Abstract
The actions of dopamine D1 family receptors (D1R) depend upon a signal transduction cascade that modulates the phosphorylation state of important effector proteins, such as glutamate receptors and ion channels. This is accomplished both through activation of protein kinase A (PKA) and the inhibition of protein phosphatase-1 (PP1). Inhibition of PP1 occurs through PKA-mediated phosphorylation of DARPP-32 or the related protein inhibitor-1 (I-1), and the availability of DARPP-32 is essential to the functional outcome of D1R activation in the basal ganglia. While D1R activation is critical for prefrontal cortex (PFC) function, especially working memory, the functional role played by DARPP-32 or I-1 is less clear. In order to examine this more thoroughly, we have utilized immunoelectron microscopy to quantitatively determine the localization of DARPP-32 and I-1 in the neuropil of the rhesus monkey PFC. Both were distributed widely in the different components of the neuropil, but were enriched in dendritic shafts. I-1 label was more frequently identified in axon terminals than was DARPP-32, and DARPP-32 label was more frequently identified in glia than was I-1. We also quantified the extent to which these proteins were found in dendritic spines. DARPP-32 and I-1 were present in small subpopulations of dendritic spines, (4.4 and 7.7% and respectively), which were substantially smaller than observed for D1R in our previous studies (20%). Double-label experiments did not find evidence for colocalization of D1R and DARPP-32 or I-1 in spines or terminals. Thus, at the least, not all prefrontal spines which contain D1R also contain I-1 or DARPP-32, suggesting important differences in D1R signaling in the PFC compared to the striatum.
Keywords: electron microscopy, dendritic spines, D1, D5, protein phosphatase-1, dopamine
The functional effects of activating D1 or D5, the dopamine D1 family receptors (D1R), depend on a complex signal transduction pathway. The basic scheme of D1R signal transduction involves the coupling of D1R to Gαs to activate adenylyl cyclase (AC) and produce cyclic AMP (cAMP) which, in turn, activates protein kinase A (PKA). PKA phosphorylates a variety of substrate proteins, and it is the altered phosphorylation of key signaling proteins that accounts for much of the effects of D1R stimulation. The actions of PKA are opposed by a variety of phosphatases, including protein phosphatase-1 (PP1), a serine/threonine phosphatase (Huang and Glinsmann, 1976; Hemmings et al., 1984). PP1 can dephosphorylate many of the downstream effectors that PKA phosphorylates, including glutamate receptors (Snyder et al., 1998; Yan et al., 1999), GABAA receptors (Yan and Surmeier, 1997; Flores-Hernandez et al., 2000), calcium channels (Surmeier et al., 1995) and cAMP response element-binding protein (Genoux et al., 2002).
D1R stimulation not only activates PKA but also can inhibit PP1. This is accomplished by PKA-mediated phosphorylation of dopamine- and cAMP-regulated phosphoprotein, 32 kDa (DARPP-32) and possibly the related protein inhibitor-1 (I-1). When DARPP-32 is phosphorylated at threonine 34 (Thr-34), or I-1 is phosphorylated at threonine 35 (Thr-35), they become potent inhibitors of PP1 (Huang et al., 1976; Hemmings et al., 1984). Many of the functional consequences of D1R activation rely on DARPP-32 modulation of PP1. For example, D1R agonist prevents the “rundown” of AMPA receptor current in striatal neurons. This effect is mimicked by inhibition of PP1 with okadaic acid or phosphorylated DARPP-32 (Yan et al., 1999). D1R agonists increase the phosphorylation of the NMDA receptor NR1 subunit in the basal ganglia of wild-type but not DARPP-32 knockout mice (Snyder et al., 1998). Activation of D1R in neostriatal neurons results in phosphorylation of GABAA receptors and enhances GABAA currents, and this action is decreased in DARPP-32 knockout mice (Flores-Hernandez et al., 2000). These data suggest that DARPP-32 mediated inhibition of PP1 is necessary for D1R actions in the striatum. However, there is growing evidence that D1R signal transduction may differ in the prefrontal cortex (PFC). For example, DARPP-32 knockout mice show no alteration in D1R stimulation effects on pyramidal cell GABAA currents in mouse frontal cortex or in or dopamine-mediated increases in firing rate of fast-spiking interneurons in frontal cortex (Trantham-Davidson et al., 2004; Trantham-Davidson et al., 2008). Furthermore, in PFC pyramidal cells, D1R activation augments NMDA receptor activity but this is not dependent on the PKA pathway (Chen et al., 2004). These data indicate that D1R signaling in the PFC may differ from the basal ganglia in being less dependent on DARPP-32. However, whether I-1 is a substitute for DARPP-32 in cortical D1R signaling is currently unknown.
Studies of DARPP-32 and I-1 distribution have identified protein-specific and regional differences in their neuropil labeling. DARPP-32 labeling is intense in the basal ganglia but relatively weak in the PFC (Hemmings et al., 1992; Ouimet et al., 1992). This stark difference between basal ganglia and cortical labeling is not seen with I-1 (Gustafson et al., 1991; Hemmings et al., 1992). In the striatum, both DARPP-32 and I-1 are found in dendrites and spines of medium spiny projection neurons (Hemmings and Greengard, 1986; Gustafson et al., 1991; Hemmings et al., 1992; Ouimet et al., 1992), but neither has been observed in cholinergic- or neuropeptide Y-containing interneurons of the striatum (Anderson and Reiner, 1991; Gustafson et al., 1991; Reiner et al., 1998). In the cortex, both DARPP-32 and I-1 have been identified in spiny pyramidal projection neurons, but also in local circuit interneurons (Berger et al., 1990; Gustafson et al., 1991; Lowenstein et al., 1995; Trantham-Davidson et al., 2008). Interestingly, the sole electron microscopic (EM) examination of either protein in the cortex suggested that I-1 is almost exclusively localized to dendrites (Lowenstein et al., 1995). Furthermore, in the striatum DARPP-32 is extensively co-localized with the D1 dopamine receptor in medium spiny neurons (Ouimet et al., 1984; Walaas and Greengard, 1984; Bateup et al., 2008; Bertran-Gonzalez et al., 2008), but confocal microscopic examination of interneuron dendrites which demonstrated electrophysiological responses to D1R agents in mouse frontal cortex did not show DARPP-32 label (Trantham-Davidson et al., 2008). Together, these studies indicate that the localization of DARPP-32 and/or I-1 is different in the PFC than the striatum, and that these localization differences impact D1R signaling in the PFC. Understanding the precise localization of both proteins using quantitative immunoelectron microscopy will help to elucidate D1R signaling differences between striatum and PFC.
We have previously quantified the distributions of D1R subtypes and PP1 isoforms, as well as the extent to which prefrontal spines and axon terminals contain these proteins (Muly et al., 2001; Bordelon et al., 2005; Bordelon-Glausier et al., 2008) We undertook the present study to determine if, despite weak labeling observed in light microscopic studies, DARPP-32 is positioned to mediate D1R signaling in the PFC, and if not, whether I-1 is positioned to take over this function in the cortex. We found that both DARPP-32 and I-1 were most frequently identified in dendritic shafts, unlike the D1R and PP1 isoforms. Moreover, DARPP-32 and I-1 were only present in approximately 4% and 8% of area 9 pyramidal cell spines, respectively. Thus, while all D1R labeled spines also contain PP1 (Muly et al., 2001; Bordelon-Glausier et al., 2008), at least some do not contain I-1 or DARPP-32. These results add to the growing literature that D1R signal transduction in the PFC differs from that in the striatum.
Experimental Procedures
Animals and preparation of tissue
Tissue from six Macaca mulatta monkeys was used for this study. The care of the animals and all anesthesia and sacrifice procedures in this study were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University. The animal used for immunoblotting was sacrificed by pentobarbital overdose (100 mg/kg), and blocks of various brain regions were frozen. The animals used for light microscopy (LM) and EM analysis were sacrificed with an overdose of pentobarbital (100mg/kg) and then perfused with a flush of Tyrode’s solution. The flush was followed by 3 to 4 liters of fixative solution of 4% paraformaldehyde/0.1–0.2% glutaraldehyde/0–0.2% picric acid in phosphate buffer (0.1M, pH 7.4; PB). The brain was blocked and post-fixed in 4% paraformaldehyde for 2–24 hours. Coronal, 50 µm thick vibratome sections of prefrontal cortical area 9 (Walker, 1940) were cut and stored frozen at −80°C in 15% sucrose until immunohistochemical experiments were performed.
