Abstract
Staphylococcal toxic shock syndrome toxin 1 (TSST-1) is the cause of menstrual toxic shock syndrome (mTSS) associated with vaginal colonization by Staphylococcus aureus. In this pilot study, we measured TSST-1 and alpha-toxin, another exotoxin, on used tampons from four healthy women with S. aureus on tampons and from two women with tampon-associated mTSS. Tampons from all six women were sectioned into approximately 0.5-cm3 pieces, some containing menstrual blood and some lacking menstrual blood. The pH of tampon sections with or without menstrual blood was neutral. S. aureus CFU were present in tampon sections at approximately equivalent counts (total counts were 1 × 108 to 2 × 109 CFU/tampon). TSST-1 (2 to 80 μg/tampon) and alpha-toxin (28 to 30 μg/tampon) were present only in the sections containing little or no menstrual blood (low hemoglobin density). In the tampons from TSS patients, the cytokine gamma interferon (IFN-γ) was detected only in menstrual-blood-containing sections, whereas the chemokines macrophage inflammatory protein 3α and interleukin-8 were detected in all sections. Thus, IFN-γ was being produced systemically, whereas the chemokines were being produced both locally by epithelial cells and systemically. The data show that S. aureus exotoxins can be identified in tampons ex vivo in sites with low hemoglobin density.
Staphylococcal toxic shock syndrome (TSS) is an acute-onset illness characterized by fever (≥102°F), vomiting and diarrhea, hypotension, and a sunburn-like rash (6, 31, 32). Menstrual TSS (mTSS) is typically associated with tampon usage by previously healthy women (6, 31). Nonmenstrual TSS is known to occur in women who are not menstruating as well as in female children and males; nonmenstrual TSS has been identified in conjunction with many bacterial infections, such as pneumonia, and skin lesions (21, 32).
One predisposing factor to developing mTSS is vaginal colonization with a superantigen-producing strain of Staphylococcus aureus (1, 2, 29). TSS toxin 1 (TSST-1) is the superantigen most commonly associated with mTSS cases (1, 2, 29). Although 10 to 30% of women are colonized vaginally with S. aureus, fewer are colonized with TSST-1-positive S. aureus, which may contribute to the rarity of the menstrual illness (18, 27, 28). Although the incidence of mTSS is low (1 to 3 in 100,000), the illness remains of interest because tampon usage is a common practice (8, 20, 27), and women have menstrual periods for 30 to 40 years of their lives; the number of staphylococcal menstrual and nonmenstrual TSS cases reported to the Centers for Disease Control and Prevention is approximately 70 to 100 per year.
The role of tampons in the pathogenesis of mTSS is incompletely understood. Although tampons are not a source for toxigenic S. aureus and do not appear to increase the S. aureus cell density vaginally, studies have shown that tampons used during menstruation can become colonized with S. aureus (17, 27, 28, 34). Using fluorescent in situ hybridization, Veeh et al. confirmed the presence of S. aureus biofilms on the vaginal mucosa of healthy women and on fibers from tampons used by the same women during menstruation (34). However, to date, limited studies have evaluated used tampons for the presence of TSST-1. No studies have evaluated tampon sections containing menstrual blood versus sections without menstrual blood for TSST-1.
There are several factors known to regulate TSST-1 production by S. aureus. In vitro studies demonstrate that O2 and CO2, iron, pH, glucose, and temperature are factors that can alter the concentration of TSST-1 produced by S. aureus (10, 12, 25, 33, 36). Recent in vitro studies have shown that both α- and β-globin chains of human hemoglobin can inhibit the production of TSST-1 without impacting growth of the organism (26). Given that menstrual blood is rich in hemoglobin, the study suggests that toxigenic S. aureus present on tampons might produce TSST-1 only in areas that lack menstrual blood. The present pilot study evaluated used tampons both from healthy women and from those diagnosed with mTSS for the presence of S. aureus and two exotoxins (TSST-1 and alpha-toxin) in areas with and without menstrual blood.
