Abstract
Measurement of antibody to cytomegalovirus (CMV) glycoprotein B (gB) is valuable in the assessment of the antibody response to infection and to gB-containing vaccines. For this purpose, an enzyme-linked immunosorbent assay (ELISA) with a recombinant CMV gB molecule as the antigen was evaluated. Sera from 168 anti-CMV IgG-positive and 100 seronegative subjects were used to evaluate the anti-gB antibody assay. A cutoff optical density (OD) that would distinguish gB antibody-positive from -negative sera was established. Titers of antibody to gB determined by endpoint dilution were compared with those calculated using regression analysis. The run-to-run and interoperator reproducibilities of results were measured. The mean OD + 5 standard deviations from 50 anti-CMV IgG antibody-negative sera (0.2472) was used as the cutoff between anti-gB antibody-positive and -negative results. All sera from 100 anti-CMV IgG-seronegative subjects were negative for antibody to gB. All but 1 of 168 sera from seropositive subjects were positive for antibody to gB. Observed antibody levels based on titration to the endpoint were very similar to results calculated using linear regression. The run-to-run consistency of endpoints was excellent, with 38 runs from one operator and 48 runs from another all giving results within 1 dilution of the mean value for each of three anti-CMV IgG antibody-positive serum pools. The geometric mean titer of antibody to gB for 99 sera from seropositive blood donors was 1/10,937. This ELISA gives accurate and reproducible results for the relative quantity of anti-CMV gB IgG in serum over a wide range of antibody levels.
Measurement of antibodies to specific cytomegalovirus (CMV) proteins is a valuable tool for the study of the host immune responses to infection and to CMV vaccines. Assessment of immune responses to viral proteins can inform an understanding of the biology of infection, refine approaches to serologic diagnosis, and identify candidate vaccine components. Measurement of antibodies to proteins that are components of CMV vaccines is of obvious importance in comparing the immunogenicities of various vaccine antigen preparations and vaccine platforms. Cytomegalovirus glycoprotein B (gB) (UL55) is a major component of the viral envelope and plays an important role in viral attachment, entry, and cell-to-cell spread and in the fusion of infected cells (2, 3, 7, 13). Indeed, gB is required for viral infectivity. Cytomegalovirus-infected humans consistently have antibody to gB, and a substantial proportion of CMV-neutralizing antibodies in human serum are specific for gB (2, 5, 6). One would expect the antibody response to gB to be an important measure of the immunogenicity of any CMV vaccine based on whole virus or containing gB. Not surprisingly, gB has been included as a component of several investigational CMV vaccines that have entered clinical trials to date, including a recombinant gB vaccine, a bivalent DNA vaccine, a gB vaccine in a canarypox virus vector, and a vaccine using an alphavirus vector (1, 4, 9, 11, 14). This report characterizes an enzyme-linked immunosorbent assay (ELISA) method for measurement of the serum antibody response to CMV gB that uses as an antigen a recombinant CMV gB molecule produced in Chinese hamster ovary cell culture. This CMV gB molecule was originally produced by Chiron as a vaccine antigen and was evaluated in a number of clinical trials (8, 12). The vaccine was acquired by Sanofi Pasteur in 2000, and a recent phase 2 clinical trial showed that the CMV gB vaccine has efficacy for the prevention of maternal CMV infection (10).
MATERIALS AND METHODS
Study sera.
A total of 268 sera (1 sample from each of 268 subjects) that had been tested for IgG antibody to CMV were used to characterize the CMV gB ELISA. In addition, 66 sera positive for anti-CMV IgG antibody were used to create pools of serum controls with low, intermediate, and high levels of antibody to CMV. Study sera included deidentified remnant diagnostic laboratory samples, deidentified sera from healthy adult blood donors kindly provided by the American Red Cross, and sera from healthy young women who volunteered for vaccine clinical trials. One hundred sera were negative for antibody to CMV, and 168 were positive for IgG antibody to CMV. All sera were from subjects participating in studies approved by the University of Alabama at Birmingham (UAB) Institutional Review Board for Human Use.
