acceptors is not well understood. Copper that is released from the transporter could freely diffuse and incorporate into the active sites of the enzymes, which have already been formed and are ready to bind the cofactor (as was shown in the case of ceruloplasmin [56]). Alternatively, the interaction between the transporter and the acceptor protein may facilitate copper release from the Cu-ATPase and promote subsequent incorporation into the acceptor. Interaction between ATP7A and SOD3, which receives its copper from Cu-ATPase ATP7A, was reported in support of this latter model [57]. However, such interaction can be transient and not always readily detected (as was observed for PAM, another ATP7A partner). Recent characterization of the disease-causing mutation in ceruloplasmin (a ferroxidase that receives copper from ATP7B) provided evidence for a ‘functional silencing’ of ATP7B by an inactive mutant of ceruloplasmin [58]. Thus, it appears that a tight coupling may exist between the biosynthesis of active cuproenzymes and transporters’ activity.
Structure & mechanism of Cu-ATPases
Several detailed reviews have been recently published describing the biochemistry of ATP7A and ATP7B. Therefore, here, we will only briefly summarize the key structural and functional characteristics of Cu-ATPases. Both ATP7A and ATP7B are 160–170-kDa membrane proteins with eight TM segments and several cytosolic domains. The cytosolic N-terminal domain contains six copper binding sites formed by GMxCxxC motifs; the ATP-binding domain (located between TM6 and TM7) consists of two parts, the N- and P-domains, and is involved in ATP binding and hydrolysis. The A-domain (located between TM4 and TM5) interacts with the ATP-binding domain and is required for conformational changes during ATP hydrolysis. The C-terminus contains structural determinants for endocytosis [59,60].
ATP7A is glycosylated (presumably in the first luminal loop), whereas ATP7B is not. Both Cu-ATPases are thought to receive copper in the cytosol from the soluble copper chaperone Atox1 and then transfer copper into the intracellular compartments (either trans-Golgi network [TGN] or vesicles, see later on Cu-ATPases compartmentalization). The sequence homology between ATP7A and ATP7B is high (~60%), however their functional properties are not identical. Comparison of the enzymatic characteristics of two ATPases under identical experimental conditions revealed that ATP7A is likely to have a faster turnover compared with ATP7B because it performs key steps of the catalytic cycle faster than ATP7B, however ATP7B may have a higher affinity for copper [61].
ATP7A and ATP7B belong to the family of P-type ATPases. The characteristic mechanistic feature of this family is the formation of a transient phosphorylated intermediate during ATP hydrolysis [62]. Phosphorylation occurs at the invariant Asp residue in the DKTG motifs located in the P-domain of the transporter. For many P-type ATPases, including ATP7A and ATP7B, the formation of a phosphointermediate and subsequent ion transport require the binding of transported ion within the membrane portion of the protein. However, recent data suggest that Cu-ATPases have a unique ability to become phosphorylated at the catalytic Asp in the absence of copper [63,64]. Physiological significance of this phenomenon for Cu-ATPase function is presently unclear. Nevertheless, it deserves attention, because recent studies provide convincing evidence that chemotherapeutic drugs, such as cisplatin, induce catalytic phosphorylation of Cu-ATPase in the absence of copper (see later for details).
The current model for ATP7A and ATP7B function is the following. Under basal conditions, these transporters are located in the TGN, where they receive copper from the copper chaperone Atox1. Atox1 transfers copper to one of the metal-binding sites in the N-terminal domain of Cu-ATPase (site 2 in ATP7B); the mutation of CxxC to AxxA in this domain completely abolishes the Atox1-dependent stimulation of ATPase activity [65]. Initial copper binding results in a conformational change that allows Atox1 to transfer copper to other sites in the N-terminal domain, such as site 4 in ATP7B or between sites [66], or even donate copper to the membrane portion, as suggested by studies in the archeal CopA [67]. Copper transfer to the TM domain is accompanied by ATP hydrolysis and phosphorylation of the catalytic Asp (see earlier). This in turn induces the change in protein conformation exposing copper to the lumen of the TGN vesicles. The low pH within the TGN may facilitate copper release, leading to Cu-ATPase dephosphorylation and return in the original state. When Cu is elevated, all sites within the N-terminal domain are saturated with copper; this results in weakening interactions between the N-terminus and the ATP-binding domain and an increased rate of catalytic phosphorylation [68]. Thus, copper is not only a transported ion but also a modulator of its own transporter activity.
Copper export is regulated through the intracellular trafficking of Cu-ATPases
Cu-ATPases perform their function in two major cellular locations. At steady state, in all cell types characterized so far, both ATP7A and ATP7B show predominantly intracellular perinuclear localization consistent with their targeting to the TGN. In this location, Cu-ATPases contribute to the biosynthesis of copper-dependent enzymes by retrieving copper from the cytosolic chaperone Atox1 and transferring copper into the lumen of the secretory pathway. It is interesting to consider what could be the role of two structurally and functionally similar Cu-ATPases in the TGN in cells in which they are co-expressed. Presently, there have been no high-resolution studies to determine whether ATP7A and ATP7B are located in the same TGN vesicles; available low-resolution experiments suggest only partial overlap and perhaps distinct compartmentalization within the TGN [69]. The available functional data also suggest that, in the TGN, one Cu-ATPase can compensate for the loss of function of the other. For example, in a cultured cell model, either ATP7A or ATP7B can transfer copper to tyrosinase (a copper-dependent enzyme involved in pigmentation). Similarly, inactivation of ATP7B prevents copper delivery to ceruloplasmin in the liver (where ATP7B is the only Cu-ATPase), but not in the cerebellum (where ATP7A and ATP7B are co-expressed) [61]. Thus, the major functional requirement in two transporters appears to be at the post-TGN level. Indeed, there is a major difference in the location and function of the two Cu-ATPases when copper is elevated.
Numerous reports have provided convincing evidence that the intracellular targeting of ATP7A and ATP7B is regulated by changing copper levels. Studies in polarized epithelial cells and in tissue illustrate that, in response to copper elevation, ATP7A traffics from the TGN to vesicles in immediate vicinity of the basolateral membrane [70], presumably to export excess copper into the blood. Direct evidence for coupling between ATP7A trafficking and copper export was recently reported in polarized Caco-2 and MDCK cells [71].
By contrast, ATP7B traffics to the apical membrane [72]. In the liver, this trafficking is necessary to export excess copper into the bile, while in mammary glands the trafficking step is associated with copper transport into milk. Mice lacking functional Atp7b (Atp7b−/− and toxic milk mice) have low copper content of their milk. This results in copper deficiency in pups and, in some cases, death. There is little doubt that copper homeostasis in different tissues is regulated with different precision and through overlapping but not identical mechanisms. In the liver, only one ATPase, ATP7B, is involved in copper export. In the placenta, excess copper is transported by two Cu-ATPases in different directions: to a fetus or back to the maternal circulation [73], and both ATP7A and ATP7B are thought to traffic from the TGN in response to copper elevation to facilitate copper efflux. In the kidney, two Cu-ATPases are present, however, the trafficking in opposite directions is not observed. Rather, in response to copper elevation, ATP7A traffics, as in other cells and tissues, to the basolateral membrane, while ATP7B does not relocalize, even when copper is highly elevated [74]. The mechanism behind the loss of trafficking is still unclear, but could be associated with a post-transcriptional modification of ATP7B mRNA or protein [74]. Thus, the role of renal ATP7B could be in copper sequestration and storage rather than apical efflux, although this hypothesis remains to be directly tested. Similar function was proposed for ATP7B in intestine, in which ATP7B traffics to vesicles [75].
