Abstract
Summary: The potential impact of the Three Gorges Dam (TGD) on schistosomiasis transmission in China has invoked considerable global concern. The TGD will result in changes in the water level and silt deposition downstream, favoring the reproduction of Oncomelania snails. Combined with blockages of the Yangtze River's tributaries, these changes will increase the schistosomiasis transmission season within the marshlands along the middle and lower reaches of the Yangtze River. The changing schistosome transmission dynamics necessitate a comprehensive strategy to control schistosomiasis. This review discusses aspects of the epidemiology and transmission of Schistosoma japonicum in China and considers the pathology, clinical outcomes, diagnosis, treatment, immunobiology, and genetics of schistosomiasis japonica together with an overview of current progress in vaccine development, all of which will have an impact on future control efforts. The use of synchronous praziquantel (PZQ) chemotherapy for humans and domestic animals is only temporarily effective, as schistosome reinfection occurs rapidly. Drug delivery requires a substantial infrastructure to regularly cover all parts of an area of endemicity. This makes chemotherapy expensive and, as compliance is often low, a less than satisfactory control option. There is increasing disquiet about the possibility that PZQ-resistant schistosomes will develop. Consequently, as mathematical modeling predicts, vaccine strategies represent an essential component in the future control of schistosomiasis in China. With the inclusion of focal mollusciciding, improvements in sanitation, and health education into the control scenario, China's target of reducing the level of schistosome infection to less than 1% by 2015 may be achievable.
INTRODUCTION
Schistosomiasis (bilharzia) is an intravascular disease caused by parasitic trematode worms of the genus Schistosoma. The burden of disease attributable to the three major human schistosome species (Schistosoma mansoni, S. haematobium, and S. japonicum) is estimated to be 1.7 to 4.5 million disability-adjusted life years (DALYs). However, reassessment of schistosome-related disability (107), particularly disability weights for S. japonicum (which are 20 to 30 times higher than those used for the calculation of the global disease burden estimate [68, 98]), combined with recent information on global prevalence (188) indicates that the true burden is substantially greater than previously appreciated (68).
In China, zoonotic schistosomiasis japonica, also called “snail fever,” is associated with chronic liver and intestinal fibrosis; it is a major health risk for more than 50 million Chinese, with approximately 1 million people and several hundred thousand livestock currently infected (12, 140, 162, 187, 195, 235, 236). Major foci of endemicity occur in the marsh and lake (Dongting Lake and Poyang Lake) regions along the Yangtze River basin, where the elimination of transmission has proven difficult (162, 235, 236). Archaeological studies have revealed that the disease has a very long history in China (162). S. japonicum eggs were identified in a female corpse dating back to the Western Han dynasty some 2,100 years ago that was exhumed in 1971 in Hunan Province. Schistosome eggs were also found in the liver of another corpse buried 100 years earlier in Jianglin Hsien, Hubei Province. In old volumes of traditional Chinese medicine, a description of clinical symptoms resembling Katayama syndrome (acute schistosomiasis; see below) can be traced back to 400 B.C.E. The first reported clinical diagnosis in modern China was made by an American physician (O. T. Logan) in 1905 in Hunan Province.
After the founding of the People's Republic of China in 1949, large-scale epidemiological surveys were carried out by Chinese scientists to determine the incidence, prevalence, and intensity of S. japonicum infections. The results revealed that schistosomiasis was endemic in 380 counties in 12 provinces, mainly south of the Yangtze River. Approximately 12 million people were infected, with an additional 100 million people at serious risk. A total of 14,000 km2 of infected Oncomelania flood plains were identified as potential transmission zones despite remarkable successes in schistosomiasis control achieved over the previous 4 decades (235). Subsequent control efforts resulted in a substantial reduction in the prevalence of infection with S. japonicum in humans, to fewer than a million cases in 2004, with the number of provinces where schistosomiasis is endemic reduced to seven (235).
Although it has weaknesses, the schistosomiasis control program for China is recognized as one of the most successful globally, with mass chemotherapy as its cornerstone (63, 235). Nevertheless, recent control efforts appear to be stagnating, and because of this and the potential for reemergence of schistosomiasis, the Chinese authorities regard the disease as one of the top public health priorities for China, on par with HIV/AIDS, tuberculosis, and hepatitis B (193). S. japonicum also debilitates infected domestic livestock which are used for food and as work animals, consequently adding to the economic burden and suffering of communities where the disease is endemic (162).
Schistosomiasis is strongly linked with poverty, and despite recent improvements in the rural Chinese economy, which have resulted in a better standard of living, the prevalence of S. japonicum and its associated morbidity have continued to rise in some localities (13, 69, 112, 197, 235). Large population movements taking place in southern China have exacerbated the schistosomiasis problem (117). The downturn in the world economy in 2007 has also induced further population movements as businesses in urban centers close and employment opportunities contract. This is likely to impact negatively schistosomiasis control efforts, with millions of residents returning from cities to their home villages in areas of endemicity to seek employment.
The threat posed by the Three Gorges Dam (TGD), which is the world's largest hydroelectric project and is aimed at developing and controlling the Yangtze River, reducing flooding in the lower plains regions, and hence ameliorating economic losses (238), will further undermine control efforts. It will change the Yangtze Basin ecology and associated schistosomiasis transmission risks over the next decade and beyond (193, 236). This important issue is covered in detail below.
LIFE CYCLE OF S. JAPONICUM
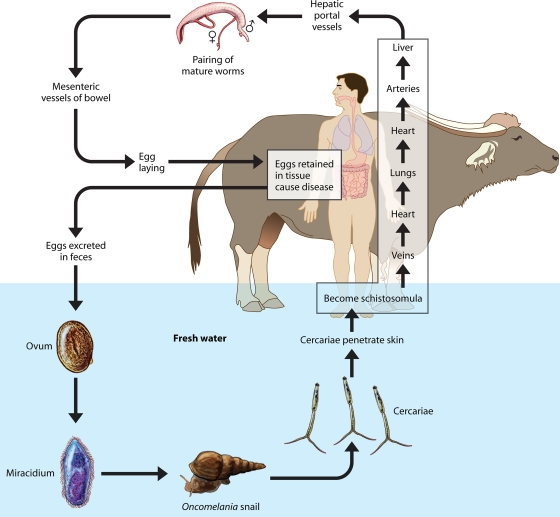
The life cycle of S. japonicum is shown in Fig. 1. The parasite is transmitted through freshwater containing free-swimming larval forms called cercariae. These penetrate the skin of humans and a wide range of other animals, including water buffaloes, cattle, rodents, dogs, sheep, pigs, and dogs, which can act as reservoirs for human transmission (67, 136, 162, 235). The cercariae shed their bifurcated tails and transform their trilaminate (single-lipid-layer) tegument into an unusual heptalaminate (double-lipid-layer) form. This is exquisitely adapted to the mammalian environment, with the ability to control water and drug uptake (64) and to modulate or subvert host immunological mediators at the schistosome surface (19). Now schistosomula, they leave the skin via the blood vessels and draining lymphatics and reach the lungs. Unlike other trematodes, schistosomes are dioecious (i.e., they have separate sexes), with the adults having a cylindrical body 7 to 20 mm in length featuring two terminal suckers, the complex tegument, a blind digestive tract, and reproductive organs. After several days the male and female worms exit the lungs and arrive in the hepatic portal system, where they mature, pair up, and migrate downstream. The worm pairs reach mucosal branches of the inferior mesenteric and superior hemorrhoidal veins, and the females then begin egg production. The process of migration and maturation takes about 4 to 5 weeks, depending on the host species involved. Many eggs pass through the intestinal wall and are discharged in the feces.
FIG. 1.
Life cycle of Schistosoma japonicum.
The eggs of S. japonicum are characteristically rounded and have a shell with a reduced lateral spine. Schistosome eggshells are quinone tanned, a chemical process in which eggshell precursors are modified under the action of tyrosinase enzymes which catalyze both the hydroxylation of tyrosine residues in eggshell precursor proteins to dihydroxyphenylalanine and the subsequent oxidation to dopaquinone. Two tyrosinases have been described in S. japonicum (29). The life cycle is completed when the eggs hatch. The contained miracidia are liberated explosively, while still encapsulated within their subshell envelopes (103), and in turn, these infect receptive amphibious Oncomelania hupensis freshwater snails. The miracidium forms a sporocyst at the site of penetration, and this produces daughter sporocysts that migrate to the snail hepatopancreas and asexually produce larval cercariae for daily release into the surrounding water.
EPIDEMIOLOGY AND TRANSMISSION
The epidemiology and transmission of schistosomiasis constitute a complicated process that is determined by many biological, ecological, social, and economic factors involving interaction between the various hosts, life cycle stages, and the external environment.
The Lakes and Marshlands of South China
The lake and marshland region is one of the three types of region, determined by eco-epidemiological and topological characteristics, where S. japonicum transmission currently occurs in China. The other two regions are the plains region, with waterway networks, and the hilly and mountainous regions (120, 133). The majority of transmission is within the lake and marshland areas, predominantly around China's two largest lakes, Poyang Lake in Jiangxi Province and Dongting Lake in Hunan Province, thus making them major foci of endemicity and representative of the lake and marshland region as a whole (81, 162, 233).
Transmission Patterns
Although the risk factors (61, 119, 166, 223) and means of becoming infected (i.e., occupation [fishing and farming], domestic duties [washing], and social activities [swimming]) are likely similar in the areas of endemicity around both Poyang and Dongting Lakes, there are subtle differences in the patterns of transmission. The areas of endemicity in the Poyang Lake region have two distinct transmission seasons. The first is in the spring/summer from April to June/July and is concurrent with the annual flood season (38, 162). As the temperature becomes warmer and the lake water starts to rise, the female Oncomelania snails commence producing masses of eggs (March/April). These hatch (April/May), and by June the number has significantly increased and there is a mix of both young and old snails releasing S. japonicum cercariae into the water in high abundance. At the same time, humans and animals are becoming exposed to the infective cercariae, as the warm weather is conducive to an increased frequency of water contact due to the favorable conditions for agriculture, fishing, and swimming. Furthermore, S. japonicum miracidia hatch from the eggs in feces deposited by infected humans and animals, and these subsequently infect Oncomelania snails (38).
By July the water levels have reached their flood peak, and this marks the end of the first transmission season (38). During the period from July to August/September, very little transmission takes place, as the cercarial density is low due to the drowning of the older snails (greater than 1 year of age), there is no agricultural work performed, and limited fecal contamination of the marshland occurs. The aquatic nature of the young snails does, however, ensure parasite survival over this period, and as the water begins to subside (up to 90% of the lake's volume) in September/October, the second transmission season begins (38). This continues through to November and is relatively short compared to the first. The end of the second transmission season coincides with the late autumn/early winter cold weather and the dry season. During this period, the young snails bury themselves underground to ensure survival (38, 162).
Transmission patterns in the Dongting Lake region are slightly different in that transmission does not occur in two distinct seasons; rather, there is continual transmission from spring to autumn but with two distinct peaks. The transmission season starts in April, coinciding with the commencement of the annual rainy season, and similar to the case for Poyang Lake, conditions become favorable for snails as the water levels rise and the temperature increases due to the onset of spring (118). The first peak in transmission is in spring/summer from May to July, when the highest numbers of snails are infected. When the water level reaches its peak in July/August, the older snails die off, and although transmission still occurs, it is quite low (118). The second peak in transmission occurs in autumn from September to November, when the water levels fall. Similar to the case for Poyang Lake, the end of the transmission season in Dongting Lake coincides with the cold weather of late autumn/early winter and the dry season, when the snails bury themselves underground in order to survive (38, 162).
Zoonotic Transmission
The zoonotic nature of S. japonicum complicates transmission and control. It is estimated that over 40 species of wild and domestic animals comprising 28 genera and seven orders can be infected (199). It was initially believed that those of public health importance were rats, dogs, pigs, sheep and goats, cattle, and water buffaloes (102, 169, 199). In China, however, dogs, pigs, rats, and goats are likely to contribute only minimally to overall transmission. This is because until recently dogs were relatively uncommon in rural China (162), pigs are short-lived and are confined to pens within the communities and so have limited water contact (162), rats (field rats [Rattus norvegicus] and albino rats [R. norvegicus albus]) produce limited amounts of feces and harbor female S. japonicum worms with few viable eggs (66, 89, 92, 102, 144, 199), and sheep and goats also have low fecal output and are present on the marshlands for only limited periods as they are sold at an early age as a food source (89, 199).
Bovines, particularly water buffaloes (Bubalus bubalis), play a major role in the transmission of S. japonicum in China (40, 44, 50, 51, 66, 77, 79, 81, 83, 89, 92, 102, 144, 162, 191, 194, 199, 236). Significantly, the daily fecal output from a water buffalo (∼25 kg) has been estimated to be at least 100 times that produced by a human individual (250 g) (89, 162). Accordingly, a recent study has shown that the environmental contamination attributable to 238 infected bovines (225 water buffaloes and 13 cattle) was approximately 28.7 million eggs/day (79), emphasizing their considerable contribution to the deposition of S. japonicum eggs into the external environment. Furthermore, a praziquantel (PZQ)-based intervention study (1998 to 2003) (83) around Poyang Lake in Jiangxi Province provided the first experimental proof that water buffaloes are major reservoir hosts for human S. japonicum infection. The trial showed that water buffalo chemotherapy affected human infection rates, by showing a greater reduction in human incidence in an intervention village (where all humans and water buffaloes were subjected to PZQ treatment) than in a control village (with human PZQ treatment only). Mathematical modeling (202) supported this conclusion and predicted that water buffaloes were responsible for approximately 75% of human transmission in this setting (83). Furthermore, a molecular field survey of S. japonicum in China using microsatellite markers (see below) showed that humans and bovines contribute considerably more to the parasite reservoir within snails than other definitive host species (196).
A similar but more stringent PZQ intervention trial in bovines was undertaken in 2004 to 2007 (77, 79); it employed a cluster-randomized design with increased power, with the additional aim of providing general applicability to the lakes and marshlands of southern China. The trial involved four matched pairs of villages in Hunan and Jiangxi Provinces, with one village within each pair randomly selected as a control (human PZQ treatment only) and the other left as the intervention group (human and bovine PZQ treatment). A sentinel cohort of people to be monitored for new infections for the duration of the study was selected from each village. Trial results showed that a combination of human and bovine chemotherapy had a greater effect on human incidence than human treatment alone. This was supported by Poisson regression analyses yielding crude and adjusted (for water contact) relative risks of 0.5 (P = < 0.001) and 0.54 (P = < 0.001). These results provided further proof that the incidence of human S. japonicum infection could be reduced through the reduction of infection rates in water buffaloes. Furthermore, the efficacy of twice-annual bovine chemotherapy for reducing human S. japonicum infection in the lakes and marshlands was calculated to be 46% (77).
Spatial Epidemiology
Spatial epidemiology, geographical information systems (GIS), remote sensing (RS), and the use of advanced Bayesian based spatial statistics have become important tools in China's national schistosomiasis control program over the past 10 to 15 years (82, 159, 173, 235). A comprehensive review of these techniques with applications to the epidemiology and control of schistosomiasis in China is available (221). Predictions on infection risk and active transmission sites are made mainly by the application of the normalized difference vegetation index (NDVI) or land surface temperature (LST) to predict Oncomelania habitats (116, 232).
One such study (82) employed RS and GIS techniques to identify habitats of O. hupensis in the Poyang Lake area. Multitemporal Landsat TM 5 satellite images were used to derive land use types from the dry and wet seasons, as well as to extract the NDVI. The derived environmental features were used to develop a composite model that predicted an estimated 709 km2 of marshlands in Poyang Lake as potential habitats for O. hupensis. In a further step, the predicted snail habitats were used as centroids, and buffer zones were established around them. Villages with an overall prevalence of S. japonicum below 3% were located more than 1.2 km away from the centroids. A gradient of high to low prevalence was observed with increasing distance from centroids. The developed model has proved to be an important tool for identifying high-risk areas for S. japonicum transmission.
Molecular Epidemiology
Current chemotherapy-based control, even when combined with large-scale mollusciciding against the Oncomelania snail intermediate hosts, will have limited further impact upon prevalence levels among humans in China. This is attributable partly to the presence of nonhuman definitive host reservoirs, particularly water buffaloes, which, as discussed above, contribute substantially to schistosomiasis transmission in the marshland and lake areas. Molecular epidemiologic studies provide the means to elucidate S. japonicum transmission patterns across multiple host species, with important implications for control, particularly in terms of identifying which nonhuman mammals are most important as zoonotic reservoirs and should thus be targeted by interventions such as chemotherapy and/or vaccination (168).
A range of different protein- and DNA-based techniques have been used to study the population genetics of S. japonicum in China (175), but the recent application of multiplexed microsatellite markers for genotyping isolates has proven especially useful (168, 175, 196, 226). One study has provided molecular evidence that bovines contribute much more than other domesticated animals such as dogs, cats, pigs, and goats toward parasite genotypes within humans (196), thereby confirming these as major reservoirs for schistosomiasis in the low-lying marshland/lake regions of China. Another study has provided an indication that, in contrast to bovines in the marshlands, rodents and dogs may feature as infection reservoirs in some of the hilly/mountainous regions of China, where bovines are scarce (168). If this proves to be the case, control of infection among wild rodent populations could be extremely challenging in these areas (168), although currently by far the majority of schistosomiasis cases in China are concentrated in the lake and marshland regions (235).
IMPACT OF THE TGD ON TRANSMISSION
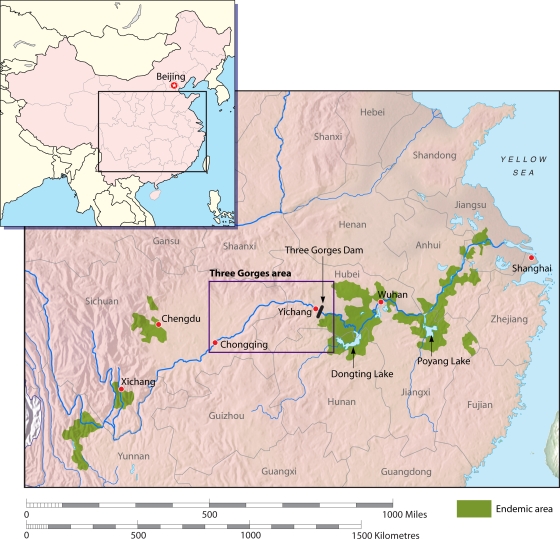
The TGD is located in the Three Gorges region in the upper reaches of the Yangtze River (the world's third-largest river [5,920 km long]) (Fig. 2). It spans the Yangtze at Sandouping Island, just west of the city of Yichang in Hubei Province. The dam was first suggested by nationalist leader Sun Yat-Sen in 1919 and later became a dream of communist leader Mao Zedong. In 1992, this dream became a reality when the Chinese government officially announced plans for construction, which commenced in 1994, and by 2003 it was close to a height of 135 m. In 2009, it reached its full height of 185 m and began to generate 18,600 MW of power for the whole of China (176). It will also help to control the lower Yangtze floods, which cause periodic disasters and which are much feared.
FIG. 2.
The Three Gorges Dam, Poyang Lake, Dongting Lake, and Hunan, Jiangxi, Hubei, Jiangsu, and Anhui Provinces. (Adapted from reference 177 with permission of the publisher.)
By 2010, the 2.3-km-long dam will result in a 600-km-long reservoir that will inundate 115,000 acres of cultivated land, requiring resettlement of approximately 2 million people (176). This critical time point marks the start of environmental changes that will have a considerable impact on S. japonicum transmission. The construction of the dam will change water and sand distributions downstream and hence have a significant impact on ecological systems, including Dongting and Poyang Lakes and the canals of Hubei (Fig. 2), where S. japonicum transmission is projected to increase. Schistosomiasis is currently not endemic in the Three Gorges region above the dam, as the rapidly flowing environment is extremely hostile for snail reproduction, and to date, no oncomelanid snails have been detected there (238). However, the 600-km lake created behind the dam, which will stretch upstream, will have shorelines suitable in many areas for snail breeding and schistosome transmission. The lake will be located between two key transmission zones of S. japonicum, Sichuan and Hubei-Hunan. The downstream schistosomiasis-free buffer is only 40 km, and the upstream buffer is 500 km. It is feared that in time these two areas of endemicity will merge into one, thus spreading the disease into a new location. It is anticipated that the spread of schistosomiasis into this area where it is currently not endemic will be expedited by infected humans traveling on the newly created waterways and by the introduction of the intermediate snail hosts. Once schistosomes are established in the lake, they would be difficult to eliminate (162).