Serologic methods.
Sera were screened for IgG antibody to CMV by using a commercial microparticle immunoassay (Axsym System CMV IgG; Abbott Laboratories, Abbott Park, IL) according to the manufacturer's instructions. The results were recorded as antibody units (AU) per milliliter. Values greater than 15 AU/ml were considered positive for anti-CMV IgG; the highest value achievable is reported by this system as >250 AU/ml. Values less than 10 AU/ml were considered negative. Results from 10 to 15 AU/ml were considered to be in an indeterminate range.
The gB ELISA was performed in 96-well microtiter plates. The procedure used is a modification of a procedure provided to us by Chiron that was used in studies of a CMV gB vaccine sponsored by Chiron (9) and a procedure kindly shared with us by Richard Ward, Director of the Laboratory for Specialized Clinical Studies at Cincinnati Children's Hospital Medical Center. Recombinant CMV gB (Sanofi Pasteur, Marcy L'Etoile, France) provided for laboratory use by the manufacturer was used as the antigen for the ELISA. The recombinant gB is a mutagenized form of CMV Towne gB in which the transmembrane portion and fusion domains have been deleted and the proteolytic cleavage site has been blocked by site-specific mutagenesis (5, 12). The molecule retains the major antigenic domains, AD-1 and AD-2, and is produced in Chinese hamster ovary cell culture as a glycosylated protein of 807 amino acids, compared with 907 amino acids for the native CMV Towne gB (5, 12). Costar clear polystyrene high-protein-binding enzyme immunoassay (EIA) plates (Corning, Lowell, MA) were coated with CMV gB (0.75 μg per ml) in a coating buffer composed of 0.015 M Na2CO3 and 0.035 M NaHCO3. After overnight incubation, the plate was washed five times with 200 μl of wash buffer (phosphate-buffered saline containing 0.25% Tween 20) using an automated plate washer (Wellwash 4 Mk2; Thermo Scientific, Waltham, MA). The plates were then blocked with wash buffer containing 0.89% bovine serum albumin at room temperature for 90 min. Serial 2-fold dilutions of sera in blocking buffer were prepared, and 100 μl of each dilution was added per well. After a 45-min incubation at 37°C, the well contents were discarded, and the wells were washed five times with wash buffer. The wells then received 50 μl of appropriately diluted horseradish peroxidase-conjugated Fc-specific goat anti-human IgG (KPL, Inc., Gaithersburg, MD). After 30 min at 37°C, the well contents were discarded, and all the wells were washed five times with wash buffer. Fifty microliters of the substrate reagent, tetramethylbenzidine (KPL, Gaithersburg, MD), was then added to each well. After 15 min at room temperature, the reaction was stopped by the addition of 50 μl of 1 M phosphoric acid. Optical densities (OD) at 450 nm were determined using an ELISA plate reader (ELX 808 microplate reader; BioTek Instruments, Inc., Winooski, VT). Results were expressed as titers or reciprocal titers.
Data analysis.
The observed endpoint titer for the anti-gB antibody assay was the highest serum dilution that yielded an OD greater than the value that defined the cutoff between positive and negative results. Calculated endpoint titers were determined by using the straight line through the OD dilution points to find the serum dilution at which the OD would equal the cutoff value. Linear regression was used to define r2 as a measure of the linear correlation between the OD and the serum dilution. For convenience, titers are expressed as reciprocal values. Descriptive statistics were used to define the central tendency and variance. The Mann-Whitney U test was used for comparison of antibody levels between operators or between endpoint determination methods. Logarithms to base 2 (log2) were used for data involving extremely high titers and for the graphical presentation of data.
For calculations using the results of the Axsym anti-CMV IgG antibody assay, values reported as >250 AU/ml were assigned a value of 251 AU/ml.