Clinical relevance & therapeutic potential of copper transporters
Interaction of copper transport with other metabolic pathways
The role of Cu-ATPases in the delivery of a cofactor to a number of important metabolic enzymes links copper transport to many key physiological processes. Thus, it is not surprising that severe copper deficiency observed in the CTR1 knockout animals and in MND patients is lethal. However, modulating copper levels (and corresponding physiological processes) may have potential therapeutic effects. For example, copper is important for angiogenesis, the process of forming new capillaries, which is essential for rapid tumor growth. Recent high-resolution measurements of copper distribution in highly vascularized ductal carcinomas revealed increased concentration of copper in putative neoangiogenic areas [76,77]. While molecular players and mechanisms that govern copper participation in angiogenesis are yet to be characterized, utilization of copper chelators to restrict copper supplies has shown promise in reducing tumor growth [78,79].
Analysis of consequences of copper elevation in the animal model of WND revealed another interesting wide-reaching role of copper. Elevated copper was found to dramatically alter hepatic lipid metabolism, in particularly cholesterol biosynthesis, which was greatly down-regulated in the ATP7B-deficient animals [80]. By contrast, dietary copper deficiency was previously shown to upregulate fatty acid synthase and enhance lipid synthesis in hepatocytes [81]. This reciprocal relationship between copper levels and lipid biosynthesis suggests a tight link between the lipid and copper metabolisms.
In a recent report, copper treatment was also shown to induce the secretion of activated sphingomyelinase (Asm) from leukocytes, leading to the release of ceramide and increasing cell apoptosis [82]. It is particularly interesting that the inhibition of Asm abrogated these effects, pointing to a specific connection between cellular copper levels and sphingomyelin production. The involvement of copper transport in the biosynthesis of another complex lipid, inositolphosphorylceramide, was previously reported in yeast, where Cu-ATPase Ccc2 was required for inositolphosphorylceramide hydroxylation [83]. These observations, along with the well-known inhibitory effect of copper deficiency on myelin formation [84], illustrate that a thorough analysis of the link between copper levels and lipid metabolism is likely to yield important and clinically relevant results.
Another interesting effect of elevated copper is on the cell cycle. In the murine Atp7b−/− livers that accumulate copper to very high levels, a number of transcripts for proteins associated with chromosome maintenance and cell cycle progression were upregulated [80]. This observation suggests that elevated copper may modulate, directly or indirectly (by inducing oxidative changes), the cell cycle machinery. Recent studies demonstrated that the effect of copper on cell growth could be mediated by the copper chaperone Atox1 [85]. In this study, the authors found that the stimulation of mouse embryonic fibroblasts with copper increased cell proliferation. The effect was associated with the upregulation of cyclin D1 expression and cell entry into S phase, which were completely abolished in the Atox1−/− fibroblasts [85].
Equally intriguing is the hormonal regulation of copper homeostasis. In lactating animals, there is a massive redirection of copper from the liver and kidney to mammary gland [86]. Copper uptake into a mammary gland is particularly high during early lactation [87]. At the cellular level, treatment with hormones (estrogen and progesterone, followed by dexamethasone, insulin and prolactin to mimic the physiological hormonal changes within the breast during lactation) induces trafficking of ATP7B towards the apical plasma membrane, consistent with its role in copper delivery to milk [88]. Another example of hormonal regulation has been reported in differentiated polarized human placental choriocarcinoma cells, Jeg-3 [71]. Insulin and estrogen were found to increase expression of ATP7A and induce trafficking of this Cu-ATPase towards the basolateral membrane in a copper-independent manner. By contrast, ATP7B was downregulated and protein was found in a tight perinuclear location. The authors proposed that insulin and estrogen stimulate copper transport to the fetus by increasing the expression and transport activity of ATP7A [71]. The mechanism through which hormones mediate their effects remains unknown, however it is worth noting that both ATP7A and ATP7B are targets of a kinase-mediated phosphorylation [89,90]. It is possible that the phosphorylation by kinases may play a role in the response of copper transporters to a hormone-mediated signaling.
Recent studies suggest that certain pathological conditions alter copper homeostasis in human cells. In RAW 264.7 macrophages, oxygen limitation stimulates expression of CTR1 and induces copper uptake [91]. Interestingly, despite an increased copper influx, the activities of cytosolic superoxide dismutase and mitochondrial cytochrome C oxidase are reduced in hypoxia. By contrast, the trafficking and presumably activity of ATP7A are stimulated [91]; this suggests the important and specific role for ATP7A in cellular response to oxygen deprivation [91].
Cisplatin mediates its toxic effect through more than one mechanism
Cisplatin or cis-diamminedichloridoplatinum (II) (cis-Pt(NH3)2Cl2) is a platinum-based drug widely used to treat various types of cancers, including sarcomas, ovarian carcinomas and lymphomas. The other members of this family of drugs are carboplatin and oxaliplatin. Once inside the cell, cisplatin undergoes hydrolysis, producing the highly reactive platinum complex [Pt(NH3)2ClH2O]+. The complex binds and induces crosslinking of DNA, ultimately triggering apoptosis [92]. In addition to binding and crosslinking DNA, cisplatin can mediate its toxic effect through upregulating the high-mobility group proteins, inducing unfolded protein response and ER stress, as well as activating complex signaling pathways [93].
The mechanism by which cisplatin-like compounds enter cells is not well understood. Recently, an intriguing possibility has emerged that copper transporters may play a role in the uptake as well as the export of cisplatin. It was found that cells resistant to high levels of cisplatin are also resistant to high copper levels and vice versa [94] and that the changes in the level of expression of copper transporters influence cells resistance to the drug. It was suggested that cisplatin and cisplatin-like drugs utilize the cellular copper-transport machinery to enter the cell (via CTR1) and to be exported from the cell (via Cu-ATPases). The apparent association between copper metabolism and cell resistance to platinum drugs opens up the possibility of modulating the activity of copper transporters in order to alleviate drug resistance and get the most benefit from the platinum-based cancer therapy.
Role of CTR1 & CTR2 in cisplatin uptake
Since the discovery of the connection between copper homoeostasis and cellular response to cisplatin [15], experimental evidence has been accumulating that CTR1 plays a role in the uptake of the drug. There is, however, a considerable dichotomy in specific results, as we now describe. Consequently, the mechanism of CTR1-facilitated uptake of cisplatin is far from being clear. Early experiments conducted in yeast revealed that cells lacking yCtr1 accumulated 50% less cisplatin compared with the wild-type strain and showed an eightfold higher resistance to the drug [95]. Similar observations were made in mammalian cells, where mouse primary embryonic cell lines lacking CTR1 showed higher resistance to cisplatin than cells expressing CTR1. It was also demonstrated that, in yeast, copper and cisplatin compete and reduce one anothers uptake [95]. These results strongly suggested that CTR1 could be a key transporter providing the pathway for the cisplatin entry into the cell.