In addition to the problems posed by the new lake, the downstream area will undergo massive change in ways that could also affect schistosome transmission. Downstream flows will be higher in winter and lower in summer, and the annual flooding will be prevented. The marshlands will expand. Silt deposition will change, and some areas will become more suitable habitats for Oncomelania snails and other areas less so. The current two transmission seasons may become one long season with the absence of the annual floods, which drown the adult snail intermediate hosts.
The transmission season may also be further extended by climate change, with the temperature remaining longer above the optimal 10°C required for miracidium release. It has also been estimated (221) that climate change has expanded the potential schistosomiasis transmission area by 41,335 km2, translating to an additional 20.7 million people at risk, via the shifting of the historical 0 to 1°C January isotherm from a latitude of 33°15′ N to 33°41′ N. Furthermore, two lakes forming part of the proposed south-north water transfer project are located within this new transmission area and may pose additional challenges for control (220). However, a study to test the survival of Oncomelania snails in the colder temperatures of northern China showed that the reproductive organs became abnormal, suggesting that climate change would have to considerably increase the winter temperature if the south-north water diversion project was to increase the schistosomiasis transmission areas to northern China (192). Nevertheless, these factors are certain to alter the distribution and endemicity of schistosome infection in ways that are unpredictable at present, calling for rigorous monitoring and surveillance of schistosomiasis in a future, warmer China (237). Seto and colleagues (173) believe that overall snail densities may decrease with lower and more stable water levels but that the density of infected snails and corresponding human infections may increase due to the colocation of bovine grazing areas, snail habitat, and human activity that may occur with the more stable water levels.
The TGD will result in large changes to the flow, depth, and sedimentation load of the Yangtze River; subsequently, the distribution and numbers of schistosome-infected snails will be altered, increasing transmission of S. japonicum and its reintroduction into areas that were previously controlled (162, 187, 238). Mathematical modeling predicts a marked elevation in snail-breeding areas, increased rates of infection with S. japonicum, and greater associated morbidity (229, 235). Further, the “Return Land to Lake” program will significantly extend the water surface area in Dongting and Poyang Lakes; thousands of high-risk farmers and fishermen are being resettled closer to lake water and snail habitats (117). This will also have an impact on schistosomiasis transmission, as water contact and the prevalence and intensity of infection will increase (117, 178, 211). Another important consideration is that migrants relocated downstream from areas upstream of the TGD where schistosomiasis is not endemic will have no immunity to an infection with S. japonicum and hence will readily acquire infection on exposure and likely will develop severe disease as a result. A description of the pathological and clinical outcomes of schistosomiasis japonica follows.
PATHOLOGY AND CLINICAL OUTCOMES
Acute Disease
In nonimmune individuals, the first clinical presentation of acute schistosomiasis is delayed by several weeks or even months. The symptom complex, known as Katayama fever and more recently termed Katayama syndrome, is an early clinical manifestation of first infection or heavy reinfection with schistosome cercariae (164). This acute form of the disease was first described in Japan over 100 years ago, yet it remains the least known of all the clinical stages of schistosomiasis.
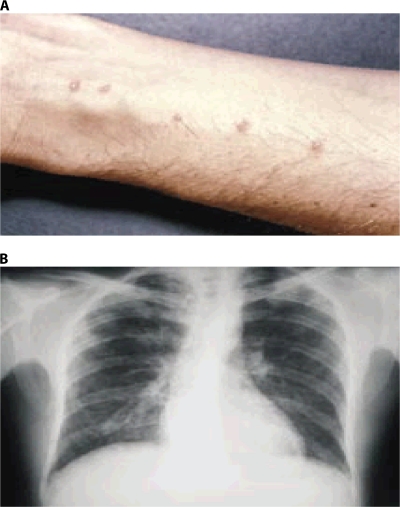
Percutaneous penetration of the cercariae can provoke a temporary urticarial rash that can manifest within hours and persist for days as maculopapular lesions (8, 111, 164, 165, 190, 225). In temperate climate zones, a similar “swimmers itch” is also frequently seen with avian trematode cercariae (23). The presentation of delayed-onset dermatitis, manifested as urticaria or angioedema, can occur within 1 to 12 weeks after heavy exposure to cercaria-infested water (22, 36, 48, 164, 165), with initial symptoms subsiding within 48 h. The skin lesions are often puritic. They are discrete erythematous raised lesions that vary in size from 1 to 3 cm (Fig. 3A) (20, 36, 48, 164, 165). Because of the temporal association with water exposure, the diagnosis is usually suspected clinically.
FIG. 3.
Clinical features of acute schistosomiasis. (A) Cercarial dermatitis. (Reprinted from the Centers for Disease Control and Prevention website [http://www.dpd.cdc.gov/dpdx/HTML/ImageLibrary/A-F/CercarialDermatitis/body_CercarialDermatitis_il2.htm].) (B) Pulmonary infiltrates. (Reprinted from the Prince Leopold Institute of Tropical Medicine website [http://www.itg.be/itg/DistanceLearning/LectureNotesVandenEndenE/imagehtml/ppages/CD_1069_051c.htm] with permission of the publisher.)
Katayama syndrome is a systemic hypersensitivity reaction against the migrating schistosomula and eggs and can occur within 14 to 84 days after a primary infection (164, 165). In many cases, acute infections are asymptomatic due to low cercarial loads and/or immunity. Symptoms that do manifest, however, are thought to be a result of an allergic reaction during larval migration and early oviposition by adult worms, and they will vary in severity depending on the infecting species (47, 91, 164, 165, 190). The delay in symptoms by weeks to months after exposure adds to the diagnostic difficulty. Disease onset is usually sudden, producing many nonspecific symptoms, such as fever, fatigue, myalgia, malaise, urticaria, nonproductive cough, eosinophilia, and patchy pulmonary infiltrates on chest radiographs (Fig. 3B) (52, 161, 164). Examples of pronounced radiological alterations include thickening of bronchial walls and beaded micronodulation in the lower pulmonary fields (161, 164). Abdominal symptoms may develop within a few weeks because of migration of juvenile worms and egg deposition of the mature worms (164, 165). High-grade nocturnal fever and eosinophilia are generally present (164). Most patients recover spontaneously after 2 to 10 weeks, whereas some develop persistent and more serious disease, with weight loss, dyspnoea, diarrhea, diffuse abdominal pain, hepatomegaly, and generalized rash.
Katayama syndrome caused by S. japonicum infections is not restricted to primary infection but also occurs in people living in areas of endemicity with a history of previous infection and a current exposure to high cercarial load. In China, for example, rebound epidemics have typically been reported in communities where the disease is endemic and that are exposed to floods (39, 162, 164).
Chronic Disease
Many of the eggs released by female S. japonicum worms are trapped in the tissues of the mammalian host instead of being excreted. Each female worm produces up to 3,500 eggs per day, and a large proportion lodge within host tissues. It is these retained eggs, which elicit intense inflammatory responses, rather than the worms themselves that cause the principal pathology associated with schistosomiasis (80, 165). The eggs contain miracidia that mature over 5 days and remain alive for up to 20 days. Chronic disease results from the host's immune response to the eggs and the granulomatous reaction evoked by the antigens (glycoproteins appear to be particularly relevant [172]) secreted by the miracidia. The mechanisms involved in schistosome-induced immunopathogenesis have been reviewed extensively (15, 26, 80, 165, 204, 213) and so will not be covered in depth here. Most of the available information comes from research with murine models, which indicates that the determinants of granuloma size may frequently dissociate from those of hepatic fibrosis (15, 26, 165, 204, 213). Metabolic profiling of a patent schistosome infection in mouse tissues (jejunum, ileum, colon, liver, spleen, and kidney) using 1H magic angle spinning nuclear magnetic resonance spectroscopy revealed, as might be expected, a clear gross disruption of metabolism at the molecular level (115).
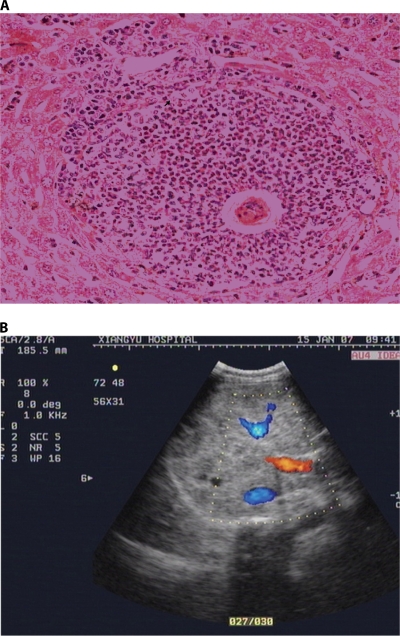
The intensity and duration of infection may determine the amount of antigen released and the severity of chronic fibro-obstructive disease. The granulomas destroy the ova but result in fibrotic deposition in host tissues. Most granulomas develop at the sites of maximal accumulation of eggs, which are the intestine and the liver in the case of S. japonicum. A characteristic perioval granuloma forms (Fig. 4A), with a necrotic center containing the egg or egg cluster surrounded by epithelioid cells, giant cells, and lymphocytes and an outer layer of plasma cells, eosinophils, and fibroblasts. Single eggs are usually reabsorbed, but the tissue damage leads to fibrosis. Large egg clusters tend to calcify.
FIG. 4.
Clinical features of chronic schistosomiasis. (A) A characteristic perioval granuloma formed around a Schistosoma japonicum egg in a mouse liver. (B) An ultrasonogram showing gross hepatic fibrosis (grade 3).
Chronic infection and granulomatous inflammation lead to the excess deposition of collagen and other extracellular matrix components. Collagen deposition, cross-linking, contraction, and reabsorption are in dynamic balance, and each component is subject to immunoregulation. Periportal collagen deposits lead to the progressive obstruction of blood flow, portal hypertension, and ultimately varices, variceal bleeding, hepatosplenomegaly, and hypersplenism. This periportal fibrosis can be seen on ultrasonography, computed tomography, or magnetic resonance imaging and is characteristic of schistosomiasis (Fig. 4B). Hepatocellular synthetic function is preserved until the very late stages of disease. Ultrasonography, in addition to clinical examination, is used to detect and quantify hepatosplenic disease based on World Health Organization criteria (165).
Other Morbidities
Neurological disease is often associated with S. japonicum infections (80, 164, 165) and is thought to occur through aberrant migration of adult worms to the brain (Fig. 5) or spinal cord. It often occurs early during an infection, but it is probably a clinical entity distinct from Katayama syndrome, since the systematic manifestations are generally absent. Transverse myelitis is the most common neurological symptom.
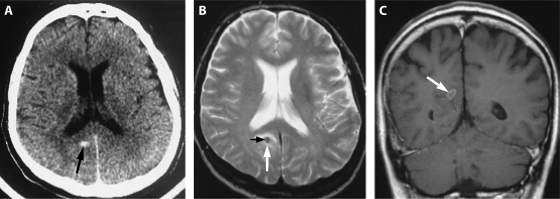
FIG. 5.
Cerebral schistosomiasis. (A) An unenhanced axial computed tomography (CT) scan shows a small, oval, hyperdense lesion (arrow) in the paraventricular zone, dorsal of the right posterior horn. (B) An axial T2-weighted (2437/90/1 [repetition time/echo time/excitations]) magnetic resonance (MR) image shows a hypointense lesion (white arrow) with a small, centrally located area with an intermediate signal (black arrow). (C) A coronal contrast-enhanced T1-weighted (600/15/2) MR image shows an oval lesion with an intermediate signal (arrow) with ringlike and septumlike contrast enhancement. (Reprinted from reference 156a with permission of the publisher. © by American Society of Neuroradiology).
Schistosome infection during childhood can cause considerable growth retardation and anemia (80, 165). Successful chemotherapy leads to substantial but incomplete catch-up growth and improvement in hemoglobin levels (80, 165). Infected children may also have cognitive impairment and memory deficits, and schistosome infection appears to have adverse effects on both maternal health and the fetus (80, 165).
DIAGNOSIS
Correct diagnosis is central to treating schistosomiasis and for its control. Case finding and community treatment, assessment of morbidity, and evaluations of control strategies all build on the results from diagnostic tests. There are four major approaches for diagnosis of S. japonicum: direct parasitological methods (detecting eggs in the stool or in tissues); indirect methods of detecting morbidity via clinical, subclinical, or biochemical markers; serological methods to detect immunological responses to antigens or the antigens themselves; and PCR-based techniques.
Parasitological Methods
The detection of S. japonicum eggs in feces is diagnostic of schistosomiasis japonica (162, 165, 167, 187, 203). The Kato-Katz thick-smear technique (105) is widely used, as it is cost-effective, easy to learn, and not technically difficult and requires limited special resources (33, 157). This rapid, simple, and inexpensive method requires 40 to 50 mg of feces and is used widely in community surveys, in field studies, and in the national control program in China to determine the burden of eggs in feces (162, 187, 228). A number of studies of the utility of the Kato-Katz technique in the diagnosis of schistosomes have shown variation in sensitivity, with various stool samples and slide preparations per stool employed. In general, as the numbers of stool samples collected and slides prepared increase, so does the sensitivity (59, 227). Studies on S. japonicum have also shown day-to-day variation in stool egg counts and also clustering of eggs within the stool (163, 227).
Although the sensitivity of the Kato-Katz technique increases with the number of stool samples and slide preparations, so does the time and manpower required. The use of formalin-based techniques for sedimentation and concentration may increase the diagnostic yield but may not be useful for patients with few eggs (124, 162). The Helmintex test for detection of schistosome eggs in feces, through their interaction with paramagnetic beads in a magnetic field, has recently been described (65). Although highly sensitive, its cost-effectiveness will need to be considered against that of the Kato-Katz technique to determine whether it has wide applicability in a variety of field settings.
The miracidium-hatching test has been used extensively by public health workers in China to rule out S. japonicum infection (162). The test is initiated by the concentration of ova from saline using fresh feces through a nylon tissue bag and suspension in distilled water. Miracidia that hatch from ova are visualized macroscopically, and their presence is diagnostic of infection. The miracidium-hatching test is also quite often used for the diagnosis of schistosomiasis in animals, and intensity of infection is subsequently assessed using a traditional Chinese sedimentation method (81). In patients with a typical clinical presentation but negative for fecal eggs, a biopsy of the rectal mucosa should be considered for diagnosis (165).
Indirect Methods
Indirect methods for diagnosing S. japonicum by the detection of morbidity via clinical, subclinical, or biochemical markers are generally not very specific given the generalized presentation of the disease. Current indirect methods include the use of clinical assessment of the patient coupled with ultrasound, liver biopsy, and subsequent histological examination and the measurement of biochemical markers (162, 165).
Hepatosplenomegaly and portal hypertension are seen with S. japonicum infection, and ultrasonography can detect hepatosplenic involvement. Detection of changes to the liver parenchyma has been shown to be associated with S. japonicum infection, but the grading standard of ultrasound needs to be improved. The use of ultrasound so far has been restricted mainly to hospitals and medical surveys and is unlikely to be used widely for control programs due to the high expense and expertise required for use (46, 104, 162, 165).
Liver biopsies and subsequent histological examination are limited to the hospital setting as they are not practical for the field, given the invasive nature and the potential for sampling error. Biochemical markers of liver fibrosis (procollagen peptide types III and IV, the P1 fragment of laminin, hyaluronic acid, fibrosin, tumor necrosis factor alpha receptor II, and sICAM-1) measured in serum have the potential to provide a highly sensitive and cost-effective method for the assessment of schistosome-induced fibrosis but are still under investigation (46, 60, 80, 104, 162, 165).
Immunological Methods
Antibody detection is quite sensitive and useful in a few specific circumstances, but its use can be limited because antibodies persist after parasitologic cure (162, 165, 187). Overviews of immunodiagnosis, including the description of new serologic diagnostic developments for schistosomiasis in China, are available (215, 239, 240). A positive serologic test may be diagnostic in patients in whom there are no eggs, such as those with Katayama syndrome (164). Furthermore, serologic testing is valuable in field studies for defining regions of low-level endemicity where individual patients have low egg burdens (187). Serologic testing may also be useful in determining whether infection has reemerged in a region after an apparently successful elimination program, and it is important for diagnosis in travelers. Commercially available immunodiagnostic kits are generally not as sensitive as multiple fecal examinations and are less specific, due to cross-reactivity with other helminths. Most techniques detect IgG, IgM, or IgE against soluble worm antigen or soluble egg antigen (SEA) by enzyme-linked immunosorbent assay (ELISA), indirect hemagglutination (IHA), or immunofluorescence (240). IHA is a simple and rapid test and continues to be applied for community diagnosis and screening of people targeted for chemotherapy in China. Pooled serum sample testing by IHA has been shown to be an acceptably sensitive method for detecting antibody in S. japonicum infections (99).
A highly practical, simple, and inexpensive dipstick immunoassay (DDIA) kit has been developed in China for the detection of antibodies against S. japonicum using SEA labeled with a colloidal dye and is particularly useful for screening in the field (239). Recombinant fructose-1,6-bisphosphate aldolase (FBPA) derived from S. japonicum from Taiwan (a zoophilic strain that infects only domestic and small animals) is a useful diagnostic antigen in ELISA for the diagnosis of both human and water buffalo schistosomiases (152, 153).
Detection of circulating adult worm or egg antigens with labeled monoclonal or polyclonal antibodies in serum, urine, or sputum in infected individuals is another promising technique that may eventually supersede traditional diagnostic methods (240). In this respect, a novel immunomagnetic bead ELISA based on IgY (egg yolk immunoglobulin) has been developed for detection of circulating egg antigen in sera of mice infected with S. japonicum (114) although it has yet to be assessed using human sera. Additional supportive clinical and laboratory findings for diagnosis of schistosomiasis include evidence of peripheral blood eosinophilia, anemia (iron deficiency anemia, anemia of chronic disease, or macrocytic anemia), hypoalbuminemia, elevated urea and creatinine levels, and hypergammaglobulinemia (165).
PCR Methods
The sensitivity of microscopic detection of eggs in stool or organ biopsy specimens is variable due to fluctuation of egg shedding. Serological tests, on the other hand, do not distinguish between active and past disease, as titers typically take 18 to 24 months to drop after chemotherapeutic cure. In patients with acute disease (Katayama syndrome), both serology and direct detection may produce false-negative results. As a consequence, several groups have developed specific and highly sensitive PCR-based assays for the detection of S. japonicum DNA in feces or serum/plasma specimens of infected animals, including mice, rabbits, and pigs, which potentially provide a test for early diagnosis and therapy evaluation in humans (74, 123, 125, 214). In a recent development, a PCR test for detection of cell-free parasite DNA (CFPD) in human plasma has been devised, which may provide a new laboratory tool for diagnosing schistosomiasis in all phases of clinical disease, including the capacity to rule out Katayama syndrome and active disease (201).
A test for the specific and quantitative detection of S. japonicum cercariae in water by real-time PCR has also been developed (97). The cercarial stage of S. japonicum exhibits high spatial and temporal variability, and current detection techniques in China rely on a sentinel mouse method, which has serious limitations in obtaining data in both time and space. The PCR-based method has a detection limit below the DNA equivalent of half of one cercaria and has the potential to be applied to environmental water samples to produce a rapid, reliable assay for cercarial location in areas of endemicity (97).
CHEMOTHERAPY
Human Treatment
Early treatments against schistosomiasis had severe and even lethal side effects that had to be weighed against the benefits for the patient; however, the 1970s brought the advent of effective and safe drugs (80).
PZQ.