RESULTS
Determination of the cutoff between results negative and positive for antibody to gB.
Fifty sera from seronegative subjects were used to define the expected OD for sera from uninfected subjects with the CMV gB ELISA. With the Axsym assay, these 50 sera had a median anti-CMV IgG level of 1.6 AU/ml, with a range from 0.0 to 8.8 AU/ml, all clearly less than the Axsym cutoff of <10.0 AU/ml for negative results. Three dilutions (starting at 1/200) of each anti-CMV IgG-negative serum sample were tested by the CMV gB ELISA. The mean OD ± 1 standard deviation (SD) for the 50 samples at a 1/200 dilution was 0.0525 ± 0.0389. The numbers of sera with OD greater than specified multiples of this SD are shown in Table 1. All 50 samples had OD that were less than 4 standard deviations above the mean OD for the group at a 1/200 dilution. Considering the relatively modest sample size for this determination and the desire to have an assay with a high predictive value for a positive result, it was decided that sera with OD at a 1/200 dilution less than 0.2472, 5 standard deviations above the mean OD for negative sera, would be considered negative for antibody to CMV gB. Fifty additional anti-CMV IgG-negative sera were then tested in order to further validate the negative criterion. These 50 sera had a median CMV IgG level (by the Axsym assay) of 0.6 AU/ml, with a range from 0.0 to 7.0 AU/ml. In the CMV gB ELISA, all 50 sera had OD of <0.2472 at a 1/200 dilution.
TABLE 1.
ELISA results for IgG antibody to CMV gB for sera from 50 donors who were negative for anti-CMV antibody by a commercial assay
| Serum dilution | OD |
Proportion of sera with OD greater than: |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean + 3 SD | Mean + 4 SD | Mean + 3 SD | Mean + 4 SD | |
| 1/200 | 0.0525 | 0.0389 | 0.1694 | 0.2083 | 2/50 | 0/50 |
| 1/400 | 0.0300 | 0.0222 | 0.0967 | 0.1190 | 1/50 | 0/50 |
| 1/800 | 0.0185 | 0.0125 | 0.0560 | 0.0685 | 1/50 | 0/50 |
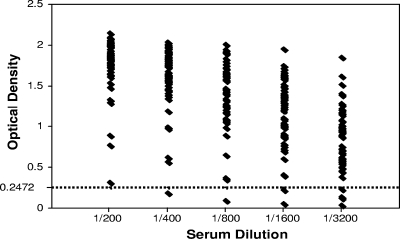
In order to determine whether an OD of ≥0.2472 would identify sera from subjects with CMV infection, 50 sera that were anti-CMV IgG positive by the Axsym assay were tested initially. CMV IgG levels ranged from 15.3 to >250 AU/ml, with a median of 145.7 AU/ml. Each of these 50 samples was diluted five times (2-fold), starting at 1/200, and was tested by the CMV gB ELISA. Each of these 50 sera had an OD above the cutoff of 0.2472 at a 1/200 dilution. The OD of these 50 samples at each dilution tested in relation to the cutoff value are displayed in Fig. 1. The highest dilution at which the OD was ≥0.2472 was considered the antibody titer (endpoint). However, a number of samples had OD well above the cutoff, even at 1/3,200, the highest dilution tested. It was clear that a method of calculating the endpoint without diluting high-titer sera to the endpoint would be useful.
FIG. 1.
Optical densities by dilution of 49 sera from CMV-seropositive donors in the CMV gB IgG ELISA, in relation to the cutoff OD of 0.2472. One of the initial 50 samples tested from seropositive donors had OD at each dilution that were too high to fit on this graph, so it was excluded.
Calculation of the endpoint.