However, other studies do not seem to strongly support this conclusion. In recent report, the complete loss of CTR1 function was associated with a more modest, twofold increase in cell survival and the amount of cisplatin entering cells was only diminished by 10–15% [44]. It is also peculiar that the overexpression of CTR1 has no effect on the intracellular accumulation of the drug [96]. This latter observation is inconsistent with the mechanism based on direct transport through CTR1; and it raises the possibility that changes in CTR1 levels may induce additional pathways that regulate the removal/detoxification of the drug (see more below).
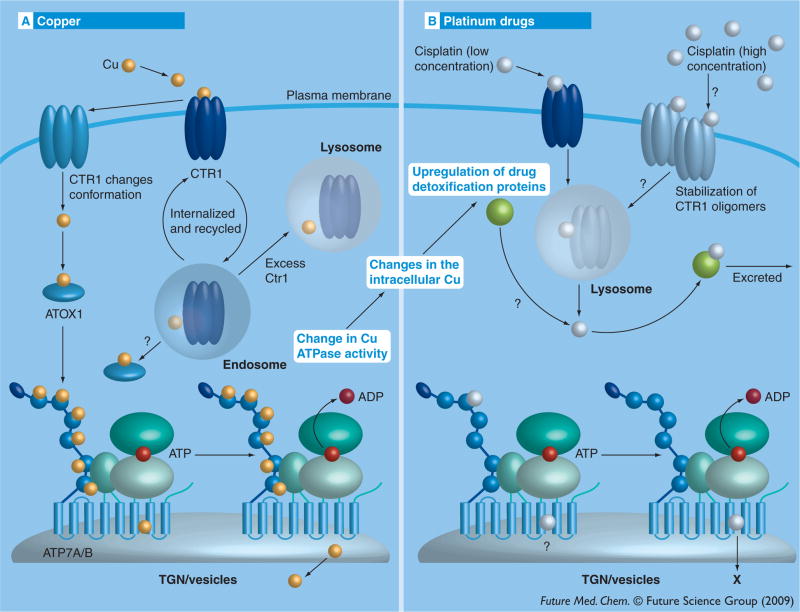
Current data indicate that copper and cisplatin interact with CTR1 at distinct sites. For example, the binding of copper and cisplatin has different effects on yCTR1 conformation, as indicated by FRET measurements [44]. In HEK293 cells, treatment with cisplatin results in the formation of a dithiotheitol-resistant trimeric form of endogenous CTR1, which can be visualized by gel electrophoresis. Such stable trimers are not observed following copper treatment of the same cells; in this case, the predominant form on a gel is a dimer [36]. Furthermore, Met to Ala substitution in both Mets motifs of hCTR1 (A7GASYA12 and A40AAAPA45) prevents the cisplatin-induced stabilization of CTR1 trimers, presumably due to loss of binding. By contrast, copper still binds and induces CTR1 endocytosis [36]. Lastly, the yCTR1 allele, M127A, defective in copper transport, showed intracellular cisplatin levels comparable to those of the WT strain [44]. Altogether, these observations suggest that cisplatin is likely to bind to the N-terminus of CTR1 and crosslink the CTR1 monomers without blocking copper binding and copper-induced endocytosis [35]. If this is the case, cisplatin can be taken into cells as a ‘passenger’ during the normal course of copper-dependent endocytosis of CTR1 (Figure 3).
Figure 3. Proposed associations between mammalian copper metabolism and the transport of cisplatin.
(A) Copper enters the cell through CTR1, which is localized on the basolateral membrane [34]. Upon release, copper is accepted by the metallochaperone ATOX1, which then transfers copper to the P-type ATPases ATP7A or ATP7B [66,67]. Hydrolysis of ATP by the ATPases is coupled with copper transport across the membrane. (B) Cisplatin entry into the cell depends on the presence of active CTR1. The exact mechanism of uptake is unknown, but it may involve stabilization of CTR1 oligomers and endocytosis. Further cisplatin interacts with ATP7B at the N-terminal part and can be partially sequestered [105,64]. Cisplatin, as with copper, induces phosphorylation of ATP7B but is not transported [64]. Cells with changes in intracellular copper (due to changes in the activity or expression of copper transporters) show upregulation of additional drug-detoxification pathways (see central part within closed dotted ellipse) [104].
It was also reported that the exposure of mouse embryo fibroblasts and ovarian carcinoma cells to low concentration of cisplatin (2 μM) triggered almost instant disappearance of CTR1 that was interpreted as protein degradation [97]. CTR1 reappeared when higher concentrations of cisplatin were used (10 and 200 μM) [97]. In the studies with HEK293 cells (see earlier) rapid degradation was not observed, possibly because a low concentration of cisplatin was not used or a different antibody was used to detect the protein [36]. If the mechanism of cisplatin uptake involves endocytosis of drug bound to CTR1 and/or degradation of the cisplatin–CTR1 complex (presumably in the lysosomes), then an additional step is needed to complete the process: cisplatin needs to be released from the endocytic vesicles/lysosomes into the cytosol (Figure 3). What molecular machinery mediates this step is currently unknown.
Alternatively, the CTR1 involvement in the uptake of cisplatin could be indirect. In this scenario, copper deficiency due to CTR1 downregulation may result in metabolic changes that facilitate entry, retention and/or efficacy of cisplatin. The plausibility of this model has been recently demonstrated [64]. Specifically, it was shown that the treatment of Huh7 cells with the copper chelator BCS, prior to treating with cisplatin, increases cell resistance to the drug [64]. The levels of CTR1 were not investigated in these experiments and it is possible that, in Huh7 cells, BCS treatment decreased the expression of CTR1, thus inducing resistance. However, in other tissues, treatment with BCS increases the CTR1 levels [26]; thus, this explanation may not hold.
More likely, copper deficiency induces diverse metabolic changes (as previously discussed), which contribute to the cisplatin resistance phenotype. It is worth noting that the down-regulation of CTR1 and the overexpression of ATP7A (observed in cisplatin-resistant cells) would both generate a copper-deficient phenotype. From this point of view, it seems particularly significant that the comparison of the DDP-resistant cells to nonresistant cells of the same origin revealed that the resistant cells have 22–56% lower basal levels of copper (i.e., they are copper deficient) [94]. While these observations are highly suggestive of a link between copper transport and resistance, it seems clear that simple models for the role of copper transporters in resistance to cisplatin are unlikely to be satisfactory. This can be illustrated by recent work [64], where both copper deficiency or copper overload were found to increase resistance to cisplatin, and treatment with copper chelator BCS had cell-specific effects (i.e., the resistance of Huh7 cells was increased in response to BCS, while HepG2 cells that have higher initial cisplatin resistance were unaffected by pretreatment with BCS).
