Abstract
Summary: Blastomycosis is endemic in regions of North America that border the Great Lakes and the St. Lawrence River, as well as in the Mississippi River and Ohio River basins. Men are affected more often than women and children because men are more likely to participate in activities that put them at risk for exposure to Blastomyces dermatitidis. Human infection occurs when soil containing microfoci of mycelia is disturbed and airborne conidia are inhaled. If natural defenses in the alveoli fail to contain the infection, lymphohematogenous dissemination ensues. Normal host responses generate a characteristic pyogranulomatous reaction. The most common sites of clinical disease are the lung and skin; osseous, genitourinary, and central nervous system manifestations follow in decreasing order of frequency. Blastomycosis is one of the great mimickers in medicine; verrucous cutaneous blastomycosis resembles malignancy, and mass-like lung opacities due to B. dermatitidis often are confused with cancer. Blastomycosis may be clinically indistinguishable from tuberculosis. Diagnosis is based on culture and direct visualization of round, multinucleated yeast forms that produce daughter cells from a single broad-based bud. Although a long course of amphotericin B is usually curative, itraconazole is also highly effective and is the mainstay of therapy for most patients with blastomycosis.
INTRODUCTION
The thermally dimorphic fungus Blastomyces dermatitidis causes blastomycosis, one of the mycoses that is endemic in North America. This illness previously was called Gilchrist's disease in honor of the man who first recognized it in Baltimore, MD, in 1894 (51). Early descriptions emphasized the cutaneous manifestations, so blastomycosis was considered primarily a localized dermatologic condition rather than a systemic infection. Initially, Gilchrist mistakenly attributed the infection to a protozoan microorganism, but later he and Stokes identified the causative fungus (52). Blastomycosis has been known as North American blastomycosis in order to distinguish it from South American blastomycosis (paracoccidioidomycosis), but this terminology was abandoned upon the realization that blastomycosis is not restricted to North America. Chicago disease is another obsolete synonym for it.
B. dermatitidis, the asexual (imperfect) form of Ajellomyces dermatitidis, exists as a mold in nature and when grown in the laboratory at room temperature. Conidia become airborne when mycelia are disturbed. Humans inhale conidia into the alveoli, where the conidia undergo transition to yeasts, the forms that are seen in mammalian tissue and in cultures at 37°C. The transition from the mycelial phase to the yeast phase is associated with a heat-related partial uncoupling of oxidative phosphorylation (82).
The clinical manifestations of infection with B. dermatitidis are protean, and blastomycosis may mimic other, more common diseases. The greatest challenge in making the diagnosis of blastomycosis is considering it in the first place. Although the disease can be fatal, most patients are cured with antifungal therapy.
This review focuses on the clinical, diagnostic, and therapeutic aspects of blastomycosis.
EPIDEMIOLOGY AND ECOLOGY OF B. DERMATITIDIS
The epidemiology of blastomycosis is described less thoroughly than the epidemiologies of the other endemic mycoses of North America, coccidioidomycosis and histoplasmosis. The absence of sensitive and specific serologic assays and the use of inadequate skin test reagents have hindered efforts to fully define the epidemiology of blastomycosis, the understanding of which is based primarily upon case series. Perhaps more importantly, B. dermatitidis has been isolated only infrequently from environmental sources, and thus the ecology of B. dermatitidis remains incompletely understood.
Blastomycosis is predominantly a disease of North America. It is endemic in southern and southeastern states that border the Ohio River and Mississippi River valleys of the United States, as well as in Midwestern states and Canadian provinces that border the Great Lakes and the Saint Lawrence Riverway. Historically, most reported cases occurred in Arkansas, Kentucky, Mississippi, North Carolina, Tennessee, Louisiana, Illinois, and Wisconsin (19). Areas in which the disease is hyperendemic exist in north-central Wisconsin and the northern region of Ontario, Canada (9, 85). In Vilas County in north-central Wisconsin, where the annual incidence is 40 cases/100,000 people, residence near a waterway is associated with an increased risk of blastomycosis (9). In this study, one-third of patients who owned a dog reported that at least one of their dogs was diagnosed with blastomycosis, usually during the 6 months before symptoms began in the human cases, reinforcing the concept that canine blastomycosis can be a harbinger of disease in humans, as had been described earlier (108). In urban Milwaukee County in southeastern Wisconsin, where the incidence of blastomycosis is much less than the disease incidence in rural areas, cases occur predominantly among people who live in watershed areas with open waterways (10).
The incidence of blastomycosis appears to be increasing in some areas. For example, in Illinois, where blastomycosis is a reportable disease, 94 cases were reported to the Illinois Department of Public Health in 2004 (12). Between 1993 and 2003, a total of 500 cases were reported, with the majority of cases coming from the northeastern part of the state (44). In almost half of these cases, symptoms first appeared between January and April (44). Cold weather seasonality in Wisconsin, Manitoba (Canada), and Ontario has been described as well (9, 36, 85). In Ontario, 309 cases were reported between 1994 and 2003, with the majority being recognized from 2001 to 2003 (85). Blastomycosis is being reported more frequently in Missouri, particularly in southeastern Mississippi County, where the incidence was 12 cases/100,000 people, with higher rates among African-Americans, from 1992 to 1999 (24). Outside the traditionally recognized areas of disease endemicity, two male coworkers developed blastomycosis, presumably as a consequence of exposure to contaminated soil during their work on a prairie dog relocation project on the eastern slope of the Rocky Mountains in Colorado (37).
Data from the 2002 Nationwide Inpatient Sample show that 703 adults and children with blastomycosis were hospitalized in U.S. hospitals in 2002 (30). The majority of hospitalizations occurred in the Midwest and the South (6.07 cases/1 million people and 3.10 cases/1 million people, respectively) (30).
All of the epidemiology described above is based on the recognition and reporting of symptomatic cases. Unlike Histoplasma capsulatum, for which skin testing reliably provided a clear picture of the prevalence and geographic distribution of human exposure, including that which is asymptomatic, knowledge regarding the epidemiology of sporadic, asymptomatic infection with B. dermatitidis is lacking. One study that used an antigen-specific lymphocyte stimulation assay identified positive responses in 30% of forestry workers in northern Minnesota and northern Wisconsin (118).
Authentic, autochthonous cases of blastomycosis also occur in widely dispersed regions of Africa, with the greatest number originating from southern Africa, specifically South Africa and Zimbabwe (6, 48). Autochthonous infections have also been acquired in India (38). When blastomycosis occurs in patients residing in parts of the world where B. dermatitidis is not thought to exist, three scenarios should be considered. First, physicians who are not familiar with the disease might be wrong about the diagnosis. Second, B. dermatitidis can be transferred via fomites from an area where it exists in nature to an area where it is not endemic, where infection may then occur. Lastly, disease can be due to endogenous reactivation after a person has moved from an area of endemicity to an area of nonendemicity (38).
Although the majority of cases are sporadic or endemic, epidemics of blastomycosis associated with exposure to a common outdoor source are documented, the most important of which, in terms of understanding the ecology of B. dermatitidis, occurred in Wisconsin in 1984 (64). Klein and colleagues were the first investigators to isolate B. dermatitidis from the soil in conjunction with a human outbreak, which involved 48 cases of blastomycosis among schoolchildren and adults who had visited a beaver dam in Eagle River, WI, in southeastern Vilas County (64). During investigation of another epidemic, this group again isolated the fungus from environmental sources (66). It is noteworthy that a subsequent study that examined a geographically diverse collection of B. dermatitidis isolates with a typing system based on PCR found that the soil isolates from Eagle River were different from the strains that caused the majority of cases (81). Nevertheless, evidence suggests that the ecological niche for B. dermatitidis is wet earth that has been enriched with animal droppings, rotting wood, and other decaying vegetable matter.
Disruption of wet soil or organic matter containing microfoci of B. dermatitidis mycelia releases infectious conidia, which are subsequently inhaled by a susceptible host. By far, the most significant route of transmission for B. dermatitidis is inhalation of airborne conidia. Less commonly, direct cutaneous inoculation via a penetrating outdoor injury, a laboratory accident, or even the bite of an infected dog occurs (54). B. dermatitidis is not transmitted from animals to humans otherwise. Shared environmental exposures explain the occurrence of disease in humans and their canine companions (108). Outside of rare instances of conjugal and intrauterine transmission (35, 119), B. dermatitidis is not transmitted from person to person, and therefore, blastomycosis is generally not contagious.
Among endemic cases, the typical American patient with blastomycosis is a middle-aged man who participates in outdoor recreational or occupational activities (18). As with the epidemics mentioned previously, endemic cases often involve exposures that occur in close proximity to bodies of freshwater. It is unclear whether this association represents a true causal link with regard to transmission risk or whether it is simply that many popular outdoor activities involve water (15). Residence near an open body of water in an area of endemicity may be at least as important a risk factor as occupational exposure (9, 10).
Of the 135 patients referred to the University of Arkansas for Medical Sciences in Little Rock, AR, for treatment of blastomycosis between 1982 and 1994, 78 (58%) were men (19). This is similar to more-recent data from Manitoba and Ontario (36, 85). The fact that men are more likely than women to participate in activities that are associated with exposure to B. dermatitidis (e.g., hunting, fishing, or forestry work) best explains the predominance of adult males having this disease. In addition, epidemiological data derived from blastomycosis studies performed in Veterans Administration (VA) hospitals, which serve a predominantly male population, skew the male-to-female ratio (11a). Blastomycosis is uncommon among children and adolescents; a retrospective review from 1983 to 1995 identified only 10 cases that were treated in Little Rock, AR (110). The racial distribution of blastomycosis in case series generally reflects the racial composition of the region from which the cases were collected, although in some regions, certain races have a higher incidence of disease, such as aboriginal people in Manitoba (36) and African-Americans in southeastern Missouri (24). One plausible explanation is that these groups have greater environmental exposures to B. dermatitidis.
In summary, sex, age, and race do not appear to affect directly the susceptibility to blastomycosis, but rather these factors represent variables that influence the likelihood of exposure to B. dermatitidis in the environment. The observation that women and children are as likely as men to be infected during an epidemic supports this conclusion.
PATHOGENESIS AND PATHOLOGY
In the lung, the phagocytic actions of alveolar macrophages, neutrophils, and monocytes provide natural resistance to infection with conidia of B. dermatitidis (41). In addition, alveolar macrophages have been shown to inhibit the transformation of conidia to the pathogenic yeast form (113). This is an important step in terms of pathogenesis, because the yeast form, which possesses a thick capsule, is very difficult for phagocytes to ingest and kill. Polymorphonuclear leukocytes, which handle inhaled conidia better than macrophages, are relatively ineffective against yeast forms (67). Proliferation of yeast forms in the alveoli signals the failure of natural resistance to B. dermatitidis.
If host responses in the lung fail to contain the infection, lymphohematogenous dissemination follows, with foci of infection potentially spreading to any organ system. In decreasing order of frequency, the skin, bones, and male genitourinary system are the most common sites of extrapulmonary disease. With the development of immunity, a pyogranulomatous response ensues at the initial site of pulmonary infection and at any sites of distant spread. Formation of noncaseating granulomas follows an initial suppurative response (107). Even if the pulmonary focus resolves initially, later endogenous reactivation of disease at any pulmonary or extrapulmonary site can occur, with or without previous therapy (70, 103).
Cell-mediated immunity is thought to be ultimately important in preventing progressive blastomycosis. However, the absence of a reliable skin test antigen has made assessment of delayed-type hypersensitivity (DTH) difficult. Much of what is known about cellular immune responses to B. dermatitidis is derived from animal models, in vitro correlates of DTH, and the assumption that host responses to B. dermatitidis are similar to those that H. capsulatum and Coccidioides species elicit (16, 67). In addition, the pyogranulomatous response seen during blastomycosis and the presence of yeast cells inside or in contact with monocytes, macrophages, and giant cells suggest an important cellular immune response (16). Humoral immune responses do not play a significant role in host defense against B. dermatitidis.