Antisera
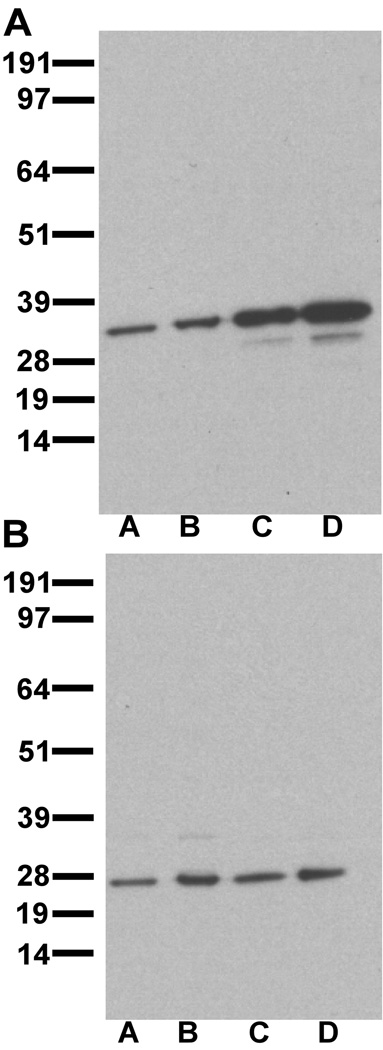
Three antibodies were used in these studies: mouse anti-DARPP-32, rabbit anti-inhibitor-1 and rat anti-D1 (Sigma-RBI, St. Louis, MO). The mouse anti-DARPP-32 antibody was raised against purified bovine DARPP-32 (Hemmings and Greengard, 1986), and does not produce labeling in DARPP-32 knock-out mice (Fienberg and Greengard, 2000). In monkey PFC and striatum, the DARPP-32 antibody labeled a predominant band of a molecular weight of approximately 32 kDa (Fig. 1A). The rabbit anti-inhibitor-1 antibody was raised against I-1 from rabbit skeletal muscle. This antibody does not recognize DARPP-32 on immunoblot, and I-1-immunoreactivity (-IR) is not seen in immunohistochemical reactions when the I-1 antibody is preadsorbed with I-1 protein (Gustafson et al., 1991). It does not produce labeling in I-1 knock-out mice (Allen et al., 2000). In monkey PFC and caudate, the I-1 antibody labels a major band at approximately 27–28 kDa (Fig. 1B), consistent with the molecular weight obtained in previous reports (Hemmings et al., 1992). The specificity and characterization of the rat anti-D1 antibody have been previously described (Hersch et al., 1995; Glausier et al., 2008).
Figure 1.
Immunoblots of DARPP-32 (A) and I-1 (B). A: DARPP-32 antibody stains a predominant band at approximately 32 kDa. B: I-1 antibody stains a predominant band at approximately 27–28 kDa. Lane A- 20 µg PFC protein, Lane B- 50µg PFC protein, Lane C- 5 µg striatum protein, Lane D- 10 µg striatum protein.
Immunoblotting
We performed immunoblotting, as described previously (Muly et al., 2004a) to assess the specificity of our antibodies to DARPP-32 and I-1. Briefly, samples of PFC and caudate from one male Macaca mulatta (aged 1.04 years) were dounce homogenized in buffer containing 140 mM KCl, 10 mM glucose, 1.2 mM MgCl2, 10mM HEPES, pH 7.4, with a cocktail of protease inhibitors added. The homogenate was centrifuged, the pellet discarded, and the supernatant was assayed for protein concentrations using a colormetric assay (Bio-Rad Laboratories, Hercules, CA). The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Each lane was loaded with protein sample, and the gel was run for 50 minutes at 200 V. The gel was then transferred to PVDF membrane. The membrane was rinsed, blocked, and probed with mouse anti-DARPP-32 (1:7,500) or rabbit anti-I-1 (1:2,000). After rinsing, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (HRP-goat anti-rabbit IgG, 1:10,000, Bio-Rad; or HRP-goat anti-mouse IgG, 1:20,000, Bio-Rad). Labeling was revealed by chemiluminescence. A ladder of markers was used to estimate the molecular weight of the labeled bands (SeeBlue plus 2, Invitrogen). Images of the immunoblots in TIFF format were imported into an image processing program (Canvas 8, Deneba, Miami, FL) where the image was cropped and labels were added.
Single-label immunohistochemistry
Single-label immunoperoxidase labeling was performed by using mouse anti-DARPP-32 at 1:12,000 dilution and rabbit anti-I-1 at 1:15,000 dilution. The single-label immunoperoxidase labeling was performed as described previously (Muly et al., 1998). Briefly, sections were thawed, incubated in blocking serum (3% normal goat serum, 1% bovine serum albumin, 0.1% glycine, 0.1% lysine in 0.01 M phosphate buffered saline, pH 7.4) for 1 hour and then placed in primary antiserum diluted in blocking serum. After 36 hours at 4°C, the sections were rinsed and placed in a 1:200 biotinylated donkey anti-mouse IgG (Jackson Immuno Research, West Grove, PA) or 1:200 dilution of biotinylated goat anti-rabbit IgG (Vector, Burlingame, CA) for 1 hour at room temperature. The sections were then rinsed, placed in avidin-biotinylated peroxidase complex (ABC Elite, Vector, Burlingame, CA) for 1 hour at room temperature, and then processed to reveal peroxidase using 3,3'-diaminobenzidine (DAB) as the chromogen. Sections for LM examination were then rinsed, mounted, dehydrated in xylene and ethanol and cover slipped. Sections for EM analysis were then post-fixed in osmium tetroxide, stained en bloc with uranyl acetate, dehydrated in ethanol and propylene oxide, and embedded in Durcupan resin (Electron Microscopy Sciences, Fort Washington, PA). Selected regions were mounted on blocks, and ultrathin sections were collected onto pioloform-coated slot grids and counterstained with lead citrate. Control sections processed as above except for the omission of the primary immunoreagent, did not contain DAB label upon examination.
Four Macaca mulatta monkeys in total were processed for DARPP-32 EM examination. Three of the four were female, and the age range was 2–5.25 years. Four Macaca mulatta monkeys in total were processed for I-1 EM examination. Two were male, and two were female; the age range was 2–5.25 years of age.
Double-label immunohistochemistry
Double-label immunoperoxidase labeling was performed by using mouse anti-DARPP-32 at 1:12,000, rabbit anti-I-1 at 1:15,000 and rat-anti D1 at 1:500. The colocalization of DARPP-32 and I-1, D1 and I-1, and D1 and DARPP-32 was examined using a standard pre-embedding immunogold/DAB protocol as described previously (Glausier et al., 2008). Two sets of experiments each using two monkeys were completed for each pair such that an antigen was revealed with immunogold in one set and DAB in the second set to eliminate any potential confounds resulting from differences in the sensitivity of immunogold and DAB.
Analysis of material
For LM examination, images were captured on a Leica DMRBE microscope using a Spot RT color digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI) and Simple PCI imaging software (version 5.3, Hamamatsu Corp., Sewickley, PA). Images in TIFF format were imported into an imaging processing program (Canvas 8, Deneba Software, Miami, FL). The contrast and brightness of the images was adjusted and labels were added.