Recently, the complexity of the picture was further illustrated by the experiments investigating the role of CTR2 in cisplatin sensitivity [98]. In these experiments, the siRNA-mediated down-regulation of CTR2 increased cisplatin uptake. This result is difficult to explain through a mechanism that involves direct transport of the drug by the transporter. Furthermore, cells lacking CTR1 (Ctr1−/− mouse fibroblasts) accumulated more cisplatin than CTR1-positive cells and showed lower survival when CTR2 in these cells was downregulated by 30–50%. These results seem to eliminate the central role of CTR1 in cisplatin uptake. Furthermore, contrary to the earlier work, the down-regulation of CTR2 increased cellular copper levels, showed no effect on vesicular accumulation and more drug was reached the nucleus. Once again, the simple mechanism involving only CTR1 and CTR2 in cisplatin uptake does not accommodate current data and further experiments are needed to establish the precise role of copper transporters in cisplatin uptake and resistance.
Role of ATP7B & ATP7A in exporting cisplatin out of the cell: direct or indirect effect?
The involvement of Cu-ATPases in modulating resistance to platinum-containing drugs was initially suggested by the observation that cancer cells overexpressing ATP7B show higher tolerance to the increasing concentration of cisplatin [15]. Of the two human copper ATPases, ATP7B seems to be the primary candidate responsible for platinum drug resistance, with ATP7A probably playing a minor role [99]. In cultured ovary cells, the downregulation of ATP7B using siRNA increases cell sensitivity to cisplatin, while the downregulation of ATP7A does not have a significant effect [100]. Furthermore, in vivo (in the mouse model of ovary cancer), the downregulation of ATP7B using the liposome-mediated delivery of siRNA decreases the size of growing tumors by 40% and increases the efficacy of the cisplatin therapy in an additive manner [100]. This first in vivo application of ATP7B downregulation illustrates the therapeutic potential of modulating ATP7B levels to combat acquired cell resistance, which is a recurring and significant problem in clinical setting.
While clinical data repeatedly show association between the levels of Cu-ATPases and resistance to cisplatin, the mechanism through which Cu-ATPases affect cell resistance is unclear. It has been suggested that cisplatin is transported by Cu-ATPases and that the ATPases efflux cisplatin out of the cells. This hypothesis did not receive sufficient experimental support, as the increase in ATP7B levels does not always reduce intracellular cisplatin. Consequently, it was proposed that ATP7B (or ATP7A) may transport and sequester cisplatin in intracellular vesicles. The idea that Cu-ATPases are specific transporters for cisplatin was met with scepticism, because Cu-ATPases are highly selective for Cu(I) and the size and chemistry of Cu(I) and cisplatin are very different. Currently, despite some suggestive data, convincing evidence that ATP7B transports cisplatin similarly to copper is lacking. Current data appear more consistent with the model where Cu-ATPases control (directly or indirectly) the availability of the drug in the cytosol rather than directly mediating transport of the drug.
The following results were used to suggest the direct involvement of Cu-ATPases in the transport of cisplatin. It was reported that the fluorescently tagged ATP7B-ECFP colocalizes with a fluorescent derivative of cisplatin in vesicles [101]. In addition, cisplatin content was compared in vesicles isolated from control cells and cells over-expressing ATP7B and the latter samples were found to have twice as much cisplatin. These data are fully consistent with the role of ATP7B in cisplatin handling; however, the results do not discriminate between the transport of the drug into vesicles and cisplatin binding at the vesicle surface (to ATP7B or other proteins).
To determine whether cisplatin is a substrate for ATP7B, the transport of the drug was assayed in vesicles isolated from the Sf9 cells infected with a baculovirus that expressed either the wild-type ATP7B or a catalytically inactive ATP7B [102]. In the absence of ATP, the vesicles containing either the wild-type or inactive mutant ATP7B have more cisplatin than vesicles without ATP7B indicating that cisplatin indeed binds to the transporter. Furthermore, at low pH (~3–4), the ATP-dependent accumulation of cisplatin was observed in vesicles containing active ATP7B. The estimated Vmax indicated that cisplatin is transported much more slowly than copper [102]. Similar experiments were later carried out closer to physiological pH (~7.0) [64]. In these studies, the ATP7B-mediated transport of copper was measured and the ability of cisplatin to compete with copper was evaluated. No competition was observed, indicating that copper and cisplatin are unlikely to be transported using the same mechanism [64]. Altogether, these experiments suggest that ATP7B may facilitate the passage of cisplatin through the membrane via some unusual ‘leak’ pathway, which could be particularly apparent at non-physiological pH, such as pH 3–4. However, alternative explanations for the role of ATP7B seem more plausible.
There is currently little doubt that when cisplatin binds to ATP7B it stimulates its catalytic phosphorylation [64,102]. It is also clear that the cisplatin-binding sites and copper-binding site are not identical, because excess cisplatin does not decrease the copper-dependent phosphorylation of ATP7B or influence copper transport [64]. Dolgova and colleagues, using a creative in vivo assay in bacterial culture, found that the domain of ATP7B alone confers cell resistance to cisplatin without active drug extrusion from the cell [103]. This study found that three cisplatin molecules bind per the ATP7B fragment containing the first four metal N-terminal binding sites. It was suggested that cisplatin interacts with the copper coordinating motifs CxxC within this fragment. However, in studies of the full-length ATP7B, it was demonstrated that mutation of these four CxxC sites to AxxA does not decrease the ability of cisplatin to stimulate catalytic phosphorylation of ATP7B [64]. Thus, the precise coordination of cisplatin by the N-terminus of ATP7B remains to be established.
In mammalian cell culture, the overexpression of the N-terminal domain of ATP7B also induced resistance to the drug; however, the effect was lower compared with the full-sized ATP7B [100], indicating that cisplatin binding to ATP7B alone may not be sufficient to explain the resistance phenotype. The involvement of several mechanisms and an indirect effect of Cu-ATPases on drug resistance are suggested by several other observations. For example, in hepatocytes, no correlation was observed between levels of endogenous ATP7B and cell resistance to cisplatin [64]. Furthermore, contrary to the results of siRNA-mediated downregulation of ATP7B in ovary cells, primary hepatocytes lacking ATP7B have higher viability at high (50–125 μm) levels of cisplatin, when compared with control hepatocytes. This unexpected observation might be explained by the fact that copper accumulation induced by ATP7B inactivation greatly upregulates expression of metallothioneins, alters the DNA-repair machinery, changes the expression levels for several ABC-type transporters and may alter the redox balance in a cell [80]. In other words, changes in copper metabolism trigger a metabolic response that involves a number of molecules implicated in resistance to cisplatin. That the copper overload may induce a wide-ranging detoxification response is also suggested by an observation from Owatari and colleagues [104]. These authors found that the CHOK1 cells and the fibroblasts isolated from MND patients transfected with ATP7A showed higher resistance not only to cisplatin but also to various nonplatinate anticancer drugs, such as vincristine, paclitaxel, 7-ethyl-10- hydroxycamptothecin (SN-38) and doxorubicin, to name a few. Clearly, a better understanding of transcriptional and metabolic changes associated with the changes in the expression and/or activity of Cu-ATPases are likely to facilitate our understanding of drug resistance and would help to utilize ‘the copper pathways’ in designing new therapeutics for cancer.