MATERIALS AND METHODS
Bacteria.
mTSS-associated S. aureus MN8 was used as the source of purified TSST-1 and as the source of culture fluids to assess the stability of chemokines to proteolysis by TSS-associated S. aureus. TSST-1 was purified from S. aureus MN8 culture fluids by precipitation with ethanol, resolubilization in water, and preparative thin-layer isoelectric focusing (4). Ampholytes were removed by dialysis for 4 days against distilled water at 4°C. Purified TSST-1 was homogeneous when 5 μg was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (14) and silver staining (Bio-Rad Laboratories, Hercules, CA). S. aureus MN8 culture fluids were obtained after growth of the organism to stationary phase in beef heart medium (4), at 37°C with shaking (200 rpm). Bacteria were removed by centrifugation (4,000 × g, 15 min) followed by filtration (0.2 μm pore size; Millipore, Cork, Ireland).
Tampons and blood from participants.
Six tampons were used in our studies. Tampons and blood samples were obtained from 4 healthy women after informed consent was obtained, according to an approved University of Minnesota institutional review board (IRB) protocol. Tampons and blood samples were obtained from 2 mTSS patients, also according to an approved University of Minnesota IRB protocol; the tampons were removed and blood was drawn as part of the patients' standard medical care, and these were provided on the same day by the patients' treating physicians in the Minneapolis-St. Paul area. The patients had physician-diagnosed mTSS. No efforts were made to establish that the patients met all criteria for TSS; however, this was not deemed to be necessary since past experience has shown that there is a high correlation of physician-diagnosed mTSS and the presence of TSST-1-positive S. aureus vaginally (24, 30).
One corner of each tampon was touched with a sterile cotton swab, which was then used to streak a blood agar plate for detection of S. aureus. The used tampons were immediately frozen at −20°C. The blood agar plates (5% sheep blood agar) were cultured overnight in a 7% CO2 incubator and then were examined for S. aureus and Escherichia coli. Standard microbiologic identifications were used for S. aureus (Gram-positive cocci, catalase positive, and coagulase positive), and presumed E. coli was subcultured onto MacConkey agar plates and identified as the dominant Gram-negative, lactose-fermenting organisms from the tampons. Subsequently, E. coli was identified as indole positive, methyl red positive, Voges-Praskauer negative, and citrate negative. The S. aureus strains from the blood agar plates were tested for TSST-1 by antibody detection (23). Once established that the women had TSST-1-positive S. aureus, the tampons were removed from the freezer and, while frozen, immediately sectioned into approximately 0.5-cm3 sections with scalpels. Each tampon section was then placed into a 3-ml syringe, 1 ml of distilled water was added, and the contents were expressed. The pH of each section was immediately determined with a standard pH meter. The absorbance at 410 nm (a measure of hemoglobin content) was determined with a spectrophotometer (Genesys 5; Thermo Spectronic, Rochester, NY). In many instances, the absorbance of the sections exceeded 2, and in such cases the samples were diluted in water until the absorbance was between 0.1 and 1.5. The hemoglobin densities of all sections were determined by multiplying the dilution factors by the absorbances. For convenience, the sections are presented in the figures as hemoglobin density values, with values of <5 meaning white in appearance and values of ≥5 meaning red in appearance. The hemoglobin density values ranged from 0 to >100 for the 6 tampons. S. aureus CFU/ml were determined by plate counts from each section. The amount of TSST-1 (μg/section) was determined by semiquantitative Western immunoblot analysis, which allows for the detection of TSST-1 in the presence of antibodies and blood components (26). The Western immunoblots were developed with alkaline phosphatase-conjugated antibodies and substrate (3). A sample Western immunoblot from the tampon sections is shown in Fig. 1B. Protein band areas from Western immunoblots were determined by use of a computer program provided by NIH (ImageJ 1.34S) at http://rsb.info.nih.gov/ij/. Band areas in tampon sections were compared to areas from standard curves generated by use of highly purified TSST-1 (Fig. 1B); the R2 values for standard curves were consistently above 0.95.
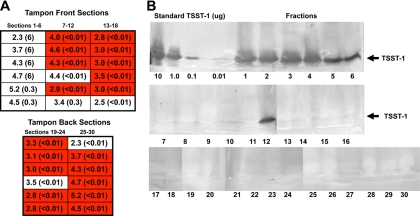
FIG. 1.
(A) Diagram of sections, listed arbitrarily as tampon front and back, of a tampon from a healthy woman with TSST-1-positive S. aureus on the tampon, showing tampon regions that are low (white) and high (red) in hemoglobin density. S. aureus CFU/ml were determined by plate counts from each section, and values (×107) for each section are listed. Amounts of TSST-1 (μg/section) were determined by quantitative Western immunoblot analysis and are given in parentheses. (B) Western immunoblot analysis of fluid extracted from the same tampon sections. See Materials and Methods for details.