Praziquantel (PZQ), a pyrazinoisoquinoline derivative, is a safe and highly effective oral drug that is active against all schistosome species; it is the mainstay of treatment and a critical part of community-based schistosomiasis control programs, including those in China (Fig. 6A) (57, 58, 162, 186, 187, 216, 236). Since its discovery in the mid-1970s, its safety and efficacy have ensured its widespread use. It is absorbed well but undergoes extensive first-pass hepatic clearance. PZQ is secreted in breast milk and is metabolized by the liver, and its (inactive) metabolites are excreted in the urine. Side effects are mild, and it can be used to treat young children and pregnant women. Schistosome antigen-specific antibody levels and PZQ-induced boosts in antibody levels have been shown to be broadly suppressed during pregnancy, but the efficacy of the drug is maintained (185). PZQ acts within 1 h of ingestion, although its precise action on adult worms is unknown. It appears to cause tetanic contractions and tegumental vacuoles, causing worms to detach from the wall of the vein and die. Schistosome calcium ion channels have been suggested as the molecular target of PZQ, but the evidence remains indirect and its precise mechanism of action remains unknown (9). A recent study implicated myosin light chain as an alternative target of PZQ (71). In animal models, the presence of host antibodies has been shown to be critical for its efficacy (57, 162).
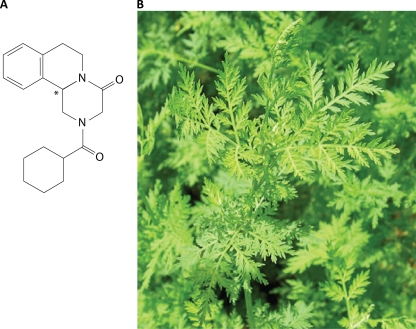
FIG. 6.
Chemotherapy. (A) Praziquantel. (B) Artemisia annua, used in the preparation of artemether and other artemisinin derivatives.
Standard treatment of chronic schistosomiasis is 60 mg/kg of PZQ (94) in divided doses; for mass chemotherapy a single dose (40 mg/kg) is used (165). Treatment failures with this dose have been reported, particularly in areas where schistosomiasis has been recently introduced. Whether this is because the drug works in concert with the host immune response, which has yet to develop, or due to migrating larvae not yet susceptible to PZQ is unknown. Susceptibility usually takes three weeks to develop. There is also some evidence, albeit controversial, that resistance to PZQ may be emerging in Africa, where there has been heavy exposure to the drug and where, worryingly, there are reports of S. mansoni and S. haematobium infections that are not responsive (80). There is laboratory evidence that schistosomes subjected to repeated in vivo PZQ treatments for several generations have reduced drug sensitivity (156) and that drug-tolerant schistosome worms have altered tegumental architecture and increased P-glycoprotein levels (143), which could limit the effectiveness of the drug. So far, however, patients in many communities have undergone multiple courses of treatment over a period of 10 years or more without a demonstrable loss of efficacy. There have been no reports of the development of tolerance/resistance of S. japonicum to PZQ in the clinical treatment of patients in China (216). In any case, because worm reproduction in the mammalian host is sexual and the generation time is relatively long, resistance is likely to take many years to become an important clinical and public health issue.
Nevertheless, resistance against PZQ in the future cannot be ruled out, and research toward development of alternative drugs, such as 4-phenyl-1,2,5-oxadiazole-3-carbonitrile-2-oxide (which has been shown to inhibit a crucial parasite enzyme, thioredoxin glutathione reductase) (170), the antimalarial mefloquine (106, 231), curcumin (a polyphenol derivative of turmeric) (6, 132), and sulfur-containing compounds, including the aminoalkanethiols, aminoalkanethiosulfuric acids, and aminoalkyl disulfides (54), should be actively encouraged. Other drugs that have been used in the treatment of schistosomiasis are oxamniquine (vansil) for S. mansoni and metrifonate (trichlorfon) for S. haematobium, but both are ineffective against S. japonicum. Potential new targets for drug development against schistosomes include peroxiredoxin-1 (110), thioredoxin glutathione reductase (174), tyrosine kinases (183), tyrosine kinase receptors (4, 5), phosphatidylinositol kinases (11), and neuropeptides (141).
A comparative chemogenomics strategy, recently described for S. mansoni, can predict additional potential new drugs targets in schistosomes (27). The approach utilized S. mansoni genome sequence information, identified putative essential genes based on similarity to experimentally determined essential genes/proteins in two model metazoans (in this case Drosophila melanogaster and Caenorhabditis elegans), and then defined a subset of potential drug targets (e.g., GTP binding protein, methionine aminopeptidase, and RacGTPase) in S. mansoni for which structural information about known target proteins, including bound ligands, exists.
ART.
PZQ cannot be used for chemoprophylaxis because of its short half-life (1 to 1.5 h) and because it cannot kill schistosomula (the migrating larvae) that are 3 to 21 days old. Artemether (ART) (and other artemisinin derivatives), which comes from the leaves of the Chinese medicinal plant Artemisia annua (Fig. 6B) and is used for the treatment of malaria, is effective against juvenile schistosomes during the first 21 days of infection in animals and humans (216), and it should kill all immature schistosomula if it is given every 2 weeks. Accordingly, it has been used as a chemoprophylactic for high-risk groups, such as flood relief workers and fishermen, in areas in China where schistosomiasis is endemic (187, 216). The doses required are lower than those required for treatment of malaria, but it is unlikely that ART would be used in other regions, such as Africa, where malaria is endemic because such use might lead to the selection of ART-resistant Plasmodium falciparum.
In animals, combination therapy with PZQ plus ART is safe and results in higher worm reduction rates than therapy with PZQ alone (186, 216). However, a randomized, double-blind, placebo-controlled human trial for evaluating combined chemotherapy with PZQ and ART at two different PZQ dosages (60 mg/kg and 120 mg/kg) in the treatment of acute schistosomiasis japonica in China failed to show improved treatment efficacy compared with PZQ alone (94).
Animal Treatment
Periodic treatment of bovines with PZQ, managed by local veterinarians, is often included in control programs for S. japonicum in China. This is because they are large animals (water buffaloes often exceed 500 kg in weight), they deposit considerable amounts of excreta near or in surface water, they live for 10 to 12 years, and they can carry large numbers of schistosomes and develop hepato-intestinal disease. In marshland areas where S. japonicum is endemic, 5 to 40% of water buffaloes and cattle are infected. PZQ is curative, but the animals become reinfected. In most areas of endemicity, a large proportion (>70%) of the environmental contamination may be traced back to bovine defecation and a high proportion of schistosome eggs (162). The treatment doses (25 mg/kg for water buffaloes and 30 mg/kg for cattle) are lower than that used for humans because the collateral effects may be fatal, which is thought to result from portal occlusion by dead worms in heavily infected animals, as noted in other forms of cattle schistosomiasis (51). Older buffaloes tend to excrete fewer viable eggs than those less than 18 months old; this could reflect self-cure, decreased egg viability, or decreased egg production by female worms, and all have been described for other forms of bovine schistosomiasis (162).
IMMUNOLOGICAL CONSIDERATIONS
Schistosomes are nonreplicating organisms in their mammalian hosts. As a result, a partial, nonsterilizing, naturally acquired or vaccine-induced immunity could potentially decrease human/animal pathology and transmission in areas where schistosomiasis is endemic. A number of recent articles and reviews have considered the immunobiology of schistosomiasis, including the nature of the host innate and adaptive responses to schistosomes, and strategies used by these parasites to manipulate such responses (1, 31, 32, 138, 146, 151, 213), so only a brief overview of the murine and human studies that have been undertaken will be given here.
Mouse Studies
Studies in murine models have established that T-cell-mediated immunity is fundamental to acquired resistance to schistosomes in mice and that the processes of immune regulation and T-cell regulation involved are complex (138). Much of this protection is mediated by activated macrophages, and this together with studies of cytokines suggests that a vaccine that can induce macrophage-activating Th1 cytokines (gamma interferon [IFN-γ] and interleukin-2 [IL-2]) may be beneficial in preventing schistosomiasis. However, repeated vaccination with irradiated cercariae produced incremental increases in Th2-mediated (IL-4 and IL-5 predominance) protection, which was transferable to nonvaccinated animals.
Studies using B-cell-deficient and cytokine-deficient mice demonstrated that successful antischistosome vaccination required induction of strong Th1 and Th2 responses. Following infection by normal or radiation-attenuated cercariae, the predominant early immune response was Th1 mediated and aimed at the adult worm. Following egg deposition in tissues (6 weeks postinfection [p.i.] for S. mansoni or 4 to 5 weeks p.i. for S. japonicum), the Th1 response diminished, being replaced by a prominent Th2-mediated phase. Indeed, it appears that egg antigens are able to directly suppress the Th1 response, a phenomenon which may also occur in humans. The Th2 response results in an increase in serum IL-5, massive bone and blood eosinophilia, and the characteristic granulomatous response aimed at the egg, resulting in collagen deposition, tissue fibrosis, and the disease manifestations of schistosomiasis. The precise role of eosinophils in the disease process in the mouse model of infection remains undetermined, although recent work suggests that macrophage migration inhibitory factor (MIF) participates in the IL-5-driven maturation of eosinophils and in tissue eosinophilia associated with schistosome infection (131).
The strong Th2 responses that peak during the acute stage of schistosome infection and then decline despite ongoing infection, in a process termed immunomodulation, minimize the Th-2 dependent immunopathology during the chronic stage of infection (181). Arginase-1 and resistin-like molecule (RELM-alpha/fizz1; retnla) derived from alternatively activated macrophages (AAMacs) appear to be critical mediators of this immune downmodulation (148, 154, 155, 181). Recent evidence also suggests that the decline in Th2 cell responsiveness that protects mice during chronic schistosomiasis is the net result of the upregulation of gene related to anergy in lymphocytes (GRAIL), an E3 ubiquitin ligase previously implicated in the development of T-cell anergy, triggered by repeated schistosome antigen stimulation over time (181). Whereas thymic stromal lymphopoietin receptor signaling plays a key role in allergen-driven Th2 responses and participates in the development of schistosome egg-induced CD4+ Th2 responses, it plays only a transient role in the development of the Th2-dependent pathology (158).
Human Studies
As with the other human schistosomes, longitudinal cohort studies of reinfection rates following curative drug treatment have shown that people living in areas where schistosomes are endemic acquire some form of protective immunity after 2 decades or more of exposure to S. japonicum (32, 80, 138, 146, 162). However, age-related innate resistance mechanisms may also play an important part in the epidemiology of schistosomiasis (31, 80, 138). Immune correlative studies in various parts of the world suggest that acquired antischistosome protective immunity after curative drug therapy is mediated (although not exclusively) by a Th2 response, orchestrated by IgE against adult and larval antigens, which stimulate eosinophils to release cytotoxins targeting schistosomula (31, 80, 138, 146). Despite the protective role of IgE, high levels of IgG4 are also produced during infection, potentially blocking the protective effects of other immunoglobulins. It has been shown that immunity to reinfection is more closely related to the IgE/IgG4 balance than to the absolute level of each isotype (32, 138, 146).
The clinical expression of immunity to schistosome infection is not, however, simply determined by the mere balance between IgE and IgG4 antibodies. It cannot exclude the participation of additional mechanisms such as a potential protective role of IgA antibodies in human schistosomiasis; the effector functions of IgA antibodies may be associated with a decrease in female worm fecundity and egg viability (31, 32, 138). It is also important to stress that the development of a vaccine for schistosomiasis that is dependent on IgE would be potentially problematic and would likely be impeded by regulatory and safety issues due to potential anaphylaxis induced by vaccination (138). Therefore, looking to the immune responses of chronically infected individuals, and even those who become refractory by producing IgE after drug treatment, should be approached with caution (138).
Although we still know very little about the protective mechanisms required to engineer an efficacious recombinant vaccine for human schistosomiasis, the analysis of human antibody and cytokine responses to candidate vaccine antigens is potentially a creditable way for establishing bona fide vaccine candidates (16). One such study, carried out over several years in areas in Egypt where schistosomiasis mansoni is endemic (7), focused on 10 of the most promising S. mansoni vaccine antigens. At various time points, immune responses against the panel of antigens in cohorts of humans living in areas where they were regularly exposed to infection were determined, and these results were compared with parasitologic diagnosis. Cellular and humoral immune responses were significantly associated with either apparent resistance or apparent susceptibility to reinfection following chemotherapy. However, only a minority of these responses produced consistent associations, and the results were seldom clear-cut.
A similar investigation, carried out in the Philippines on S. japonicum (3), essentially corroborated the Egyptian findings, although a straightforward comparison was not possible because some of the antigens tested were different in the two studies. A more recent study on S. japonicum in the Philippines showed that IgE responses to paramyosin predicted resistance to reinfection and were attenuated by IgG4 (101), thereby corroborating protective cytokine responses previously described by the same group (113). Whereas these data add support for paramyosin as a vaccine candidate for human schistosomiasis japonica (100, 138) and underscore the importance of careful adjuvant selection to avoid the generation of blocking IgG4 antibody responses, the risk of inducing allergic reactions in previously exposed and sensitized individuals warrants careful future consideration if paramyosin is to be pursued as a vaccine target.
The discovery of the surface-located tetraspanins Sm-TSP-1 and TSP-2 as major candidate vaccine antigens resulted from a combination of protective efficacy data obtained in the S. mansoni murine challenge model with their recognition by IgG1 and IgG3 antibodies from humans exposed but resistant to schistosomiasis (184). Human studies are thus instructive not only for identifying the few antigens directly and exclusively associated with resistance but also for indicating which of these components can be formulated with adjuvants to generate protective responses in animal models (139).
VACCINE DEVELOPMENT
The control of schistosomiasis requires large-scale population-based chemotherapy in addition to environmental and behavioral modification, but it is difficult and costly to sustain such a program (16). Consequently, there is a need for a vaccine for long-term prevention. Development of vaccines against S. mansoni and S. haematobium necessitates the use of clinical vaccines for human application. The zoonotic transmission of schistosomiasis japonica allows for a complementary approach for S. japonicum, involving the development and deployment of a transmission-blocking veterinary vaccine in livestock animals, particularly bovines, prior to developing a human vaccine should this be necessary. The vaccine would be used in reservoir hosts of S. japonicum to potentially reduce transmission to humans. Bovines (cattle and water buffaloes) are the major reservoirs for S. japonicum infection in China. Estimates that 75 to 90% of egg contamination comes from this source have been made through drug intervention studies and mathematical modeling, underpinning the rationale for developing a veterinary vaccine against S. japonicum (77, 78, 79, 83, 202). The possibility that this strategy could pay off already is supported by Chinese studies showing that the animal-snail-human transmission cycle is more prominent than the human-snail-human one in sustaining the infection (77, 79, 210).
Schistosomiasis japonica was once highly prevalent in China in other domestic animals such as pigs, but in recent years these animals have been considered of less importance because they are usually restricted to pens, with limited access to the marshland areas (78). Sheep and goats are also infected but to a far lesser extent, and as wild animals become rarer, their involvement in transmission can probably be ignored (78).
A high level of protection against S. japonicum infection has been attained in mice, water buffaloes, and pigs when the animals were immunized with irradiated cercariae, with both type 1 and type 2 helper T-cell responses likely contributing to protection (139). Vaccination can be targeted either to the prevention of schistosome infection or to the reduction of parasite fecundity. A reduction in worm numbers is the gold standard for antischistosome vaccine development, with the migrating schistosomulum stage likely to be the major vaccine target of protective immune responses (137, 139, 206). However, as schistosome eggs are responsible for both pathology and transmission, a vaccine targeting parasite fecundity and/or egg viability is also relevant (31, 32, 137, 139).
Coordinated laboratory and field research has identified a set of well-defined S. japonicum molecules with vaccine potential. Some of the lead S. japonicum vaccine candidates (as recombinant protein and/or DNA vaccines) are presented in Table 1. Many are membrane proteins, muscle components, or enzymes, and further details of the characteristics and efficacies, where tested, of these and other vaccine candidates can be found elsewhere (96, 137, 138, 139, 180, 212). Recent studies in water buffaloes of the protection afforded by S. japonicum recombinant proteins (e.g., paramyosin [Sj-97] and GST-26 [Sj-GST26] [139]) and DNA plasmids (triose phosphate isomerase [Sj-TP1], 23-kDa integral membrane [tetraspanin] protein [Sj-23] [49]) have yielded encouraging results, indicating the feasibility of developing a vaccine that blocks schistosome transmission for use in reservoir hosts.
TABLE 1.
Some of the leading S. japonicum vaccine candidates that have shown efficacy in the mouse model and in reservoir hosts of schistosomiasis japonicaa
| Antigen (as recombinant protein or DNA plasmid) | Abbreviation | Size (kDa) | Stage(s) in which expressed | Biological function | Worm burden reduction (%) inc: |
|
|---|---|---|---|---|---|---|
| Mouse | Other hosts | |||||
| Paramyosin | Sj97 | 97 | Schistosomula, adults | Contractile protein and others | 20-86 | 17-60 (buffalo/cattle/pig/sheep) |
| Triose phosphate isomerase | SjTPI | 28 | All | Enzyme | 21-33 | 42-60 (buffalo/pig) |
| 23-kDa integral membrane protein | Sj23 | 23 | Adults | Membrane protein | 27-35 | 0-59 (water buffalo/cattle/sheep) |
| Aspartic protease | SjASP | 46 | All | Digestion of hemoglobin | 21-40 | |
| Calpain large subunit | Sjcalpain | 80 | All | Protease | 40-41 | |
| 28-kDa glutathione S-transferase | Sj28GST | 28 | All | Enzyme | 0-35 | 16-69 (water buffalo/cattle/sheep) |
| 26-kDa glutathione S-transferase | Sj26GST | 26 | All | Enzyme | 24-30 | 25-62 (water buffalo/cattle/pig/sheep) |
| Signaling protein 14-3-3 | Sj14-3-3 | 30 | All? | Molecular chaperone | 26-32 | |
| Fatty acid binding protein | Sj14 | 14 | All? | Binds fatty acids | 34-49 | 32-59 (rat/sheep) |
| Serpin | Sjserpin | 45 | Adults | Serine proteinase inhibitor | 36 | |
| Very-low-density lipoprotein binding protein | SjSVLBP | 20 | Adult males | Binds lipoproteins | 34 | |
| Ferritin | SjFer | 450 | All? | Iron storage | 35 | |
| Calcium binding proteinb | Sj8 | 8 | Cercariae, schistosomula | Binds calcium | 50 | |
Adapted from reference 140 with permission of the publisher.
Data are from reference 129.
Egg reduction (in feces and/or liver) was also recorded with many of the candidates. When evaluated, reduced egg-hatching capacity of S. japonicum eggs into viable miracidia was observed with some vaccines. Cocktails of several of the leading candidates have also been tested.
Despite this wealth of information on vaccine discovery and efficacy, the current S. japonicum vaccine candidates may prove not to be the most effective, and it is therefore important to continue to identify new target antigens. The landmark availability of the complete sequences of the S. japonicum (171) and S. mansoni (18) genomes will provide the necessary ancillary information. As well, new approaches in antigen discovery through the generation of a large schistosome transcriptome database, gene finding, and the explosion in postgenome technologies, including DNA microarray profiling, proteomics, glycomics, immunomics, and the application of RNA interference (RNAi) and novel imaging techniques (2, 24, 25, 41, 53, 72, 73, 75, 76, 85, 95, 109, 126, 127, 179, 189, 197, 205, 219, 224, 230), provide an unprecedented opportunity to identify a new generation of vaccine target molecules that may induce greater potency than the current candidate schistosome antigens (138).
Molecules containing signal peptides and signal anchors as predictors of excretory-secretory products, including enzymes and components exposed on the schistosome tegument (including receptors) that interact directly with the host immune system, are highly relevant targets for study (128). Some caution is necessary, however, as the S. japonicum homologue of the surface-located tetraspanin Sm-TSP-2, discussed above and regarded as a major candidate vaccine antigen for S. mansoni (184), showed at best only very moderate protection in immunized mice. This is due likely to the high level of polymorphism of this molecule, which reduces its potential as a vaccine candidate, at least against S. japonicum (28).
The selection of a suitable adjuvant and delivery system to aid in the stimulation of the appropriate immune response are also critical steps in the path to the development and employment of successful antischistosome vaccines, and a number of different approaches have been tested with some success (17, 138). Immunogenicity relies on antigenic access to particular pathways promoting the secretion of cytokines from antigen-presenting and other cells. This can now be achieved artificially by using immunomodulating agents, live microbiological carriers, or DNA technology. The new lines of immunomodulators include a variety of agents, such as lipopeptides, saponins, muramyl dipeptides, CpG oligodeoxynucleotides, and lipopolysaccharide (LPS), which could provide clues as to how vaccines should be formulated (17).