CLINICAL MANIFESTATIONS
The consequences of infection with B. dermatitidis are variable and range from subclinical infection to fatal disseminated disease. Symptoms of acute blastomycosis include an influenza-like illness with fever, cough, myalgia, arthralgia, and pleurisy (109). Cases in which acute pneumonia resolves spontaneously are documented (64, 103, 109). Without treatment, the prognosis is grave when infection spreads beyond the lungs; the case fatality rate for disseminated blastomycosis was 78% prior to the availability of specific antifungal therapy (79). Among ill patients, constitutional symptoms such as weight loss, malaise, and fatigue are common but are too nonspecific to be helpful diagnostically (16). Forty-seven percent of patients seen in Little Rock had extrapulmonary disease, and 53% had only pulmonary involvement (19). In other series, disease was limited to the lungs in about 75% of cases (9, 26). Most patients with osseous, genitourinary, or central nervous system (CNS) sites of disease also have active pulmonary or cutaneous involvement.
Pulmonary Blastomycosis
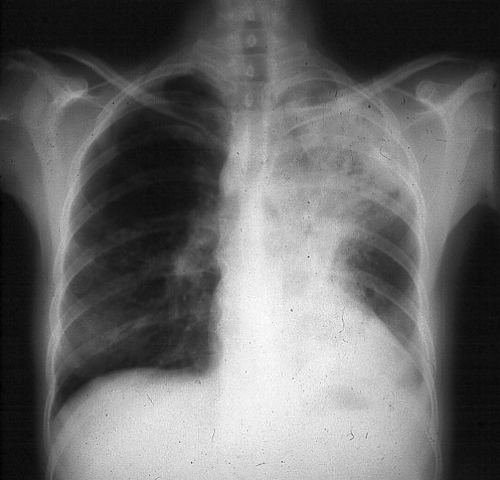
The majority of patients with blastomycosis, whether symptomatic or not, have an alveolar or mass-like infiltrate on chest radiography (Fig. 1) (14). The specific location of the infiltrate is not particularly helpful diagnostically, although the upper lobes may be involved most often (98). Mass lesions mimic malignancy, so physicians in areas of endemicity must include blastomycosis in the initial differential diagnosis for a patient with presumed lung cancer. Reticulonodular and miliary radiographic patterns and solitary nodules are seen less often than alveolar and mass-like infiltrates. Cavitation of the lung parenchyma is uncommon relative to that seen with tuberculosis, histoplasmosis, and coccidioidomycosis. Although small pleural effusions may be present, massive effusions are uncommon (14).
FIG. 1.
A chest radiograph from a patient with pulmonary blastomycosis showing opacification in the left upper lobe. (Photograph courtesy of Robert W. Bradsher, University of Arkansas for Medical Sciences, Department of Internal Medicine, Division of Infectious Diseases.)
Pulmonary blastomycosis presents clinically as acute pneumonia, chronic pneumonia, or an asymptomatic radiographic abnormality. Fever, chills, and productive cough, with or without hemoptysis, characterize acute pneumonia. This syndrome is indistinguishable from acute bacterial community-acquired pneumonia, and many patients receive antibacterial medications before the diagnosis of blastomycosis is considered (16). Among the subset of patients with acute pulmonary blastomycosis whose disease is self-limited, clinical improvement and resolution of pneumonia may be attributed mistakenly to effective antibacterial therapy.
A 2- to 6-month illness with weight loss, fever, night sweats, cough with sputum, and chest pain typifies chronic pneumonia (16). Mass-like pulmonary infiltrates may be more common in patients with chronic pneumonia (98). This illness may be misdiagnosed as pulmonary tuberculosis, chronic pulmonary histoplasmosis, or malignancy.
Miliary blastomycosis or endobronchial spread of infection can cause the adult respiratory distress syndrome (ARDS) with diffuse bilateral pulmonary infiltrates and noncardiogenic pulmonary edema (3, 71, 83). ARDS caused by pulmonary blastomycosis is associated with high mortality (83). In a series from Mississippi, 9 (∼8%) of 107 patients with pulmonary blastomycosis had ARDS, and 7 (78%) of this number died of respiratory failure over a median course of about a week (71).
Extrapulmonary Blastomycosis
Cutaneous.
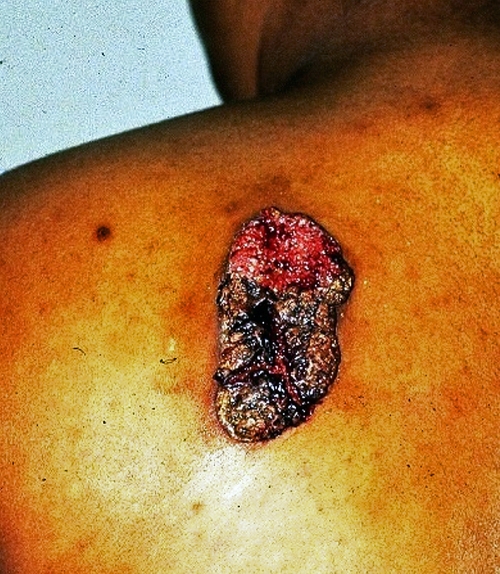
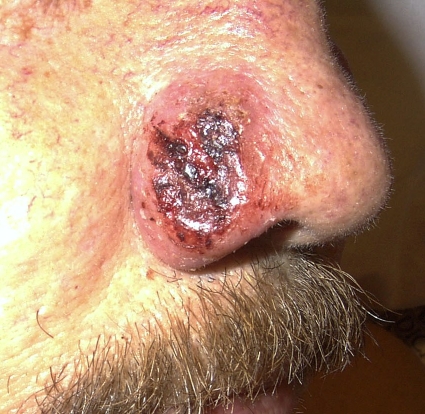
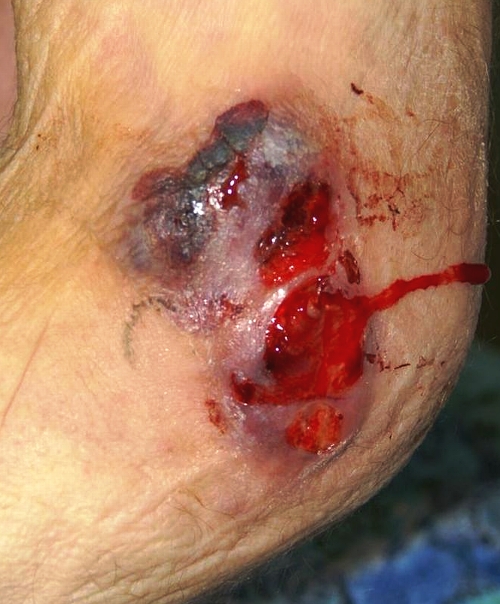
Skin involvement has been reported in 40% to 80% of cases (1, 11a, 23, 28, 43, 76, 121). The cutaneous manifestations of blastomycosis come in two forms, verrucous and ulcerative. Verrucous skin lesions, which lie above subcutaneous abscesses, are raised and crusted with an irregular shape and sharp borders (Fig. 2). The gross appearance of these lesions can mimic basal cell cancer, squamous cell cancer, and giant keratoacanthoma (17). Histologically, there is papillomatosis with downward proliferation of the epidermis and formation of microabscesses (111). The presence of pseudoepitheliomatous hyperplasia and acanthosis (hyperplasia and diffuse thickening of the stratum spinosum of the epidermis) can be misleading, so it is important to look for yeast forms in biopsy specimens with a special stain, such as Grocott-Gomori methenamine silver nitrate (GMS) (16). A cutaneous ulcer occurs when a subcutaneous abscess drains out through the skin (Fig. 3 and 4). These ulcers have heaped-up borders with or without an exudative base. Microabscesses are present, even in lesions without overt clinical evidence of inflammation. Although less common than with disseminated histoplasmosis, mucosal lesions of the nose, mouth, or larynx can occur (112). Verrucous lesions of the pharynx may be confused with squamous cell cancer.
FIG. 2.
A verrucous cutaneous lesion due to blastomycosis. Note the irregular shape and crusting. (Photograph courtesy of Robert W. Bradsher, University of Arkansas for Medical Sciences, Department of Internal Medicine, Division of Infectious Diseases.)
FIG. 3.
A cutaneous ulcer without exudate on the nose of a renal transplant recipient with blastomycosis. Note the heaped-up borders. (Photograph courtesy of Robert W. Bradsher, University of Arkansas for Medical Sciences, Department of Internal Medicine, Division of Infectious Diseases.)
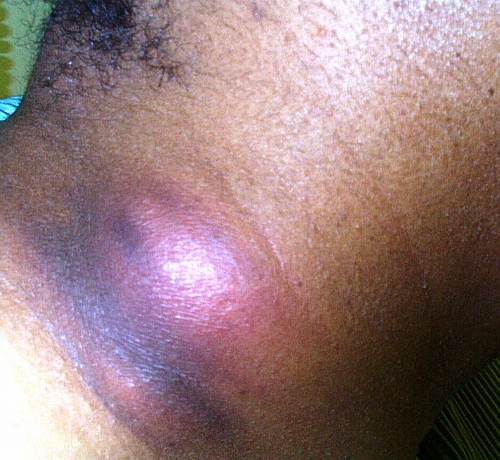
FIG. 4.
An exudative, ulcerative cutaneous lesion on medial aspect of the right elbow of a man with blastomycosis. (Photograph courtesy of J. Ryan Bariola, University of Arkansas for Medical Sciences, Department of Internal Medicine, Division of Infectious Diseases.)
Osseous.
After the lungs and skin, bone is the third most common site of disease. Among patients with blastomycosis described in case series, the prevalence of bone disease ranges from approximately 6% to 48% (1, 11a, 23, 28, 43, 76, 121). On average, osteomyelitis is present in approximately 25% of cases with extrapulmonary manifestations (16). About 75% of patients with osseous blastomycosis have pulmonary disease at the time of presentation (50). Although essentially any bone can be involved, the vertebrae, ribs, skull, and long bones are affected most commonly (8, 32, 50, 121). Radiographically, osteomyelitis can appear as a clearly demarcated osteolytic lesion without periosteal involvement or as a diffuse, destructive process with periosteal new bone formation (Fig. 5) (50). Infection can spread into an adjacent joint, causing purulent arthritis (106). Extension into the soft tissues causes a painful subcutaneous abscess with draining sinuses.
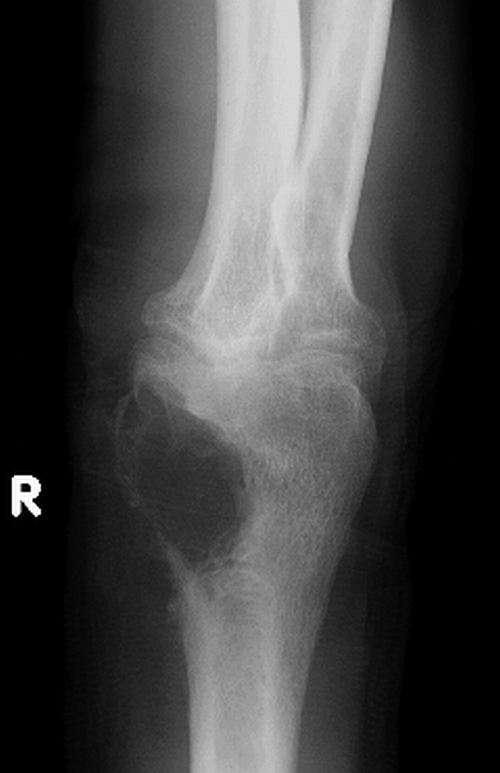
FIG. 5.