Future perspective
In the past decade, the field of copper transport has undergone a remarkable expansion and transformation. The family of proteins that regulate copper homeostasis continues to grow and our knowledge of copper transport mechanisms is becoming more sophisticated. Yet, much remains to be learned. The mechanism of dietary copper acquisition, the molecular basis of copper delivery to tissues and the control of copper distribution within the cells are exciting topics that await detailed dissection in the near future. It seems likely that the next few years will uncover cell- and tissue-specific mechanisms regulating copper homeostasis and bring a better understanding of the role of copper transporters in response to pathological conditions. In-depth exploration of the link between copper homeostasis to other physiological processes (e.g., lactation, inflammation, lipid metabolism and angiogenesis) is an avenue that promises to fully utilize the therapeutic potential of regulating human copper transport.
Executive summary
Available clinical data illustrate the growing need to better understand the regulation of copper acquisition and distribution in cells and tissues.
The high-affinity copper transporter CTR1 may have more than one function in intestinal cells and act in more than one cellular location.
The copper transport through CTR1 involves coordinated changes of the extracellular and cytosolic portions of the transporter.
Specific function of Cu-ATPases ATP7A and ATP7B in a cell is determined by their intracellular localization.
Alterations in the expression of copper transporters modulate the resistance of tumor cells to cisplatin-like drugs; the mechanism behind this important phenomenon needs further study.
Copper homeostasis is linked to important metabolic pathways and influences lipid metabolism and cell cycle.
Elucidating the connections of human copper transport to other metabolic pathways will help to fully utilize the therapeutic potential of regulating copper transporters.
Acknowledgments
We thank Amanda Barry for careful reading of the manuscript and helpful comments.
- ATP7A
Copper-transporting ATPase that mediates transfer of copper from the cytosol to several cuproenzymes in the secretory pathways and facilitates export of copper out of the cell. Genetic mutations in ATP7A result in Menkes disease
- ATP7B
Copper-transporting ATPase with the major function in regulating copper homeostasis in the liver. Mutations in ATP7B are associated with Wilson’s disease
- Cisplatin
cis-diamminedichloroplatinum II, the major chemotherapeutic drug widely used for the treatment of ovary, testicular, lung and other cancers
- CTR1
Transporter that is essential for high-mammalian cells
- CTR2
Low-affinity copper transporter of unknown physiological function
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
This work was funded by the NIH grant R01 DK071865 to Svetlana Lutsenko. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Nelson KT, Prohaska JR. Copper deficiency in rodents alters dopamine β-mono-oxygenase activity, mRNA and protein level. Br J Nutr. 2008;102(1):18–28. doi: 10.1017/S0007114508162961. [DOI] [PubMed] [Google Scholar]
- 2.Johnson WT, Anderson CM. Cardiac cytochrome C oxidase activity and contents of subunits 1 and 4 are altered in offspring by low prenatal copper intake by rat dams. J Nutr. 2008;138(7):1269–1273. doi: 10.1093/jn/138.7.1269. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Z, Johnson WT, Kang YJ. Regression of copper-deficient heart hypertrophy: reduction in the size of hypertrophic cardiomyocytes. J Nutr Biochem. 2008;20(8):621–628. doi: 10.1016/j.jnutbio.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AD, Botero S, Levander OA. Copper deficiency increases the virulence of amyocarditic and myocarditic strains of coxsackievirus B3 in mice. J Nutr. 2008;138(5):849–855. doi: 10.1093/jn/138.5.849. [DOI] [PubMed] [Google Scholar]
- 5.Gambling L, Andersen HS, McArdle HJ. Iron and copper, and their interactions during development. Biochem Soc Trans. 2008;36(6):1258–1261. doi: 10.1042/BST0361258. [DOI] [PubMed] [Google Scholar]
- 6.Keen CL, Uriu-Hare JY, Hawk SN, et al. Effect of copper deficiency on prenatal development and pregnancy outcome. Am J Clin Nutr. 1998;67(Suppl 5):S1003–S1011. doi: 10.1093/ajcn/67.5.1003S. [DOI] [PubMed] [Google Scholar]
- 7.Kaler SG, Holmes CS, Goldstein DS, et al. Neonatal diagnosis and treatment of Menkes disease. N Engl J Med. 2008;358(6):605–614. doi: 10.1056/NEJMoa070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Luis DA, Pacheco D, Izaola O, Terroba MC, Cuellar L, Martin T. Clinical results and nutritional consequences of biliopancreatic diversion: three years of follow-up. Ann Nutr Metab. 2008;53(3–4):234–239. doi: 10.1159/000185641. [DOI] [PubMed] [Google Scholar]
- 9.Ernst B, Thurnheer M, Schmid SM, Schultes B. Evidence for the necessity to systematically assess micronutrient status prior to bariatric surgery. Obes Surg. 2009;19(1):66–73. doi: 10.1007/s11695-008-9545-4. [DOI] [PubMed] [Google Scholar]
- 10.Halfdanarson TR, Kumar N, Li CY, Phyliky RL, Hogan WJ. Hematological manifestations of copper deficiency: a retrospective review. Eur J Haematol. 2008;80(6):523–531. doi: 10.1111/j.1600-0609.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 11.Marinella MA. Anemia following Roux-en-Y surgery for morbid obesity: a review. South Med J. 2008;101(10):1024–1031. doi: 10.1097/SMJ.0b013e31817cf7b7. [DOI] [PubMed] [Google Scholar]
- 12.Halfdanarson TR, Kumar N, Hogan WJ, Murray JA. Copper deficiency in celiac disease. J Clin Gastroenterol. 2009;43(2):162–164. doi: 10.1097/MCG.0b013e3181354294. [DOI] [PubMed] [Google Scholar]
- 13.Kumar N. Copper deficiency myelopathy (human swayback) Mayo Clin Proc. 2006;81(10):1371–1384. doi: 10.4065/81.10.1371. [DOI] [PubMed] [Google Scholar]
- 14.Das SK, Ray K. Wilson’s disease: an update. Nat Clin Pract Neurol. 2006;2(9):482–493. doi: 10.1038/ncpneuro0291. [DOI] [PubMed] [Google Scholar]
- 15.Komatsu M, Sumizawa T, Mutoh M, et al. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 2000;60(5):1312–1316. [PubMed] [Google Scholar]
- 16.Safaei R, Howell SB. Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol Hematol. 2005;53(1):13–23. doi: 10.1016/j.critrevonc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Lim CM, Cater MA, Mercer JF, La Fontaine S. Copper-dependent interaction of dynactin subunit p62 with the N terminus of ATP7B but not ATP7A. J Biol Chem. 2006;281(20):14006–14014. doi: 10.1074/jbc.M512745200. [DOI] [PubMed] [Google Scholar]
- 18.Holloway ZG, Grabski R, Szul T, et al. Activation of ADP-ribosylation factor regulates biogenesis of the ATP7A-containing trans-Golgi network compartment and its Cu-induced trafficking. Am J Physiol Cell Physiol. 2007;293(6):C1753–C1767. doi: 10.1152/ajpcell.00253.2007. [DOI] [PubMed] [Google Scholar]
