Abstract
Rapid, simple, and accurate antemortem tests for tuberculosis (TB) in cattle need to be developed in order to augment the existing screening methods. In particular, as cattle vaccines are developed, such tests would allow the continuation of test-and-slaughter policies alongside vaccination. Therefore, the development of an assay that distinguishes infected from vaccinated animals (a DIVA test) is an urgent research requirement. In this study, we assessed the performance of a novel multiplex serological test with sera collected from 96 skin-tested animals with bovine tuberculosis, 93 TB-free animals, and 39 cattle vaccinated with Mycobacterium bovis BCG. Our results indicate that the test has a relative sensitivity range of 77.0% to 86.5% at corresponding specificity levels of 100.0% to 77.6%. Comparison with the Bovigam gamma interferon antemortem test revealed that this serology test was significantly more sensitive at specificities above 97.9%, while the Bovigam test was, on average, about 10% more sensitive when the test specificity was set below 97%. Importantly, this serological multiplex assay does not react with sera from BCG-vaccinated calves and is therefore suitable as a DIVA test alongside BCG-based vaccine strategies.
Bovine tuberculosis (bTB) is a zoonotic disease caused by the bacterial pathogen Mycobacterium bovis. Although the main host is cattle, M. bovis can infect many species, including wildlife and humans. In developed countries where the control and eradication of bTB continue to be a problem, the impact of bTB on the farming community and government control agencies is primarily of economic significance (1, 20; http://www.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/tb/documents/expenditure-stats.pdf). However, in the presence of wildlife reservoirs, such as the possum in New Zealand or the badger in Great Britain and Ireland, bTB has been difficult to eradicate. The primary bTB screening and control tool is the tuberculin skin test (TST), with the removal of animals found to be positive (reactors). The TST as applied in Great Britain and Ireland is called the single intradermal comparative cervical tuberculin test (SICCT) (14). It involves the injection of bovine tuberculin (a purified protein derivative [PPD] prepared from M. bovis strain AN5) and PPD tuberculin from Mycobacterium avium into the skin of the neck. Animals with bovine PPD (PPD-B)-biased responses are then removed.
A widely used ancillary in vitro test for TB is a gamma interferon (IFN-γ) release assay (IGRA) that also employs avian and bovine tuberculin (Bovigam) (18, 28, 30). Both the TST and the Bovigam assay probe cell-mediated immune (CMI) responses (16). Questions have been raised with regard to both the sensitivity (proportion of true positives) and specificity (proportion of true negatives) of tuberculin-based tests, for example, for herds that are coinfected with Mycobacterium avium subsp. paratuberculosis (2, 3), or in the context of vaccine strategies under development that involve the use of M. bovis bacillus Calmette-Guérin (BCG) (4, 23, 24). Therefore, research has been directed toward the diagnosis of bTB using specific and defined antigens that will improve test specificity and enable tests to function as DIVA tests. This has been particularly successful when such antigens are used in combination with IGRAs as DIVA tests (5, 8, 19, 23, 25).
Serological tests have logistical and financial advantages over assays based on cell-mediated immunity. Yet in the past they have suffered from relatively poor sensitivity (26, 29, 31) and therefore were not considered the method of choice for the delivery of highly sensitive tests for bTB. However, recent advances in antigen discovery and the development of novel and more-sensitive detection systems have led to serological assays that show promise in delivering highly sensitive tests (10, 27). One recently described assay (the Enferplex TB assay) is a chemiluminescent multiplex system that can simultaneously detect and analyze antibody responses to multiple antigens spotted in a single well of a 96-well plate (27). The sensitivity and specificity of the Enferplex TB assay have been reported as 93.1% and 98.4%, respectively, in a nonblinded Irish study (27).
The present study was conducted to extend these findings by determining the relative sensitivity and specificity of the Enferplex TB serum assay in comparison to those of the SICCT and the Bovigam IGRA in a blinded study with serum samples from cattle from Great Britain with and without bTB. In contrast to the method of another study (T. Clegg, A. Duignan, C. Whelan, E. Gormley, M. Good, J. Clarke, N. Toft, and S. J. More, submitted for publication), which assessed nonoverlapping populations by latent class analysis to compare tests, the same animals in the present study were tested with the Enferplex TB assay, the SICCT, and the Bovigam IGRA (a subset of 30 only). Further, we assessed the performance of this serological assay as a DIVA test compared to those of the SICCT and the Bovigam IGRA by using sera from BCG-vaccinated cattle.
MATERIALS AND METHODS
Serum samples.
Serum samples were collected from 96 SICCT-positive cattle from herds in Great Britain with known bTB incidence. All animals presented with visible lesions typical of bTB at slaughter and were culture positive. These animals were sampled as part of two studies funded by the Department for Environment, Food and Rural Affairs (Defra) (SE3013 and SE3227). In addition, 93 sera were collected from skin test-negative animals from herds with no recent TB history located in regions of Great Britain where bTB is not endemic. A group of 39 male calves (Holstein or Holstein crosses) were neonatally vaccinated with a Danish BCG strain (Statens Serum Institut, Copenhagen, Denmark), and serum was collected 6 months postvaccination. Serum samples from all animal groups were drawn between 2 and 5 weeks after the SICCT was performed.