Understanding the mechanisms whereby these novel adjuvants work will no doubt facilitate the production of more effective vaccines. In this respect, a polyvalent subunit vaccine, SjGP-3, comprised of an individual fragment of paramyosin fused to Sj26GST, was recently formulated in various adjuvants (complete Freund adjuvant [CFA], ISA 206, IMS 1312, and ISA 70 M) and tested for vaccine efficacy against S. japonicum in mice (218). SjGP-3 formulated in the veterinary adjuvant ISA 70 M induced a lasting polarized Th1 immune response, whereas the other adjuvants, including CFA, ISA 206, and IMS 1312, generated a moderate mixed Th1/Th2 response after immunization, but all except IMS 1312 shifted to a Th2 response after the onset of egg production. More importantly, the SjGP-3/70 M formulation induced significant reductions (47.0 to 50.3%) in liver egg deposition and in the number of liver eggs per female (34.5 to 37.2%) although worm burden reductions (17.3 to 23.1%) were less pronounced. There was no effect of the formulations with the other adjuvants on the number of liver eggs per female, and thus there was a direct correlation of the polarized Th1 response with reduction of liver egg burdens, thereby lending support to an immune deviation strategy for schistosomiasis japonica vaccine development.
ALLERGY, AUTOIMMUNE DISEASE, AND SCHISTOSOME INFECTION
In areas where schistosomiasis is endemic, there is a negative correlation between atopy and infection, which is associated with a low prevalence of asthma. Thus, a number of groups have hypothesized that Schistosoma egg antigens can induce production of CD4+ CD25+ regulatory T cells, downmodulating Th2 allergic airway inflammation and inhibiting asthma development. Indeed, this appears to be the case in experiments with murine models, which have revealed novel aspects of protection against asthma and suggest a mechanistic explanation for the protective effect of schistosome infection on its development and other allergic responses as well (87, 145, 150, 222).
These findings can help explain reactions found in humans, since individuals with active schistosome and other helminth infections appear to be less responsive to allergen provocation than individuals who are free of infection (93, 150). Schistosome infection or administration of schistosome proteins also has been shown to modulate the progression of a number of progressive experimental autoimmune diseases in mice, including colitis, arthritis, and diabetes (149, 182, 234). The identification of the key schistosome molecules that induce protection against allergic and autoimmune diseases points to the exciting development of new therapies, including vaccines, against these chronic conditions.
GENETICS AND SUSCEPTIBILITY TO SCHISTOSOME INFECTION
Genetic background appears to play a critical role in influencing aspects of host immunity and hence in determining the susceptibility to and outcome of schistosome infections (30).
Susceptibility to Infection and Reinfection
Segregation analysis revealed that susceptibility to infection in schistosomiasis mansoni is controlled by a gene locus (the SM1 [S. mansoni 1] gene) linked to chromosome 5 in the 5q31-q33 region (134, 135). This region contains the genes encoding IL-3, IL-4, IL-5, IL-9, IL-13, and IgE, which are associated with the regulation of Th2-type responses. Polymorphisms in these genes that lead to an increase or decrease in cytokine levels could influence the antibody isotypes and cellular interactions that in turn may contribute to resistance or susceptibility of individuals to reinfection with schistosomiasis (70). Indeed, analysis of the 5q31-q33 locus showed that subjects bearing the IL-13-1055T/T genotype were on average much less likely to be infected with S. haematobium than individuals with other genotypes (108). This observation mirrors studies on asthma indicating that the IL-13-1055T allele increases gene transcription, and it is in agreement with the fact that IL-13 appears to enhance resistance to infection by schistosomes in humans (108).
A study on S. mansoni confirmed these findings and showed as well that individuals with certain genotypic patterns of single-nucleotide polymorphisms (SNPs) in IL-4 and IFN-γ were also more likely to be resistant to reinfection than those with different patterns (70). Other work has shown that polymorphisms in the STAT6 gene may also be important and is consistent with the Th2 cytokine pathway enhancing resistance to schistosome infection in humans (88). The low-affinity IgE receptor (CD23) has also been implicated in the development of resistance to schistosome infection through its role in IgE regulation (147). Two SNPs within IL-5 have been associated with the development of symptomatic infection with S. japonicum in China (61, 62).
Susceptibility to Severe Disease
Segregation of a codominant gene (SM2) has been implicated in the familial distribution of severe schistosomiasis mansoni, with linkage analysis indicating that this gene occurs within the 6q22-q23 region and that it is closely linked to the gamma interferon (IFN-γ) receptor 1 gene encoding the receptor of the strongly antifibrogenic cytokine IFN-γ (56). A later study confirmed linkage of severe schistosome-induced hepatic fibrosis with the IFN-γ receptor 1 locus and also suggested a possible association with the IL-13/IL-4 region and the transforming growth factor β1 (TGF-β1) gene (21). Further, IFN-γ production by egg antigen-stimulated peripheral blood mononucleocytes correlates with protection against severe schistosomiasis mansoni (90) and two polymorphisms in IFN-γ have been found to be associated with advanced hepatic disease (42), consistent with the antifibrogenic role of IFN-γ and the low IFN-γ production by subjects with severe disease (42, 43). Whether the severe fibrosis in subjects infected with S. japonicum is controlled by the same dominant gene has yet to be determined. However, IFN-γ has been shown to be linked with protection against peripheral fibrosis in humans with schistosomiasis japonica whereas IL-10 protects against severe hepatic central fibrosis, which suggests that the two fibrotic outcomes are under different genetic control (10). Variants of connective tissue growth factor (CTGF), which is also located close to the IFN-γ receptor 1 gene on 6q23, have also been shown to be associated with severe fibrosis in Chinese, Sudanese, and Brazilians infected with schistosomes (55).
There are several reported associations between the clinical manifestations of chronic schistosomiasis and gene alleles within the major histocompatibility complex (MHC), although no consistent picture has emerged from these studies (30).
MATHEMATICAL MODELING
The modeling of schistosomiasis has important implications when considering different options for control. Models can predict the spread of disease, the utility of various strategies for treatment coverage, and the impact of vaccines. The future elimination of schistosomiasis in China will no doubt rely on a combination of various control options, such as drug treatment regimens, mollusciciding, vaccination, environmental modification, and improved sanitation. Mathematical models will assist in estimating the effects of these combined strategies and the costs of control.
The first mathematical models developed for schistosomiasis were those by Hairston (86) and MacDonald (130). Since then, a number of transmission dynamics models have been developed and published, with the majority focusing on the African schistosomes (34, 35, 37, 84, 142, 207, 208, 209). Liang et al. and Spear et al. (121, 122, 177) developed mathematical models for schistosomiasis japonica in China, although they did not model transmission in the lake and marshland areas, where the majority of transmission occurs, nor did they model the involvement of bovine hosts in the transmission. Riley et al. (160) developed a S. japonicum transmission dynamics model for the Philippines that tried to account for the additional mammalian hosts and zoonotic transmission. However, there is some conjecture over the parameterization of the model (78) due to the potential underestimation of the bovine S. japonicum prevalence in the Philippines, as discussed above.
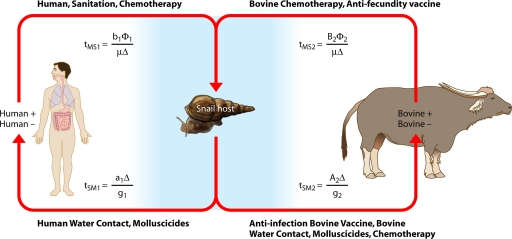
A model by Williams et al. (202) (Fig. 7) was developed to simulate transmission dynamics of schistosomiasis japonica in the lake and marshland areas of China and to predict the effects of different control strategies. It extended the two-host model of Barbour (14) to allow for heterogeneity within human and bovine definitive hosts. It consists of a set of simultaneous equations which model the rate of change in prevalence over time: prevalence in definitive host class i, dPi/dt = gi[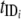 y(1 − Pi) − Pi]; prevalence in intermediate host, dy/dt = μ[Σ(
y(1 − Pi) − Pi]; prevalence in intermediate host, dy/dt = μ[Σ(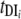 Pi)(1 − y) − y] (where i = 1, 2, … n).
Pi)(1 − y) − y] (where i = 1, 2, … n). 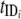 and
and 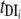 are composite transmission parameters for transmission from the intermediate host to the ith definitive host and for transmission from the ith definitive host to the intermediate host, respectively, which depend upon the duration of infection in the respective hosts, snail and definitive host densities, and force of infection per unit host (202). The model is parameterized using data of S. japonicum epidemiology, including the distribution of endemic prevalences within host classes, and infection duration. Interventions can then be imposed on the system, and equations can be solved numerically to predict the consequences for prevalence and incidence.
are composite transmission parameters for transmission from the intermediate host to the ith definitive host and for transmission from the ith definitive host to the intermediate host, respectively, which depend upon the duration of infection in the respective hosts, snail and definitive host densities, and force of infection per unit host (202). The model is parameterized using data of S. japonicum epidemiology, including the distribution of endemic prevalences within host classes, and infection duration. Interventions can then be imposed on the system, and equations can be solved numerically to predict the consequences for prevalence and incidence.
FIG. 7.
Mathematical model of Schistosoma japonicum transmission and control. tMS1, tSM1, tMS2, and tSM2 are transmission parameters, which are described generally in the text as 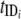 and
and 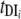 . (Adapted from reference 202 with permission of Elsevier.)
. (Adapted from reference 202 with permission of Elsevier.)
Simulation of transmission and control using this model has predicted that bovines are major reservoirs for human infection in the lake and marshland areas of China (78, 83), that human mass chemotherapy alone is not sufficient for long-term sustainable control (83), and that an integrated approach, such as a combination of human chemotherapy and bovine vaccination, is required for long-term sustainable control in China (49, 78, 202). With regard to the last scenario, the model predicts that a hypothetical S. japonicum vaccine capable of reducing the fecal egg output of water buffaloes by 45% alone or in conjunction with PZQ treatment will lead to a significant reduction in transmission (202). In fact, the two DNA plasmid vaccines, SjCTPI-Hsp70 and SjC23-Hsp70, developed and tested experimentally by Da'Dara et al. (49) both exceeded this hypothetical level. Indeed, mathematical modeling of SjCTPI-Hsp70 and SjC23-Hsp70 alone and in conjunction with human chemotherapy showed a significant reduction in transmission, almost to the point of elimination (Fig. 8).
FIG. 8.
Mathematical modeling of two Schistosoma japonicum DNA vaccine constructs. The DNA vaccine constructs SjC23-Hsp70 (A) and SjTPI-Hsp70 (B) were modeled alone and as an intervention (in conjunction with annual human mass treatment and an initial water buffalo treatment) and compared to a hypothetical vaccine providing 45% efficacy.
CONTROL
The collective efforts to control schistosomiasis japonica in China over the past 50 years have been enormous. In those areas where it was possible, the transmission environment was modified to make it insusceptible to Oncomelania snails. Toxic chemicals were used to cure infection, and those infected were followed for years until cure was certain. Great progress was made, and the huge toll on affected human populations now has been reduced greatly. Dwarfism, impoverishing incapacity to work in rural areas, and widespread premature death are now gone. The tenacity and dedication of the thousands of schistosomiasis control workers, farmers, government cadres, and public health officials were central to this massive control effort.
Current Control Options
Despite these considerable achievements, progress appears to be stagnating, as national surveys of schistosomiasis have shown that the prevalence in humans in areas of endemicity did not substantially change from 1995 (4.9%) to 2004 (5.1%) (198). Past efforts to control the amphibious Oncomelania snail populations through chemical mollusciciding or modification of snail habitats have often resulted in environmental pollution (198). After over 20 years of experience, it is generally agreed that morbidity control through population-based PZQ chemotherapy, although the mainstay of current schistosomiasis control programs in China and elsewhere, does have limitations. PZQ compliance can be erratic, and mass treatment does not prevent reinfection. This occurs rapidly in exposed populations with a history of endemicity, such that within a period of 18 to 24 months following chemotherapy, the prevalence returns to its baseline level (165). A recent study from Mali in Africa has emphasized the inability of PZQ to prevent reinfection, as schistosomiasis prevalence has returned to baseline levels following almost a decade of mass chemotherapy (45). Furthermore, efficient drug delivery can require a substantial infrastructure to regularly mass treat populations in areas of endemicity. This can make chemotherapy an expensive and often impractical approach. In China, there is the additional challenge that transmission control necessitates interventions targeting animal reservoirs, particularly buffaloes (77, 78, 79, 210). Moreover, in situations of ongoing high transmission and interrupted chemotherapy campaigns, severe “rebound morbidity” in terms of hepatosplenic disease is now well documented for schistosomiasis, contributing to the disease burden (68, 165).
The inability to eliminate S. japonicum from large areas of the lake and marshland zones of the middle Yangtze River and its upper reaches, together with ecological transformations soon to arise throughout the Yangtze basin due to the complete closure of the TGD in 2009, makes it imperative to develop new integrated, cost-effective, and sustainable strategies for control.
Future Integrated Control
An integrated, sustainable approach for control of schistosomiasis is required and will rely on the disruption of transmission across the multiple transmission pathways. Combinations of the following strategies are probably the way forward in China: human chemotherapy (human-snail pathway), bovine chemotherapy (bovine-snail pathway), health education/promotion (snail-human pathway), bovine vaccination (anti-infection vaccine, snail-bovine pathway; antifecundity vaccine, bovine-snail pathway), improved sanitation (e.g., provision of toilets for mobile populations such as fishermen) and agricultural practices (human-snail pathway), and mollusciciding (snail-human and snail-bovine pathways). Mathematical modeling has already shown that integrated control strategies (including combinations of treatment, bovine vaccination, improved sanitation, and reduced water contact) are indeed effective for short- and long-term control (49, 202).
A pilot study for integrated control of schistosomiasis has recently been undertaken in China; this involved a multipronged approach of removing bovines from snail-infested grasslands, providing farmers with mechanized farm equipment, improving sanitation by supplying tap water, building lavatories and latrines, providing boats with fecal-matter containers, and implementing an intensive health education program (197). This comprehensive control strategy based on interventions to reduce the rate of transmission of S. japonicum infection from cattle and humans to snails was highly effective. However, it has some drawbacks in that due to the difficult terrain, the replacement of bovines with farm machines may not be feasible in many areas of the lakes/marshlands or the mountainous regions of China where schistosomes are endemic, and the costs involved may be prohibitive.
Health education interventions are often characterized as being longer term and too costly, but an integrated public health approach to the elimination of schistosomiasis requires action through multiple control strategies, of which health education is an essential part. School-aged children have been recognized as a high-risk population for schistosome infection (200), and a focus on this population is seen to yield benefits for both this generation and the next. In support of overall community involvement, the school is clearly an important setting for integrating both health education and other efforts toward control of schistosomiasis. There is greatly increased understanding of program implementation focusing on the whole school community, and both efficiencies and logistics have been addressed by working through school structures.
The school has proven to be a very effective setting for community-based initiatives designed to reduce parasite infection in China, drawing together not only the immediate school participants, such as students, school principals, and teachers, but also government officials across different sectors, health professionals, and the wider community (217). It allows a comprehensive or integrated approach for the control of schistosomiasis and other neglected tropical diseases that builds supportive structures and strategies rather than single, isolated approaches. Such a “joined-up” approach ensures that all the parties involved in various important prevention and control measures can be confident that their work is being supported and reinforced across the community. Thus, health professionals involved in drug treatment, environmental use of molluscicides, vaccination, provision of potable water supplies, and appropriate treatment of human waste, together with community education about hygiene and personal health, can be coordinated and supportive.
Past efforts to control Oncomelania snail populations through the use of chemical molluscicides or alteration of their habitats have resulted in environmental pollution and damage (198). Recent developments in spatial epidemiology now allow the use of targeted mollusciciding, thus increasing its success and limiting the environmental pollution. GIS and RS can be used as tools to identify active transmission sites and snail “hot spots” (areas that are close to human habitation, where there is maximal and daily visitation by humans and buffaloes in an environment continuously supporting snails, resulting in high focal transmission [50]), which can then be targeted with molluscicides.
There is a strong argument to consider including vaccination of domestic livestock as a control intervention in China, and given the national and international efforts to generate antischistosome vaccines (138), there is enhanced optimism about possible future success. However, such a vaccine will be only one component of an integrated schistosomiasis control program. The linking of vaccination with chemotherapy would reduce overall morbidity in conjunction with limiting the impact of reinfection. Although induction of consistent, high-level protection has not been recorded for any of the current bovine vaccine candidates, the reported range of around 50% protection should be sufficient for a combined chemotherapy-bovine vaccination approach that could provide an effective control strategy for schistosomiasis.
FINAL COMMENTS
The potential impact of the TGD on S. japonicum transmission has caused considerable global concern (238). Schistosomiasis emergences or reemergences have resulted from other large-scale hydroprojects, such as the Gezira-Managil Dam in Sudan, the Aswan Dam in Egypt, the Melkasadi Dam in Ethiopia, and the Danling and Huangshi Dams in China (80). The TGD will result in changes in water level and silt deposition downstream, favoring the reproduction of Oncomelania snails. Combined with blockages of the Yangtze River's tributaries, these changes will increase the schistosomiasis transmission season within the marshlands along the middle and lower reaches of the Yangtze River. The changing schistosome transmission dynamics necessitate a comprehensive strategy to control schistosomiasis.
The use of synchronous chemotherapy for humans and domestic animals is only temporarily effective, as schistosome reinfection occurs rapidly. Drug delivery requires a substantial infrastructure to regularly cover all parts of an area of endemicity. This makes chemotherapy expensive and, as compliance is often low, a less than satisfactory control option. There is increasing concern about schistosomes developing resistance to PZQ. Consequently, as mathematical modeling predicts, vaccine strategies represent an essential component for the future control of schistosomiasis in China. With the inclusion of focal mollusciciding, improvements in sanitation, and health education into the control scenario, China's target of reducing the level of schistosome infection to less than 1% by 2015 (187) may be achievable.
Acknowledgments
Our studies on schistosomiasis have received financial support from various sources, including the UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases, the National Health and Medical Research Council of Australia, the Wellcome Trust (United Kingdom), the Sandler Foundation (United States), the Dana Foundation (United States), and the National Institute of Allergy and Infectious Diseases (NIAID).
We thank all our colleagues in Australia and our collaborators in China, who have provided us with much invaluable support during the course of our research. We also thank Mimi Kersting for preparing Fig. 1.
Biography
 Donald P. McManus, B.Sc., Ph.D., D.Sc., joined Imperial College upon completion of his doctorate in the United Kingdom, researching the molecular biology/immunology of parasitic worms. He moved to Queensland Institute of Medical Research, Australia, as Research Coordinator/Deputy Director of the Tropical Health Program. Currently, he heads the molecular parasitology laboratory. Professor McManus has published over 400 articles, which have been cited over 6,000 times. He was coauthor of a landmark paper describing the complete genomic sequence of Schistosoma japonicum (Nature 460:345-352, 2009). He is a member of the South East Asian Regional Network on Schistosomiasis, a continuing member of the WHO Expert Advisory Panel on Parasitic Diseases (Schistosomiasis), and a member of the WHO Disease Reference Group on Zoonoses and Marginalized Infectious Diseases of Poverty. He is an editorial board member for 12 journals. He has presented plenary/keynote addresses at over 50 national/international meetings in the past 10 years. He currently holds grants from Dana Foundation USA and the Australian NHMRC.
Donald P. McManus, B.Sc., Ph.D., D.Sc., joined Imperial College upon completion of his doctorate in the United Kingdom, researching the molecular biology/immunology of parasitic worms. He moved to Queensland Institute of Medical Research, Australia, as Research Coordinator/Deputy Director of the Tropical Health Program. Currently, he heads the molecular parasitology laboratory. Professor McManus has published over 400 articles, which have been cited over 6,000 times. He was coauthor of a landmark paper describing the complete genomic sequence of Schistosoma japonicum (Nature 460:345-352, 2009). He is a member of the South East Asian Regional Network on Schistosomiasis, a continuing member of the WHO Expert Advisory Panel on Parasitic Diseases (Schistosomiasis), and a member of the WHO Disease Reference Group on Zoonoses and Marginalized Infectious Diseases of Poverty. He is an editorial board member for 12 journals. He has presented plenary/keynote addresses at over 50 national/international meetings in the past 10 years. He currently holds grants from Dana Foundation USA and the Australian NHMRC.