- 19.Burkhead JL, Morgan CT, Shinde U, Haddock G, Lutsenko S. COMMD1 forms oligomeric complexes targeted to the endocytic membranes via specific interactions with phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2009;284(1):696–707. doi: 10.1074/jbc.M804766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4(3):176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 21.Maryon EB, Molloy SA, Zimnicka AM, Kaplan JH. Copper entry into human cells: progress and unanswered questions. Biometals. 2007;20(3–4):355–364. doi: 10.1007/s10534-006-9066-3. [DOI] [PubMed] [Google Scholar]
- 22.Dancis A, Yuan DS, Haile D, et al. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994;76(2):393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA. 1997;94(14):7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moller LB, Petersen C, Lund C, Horn N. Characterization of the hCTR1 gene: genomic organization, functional expression, and identification of a highly homologous processed gene. Gene. 2000;257(1):13–22. doi: 10.1016/s0378-1119(00)00394-2. [DOI] [PubMed] [Google Scholar]
- 25.Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA. 2001;98(12):6836–6841. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr. 2006;136(1):21–26. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27 ▪.Lee J, Pena MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem. 2002;277(6):4380–4387. doi: 10.1074/jbc.M104728200. This study provided the first characterization of transport characteristics of CTR1 and identified silver as a specific inhibitor. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem. 2002;277(43):40253–40259. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- 29.Kim H, Son HY, Bailey SM, Lee J. Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):G356–G364. doi: 10.1152/ajpgi.90632.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA. 2001;98(12):6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haremaki T, Fraser ST, Kuo YM, Baron MH, Weinstein DC. Vertebrate Ctr1 coordinates morphogenesis and progenitor cell fate and regulates embryonic stem cell differentiation. Proc Natl Acad Sci USA. 2007;104(29):12029–12034. doi: 10.1073/pnas.0701413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32 ▪▪.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4(3):235–244. doi: 10.1016/j.cmet.2006.08.009. Pioneering experiments on tissue-specific inactivation of CTR1 that demonstrated the important role of CTR1 in intestinal absorption and revealed the unexpected function of intestinal CTR1 in mediating availability of absorbed copper to cytosolic proteins. [DOI] [PubMed] [Google Scholar]
- 33 ▪▪.Zimnicka AM, Maryon EB, Kaplan JH. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J Biol Chem. 2007;282(36):26471–26480. doi: 10.1074/jbc.M702653200. First demonstration that CTR1 may function in copper uptake at the blood site of various cells. [DOI] [PubMed] [Google Scholar]
- 34.Zerounian NR, Redekosky C, Malpe R, Linder MC. Regulation of copper absorption by copper availability in the Caco-2 cell intestinal model. Am J Physiol Gastrointest Liver Physiol. 2003;284(5):G739–G747. doi: 10.1152/ajpgi.00415.2002. [DOI] [PubMed] [Google Scholar]
- 35.Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem. 2004;279(17):17428–17433. doi: 10.1074/jbc.M401493200. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Smith K, Petris MJ. Cisplatin stabilizes a multimeric complex of the human Ctr1 copper transporter: requirement for the extracellular methionine-rich clusters. J Biol Chem. 2004;279(45):46393–46399. doi: 10.1074/jbc.M407777200. [DOI] [PubMed] [Google Scholar]
- 37.Eisses JF, Kaplan JH. The mechanism of copper uptake mediated by human CTR1: a mutational analysis. J Biol Chem. 2005;280(44):37159–37168. doi: 10.1074/jbc.M508822200. [DOI] [PubMed] [Google Scholar]
- 38.De Feo CJ, Aller SG, Unger VM. A structural perspective on copper uptake in eukaryotes. Biometals. 2007;20(3–4):705–716. doi: 10.1007/s10534-006-9054-7. [DOI] [PubMed] [Google Scholar]
- 39.Maryon EB, Molloy SA, Kaplan JH. O-linked glycosylation at threonine 27 protects the copper transporter hCTR1 from proteolytic cleavage in mammalian cells. J Biol Chem. 2007;282(28):20376–20387. doi: 10.1074/jbc.M701806200. [DOI] [PubMed] [Google Scholar]
- 40.Jiang J, Nadas IA, Kim MA, Franz KJ. A Mets motif peptide found in copper transport proteins selectively binds Cu(I) with methionine-only coordination. Inorg Chem. 2005;44(26):9787–9794. doi: 10.1021/ic051180m. [DOI] [PubMed] [Google Scholar]
- 41 ▪▪.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA. 2009;106(11):4237–4242. doi: 10.1073/pnas.0810286106. Structural study offering important insights into the mechanism of copper entry into human cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem. 2002;277(29):26021–26030. doi: 10.1074/jbc.M202547200. [DOI] [PubMed] [Google Scholar]
- 43.Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem. 2002;277(32):29162–29171. doi: 10.1074/jbc.M203652200. [DOI] [PubMed] [Google Scholar]
- 44.Sinani D, Adle DJ, Kim H, Lee J. Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J Biol Chem. 2007;282(37):26775–26785. doi: 10.1074/jbc.M703973200. [DOI] [PubMed] [Google Scholar]
- 45.Wu X, Sinani D, Kim H, Lee J. Copper transport activity of yeast Ctr1 is down-regulated via its C terminus in response to excess copper. J Biol Chem. 2009;284(7):4112–4122. doi: 10.1074/jbc.M807909200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao Z, Wedd AG. A C-terminal domain of the membrane copper pump Ctr1 exchanges copper(I) with the copper chaperone Atx1. Chem Commun (Camb) 2002;6:588–589. doi: 10.1039/b111180a. [DOI] [PubMed] [Google Scholar]
- 47 ▪.Chen HH, Song IS, Hossain A, et al. Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1. Mol Pharmacol. 2008;74(3):697–704. doi: 10.1124/mol.108.047969. Important study demonstrating the link between glutathione biosynthesis, CTR1 expression and resistance of cells to cisplatin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gybina AA, Prohaska JR. Variable response of selected cuproproteins in rat choroid plexus and cerebellum following perinatal copper deficiency. Genes Nutr. 2006;1(1):51–59. doi: 10.1007/BF02829936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klomp AE, Tops BB, Van Denberg IE, Berger R, Klomp LW. Biochemical characterization and subcellular localization of human copper transporter 1 (hCTR1) Biochem J. 2002;364(2):497–505. doi: 10.1042/BJ20011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Berghe PV, Folmer DE, Malingre HE, et al. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J. 2007;407(1):49–59. doi: 10.1042/BJ20070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertinato J, Swist E, Plouffe LJ, Brooks SP, L’Abbe MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J. 2008;409(3):731–740. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- 52.Rees EM, Thiele DJ. Identification of a vacuole-associated metalloreductase and its role in Ctr2-mediated intracellular copper mobilization. J Biol Chem. 2007;282(30):21629–21638. doi: 10.1074/jbc.M703397200. [DOI] [PubMed] [Google Scholar]