Radiograph of the right arm shown in Fig. 4 that reveals a large osteolytic lesion of the distal humerus due to osteomyelitis. R, right. (Photograph courtesy of J. Ryan Bariola, University of Arkansas for Medical Sciences, Department of Internal Medicine, Division of Infectious Diseases.)
Vertebral osteomyelitis due to blastomycosis most often affects the lower thoracic and lumbar regions of the spine although cervical, upper thoracic, and sacral involvement has been reported (90, 105). Extension of infection from the spine, causing paravertebral or psoas abscess, is a frequent complication (8, 90, 121). All 8 patients with vertebral blastomycosis in one report had evidence of contiguous abscesses (105). As these abscesses track through soft tissues, subcutaneous masses can appear at any spinal level, in the flank, or in the thigh when a psoas abscess extends beneath the inguinal ligament. These purulent subcutaneous collections often drain through the skin, causing a chronic, discharging sinus or ulcer.
Genitourinary.
Involvement of the genitourinary system is recognized in 10% to 30% of cases (1, 11a, 23, 28, 43, 76, 121). Because men are more likely than women to have extrapulmonary blastomycosis, the most common genitourinary sites of disease are the prostate, the testicle, and the epididymis (107, 121). Epididymo-orchitis causes swelling and pain, and prostatitis is associated with dysuria and symptoms of obstruction. Prostatic massage prior to collection of urine may enhance the yield of urine culture (60). Reported cases of female genitourinary blastomycosis include endometrial infection that occurred via transmission from a man with cutaneous blastomycosis of the penis (46) and tubo-ovarian abscess that occurred in the setting of reactivation disease (88). Peritoneal seeding with ascites may accompany tubo-ovarian abscess (87).
Central nervous system.
CNS infection, in the form of meningitis or mass lesions, is apparent in 5% to 10% of cases of disseminated blastomycosis (68, 104). Central diabetes insipidus has occurred in patients with blastomycosis (62). Pituitary involvement in conjunction with chronic meningitis, chronic pneumonia, and genitourinary infection resembles tuberculosis (86). The cerebrospinal fluid white cell differential may show neutrophilic predominance in patients with meningitis (57). Making the diagnosis of CNS blastomycosis is challenging; for example, in one series of patients with meningitis, culture of cerebrospinal fluid obtained via lumbar puncture yielded the diagnosis for only 2 of 22 patients (68). More-invasive procedures, such as brain biopsy and ventricular tap, are more likely to provide sufficient material for diagnosis (68, 104). It is important to look for more-accessible sites of disease, such as the skin or lung, from which to obtain specimens for microscopic examination and culture.
Other sites of disease.
B. dermatitidis can disseminate to virtually any organ system. As already mentioned, subcutaneous abscesses present as masses growing from deeper foci (Fig. 6). Involvement of the reticuloendothelial system with lymphadenopathy or abscesses in the liver or spleen is well documented (1, 11a, 28, 121). Other uncommonly affected sites include the eye (78), the middle ear (61), the paranasal sinuses (123), breast tissue (47), the myocardium, the pericardium, and the gastrointestinal tract, as well as the thyroid and adrenal glands (17).
FIG. 6.
A subcutaneous neck mass with overlying cutaneous erythema due to blastomycosis. (Photograph courtesy of Robert W. Bradsher, University of Arkansas for Medical Sciences, Department of Internal Medicine, Division of Infectious Diseases.)
Blastomycosis in Immunocompromised Patients
Unlike H. capsulatum, Coccidioides immitis/C. posadasii, Cryptococcus neoformans, and other opportunistic fungal pathogens, B. dermatitidis appears no more likely to cause disease in a person with compromised cellular immunity than to cause disease in a normal host. The presence of natural defenses against inhaled conidia in the lung may explain this observation. When blastomycosis does occur in immunocompromised patients, the disease is more aggressive than in immunocompetent hosts (95). In one series, 30% of patients died of blastomycosis, and disseminated disease and involvement of the CNS were relatively common (95). Among patients with AIDS, blastomycosis tends to be a severe illness with widespread dissemination, often including the CNS, and is associated with a high rate of early mortality (94, 122). Miliary disease has occurred in the setting of HIV infection (59).
DIAGNOSIS
No clinical or radiographic abnormalities are absolutely diagnostic of blastomycosis. The spectrum of disease that B. dermatitidis causes overlaps with those of other fungal pathogens and malignancy, and the clinical picture may be indistinguishable from tuberculosis. Therefore, visualization of characteristic yeast forms or growth of the fungus in culture is necessary to diagnose blastomycosis definitively.
Culture
Various specimens may be submitted for culture diagnosis of blastomycosis, based on the clinical manifestations (Table 1). For cutaneous lesions, it is important to obtain the specimen from the active, leading edge, and biopsy specimens should include the full thickness of the lesion, if possible.
TABLE 1.
Specimens for diagnosis of blastomycosis
| Infection | Specimen(s) |
|---|---|
| Pneumoniaa | Sputum, bronchial washings, bronchoalveolar lavage |
| Cutaneous/subcutaneous | Deep tissue biopsy specimen, scrapings, exudate |
| Osteoarticular | Joint fluid, synovial tissue biopsy specimen, bone biopsy specimen |
| Genitourinary | Prostate biopsy specimen, urine |
| Meningitis | Cerebrospinal fluid |
| Disseminated disease | Bone marrow |
Martynowicz et al. showed that the diagnostic yield (culture positivity rate) was highest for bronchial washings, followed by sputum (80).
For most specimens, direct examination should be performed in addition to culture. The presence of characteristic yeast cells (described in detail in the “Histopathology/Cytopathology” section) allows a rapid diagnosis; however, because direct examination is less sensitive than culture, a negative result does not exclude the possibility of blastomycosis. A wet preparation is the simplest method for direct examination; however, yeast cells may be difficult to find in specimens that contain distracting tissue debris and cells, such as sputum and skin scrapings. Techniques that enhance visibility of fungal elements include use of a potassium hydroxide (KOH) solution, which dissolves tissue material. KOH can be used with or without the addition of calcofluor white, a fluorochrome compound that binds to chitin in the walls of fungal cells and fluoresces when exposed to short-wavelength UV light from a fluorescence microscope.
Culture, using a variety of media, currently is the most sensitive method for diagnosis of blastomycosis. All specimens should be inoculated into a general-purpose growth medium, such as Sabouraud dextrose agar, potato dextrose agar, potato flake agar, or inhibitory mold agar. For specimens that are likely to be contaminated with saprophytic fungi and/or bacteria, both a nonselective growth medium and a selective medium containing cycloheximide to inhibit saprophytic fungi and antibacterial agents should be inoculated. For tissue specimens, adding an enriched medium like brain heart infusion agar with blood and supplemented with antibacterial agents is recommended. All plates are incubated at 25 to 30°C for 4 to 6 weeks.
Growth of B. dermatitidis generally is apparent on fungal isolation media in 5 to 10 days; however, if there are few organisms in the specimen, colonies may not appear for 30 days. Colonies initially are white to off-white and glabrous or waxy in appearance, becoming gray to brown as aerial hyphae develop with age. Microscopically, the mold form of B. dermatitidis is characterized by delicate, septate hyphae, 1 to 2 μm in diameter, and most important diagnostically, oval or pyriform single-celled conidia, 2 to 4 μm in diameter, found singly at the tips of short or long conidiophores, resembling lollipops. The presence of “lollipops,” however, is not diagnostic of B. dermatitidis. Other molds (e.g., Pseudallescheria boydii and Chrysosporium spp.) have a similar appearance; therefore, identification requires additional testing. P. boydii and many Chrysosporium spp. do not grow on media containing cycloheximide, whereas B. dermatitidis does. Additionally, Chrysosporium generally does not grow at 37°C, but B. dermatitidis will (ultimately as a yeast). Although these are useful features, confirmatory testing is necessary.
The test most commonly used to confirm an identification of B. dermatitidis is the commercially available chemiluminescent DNA probe (AccuProbe; GenProbe Inc., San Diego, CA). This test provides results within hours once there is sufficient growth, but it has limitations. It produces a positive result with all Paracoccidioides brasiliensis isolates, but in most cases, this is not problematic. Paracoccidioidomycosis occurs almost exclusively in South America and Central America; it is extremely rare in the Northern Hemisphere. P. brasiliensis may be distinguished from B. dermatitidis based on the microscopic appearance of the yeast phase; however, conversion to the yeast phase may take weeks. The yeast phase of P. brasiliensis is characterized by multiple buds from a single thin-walled cell, whereas B. dermatitidis typically is a thick-walled yeast with a single broad-based bud. The probe also produces a positive result with Gymnascella hyalinospora and Emmonsia parva, both of which are infrequently isolated in the clinical laboratory and are rare human pathogens.
Other ways to confirm an identification of B. dermatitidis are conversion of the mold to the yeast form, which can be very time-consuming and therefore is done rarely in the clinical laboratory, and repetitive-sequence-based PCR, which is an option for laboratories that use the commercially available DiversiLab system (bioMerieux, Durham, NC) for bacterial strain typing (102). Nucleic acid sequencing has been investigated (11) but remains infrequently used, although it may become a useful alternative method for fungal identification as databases become more reliable and commercial kits are more readily available.
Histopathology/Cytopathology
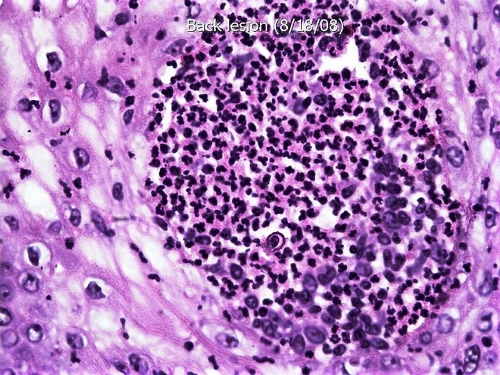
The characteristic host response to infection with B. dermatitidis is a mixed inflammatory reaction with clusters of polymorphonuclear leukocytes and granulomas, usually noncaseating, with epitheloid histiocytes and giant cells, primarily the foreign body type. Early in the infection, polymorphonuclear leukocytes predominate and organisms generally are found easily. As granulomas form, the number of organisms tends to decrease. In immunocompromised persons, many organisms are seen in tissue, with minimal or no surrounding inflammation, and in immunocompetent persons who develop rapidly progressive and fatal disease, the inflammatory response may consist only of neutrophils. Infection of the skin and mucosal surfaces is characterized by prominent pseudoepitheliomatous hyperplasia that histologically may resemble squamous cell carcinoma, with microabscess formation (Fig. 7).
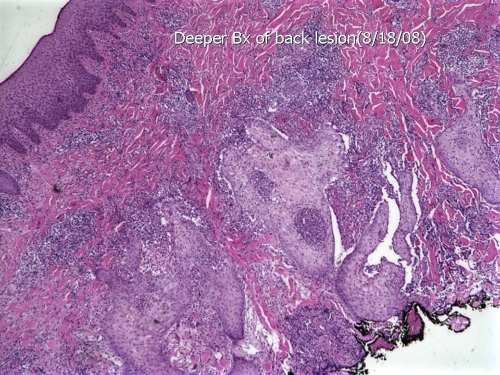
FIG. 7.
Hematoxylin-and-eosin-stained section of an ulcerated lesion on the thigh shows pseudoepitheliomatous hyperplasia, epidermal abscesses, and a prominent inflammatory infiltrate in the dermis (low-power magnification). Bx, biopsy. (Photograph courtesy of Jameel Ahmad Brown, University of Arkansas for Medical Sciences, Department of Pathology.)