A further group of 21 animals, composed of Holstein or Holstein-cross male calves, was experimentally infected via the intratracheal route with 2,000 CFU of the Great Britain M. bovis field isolate AF2122/97. The animals were approximately 6 months old at the time of infection, and monthly serum samples were collected over a 16-week period. All animals presented with visible lesions and were culture positive when postmortem examinations were carried out, 17 weeks postinfection.
Project license.
All animal experimentation was carried out under a Great Britain Home Office animal project license granted under the Animals (Scientific Procedures) Act of 1984. This project license was approved by the local Ethical Review Board before its submission to the Home Office.
Enferplex TB serum assay.
The multiplex immunoassay (Enferplex TB assay; Enfer Scientific, Naas, County Kildare, Ireland) was conducted using methods described previously (27). Briefly, a multiplex chemiluminescent immunoassay was developed to simultaneously detect antibody recognition of as many as 25 antigens in a single well in a 96-well plate array format. In this assay, the chemiluminescent signal is captured with a digital imaging system and is analyzed with bespoke software based on Excel (Enfer Scientific) that tracks each serum sample for its pattern of antibody recognition of M. bovis antigens. Samples of known provenance were used to set different levels of specificity and sensitivity based on patterns of antibody recognition. Five different cutoff levels with different sensitivities and specificities were used in the current study (Enferplex TB cutoff levels 1 to 5).
Bovigam IGRA.
Blood was collected from a subset of 30 of the cattle with bTB, and the Bovigam IGRA was performed as in the routine application of this test in Great Britain. Briefly, heparinized blood was collected, and whole blood cultures were initiated on the day following sampling. Blood cells were stimulated with avian and bovine PPDs (Veterinary Laboratories Agency [VLA]—Weybridge, Addlestone, United Kingdom) at concentrations of 10 μg/ml. Only results that met the positive-control criteria were used (optical density at 450 nm [OD450], >0.5 by use of staphylococcal enterotoxin B [Sigma-Aldrich, Poole, United Kingdom] at 1 μg/ml). Plasma supernatants were harvested 20 to 24 h later, and the IFN-γ contents of the supernatants were determined with the Bovigam IFN-γ kit (Prionics, Schlieren, Switzerland) according to the manufacturer's instructions. Cutoffs at specificity levels corresponding to those calculated for the different interpretations of the Enferplex TB assay were obtained for the Bovigam IGRA by using receiver operating characteristic (ROC) curve analysis as described elsewhere (21a). ROC curves are graphical plots of sensitivity versus (1 − specificity) for a binary classifier system as its discrimination threshold is varied, and they can be used to select optimal cutoffs for test positivity.
Statistical analysis.
The performances of the IGRA and the multiplex assay were compared using either the Mann-Whitney test or Fisher's exact test with significance levels set at 5%. Analysis was performed using Instat, version 3 (GraphPad, San Diego, CA).
RESULTS AND DISCUSSION
Sera from 189 animals (96 SICCT reactor animals with visible tuberculous lesions and culture-confirmed bTB; 93 bTB-free animals) were analyzed using the Enferplex TB assay. The disease statuses of these animals were unknown to the operators of the test, and the code was broken only after the test results were interpreted. Five interpretation criteria representing increasing specificity settings were applied. The results shown in Table 1 demonstrated the high accuracy of the test, with relative sensitivities ranging from 86.5% at the lowest specificity setting of 79.6% (Enferplex TB cutoff level 1) to 77.1% at the most stringent specificity setting (100% [Enferplex TB cutoff level 5]).
TABLE 1.
Performance of the Enferplex TB multiplex immunoassay on field animals
| Enferplex TB cutoff level | Sensitivitya | Specificityb |
|---|---|---|
| 5 | 77.1 (67.4, 85.0) | 100 (96.1, 100) |
| 4 | 79.2 (69.7, 86.8) | 97.9 (92.4, 99.7) |
| 3 | 81.3 (72.0, 88.5) | 94.6 (87.9, 98.2) |
| 2 | 82.3 (73.1, 89.3) | 91.4 (83.8, 96.2) |
| 1 | 86.5 (78.0, 92.6) | 79.6 (70.0, 87.23) |
Measured for 96 SICCT reactor animals, with visible lesions and positive M. bovis cultures. Both sensitivity and specificity are expressed as percentages, with 95% confidence intervals in parentheses.
Measured for 93 skin test-negative cattle from TB-free herds.
The sensitivity of the test in this study is significantly lower at comparable specificity levels than the sensitivity obtained in the study testing Irish cattle that was previously reported by Whelan et al. in 2008 (27) (79.2% versus 93.1% at specificities of 97.9 and 98.4%, respectively; P, <0.0001 by Fisher's exact test). The difference in sensitivity could be due to a different disease status of the Irish cattle, to differences in populations, or to the fact that the samples in the present study were drawn after skin test injection. However, the latter hypothesis does not agree with published data demonstrating that tuberculin injection boosts serum responses, thus increasing test sensitivity (12, 13, 26).