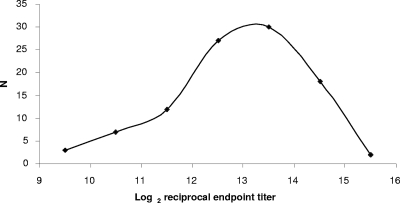
Regression analysis with data points from the more vertical portion of the OD dilution curve to define a straight line was used to determine the intersection between this line and the cutoff OD. To validate this approach, sera from 100 seropositive subjects were tested using 8 serial 2-fold dilutions from 1/200. Axsym anti-CMV IgG levels for these 100 sera ranged from 18.7 to 251 AU/ml, with a median of 212.4 AU/ml. With the CMV gB ELISA, 99/100 (99.0%) sera had OD above the cutoff (0.2472) at a 1/200 dilution. The single CMV-seropositive subject whose sample was not positive by the anti-CMV gB IgG ELISA had a very low anti-CMV IgG level (18.7 AU/ml), barely above the lower limit of the Axsym assay for positive results (15.0 AU/ml). Endpoints were calculated for the 99 subjects whose IgG levels were above the cutoff by using regression analysis. For each serum sample, results from at least 4 dilutions that fell on the more vertical portion of the OD dilution curve were used to calculate the dilution at which the OD would equal 0.2472. A frequency distribution for the calculated log2 endpoint titers is shown in Fig. 2. The geometric mean endpoint titer for sera from these 99 blood donors was 10,937. There were 79 sera in this group that had OD of <0.2472 at their highest dilutions. For these 79 sera, the median calculated endpoint was 6,457 (range, 559 to 20,959). If the highest dilution for each sample at which the OD was >0.2472 was considered the endpoint, the median observed endpoint was 6,400 (range, 800 to 12,800), not significantly different from the median calculated endpoint (P, 0.46).
FIG. 2.
Frequency distribution of anti-CMV gB IgG endpoint titers of sera from 99 CMV-seropositive blood donors. N, number of serum samples.
To further validate the calculation method, sera from 18 subjects who had extremely high levels of antibody to gB in serum were studied. Using 8 serial 2-fold dilutions starting at 1/800, the endpoints calculated from the OD dilution curve ranged from 117,181 (log2, 16.84) to 3,286,879 (log2, 21.65). The titration was repeated using 8 serial 2-fold dilutions starting at 1/12,800. Each serum sample tested in this manner had an OD of <0.2472 for at least the highest dilution, and the highest dilution at which the OD was above this cutoff was considered the observed endpoint titer. The log2 median observed endpoint was 17.64 (range, 16.64 to 19.64). Endpoint titers were then calculated by regression analysis of the OD dilution data obtained for 8 dilutions starting at 1/12,800. The log2 median calculated endpoint was 18.30 (range, 16.62 to 19.58). Statistical analysis comparing the 1/800 starting-dilution endpoint data to the observed endpoints and the 1/12,800 starting-dilution data to the observed endpoints yielded P values of 0.10 and 0.39, respectively. Although the difference between the calculated and observed endpoints was not statistically significant for either dilution set, the results were in better agreement when the data used came from serum dilutions that covered the range of the observed endpoints. None of the endpoints calculated from the 1/12,800 starting-dilution data differed from the observed endpoint by more than a log2 of 0.78, less than 1 dilution. With the 1/800 starting-dilution data, 4/18 results were more than 1 dilution (log2 of difference, >1.00) greater than the observed endpoint.
Observed versus calculated endpoints.
The calculation of endpoints creates the opportunity for additional errors besides those that could affect the accuracy of any ELISA method, such as variation in the quality and consistency of reagents and errors or inconsistency in incubation times, pipetting, blocking, or washing. Calculation errors arise mainly from using OD dilution data that are not on the falling portion of the curve and from a failure of the data points to describe a straight line. Based on the experience gained testing the first 100 anti-CMV IgG-positive sera, it was determined that the criteria for a valid run should include the following. (i) At the highest dilution used in endpoint calculation, the OD must be ≤1.1, or the difference in the OD between the lowest and highest dilutions used in endpoint calculation must be ≥0.9. (ii) The difference in the OD between the lowest dilution used for endpoint calculation and the next-to-lowest dilution must be ≥0.15, and the difference in the OD between the highest and next-to-highest dilutions used must be ≥0.15. (iii) The r2 for the straight line used to determine the endpoint must be ≥0.95.