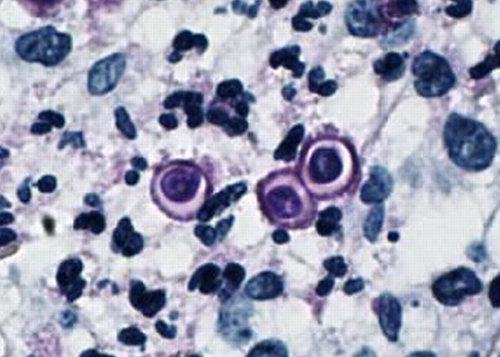
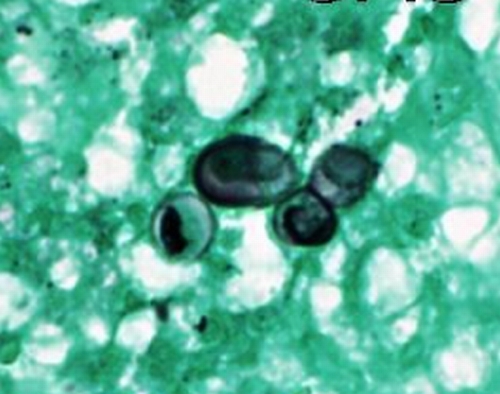
The classic appearance of B. dermatitidis in direct preparations of clinical specimens submitted to the microbiology laboratory, cytologic preparations (e.g., of sputum or material from fine needle aspirates), or sections of formalin-fixed and paraffin-embedded tissue is a round to oval, multinucleate yeast cell, 8 to 15 μm in diameter, with a single broad-based bud (Fig. 8). The yeasts have thick, refractile cell walls and are easily seen in tissue sections stained with hematoxylin and eosin (Fig. 9). Other useful tissue stains are periodic acid-Schiff stain (PAS) (Fig. 10), PAS with hematoxylin counterstain, and GMS, which provides excellent contrast (Fig. 11). Organisms may be located intra- or extracellularly.
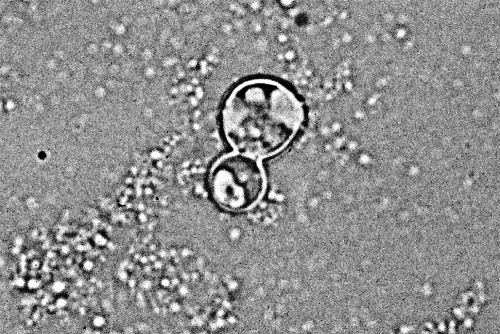
FIG. 8.
KOH preparation of clinical material showing the typical appearance of B. dermatitidis. Note the round shape, doubly refractile wall, and single broad-based bud. (Photograph courtesy of Robert W. Bradsher, University of Arkansas for Medical Sciences, Department of Internal Medicine, Division of Infectious Diseases.)
FIG. 9.
High-power magnification of the section illustrated in Fig. 7 shows a microabscess with a nonbudding yeast cell of B. dermatitidis. Note the characteristic thick cell wall and centrally retracted cytoplasm (high-power magnification). (Photograph courtesy of Jameel Ahmad Brown, University of Arkansas for Medical Sciences, Department of Pathology.)
FIG. 10.
PAS-stained section of the lesion illustrated in Fig. 7 shows a budding yeast of B. dermatitidis with the characteristic broad-based bud, as well as a single nonbudding yeast cell (high-power magnification). (Photograph courtesy of Jameel Ahmad Brown, University of Arkansas for Medical Sciences, Department of Pathology.)
FIG. 11.
GMS-stained section of the lesion illustrated in Fig. 7 shows a budding yeast of B. dermatitidis with the characteristic broad-based bud, as well as two slightly misshapen single nonbudding yeast cells (high-power magnification). (Photograph courtesy of Jameel Ahmad Brown, University of Arkansas for Medical Sciences, Department of Pathology.)
When classic multinucleate yeast forms with diagnostic broad-based buds are seen, B. dermatitidis can be identified with confidence. If these forms are not present, B. dermatitidis may be confused with other fungi. Large, nonbudding cells of B. dermatitidis, especially those with poorly stained inner contents, may be confused with C. neoformans or immature spherules of C. immitis/C. posadasii (124). The mucicarmine stain intensely colors the mucopolysaccharide of the cryptococcal capsule and, therefore, will differentiate encapsulated strains of C. neoformans from B. dermatitidis, the cell walls of which are often weakly mucicarmine positive. Capsule-deficient forms of C. neoformans can be differentiated based on positive staining with melanin stains (B. dermatitidis is negative). To distinguish B. dermatitidis from immature spherules of C. immitis/C. posadasii, Alcian blue (pH 2.5) and/or acid-fast stains may be helpful (B. dermatitidis may be weakly positive, C. immitis/C. posadasii is negative). Additionally, close examination of serial sections for diagnostic forms of either fungus is recommended.
Atypical forms of B. dermatitidis infrequently are encountered in tissue. These include very small yeast cells, 2 to 4 μm in diameter, which may be confused with H. capsulatum, and rarely, hyphal or filamentous forms. One technique that may aid in identification of atypical forms is staining with Congo red, which helps distinguish microforms of B. dermatitidis (positive) from H. capsulatum (negative) (5). In situ hybridization has been shown to accurately identify B. dermatitidis in tissue (58); however, because probes are not commercially available, this is not a viable option for most laboratories.
Antibody Tests
Tests for detection of antibodies to B. dermatitidis have been commercially available for many years, but unfortunately, they are generally considered inadequate for diagnosis of blastomycosis. Complement fixation using yeast-phase antigens, which has been in use the longest, has the worst performance characteristics. Its sensitivity and specificity are too low (57% and 30%, respectively, in one study [117]) to be of diagnostic value. In fact, data from one report showed that patients with culture-confirmed blastomycosis were as likely to have complement-fixing antibodies against histoplasmin as they were to have such antibodies against blastomycin (107). Immunodiffusion using purified B. dermatitidis A antigen was introduced next and proved to be more sensitive and specific than complement fixation. A positive immunodiffusion result is specific for blastomycosis, but a negative test does not exclude the diagnosis, because the test sensitivity is 65% to 80% (63, 117). Enzyme immunoassays, the most recently developed antibody detection tests, are more sensitive than immunodiffusion for diagnosis of blastomycosis but less specific (65).
Antigen Detection
A test for detection of B. dermatitidis antigen in human specimens currently is offered by one reference laboratory in the United States. The performance characteristics of the assay have been studied predominantly with urine, although cerebrospinal fluid, bronchoalveolar lavage fluid, serum, and other sterile body fluids (except EDTA-separated plasma) also may be tested. In the largest published evaluation (42), antigen was detected in the urine of 92.9% of 42 patients with culture- or histologically proven blastomycosis, including 25 (89.3%) of 28 with disseminated disease and all 14 patients with pulmonary disease. However, in that study, assay specificity was a problem; B. dermatitidis antigen also was detected in the urine of 26 (96.3%) of 27 of patients with histoplasmosis, all 10 patients with paracoccidioidomycosis, and 7 (70%) of 10 patients with penicilliosis. A few case reports describing the usefulness of repeated antigen testing during therapy to monitor response have been published (84, 116); however, additional data are needed to confirm these results. The urine of patients with blastomycosis may contain cross-reactive or shared antigens with H. capsulatum var. capsulatum; in one study, 12 of 19 patients with blastomycosis tested positive with the H. capsulatum urine antigen assay (120).
Although not an antigen test, detection of (1→3)-β-d-glucan in serum has been shown to be a useful adjunct for diagnosis of invasive fungal infections, especially candidiasis, aspergillosis, and histoplasmosis (91, 92, 100). In contrast, preliminary data suggest that the test is not of value with patients with blastomycosis. In a very small study of four patients with microbiologically proven B. dermatitidis infection, β-d-glucan was detected in only one patient, who had chronic disseminated disease with multiple skin lesions (53).
Nucleic Acid Amplification
There are no commercially available nucleic acid amplification tests for direct detection of B. dermatitidis in clinical specimens. Nested PCR using fixed tissue has shown promise (11). Methods based on PCR have been useful in defining the molecular epidemiology of blastomycosis (81).
Skin Tests
No skin test antigens for blastomycosis are commercially available. Many years ago, blastomycin, a crude mycelial filtrate, was investigated as a possible skin test antigen, but it proved to be neither sensitive nor specific. In two studies of patients with culture-confirmed blastomycosis, 59% and 100% of those evaluated were skin test negative (11a, 121).
Susceptibility Testing
Although standardized guidelines have been developed for in vitro susceptibility testing of certain fungi, such guidelines do not exist for the testing of dimorphic fungi.
TREATMENT
Treatment Options
All patients with chronic pulmonary or disseminated extrapulmonary blastomycosis and all immunocompromised patients with blastomycosis should receive antifungal therapy. The site(s) of disease, the severity of illness, and the presence or absence of underlying immunosuppression influence the choice of antifungal drug and the duration of treatment. Other considerations include the potential for drug toxicity, possible drug-drug interactions, and pregnancy. Currently, in vitro antifungal susceptibility testing has no role in the selection of antifungal therapy for the treatment of infections due to dimorphic fungi due to lack of standardization and lack of knowledge regarding susceptibility breakpoints. As previously mentioned, acute blastomycosis may resolve spontaneously. However, it is common practice to treat patients with this diagnosis because effective, relatively safe oral antifungal drugs are available and because it is impossible to predict which patients with acute infection will spontaneously recover and which will develop extrapulmonary disease later. Patients with acute blastomycosis from whom therapy is withheld should be monitored closely for years. The drugs that are available for the treatment of blastomycosis are shown in Table 2.
TABLE 2.
Options for treatment of blastomycosis
| Agent | Comments |
|---|---|
| Amphotericin B | Used for patients with moderately severe to severe disease, those with central nervous system involvement, those who are immunocompromised, and those who are pregnant; lipid formulations are preferable in some situations |
| Itraconazole | Preferred agent for patients with mild to moderate disease and as step-down therapy for those with more severe disease who have received amphotericin B; oral solution provides more consistent serum levels than capsules |
| Fluconazole | A second-line agent that is less efficacious than itraconazole; an option for step-down therapy after a course of amphotericin B for central nervous system infection |
| Ketoconazole | First azole proven to be effective; rarely used now |
| Voriconazole | Case reports, in vitro studies, and animal studies support its use; no prospective human trials; an option for step-down therapy after a course of amphotericin B for central nervous system infection |
| Posaconazole | May be effective, but there is very little clinical experience |
| Echinocandins | Poor to intermediate in vitro activity; should not be used |
The Infectious Diseases Society of America (IDSA) has published clinical practice guidelines that are based upon open-label studies, case reports, and expert opinion (27). There has never been a clinical trial to compare amphotericin B to azole therapy or one azole to another for the treatment of blastomycosis. The IDSA recommendations for treatment of various clinical forms of blastomycosis are shown in Table 3.
TABLE 3.