The cytolysin alpha-toxin was quantified by the ability to lyse rabbit erythrocytes (26). Briefly, rabbit erythrocytes were separated from blood and washed with phosphate-buffered saline (PBS) by centrifugation (400 × g, 10 min). After the final wash of PBS was decanted, 30 μl of pelleted erythrocytes was suspended in 10 ml melted agarose (0.85% in PBS). Microscope slides were coated with 4 ml of the erythrocyte suspension, and once solidified, 4-mm wells were cut in the agarose. Purified alpha-toxin (0.05, 0.5, and 5 μg/ml) and extracts from tampons (10 μl diluted 1/2 in 2× PBS) were added in 20-μl volumes to the wells. The slides were incubated for 4 h at 37°C, and then zones of erythrocyte lysis were measured and compared to alpha-toxin standards.
Enzyme-linked immunosorbent assay (ELISA) kits for human gamma interferon (IFN-γ), macrophage inflammatory protein 3α (MIP-3α), and interleukin-8 (IL-8) were purchased from R&D Systems, Minneapolis, MN, and used according to the manufacturer's instructions.
At the time the above-described tampon studies were being performed, a serum sample from each participant was evaluated by ELISA for total antibodies, which included IgM, IgG, and IgA, to TSST-1 (26). The 4 healthy participants were informed of their immunity status with respect to TSST-1. The two without demonstrable antibodies were advised to consult their physicians concerning the significance of the finding.
Protease assay.
Purified MIP-3α and IL-8 were purchased from R&D Systems. These proteins were adjusted to 1,000 pg/ml and then diluted 50% with sterile culture fluids from S. aureus MN8 or sterile uncultured media. At designated times, duplicate samples of each were removed, and the remaining chemokines were quantified by ELISA (R&D Systems).
RESULTS
There have been limited studies performed with used tampons to estimate the amount of TSST-1 produced by S. aureus vaginally. In the present study, tampons and blood samples were obtained from individual menstruating women with TSST-1-positive S. aureus present vaginally, and the tampons were evaluated for the presence of TSST-1 by use of a Western immunoblotting procedure (26). Two of the women were healthy and lacked detectable anti-TSST-1 antibodies (titers of <10, where the titer is defined as the reciprocal of the dilution of the last positive ELISA wells) while being colonized vaginally with TSST-1-positive S. aureus (as assessed by its presence on tampons). Two women were healthy, were colonized vaginally with TSST-1-positive S. aureus, and had antibodies to TSST-1 (titers of 160); no efforts were made to separate the contributions of IgM, IgG, and IgA to these titers. Two women had mTSS as determined by their attending physicians, lacked anti-TSST-1 antibodies, and were colonized vaginally with TSST-1-positive S. aureus. (It is noteworthy that it is exceptionally difficult to obtain used tampons from mTSS patients, since women who suspect they have TSS are advised to remove tampons immediately and then seek medical attention; the tampons are typically discarded prior to seeking help.) Interestingly, the tampons from the 4 healthy women (with or without antibodies to TSST-1) were positive for S. aureus and negative for E. coli. The two mTSS patients were positive for both S. aureus (approximately 75% of the colonies) and E. coli (approximately 25% of the colonies).
Healthy women with TSST-1-positive S. aureus vaginally.
It was thought that menstruating women who lack demonstrably circulating antibodies to TSST-1 in the presence of TSST-1-positive S. aureus vaginally would uniformly develop mTSS. This does not appear to be the case.
The results of the tampon dissection from one of the four healthy women with TSST-1-positive S. aureus vaginally (this participant lacked detectable circulating antibodies against TSST-1) are shown in Fig. 1A and B. The tampon, obtained on day 2 of menses, was sectioned into 30 pieces. All sections were extracted with water and assayed for amount of hemoglobin by absorbance at a wavelength of 410 nm, number of S. aureus CFU by plate counts, pH, and TSST-1 by semiquantitative Western blotting. S. aureus CFU were relatively equally distributed among all sections of the tampon. The total numbers of S. aureus CFU were approximately 1 × 109/tampon. Many sections of the tampon had little or no menstrual blood (low hemoglobin density [Fig. 1A]), whereas others were saturated with menstrual blood (high hemoglobin density [Fig. 1A]). The pH of all sections from the tampon was approximately 7; this was the case for all tampons studied, whether from healthy women or women with mTSS, and thus will not be addressed further. Finally, the regions of the tampon that were TSST-1 positive were those sections that lacked or contained only very small amounts of menstrual blood (low hemoglobin density values [note that tampon sections with hemoglobin density values of <5 appear white because hemoglobin in blood absorbs very strongly at 410 nm]). A total of 25 μg of TSST-1/tampon was detected by the Western immunoblotting technique with determination of band area (Fig. 1B).