For EM analysis, the single DAB-labeled material was analyzed as previously described (Muly et al., 2003). Blocks of tissue from layer III of cortical area 9 were made and cut in ultrathin sections that were examined using a Zeiss EM10C electron microscope. Layer III was chosen because both D1R subtypes are found in dendritic structures more frequently there (Bordelon-Glausier et al., 2008). Regions of the grids containing neuropil were selected based on the presence of label and adequate ultrastructural preservation. Fields of immunoreactive elements in the neuropil were randomly selected, and images were collected at a magnification of 31,500 using a Dualvision cooled CCD camera (1300 × 1030 pixels) and Digital Micrograph software (version 3.7.4, Gatan, Inc., Pleasanton, CA). For DARPP-32, a total of 273 micrographs were analyzed, representing 1,665 µm2. Four hundred forty-five profiles were counted, and each monkey contributed 105–117 profiles. For I-1, a total of 172 micrographs were analyzed, representing 1,050 µm2. Five hundred profiles were counted, and each monkey contributed 111–148 profiles. On each micrograph, DAB-labeled profiles were identified and classified as spines, dendrites, terminals, axons or glia based on ultrastructural criteria (Peters et al., 1991) as previously described (Muly et al., 2003). Profiles that could not be clearly characterized based on these criteria were considered unknown profiles. The number of immunoreactive profiles was tabulated and the distributions (excluding the unknown profiles) compared with a Chi-square analysis. All p-values are reported as Fisher’s exact p-value. The double-label material was analyzed as previously described (Glausier et al., 2008). Briefly, spines, terminals and dendrites containing immunogold label were imaged and then the presence or absence of DAB label was determined. For DARPP-32/I-1 double-label, 126 micrographs were analyzed, and a total of 160 micrographs were analyzed for D1/I-1 and D1/DARPP-32 double-label experiments.
To determine the percentage of spines which contain DARPP-32 or I-1, fields of neuropil were randomly selected, and images were collected at a magnification of 20,000. An ANOVA sample size analysis (SigmaStat, Version 2.03, SPSS Inc.) indicated that the minimum sample size required to have a statistical power of 80% and a minimal detectable difference in the two group means of 5 was 129 images; therefore 134 images from three monkeys in the DARPP-32 condition and 135 images from three monkeys in the I-1 condition were analyzed. In each experimental condition, the number of micrographs analyzed from each monkey was similar. On each micrograph, spines were identified using the previously described ultrastructural criteria (Peters et al., 1991), then classified as immunopositive or immunonegative. The mean percentage of immunopositive spines was tabulated for both conditions and compared using an ANOVA. The results are reported as mean ± standard error. The percentage of spines and axon terminals labeled for DARPP-32 and I-1 was compared to the percentage labeled by D1, D5, PP1α and PP1γ1. These data are from two previous studies from our laboratory (Muly et al., 2001; Bordelon-Glausier et al., 2008) in which the data were collected and analyzed in the same manner as described in the current study.
Results
Light microscopic distribution of I-1 and DARPP-32
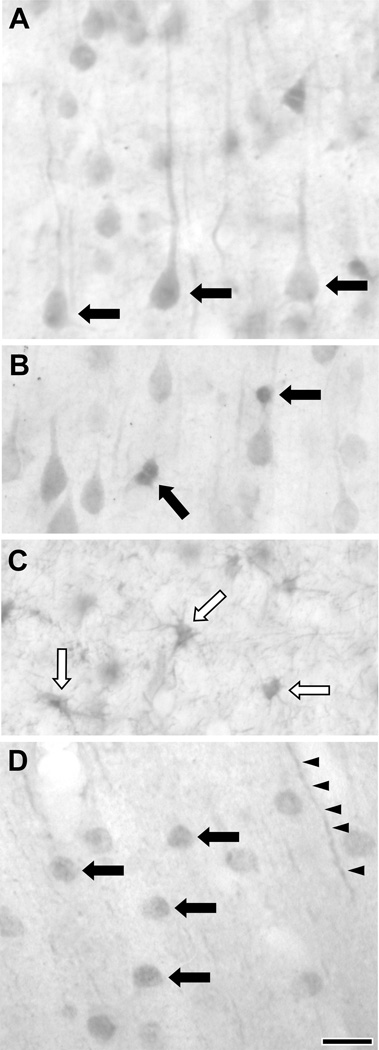