Recommendations for treatment of blastomycosisa
| Disease manifestation/patient type | Preferred treatment | Comments |
|---|---|---|
| Mild to moderate pulmonary or disseminated disease | 200 mg itraconazole orally once or twice per dayb for 6-12 mo | Treat osteoarticular disease for 12 mo |
| Moderately severe to severe pulmonary or disseminated disease, but not in the central nervous system | 0.7-1.0 mg amphotericin B deoxycholate/kg per day, or 3-5 mg lipid amphotericin B/kg per day for 1-2 wk, followed by 200 mg itraconazole twice per dayb for 6-12 mo (pulmonary) or 12 mo (disseminated) | Amphotericin B deoxycholate given at a total dose of 2 g can be used for the entire course of treatment, but most clinicians prefer to step-down to itraconazole after initial improvement; lipid amphotericin B products have fewer adverse effects than deoxycholate |
| Central nervous system disease | 5 mg lipid amphotericin B/kg per day for 4-6 wk, followed by an oral azole for at least 1 yr | Azole options for step-down therapy include 200 mg itraconazole twice or thrice per day, 800 mg fluconazole per day, and 200-400 mg voriconazole twice per day |
| Immunosuppressed patients | 0.7-1.0 mg amphotericin B deoxycholate/kg per day, or 3-5 mg lipid amphotericin B/kg per day for 1-2 wk, followed by 200 mg itraconazole twice per dayb for 12 mo | Lifelong suppressive therapy may be necessary for patients whose immunocompetence does not improve |
| Pregnant patients | 3-5 mg lipid amphotericin B/kg per day | Systemic azole therapy is contraindicated in pregnancy |
Adapted from reference 27 with permission of The University of Chicago Press.
The dose of itraconazole for pediatric patients is 10 mg/kg per day, not to exceed 400 mg per day.
Amphotericin B.
In 1967, Abernathy published his experience that showed that a total dose of 1.0 to 2.0 g of amphotericin B cures most patients with blastomycosis (2). In a comparative trial performed a few years later, treatment was successful for 91% of patients who received amphotericin B, compared with 72% of patients who were treated with 2-hydroxystilbamidine (23). Amphotericin B, whose efficacy was shown in this study, remains the gold standard against which all other regimens for the treatment of blastomycosis are compared (20). Relapse is rare for patients who have received at least 1.5 g. Despite proven efficacy and decades of experience with amphotericin B, use of this agent for the entire course of treatment of blastomycosis became a rarity once it was shown that orally administered, less-toxic azole antifungal drugs could be used to treat most patients, either as initial therapy or as step-down therapy after a short course of amphotericin B.
Amphotericin B deoxycholate (henceforth, conventional amphotericin B) or a lipid amphotericin B product (henceforth, lipid amphotericin B) is the preferred initial drug for patients with blastomycosis who are severely ill, who have life-threatening disease, who have CNS involvement, or who are immunocompromised (27). In addition, amphotericin B is the best option for patients whose disease progresses during azole therapy and for those who cannot take an azole. Adverse reactions are well known and include infusion-related side effects, decrease in glomerular filtration rate, hypokalemia, hypomagnesemia, and anemia. Pre- and postdose hydration with normal saline is useful for preventing or lessening the severity of nephrotoxicity (21). Premedication with acetaminophen and diphenhydramine may abrogate infusion-related side effects, but there are no data to support the routine use of premedications (40). Meperidine is used to treat rigors that can occur during infusion of amphotericin B.
Lipid amphotericin B can be used instead of conventional amphotericin B (27). Although it is much more expensive, lipid amphotericin B is associated with fewer infusion-related side effects and is less likely to cause renal dysfunction than the conventional formulation. Many experts choose lipid amphotericin B for patients with underlying kidney disease and for those at increased risk of developing nephrotoxicity. However, support for its use in blastomycosis comes from animal models and anecdotal clinical experience (27, 29, 31, 34, 75, 93, 99).
With the availability of less-toxic oral azoles, it has become standard practice to switch from amphotericin B to an azole after initial improvement or, in less-ill immunocompetent patients without CNS disease, to begin therapy with an azole instead of amphotericin B.
Azoles.
Ketoconazole, itraconazole, fluconazole, voriconazole, and posaconazole are the azole antifungal drugs that are available for the treatment of blastomycosis. Ketoconazole was the first azole that was proven to be effective for the treatment of mild to moderate blastomycosis in immunocompetent patients (14, 89). Itraconazole later replaced ketoconazole as the azole of choice, whether given as primary therapy for stable patients or following an initial course of amphotericin B. Based on historical comparison, itraconazole is better tolerated and more effective than ketoconazole. In a study the Mycoses Study Group of the National Institute of Allergy and Infectious Diseases (NIAID) performed, treatment with itraconazole at 200 to 400 mg per day cured 43 (90%) of 48 patients with non-life-threatening blastomycosis; among those who adherently took the drug for more than 2 months, 39 (95%) of 40 were cured (39). Bradsher had similar success with itraconazole in a single-center observational study (18). The usual dose is 200 mg once or twice per day; patients with disease that persists or progresses while on the once-daily dose should be treated with 200 mg twice per day.
Optimal absorption of itraconazole capsules and ketoconazole requires gastric acidity, and these medications should be taken with food (33). Medications that increase gastric pH such as antacids, histamine type II receptor blockers, and proton pump inhibitors should be avoided during therapy with either itraconazole capsules or ketoconazole. Itraconazole is also available as a solution at a concentration of 10 mg/ml; this formulation does not have to be taken with food, and gastric pH does not affect its absorption (77). Because the itraconazole concentrations achieved in serum with the solution are higher than those achieved with capsules, the solution formulation is preferred (27). Measured serum levels of itraconazole are quite variable even with the oral solution; for this reason, therapeutic drug monitoring, with the goal of maintaining serum levels between 1.0 and 10.0 μg/ml, is recommended (27). Measurement of serum itraconazole levels is unlikely to provide information that is clinically useful concerning patients whose disease manifestations, for example, a skin lesion or pulmonary opacity, are improving with therapy.
Fluconazole at a dose of 800 mg per day is an alternative for immunocompetent patients with mild to moderate blastomycosis who cannot absorb or tolerate itraconazole or to avoid drug-drug interactions (27). In a pilot study, fluconazole at 200 to 400 mg per day achieved success for only 65% of 23 patients who took the drug for at least 6 months (96). A later study used 400 to 800 mg per day, which was successful for 34 (87%) of 39 patients who took the drug for a median of about 9 months (97). Fluconazole penetrates well into the CNS and is absorbed well regardless of gastric pH (33).
Voriconazole and posaconazole have activity against B. dermatitidis in vitro and in animal models (45, 74, 114, 115). Anecdotal reports support the use of voriconazole as an alternative agent, particularly in the setting of CNS blastomycosis (7, 13, 64, 73, 93).
All azole antifungal drugs can cause hepatic toxicity, which is most often in the form of asymptomatic elevation of aminotransferases. This occurs in up to 5% of patients who take itraconazole (33). Clinically important hepatitis, which is rare, usually occurs during the first 2 months of treatment (33). Hepatic aminotransferases should be checked at the baseline, after 2 to 4 weeks of therapy, and every 3 months thereafter (27). Gastrointestinal upset, including nausea and vomiting, affects up to 10% of patients (33). Diarrhea, due to the excipient used to solubilize the parent compound, can be sufficiently severe to force discontinuation of the solution formulation of itraconazole (4). Itraconazole has significantly less effect on mammalian steroidogenesis than ketoconazole; adrenal insufficiency, gynecomastia, decreased libido, impotence, and menstrual irregularities are associated with ketoconazole but not with itraconazole (33). Like itraconazole, fluconazole has little effect on mammalian steroid synthesis. Through an unknown mechanism, itraconazole can cause a syndrome of hypertension, edema, and hypokalemia (4). The drug should be used with caution in patients with congestive heart failure.
All azoles also inhibit mammalian P-450 enzymes to some degree, forming the basis for numerous drug-drug interactions. Itraconazole is both an inhibitor and a substrate of the cytochrome P-450 enzyme system, in particular, the 3A4 isoform (CYP3A4). Administration of itraconazole with another CYP3A4 substrate increases the plasma concentration of the coadministered drug to levels that are potentially toxic. A number of different drugs increase the metabolism of itraconazole, lowering blood levels, which may cause treatment to fail. It is important to consider drug-drug interactions whenever azoles are prescribed to a patient who takes other medications.
Echinocandins.
Caspofungin, micafungin, and anidulafungin possess, at best, intermediate activity against B. dermatitidis in vitro and should not be used to treat blastomycosis (45).
Surgery.
Antifungal therapy alone is effective for the vast majority of cases of blastomycosis. Occasionally, drainage of large abscesses, in conjunction with appropriate antifungal therapy, may be necessary for optimal management. Surgical intervention may be important in the management of brain abscess or epidural abscess when these foci cause neurologic deficits (27). Debridement of bone is necessary only when osteomyelitis fails to respond to medical management; this is uncommon. Percutaneous, computed tomography (CT)-guided aspiration of soft tissue abscesses is an effective approach for the management of paraspinal abscess that may complicate vertebral blastomycosis (25, 105).
Mild to Moderate Pulmonary and Extrapulmonary Blastomycosis
Standardized criteria by which the severity of blastomycosis can be determined do not exist. Clinical judgment must be applied on a case-by-case basis. Many patients with mild disease are treated in the outpatient setting. Preferred therapy consists of 200 mg itraconazole orally once or twice per day, although 200 mg is sometimes given thrice per day for 3 days to reach steady-state serum levels more quickly (27). Serum itraconazole levels can be checked after 2 weeks of therapy. Fluconazole at a dose of 400 to 800 mg per day is a less-effective option for those patients who are unable to tolerate itraconazole or for whom drug-drug interactions preclude the use of itraconazole. The recommended duration of therapy with itraconazole is at least 6 to 12 months and 3 months beyond the resolution of all signs, symptoms, and radiographic abnormalities. Those with osteoarticular disease, which is more likely to relapse, should be treated for 12 months (27).
Moderately Severe to Severe Pulmonary and Extrapulmonary Blastomycosis
Patients whose illness is so severe that they require admission to the hospital fall into this category. This includes patients with extensive pneumonia, multiorgan disease, respiratory failure due to ARDS, other organ failure, or hemodynamic instability. The preferred initial therapy for these patients is conventional amphotericin B at 0.7 to 1.0 mg/kg of body weight per day or lipid amphotericin B at 3 to 5 mg/kg per day for 1 to 2 weeks or until clinical improvement occurs (27). Thereafter, therapy is switched to itraconazole as described for patients with milder disease. Patients with severe extrapulmonary blastomycosis should be treated for at least 12 months (27). Corticosteroids have been given to patients with ARDS, with apparent success in two cases (69).
Central Nervous System Blastomycosis
Lipid amphotericin B at a dose of 5 mg/kg per day for 4 to 6 weeks should be given to patients with disseminated extrapulmonary blastomycosis that involves the CNS (27). Lipid amphotericin B is preferred in this situation because it is less likely than conventional amphotericin B to cause significant adverse events over the prolonged course of therapy that is required. In addition, data from an animal model suggest that liposomal amphotericin B achieves a greater concentration in the CNS than conventional amphotericin B (56). After the 4- to 6-week course of lipid amphotericin B is completed, an azole is given for at least 12 months and until objective evidence of active CNS infection (e.g., abnormality of cerebrospinal fluid or mass lesion) is no longer present. There is uncertainty regarding the optimal azole to use as step-down therapy for CNS blastomycosis. Itraconazole has better intrinsic activity against B. dermatitidis than fluconazole, but fluconazole penetrates better into the CNS than itraconazole. Voriconazole possesses both of these important characteristics (intrinsic activity and capability for CNS penetration), but clinical experience with this agent in patients with blastomycosis is limited (49). Itraconazole at 200 mg twice or thrice per day, fluconazole at 800 mg per day, and voriconazole at 200 to 400 mg twice per day are options for step-down therapy (27). Azoles should not be used as initial therapy for patients with CNS blastomycosis.
Blastomycosis in Special Hosts
Immunocompromised.