Day 2 tampons from one additional healthy woman lacking serum antibodies to TSST-1 and two healthy women who had serum antibodies to TSST-1 were analyzed, with similar results regarding even distribution of S. aureus CFU numbers within tampon sections and association of TSST-1 production with lack of menstrual blood. The results from our analyses of all used tampons, including those from healthy women and women with mTSS, are summarized in Table 1.
TABLE 1.
TSST-1 antibody titers, S. aureus CFU, and amounts of TSST-1 and alpha-toxin proteins present in used tampons from four healthy women and two women with mTSS
| Tampon source | Serum antibody titera | S. aureus CFU/tampon (×108) | Total amt, in μg, of TSST-1 (alpha-toxin) in sectionsb with: |
|
|---|---|---|---|---|
| Low hemoglobin density | High hemoglobin density | |||
| Healthy subjects | ||||
| 1 | <10 | 10 | 25 | NDc |
| 2 | <10 | 1.5 | 2 | ND |
| 3 | 160 | 4.0 | 9 | ND |
| 4 | 160 | 5.0 | 15 | ND |
| Avg | 5.1 | 13d | ND | |
| Subjects with mTSS | ||||
| 1 | <10 | 27 | 69 (28) | ND (ND) |
| 2 | <10 | 5 | 80 (30) | ND (ND) |
| Avg | 16 | 74d (29) | ND (ND) | |
Titer of serum antibody against TSST-1.
Low hemoglobin density refers to tampon sections that appear white, whereas high hemoglobin density refers to sections that appear red.
ND, none detected.
The total amounts of TSST-1 were considered significantly different (P < 0.002) by the Student t test.
The women who lacked antibody to TSST-1 were informed of their lack of serum antibodies to TSST-1 and the presence of TSST-1-positive S. aureus vaginally and were advised to consult their physicians. The two women with TSST-1-positive S. aureus vaginally and with serum antibodies had a titer against TSST-1 of 160 (including IgM, IgG, and IgA), a value that is typical of antibody-positive individuals (18).
mTSS patients with TSST-1-positive S. aureus vaginally.
The tampons from both women with mTSS were obtained and sectioned (Fig. 2A and B). One section of the tampon from one patient (day 4 of menstruation) had a high hemoglobin density and appeared red, and all of the remaining sections had a low hemoglobin density and appeared white (Fig. 2A); the pH was neutral throughout the tampon. This woman was colonized with TSST-1-positive S. aureus, with approximately 2.7 × 109 CFU/tampon. TSST-1 was present within the areas with low hemoglobin density, with 69 μg of TSST-1/tampon. Due to recent data suggesting that the staphylococcal exotoxin alpha-toxin may facilitate TSST-1 penetration across the mucosal barrier (19), the concentrations of alpha-toxin present in all sections of the tampon were quantified; alpha-toxin was present in this tampon only in the low-hemoglobin-density areas, at a total concentration of approximately 28 μg/tampon (data for each section not shown). The tampons from the healthy women were not evaluated for alpha-toxin since those tampons were studied prior to recognition of the possible role of alpha-toxin in TSST-1 penetration of the vaginal mucosa.
FIG. 2.
Diagrams of sections, listed arbitrarily as tampon front and back, of tampons from women with mTSS, showing regions of the tampons with low hemoglobin density (white) and high hemoglobin density (red). S. aureus CFU/ml were determined by plate counts from each section, and values (×107) for each section are listed. Amounts of TSST-1 (μg/section) were determined by quantitative Western immunoblot analysis and are given in parentheses.
The tampon from the second woman with mTSS was obtained on day 3 of menses and sectioned (Fig. 2B). Multiple sections had high hemoglobin density (≥5), and others had low hemoglobin density (<5); the pH was 7 throughout the tampon. This woman was colonized with TSST-1-positive S. aureus, with approximately 5 × 108 CFU/tampon. TSST-1 was detectably present almost entirely within the areas of low hemoglobin density, with approximately 80 μg of TSST-1/tampon. The cytolysin alpha-toxin was present in the low-hemoglobin-density areas of the tampon at approximately 30 μg/tampon (data for each section not shown).