- 53 ▪.Nyasae L, Bustos R, Braiterman L, Eipper B, Hubbard A. Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: copper-dependent redistribution between two intracellular sites. Am J Physiol Gastrointest Liver Physiol. 2007;292(4):G1181–G1194. doi: 10.1152/ajpgi.00472.2006. This study offers convincing evidence for a predominantly intracellular localization and, hence, function of ATP7A. [DOI] [PubMed] [Google Scholar]
- 54.Pase L, Voskoboinik I, Greenough M, Camakaris J. Copper stimulates trafficking of a distinct pool of the Menkes copper ATPase (ATP7A) to the plasma membrane and diverts it into a rapid recycling pool. Biochem J. 2004;378(3):1031–1037. doi: 10.1042/BJ20031181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlief ML, Craig AM, Gitlin JD. NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J Neurosci. 2005;25(1):239–246. doi: 10.1523/JNEUROSCI.3699-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hellman NE, Kono S, Mancini GM, Hoogeboom AJ, De Jong GJ, Gitlin JD. Mechanisms of copper incorporation into human ceruloplasmin. J Biol Chem. 2002;277(48):46632–46638. doi: 10.1074/jbc.M206246200. [DOI] [PubMed] [Google Scholar]
- 57.Qin Z, Itoh S, Jeney V, Ushio-Fukai M, Fukai T. Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. Faseb J. 2006;20(2):334–336. doi: 10.1096/fj.05-4564fje. [DOI] [PubMed] [Google Scholar]
- 58 ▪.di Patti MC, Maio N, Rizzo G, et al. Dominant mutants of ceruloplasmin impair the copper loading machinery in aceruloplasminemia. J Biol Chem. 2009;284(7):4545–4554. doi: 10.1074/jbc.M805688200. The first direct demonstration of a tight functional link between copper transporter and acceptor protein. [DOI] [PubMed] [Google Scholar]
- 59.Stephenson SE, Dubach D, Lim CM, Mercer JF, La Fontaine S. A single PDZ domain protein interacts with the Menkes copper ATPase, ATP7A. A new protein implicated in copper homeostasis. J Biol Chem. 2005;280(39):33270–33279. doi: 10.1074/jbc.M505889200. [DOI] [PubMed] [Google Scholar]
- 60.Francis MJ, Jones EE, Levy ER, Martin RL, Ponnambalam S, Monaco AP. Identification of a dileucine motif within the C terminus domain of the Menkes disease protein that mediates endocytosis from the plasma membrane. J Cell Sci. 1999;112(11):1721–1732. doi: 10.1242/jcs.112.11.1721. [DOI] [PubMed] [Google Scholar]
- 61.Barnes N, Tsivkovskii R, Tsivkovskaia N, Lutsenko S. The copper-transporting ATPases, menkes and wilson disease proteins, have distinct roles in adult and developing cerebellum. J Biol Chem. 2005;280(10):9640–9645. doi: 10.1074/jbc.M413840200. [DOI] [PubMed] [Google Scholar]
- 62.Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol. 2004;5(4):282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- 63.Hatori Y, Hirata A, Toyoshima C, Lewis D, Pilankatta R, Inesi G. Intermediate phosphorylation reactions in the mechanism of ATP utilization by the copper ATPase (CopA) of Thermotoga maritima. J Biol Chem. 2008;283(33):22541–22549. doi: 10.1074/jbc.M802735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leonhardt K, Gebhardt R, Mossner J, Lutsenko S, Huster D. Functional interactions of Cu-ATPase ATP7B with cisplatin and the role of ATP7B in the resistance of cells to the drug. J Biol Chem. 2009;284(12):7793–7802. doi: 10.1074/jbc.M805145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker JM, Huster D, Ralle M, Morgan CT, Blackburn NJ, Lutsenko S. The N-terminal metal-binding site 2 of the Wilson’s disease protein plays a key role in the transfer of copper from Atox1. J Biol Chem. 2004;279(15):15376–15384. doi: 10.1074/jbc.M400053200. [DOI] [PubMed] [Google Scholar]
- 66.Achila D, Banci L, Bertini I, Bunce J, Ciofi-Baffoni S, Huffman DL. Structure of human Wilson protein domains 5 and 6 and their interplay with domain 4 and the copper chaperone HAH1 in copper uptake. Proc Natl Acad Sci USA. 2006;103(15):5729–5734. doi: 10.1073/pnas.0504472103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez-Guerrero M, Arguello JM. Mechanism of Cu+-transporting ATPases: soluble Cu+ chaperones directly transfer Cu+ to transmembrane transport sites. Proc Natl Acad Sci USA. 2008;105(16):5992–5997. doi: 10.1073/pnas.0711446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huster D, Lutsenko S. The distinct roles of the N-terminal copper-binding sites in regulation of catalytic activity of the Wilson’s disease protein. J Biol Chem. 2003;278(34):32212–32218. doi: 10.1074/jbc.M305408200. [DOI] [PubMed] [Google Scholar]
- 69.Linz R, Lutsenko S. Copper-transporting ATPases ATP7A and ATP7B: cousins, not twins. J Bioenerg Biomembr. 2007;39(5–6):403–407. doi: 10.1007/s10863-007-9101-2. [DOI] [PubMed] [Google Scholar]
- 70.Monty JF, Llanos RM, Mercer JF, Kramer DR. Copper exposure induces trafficking of the menkes protein in intestinal epithelium of ATP7A transgenic mice. J Nutr. 2005;135(12):2762–2766. doi: 10.1093/jn/135.12.2762. [DOI] [PubMed] [Google Scholar]
- 71.Greenough M, Pase L, Voskoboinik I, Petris MJ, O’Brien AW, Camakaris J. Signals regulating trafficking of Menkes (MNK; ATP7A) copper-translocating P-type ATPase in polarized MDCK cells. Am J Physiol Cell Physiol. 2004;287(5):C1463–C1471. doi: 10.1152/ajpcell.00179.2004. [DOI] [PubMed] [Google Scholar]
- 72.Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology. 2000;119(3):782–793. doi: 10.1053/gast.2000.17834. [DOI] [PubMed] [Google Scholar]
- 73.Hardman B, Michalczyk A, Greenough M, Camakaris J, Mercer JF, Ackland ML. Hormonal regulation of the Menkes and Wilson copper-transporting ATPases in human placental Jeg-3 cells. Biochem J. 2007;402(2):241–250. doi: 10.1042/BJ20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barnes N, Bartee MY, Braiterman L, et al. Cell-specific trafficking suggests a new role for renal ATP7B in the intracellular copper storage. Traffic. 2009;10(6):767–779. doi: 10.1111/j.1600-0854.2009.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiss KH, Wurz J, Gotthardt D, Merle U, Stremmel W, Fullekrug J. Localization of the Wilson disease protein in murine intestine. J Anat. 2008;213(3):232–240. doi: 10.1111/j.1469-7580.2008.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finney L, Mandava S, Ursos L, et al. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc Natl Acad Sci USA. 2007;104(7):2247–2252. doi: 10.1073/pnas.0607238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finney L, Vogt S, Fukai T, Glesne D. Copper and angiogenesis: unravelling a relationship key to cancer progression. Clin Exp Pharmacol Physiol. 2009;36(1):88–94. doi: 10.1111/j.1440-1681.2008.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodman VL, Brewer GJ, Merajver SD. Copper deficiency as an anti-cancer strategy. Endocr Relat Cancer. 2004;11(2):255–263. doi: 10.1677/erc.0.0110255. [DOI] [PubMed] [Google Scholar]