The preferred treatment of blastomycosis for immunocompromised patients, including solid organ and stem cell recipients, those with AIDS, and those taking glucocorticoids chronically, is similar to that described above for patients with severe disease. That is, initial therapy in this patient population should be conventional or lipid amphotericin B for 1 to 2 weeks or until there is clinical improvement; thereafter, a switch to itraconazole should be made (27). The total duration of therapy should be at least 12 months but is usually longer for patients with ongoing immunosuppression; in some cases, this translates to lifelong chronic suppressive therapy. For AIDS patients who complete 12 months of antifungal therapy and attain a CD4 T-lymphocyte count above 150 cells/μl for at least 6 months while taking highly active antiretroviral therapy, it is reasonable to consider discontinuation of azole therapy. This suggestion is based upon the experience with disseminated histoplasmosis in the population with AIDS (55).
Pregnancy.
There are few reports of blastomycosis during pregnancy (72, 101). Treatment in this setting has 2 goals: to cure the patient and to prevent transplacental transmission of infection to the fetus. Systemic azoles, which have teratogenic potential, are contraindicated during pregnancy. The IDSA guidelines recommend lipid amphotericin B at a dose of 3 to 5 mg/kg/day for pregnant women with blastomycosis (27).
Pediatric.
Although blastomycosis is not commonly diagnosed in children, when it does occur, the clinical spectrum of disease resembles that which occurs in adults (110). Therapy in the pediatric population is similar to that which is recommended for adults. Children are more likely than adults to tolerate conventional amphotericin B, so there may be less need to use lipid amphotericin B. Itraconazole should be given to children at a dose of 10 mg/kg per day, up to a maximum of 400 mg per day.
PREVENTION
There are no data upon which to base recommendations pertaining to avoidance of exposure to B. dermatitidis. The disease is uncommon, even in areas in which the disease is endemic, and there is nothing to suggest that modifying individual behavior would change the incidence of the blastomycosis (20). A vaccine to prevent blastomycosis is not available.
Biography
 Michael Saccente, M.D., is Associate Professor of Medicine in the Division of Infectious Diseases and Director of the Internal Medicine Residency Program at the University of Arkansas for Medical Sciences and the Central Arkansas Veterans Healthcare System in Little Rock, AR, where he has been on the faculty since 1996. He earned his undergraduate degree at the University of Miami in Florida and received his M.D. degree from the Medical University of South Carolina in Charleston, SC. Dr. Saccente subsequently completed his residency training in internal medicine and his fellowship training in infectious diseases at the University of Alabama at Birmingham, AL. His clinical interests include endemic mycoses and HIV/AIDS.
Michael Saccente, M.D., is Associate Professor of Medicine in the Division of Infectious Diseases and Director of the Internal Medicine Residency Program at the University of Arkansas for Medical Sciences and the Central Arkansas Veterans Healthcare System in Little Rock, AR, where he has been on the faculty since 1996. He earned his undergraduate degree at the University of Miami in Florida and received his M.D. degree from the Medical University of South Carolina in Charleston, SC. Dr. Saccente subsequently completed his residency training in internal medicine and his fellowship training in infectious diseases at the University of Alabama at Birmingham, AL. His clinical interests include endemic mycoses and HIV/AIDS.
 Gail L. Woods, M.D., is Professor of Pathology at the University of Arkansas for Medical Sciences and Chief of Pathology and Laboratory Medicine Service at the Central Arkansas Veterans Healthcare System in Little Rock, AR, where she has been on the faculty since 2005. She earned her undergraduate degree at Beloit College in Wisconsin and received her M.D. degree from Indiana University Medical School in Indianapolis, IN. Dr. Woods subsequently completed her residency training in pathology at the University of South Florida in Tampa, FL, and her fellowship training in medical microbiology at the Cleveland Clinic. Her clinical interests include endemic mycoses, mycobacterial infections, and molecular diagnostics.
Gail L. Woods, M.D., is Professor of Pathology at the University of Arkansas for Medical Sciences and Chief of Pathology and Laboratory Medicine Service at the Central Arkansas Veterans Healthcare System in Little Rock, AR, where she has been on the faculty since 2005. She earned her undergraduate degree at Beloit College in Wisconsin and received her M.D. degree from Indiana University Medical School in Indianapolis, IN. Dr. Woods subsequently completed her residency training in pathology at the University of South Florida in Tampa, FL, and her fellowship training in medical microbiology at the Cleveland Clinic. Her clinical interests include endemic mycoses, mycobacterial infections, and molecular diagnostics.
REFERENCES
- 1.Abernathy, R. S. 1959. Clinical manifestations of pulmonary blastomycosis. Ann. Intern. Med. 51:707-727. [DOI] [PubMed] [Google Scholar]
- 2.Abernathy, R. S. 1967. Amphotericin therapy of North American blastomycosis. Antimicrob. Agents Chemother. 3:208-211. [PubMed] [Google Scholar]
- 3.Amini, M., W. E. Shams, T. Barklow, and F. A. Sarubbi. 2007. A 45-year-old man with fever and adult respiratory distress syndrome. Clin. Infect. Dis. 44:566-567, 615-616. [DOI] [PubMed] [Google Scholar]
- 4.Ashley, E. S. D., R. Lewis, J. S. Lewis, C. Martin, and D. Andes. 2006. Pharmacology of systemic antifungal agents. Clin. Infect. Dis. 43:S28-S39. [Google Scholar]
- 5.Axelson, G. K., T. Giorgadze, and G. A. Youngberg. 2008. Evaluation of the use of Congo red staining in the differential diagnosis of Candida vs. various other yeast-form fungal organisms. J. Cutan. Pathol. 35:27-30. [DOI] [PubMed] [Google Scholar]
- 6.Baily, G. G., V. J. Robertson, P. Neill, P. Garrido, and L. F. Levy. 1991. Blastomycosis in Africa: clinical features, diagnosis, and treatment. Rev. Infect. Dis. 13:1005-1008. [DOI] [PubMed] [Google Scholar]
- 7.Bakleh, M., A. J. Aksamit, I. M. Tleyjeh, and W. F. Marshall. 2005. Successful treatment of cerebral blastomycosis with voriconazole. Clin. Infect. Dis. 40:e69-e71. [DOI] [PubMed] [Google Scholar]
- 8.Bassett, F. H., and J. P. Tindall. 1972. Blastomycosis of bone. South. Med. J. 65:547-555. [DOI] [PubMed] [Google Scholar]
- 9.Baumgardner, D. J., B. P. Buggy, B. J. Mattson, J. S. Burdick, and D. Ludwig. 1992. Epidemiology of blastomycosis in a region of high endemicity in north central Wisconsin. Clin. Infect. Dis. 15:629-635. [DOI] [PubMed] [Google Scholar]
- 10.Baumgardner, D. J., E. M. Knavel, D. Steber, and G. R. Swain. 2006. Geographic distribution of human blastomycosis cases in Milwaukee, Wisconsin, U. S. A.: association with urban watersheds. Mycopathologia 161:275-282. [DOI] [PubMed] [Google Scholar]
- 11.Bialek, R., G. M. Gonzalez, D. Begerow, and U. E. Zelck. 2005. Coccidioidomycosis and blastomycosis: advances in molecular diagnosis. FEMS Immunol. Med. Microbiol. 45:355-360. [DOI] [PubMed] [Google Scholar]
- 11a.Blastomycosis Cooperative Study of the Veterans Administration. 1964. Blastomycosis: a review of 198 collected cases in Veterans Administration hospitals. Am. Rev. Respir. Dis. 89:659-672. [DOI] [PubMed] [Google Scholar]
- 12.Borchardt, S. M. 2005. Awareness of blastomycosis in Illinois needs to increase. Ill. Infect. Dis. Rep. 3:1-2. [Google Scholar]
- 13.Borgia, S. M., J. D. Fuller, A. Sarabia, and P. El-Helou. 2006. Cerebral blastomycosis: a case series incorporating voriconazole in the treatment regimen. Med. Mycol. 44:659-664. [DOI] [PubMed] [Google Scholar]
- 14.Bradsher, R. W., D. C. Rice, and R. S. Abernathy. 1985. Ketoconazole therapy for endemic blastomycosis. Ann. Intern. Med. 103:872-879. [DOI] [PubMed] [Google Scholar]
- 15.Bradsher, R. W. 1987. Water and blastomycosis: don't blame beaver. Am. Rev. Respir. Dis. 136:1324-1326. (Editorial.) [DOI] [PubMed] [Google Scholar]
- 16.Bradsher, R. W. 1988. Blastomycosis. Infect. Dis. Clin. North Am. 2:877-898. [PubMed] [Google Scholar]
- 17.Bradsher, R. W., M. R. Martin, T. D. Wilkes, C. Waltman, and K. Bolyard. 1990. Unusual presentations of blastomycosis: ten case summaries. Infect. Med. 7:10-19. [Google Scholar]
- 18.Bradsher, R. W. 1992. Blastomycosis. Clin. Infect. Dis. 14(Suppl. 1):S82-S90. [DOI] [PubMed] [Google Scholar]
- 19.Bradsher, R. W. 1996. Histoplasmosis and blastomycosis. Clin. Infect. Dis. 22:S102-S111. [DOI] [PubMed] [Google Scholar]
- 20.Bradsher, R. W., S. W. Chapman, and P. G. Pappas. 2003. Blastomycosis. Infect. Dis. Clin. North Am. 17:21-40. [DOI] [PubMed] [Google Scholar]
- 21.Branch, R. A. 1988. Prevention of amphotericin B-induced renal impairment: review on the use of sodium supplementation. Arch. Intern. Med. 148:2389-2394. [PubMed] [Google Scholar]
- 22.Reference deleted.
- 23.Busey, J. F. 1972. Blastomycosis: a comparative study of 2-hydroxystilbamidine and amphotericin B therapy. Am. Rev. Respir. Dis. 105:812-818. [DOI] [PubMed] [Google Scholar]
- 24.Cano, M. V., G. F. Ponce-de-Leon, S. Tippen, M. D. Lindsley, M. Warwick, and R. A. Hajjeh. 2003. Blastomycosis in Missouri: epidemiology and risk factors for endemic disease. Epidemiol. Infect. 131:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Challapalli, M., and D. G. Cunningham. 1996. North American blastomycosis of the vertebrae in an adolescent. Clin. Infect. Dis. 23:853-854. [DOI] [PubMed] [Google Scholar]
- 26.Chapman, S. W., A. C. Lin, K. A. Hendricks, R. L. Nolan, M. M. Currier, K. R. Morris, and H. R. Turner. 1997. Endemic blastomycosis in Mississippi: epidemiologic and clinical studies. Semin. Respir. Infect. 12:219-228. [PubMed] [Google Scholar]
- 27.Chapman, S. W., W. E. Dismukes, L. A. Proia, R. W. Bradsher, G. Campbell, P. G. Pappas, M. G. Threlkeld, and C. A. Kauffman. 2008. Practice guidelines for the management of patients with blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 46:1801-1812. [DOI] [PubMed] [Google Scholar]
- 28.Cherniss, E. I., and B. A. Waisbren. 1956. North American blastomycosis: a clinical study of 40 cases. Ann. Intern. Med. 44:105-123. [DOI] [PubMed] [Google Scholar]
- 29.Chowfin, A., R. Tight, and S. Mitchell. 2000. Recurrent blastomycosis of the central nervous system: case report and review. Clin. Infect. Dis. 30:969-971. [DOI] [PubMed] [Google Scholar]
- 30.Chu, J. H., C. Feudtner, K. Heydon, T. J. Walsh, and T. E. Zaoutis. 2006. Hospitalizations for endemic mycoses: a population-based national study. Clin. Infect. Dis. 42:822-825. [DOI] [PubMed] [Google Scholar]
- 31.Clemons, K. V., and D. A. Stevens. 1991. Comparative efficacies of amphotericin B lipid complex and amphotericin B deoxycholate suspension against murine blastomycosis. Antimicrob. Agents Chemother. 35:2144-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colonna, P. C., and T. Gucker. 1944. Blastomycosis of the skeletal system. J. Bone Joint Surg. 26:322-328. [Google Scholar]
- 33.Como, J. A., and W. E. Dismukes. 1994. Oral azole drugs as systemic antifungal therapy. N. Engl. J. Med. 330:263-272. [DOI] [PubMed] [Google Scholar]
- 34.Cook, P. P. 2001. Amphotericin B lipid complex for the treatment of recurrent blastomycosis of the brain in a patient previously treated with itraconazole. South. Med. J. 94:548-549. [PubMed] [Google Scholar]
- 35.Craig, M. W., W. N. Davey, and R. A. Green. 1970. Conjugal blastomycosis. Am. Rev. Respir. Dis. 102:86-90. [DOI] [PubMed] [Google Scholar]
- 36.Crampton, T. L., R. B. Light, G. M. Berg, M. P. Meyers, G. C. Schroeder, E. S. Hershfield, and J. M. Embil. 2002. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin. Infect. Dis. 34:1310-1316. [DOI] [PubMed] [Google Scholar]
- 37.De Groote, M. A., R. Bjerke, H. Smith, and L. V. Rhodes III. 2000. Expanding epidemiology of blastomycosis: clinical features and investigation of 2 cases in Colorado. Clin. Infect. Dis. 30:582-584. [DOI] [PubMed] [Google Scholar]
- 38.DiSalvo, A. F. 1992. The ecology of Blastomyces dermatitidis, p. 43-73. In Y. Al-Doory and A. F. DiSalvo (ed.), Blastomycosis. Plenum Publishing Corporation, New York, NY.