Both TSST-1 and alpha-toxin induce cytokines from lymphocytes and antigen-presenting cells (5, 15, 16, 19). For tampons from both of the mTSS patients, amounts of the T-lymphocyte cytokine IFN-γ and two chemokines, MIP-3α and IL-8, were quantified (Table 2). IFN-γ was chosen since this cytokine is produced by CD4+ T lymphocytes, a major target of TSST-1 superantigen activity. The two chemokines were chosen based on prior analyses of the responses of an immortalized human vaginal epithelial cell line to TSS-associated S. aureus and TSST-1 (19). Cytokine and chemokine amounts in the tampon regions with high hemoglobin density may be measures of both local and systemic production. In contrast, the amounts in the tampon regions of low hemoglobin density are likely to represent production locally, primarily by epithelial cells. Hypothetically, cytokines and chemokines could diffuse into the low-hemoglobin-density areas of tampons from the high-hemoglobin-density areas and thus not reflect local production. However, TSST-1 was consistently observed in the low-hemoglobin-density areas but not in the high-hemoglobin-density areas of tested tampons, suggesting that protein diffusion is not significant.
TABLE 2.
Total amounts of IFN-γ, MIP-3α, and IL-8 quantified by ELISA performed with used tampons from two women with mTSS
| mTSS patient | Section typea (no. of sections) | Amtb (ng/ml) of: |
||
|---|---|---|---|---|
| IFN-γ | MIP-3α | IL-8 | ||
| 1 | High hemoglobin density (1) | 16 | 221 | 356 |
| Low hemoglobin density (47) | NDc (<0.03) | 335 ± 200 | 656 ± 120 | |
| 2 | High hemoglobin density (17) | 723 ± 110 | 157 ± 51 | 877 ± 136 |
| Low hemoglobin density (31) | ND (<0.03) | 73 ± 23 | 173 ± 63 | |
High hemoglobin density refers to tampon sections that appear red, whereas low hemoglobin density refers to sections that appear white.
Values for more than one section indicate averages ± standard deviations.
ND, none detected.
IFN-γ was detected only in the high-hemoglobin-density areas of tampons in both TSS patients and not in the low-hemoglobin-density areas, suggesting that CD4+ T lymphocytes produced IFN-γ systemically but not vaginally. We have shown previously that isolated human vaginal epithelial cells do not produce IFN-γ in response to TSST-1 and alpha-toxin (19). Both high- and low-hemoglobin-density sections of the tampons contained MIP-3α and IL-8.
It was noted in prior analyses that MIP-3α gene upregulation in isolated human vaginal epithelial cells is greater than IL-8 gene upregulation in response to TSST-1-positive S. aureus (19). However, as indicated in Table 2, levels of MIP-3α protein in tampons from mTSS patients are lower than levels of IL-8 protein. We hypothesized that staphylococcal proteases may degrade MIP-3α vaginally more quickly than they degrade IL-8. This was observed when purified MIP-3α and IL-8 were exposed to S. aureus MN8 culture fluids (Table 3). By 6 h after exposure to culture fluids, MIP-3α was 93% degraded, compared to only 28% for IL-8.
TABLE 3.
S. aureus MN8 protease degradation of purified MIP-3α and IL-8
| Chemokine (500 pg/ml) | Incubation time (h) with S. aureus supernate | % of chemokine remaininga |
|---|---|---|
| MIP-3α | 0 | 100 |
| 1 | 81 | |
| 3 | 40 | |
| 6 | 7 | |
| IL-8 | 0 | 100 |
| 1 | 100 | |
| 3 | 89 | |
| 6 | 72 |
Results are representative of duplicate samples.
DISCUSSION
For vaginal mTSS to occur, the following stepwise events appear to take place: (i) women become colonized vaginally with TSST-1-positive S. aureus (1, 29); (ii) TSST-1 and other virulence factors required for penetration of TSST-1 across the vaginal mucosa are produced, as the organism usually remains localized on the mucosal surface (6, 31); (iii) TSST-1 gains access to the submucosa or circulation; and (iv) TSST-1 in women lacking neutralizing antibodies to TSST-1 (1, 11) interacts with T cells and macrophages, generating cytokines that result in the clinical features of mTSS (15, 16).