 Darren Gray is a Research Fellow at Griffith University, Australia, a Visiting Scientist within the Molecular Parasitology Laboratory at the Queensland Institute of Medical Research, and a Lecturer within the School of Population Health, the University of Queensland. He is an experienced infectious disease epidemiologist with a track record in epidemiology, parasitology, schistosomiasis, infectious disease transmission dynamics, clinical and field-based research in China, statistical and mathematical modeling, and data management. Dr. Gray has been working on schistosomiasis japonica since 2002.
Darren Gray is a Research Fellow at Griffith University, Australia, a Visiting Scientist within the Molecular Parasitology Laboratory at the Queensland Institute of Medical Research, and a Lecturer within the School of Population Health, the University of Queensland. He is an experienced infectious disease epidemiologist with a track record in epidemiology, parasitology, schistosomiasis, infectious disease transmission dynamics, clinical and field-based research in China, statistical and mathematical modeling, and data management. Dr. Gray has been working on schistosomiasis japonica since 2002.
 Yuesheng Li is the director of the Hunan Institute of Parasitic Diseases, Hunan Province, China (WHO Collaborating Centre for Schistosomiasis Control), and a Future Fellow within the Molecular Parasitology Laboratory, the Queensland Institute of Medical Research. He is also a member of the Schistosomiasis Control Steering Committee for the Chinese Ministry of Health. Professor Li is a clinician, an epidemiologist, and an expert on schistosomiasis transmission and control. He was the first Chinese scientist to be awarded the prestigious International Research Scholarship from the Howard Hughes Medical Institute. Professor Li has been working on schistosomiasis japonica for over 20 years.
Yuesheng Li is the director of the Hunan Institute of Parasitic Diseases, Hunan Province, China (WHO Collaborating Centre for Schistosomiasis Control), and a Future Fellow within the Molecular Parasitology Laboratory, the Queensland Institute of Medical Research. He is also a member of the Schistosomiasis Control Steering Committee for the Chinese Ministry of Health. Professor Li is a clinician, an epidemiologist, and an expert on schistosomiasis transmission and control. He was the first Chinese scientist to be awarded the prestigious International Research Scholarship from the Howard Hughes Medical Institute. Professor Li has been working on schistosomiasis japonica for over 20 years.
 Feng Zheng, former Director of the Institute of Parasitic Diseases-Centre for Disease Control, Shanghai, China, is currently a senior consultant to the WHO Collaborating Centre for Malaria, Schistosomiasis and Filariasis within the Institute of Parasitic Diseases, Chinese Centre for Disease Control and Prevention. Professor Zheng is a highly experienced clinician and molecular parasitologist and an expert on schistosomiasis japonica, on which he has been working for over 30 years.
Feng Zheng, former Director of the Institute of Parasitic Diseases-Centre for Disease Control, Shanghai, China, is currently a senior consultant to the WHO Collaborating Centre for Malaria, Schistosomiasis and Filariasis within the Institute of Parasitic Diseases, Chinese Centre for Disease Control and Prevention. Professor Zheng is a highly experienced clinician and molecular parasitologist and an expert on schistosomiasis japonica, on which he has been working for over 30 years.
 Gail Williams is a Professor of International Health Statistics and Head of the Epidemiology and Biostatistics Discipline in the School of Population Health, the University of Queensland. She has extensive experience in statistical and epidemiological methods applied to population health. Professor Williams has held positions within the Queensland Centre for Public Health (QCPH) and the Australian Centre for International and Tropical Health and Nutrition (ACITHN). Her specific statistical expertise lies in the area of longitudinal studies, specifically population-based intervention studies, long-term cohort studies, and time series studies. As part of this work, she has led a team of statisticians who developed innovative ways of analyzing data derived from longitudinal studies. She has been involved in a series of projects related to the control of schistosomiasis in China, where she published the first mathematical model of schistosomiasis transmission in China. She has been working on schistosomiasis japonica for the past 15 years.
Gail Williams is a Professor of International Health Statistics and Head of the Epidemiology and Biostatistics Discipline in the School of Population Health, the University of Queensland. She has extensive experience in statistical and epidemiological methods applied to population health. Professor Williams has held positions within the Queensland Centre for Public Health (QCPH) and the Australian Centre for International and Tropical Health and Nutrition (ACITHN). Her specific statistical expertise lies in the area of longitudinal studies, specifically population-based intervention studies, long-term cohort studies, and time series studies. As part of this work, she has led a team of statisticians who developed innovative ways of analyzing data derived from longitudinal studies. She has been involved in a series of projects related to the control of schistosomiasis in China, where she published the first mathematical model of schistosomiasis transmission in China. She has been working on schistosomiasis japonica for the past 15 years.
 Donald Stewart was educated in the United Kingdom (Durham, Oxford, Leicester), New Zealand (Otago), and Australia (UNSW). Currently Professor of Health Promotion and Head of School, Public Health, Griffith University, and Vice President of the South West Pacific Region of the IUHPE, he has over 35 years of international experience in the fields of public health, education, and community development. He is the recipient of three Australian Research Council grants for HIV prevention as well as a major research grant from Health Promotion Queensland for “Resilient Children and Communities.” Since 1995, his recognition by the WHO as a leader in comprehensive, school-based health promotion has led to appointments as a consultant in Malaysia, Vietnam, China (Hunan, Yunnan, Szechuan, Fujian, Heilongjiang, and Beijing), and Papua New Guinea. His work in China and Vietnam has focused on intersectoral health promotion using the health-promoting school approach to prevent infection by soil-transmitted helminths.
Donald Stewart was educated in the United Kingdom (Durham, Oxford, Leicester), New Zealand (Otago), and Australia (UNSW). Currently Professor of Health Promotion and Head of School, Public Health, Griffith University, and Vice President of the South West Pacific Region of the IUHPE, he has over 35 years of international experience in the fields of public health, education, and community development. He is the recipient of three Australian Research Council grants for HIV prevention as well as a major research grant from Health Promotion Queensland for “Resilient Children and Communities.” Since 1995, his recognition by the WHO as a leader in comprehensive, school-based health promotion has led to appointments as a consultant in Malaysia, Vietnam, China (Hunan, Yunnan, Szechuan, Fujian, Heilongjiang, and Beijing), and Papua New Guinea. His work in China and Vietnam has focused on intersectoral health promotion using the health-promoting school approach to prevent infection by soil-transmitted helminths.
 Jose Rey-Ladino completed a Ph.D. in parasitology from the St. Petersburg Veterinary Institute, Russia. Later, he joined the Tropical Medicine Research Institute (Tulane University-Colciencias) in Colombia, where he worked on Leishmania pathogenesis. He then received a fellowship from the WHO and completed a Ph.D. in immunology and microbiology at the UBC, Canada. He joined the Cancer Research Center and became Senior Research Scientist at CDC, Canada, where he developed research on Chlamydia vaccine development. Presently, Dr. Rey-Ladino is a Senior Research Fellow at Griffith University, and his research interests lie broadly in the realm of tropical infectious diseases, immunology, and vaccine development. His current research is focused on the identification of immune components resulting from the interaction of pathogens and dendritic cells (DCs) and their relevance for vaccine development. Dr. Rey-Ladino is currently implementing this approach to identify novel vaccine candidate antigens from the surface MHC of DCs pulsed with lysates from pathogens.
Jose Rey-Ladino completed a Ph.D. in parasitology from the St. Petersburg Veterinary Institute, Russia. Later, he joined the Tropical Medicine Research Institute (Tulane University-Colciencias) in Colombia, where he worked on Leishmania pathogenesis. He then received a fellowship from the WHO and completed a Ph.D. in immunology and microbiology at the UBC, Canada. He joined the Cancer Research Center and became Senior Research Scientist at CDC, Canada, where he developed research on Chlamydia vaccine development. Presently, Dr. Rey-Ladino is a Senior Research Fellow at Griffith University, and his research interests lie broadly in the realm of tropical infectious diseases, immunology, and vaccine development. His current research is focused on the identification of immune components resulting from the interaction of pathogens and dendritic cells (DCs) and their relevance for vaccine development. Dr. Rey-Ladino is currently implementing this approach to identify novel vaccine candidate antigens from the surface MHC of DCs pulsed with lysates from pathogens.
 Allen Ross, M.D., Ph.D., is a Physician Scientist and currently Chair of Public Health and Director of Population Health Research at Griffith University, Australia. His expertise and research interests lie in the realm of public heath medicine, infectious diseases, disease control, and vaccination. He has designed, implemented, and coordinated multifaceted infectious disease projects in various locations in the developed and developing worlds. Dr. Ross completed a Bachelor and Masters of Science degree in Canada before proceeding to Brisbane, Australia, where he completed a Doctorate in Tropical Health with Distinction and a Doctorate of Medicine. He is a committee member of the Australasian Faculty of Public Health Medicine (AFPHM) Accreditation Subcommittee and a member of the South East Asian Regional Network on Schistosomiasis. He has worked on the control of Oriental schistosomiasis for the past 15 years.
Allen Ross, M.D., Ph.D., is a Physician Scientist and currently Chair of Public Health and Director of Population Health Research at Griffith University, Australia. His expertise and research interests lie in the realm of public heath medicine, infectious diseases, disease control, and vaccination. He has designed, implemented, and coordinated multifaceted infectious disease projects in various locations in the developed and developing worlds. Dr. Ross completed a Bachelor and Masters of Science degree in Canada before proceeding to Brisbane, Australia, where he completed a Doctorate in Tropical Health with Distinction and a Doctorate of Medicine. He is a committee member of the Australasian Faculty of Public Health Medicine (AFPHM) Accreditation Subcommittee and a member of the South East Asian Regional Network on Schistosomiasis. He has worked on the control of Oriental schistosomiasis for the past 15 years.
REFERENCES
- 1.Abath, F. G., C. N. Morais, C. E. Montenegro, T. A. Wynn, and S. M. Montenegro. 2006. Immunopathogenic mechanisms in schistosomiasis: what can be learnt from human studies? Trends Parasitol. 22:85-91. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Hafeez, E. H., M. Kikuchi, K. Watanabe, T. Ito, C. Yu, H. Chen, T. Nara, T. Arakawa, Y. Aoki, and K. Hirayama. 2009. Proteome approach for identification of schistosomiasis japonica vaccine candidate antigen. Parasitol. Int. 58:36-44. [DOI] [PubMed] [Google Scholar]
- 3.Acosta, L. P., G. Waine, G. D. Aligui, W. U. Tiu, R. M. Olveda, and D. P. McManus. 2002. Immune correlate study on human Schistosoma japonicum in a well-defined population in Leyte, Philippines. II. Cellular immune responses to S. japonicum recombinant and native antigens. Acta Trop. 84:137-149. [DOI] [PubMed] [Google Scholar]
- 4.Ahier, A., N. Khayath, J. Vicogne, and C. Dissous. 2008. Insulin receptors and glucose uptake in the human parasite Schistosoma mansoni. Parasite 15:573-579. [DOI] [PubMed] [Google Scholar]
- 5.Ahier, A., P. Rondard, N. Gouignard, N. Khayath, S. Huang, J. Trolet, D. J. Donoghue, M. Gauthier, J. P. Pin, and C. Dissous. 2009. A new family of receptor tyrosine kinases with a venus flytrap binding domain in insects and other invertebrates activated by aminoacids. PLoS One 4:e5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allam, G. 2009. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology 214:712-727. [DOI] [PubMed] [Google Scholar]
- 7.Al-Sherbiny, M., A. Osman, R. Barakat, H. El Morshedy, R. Bergquist, and R. Olds. 2003. In vitro cellular and humoral responses to Schistosoma mansoni vaccine candidate antigens. Acta Trop. 88:117-130. [DOI] [PubMed] [Google Scholar]
- 8.Appleton, C. C. 1984. Schistosome dermatitis—an unrecognised problem in South Africa. S. Afr. Med. J. 65:467-469. [PubMed] [Google Scholar]
- 9.Aragon, A. D., R. A. Imani, V. R. Blackburn, P. M. Cupit, S. D. Melman, T. Goronga, T. Webb, E. S. Loker, and C. Cunningham. 2009. Towards an understanding of the mechanism of action of praziquantel. Mol. Biochem. Parasitol. 164:57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnaud, V., J. Li, Y. Wang, X. Fu, S. Mengzhi, X. Luo, X. Hou, H. Desseing, Z. Jie, Y. Xin-Ling, H. He, D. P. McManus, Y. Li, and A. Dessein. 2008. Regulatory role of interleukin-10 and interferon-γ in severe hepatic central and peripheral fibrosis in humans infected with Schistosoma japonicum. J. Infect. Dis. 198:418-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahia, D., L. M. Oliveira, R. A. Mortara, and J. C. Ruiz. 2009. Phosphatidylinositol- and related kinases: a genome-wide survey of classes and subtypes in the Schistosoma mansoni genome for designing subtype-specific inhibitors. Biochem. Biophys. Res. Commun. 380:525-530. [DOI] [PubMed] [Google Scholar]
- 12.Balen, J., Z. F. Zhao, G. M. Williams, D. P. McManus, G. Raso, J. Utzinger, J. Zhou, and Y. S. Li. 2007. Prevalence, intensity and associated morbidity of Schistosoma japonicum infection in the Dongting Lake region, China. Bull. World Health Organ. 85:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banister, J., and X. B. Zhang. 2005. China, economic development and mortality decline. World Dev. 33:21-41. [Google Scholar]
- 14.Barbour, A. D. 1996. Modeling the transmission of schistosomiasis: an introductory view. Am. J. Trop. Med. Hyg. 55(Suppl.):135-143. [DOI] [PubMed] [Google Scholar]
- 15.Bartley, P. B., G. A. Ramm, M. K. Jones, R. G. Ruddell, Y. Li, and D. P. McManus. 2006. A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int. J. Parasitol. 36:993-1001. [DOI] [PubMed] [Google Scholar]
- 16.Bergquist, R., J. Utzinger, and D. P. McManus. 2008. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS. Negl. Trop. Dis. 2:e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergquist, N. R., L. R. Leonardo, and G. F. Mitchell. 2005. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 21:112-117. [DOI] [PubMed] [Google Scholar]
- 18.Berriman, M., B. J. Haas, P. T. LoVerde, R. A. Wilson, G. P. Dillon, G. C. Cerqueira, S. T. Mashiyama, B. Al-Lazikani, L. F. Andrade, P. D. Ashton, M. A. Aslett, D. C. Bartholomeu, G. Blandin, C. R. Caffrey, A. Coghlan, R. Coulson, T. A. Day, A. Delcher, R. DeMarco, A. Djikeng, T. Eyre, J. A. Gamble, E. Ghedin, Y. Gu, C. Hertz-Fowler, H. Hirai, Y. Hirai, R. Houston, A. Ivens, D. A. Johnston, D. Lacerda, C. D. Macedo, P. McVeigh, Z. Ning, G. Oliveira, J. P. Overington, J. Parkhill, M. Pertea, R. J. Pierce, A. V. Protasio, M. A. Quail, M. A. Rajandream, J. Rogers, M. Sajid, S. L. Salzberg, M. Stanke, A. R. Tivey, O. White, D. L. Williams, J. Wortman, W. Wu, M. Zamanian, A. Zerlotini, C. M. Fraser-Liggett, B. G. Barrell, and N. M. El-Sayed. 2009. The genome of the blood fluke Schistosoma mansoni. Nature 460:352-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhardwaj, R., and P. J. Skelly. 2009. Purinergic signaling and immune modulation at the schistosome surface? Trends Parasitol. 25:256-260. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard, T. J. 2004. Schistosomiasis. Travel Med. Infect. Dis. 2:5-11. [DOI] [PubMed] [Google Scholar]
- 21.Blanton, R. E., E. A. Salam, A. Ehsan, C. H. King, and K. A. Goddard. 2005. Schistosomal hepatic fibrosis and the interferon gamma receptor: a linkage analysis using single-nucleotide polymorphic markers. Eur. J. Hum. Genet. 13:660-668. [DOI] [PubMed] [Google Scholar]
- 22.Bottieau, E., J. Clerinx, M. R. de Vega, E. Van den Enden, R. Colebunders, M. Van Esbroeck, T. Vervoort, A. Van Gompel, and J. Van den Ende. 2006. Imported Katayama fever: clinical and biological features at presentation and during treatment. J. Infect. 52:339-345, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Bouree, P., and E. Caumes. 2004. Cercarial dermatitis. Presse Med. 33:490-493. (In French.) [DOI] [PubMed] [Google Scholar]
- 24.Brejová, B., T. Vinar, Y. Chen, S. Wang, G. Zhao, D. G. Brown, M. Li, and Y. Zhou. 2009. Finding genes in Schistosoma japonicum: annotating novel genomes with help of extrinsic evidence. Nucleic Acids Res. 37:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brindley, P. J., and E. J. Pearce. 2007. Genetic manipulation of schistosomes. Int. J. Parasitol. 37:465-473. [DOI] [PubMed] [Google Scholar]
- 26.Burke, M. L., M. K. Jones, G. N. Gobert, Y. S. Li, M. K. Ellis, and D. P. McManus. 2009. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 31:163-176. [DOI] [PubMed] [Google Scholar]
- 27.Caffrey, C. R., A. Rohwer, F. Oellien, R. J. Marhöfer, S. Braschi, G. Oliveira, J. H. McKerrow, and P. M. Selzer. 2009. A comparative chemogenomics strategy to predict potential drug targets in the metazoan pathogen Schistosoma mansoni. PLoS One 4:e4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai, P., L. Bu, J. Wang, Z. Wang, X. Zhong, and H. Wang. 2008. Molecular characterization of Schistosoma japonicum tegument protein tetraspanin-2: sequence variation and possible implications for immune evasion. Biochem. Biophys. Res. Commun. 372:197-202. [DOI] [PubMed] [Google Scholar]
- 29.Cai, G., Y. Bae, Y. Zhang, Y. He, M. Jiang, and L. He. 2009. Expression and characterization of two tyrosinases from the trematode Schistosoma japonicum. Parasitol. Res. 104:601-609. [DOI] [PubMed] [Google Scholar]
- 30.Campino, S., D. Kwiatkowski, and A. Dessein. 2006. Mendelian and complex genetics of susceptibility and resistance to parasitic infections. Semin. Immunol. 18:411-422. [DOI] [PubMed] [Google Scholar]
- 31.Capron, A. G., G. J. Riveau, M. Capron, and F. Trottein. 2005. Schistosomes: the road from host-parasite interactions to vaccines in clinical trials. Trends Parasitol. 21:143-149. [DOI] [PubMed] [Google Scholar]
- 32.Capron, A., G. J. Riveau, P. B. Bartley, and D. P. McManus. 2002. Prospects for a schistosome vaccine. Curr. Drug Targets Immune Endocr. Metab. Disord. 2:281-290. [DOI] [PubMed] [Google Scholar]
- 33.Carabin, H., E. Balolong, L. Joseph, S. T. McGarvey, M. V. Johansen, T. Fernandez, A. L. Willingham, and R. Olveda. 2005. Estimating sensitivity and specificity of a faecal examination method for Schistosoma japonicum infection in cats, dogs, water buffaloes, pigs, and rats in Western Samar and Sorsogon Provinces, The Philippines. Int. J. Parasitol. 35:1517-1524. [DOI] [PubMed] [Google Scholar]
- 34.Carabin, H., H. L. Guyatt, and D. Engels. 2000. A comparative analysis of the cost-effectiveness of treatment based on parasitological and symptomatic screening for Schistosoma mansoni in Burundi. Trop. Med. Int. Health 5:192-202. [DOI] [PubMed] [Google Scholar]
- 35.Carabin, H., M. S. Chan, and H. L. Guyatt. 2000. A population dynamic approach to evaluating the impact of school attendance on the unit cost and effectiveness of schoolbased schistosomiasis chemotherapy programmes. Parasitology 121:171-183. [DOI] [PubMed] [Google Scholar]
- 36.CDC. 2005. Travelers' health: yellow book, health information for international travel, 2005-2006, p. 326. CDC, Atlanta, GA.