The localization of DARPP-32 has been previously characterized with light microscopy in the primate PFC (Berger et al., 1990). Briefly, DARPP-32-immunoreactive (-IR) cell bodies were generally pyramidal in shape, and DARPP-32-IR glia were identified. Our examination of DARPP-32 is consistent with this report. Somatodendritic labeling in pyramidal shaped neurons was observed in layers II-VI (Fig. 2A). Smaller, multipolar cells showing DARPP-32 immunoreactivity were also observed in all cortical layers (Fig. 2B). In addition, labeled glial cells were observed in the subcortical white matter (Fig. 2C), consistent with previous reports (Ouimet et al., 1992). The localization of I-1 in the rat, cat and ferret frontal cortex has been previously described at the LM level (Gustafson et al., 1991; Lowenstein et al., 1995). Each study found somewhat different distributions of I-1-IR cells, though both identified I-1 in pyramidal and non-pyramidal cells. We found that in monkey PFC, neuronal I-1 label was observed in layers II-VI. Cellular labeling appeared to be mainly in nuclei (Fig. 2D), making it difficult to determine if these were pyramidal or non-pyramidal neurons. However, labeled processes in the neuropil that appeared to be apical dendrites could be identified, suggesting that at least some of these labeled neurons were pyramidal cells. In addition to labeled processes, I-1 label was also seen in a light punctate pattern throughout the neuropil.
Figure 2.
Light microscopic images of DARPP-32 and I-1 labeling in layer III of the macaque PFC. A–C: DARPP-32 labeled pyramidal cell bodies (A, black arrows), as well as small multipolar cells (B, black arrows) in the grey matter. Glia were immunoreactive in the subcortical white matter as well (C, white arrows). D: I-1 label stained the nucleus of neurons (black arrows) and the PFC neuropil (black arrowheads). Scale bar is 20 µm.
Subcellular localization of I-1 and DARPP-32
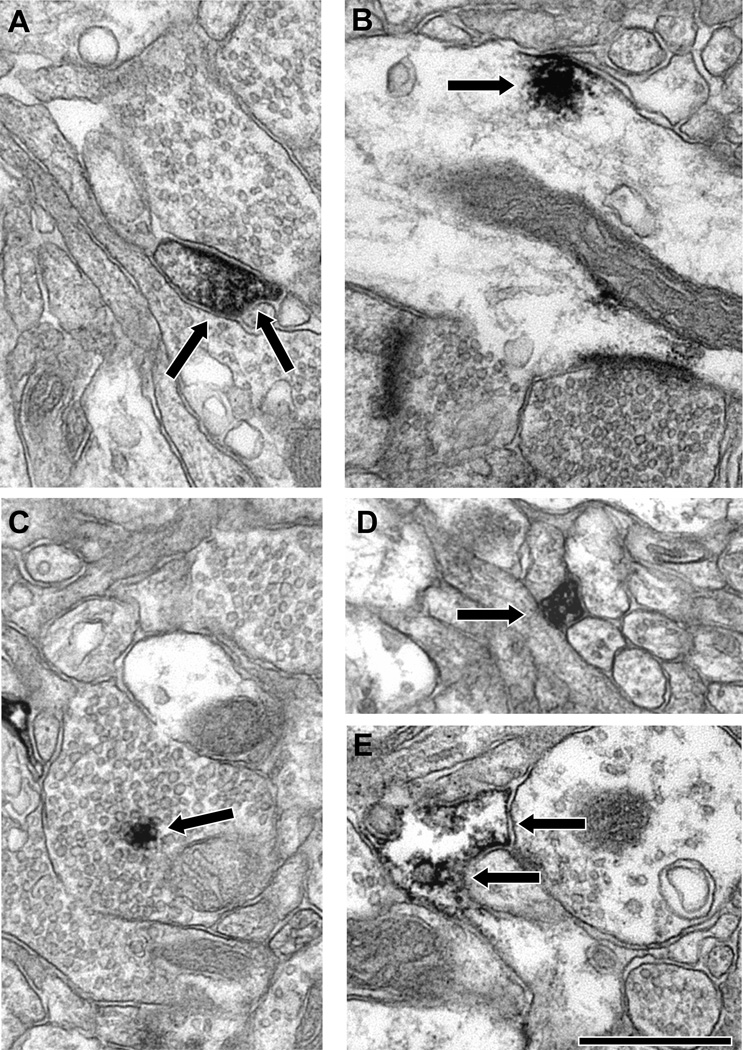
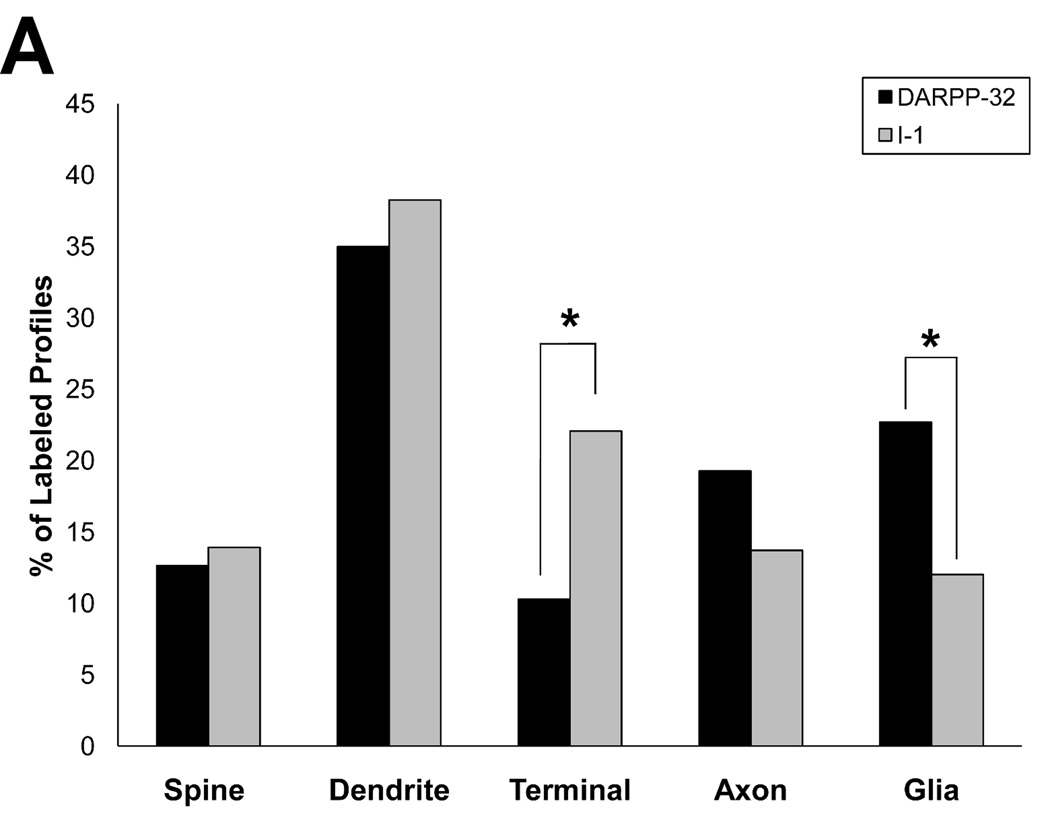
We next examined the localization of these two proteins in layer III neuropil using material prepared for EM examination. Both DARPP-32 (Fig. 3) and I-1 (Fig. 4) were widely distributed, being found in dendritic spines, dendritic shafts, axon terminals, pre-terminal axons and glia. Our qualitative impression was that dendritic shafts were the most commonly observed labeled element for both signaling proteins. In order to test this impression and to determine if the distributions of these two functionally related proteins differed, we quantified the neuropil distribution of DARPP-32 and I-1. We confirmed that dendrites are the most commonly labeled type of element for both DARPP-32 and I-1, and the overall distribution of both proteins did differ significantly (Fig. 5; χ2 = 41.237; p < 0.0001). Post-hoc testing revealed that a significantly larger proportion of I-1 labeled profiles were axon terminals (22.1%) relative to DARPP-32 (10.3%); and a significantly larger proportion of DARPP-32 labeled profiles were glia (22.7%) relative to I-1 (12.0%).
Figure 3.
Electron micrographs illustrating the localization of DARPP-32 in the neuropil of PFC area 9. DAB label (arrows) was identified in spines (A), dendritic shafts (B), axon terminals (C), pre-terminal axons (D) and glia (E). Both the labeled spine (A) and dendrite (B) are receiving asymmetric synapses. Scale bar is 500 nm in C and E and 750 nm in A, B and D.
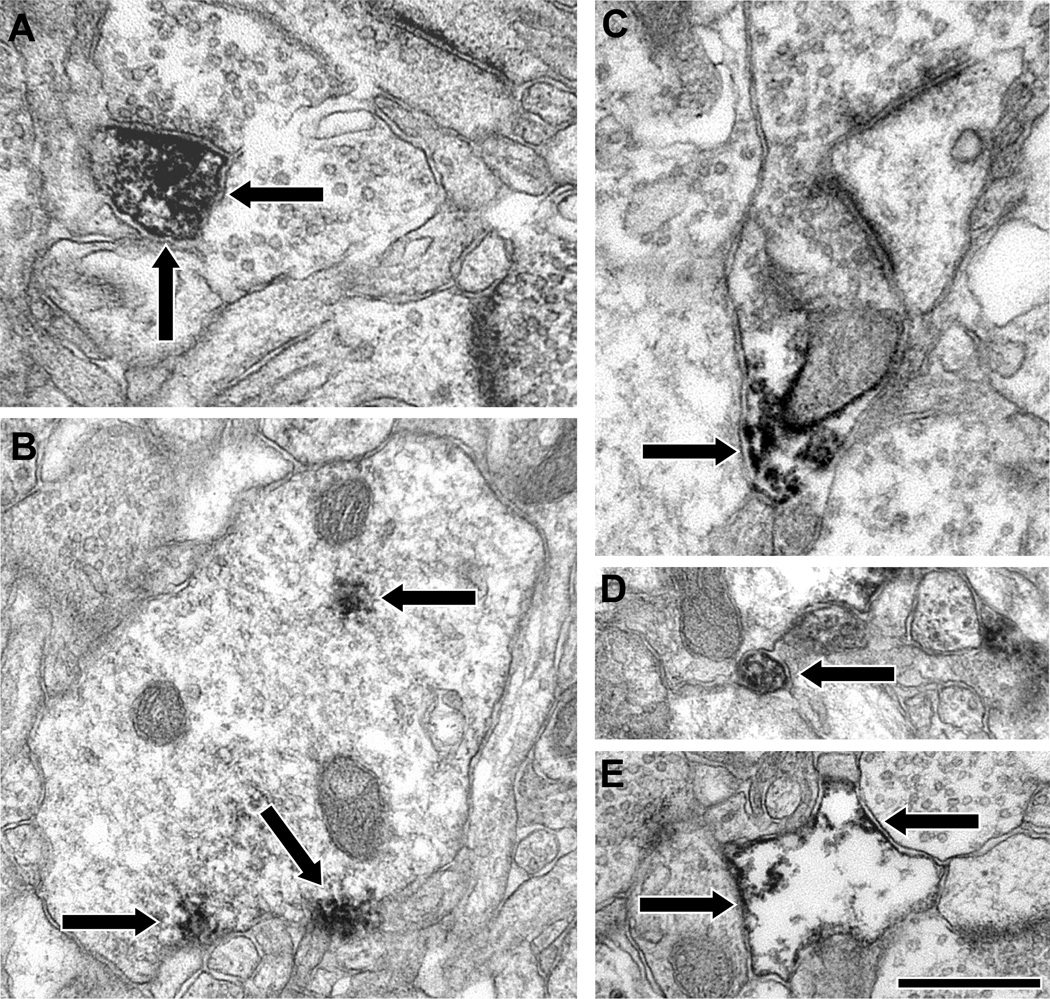
Figure 4.
Electron micrographs illustrating the localization of I-1 in the neuropil of PFC area 9. DAB label (arrows) was identified in spines (A), dendritic shafts (B), axon terminals (C), pre-terminal axons (D) and glia (E). The labeled spine in A is receiving an asymmetric synapse, and the labeled axon terminal in C is making a perforated asymmetric synapse. Scale bar is 500 nm for A, C and D and 750 nm for B and E.
Figure 5.
Histogram showing the relative abundance of labeled elements for DARPP-32 and I-1 in the neuropil of monkey PFC area 9. The distribution of immunoreactive profiles is significantly different between the proteins (χ2 = 41.237; p < 0.0001). Post hoc analysis revealed that a larger percentage of I-1 label is found in axon terminals, while DARPP-32 label is found more frequently in glia.