Reproducibility.
Three pools of sera were prepared for use as a quality control measure and for assessing the run-to-run and interoperator consistency of the assay results. Twenty sera with Axsym anti-CMV IgG levels from 15 to 89 AU/ml were mixed to form a low-positive control; this pool had an anti-CMV IgG level of 77.6 AU/ml. Twenty-five sera with anti-CMV IgG levels from 90 to 199 AU/ml were mixed to form an intermediate positive control; this pool had an anti-CMV IgG level of 137.4 AU/ml. Twenty-one sera with anti-CMV IgG levels from 200 to >250 AU/ml were mixed to form a high-positive control; this pool had an anti-CMV IgG level of >250 AU/ml.
The run-to-run reproducibility of assay results was assessed for each of two operators and between the two operators. For operator 1, mean log2 endpoints ± 1 SD (for 38 runs) for low-, intermediate, and high-positive controls were, respectively, 12.38 ± 0.28, 12.68 ± 0.43, and 13.89 ± 0.33. For operator 2, the corresponding results (for 48 runs) were 12.23 ± 0.42, 12.65 ± 0.36, and 13.96 ± 0.48. Results within 1 dilution (log2, 1.0) were considered acceptable in terms of reproducibility. For each operator, 100% of the results with positive controls from the runs discussed above were within ±1.0 log2 (one 2-fold dilution) of the operator's mean. In addition, statistical comparison of results showed that there was no statistically significant difference between the two operators for low (P, 0.12)-, intermediate (P, 0.96), or high (P, 0.61)-positive-control serum results.
DISCUSSION
This ELISA method for measuring IgG antibody to CMV gB allows calculation of the endpoint titer by using regression analysis of the more vertical portion of the OD-dilution curve. The calculated endpoints are very similar over a wide range of anti-gB antibody levels to endpoints observed by diluting sera to the point where the OD is below the cutoff value indicating no detectable antibody to gB. The assay provides excellent run-to-run and interoperator reproducibility. A similar ELISA method was used at Chiron (now Novartis) to measure levels of antibody to CMV gB in sera from vaccine trial participants and controls (9). The geometric mean titer (GMT) of anti-gB antibody reported for 200 seropositive adults tested at Chiron was 3,186, compared with a GMT of 10,937 reported here for 99 seropositive blood donors.
The anti-gB antibody assay is able to distinguish sera that are positive or negative for anti-CMV antibody nearly as well as a commercial assay that uses whole virus as an antigen, based on results with sera from 168 anti-CMV antibody-positive subjects. However, without further evaluation using a larger sample size, the gB ELISA cannot be recommended for this purpose. The antigen used for this assay is the same molecule used as an antigen in an investigational CMV gB vaccine. The availability of a purified recombinant gB molecule very likely contributed to the sensitivity and specificity of this assay for the detection of persons with antibody to CMV. The fact that all but 1 of 168 anti-CMV antibody-positive subjects tested exhibited antibody to gB provides further evidence that the recombinant gB molecule used as an antigen contains highly conserved immunogenic domains that are recognized by antibodies stimulated by infection by all CMV strains.