- 37.Chan, M. S., and D. A. P. Bundy. 1997. Modelling the dynamic effects of community chemotherapy on the patterns of morbidity due to Schistosoma mansoni. Trans. R. Soc. Trop. Med. Hyg. 91:216-220. [DOI] [PubMed] [Google Scholar]
- 38.Chen, H., and D. Lin. 2004. The prevalence and control of schistosomiasis in Poyang Lake region, China. Parasitol. Int. 53:115-125. [DOI] [PubMed] [Google Scholar]
- 39.Chen, M. G. 1993. Schistosoma japonicum and S japonicum-like infections: epidemiology, clinical and pathological aspects, p. 237-270. In P. Jordan, G. Webbe, and F. S. Sturrock (ed.), Human schistosomiasis. CAB International, Wallingford, United Kingdom.
- 40.Chen, M. G., and Z. Feng. 1999. Schistosomiasis control in China. Parasitol. Int. 48:11-19. [DOI] [PubMed] [Google Scholar]
- 41.Cheng, G., Z. Fu, J. Lin, Y. Shi, Y. Zhou, Y. Jin, and Y. Cai. 2009. In vitro and in vivo evaluation of small interference RNA-mediated gynaecophoral canal protein silencing in Schistosoma japonicum. J. Gene Med. 11:412-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chevillard, C., C. E. Moukoko, N. E. Elwali, J. H. Bream, B. Kouriba, L. Argiro, S. Rahoud, A. Mergnai, S. Henri, J. Gaudart, Q. Mohamed-Ali, H. A. Young, and A. J. Dessein. 2003. IFN-gamma polymorphisms (IFN-gamma +2109 and IFN-gamma +3810) are associated with severe hepatic fibrosis in human hepatic schistosomiasis (Schistosoma mansoni). J. Immunol. 171:5596-5601. [DOI] [PubMed] [Google Scholar]
- 43.Chevillard, C., S. Henri, F. Stefani, D. Parzy, and A. Dessein. 2002. Two new polymorphisms in the human interferon gamma (IFN-gamma) promoter. Eur. J. Immunogenet. 29:53-56. [DOI] [PubMed] [Google Scholar]
- 44.Chinese Expert Advisory Committee on Schistosomiasis. 1998. An introduction of a nationwide sampling survey on schistosomiasis in China. Chin. J. Parasitol. Parasitic Dis. 16:84-88. (In Chinese.) [PubMed] [Google Scholar]
- 45.Clements, A. C. A., E. Bosque-Oliva, M. Sacko, A. Landoure, R. Dembele, M. Traore, G. Coulibaly, A. F. Gabrielli, A. Fenwick, and S. Brooker. 2009. A comparative study of the spatial distribution of schistosomiasis in Mali in 1984-1989 and 2004-2006. PLoS Negl. Trop. Dis. 3:e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook, G. 1996. Manson's tropical diseases, 20th ed. W. B. Saunders, London, United Kingdom.
- 47.Cooke, G. S., A. Lalvani, F. V. Gleeson, and C. P. Conlon. 1999. Acute pulmonary schistosomiasis in travellers returning from Lake Malawi, sub-Saharan Africa. Clin. Infect. Dis. 29:836-839. [DOI] [PubMed] [Google Scholar]
- 48.Corachan, C. 2002. Schistosomiasis and international travel. Clin. Infect. Dis. 35:446-450. [DOI] [PubMed] [Google Scholar]
- 49.Da'Dara, A. A., Y. S. Li, T. Xiong, J. Zhou, G. M. Williams, D. P. McManus, Z. Feng, X. L. Yu, D. J. Gray, and D. A. Harn. 2008. DNA-based vaccine protects against zoonotic schistosomiasis in water buffalo. Vaccine 26:3617-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis, G. M., W. Wu, H. Chen, H. Liu, J. Guo, D. D. Lin, S. B. Lu, G. Williams, A. Sleigh, Z. Feng, and D. P. McManus. 2002. A baseline study of importance of bovines for human Schistosoma japonicum infections around Poyang Lake, China: villages studied and snail sampling strategy. Am. J. Trop. Med. Hyg. 66:359-371. [DOI] [PubMed] [Google Scholar]
- 51.De Bont, J., and J. Vercruysse. 1997. The epidemiology and control of cattle schistosomiasis. Parasitol. Today 13:255-262. [DOI] [PubMed] [Google Scholar]
- 52.de Jesus, A. R., A. Silva, L. B. Santana, A. Magalhaes, A. A. de Jesus, R. P. de Almeida, M. A. Rego, M. N. Burattini, E. J. Pearce, and E. M. Carvalho. 2002. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J. Infect. Dis. 185:98-105. [DOI] [PubMed] [Google Scholar]
- 53.De Marco, R., and S. Verjovski-Almeida. 2009. Schistosomes—proteomics studies for potential novel vaccines and drug targets. Drug Discov. Today 14:472-478. [DOI] [PubMed] [Google Scholar]
- 54.de Oliveira Penido, M. L., P. M. Zech Coelho, R. T. de Mello, D. Piló-Veloso, M. C. de Oliveira, J. R. Kusel, and D. L. Nelson. 2008. Antischistosomal activity of aminoalkanethiols, aminoalkanethiosulfuric acids and the corresponding disulfides. Acta Trop. 108:249-255. [DOI] [PubMed] [Google Scholar]
- 55.Dessein, A., C. Chevillard, V. Arnaud, X. Hou, A. A. Hamdoun, H. Dessein, H. He, S. A. Abdelmaboud, X. Luo, J. Li, A. Varoquaux, A. Mergani, M. Abdelwahed, J. Zhou, A. Monis, M. G. Pitta, N. Gasmelseed, S. Cabantous, Y. Zhao, A. Prata, C. Brandt, N. E. Elwali, L. Argiro, and Y. Li. 2009. Variants of CTGF are associated with hepatic fibrosis in Chinese, Sudanese, and Brazilians infected with schistosomes. J. Exp. Med. 206:2321-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dessein, A. J., D. Hillaire, N. E. Elwali, S. Marquet, Q. Mohamed-Ali, A. Mirghani, S. Henri, A. A. Abdelhameed, O. K. Saeed, M. M. Magzoub, and L. Abel. 1999. Severe hepatic fibrosis in Schistosoma mansoni infection is controlled by a major locus that is closely linked to the interferon-gamma receptor gene. Am. J. Hum. Genet. 65:709-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doenhoff, M. J., D. Cioli, and J. Utzinger. 2008. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 21:659-667. [DOI] [PubMed] [Google Scholar]
- 58.Doenhoff, M. J., and L. Pica-Mattoccia. 2006. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev. Anti-Infect. Ther. 4:199-210. [DOI] [PubMed] [Google Scholar]
- 59.Ebrahim, A., H. El-Morshedy, E. Omer, S. El-Daly, and R. Barakat. 1997. Evaluation of the Kato Katz thick smear and formol ether sedimentation techniques for quantitative diagnosis of Schistosoma mansoni infection. Am. J. Trop. Med. Hyg. 57:706-708. [DOI] [PubMed] [Google Scholar]
- 60.Ellis, M. K., Y. Li, X. Hou, H. Chen, and D. P. McManus. 2008. sTNFR-II and sICAM-1 are associated with acute disease and hepatic inflammation in schistosomiasis japonica. Int. J. Parasitol. 38:717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellis, M. K., and D. P. McManus. 2009. Familial aggregation of human helminth infection in the Poyang Lake region of China with a focus on genetic susceptibility to schistosomiasis japonica and associated markers of disease. Parasitology 136:699-712. [DOI] [PubMed] [Google Scholar]
- 62.Ellis, M. K., Z. Z. Zhao, H. G. Chen, G. W. Montgomery, Y. S. Li, and D. P. McManus. 2007. Analysis of the 5q31-q33 locus shows an association between single nucleotide polymorphism variants in the IL-5 gene and symptomatic infection with the human blood fluke, Schistosoma japonicum. J. Immunol. 179:8366-8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engels, D., L. Chitsulo, A. Montresor, and L. Savioli. 2002. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 82:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faghiri, Z., and P. J. Skelly. 2009. The role of tegumental aquaporin from the human parasitic worm, Schistosoma mansoni, in osmoregulation and drug uptake. FASEB J. 23:2780-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fagundes Teixeira, C., E. Neuhauss, R. Ben, J. Romanzini, and C. Graeff-Teixeira. 2007. Detection of Schistosoma mansoni eggs in feces through their interaction with paramagnetic beads in a magnetic field. PLoS Negl. Trop. Dis. 1:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fedorko, J. M. 1999. Schistosoma japonicum in the black rat, Rattus rattus mindanensis, from Leyte, Philippines in relation to Oncomelania snail colonies with reference to other endoparasites. Southeast Asian J. Trop. Med. Public Health 30:343-349. [PubMed] [Google Scholar]
- 67.Fernandez, T. J., M. R. Tarafder, E. Balolong, L. Joseph, A. L. Willingham, P. Belisle, J. P. Webster, R. M. Olveda, S. T. McGarvey, and H. Carabin. 2007. Prevalence of Schistosoma japonicum infection among animals in fifty villages of Samar Province, The Philippines. Vector Borne Zoonotic Dis. 7:147-155. [DOI] [PubMed] [Google Scholar]
- 68.Finkelstein, J. L., M. D. Schleinitz, H. Carabin, and S. T. McGarvey. 2008. Decision-model estimation of the age-specific disability weight for schistosomiasis japonica: a systematic review of the literature. PLoS Negl. Trop. Dis. 2:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao, J., J. Qian, S. Tang, B. O. Eriksson, and E. Blas. 2002. Health equity in transition from planned to market economy in China. Health Policy Plann. 17:20-29. [DOI] [PubMed] [Google Scholar]
- 70.Gatlin, M. R., C. L. Black, P. N. Mwinzi, W. E. Secor, D. M. Karanja, and D. G. Colley. 2009. Association of the gene polymorphisms IFN-gamma +874, IL-13-1055 and IL-4-590 with patterns of reinfection with Schistosoma mansoni. PLoS Negl. Trop. Dis. 3:e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gnanasekar, M., A. M. Salunkhe, A. K. Mallia, Y. X. He, and R. Kalyanasundaram. 2009. Praziquantel affects the regulatory myosin light chain of Schistosoma mansoni. Antimicrob. Agents Chemother. 53:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gobert, G. N., D. P. McManus, S. Nawaratna, L. Moertel, J. Mulvenna, and M. K. Jones. 2009b. Tissue specific profiling of females of Schistosoma japonicum by integrated laser microdissection microscopy and microarray analysis. PLoS Negl. Trop. Dis. 3:e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gobert, G. N., L. Moertel, P. J. Brindley, and D. P. McManus. 2009. Developmental gene expression profiles of the human pathogen Schistosoma japonicum. BMC Genomics 10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gobert, G. N., M. Chai, M. Duke, and D. P. McManus. 2005. Copro-PCR based detection of Schistosoma eggs using mitochondrial DNA markers. Mol. Cell. Probes 19:250-254. [DOI] [PubMed] [Google Scholar]
- 75.Gobert, G. N., and M. K. Jones. 2008. Discovering new schistosome drug targets: the role of transcriptomics. Curr. Drug Targets 11:922-930. [DOI] [PubMed] [Google Scholar]
- 76.Gomes, M. S., F. J. Cabral, L. K. Jannotti-Passos, O. Carvalho, V. Rodrigues, E. H. Baba, and R. G. Sá. 2009. Preliminary analysis of miRNA pathway in Schistosoma mansoni. Parasitol. Int. 58:61-68. [DOI] [PubMed] [Google Scholar]
- 77.Gray, D. J., G. M. Williams, Y. S. Li, H. G. Chen, R. S. Li, S. J. Forsyth, A. G. Barnett, J. G. Guo, A. G. Ross, Z. Feng, and D. P. McManus. 2009. A cluster-randomized bovine intervention trial against S. japonicum in the Peoples' Republic of China; bovine and human transmission. PLoS One 4:e5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray, D. J., G. M. Williams, Y. S. Li, and D. P. McManus. 2008. Transmission dynamics of Schistosoma japonicum in the lake and marshlands region of China. PLoS One 3:e4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gray, D. J., G. M. Williams, Y. S. Li, H. Chen, R. S. Li, S. J. Forsyth, A. G. Barnett, J. Guo, Z. Feng, and D. P. McManus. 2007. A cluster-randomized bovine intervention trial against Schistosoma japonicum in the People's Republic of China: design and baseline results. Am. J. Trop. Med. Hyg. 77:866-874. [PMC free article] [PubMed] [Google Scholar]
- 80.Gryseels, B., K. Polman, J. Clerinx, and L. Kestens. 2006. Human schistosomiasis. Lancet 368:1106-1118. [DOI] [PubMed] [Google Scholar]
- 81.Guo, J. G., A. G. Ross, D. D. Lin, G. M. Williams, H. G. Chen, Y. Li, G. M. Davis, Z. Feng, D. P. McManus, and A. C. Sleigh. 2001. A baseline study on the importance of bovines for human Schistosoma japonicum infection around Poyang Lake, China. Am. J. Trop. Med. Hyg. 65:272-278. [DOI] [PubMed] [Google Scholar]
- 82.Guo, J., G. P. Vounatsou, C. L. Cao, J. Utzinger, H. Q. Zhu, D. Anderegg, R. Zhu, Z. Y. He, D. Li, F. Hu, M. G. Chen, and M. Tanner. 2005. A geographic information and remote sensing-based model for prediction of Oncomelania hupensis habitats in the Poyang Lake area, China. Acta Trop. 96:213-222. [DOI] [PubMed] [Google Scholar]
- 83.Guo, J., Y. Li, D. Gray, A. Ning, G. Hu, H. Chen, G. M. Davis, A. C. Sleigh, F. Zheng, D. P. McManus, and G. M. Williams. 2006. A drug-based intervention study on the importance of buffaloes for human Schistosoma japonicum infection around Poyang Lake, People's Republic of China. Am. J. Trop. Med. Hyg. 74:335-341. [PubMed] [Google Scholar]
- 84.Guyatt, H. L., and M. Tanner. 1996. Different approaches to modeling the cost-effectivenss of schistosomiasis control. Am. J. Trop. Med. Hyg. 55(5 Suppl.):159-164. [DOI] [PubMed] [Google Scholar]
- 85.Haas, B. J., M. Berriman, H. Hirai, G. G. Cerqueira, P. T. Loverde, and N. M. El-Sayed. 2007. The Schistosoma mansoni genome: closing in on a final gene set. Exp. Parasitol. 117:225-228. [DOI] [PubMed] [Google Scholar]
- 86.Hairston, N. G. 1965. On the mathematical analysis of schistosome populations. Bull. World Health Organ. 33:45-62. [PMC free article] [PubMed] [Google Scholar]
- 87.Hattori, H., L. E. Rosas, M. Okano, J. E. Durbin, K. Nishizaki, and A. R. Satoskar. 2007. STAT1 is involved in the pathogenesis of murine allergic rhinitis. Am. J. Rhinol. 21:241-247. [DOI] [PubMed] [Google Scholar]
- 88.He, H., A. Isnard, B. Kouriba, S. Cabantous, A. Dessein, O. Doumbo, and C. Chevillard. 2008. A STAT6 gene polymorphism is associated with high infection levels in urinary schistosomiasis. Genes Immun. 9:195-206. [DOI] [PubMed] [Google Scholar]
- 89.He, Y. X., B. Salafsky, and K. Ramaswamy. 2001. Host-parasite relationships of Schistosoma japonicum in mammalian hosts. Trends Parasitol. 17:320-324. [DOI] [PubMed] [Google Scholar]
- 90.Henri, S., C. Chevillard, A. Mergani, P. Paris, J. Gaudart, C. Camilla, H. Dessein, F. Montero, N. E. Elwali, O. K. Saeed, M. Magzoub, and A. J. Dessein. 2002. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN-gamma is associated with protection against fibrosis and TNF-alpha with aggravation of disease. J. Immunol. 169:929-936. [DOI] [PubMed] [Google Scholar]
- 91.Hiatt, R. A., Z. R. Sotomayor, G. Sanchez, M. Zambrana, and W. B. Knight. 1979. Factors in pathogenesis of acute schistosomiasis mansoni. J. Infect. Dis. 139:659-666. [DOI] [PubMed] [Google Scholar]
- 92.Ho, Y. H., and Y. X. He. 1963. On the host specificity of Schistosoma japonicum. Chin. Med. J. 82:403-414. [PubMed] [Google Scholar]
- 93.Hopkin, J. 2009. Immune and genetic aspects of asthma, allergy and parasitic worm infections: evolutionary links. Parasite Immunol. 31:267-273. [DOI] [PubMed] [Google Scholar]
- 94.Hou, X. Y., D. P. McManus, D. J. Gray, J. Balen, X. S. Luo, Y. K. He, M. K. Ellis, G. M. Williams, and Y. S. Li. 2008. A randomised, double-blind, placebo-controlled trial on the safety and efficacy of combined praziquantel and artemether treatment for acute schistosomiasis japonica in China. Bull. World Health Organ. 86:788-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu, W., Q. Yan, D. K. Shen, F. Liu, Z. D. Zhu, H. D. Song, X. R. Xu, Z. J. Wang, Y. P. Rong, L. C. Zeng, J. Wu, X. Zhang, J. J. Wang, X. N. Xu, S. Y. Wang, G. Fu, X. L. Zhang, Z. Q. Wang, P. J. Brindley, D. P. McManus, C. L. Xue, Z. Feng, Z. Chen, and Z. G. Han. 2003. Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNA resource. Nat. Genet. 35:139-147. [DOI] [PubMed] [Google Scholar]
- 96.Hu, S., Z. Wu, L. Yang, and M. C. Fung. 2009. Molecular cloning and expression of a functional anti-inflammatory protein, Sj16, of Schistosoma japonicum. Int. J. Parasitol. 39:191-200. [DOI] [PubMed] [Google Scholar]
- 97.Hung, Y. W., and J. Remais. 2008. Quantitative detection of Schistosoma japonicum cercariae in water by real-time PCR. PLoS Negl. Trop. Dis. 2:e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jia, T. W., X. N. Zhou, X. H. Wang, J. Utzinger, P. Steinmann, and X. H. Wu. 2007. Assessment of the age-specific disability weight of chronic schistosomiasis japonica. Bull. World Health Organ. 85:458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jia, X. M., H. Sriplung, V. Chongsuvivatwong, and A. Geater. 2009. Sensitivity of pooled serum testing for screening antibody of schistosomiasis japonica by IHA in a mountainous area of Yunnan, China. Parasitology 136:267-272. [DOI] [PubMed] [Google Scholar]
- 100.Jiz, M., H. W. Wu, R. Meng, S. Pond-Tor, M. Reynolds, J. F. Friedman, R. Olveda, L. Acosta, and J. D. Kurtis. 2008. Pilot-scale production and characterization of paramyosin, a vaccine candidate for schistosomiasis japonica. Infect. Immun. 76:3164-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiz, M., J. F. Friedman, T. Leenstra, B. Jarilla, A. Pablo, G. Langdon, S. Pond-Tor, H. W. Wu, D. Manalo, R. Olveda, L. Acosta, and J. D. Kurtis. 2009. Immunoglobulin E (IgE) responses to paramyosin predict resistance to reinfection with Schistosoma japonicum and are attenuated by IgG4. Infect. Immun. 77:2051-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johansen, M. V., H. O. Bøgh, P. Nansen, and N. O. Christensen. 2000. Schistosoma japonicum infection in the pig as a model for human schistosomiasis japonica. Acta Trop. 76:85-99. [DOI] [PubMed] [Google Scholar]
- 103.Jones, M. K., S. H. Bong, K. M. Green, P. Holmes, M. Duke, A. Loukas, and D. P. McManus. 2008. Correlative and dynamic imaging of the hatching biology of Schistosoma japonicum from eggs prepared by high pressure freezing. PLoS Negl. Trop. Dis. 2:e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jordan, P., G. Webbe, and R. F. Sturrock. 1993. Human schistosomiasis. CAB International, Wallingford, United Kingdom.