Studies have shown that women can be colonized vaginally with TSST-1-positive S. aureus during menstruation; more than 75% of these women are expected to have circulating antibodies to TSST-1 (18, 27, 28). Similarly, it is well established that the in vitro environmental conditions that favor TSST-1 production can be present vaginally in menstruating women (10, 12, 25, 33). However, only a single study has shown that TSST-1 can be produced vaginally (22). The present study confirms that TSST-1 can be present vaginally in tampons but demonstrates the presence of the toxin both in four healthy women and in two patients with mTSS. Although it has not been established how much TSST-1 is required vaginally to cause mTSS, the concentrations demonstrated in this study are in excess of amounts of superantigens necessary to cause illness when a superantigen, such as staphylococcal enterotoxin A (SEA), is administered intravenously (9).
Our pilot study is important for other reasons. We established that TSST-1 causation of mTSS is more complicated than previously thought. It has been assumed that TSST-1 is produced in menstrual blood. This study demonstrates that TSST-1 is located primarily in tampon regions that have little or no menstrual blood. This finding is consistent with previous studies showing that the α- and β-globin chains of hemoglobin inhibit TSST-1 production (26). The present studies also suggest that vaginal secretions in low-hemoglobin-density regions of tampons contain factors required for exotoxin production, including neutral pH and protein (25). We measured pH and show that all parts of tampons from the studied menstruating women have relatively neutral pH, whether containing menstrual blood or not. We also have demonstrated that sections of tampons that have a low hemoglobin density contain serum proteins at about 1% of the level of those found in blood (unpublished data). The other major factor thought to be necessary for TSST-1 production is the presence of an aerobic environment, and we have previously established that such an environment is present vaginally through the oxygen in menstrual blood as well as through the inherent oxygen in tampons (10).
It has also been suggested that women with TSST-1-positive S. aureus present vaginally during menstruation develop mTSS in the absence of neutralizing circulating serum antibodies (1, 11, 18, 35). Our studies suggest that there is another possible outcome, i.e., women may lack circulating TSST-1 antibodies, have TSST-1 present vaginally, and not develop TSS. It is hypothesized that there may be a novel epithelial receptor that interacts with TSST-1 or other staphylococcal factors that is required for mucosal penetration of TSST-1. It is possible that this receptor either exists in a form unable to bind TSST-1 or is not present in these women. The differential reactivity of host receptors to superantigens has been demonstrated previously to explain the spectrum of group A streptococcus superantigen-associated illnesses, in which polymorphisms in major histocompatibility complex class II (MHC-II) molecules on antigen-presenting cells explain different disease outcomes in affected individuals (13).
Our studies also demonstrate that vaginal cytokine responses, including responses to the chemokines IL-8 and MIP-3α, may originate from epithelial cells. It is well established that epithelial cells present in the vagina far outnumber both classical antigen-presenting cells and T lymphocytes. It makes sense that these epithelial cells would be components of the immune system, since they contain MHC-II molecules on their surfaces, usually as a result of exposure to IFN-γ (7). Our studies show that the low-hemoglobin-density sections of tampons have IL-8 and MIP-3α in the absence of IFN-γ, suggesting that the dominant cells producing these chemokines are epithelial cells. Thus, there appears to be another undefined receptor that is stimulated by vaginal TSST-1-positive S. aureus, leading to chemokine production.
Acknowledgments
This work was supported by USPHS research grant AI074283 from the National Institute of Allergy and Infectious Diseases and a grant from the Procter and Gamble Company awarded to the University of Minnesota.
Footnotes
Published ahead of print on 24 March 2010.