Acknowledgments
This work was supported by Public Health Service grants from the Division of Microbiology and Infectious Diseases, National Institutes of Allergy and Infectious Diseases (P01-AI43681 and U01-AI063565), and by grant 5UL1 RR025777-02 from the NIH National Center for Research Resources. Research support was also provided by Sanofi Pasteur, Marcy L'Etoile, France. D. J. Hackett was the recipient of an Infectious Diseases Society of America Education and Research Foundation Medical Scholars award in July 2009.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Bernstein, D. I., M. R. Schleiss, K. Berencsi, E. Gonczol, M. Dickey, P. Khoury, C. Cadoz, C. Meric, J. Zahradnik, A.-M. Duliege, and S. A. Plotkin. 2002. Effect of previous or simultaneous immunization with canarypox expressing cytomegalovirus (CMV) glycoprotein B (gB) on response to subunit gB vaccine plus MF59 in healthy CMV-seronegative adults. J. Infect. Dis. 185:686-690. [DOI] [PubMed] [Google Scholar]
- 2.Britt, W., and M. Mach. 1996. Human cytomegalovirus glycoproteins. Intervirology 39:401-412. [DOI] [PubMed] [Google Scholar]
- 3.Compton, T., D. M. Nowlin, and N. R. Cooper. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface haparan sulfate. Virology 193:834-841. [DOI] [PubMed] [Google Scholar]
- 4.Frey, S. E., H. Harrison, R. F. Pass, E. Yang, D. Boken, R. E. Sekulovich, S. Percell, A. E. Izu, S. Hirabayashi, R. L. Burke, and A.-M. Duliege. 1999. Effects of antigen dose and immunization regimens on antibody responses to a cytomegalovirus glycoprotein B subunit vaccine. J. Infect. Dis. 180:1700-1703. [DOI] [PubMed] [Google Scholar]
- 5.Marshall, G. S., G. P. Rabalais, G. G. Stout, and S. L. Waldeyer. 1992. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J. Infect. Dis. 165:381-384. [DOI] [PubMed] [Google Scholar]
- 6.Navarro, D., E. Lennette, S. Tugizov, and L. Pereira. 1997. Humoral immune response to functional regions of human cytomegalovirus glycoprotein B. J. Med. Virol. 52:451-459. [PubMed] [Google Scholar]
- 7.Navarro, D., P. Paz, S. Tugizov, K. Topp, J. LaVail, and L. Pereira. 1993. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology 197:143-158. [DOI] [PubMed] [Google Scholar]
- 8.Pass, R. F., and R. L. Burke. 2002. Development of cytomegalovirus vaccines: prospects for prevention of congenital CMV infection. Semin. Pediatr. Infect. Dis. 13:196-204. [DOI] [PubMed] [Google Scholar]
- 9.Pass, R. F., A.-M. Duliege, S. Boppana, R. Sekulovich, S. Percell, W. Britt, and R. L. Burke. 1999. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J. Infect. Dis. 180:970-975. [DOI] [PubMed] [Google Scholar]
- 10.Pass, R. F., C. Zhang, A. Evans, T. Simpson, W. Andrews, M.-L. Huang, L. Corey, J. Hill, E. Davis, C. Flanigan, and G. Cloud. 2009. Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 360:1191-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reap, E. A., S. A. Dryga, J. Morris, B. Rivers, P. Norberg, R. A. Olmsted, and J. D. Chulay. 2007. Cellular and humoral immune responses to alphavirus replicon vaccines expressing cytomegalovirus pp65, IE1 and gB proteins. Clin. Vaccine Immunol. 14:748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaete, R. R. 1991. A recombinant subunit vaccine approach to HCMV vaccine development. Transplant. Proc. 23:90-96. [PubMed] [Google Scholar]
- 13.Tugizov, S., D. Navarro, P. Paz, Y. Wang, I. Qadri, and L. Pereira. 1994. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology 201:263-276. [DOI] [PubMed] [Google Scholar]
- 14.Wloch, M. K., L. R. Smith, B. Souphaphone, L. Reyes, C. Han, J. Kehler, H. D. Smith, L. Selk, R. Nakamura, J. M. Brown, T. Marbury, A. Wald, A. Rolland, D. Kaslow, T. Evans, and M. Boeckh. 2008. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J. Infect. Dis. 197:1634-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]