- 39.Dismukes, W. E., R. W. Bradsher, G. A. Cloud, C. A. Kauffman, S. W. Chapman, R. B. George, D. A. Stevens, W. M. Girard, M. S. Saag, C. Bowles-Patton, and the NIAID Mycoses Study Group. 1992. Itraconazole therapy for blastomycosis and histoplasmosis. Am. J. Med. 93:489-497. [DOI] [PubMed] [Google Scholar]
- 40.Dismukes, W. E. 2000. Introduction to antifungal drugs. Clin. Infect. Dis. 30:653-657. [DOI] [PubMed] [Google Scholar]
- 41.Drutz, D. J., and C. L. Frey. 1985. Intracellular and extracellular defenses against Blastomyces dermatitidis conidia and yeasts. J. Lab. Clin. Med. 105:737-750. [PubMed] [Google Scholar]
- 42.Durkin, M., J. Witt, A. LeMonte, B. Wheat, and P. Connolly. 2004. Antigen assay with the potential aid in diagnosis of blastomycosis. J. Clin. Microbiol. 42:4873-4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duttera, M. J., and S. Osterhout. 1969. North American blastomycosis: a survey of 63 cases. South. Med. J. 62:295-301. [PubMed] [Google Scholar]
- 44.Dworkin, M. S., A. N. Duckro, L. Proia, J. D. Semel, and G. Huhn. 2005. The epidemiology of blastomycosis in Illinois and factors associated with death. Clin. Infect. Dis. 41:e107-e111. [DOI] [PubMed] [Google Scholar]
- 45.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farber, E. R., M. S. Leahy, and T. R. Meadows. 1968. Endometrial blastomycosis acquired by sexual contact. Obstet. Gynecol. 32:195-199. [PubMed] [Google Scholar]
- 47.Farmer, C., M. W. Stanley, R. H. Bardales, S. Korourian, H. Shah, R. Bradsher, and V. S. Klimberg. 1995. Mycoses of the breast: diagnosis by fine-needle aspiration. Diagn. Cytopathol. 12:51-55. [DOI] [PubMed] [Google Scholar]
- 48.Frean, J. A., W. F. Carman, H. H. Crewe-Brown, G. A. Culligan, and C. N. Young. 1989. Blastomyces dermatitidis infections in the RSA. S. Afr. Med. J. 76:13-16. [PubMed] [Google Scholar]
- 49.Freifeld, A., L. Proia, D. Andes, L. M. Baddour, J. Blair, B. Spellberg, S. Arnold, A. Lentnek, and L. J. Wheat. 2009. Voriconazole use for endemic fungal infections. Antimicrob. Agents Chemother. 53:1648-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gehweiler, J. A., M. P. Capp, and E. W. Chick. 1970. Observations on the roentgen patterns in blastomycosis of bone. Am. J. Roentgenol. 108:497-510. [PubMed] [Google Scholar]
- 51.Gilchrist, T. C. 1894. Protozoan dermatitis. J. Cutan. Gen. Dis. 12:496-499. [Google Scholar]
- 52.Gilchrist, T. C., and W. R. Stokes. 1898. Cases of pseudo-lupus vularis caused by blastomyces. J. Exp. Med. 3:53-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Girouard, G., C. Lachance, and R. Pelletier. 2007. Observations on (1-3)-β-D-glucan detection as a diagnostic tool in endemic mycosis caused by Histoplasma or Blastomyces. J. Med. Microbiol. 56:1001-1002. [DOI] [PubMed] [Google Scholar]
- 54.Gnann, J. W., Jr., G. S. Bressler, C. A. Bodet III, and C. K. Avent. 1983. Human blastomycosis after a dog bite. Ann. Intern. Med. 98:48-49. [DOI] [PubMed] [Google Scholar]
- 55.Goldman, M., R. Zackin, C. J. Fichtenbaum, D. J. Skiest, S. L. Koletar, R. Hafner, L. J. Wheat, P. M. Nyangweso, C. T. Yiannoutsos. C. T. Schnizlein-Bick, S. Owens, J. A. Aberg, and AIDS Clinical Trials Group A5038 Study Group. 2004. Safety of discontinuation of maintenance therapy for disseminated histoplasmosis after immunologic response to antiretroviral therapy. Clin. Infect. Dis. 38:1485-1489. [DOI] [PubMed] [Google Scholar]
- 56.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Candelario, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 57.Harley, W. B., M. Lomis, and D. W. Haas. 1994. Marked polymorphonuclear pleocytosis due to blastomycotic meningitis: case report and review. Clin. Infect. Dis. 18:816-818. [DOI] [PubMed] [Google Scholar]
- 58.Hayden, R. T., X. Qian, G. D. Roberts, and R. V. Lloyd. 2001. In situ hybridization for the identification of yeastlike organisms in tissue section. Diagn. Mol. Pathol. 10:15-23. [DOI] [PubMed] [Google Scholar]
- 59.Herd, A. M., S. B. Greenfield, G. W. S. Thompson, and R. C. Brunham. 1990. Miliary blastomycosis and HIV infection. Can. Med. Assoc. J. 143:1329-1330. [PMC free article] [PubMed] [Google Scholar]
- 60.Inoshita, T., G. A. Youngberg, L. J. Boelen, and J. Langston. 1983. Blastomycosis presenting with prostatic involvement: report of 2 cases and review of the literature. J. Urol. 130:160-162. [DOI] [PubMed] [Google Scholar]
- 61.Istorico, L. J., M. Sanders, R. F. Jacobs, S. Gilleon, C. Glasier, and R. W. Bradsher. 1992. Otitis media due to blastomycosis: report of two cases. Clin. Infect. Dis. 14:355-358. [DOI] [PubMed] [Google Scholar]
- 62.Kelly, P. M. 1982. Systemic blastomycosis with associated diabetes insipidus. Ann. Intern. Med. 96:66-67. [DOI] [PubMed] [Google Scholar]
- 63.Klein, B. S., J. N. Kuritsky, and W. A. Chappell. 1986. Comparison of the enzyme immunoassay, immunodiffusion, and complement fixation in detecting antibody in human serum to A antigen of Blastomyces dermatitidis. Am. Rev. Respir. Dis. 133:144-148. [DOI] [PubMed] [Google Scholar]
- 64.Klein, B. S., J. M. Vergeront, R. J. Weeks, U. N. Kumar, G. Mathai, B. Varkey, L. Kaufman, R. W. Bradsher, J. F. Stoebig, and J. P. Davis. 1986. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N. Engl. J. Med. 314:529-534. [DOI] [PubMed] [Google Scholar]
- 65.Klein, B. S., J. M. Vergeront, and L. Kaufman. 1987. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J. Infect. Dis. 155:262-268. [DOI] [PubMed] [Google Scholar]
- 66.Klein, B. S., J. M. Vergeront, A. F. DiSalvo, L. Kaufman, and J. P. Davis. 1987. Two outbreaks of blastomycosis along rivers in Wisconsin: isolation of Blastomyces dermatitidis from riverbank soil and evidence of its transmission along waterways. Am. Rev. Respir. Dis. 136:1333-1338. [DOI] [PubMed] [Google Scholar]
- 67.Klein, B. S. 1992. Immunology of blastomycosis, p. 133-163. In Y. Al-Doory and A. F. DiSalvo (ed.), Blastomycosis. Plenum Publishing Corporation, New York, NY.