Distribution of I-1 and DARPP-32 in spines and terminals
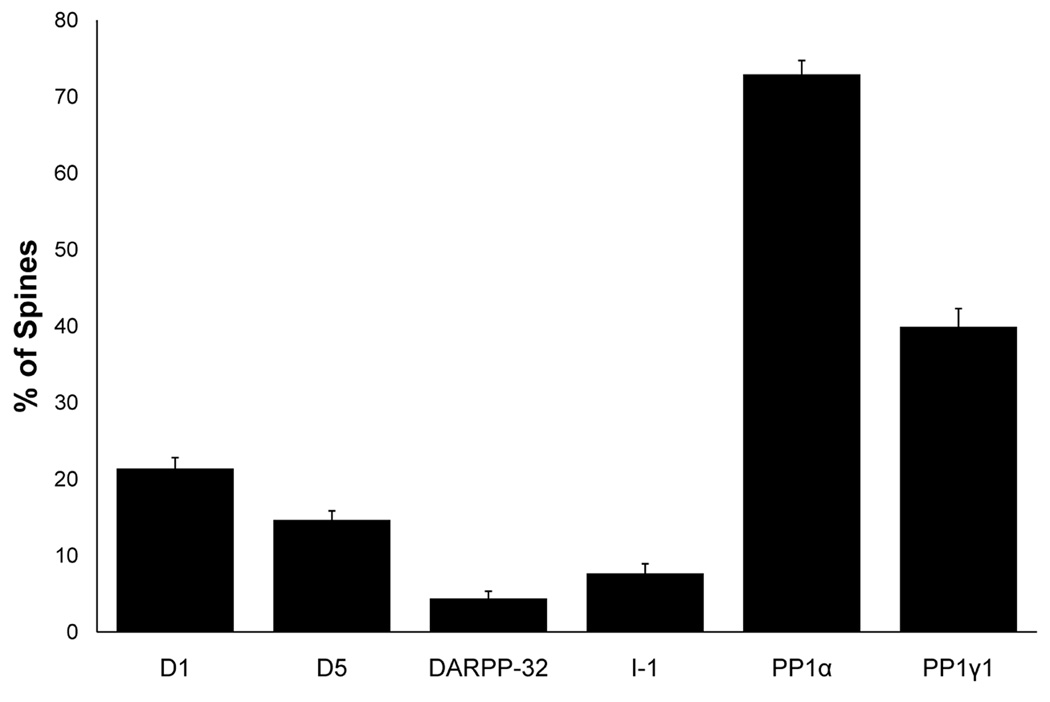
While dendritic shaft labeling predominated for both DARPP-32 and I-1, labeled spines were also observed. Because spines are the site of most excitatory synaptic inputs to pyramidal cells (Harris and Kater, 1994), we wanted to determine the percentage of spines which contain DARPP-32 or I-1 label to compare with the percentage of D1R and PP1 labeled spines (data previously published in Muly et al., 2001; Bordelon-Glausier et al., 2008). The vast majority of spines were not immunoreactive for either DARPP-32 or I-1, such that they only labeled 4.4% and 7.7% of spines, respectively. When compared to the percentage of spines labeled for D1, D5, PP1α or PP1γ1, four proteins that extensively co-localize in the spines of primate PFC (data previously published in Muly et al., 2001; Bordelon-Glausier et al., 2008), there was a main effect of protein type (F5,919 = 164.333, p < 0.001). Post-hoc Scheffe tests revealed that D1, D5, PP1α and PP1γ1 individually labeled more spines than either DARPP-32 or I-1 (Fig. 6).
Figure 6.
Histogram showing the percentage of area 9 pyramidal cell spines which contain label for D1, D5, DARPP-32, I-1, PP1α and PP1γ1 (previously published in Muly et al., 2001; Bordelon-Glausier et al., 2008). For DARPP-32, 134 micrographs containing 644 spines were analyzed, and 135 micrographs containing 624 spines were analyzed for I-1. The percentage of spines labeled by DARPP-32 (4.4% ± 0.938%) and I-1 (7.7%) ± 1.25%) was significantly less than the percentage labeled for D1, D5, PP1α and PP1γ1 (F5,979 = 164.333, p < 0.0001).
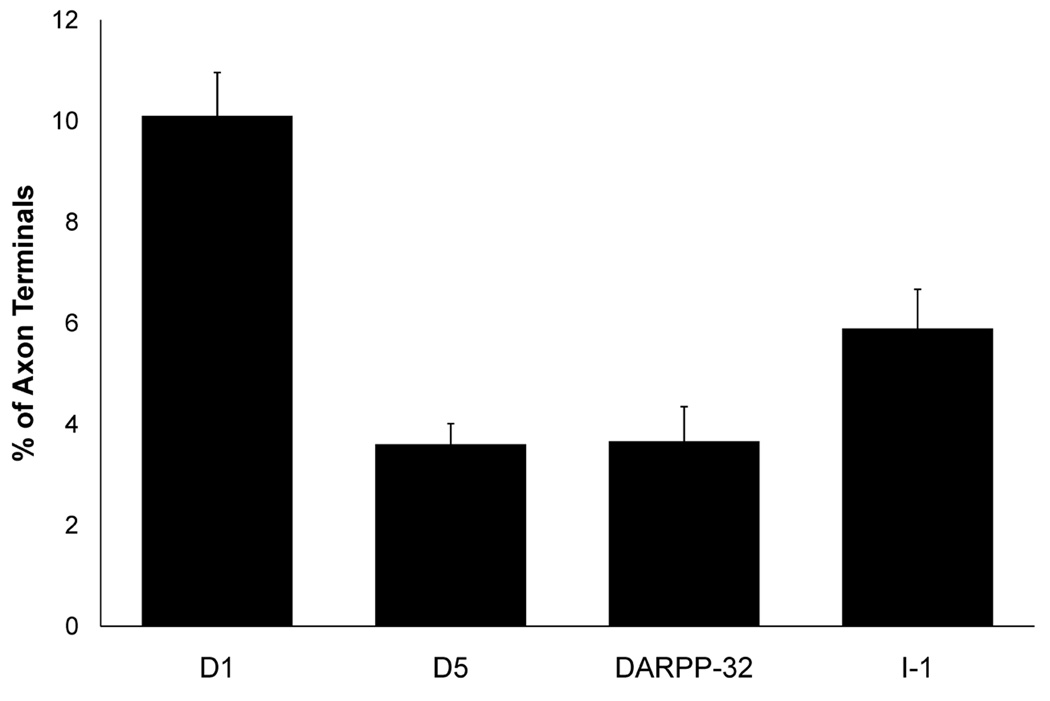
Within the neuropil, axon terminal labeling was also identified for DARPP-32 and I-1, and I-1 label was more frequently identified in axon terminals than DARPP-32 label. We wanted to determine the percentage of axon terminals which contain DARPP-32 or I-1 label to compare with the percentage labeled for D1 or D5 (Bordelon-Glausier et al., 2008). The vast majority of layer III area 9 axon terminals did not contain label for either DARPP-32 (3.7%) or I-1 (5.9%). When compared to D1 or D5, there was a main effect of protein type (F3,795 = 22.429, p < 0.0001). Post-hoc Scheffe tests revealed that D1 labeled significantly more axon terminals than DARPP-32 and I-1, but there was no difference in the percentage of terminals labeled for D5, DARPP-32 or I-1 (Fig. 7).
Figure 7.
Histogram showing the percentage of area 9 axon terminals which contain label for D1, D5, DARPP-32 and I-1 (previously published in Muly et al., 2001; Bordelon-Glausier et al., 2008). For DARPP-32, 134 micrographs containing 1,121 terminals were analyzed, and 135 micrographs containing 1,145 terminals were analyzed for I-1. The percentage of terminals labeled by DARPP-32 (3.7% ± 0.69%) and I-1 (5.9% ± 0.78%) was significantly less than the percentage labeled for D1, but not D5 (F3,795 = 22.429, p = 0.0001).