- 105.Katz, N., A. Chaves, and J. Pellegrino. 1972. A simple device for quantitative stool thick-smear technique for schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 14:397-400. [PubMed] [Google Scholar]
- 106.Keiser, J., J. Chollet, S. H. Xiao, J. Y. Mei, P. Y. Jiao, J. Utzinger, and M. Tanner. 2009. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis. 3:e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.King, C. H., K. Dickman, and D. J. Tisch. 2005. Reassessment of the cost of chronic helminthic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 365:1561-1569. [DOI] [PubMed] [Google Scholar]
- 108.Kouriba, B., C. Chevillard, J. H. Bream, L. Argiro, H. Dessein, V. Arnaud, L. Sangare, A. Dabo, A. H. Beavogui, C. Arama, H. A. Traoré, O. Doumbo, and A. Dessein. 2005. Analysis of the 5q31-q33 locus shows an association between IL13-1055C/T IL-13-591A/G polymorphisms and Schistosoma haematobium infections. J. Immunol. 174:6274-6281. [DOI] [PubMed] [Google Scholar]
- 109.Krautz-Peterson, G., D. Ndegwa, K. Vasquez, H. Korideck, J. Zhang, J. D. Peterson, and P. J. Skelly. 2009. Imaging schistosomes in vivo. FASEB J. 23:2673-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kumagai, T., Y. Osada, N. Ohta, and T. Kanazawa. 2009. Peroxiredoxin-1 from Schistosoma japonicum functions as a scavenger against hydrogen peroxide but not nitric oxide. Mol. Biochem. Parasitol. 164:26-31. [DOI] [PubMed] [Google Scholar]
- 111.Lambertucci, J. R., A. A. Rayes, C. H. Barata, R. Teixeira, and R. Gerspacher-Lara. 1997. Acute schistosomiasis: report on five singular cases. Mem. Inst. Oswaldo Cruz 92:631-635. [DOI] [PubMed] [Google Scholar]
- 112.Lee, L. 2004. The current state of public health in China. Annu. Rev. Public Health 25:327-339. [DOI] [PubMed] [Google Scholar]
- 113.Leenstra, T., L. P. Acosta, H. W. Wu, G. C. Langdon, J. S. Solomon, D. L. Manalo, L. Su, M. Jiz, B. Jarilla, A. O. Pablo, S. T. McGarvey, R. M. Olveda, J. F. Friedman, and J. D. Kurtis. 2006. T-helper-2 cytokine responses to Sj97 predict resistance to reinfection with Schistosoma japonicum. Infect. Immun. 74:370-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lei, J. H., W. Q. Liu, C. S. Sun, C. L. Tang, M. J. Li, Y. L. Chen, and Y. L. Li. 2009. Detection of circulating antigen in serum of mice infected with Schistosoma japonicum by immunomagnetic bead ELISA based on IgY. Acta Trop. 111:39-43. [DOI] [PubMed] [Google Scholar]
- 115.Li, J. V., E. Holmes, J. Saric, J. Keiser, S. Dirnhofer, J. Utzinger, and Y. Wang. 2009. Metabolic profiling of a Schistosoma mansoni infection in mouse tissues using magic angle spinning-nuclear magnetic resonance spectroscopy. Int. J. Parasitol. 39:547-558. [DOI] [PubMed] [Google Scholar]
- 116.Li, Y. S., A. C. Sleigh, A. G. P. Ross, G. M. Williams, S. J. Forsyth, M. Tanner, and D. P. McManus. 1999. A two-year epidemiological survey in China provides epidemiological evidence for human resistance to re-infection by Schistosoma japonicum. Ann. Trop. Med. Parasitol. 93:629-642. [DOI] [PubMed] [Google Scholar]
- 117.Li, Y. S., G. Raso, Z. Y. Zhao, Y. K. He, M. K. Ellis, and D. P. McManus. 2007. Large water management projects and schistosomiasis control, Dongting Lake region, China. Emerg. Infect. Dis. 13:973-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li, Y., A. C. Sleigh, A. G. P. Ross, G. M. Williams, M. Tanner, and D. P. McManus. 2000. Epidemiology of Schistosoma japonicum in China: morbidity and strategies for control in the Dongting Lake region. Int. J. Parasitol. 30:273-281. [DOI] [PubMed] [Google Scholar]
- 119.Li, Y., A. C. Sleigh, G. M. Williams, A. G. P. Ross, S. J. Forsyth, M. Tanner, and D. P. McManus. 2000. Measuring exposure to Schistosoma japonicum in China. III. Activity diaries, snail and human infection, transmission ecology and options for control. Acta Trop. 75:279-289. [DOI] [PubMed] [Google Scholar]
- 120.Li, Y., Y. Zhao, M. Ellis, and D. P. McManus. 2005. Applications and outcomes of periodic epidemiological surveys for schistosomiasis and related economic evaluation in the People's Republic of China. Acta Trop. 96:266-275. [DOI] [PubMed] [Google Scholar]
- 121.Liang, S., E. Seto, J. V. Remais, B. Zhong, C. Yang, A. Hubbard, G. M. Davis, X. Gu, D. Qiu, and R. C. Spear. 2007. Environmental effects on parasitic disease transmission exemplified by schistosomiasis in western China. Proc. Natl. Acad. Sci. U. S. A. 104:7110-7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liang, S., R. C. Spear, E. Seto, A. Hubbard, and D. Qiu. 2005. A multi-group model of Schistosoma japonicum transmission dynamics and control: model calibration and control prediction. Trop. Med. Int. Health 10:263-278. [DOI] [PubMed] [Google Scholar]
- 123.Lier, T., G. S. Simonsen, H. Haaheim, S. O. Hjelmevoll, B. J. Vennervald, and M. V. Johansen. 2006. Novel real-time PCR for detection of Schistosoma japonicum in stool. Southeast Asian J. Trop. Med. Public Health 37:257-264. [PubMed] [Google Scholar]
- 124.Lier, T., G. S. Simonsen, T. Wang, D. Lu, H. H. Haukland, B. J. Vennervald, and M. V. Johansen. 2009. Low sensitivity of the formol-ethyl acetate sedimentation concentration technique in low-intensity Schistosoma japonicum infections. PLoS Negl. Trop. Dis. 3:e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lier, T., M. V. Johansen, S. O. Hjelmevoll, B. J. Vennervald, and G. S. Simonsen. 2008. Real-time PCR for detection of low intensity Schistosoma japonicum infections in a pig model. Acta Trop. 105:74-80. [DOI] [PubMed] [Google Scholar]
- 126.Liu, F., J. Lu, W. Hu, S. Y. Wang, S. J. Cui, M. Chi, Q. Yan, X. R. Wang, H. D. Song, X. N. Xu, J. J. Wang, X. L. Zhang, X. Zhang, Z. Q. Wang, C. L. Xue, P. J. Brindley, D. P. McManus, P. Y. Yang, Z. Feng, Z. Chen, and Z. G. Han. 2006. New perspectives on host parasite interplay by comparative transcriptomic and proteomic analyses of the human blood fluke Schistosoma japonicum. PLoS Pathol. 2:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu, F., S. J. Cui, W. Hu, Z. Feng, Z. Q. Wang, and Z. G. Han. 2009. Excretory/secretory proteome of the adult developmental stage of human blood fluke, Schistosoma japonicum. Mol. Cell Proteomics 8:1236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Loukas, A., M. Tran, and M. S. Pearson. 2007. Schistosome membrane proteins as vaccines. Int. J. Parasitol. 37:257-263. [DOI] [PubMed] [Google Scholar]
- 129.Lv, Z. Y., L. L. Yang, S. M. Hu, X. Sun, H. J. He, Z. Y. Li, Y. P. Zhou, M. C. Fung, X. B. Yu, H. Q. Zheng, A. L. Cao, and Z. D. Wu. 2009. Expression profile, localization of an 8-kDa calcium-binding protein from Schistosoma japonicum (SjCa8), and vaccine potential of recombinant SjCa8 (rSjCa8) against infections in mice. Parasitol. Res. 104:733-743. [DOI] [PubMed] [Google Scholar]
- 130.Macdonald, G. 1965. The dynamics of helminth infections, with special reference to schistosomes. Trans. R. Soc. Trop. Med. Hyg. 59:489-506. [DOI] [PubMed] [Google Scholar]
- 131.Magalhães, E. S., C. N. Paiva, H. S. Souza, A. S. Pyrrho, D. Mourão-Sá, R. T. Figueiredo, A. Vieira-de-Abreu, H. S. Dutra, M. S. Silveira, M. I. Gaspar-Elsas, P. Xavier-Elsas, P. T. Bozza, and M. T. Bozza. 2009. Macrophage migration inhibitory factor is critical to interleukin-5-driven eosinophilopoiesis and tissue eosinophilia triggered by Schistosoma mansoni infection. FASEB J. 23:1262-1271. [DOI] [PubMed] [Google Scholar]
- 132.Magalhães, L. G., C. B. Machado, E. R. Morais, E. Bueno de Carvalho Moreira, C. S. Soares, S. H. da Silva, A. A. Da Silva Filho, and V. Rodrigues. 2009. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol. Res. 104:1197-1201. [DOI] [PubMed] [Google Scholar]
- 133.Mao, S. B. 1990. Schistosomiasis biology and schistosomiasis control, p. 624-630, 643-651. People's Press of Health, Beijing, China. (In Chinese.)
- 134.Marquet, S., L. Abel, D. Hillaire, and A. Dessein. 1999. Full results of the genome-wide scan which localises a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Eur. J. Hum. Genet. 7:88-97. [DOI] [PubMed] [Google Scholar]
- 135.Marquet, S., L. Abel, D. Hillaire, H. Dessein, J. Kalil, J. Feingold, J. Weissenbach, and A. J. Dessein. 1996. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat. Genet. 14:181-184. [DOI] [PubMed] [Google Scholar]
- 136.McGarvey, S. T., H. Carabin, E. Balolong, P. Belisle, T. Fernandez, L. Joseph, V. Tallo, R. Gonzales, M. K. Tarafder, T. Alday, A. L. Willingham, and R. Olveda. 2006. Cross-sectional associations between intensity of animal and human infection with Schistosoma japonicum in Western Samar Province, Philippines. Bull. World Health Organ. 84:446-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McManus, D. P. 2005. Prospects for development of a transmission blocking vaccine against Schistosoma japonicum. Parasite Immunol. 27:297-308. [DOI] [PubMed] [Google Scholar]
- 138.McManus, D. P., and A. Loukas. 2008. Current status of vaccines for schistosomiasis. Clin. Microbiol. Rev. 21:225-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McManus, D. P., and J. P. Dalton. 2006. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology 133:S43-S61. [DOI] [PubMed] [Google Scholar]
- 140.McManus, D. P., Y. Li, D. J. Gray, and A. G. Ross. 2009. Conquering ‘snail fever’: schistosomiasis and its control in China. Expert Rev. Anti-Infect. Ther. 7:473-485. [DOI] [PubMed] [Google Scholar]
- 141.McVeigh, P., G. R. Mair, L. Atkinson, P. Ladurner, M. Zamanian, E. Novozhilova, N. J. Marks, T. A. Day, and A. G. Maule. 2009. Discovery of multiple neuropeptide families in the phylum Platyhelminthes. Int. J. Parasitol. 39:1243-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Medley, G. F., and D. A. P. Bundy. 1996. Dynamic modeling ofepidemiologic patterns of schistosomiasis morbidity. Am. J. Trop. Med. Hyg. 55:149-158. [DOI] [PubMed] [Google Scholar]
- 143.Messerli, S. M., R. S. Kasinathan, W. Morgan, S. Spranger, and R. M. Greenberg. 2009. Schistosoma mansoni P-glycoprotein levels increase in response to praziquantel exposure and correlate with reduced praziquantel susceptibility. Mol. Biochem. Parasitol. 167:54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mitchell, G. F., E. G. Garcia, S. M. Wood, R. Diasanta, R. Almonte, E. Calica, K. M. Davern, and W. U. Tiu. 1990. Studies on the sex ratio of worms in schistosome infection. Parasitology 101:27-34. [DOI] [PubMed] [Google Scholar]
- 145.Mo, H. M., J. H. Lei, Z. W. Jiang, C. Z. Wang, Y. L. Cheng, Y. L. Li, and W. Q. Liu. 2008. Schistosoma japonicum infection modulates the development of allergen-induced airway inflammation in mice. Parasitol. Res. 103:1183-1189. [DOI] [PubMed] [Google Scholar]
- 146.Mountford, A. P. 2005. Immunological aspects of schistosomiasis. Parasite Immunol. 27:243-246. [DOI] [PubMed] [Google Scholar]
- 147.Mwinzi, P. N., L. Ganley-Leal, C. L. Black, W. E. Secor, D. M. Karanja, and D. G. Colley. 2009. Circulating CD23+ B cell subset correlates with the development of resistance to Schistosoma mansoni reinfection in occupationally exposed adults who have undergone multiple treatments. J. Infect. Dis. 199:272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nair, M. G., Y. Du, J. G. Perrigoue, C. Zaph, J. J. Taylor, M. Goldschmidt, G. P. Swain, G. D. Yancopoulos, D. M. Valenzuela, A. Murphy, M. Karow, S. Stevens, E. J. Pearce, and D. Artis. 2009. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206:937-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Osada, Y., S. Shimizu, T. Kumagai, S. Yamada, and T. Kanazawa. 2009. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int. J. Parasitol. 39:457-464. [DOI] [PubMed] [Google Scholar]
- 150.Pacífico, L. G., F. A. Marinho, C. T. Fonseca, M. M. Barsante, V. Pinho, P. A. Sales-Junior, L. S. Cardoso, M. I. Araújo, E. M. Carvalho, G. D. Cassali, M. M. Teixeira, and S. C. Oliveira. 2009. Schistosoma mansoni antigens modulate experimental allergic asthma in a murine model: a major role for CD4+ CD25+ Foxp3+ T cells independent of interleukin-10. Infect. Immun. 77:98-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pearce, E. J., and A. S. MacDonald. 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2:499-511. [DOI] [PubMed] [Google Scholar]
- 152.Peng, S. Y., J. C. Tsaihong, P. C. Fan, and K. M. Lee. 2009. Diagnosis of schistosomiasis using recombinant fructose-1,6-bisphosphate aldolase from a Formosan strain of Schistosoma japonicum. J. Helminthol. 83:211-218. [DOI] [PubMed] [Google Scholar]
- 153.Peng, S. Y., K. M. Lee, J. C. Tsaihong, P. C. Cheng, and P. C. Fan. 2008. Evaluation of recombinant fructose-1,6-bisphosphate aldolase ELISA test for the diagnosis of Schistosoma japonicum in water buffaloes. Res. Vet. Sci. 85:527-533. [DOI] [PubMed] [Google Scholar]
- 154.Pesce, J. T., T. R. Ramalingam, M. M. Mentink-Kane, M. S. Wilson, K. C. El Kasmi, A. M. Smith, R. W. Thompson, A. W. Cheever, P. J. Murray, and T. A. Wynn. 2009. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5:e1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pesce, J. T., T. R. Ramalingam, M. S. Wilson, M. M. Mentink-Kane, R. W. Thompson, A. W. Cheever, J. F. Jr. Urban, and T. A. Wynn. 2009. Retnla (relmalpha/fizz1) suppresses helminth-induced th2-type immunity. PLoS Pathog. 5:e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pica-Mattoccia, L., M. J. Doenhoff, C. Valle, A. Basso, A. R. Troiani, P. Liberti, A. Festucci, A. Guidi, and D. Cioli. 2009. Genetic analysis of decreased praziquantel sensitivity in a laboratory strain of Schistosoma mansoni. Acta Trop. 111:82-85. [DOI] [PubMed] [Google Scholar]
- 156a.Preidler, K. W., T. Riepl, D. Szolar, and G. Ranner. 1996. Cerebral schistosomiasis: MR and CT appearance. AJNR Am. J. Neuroradiol. 17:1598-1600. [PMC free article] [PubMed] [Google Scholar]
- 157.Rabello, A. 1997. Diagnosing schistosomiasis. Mem. Inst. Oswaldo Cruz 92:669-676. [DOI] [PubMed] [Google Scholar]
- 158.Ramalingam, T. R., J. T. Pesce, M. M. Mentink-Kane, S. Madala, A. W. Cheever, M. R. Comeau, S. F. Ziegler, and T. A. Wynn. 2009. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J. Immunol. 182:6452-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Raso, G., B. Matthys, E. K. N′Goran, M. Tanner, P. Vounatsou, and J. Utzinger. 2005. Spatial risk prediction and mapping of Schistosoma mansoni infections among schoolchildren living in western Côte d'Ivoire. Parasitology 131:97-108. [DOI] [PubMed] [Google Scholar]
- 160.Riley, S., H. Carabin, P. Belisle, L. Joseph, V. Tallo, E. Balabong, A. L. Willingham, T. J. Fernandez, R. O. Gonzales, R. Olveda, and S. T. McGarvey. 2008. Multi-host transmission dynamics of Schistosoma japonicum in Samar Province, the Philippines. PLoS Med. 5:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rocha, M. O., R. L. Rocha, E. R. Pedroso, D. B. Greco, C. S. Ferreira, J. R. Lambertucci, N. Katz, R. S. Rocha, D. F. Rezende, and J. Neves. 1995. Pulmonary manifestations in the initial phase of Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 37:311-318. [DOI] [PubMed] [Google Scholar]
- 162.Ross, A. G. P., A. C. Sleigh, Y. Li, G. M. Davis, G. W. Williams, Z. Jiang, Z. Feng, and D. P. McManus. 2001. Schistosomiasis in the People's Republic of China: prospects and challenges for the 21st century. Clin. Microbiol. Rev. 14:270-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ross, A. G. P., A. C. Sleigh, Y. Li, G. M. Williams, G. J. Waine, S. J. Forsyth, Y. Li, G. F. Hartel, and D. P. McManus. 1998. Measuring exposure to S. japonicum in China. II. Activity diaries, pathways to infection and immunological correlates. Acta Trop. 71:229-236. [DOI] [PubMed] [Google Scholar]
- 164.Ross, A. G., D. Vickers, G. R. Olds, S. M. Shah, and D. P. McManus. 2007. Katayama syndrome. Lancet Infect. Dis. 7:218-224. [DOI] [PubMed] [Google Scholar]
- 165.Ross, A. G. P., P. B. Bartley, A. C. Sleigh, R. G. Olds, Y. Li, G. W. Williams, and D. P. McManus. 2002. Schistosomiasis. N. Engl. J. Med. 346:1212-1219. [DOI] [PubMed] [Google Scholar]
- 166.Ross, A. G. P., Y. Li, A. C. Sleigh, G. M. Williams, G. F. Hartel, S. J. Forsyth, Y. Li, and D. P. McManus. 1998. Measuring exposure to S. japonicum in China. I. Activity diaries to assess water contact and comparison to other measures. Acta Trop. 71:213-228. [DOI] [PubMed] [Google Scholar]
- 167.Ross, A. G. P., Y. Li, A. C. Sleigh, G. M. Williams, and D. P. McManus. 1998. Faecal egg aggregation in humans infected with Schistosoma japonicum in China. Acta Trop. 70:205-210. [DOI] [PubMed] [Google Scholar]
- 168.Rudge, J. W., D. B. Lu, G. R. Fang, T. P. Wang, M. G. Basáñez, and J. P. Webster. 2009. Parasite genetic differentiation by habitat type and host species: molecular epidemiology of Schistosoma japonicum in hilly and marshland areas of Anhui Province, China. Mol. Ecol. 18:2134-2147. [DOI] [PubMed] [Google Scholar]