- 79.Goodman VL, Brewer GJ, Merajver SD. Control of copper status for cancer therapy. Curr Cancer Drug Targets. 2005;5(7):543–549. doi: 10.2174/156800905774574066. [DOI] [PubMed] [Google Scholar]
- 80 ▪.Huster D, Purnat TD, Burkhead JL, et al. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem. 2007;282(11):8343–8355. doi: 10.1074/jbc.M607496200. Demonstration of a specific effect of copper overload on lipid biosynthesis in the liver. [DOI] [PubMed] [Google Scholar]
- 81.Tang Z, Gasperkova D, Xu J, Baillie R, Lee JH, Clarke SD. Copper deficiency induces hepatic fatty acid synthase gene transcription in rats by increasing the nuclear content of mature sterol regulatory element binding protein 1. J Nutr. 2000;130(12):2915–2921. doi: 10.1093/jn/130.12.2915. [DOI] [PubMed] [Google Scholar]
- 82.Lang PA, Schenck M, Nicolay JP, et al. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med. 2007;13(2):164–170. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- 83.Dunn TM, Haak D, Monaghan E, Beeler TJ. Synthesis of monohydroxylated inositolphosphorylceramide (IPC-C) in Saccharomyces cerevisiae requires Scs7p, a protein with both a cytochrome b5-like domain and a hydroxylase/desaturase domain. Yeast. 1998;14(4):311–321. doi: 10.1002/(SICI)1097-0061(19980315)14:4<311::AID-YEA220>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 84.Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11(1):107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85 ▪.Itoh S, Kim HW, Nakagawa O, et al. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem. 2008;283(14):9157–9167. doi: 10.1074/jbc.M709463200. Describes an intriguing mechanism through which changes in copper levels may alter cell proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donley SA, Ilagan BJ, Rim H, Linder MC. Copper transport to mammary gland and milk during lactation in rats. Am J Physiol Endocrinol Metab. 2002;283(4):E667–E675. doi: 10.1152/ajpendo.00115.2002. [DOI] [PubMed] [Google Scholar]
- 87.Kelleher SL, Lonnerdal B. Mammary gland copper transport is stimulated by prolactin through alterations in Ctr1 and Atp7A localization. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1181–R1191. doi: 10.1152/ajpregu.00206.2005. [DOI] [PubMed] [Google Scholar]
- 88.Michalczyk A, Bastow E, Greenough M, et al. ATP7B expression in human breast epithelial cells is mediated by lactational hormones. J Histochem Cytochem. 2008;56(4):389–399. doi: 10.1369/jhc.7A7300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vanderwerf SM, Cooper MJ, Stetsenko IV, Lutsenko S. Copper specifically regulates intracellular phosphorylation of the Wilson’s disease protein, a human copper-transporting ATPase. J Biol Chem. 2001;276(39):36289–36294. doi: 10.1074/jbc.M102055200. [DOI] [PubMed] [Google Scholar]
- 90.Voskoboinik I, Fernando R, Veldhuis N, et al. Protein kinase-dependent phosphorylation of the Menkes copper P-type ATPase. Biochem Biophys Res Commun. 2003;303(1):337–342. doi: 10.1016/s0006-291x(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 91 ▪.White C, Kambe T, Fulcher YG, et al. Copper transport into the secretory pathway is regulated by oxygen in macrophages. J Cell Sci. 2009;122(9):1315–1321. doi: 10.1242/jcs.043216. Direct demonstration of a specific role for ATP7A in cell response to pathological conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reedijk J. Why does Cisplatin reach Guanine-n7 with competing S-donor ligands available in the cell? Chem Rev. 1999;99(9):2499–2510. doi: 10.1021/cr980422f. [DOI] [PubMed] [Google Scholar]
- 93.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33(1):9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katano K, Kondo A, Safaei R, et al. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002;62(22):6559–6565. [PubMed] [Google Scholar]
- 95.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA. 2002;99(22):14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rabik CA, Maryon EB, Kasza K, Shafer JT, Bartnik CM, Dolan ME. Role of copper transporters in resistance to platinating agents. Cancer Chemother Pharmacol. 2008;64(1):133–142. doi: 10.1007/s00280-008-0860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Larson CA, Blair BG, Safaei R, Howell SB. The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol. 2009;75(2):324–330. doi: 10.1124/mol.108.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blair BG, Larson CA, Safaei R, Howell SB. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of cisplatin and carboplatin. Clin Cancer Res. 2009;15(13):4312–4321. doi: 10.1158/1078-0432.CCR-09-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Samimi G, Safaei R, Katano K, et al. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin Cancer Res. 2004;10(14):4661–4669. doi: 10.1158/1078-0432.CCR-04-0137. [DOI] [PubMed] [Google Scholar]
- 100 ▪.Mangala LS, Zuzel V, Schmandt R, et al. Therapeutic targeting of ATP7B: reversing cisplatin resistance in ovarian carcinoma. Cancer Res Clin. 2009;15(11):3770–3780. doi: 10.1158/1078-0432.CCR-08-2306. Direct demonstration in vivo of the therapeutic effect of ATP7B downregulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101 ▪.Katano K, Safaei R, Samimi G, et al. Confocal microscopic analysis of the interaction between cisplatin and the copper transporter ATP7B in human ovarian carcinoma cells. Clin Cancer Res. 2004;10(13):4578–4588. doi: 10.1158/1078-0432.CCR-03-0689. Important demonstration of a link between cellular copper homeostasis and resistance to cisplatin. [DOI] [PubMed] [Google Scholar]
- 102.Safaei R, Otani S, Larson BJ, Rasmussen ML, Howell SB. Transport of cisplatin by the copper efflux transporter ATP7B. Mol Pharmacol. 2008;73(2):461–468. doi: 10.1124/mol.107.040980. [DOI] [PubMed] [Google Scholar]
- 103.Dolgova NV, Olson D, Lutsenko S, Dmitriev OY. The soluble metal-binding domain of the copper transporter ATP7B binds and detoxifies cisplatin. Biochem J. 2009;419(1):51–56. doi: 10.1042/BJ20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Owatari S, Akune S, Komatsu M, et al. Copper-transporting P-type ATPase, ATP7A, confers multidrug resistance and its expression is related to resistance to SN-38 in clinical colon cancer. Cancer Res. 2007;67(10):4860–4868. doi: 10.1158/0008-5472.CAN-06-3096. [DOI] [PubMed] [Google Scholar]