Colocalization of D1, DARPP-32 and I-1
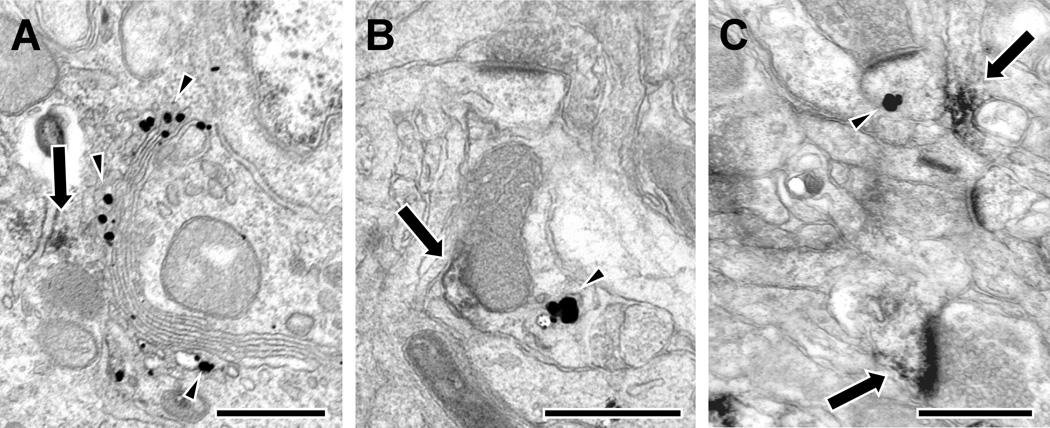
The impression from these analyses is that there is insufficient DARPP-32 or I-1 in dendritic spines or axon terminals to be available to all of the D1R found in those compartments. In order to more directly test this possibility, we used double-label EM to assess whether DARPP-32 and I-1 are colocalized with each other and with the D1 dopamine receptor. We examined DARPP-32 and I-1 relative to the D1 receptor subtype because our previous work has shown that the D5 subtype is less common in both spines and terminals and always found with D1 receptors (Bordelon-Glausier et al., 2008). Cell somata double-labeled for DARPP-32/I-1, D1/I-1 and D1/DARPP-32 (Fig. 8A) were identified, confirming that cortical neurons co-express these proteins. Examination of the neuropil found that 6% of 33 gold labeled dendritic shafts showed evidence of colocalization of DARPP-32 and I-1 (Fig. 8B), 13% of 15 gold labeled dendrites showed evidence of colocalization of D1 and DARPP-32, and 29% of 24 gold labeled dendrites showed evidence of colocalization of D1 and I-1. However, consistent with the immunoperoxidase analysis, DARPP-32 and I-1, D1 and DARPP-32, and D1 and I-1 were never colocalized in dendritic spines or axon terminals. Figure 8C illustrates a typical example of a single I-1 immunogold-labeled spine near a D1 DAB-labeled dendritic shaft and spine. Thus, DARPP-32 and I-1 are located in D1-containing neurons, but these proteins do not appear to be colocalized in dendritic spines or axon terminals.
Figure 8.
Electron micrographs illustrating selective colocalization of the dopamine D1 receptor, DARPP-32 and I-1in area 9 of monkey PFC. A: Cell soma double-labeled for DARPP-32 (DAB, arrows) and D1 (immunogold, arrowheads, found stereotypically in the Golgi apparatus). B: A dendritic shaft expressing both DARPP-32 (arrows) and I-1 (arrowheads). C: A dendritic spine and shaft single-labeled for D1 (arrows) near a separate dendritic spine single-labeled for I-1 (arrowheads). Scale bar is 500 nm.
Discussion
In the present study, we have determined that DARPP-32 and I-1 are distinctly localized in layer III neuropil of area 9 in the Macaca mulatta prefrontal cortex. Although both DARPP-32 and I-1 are potent inhibitors of PP1 when phosphorylated at Thr-34 and Thr-35, respectively (Hemmings et al., 1984), their localization in the PFC, hippocampus and amygdala (Barbas et al., 1993) and the cerebellum (Alder and Barbas, 1995) suggests that DARPP-32 and I-1 can perform distinct functions in regulating neuronal signaling. Indeed, functional differences between the two are beginning to be elucidated. For example, cyclin-dependent kinase 5 (Cdk5) can phosphorylate DARPP-32 at Thr-75, turning DARPP-32 into a PKA inhibitor (Bibb et al., 1999). However, Cdk5 phosphorylation of I-1 at serine (Ser) 6 or Ser-67 enhances PKA signaling (Nguyen et al., 2007). I-1 can also be phosphorylated by PKC at Ser-65, and phosphorylation at this site does not result in I-1 inhibition of PP1 activity (Sahin et al., 2006). Extracellular signal-related kinase (ERK) activation depends on DARPP-32, but not I-1 availability in the striatum (Valjent et al., 2005). Thus, the preferential availability of I-1 in axon terminals and DARPP-32 in glia may indicate different signaling capabilities in these compartments. There are also data suggesting functional differences regarding somatic functions, as supported by our LM observations. PP1 isoforms are located in the nucleus (Andreassen et al., 1998; Muly et al., 2001), and there is emerging evidence indicating a role for the PKA/I-1/PP1 pathway during mitotic exit (Wu et al., 2009).
Many receptors utilize the signal transduction pathway that includes I-1, DARPP-32 and PP1 (Svenningsson et al., 1998; Svenningsson et al., 2002a; Svenningsson et al., 2002b; Yi et al., 2005; Lhuillier et al., 2007), allowing them to modulate glutamatergic and GABAergic currents and calcium signaling (Wang et al., 1994; Westphal et al., 1999; Yan et al., 1999; Tang and Bezprozvanny, 2004; Terunuma et al., 2004). These studies have focused primarily on medium spiny neurons in the striatum and have emphasized the role of DARPP-32 in this signal transduction pathway. However, our results demonstrate that DARPP-32 and I-1 are located in far fewer prefrontal cortical spines (4% and 8% respectively) than PP1α or PP1γ1 (73% and 37% respectively) (Muly et al., 2001). These data suggest that DARPP-32 regulation of PP1 is a relatively minor phenomenon in pyramidal cell spines of the PFC, and that this regulation is not completely compensated for by I-1. One possibility is that PP1 in cortical spines is not subjected to inhibitory control as it is in the striatum. Alternately, PP1 may be regulated by some other currently uncharacterized phosphoprotein in the PFC (Walaas et al., 1983). Another established mechanism for regulating PP1 activity is through binding to scaffolding proteins. These scaffolding proteins, which include spinophilin, neurabin and yotiao, modulate PP1 activity generally and selectively target its activity to certain substrates (reviewed in Aggen et al., 2000; Cohen, 2002; Sarrouilhe et al., 2006). We have previously shown that the PP1 scaffolding proteins spinophilin and neurabin are found in the vast majority of prefrontal spines (Muly et al., 2004a; Muly et al., 2004b), and therefore are available to bind to and regulate PP1 activity and target proteins. There is also the possibility for activity-dependent changes in DARPP-32 and/or I-1 localization between dendritic shafts and spines, as observed for other signal transduction proteins (Muly et al., 2008). Thus, while DARPP-32 and I-1 are not constitutively available in large numbers of dendritic spines in PFC, there are a number of possible mechanisms for regulation of PP1 that will need to be explored.