REFERENCES
- 1.Bergdoll, M. S., B. A. Crass, R. F. Reiser, R. N. Robbins, and J. P. Davis. 1981. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet i:1017-1021. [DOI] [PubMed] [Google Scholar]
- 2.Bergdoll, M. S., and P. M. Schlievert. 1984. Toxic-shock syndrome toxin. Lancet ii:691. [Google Scholar]
- 3.Blake, M. S., K. H. Johnston, G. J. Russell-Jones, and E. C. Gotschlich. 1984. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal. Biochem. 136:175-179. [DOI] [PubMed] [Google Scholar]
- 4.Blomster-Hautamaa, D. A., and P. M. Schlievert. 1988. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 165:37-43. [DOI] [PubMed] [Google Scholar]
- 5.Davis, C. C., M. J. Kremer, P. M. Schlievert, and C. A. Squier. 2003. Penetration of toxic shock syndrome toxin-1 across porcine vaginal mucosa ex vivo: permeability characteristics, toxin distribution, and tissue damage. Am. J. Obstet. Gynecol. 189:1785-1791. [DOI] [PubMed] [Google Scholar]
- 6.Davis, J. P., P. J. Chesney, P. J. Wand, and M. LaVenture. 1980. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N. Engl. J. Med. 303:1429-1435. [DOI] [PubMed] [Google Scholar]
- 7.Fichorova, R. N., and D. J. Anderson. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60:508-514. [DOI] [PubMed] [Google Scholar]
- 8.Gaventa, S., A. L. Reingold, A. W. Hightower, C. V. Broome, B. Schwartz, C. Hoppe, J. Harwell, L. K. Lefkowitz, S. Makintubee, D. R. Cundiff, et al. 1989. Active surveillance for toxic shock syndrome in the United States, 1986. Rev. Infect. Dis. 11:S28-S34. [DOI] [PubMed] [Google Scholar]
- 9.Giantonio, B. J., R. K. Alpaugh, J. Schultz, C. McAleer, D. W. Newton, B. Shannon, Y. Guedez, M. Kotb, L. Vitek, R. Persson, P. O. Gunnarsson, T. Kalland, M. Dohlsten, B. Persson, and L. M. Weiner. 1997. Superantigen-based immunotherapy: a phase I trial of PNU-214565, a monoclonal antibody-staphylococcal enterotoxin A recombinant fusion protein, in advanced pancreatic and colorectal cancer. J. Clin. Oncol. 15:1994-2007. [DOI] [PubMed] [Google Scholar]
- 10.Hill, D. R., M. E. Brunner, D. C. Schmitz, C. C. Davis, J. A. Flood, P. M. Schlievert, S. Z. Wang-Weigand, and T. W. Osborn. 2005. In vivo assessment of human vaginal oxygen and carbon dioxide levels during and post menses. J. Appl. Physiol. 99:1582-1591. [DOI] [PubMed] [Google Scholar]
- 11.Kansal, R., C. Davis, M. Hansmann, J. Seymour, J. Parsonnet, P. Modern, S. Gilbert, and M. Kotb. 2007. Structural and functional properties of antibodies to the superantigen TSST-1 and their relationship to menstrual toxic shock syndrome. J. Clin. Immunol. 27:327-338. [DOI] [PubMed] [Google Scholar]
- 12.Kass, E. H., M. I. Kendrick, Y. C. Tsai, and J. Parsonnet. 1987. Interaction of magnesium ion, oxygen tension, and temperature in the production of toxic-shock-syndrome toxin-1 by Staphylococcus aureus. J. Infect. Dis. 155:812-815. [DOI] [PubMed] [Google Scholar]
- 13.Kotb, M., A. Norrby-Teglund, A. McGeer, H. El-Sherbini, M. T. Dorak, A. Khurshid, K. Green, J. Peeples, J. Wade, G. Thomson, B. Schwartz, and D. E. Low. 2002. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat. Med. 8:1398-1404. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 15.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-711. [DOI] [PubMed] [Google Scholar]
- 16.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 17.Onderdonk, A. B., G. R. Zamarchi, M. L. Rodriguez, M. L. Hirsch, A. Munoz, and E. H. Kass. 1987. Quantitative assessment of vaginal microflora during use of tampons of various compositions. Appl. Environ. Microbiol. 53:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsonnet, J., M. A. Hansmann, M. L. Delaney, P. A. Modern, A. M. Dubois, W. Wieland-Alter, K. W. Wissemann, J. E. Wild, M. B. Jones, J. L. Seymour, and A. B. Onderdonk. 2005. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J. Clin. Microbiol. 43:4628-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson, M., K. Ault, M. J. Kremer, A. J. Klingelhutz, C. C. Davis, C. A. Squier, and P. M. Schlievert. 2005. The innate immune system is activated by stimulation of vaginal epithelial cells with Staphylococcus aureus and toxic shock syndrome toxin 1. Infect. Immun. 73:2164-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reingold, A. L., C. V. Broome, S. Gaventa, and A. W. Hightower. 1989. Risk factors for menstrual toxic shock syndrome: results of a multistate case-control study. Rev. Infect. Dis. 11:S35-S41. [DOI] [PubMed] [Google Scholar]