- 169.Rudge, J. W., H. Carabin, E. Balolong, V. Tallo, J. Shrivastava, D. B. Lu, M. G. Basáñez, R. Olveda, S. T. McGarvey, and J. P. Webster. 2008. Population genetics of Schistosoma japonicum within the Philippines suggest high levels of transmission between humans and dogs. PLoS Negl. Trop. Dis. 2:e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sayed, A. A., A. Simeonov, C. J. Thomas, J. Inglese, C. P. Austin, and D. L. Williams. 2008. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat. Med. 14:407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium. 2009. The Schistosoma japonicum genome reveals unique features of host-parasite interplay. Nature 460:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schramm, G., J. V. Hamilton, C. I. Balog, M. Wuhrer, A. Gronow, S. Beckmann, V. Wippersteg, C. G. Grevelding, T. Goldmann, E. Weber, N. W. Brattig, A. M. Deelder, D. W. Dunne, C. H. Hokke, H. Haas, and M. J. Doenhoff. 2009. Molecular characterisation of kappa-5, a major antigenic glycoprotein from Schistosoma mansoni eggs. Mol. Biochem. Parasitol. 166:4-14. [DOI] [PubMed] [Google Scholar]
- 173.Seto, E. Y., W. Wu, H. Y. Liu, H. G. Chen, A. Hubbard, A. Holt, and G. M. Davis. 2008. Impact of changing water levels and weather on Oncomelania hupensis hupensis populations, the snail host of Schistosoma japonicum, downstream of the Three Gorges Dam. Ecohealth 5:149-158. [DOI] [PubMed] [Google Scholar]
- 174.Sharma, M., S. Khanna, G. Bulusu, and A. Mitra. 2009. Comparative modeling of thioredoxin glutathione reductase from Schistosoma mansoni: a multifunctional target for antischistosomal therapy. J. Mol. Graph. Model. 27:665-675. [DOI] [PubMed] [Google Scholar]
- 175.Shrivastava, J., B. Z. Qian, G. Mcvean, and J. P. Webster. 2005. An insight into the genetic variation of Schistosoma japonicum in mainland China using DNA microsatellite markers. Mol. Ecol. 14:839-849. [DOI] [PubMed] [Google Scholar]
- 176.Sleigh, A., and S. Jackson. 2001. Dams, development, and health: a missed opportunity. Lancet 357:570-571. [DOI] [PubMed] [Google Scholar]
- 177.Spear, R. C., A. Hubbard, S. Liang, and E. Seto. 2002. Disease transmission models for public health decision making: toward an approach for designing intervention strategies for schistosomiasis japonica. Environ. Health Perspect. 110:907-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Steinmann, P., J. Keiser, R. Bos, M. Tanner, and J. Utzinger. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6:411-425. [DOI] [PubMed] [Google Scholar]
- 179.Taft, A. S., J. J. Vermeire, J. Bernier, S. R. Birkeland, M. J. Cipriano, A. R. Papa, A. G. McArthur, and T. P. Yoshino. 2009. Transcriptome analysis of Schistosoma mansoni larval development using serial analysis of gene expression (SAGE). Parasitology 136:469-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tao, F. F., Y. F. Yang, H. Wang, X. J. Sun, J. Luo, X. Zhu, F. Liu, Y. Wang, C. Su, H. W. Wu, and Z. S. Zhang. 2009. Th1-type epitopes-based cocktail PDDV attenuates hepatic fibrosis in C57BL/6 mice with chronic Schistosoma japonicum infection. Vaccine 27:4110-4117. [DOI] [PubMed] [Google Scholar]
- 181.Taylor, J. J., C. M. Krawczyk, M. Mohrs, and E. J. Pearce. 2009. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression. J. Clin. Invest. 119:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thabet, H. S., N. K. Saleh, S. S. Thabet, M. Abdel-Aziz, and N. K. Kalleny. 2008. Decreased basal non-insulin-stimulated glucose uptake by diaphragm in streptozotocin-induced diabetic mice infected with Schistosoma mansoni. Parasitol. Res. 103:595-601. [DOI] [PubMed] [Google Scholar]
- 183.Ting-An, W., and Z. Hong-Xiang. 2009. PTK-pathways and TGF-beta signaling pathways in schistosomes. J. Basic Microbiol. 49:25-31. [DOI] [PubMed] [Google Scholar]
- 184.Tran, M. H., M. S. Pearson, J. M. Bethony, D. J. Smyth, M. K. Jones, M. Duke, T. A. Don, D. P. McManus, R. Correa-Oliveira, and A. Loukas. 2006. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat. Med. 12:835-840. [DOI] [PubMed] [Google Scholar]
- 185.Tweyongyere, R., P. A. Mawa, N. O. Emojong, H. Mpairwe, F. M. Jones, T. Duong, D. W. Dunne, B. J. Vennervald, E. Katunguka-Rwakishaya, and A. M. Elliott. 2009. Effect of praziquantel treatment of Schistosoma mansoni during pregnancy on intensity of infection and antibody responses to schistosome antigens: results of a randomised, placebo-controlled trial. BMC Infect. Dis. 9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Utzinger, J., S. H. Xiao, M. Tanner, and J. Keiser. 2007. Artemisinins for schistosomiasis and beyond. Curr. Opin. Invest. Drugs 8:105-116. [PubMed] [Google Scholar]
- 187.Utzinger, J., X. N. Zhou, M. G. Chen, and R. Bergquist. 2005. Conquering schistosomiasis in China: the long march. Acta Trop. 96:69-96. [DOI] [PubMed] [Google Scholar]
- 188.van der Werf, M. J., S. J. de Vlas, S. Brooker, C. W. Looman, N. J. Nagelkerke, J. D. Habbema, and D. Engels. 2003. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 86:125-139. [DOI] [PubMed] [Google Scholar]
- 189.Verjovski-Almeida, S., R. DeMarco, E. A. Martins, P. E. Guimaraes, E. P. Ojopi, A. C. Paquola, J. P. Piazza, M. Y. Nishiyama Jr., J. P. Kitajima, R. E. Adamson, P. D. Ashton, M. F. Bonaldo, P. S. Coulson, G. P. Dillon, L. P. Farias, S. P. Gregorio, P. L. Ho, R. A. Leite, L. C. Malaquias, R. C. Marques, P. A. Miyasato, A. L. Nascimento, F. P. Ohlweiler, E. M. Reis, M. A. Ribeiro, R. G. Sa, G. C. Stukart, M. B. Soares, C. Gargioni, T. Kawano, V. Rodrigues, A. M. Madeira, R. A. Wilson, C. F. Menck, J. C. Sebutal, L. C. Leite, and E. Dias-Neto. 2003. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nat. Genet. 35:148-157. [DOI] [PubMed] [Google Scholar]
- 190.Visser, L. G., A. M. Polderman, and P. C. Stuiver. 1995. Outbreak of schistosomiasis among travellers returning from Mali, West Africa. Clin. Infect. Dis. 20:280-285. [DOI] [PubMed] [Google Scholar]
- 191.Wang, L. D. 2006. The epidemic status of schistosomiasis in China: results from the third nationwide sampling survey in 2004. Shanghai Scientific and Technological Literature Publishing House, Shanghai, China.
- 192.Wang, L. D., H. G. Chen, J. G. Guo, X. J. Zeng, X. L. Hong, J. J. Xiong, X. H. Wu, X. H. Wang, L. Y. Wang, G. Xia, Y. Hao, D. P. Chin, and X. N. Zhou. 2009. A strategy to control transmission of Schistosoma japonicum in China. N. Engl. J. Med. 360:121-128. [DOI] [PubMed] [Google Scholar]
- 193.Wang, L., J. Utzinger, and X. N. Zhou. 2008. Schistosomiasis control: experiences and lessons from China. Lancet 372:1793-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang, T., M. V. Johansen, S. Zhang, F. Wang, W. Wu, G. H. Zhang, X. P. Pan, Y. Ju, and N. Ornbjerg. 2005. Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River valley, Anhui Province, China. Acta Trop. 96:198-204. [DOI] [PubMed] [Google Scholar]
- 195.Wang, T., S. Zhang, W. Wu, G. Zhang, D. Lu, N. Ornbjerg, and M. V. Johansen. 2006. Treatment and reinfection of water buffaloes and cattle infected with Schistosoma japonicum in Yangtze River valley, Anhui Province, China. J. Parasitol. 92:1088-1091. [DOI] [PubMed] [Google Scholar]
- 196.Wang, T. P., J. Shrivastava, M. V. Johansen, S. Q. Zhang, F. F. Wang, and J. P. Webster. 2006. Does multiple hosts mean multiple parasites? Population genetic structure of Schistosoma japonicum between definitive host species. Int. J. Parasitol. 36:1317-1325. [DOI] [PubMed] [Google Scholar]
- 197.Wang, W., J. R. Dai, Y. S. Liang, Y. X. Huang, and G. C. Coles. 2009. Impact of the south-north water diversion project on the transmission of Schistosoma japonicum in China. Ann. Trop. Med. Parasitol. 103:17-29. [DOI] [PubMed] [Google Scholar]
- 198.Wang, X., G. N. Gobert, X. Feng, Z. Fu, Y. Jin, J. Peng, and J. Lin. 2009. Analysis of early hepatic stage schistosomula gene expression by subtractive expressed sequence tags library. Mol. Biochem. Parasitol. 166:62-69. [DOI] [PubMed] [Google Scholar]
- 199.WHO. 1993. The control of schistosomiasis. Second report of the WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 830:1-86. [PubMed] [Google Scholar]
- 200.WHO Expert Committee. 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ. Tech. Rep. Ser. 912:1-57. [PubMed] [Google Scholar]
- 201.Wichmann, D., M. Panning, T. Quack, S. Kramme, G. D. Burchard, C. Grevelding, and C. Drosten. 2009. Diagnosing schistosomiasis by detection of cell-free parasite DNA in human plasma. PLoS Negl. Trop. Dis. 3:e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Williams, G. M., A. C. Sleigh, Y. Li, Z. Feng, G. M. Davis, H. Chen, A. G. Ross, R. Bergquist, and D. P. McManus. 2002. Mathematical modelling of schistosomiasis japonica: comparison of control strategies in the Peoples' Republic of China. Acta Trop. 82:253-262. [DOI] [PubMed] [Google Scholar]
- 203.Willingham, A. L., M. V. Johansen, and E. H. Barnes. 1998. A new technique for counting Schistosoma japonicum eggs in pig faeces. Southeast Asian J. Trop. Med. Public Health 29:128-130. [PubMed] [Google Scholar]
- 204.Wilson, M. S., M. M. Mentink-Kane, J. T. Pesce, T. R. Ramalingam, R. Thompson, and T. A. Wynn. 2007. Immunopathology of schistosomiasis. Immunol. Cell Biol. 85:148-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wilson, R. A., P. D. Ashton, S. Braschi, G. P. Dillon, M. Berriman, and A. Ivens. 2007. 'Oming in on schistosomes: prospects and limitations for postgenomics. Trends Parasitol. 23:14-20. [DOI] [PubMed] [Google Scholar]
- 206.Wilson, R. A., and P. S. Coulson. 2006. Schistosome vaccines: a critical appraisal. Mem. Inst. Oswaldo Cruz 101:13-20. [DOI] [PubMed] [Google Scholar]
- 207.Woolhouse, M. E. J. 1991. On the application of mathematical models of schistosome transmission dynamics. I. Natural transmission. Acta Trop. 49:241-270. [DOI] [PubMed] [Google Scholar]
- 208.Woolhouse, M. E. J. 1992. On the application of mathematical models of schistosome transmission dynamics. II. Control. Acta Trop. 50:189-204. [DOI] [PubMed] [Google Scholar]
- 209.Woolhouse, M. E. J. 1996. Mathematical models of transmission dynamics and control of schistosomiasis. Am. J. Trop. Med. Hyg. 55(Suppl.):144-148. [DOI] [PubMed] [Google Scholar]
- 210.Wu, X. H., X. H. Wang, J. Utzinger, K. Yang, T. K. Kristensen, R. Berquist, G. M. Zhao, H. Dang, and X. N. Zhou. 2007. Spatio-temporal correlation between human and bovine schistosomiasis in China: insight from three nationwide surveys. Geospat. Health 2:75-84. [DOI] [PubMed] [Google Scholar]
- 211.Wu, Z., K. Bu, L. Yuan, G. Yang, J. Zhu, and Q. Liu. 1993. Factors contributing to reinfection with schistosomiasis japonica after treatment in the lake region of China. Acta Trop. 54:83-88. [DOI] [PubMed] [Google Scholar]
- 212.Wu, Z. D., Z. Y. Lü, and X. B. Yu. 2005. Development of a vaccine against Schistosoma japonicum in China: a review. Acta Trop. 96:106-116. [DOI] [PubMed] [Google Scholar]
- 213.Wynn, T. A., R. W. Thompson, A. W. Cheever, and M. M. Mentink-Kane. 2004. Immunopathogenesis of schistosomiasis. Immunol. Rev. 201:156-167. [DOI] [PubMed] [Google Scholar]
- 214.Xia, C. M., R. Rong, Z. X. Lu, C. J. Shi, J. Xu, H. Q. Zhang, W. Gong, and W. Luo. 2009. Schistosoma japonicum: a PCR assay for the early detection and evaluation of treatment in a rabbit model. Exp. Parasitol. 121:175-179. [DOI] [PubMed] [Google Scholar]
- 215.Xiang, X., W. Tianping, and T. Zhigang. 2003. Development of a rapid, sensitive, dye immunoassay for schistosomiasis diagnosis: a colloidal dye immunofiltration assay. J. Immunol. Methods 280:49-57. [DOI] [PubMed] [Google Scholar]
- 216.Xiao, S. H. 2005. Development of antischistosomal drugs in China, with particular consideration to praziquantel and the artemesinins. Acta Trop. 96:153-167. [DOI] [PubMed] [Google Scholar]
- 217.Xu, L. S., B. J. Pan, J. X. Lin, L. P. Chen, S. H. Yu, and J. Jones. 2000. Creating health-promoting schools in rural China: a project started from deworming. Health Promot. Int. 15:197-206. [Google Scholar]
- 218.Xu, X., D. Zhang, W. Sun, Q. Zhang, J. Zhang, X. Xue, L. Shen, and W. Pan. 2009. A Schistosoma japonicum chimeric protein with a novel adjuvant induced a polarized Th1 immune response and protection against liver egg burdens. BMC Infect. Dis. 9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xue, X., J. Sun, Q. Zhang, Z. Wang, Y. Huang, and W. Pan. 2008. Identification and characterization of novel microRNAs from Schistosoma japonicum. PLoS One 3:e4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yang, G. J., P. Vounatsou, X. N. Zhou, J. Utzinger, and M. Tanner. 2005. A review of geographic information systems and remote sensing with applications to the epidemiology and control of schistosomiasis in China. Acta Trop. 96:117-129. [DOI] [PubMed] [Google Scholar]
- 221.Yang, G. J., P. Vounatsou, X. N. Zhou, M. Tanner, and J. Utzinger. 2005. A potential impact of climate change and water resource development on the transmission of Schistosoma japonicum in China. Parassitologia 47:127-134. [PubMed] [Google Scholar]
- 222.Yang, J., J. Zhao, Y. Yang, L. Zhang, X. Yang, X. Zhu, M. Ji, N. Sun, and C. Su. 2007. Schistosoma japonicum egg antigens stimulate CD4 CD25 T cells and modulate airway inflammation in a murine model of asthma. Immunology 120:8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yang, J., Z. Zhao, Y. Li, D. Krewski, and S. W. Wen. 2009. A multi-level analysis of risk factors for Schistosoma japonicum infection in China. Int. J. Infect. Dis. 13:e407-e412. [DOI] [PubMed] [Google Scholar]
- 224.Yang, L. L., Z. Y. Lv, S. M. Hu, S. J. He, Z. Y. Li, S. M. Zhang, H. Q. Zheng, M. T. Li, X. B. Yu, M. C. Fung, and Z. D. Wu. 2009. Schistosoma japonicum: proteomics analysis of differentially expressed proteins from ultraviolet-attenuated cercariae compared to normal cercariae. Parasitol. Res. 105:237-248. [DOI] [PubMed] [Google Scholar]
- 225.Yasawy, M. I. 2004. Katayama syndrome. Saudi Med. J. 25:234-236. [PubMed] [Google Scholar]
- 226.Yin, M., W. Hu, X. Mo, S. Wang, P. J. Brindley, D. P. McManus, G. M. Davis, Z. Feng, and D. Blair. 2008. Multiple near-identical genotypes of Schistosoma japonicum can occur in snails and have implications for population-genetic analyses. Int. J. Parasitol. 38:1681-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yu, J. M., S. J. De Vlas, H. C. Yuan, and B. Gryseels. 1998. Variations in faecal Schistosoma japonicum egg counts. Am. J. Trop. Med. Hyg. 59:370-375. [DOI] [PubMed] [Google Scholar]
- 228.Yu, J. M., S. J. de Vlas, Q. W. Jiang, and B. Gryseels. 2007. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol. Int. 56:45-49. [DOI] [PubMed] [Google Scholar]
- 229.Yuan, H. C. 1992. Epidemiological features and control of schistosomiasis japonica in China. Mem. Inst. Oswaldo Cruz 87(Suppl. 4):241. [PubMed] [Google Scholar]
- 230.Zerlotini, A., M. Heiges, H. Wang, R. L. Moraes, A. J. Dominitini, J. C. Ruiz, J. C. Kissinger, and G. Oliveira. 2009. SchistoDB: a Schistosoma mansoni genome resource. Nucleic Acids Res. 37(Database issue):D579-D582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang, C. W., S. H. Xiao, J. Utzinger, J. Chollet, J. Keiser, and M. Tanner. 2009. Histopathological changes in adult Schistosoma japonicum harbored in mice treated with a single dose of mefloquine. Parasitol. Res. 104:1407-1416. [DOI] [PubMed] [Google Scholar]
- 232.Zhang, Z. J., T. E. Carpenter, L. S. Lynn, Y. Chen, R. Bivand, A. B. Clark, F. M. Hui, W. X. Peng, Y. B. Zhou, G. M. Zhao, and Q. W. Jiang. 2009. Location of active transmission sites of Schistosoma japonicum in lake and marshland regions in China. Parasitology 136:737-746. [DOI] [PubMed] [Google Scholar]
- 233.Zheng, J. 2003. Schistosomiasis control in China: progress and challenges. Chin. J. Parasitol. Parasit. Dis. 21:4-5. (In Chinese.) [PubMed] [Google Scholar]
- 234.Zheng, X., X. Hu, G. Zhou, Z. Lu, W. Qiu, J. Bao, and Y. Dai. 2008. Soluble egg antigen from Schistosoma japonicum modulates the progression of chronic progressive experimental autoimmune encephalomyelitis via Th2-shift response. J. Neuroimmunol. 194:107-114. [DOI] [PubMed] [Google Scholar]
- 235.Zhou, X. N., J. Guo, X. H. Wu, Q. W. Jiang, J. Zheng, H. Dang, X. H. Wang, J. Xu, H. Q. Zhu, G. L. Wu, Y. S. Li, X. J. Xu, H. G. Chen, T. P. Wang, Y. C. Zhu, D. C. Qiu, X. Q. Dong, G. M. Zhao, S. J. Zhang, N. Q. Zhao, G. Xia, L. Y. Wang, S. Q. Zhang, D. D. Lin, M. G. Chen, and Y. Hao. 2007. Epidemiology of schistosomiasis in the Peoples' Republic of China, 2004. Emerg. Infect. Dis. 13:1470-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhou, X. N., L. Y. Wang, M. G. Chen, X. H. Wu, Q. W. Jiang, X. Y. Chen, J. Zheng, and J. Utzinger. 2005. The public health significance and control of schistosomiasis in China—then and now. Acta Trop. 96:97-105. [DOI] [PubMed] [Google Scholar]
- 237.Zhou, X. N., G. J. Yang, K. Yang, X. H. Wang, Q. B. Hong, L. P. Sun, J. B. Malone, T. K. Kristensen, N. R. Bergquist, and J. Utzinger. 2008. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 78:188-194. [PubMed] [Google Scholar]
- 238.Zhu, H. M., S. Xiang, K. Yang, X. H. Wu, and X. N. Zhou. 2008. Three Gorges Dam and its impact on the potential transmission of schistosomiasis in regions along the Yangtze River. Ecohealth 5:137-148. [DOI] [PubMed] [Google Scholar]
- 239.Zhu, Y., W. He, Y. Liang, M. Xu, C. Yu, W. Hua, and G. Cao. 2002. Development of a rapid, simple dipstick dye immunoassay for schistosomiasis diagnosis. J. Immunol. Methods 266:1-5. [DOI] [PubMed] [Google Scholar]
- 240.Zhu, Y. C. 2005. Immunodiagnosis and its role in schistosomiasis control in China: a review. Acta Trop. 96:130-136. [DOI] [PubMed] [Google Scholar]