D1R signaling is critical for PFC functioning (reviewed in Goldman-Rakic et al., 2000), and the molecular mechanism underlying the effects of D1R on working memory may include DARPP-32 regulation (Hotte et al., 2006). Previous studies in rodents identified relatively weak DARPP-32 and I-1 staining in the frontal cortex (Ouimet et al., 1984; Gustafson et al., 1991; Lowenstein et al., 1995), and a qualitative electron microscopic analysis of cat cortex reported I-1 labeling in dendrites but not spines (Lowenstein et al., 1995). In PFC spines, the D1R have access to both PP1α and PP1γ1 (Muly et al., 2001). However, data presented here demonstrate that the prevalence of I-1 and DARPP-32 in prefrontal spines is more restricted. At a maximum, 12% of PFC spines contain either I-1 or DARPP-32, while 20% contain D1 and/or D5 (Bordelon-Glausier et al., 2008). Furthermore, our double-label experiments suggest that DARPP-32 and I-1 are not typically found in spines with D1R. Therefore, D1R signaling in prefrontal spines must differ, at least partially, compared to the signal transduction scheme established in the striatum (reviewed in Greengard et al., 1999). In addition to possibly utilizing other phosphoproteins or specific targeting of PP1, D1R may also be signaling via other pathways in the spines which do not contain DARPP-32 or I-1. D1R has been shown to couple to the ERK cascade (Runyan and Dash, 2004; Nagai et al., 2007) or via the PKC/Ca2+/calmodulin (CaM) cascade (Nishi et al., 1999; Chen et al., 2004; Chen et al., 2007). Such signaling may allow D1R in prefrontal spines to have effects despite the relative lack of DARPP-32 or I-1. For example, D1R do not require DARPP-32 when signaling via the ERK cascade in the PFC (Valjent et al., 2005), and D1R mediated long-term potentiation of intrinsic excitability of PFC pyramidal cells utilizes the PKC/Ca2+/CaM cascade (Chen et al., 2007). These data, combined with the present study suggest that the D1R may utilize different signal transduction pathways in the PFC than those which have been determined in the striatum.
While dendritic spines are a potential site for DARPP-32 and I-1 to mediate D1R effects on glutamatergic inputs to pyramidal cells of the PFC (Goldman-Rakic et al., 1989; Krimer et al., 1997; Muly et al., 2001; Bordelon-Glausier et al., 2008), both DARPP-32 and I-1 were primarily identified in dendritic shafts, colocalized with each other and the D1 receptor. Both D1R subtypes have been identified in the dendritic shafts of pyramidal cells and interneurons (Bergson et al., 1995; Bordelon-Glausier et al., 2008; Glausier et al., 2008). D1R located on pyramidal cell dendritic shafts would be well positioned to modulate voltage gated calcium channels (Yang and Seamans, 1996; Seamans et al., 1997) or potassium channels (Yang and Seamans, 1996; Dong and White, 2003) which are important for integrating inputs to pyramidal cells (Seamans and Yang, 2004). D1R in dendritic shafts would have access to a signal transduction pathway including DARPP-32 and I-1, as well as PP1β (Bordelon et al., 2005). Further work will be required to determine which cellular populations’ dendrites express DARPP-32 and I-1 and which of DIR’s actions in the PFC are dependent on PP1 and PP1 inhibition by DARPP-32 or I-1.
Acknowledgments
This work was supported by MH076372 from NIMH to JRBNS56315 from NIH to HCHDA10044 from NIH to ACNMH074866 from NIMH to ACN and PG; RR00165 from NIH, a Merit Award from the Office of Research and Development, Department of Veterans Affairs, a University Research Committee grant from Emory University and MH068789 from NIMH to ECM.
List of abbreviations
- D1R
Dopamine D1 family receptors
- AC
Adenylyl cyclase
- PKA
Protein kinase A
- PP1
Protein phosphatase 1
- I-1
Inhibitor-1
- cAMP
Cyclic AMP
- DARPP-32
Dopamine- and cAMP-regulated phosphoprotein, 32 kDa
- PFC
Prefrontal cortex
- GABA
Gamma-Aminobutyric acid
- Thr
Threonine
- Ser
Serine
- AMPA
Alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- NMDA
N-methyl-D-aspartic acid
- IR
Immunoreactivity
- PVDF
Polyvinylidene fluoride
- HRP
Horseradish peroxidase
- DAB
3,3’-diaminobenzidine
- EM
Electron microscopy
- LM
Light microscopy
- ANOVA
Analysis of variance
- kDa
KiloDalton
- Cdk5
Cyclin-dependent kinase 5
- ERK
Extracellular signal-related kinase
- LTP
Long term potentiation
- CaM
Calmodulin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggen JB, Nairn AC, Chamberlin R. Regulation of protein phosphatase-1. Chem Biol. 2000;7:R13–R23. doi: 10.1016/s1074-5521(00)00069-7. [DOI] [PubMed] [Google Scholar]
- Alder R, Barbas H. Complementary distribution of the phosphoproteins DARPP-32 and I-1 in the cerebellar system. Neuroreport. 1995;6:2368–2372. doi: 10.1097/00001756-199511270-00022. [DOI] [PubMed] [Google Scholar]
- Allen PB, Hvalby O, Jensen V, Errington ML, Ramsay M, Chaudhry FA, Bliss TV, Storm-Mathisen J, Morris RG, Andersen P, Greengard P. Protein phosphatase-1 regulation in the induction of long-term potentiation: heterogeneous molecular mechanisms. J Neurosci. 2000;20:3537–3543. doi: 10.1523/JNEUROSCI.20-10-03537.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KD, Reiner A. Immunohistochemical localization of DARPP-32 in striatal projection neurons and striatal interneurons: implications for the localization of D1-like dopamine receptors on different types of striatal neurons. Brain Res. 1991;568:235–243. doi: 10.1016/0006-8993(91)91403-n. [DOI] [PubMed] [Google Scholar]
- Andreassen PR, Lacroix FB, Villa-Moruzzi E, Margolis RL. Differential subcellular localization of protein phosphatase-1 alpha, gamma1, and delta isoforms during both interphase and mitosis in mammalian cells. J Cell Biol. 1998;141:1207–1215. doi: 10.1083/jcb.141.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Gustafson EL, Greengard P. Comparison of the immunocytochemical localization of DARPP-32 and I-1 in the amygdala and hippocampus of the rhesus monkey. J Comp Neurol. 1993;334:1–18. doi: 10.1002/cne.903340102. [DOI] [PubMed] [Google Scholar]
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci. 2008;11:932–939. doi: 10.1038/nn.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Febvret A, Greengard P, Goldman-Rakic PS. DARPP-32, a phosphoprotein enriched in dopaminoceptive neurons bearing dopamine D1 receptors: distribution in the cerebral cortex of the newborn and adult rhesus monkey. J Comp Neurol. 1990;299:327–348. doi: 10.1002/cne.902990306. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Bordelon-Glausier JR, Khan ZU, Muly EC. Quantification of D1 and D5 dopamine receptor localization in layers I, III, and V of Macaca mulatta prefrontal cortical area 9: coexpression in dendritic spines and axon terminals. J Comp Neurol. 2008;508:893–905. doi: 10.1002/cne.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordelon JR, Smith Y, Nairn AC, Colbran RJ, Greengard P, Muly EC. Differential Localization of Protein Phosphatase-1{alpha}, {beta} and {gamma} 1 Isoforms in Primate Prefrontal Cortex. Cereb Cortex. 2005 doi: 10.1093/cercor/bhi070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Greengard P, Yan Z. Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:2596–2600. doi: 10.1073/pnas.0308618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bohanick JD, Nishihara M, Seamans JK, Yang CR. Dopamine D1/5 receptor-mediated long-term potentiation of intrinsic excitability in rat prefrontal cortical neurons: Ca2+-dependent intracellular signaling. J Neurophysiol. 2007;97:2448–2464. doi: 10.1152/jn.00317.2006. [DOI] [PubMed] [Google Scholar]
- Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Dong Y, White FJ. Dopamine D1-class receptors selectively modulate a slowly inactivating potassium current in rat medial prefrontal cortex pyramidal neurons. J Neurosci. 2003;23:2686–2695. doi: 10.1523/JNEUROSCI.23-07-02686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg AA, Greengard P. The DARPP-32 knockout mouse. Brain Res Brain Res Rev. 2000;31:313–319. doi: 10.1016/s0165-0173(99)00047-8. [DOI] [PubMed] [Google Scholar]