- 21.Reingold, A. L., N. T. Hargrett, B. B. Dan, K. N. Shands, B. Y. Strickland, and C. V. Broome. 1982. Nonmenstrual toxic shock syndrome: a review of 130 cases. Ann. Intern. Med. 96:871-874. [DOI] [PubMed] [Google Scholar]
- 22.Rosten, P. M., K. H. Bartlett, and A. W. Chow. 1987. Detection and quantitation of toxic shock syndrome toxin 1 in vitro and in vivo by noncompetitive enzyme-linked immunosorbent assay. J. Clin. Microbiol. 25:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlievert, P. M. 1988. Immunochemical assays for toxic shock syndrome toxin-1. Methods Enzymol. 165:339-344. [DOI] [PubMed] [Google Scholar]
- 24.Schlievert, P. M. 1986. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet i:1149-1150. [DOI] [PubMed] [Google Scholar]
- 25.Schlievert, P. M., and D. A. Blomster. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J. Infect. Dis. 147:236-242. [DOI] [PubMed] [Google Scholar]
- 26.Schlievert, P. M., L. C. Case, K. A. Nemeth, C. C. Davis, Y. Sun, W. Qin, F. Wang, A. J. Brosnahan, J. A. Mleziva, M. L. Peterson, and B. E. Jones. 2007. Alpha and beta chains of hemoglobin inhibit production of Staphylococcus aureus exotoxins. Biochemistry 46:14349-14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlievert, P. M., L. C. Case, K. L. Strandberg, T. J. Tripp, Y. C. Lin, and M. L. Peterson. 2007. Vaginal Staphylococcus aureus superantigen profile shift from 1980 and 1981 to 2003, 2004, and 2005. J. Clin. Microbiol. 45:2704-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlievert, P. M., M. T. Osterholm, J. A. Kelly, and R. D. Nishimura. 1982. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann. Intern. Med. 96:937-940. [DOI] [PubMed] [Google Scholar]
- 29.Schlievert, P. M., K. N. Shands, B. B. Dan, G. P. Schmid, and R. D. Nishimura. 1981. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J. Infect. Dis. 143:509-516. [DOI] [PubMed] [Google Scholar]
- 30.Schlievert, P. M., T. J. Tripp, and M. L. Peterson. 2004. Reemergence of staphylococcal toxic shock syndrome in Minneapolis-St. Paul, Minnesota, during the 2000-2003 surveillance period. J. Clin. Microbiol. 42:2875-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shands, K. N., G. P. Schmid, B. B. Dan, D. Blum, R. J. Guidotti, N. T. Hargrett, R. L. Anderson, D. L. Hill, C. V. Broome, J. D. Band, and D. W. Fraser. 1980. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N. Engl. J. Med. 303:1436-1442. [DOI] [PubMed] [Google Scholar]
- 32.Todd, J. K., F. A. Kapral, M. Fishaut, and T. R. Welch. 1978. Toxic shock syndrome associated with phage group 1 staphylococci. Lancet ii:1116-1118. [DOI] [PubMed] [Google Scholar]
- 33.Todd, J. K., B. H. Todd, A. Franco-Buff, C. M. Smith, and D. W. Lawellin. 1987. Influence of focal growth conditions on the pathogenesis of toxic shock syndrome. J. Infect. Dis. 155:673-681. [DOI] [PubMed] [Google Scholar]
- 34.Veeh, R. H., M. E. Shirtliff, J. R. Petik, J. A. Flood, C. C. Davis, J. L. Seymour, M. A. Hansmann, K. M. Kerr, M. E. Pasmore, and J. W. Costerton. 2003. Detection of Staphylococcus aureus biofilm on tampons and menses components. J. Infect. Dis. 188:519-530. [DOI] [PubMed] [Google Scholar]
- 35.Vergeront, J. M., S. J. Stolz, B. A. Crass, D. B. Nelson, J. P. Davis, and M. S. Bergdoll. 1983. Prevalence of serum antibody to staphylococcal enterotoxin F among Wisconsin residents: implications for toxic-shock syndrome. J. Infect. Dis. 148:692-698. [DOI] [PubMed] [Google Scholar]
- 36.Yarwood, J. M., and P. M. Schlievert. 2000. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J. Clin. Microbiol. 38:1797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]