- 68.Kravitz, G. R., S. F. Davies, M. R. Eckman, and G. A. Sarosi. 1981. Chronic blastomycosis meningitis. Am. J. Med. 71:501-505. [DOI] [PubMed] [Google Scholar]
- 69.Lahm, T., S. Neese, A. T. Thornburg, M. D. Ober, G. A. Sarosi, and C. A. Hage. 2008. Corticosteroids for blastomycosis-induced ARDS. Chest 133:1478-1480. [DOI] [PubMed] [Google Scholar]
- 70.Landis, F. B., and B. Varkey. 1976. Late relapse of pulmonary blastomycosis after adequate treatment with amphotericin B: a case report. Am. Rev. Respir. Dis. 113:77-81. [DOI] [PubMed] [Google Scholar]
- 71.Lemos, L. B., M. Baliga, and M. Guo. 2001. Acute respiratory distress syndrome and blastomycosis: presentation of nine cases and review of the literature. Ann. Diagn. Pathol. 5:1-9. [DOI] [PubMed] [Google Scholar]
- 72.Lemos, L. B., M. Soofi, and E. Amir. 2002. Blastomycosis and pregnancy. Ann. Diagn. Pathol. 6:211-215. [DOI] [PubMed] [Google Scholar]
- 73.Lentnek, A. L., and I. A. Lentnek. 2006. Successful management of Blastomyces dermatitidis meningitis. Infect. Med. 23:39-41. [Google Scholar]
- 74.Li, R. K., M. A. Ciblak, N. Nordoff, L. Pasarell, D. W. Warnock, and M. R. McGinnis. 2000. In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob. Agents Chemother. 44:1734-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Linden, P., P. Williams, and K. M. Chan. 2000. Efficacy and safety of amphotericin B lipid complex injection (ABLC) in solid organ transplant recipients with invasive fungal infections. Clin. Transplant. 14:329-339. [DOI] [PubMed] [Google Scholar]
- 76.Lockwood, W. R., F. Allison, Jr., B. E. Batson, and J. F. Busey. 1969. The treatment of North American blastomycosis: ten years' experience. Am. Rev. Respir. Dis. 100:314-320. [DOI] [PubMed] [Google Scholar]
- 77.Lomaestro, B. M., and M. A. Piatek. 1998. Update on drug interactions with azole antifungal agents. Ann. Pharmacother. 32:915-928. [DOI] [PubMed] [Google Scholar]
- 78.Lopez, R., J. O. Mason, J. S. Parker, and P. G. Pappas. 1994. Intraocular blastomycosis: case report and review. Clin. Infect. Dis. 18:805-807. [DOI] [PubMed] [Google Scholar]
- 79.Martin, D. S., and D. T. Smith. 1939. Blastomycosis. I. A review of the literature. Am. Rev. Tuber. 39:275-304. [Google Scholar]
- 80.Martynowicz, M. A., and U. B. S. Prakash. 2002. Pulmonary blastomycosis: an appraisal of diagnostic techniques. Chest 121:768-773. [DOI] [PubMed] [Google Scholar]
- 81.McCullough, M. J., A. F. DiSalvo, K. V. Clemons, P. Park, and D. A. Stevens. 2000. Molecular epidemiology of Blastomyces dermatitidis. Clin. Infect. Dis. 30:328-335. [DOI] [PubMed] [Google Scholar]
- 82.Medoff, G., A. Painter, and G. S. Kobayashi. 1987. Mycelial to yeast-phase transitions of the dimorphic fungi, Blastomyces dermatitidis and Paracoccidioides brasiliensis. J. Bacteriol. 169:4055-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyer, K. C., E. J. McManus, and D. G. Maki. 1993. Overwhelming pulmonary blastomycosis associated with the adult respiratory distress syndrome. N. Engl. J. Med. 329:1231-1236. [DOI] [PubMed] [Google Scholar]
- 84.Mongkolrattanothai, K., M. Peev, L. J. Wheat, and J. Marcinak. 2006. Urine antigen detection of blastomycosis in pediatric patients. Ped. Infect. Dis. J. 25:1076-1078. [DOI] [PubMed] [Google Scholar]
- 85.Morris, S. K., J. Brophy, S. E. Richardson, R. Summerbell, P. C. Parkin, F. Jamieson, B. Limerick, L. Wiebe, and E. L. Ford-Jones. 2006. Blastomycosis in Ontario, 1994-2003. Emerg. Infect. Dis. 12:274-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morse, H. G., W. P. Nichol, D. M. Cook, N. K. Blank, and T. T. Ward. 1983. Central nervous system and genitourinary blastomycosis: confusion with tuberculosis. West. J. Med. 139:99-103. [PMC free article] [PubMed] [Google Scholar]
- 87.Mouzin, E. L., and M. A. Beilke. 1996. Female genitourinary blastomycosis: case report and review. Clin. Infect. Dis. 22:718-719. [DOI] [PubMed] [Google Scholar]
- 88.Murray, J. J., C. A. Clark, R. H. Lands, C. R. Heim, and L. S. Burnett. 1984. Reactivation blastomycosis presenting as a tuboovarian abscess. Obstet. Gynecol. 64:828-830. [PubMed] [Google Scholar]
- 89.National Institute of Allergy and Infectious Diseases Mycoses Study Group. 1985. Treatment of blastomycosis and histoplasmosis with ketoconazole: results of a prospective randomized clinical trial. Ann. Intern. Med. 103:861-872. [PubMed] [Google Scholar]
- 90.Nokes, S. R., J. Adametz, G. Gardner, and J. N. Beaton. 1995. Radiology case of the month: blastomycosis osteomyelitis with epidural and retropharyngeal abscess. J. Ark. Med. Soc. 92:253-254. [PubMed] [Google Scholar]
- 91.Odabasi, Z., G. Mattiuzzi, E. Estey, H. Kantarjian, F. Saeki, R. J. Ridge, P. A. Ketchum, M. A. Finkelman, J. H. Rex, and L. Ostrosky-Zeichner. 2004. β-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199-205. [DOI] [PubMed] [Google Scholar]
- 92.Ostrosky-Zeichner, L., B. D. Alexander, D. H. Kett, J. Vasquez, P. G. Pappas, F. Saeki, P. A. Ketchum, J. Wingard, R. Schiff, H. Tamura, M. A. Finkelman, and J. H. Rex. 2005. Multicenter clinical evaluation of the (1→3)-β-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654-659. [DOI] [PubMed] [Google Scholar]
- 93.Panicker, J., T. Walsh, and N. Kamani. 2006. Recurrent central nervous system blastomycosis in an immunocompetent child treated successfully with sequential liposomal amphotericin B and voriconazole. Pediatr. Infect. Dis. J. 25:377-379. [DOI] [PubMed] [Google Scholar]
- 94.Pappas, P. G., J. C. Pottage, W. G. Powderly, V. J. Fraser, C. W. Stratton, S. McKenzie, M. L. Tapper, H. Chmel, F. C. Bonebrake, R. Blum, R. W. Shafer, C. King, and W. E. Dismukes. 1992. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann. Intern. Med. 116:847-853. [DOI] [PubMed] [Google Scholar]
- 95.Pappas, P. G., M. G. Threlkeld, G. D. Bedsole, K. O. Cleveland, M. S. Gelfand, and W. E. Dismukes. 1993. Blastomycosis in immunocompromised patients. Medicine (Baltimore) 72:311-325. [DOI] [PubMed] [Google Scholar]
- 96.Pappas, P. G., R. W. Bradsher, S. W. Chapman, C. A. Kauffman, A. Dine, G. A. Cloud, W. E. Dismukes, and the National Institute of Allergy and Infectious Diseases Mycoses Study Group. 1995. Treatment of blastomycosis with fluconazole: a pilot study. Clin. Infect. Dis. 20:267-271. [DOI] [PubMed] [Google Scholar]
- 97.Pappas, P. G., R. W. Bradsher, C. A. Kauffman, G. A. Cloud, C. J. Thomas, G. D. Campbell, Jr., S. W. Chapman, C. Newman, W. E. Dismukes, and the National Institute of Allergy and Infectious Diseases Mycoses Study Group. 1997. Treatment of blastomycosis with higher doses of fluconazole. Clin. Infect. Dis. 25:200-205. [DOI] [PubMed] [Google Scholar]
- 98.Patel, R. G., B. Patel, M. F. Petrini, R. R. Carter, and J. Griffith. 1999. Clinical presentation, radiographic findings, and diagnostic methods of pulmonary blastomycosis: a review of 100 consecutive cases. South. Med. J. 92:289-295. [DOI] [PubMed] [Google Scholar]
- 99.Perfect, J. R. 2005. Treatment of non-Aspergillus moulds in immunocompromised patients, with amphotericin B lipid complex. Clin. Infect. Dis. 40(Suppl. 6):S401-S408. [DOI] [PubMed] [Google Scholar]
- 100.Pickering, J. W., H. W. Sant, C. A. P. Bowles, W. L. Roberts, and G. L. Woods. 2005. Evaluation of a (1→3)-β-D-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 43:5957-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pipitone, M. A., and H. M. Gloster. 2005. A case of blastomycosis in pregnancy. J. Am. Acad. Dermatol. 53:740-741. [DOI] [PubMed] [Google Scholar]
- 102.Pounder, J. I., D. Hansen, and G. L. Woods. 2006. Identification of Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides species by repetitive-sequence-based PCR. J. Clin. Microbiol. 44:2977-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Recht, L. D., J. R. Philips, M. R. Eckman, and G. A. Sarosi. 1979. Self-limited blastomycosis: a report of thirteen cases. Am. Rev. Respir. Dis. 120:1109-1111. [DOI] [PubMed] [Google Scholar]
- 104.Roos, K. L., J. P. Bryan, W. W. Maggio, J. A. Jane, and W. M. Scheld. 1979. Intracranial blastomycosis. Medicine (Baltimore) 66:224-235. [DOI] [PubMed] [Google Scholar]
- 105.Saccente, M., R. S. Abernathy, P. G. Pappas, H. R. Shah, and R. W. Bradsher. 1998. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin. Infect. Dis. 26:413-418. [DOI] [PubMed] [Google Scholar]
- 106.Sanders, L. L. 1967. Blastomycosis arthritis. Arthritis Rheum. 10:91-98. [DOI] [PubMed] [Google Scholar]
- 107.Sarosi, G. A., and S. F. Davies. 1979. Blastomycosis: state of the art. Am. Rev. Respir. Dis. 120:911-938. [DOI] [PubMed] [Google Scholar]
- 108.Sarosi, G. A., M. R. Eckman, S. F. Davies, and W. K. Laskey. 1979. Canine blastomycosis as a harbinger of human disease. Ann. Intern. Med. 91:733-735. [DOI] [PubMed] [Google Scholar]
- 109.Sarosi, G. A., K. J. Hammerman, F. E. Tosh, and R. S. Kronenberg. 1974. Clinical features of acute pulmonary blastomycosis. N. Engl. J. Med. 290:540-543. [DOI] [PubMed] [Google Scholar]
- 110.Schutze, G. E., S. L. Hickerson, E. M. Fortin, D. E. Schellhase, T. Darville, P. O. Gubbins, and R. F. Jacobs. 1996. Blastomycosis in children. Clin. Infect. Dis. 22:496-502. [DOI] [PubMed] [Google Scholar]
- 111.Schwarz, J., and K. Salfelder. 1977. Blastomycosis: a review of 152 cases. Curr. Top. Pathol. 65:165-200. [PubMed] [Google Scholar]
- 112.Suen, J. Y., S. J. Wetmore, and W. J. Wetzel. 1980. Blastomycosis of the larynx. Ann. Otol. 89:563-566. [DOI] [PubMed] [Google Scholar]
- 113.Sugar, A. M., and M. Picard. 1991. Macrophage- and oxidant-mediated inhibition of the ability of live Blastomyces dermatitidis conidia to transform to the pathogenic yeast phase: implications for the pathogenesis of dimorphic fungal infections. J. Infect. Dis. 163:371-375. [DOI] [PubMed] [Google Scholar]
- 114.Sugar, A. M., and X. P. Liu. 1996. In vitro and in vivo activities of SCH 56592 against Blastomyces dermatitidis. Antimicrob. Agents Chemother. 40:1314-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sugar, A. M., and X. P. Liu. 2001. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob. Agents Chemother. 45:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tarr, M., J. Marcinak, and K. Mongkolrattanothai. 2007. Blastomyces antigen detection for monitoring progression of blastomycosis in a pregnant adolescent. Infect. Dis. Obstet. Gynecol. 2007:89059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turner, S., L. Kaufman, and M. Jalbert. 1986. Diagnostic assessment of an enzyme-linked immunosorbent assay for human and canine blastomycosis. J. Clin. Microbiol. 23:294-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vaaler, A. K., R. W. Bradsher, and S. F. Davies. 1990. Evidence of subclinical blastomycosis in forestry workers in northern Minnesota and northern Wisconsin. Ann. Intern. Med. 89:470-476. [DOI] [PubMed] [Google Scholar]
- 119.Watts, E. A., P. D. Gard, Jr., and S. W. Tuthill. 1983. First reported case of intrauterine transmission of blastomycosis. Pediatr. Infect. Dis. 2:308-310. [DOI] [PubMed] [Google Scholar]
- 120.Wheat, J., H. Wheat, P. Connolly, M. Kleiman, K. Supparatpinyo, K. Nelson, R. Bradsher, and A. Restrepo. 1997. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin. Infect. Dis. 24:1169-1171. [DOI] [PubMed] [Google Scholar]
- 121.Witorsch, P., and J. P. Utz. 1968. North American blastomycosis: a study of 40 patients. Medicine 47:169-200. [DOI] [PubMed] [Google Scholar]
- 122.Witzig, R. S., D. J. Hoadley, D. L. Greer, K. P. Abriola, and R. L. Hernandez. 1994. Blastomycosis and human immunodeficiency virus: three new cases and review. South. Med. J. 87:715-719. [DOI] [PubMed] [Google Scholar]
- 123.Witzig, R. S., E. M. Quimosing, W. L. Campbell, D. L. Greer, and R. A. Clark. 1994. Blastomyces dermatitidis infection of the paranasal sinuses. Clin. Infect. Dis. 18:267-268. [DOI] [PubMed] [Google Scholar]
- 124.Wu, S. J., T. Valyi-Nagy, H. H. Engelhard, M. A. Do, and W. M. Janda. 2005. Secondary intracerebral blastomycosis with giant yeast forms. Mycopathologia 160:253-257. [DOI] [PubMed] [Google Scholar]