- Flores-Hernandez J, Hernandez S, Snyder GL, Yan Z, Fienberg AA, Moss SJ, Greengard P, Surmeier DJ. D(1) dopamine receptor activation reduces GABA(A) receptor currents in neostriatal neurons through a PKA/DARPP-32/PP1 signaling cascade. J Neurophysiol. 2000;83:2996–3004. doi: 10.1152/jn.2000.83.5.2996. [DOI] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Khan ZU, Muly EC. Dopamine D1 and D5 Receptors Are Localized to Discrete Populations of Interneurons in Primate Prefrontal Cortex. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci U S A. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Girault JA, Hemmings HC, Jr, Nairn AC, Greengard P. Immunocytochemical localization of phosphatase inhibitor-1 in rat brain. J Comp Neurol. 1991;310:170–188. doi: 10.1002/cne.903100204. [DOI] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Girault JA, Nairn AC, Bertuzzi G, Greengard P. Distribution of protein phosphatase inhibitor-1 in brain and peripheral tissues of various species: comparison with DARPP-32. J Neurochem. 1992;59:1053–1061. doi: 10.1111/j.1471-4159.1992.tb08347.x. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distribution. J Neurosci. 1986;6:1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P, Tung HY, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotte M, Thuault S, Lachaise F, Dineley KT, Hemmings HC, Nairn AC, Jay TM. D1 receptor modulation of memory retrieval performance is associated with changes in pCREB and pDARPP-32 in rat prefrontal cortex. Behav Brain Res. 2006;171:127–133. doi: 10.1016/j.bbr.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Huang FL, Glinsmann WH. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur J Biochem. 1976;70:419–426. doi: 10.1111/j.1432-1033.1976.tb11032.x. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Jakab RL, Goldman-Rakic PS. Quantitative three-dimensional analysis of the catecholaminergic innervation of identified neurons in the macaque prefrontal cortex. J Neurosci. 1997;17:7450–7461. doi: 10.1523/JNEUROSCI.17-19-07450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhuillier L, Mombereau C, Cryan JF, Kaupmann K. GABA(B) receptor-positive modulation decreases selective molecular and behavioral effects of cocaine. Neuropsychopharmacology. 2007;32:388–398. doi: 10.1038/sj.npp.1301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein PR, Shering AF, MacDougall LK, Cohen P. Immunolocalisation of protein phosphatase inhibitor-1 in the cerebral cortex of the rat, cat and ferret. Brain Res. 1995;676:80–92. doi: 10.1016/0006-8993(95)00091-4. [DOI] [PubMed] [Google Scholar]
- Muly EC, Allen P, Mazloom M, Aranbayeva Z, Greenfield AT, Greengard P. Subcellular distribution of neurabin immunolabeling in primate prefrontal cortex: comparison with spinophilin. Cereb Cortex. 2004a;14:1398–1407. doi: 10.1093/cercor/bhh101. [DOI] [PubMed] [Google Scholar]
- Muly EC, Greengard P, Goldman-Rakic PS. Distribution of protein phosphatases-1 alpha and −1 gamma 1 and the D(1) dopamine receptor in primate prefrontal cortex: Evidence for discrete populations of spines. J Comp Neurol. 2001;440:261–270. doi: 10.1002/cne.1384. [DOI] [PubMed] [Google Scholar]
- Muly EC, Maddox M, Smith Y. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol. 2003;467:521–535. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- Muly EC, Nairn AC, Greengard P, Rainnie DG. Subcellular distribution of the Rho-GEF Lfc in primate prefrontal cortex: effect of neuronal activation. J Comp Neurol. 2008;508:927–939. doi: 10.1002/cne.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muly EC, Smith Y, Allen P, Greengard P. Subcellular distribution of spinophilin immunolabeling in primate prefrontal cortex: localization to and within dendritic spines. J Comp Neurol. 2004b;469:185–197. doi: 10.1002/cne.11001. [DOI] [PubMed] [Google Scholar]
- Muly EC, Szigeti K, Goldman-Rakic PS. D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization. J Neurosci. 1998;18:10553–10565. doi: 10.1523/JNEUROSCI.18-24-10553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, Nakanishi Y, Murai M, Mizoguchi H, Nabeshima T, Yamada K. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem. 2007;14:117–125. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, Nishi A, Kansy JW, Fernandez J, Hayashi K, Gillardon F, Hemmings HC, Jr, Nairn AC, Bibb JA. Regulation of protein phosphatase inhibitor-1 by cyclin-dependent kinase 5. J Biol Chem. 2007;282:16511–16520. doi: 10.1074/jbc.M701046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Fisone G, Snyder GL, Dulubova I, Aperia A, Nairn AC, Greengard P. Regulation of Na+, K+-ATPase isoforms in rat neostriatum by dopamine and protein kinase C. J Neurochem. 1999;73:1492–1501. doi: 10.1046/j.1471-4159.1999.0731492.x. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, LaMantia AS, Goldman-Rakic P, Rakic P, Greengard P. Immunocytochemical localization of DARPP-32, a dopamine and cyclic-AMP-regulated phosphoprotein, in the primate brain. J Comp Neurol. 1992;323:209–218. doi: 10.1002/cne.903230206. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Hemmings HC, Jr, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The fine structure of the nervous system: neurons and their supporting cells. New York: Oxford University Press; 1991. [Google Scholar]
- Reiner A, Perera M, Paullus R, Medina L. Immunohistochemical localization of DARPP32 in striatal projection neurons and striatal interneurons in pigeons. J Chem Neuroanat. 1998;16:17–33. doi: 10.1016/s0891-0618(98)00056-8. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiol Learn Mem. 2004;82:65–70. doi: 10.1016/j.nlm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Sahin B, Shu H, Fernandez J, El-Armouche A, Molkentin JD, Nairn AC, Bibb JA. Phosphorylation of protein phosphatase inhibitor-1 by protein kinase C. J Biol Chem. 2006;281:24322–24335. doi: 10.1074/jbc.M603282200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrouilhe D, di Tommaso A, Metaye T, Ladeveze V. Spinophilin: from partners to functions. Biochimie. 2006;88:1099–1113. doi: 10.1016/j.biochi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova NA, Yang CR. Contributions of voltage-gated Ca2+ channels in the proximal versus distal dendrites to synaptic integration in prefrontal cortical neurons. J Neurosci. 1997;17:5936–5948. doi: 10.1523/JNEUROSCI.17-15-05936.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Rognoni F, Fredholm BB, Greengard P, Fisone G. Activation of adenosine A2A and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARPP-32 in distinct populations of striatal projection neurons. Neuroscience. 1998;84:223–228. doi: 10.1016/s0306-4522(97)00510-1. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Liu F, Fienberg AA, Nomikos GG, Greengard P. DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc Natl Acad Sci U S A. 2002a;99:3188–3193. doi: 10.1073/pnas.052712699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Witkin JM, Fienberg AA, Nomikos GG, Greengard P. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proc Natl Acad Sci U S A. 2002b;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Bezprozvanny I. Dopamine receptor-mediated Ca(2+) signaling in striatal medium spiny neurons. J Biol Chem. 2004;279:42082–42094. doi: 10.1074/jbc.M407389200. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Jang IS, Ha SH, Kittler JT, Kanematsu T, Jovanovic JN, Nakayama KI, Akaike N, Ryu SH, Moss SJ, Hirata M. GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J Neurosci. 2004;24:7074–7084. doi: 10.1523/JNEUROSCI.1323-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Kroner S, Seamans JK. Dopamine modulation of prefrontal cortex interneurons occurs independently of DARPP-32. Cereb Cortex. 2008;18:951–958. doi: 10.1093/cercor/bhm133. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–10659. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3’:5’-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Regional and cellular distribution in the rat brain. J Neurosci. 1984;4:84–98. doi: 10.1523/JNEUROSCI.04-01-00084.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas SI, Nairn AC, Greengard P. Regional distribution of calcium- and cyclic adenosine 3’:5’-monophosphate-regulated protein phosphorylation systems in mammalian brain. II. Soluble systems. J Neurosci. 1983;3:302–311. doi: 10.1523/JNEUROSCI.03-02-00302.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol. 1940;73:59–86. [Google Scholar]
- Wang LY, Orser BA, Brautigan DL, MacDonald JF. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature. 1994;369:230–232. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- Wu JQ, Guo JY, Tang W, Yang CS, Freel CD, Chen C, Nairn AC, Kornbluth S. PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat Cell Biol. 2009;11:644–651. doi: 10.1038/ncb1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron. 1997;19:1115–1126. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V-VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KD, Chung J, Pang P, Simpkins JW. Role of protein phosphatases in estrogen-mediated neuroprotection. J Neurosci. 2005;25:7191–7198. doi: 10.1523/JNEUROSCI.1328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]