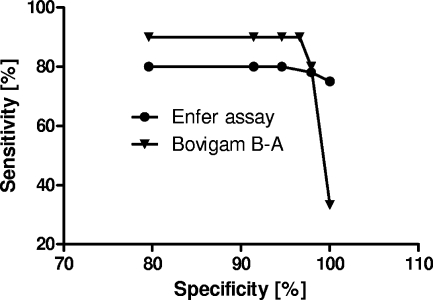
For a subset of 30 cattle with bTB, corresponding Bovigam IGRA data were available. The IGRA was performed according to the routine application of this assay in Great Britain as an ancillary test to the SICCT (http://www.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/tb/control/gamma-criteria.htm). To allow a full comparison between the tests, we used ROC analysis of a Bovigam test data set from 1,198 cattle, available through the various Great Britain validation studies undertaken since 2002 (21a). This was used to determine the Bovigam test cutoffs that corresponded to the 5 multiplex test specificity settings shown in Table 1. Using these cutoffs, relative sensitivity rates were calculated for the Bovigam assay by subtracting the OD450 obtained with the avian PPD (PPD-A) from the OD450 obtained with the bovine PPD, and these were compared to the corresponding results obtained with sera from these 30 cows in the multiplex assay. In addition, we included the relative Bovigam sensitivity for these animals at the specificity setting used in the routine Bovigam assay in Great Britain (approximately 97% [25a]). As the data in Fig. 1 indicate, the Bovigam IGRA was about 10% more sensitive than the serology test up to specificity settings of 97%. This difference was significant at these specificities (P, <0.05 by the Mann-Whitney test). However, if the specificity was maximized to 100%, the Enferplex TB immunoassay was significantly more sensitive than the IGRA (33% versus 73.3%, respectively; P, 0.004 by Fisher's exact test). Therefore, the present data demonstrate that the Enferplex TB immunoassay can be optimized to address particular surveillance requirements to a degree that the Bovigam IGRA cannot. However, the data also demonstrate that cell-mediated tests provide sensitivity advantages when lower specificity values are acceptable, thus maximizing the number of diseased animals that can be detected.
FIG. 1.
Comparison of the sensitivities of the Bovigam IGRA and the Enferplex TB multiplex immunoassay. The analysis is based on the testing of 30 animals with confirmed bTB. The interpretation of the Bovigam assay is based on the differences between the OD450 values obtained after stimulation with bovine versus avian PPD. The following cutoffs were used to determine the relative sensitivity of the Bovigam IGRA: 79.6% specificity corresponds to a difference of 0.016 OD450 unit; 91.4% specificity, to a >0.045-OD450 difference; 94.6% specificity, to a >0.076-OD450 difference; 97% specificity, to a >0.110-OD450 difference; 97.8% specificity, to a >0.178-OD difference; and 100% specificity, to a >1.903-OD450 difference.
Next, we assessed the performance of the multiplex assay for its potential as a DIVA test and compared it to the Bovigam IGRA and the SICCT (Table 2). Sera from 39 animals vaccinated neonatally with BCG were collected about 6 months postvaccination. None of these sera yielded a positive result with the Enferplex TB assay at any of the 5 specificity settings (Table 2). The results show that the Enferplex TB assay has potential as a DIVA test in the context of BCG vaccination based on the samples investigated. However, further studies need to be conducted in order to determine the degree to which our findings can be extrapolated to the wider population. In contrast, 66.6% (26/39) of the calves tested positive with the SICCT when the standard interpretation criteria (bovine PPD response minus avian PPD response, >4 mm) were used, and 87.2% (34/39) tested positive when the severe SICCT interpretation criteria (bovine minus avian PPD response, >2 mm) were applied (Table 2). We also performed an IGRA in order to obtain comparative test information. With the Bovigam IGRA, 38.5% (15/39) of these animals tested positive by using an OD450 differential (bovine minus avian PPD) of >0.1 as the cutoff for positivity (Table 2), confirming that the specificity of tuberculin-based tests is compromised after BCG vaccination. DIVA reagents based on the Bovigam IGRA are currently being developed and include antigens such as ESAT-6 and CFP-10, whose genes are deleted from the genome of BCG (5, 15, 21, 24). When we used these two antigens in the form of a synthetic peptide pool in the Bovigam assay (22) for these 39 animals, we found weak but positive responses for 7.7% (3/39) of the BCG-vaccinated animals by using an OD450 differential of >0.1 (ESAT-6/CFP-10 minus medium only) as the cutoff (Table 2). Thus, the Enferplex TB assay is at least as specific a DIVA test as the Bovigam IGRA with ESAT-6 and CFP-10. When we calculated the sensitivity of the Bovigam assay relative to that of the SICCT for the 30 animals with bTB assessed as described above by using ESAT-6 and CFP-10 as IGRA antigens, we found that it was lower than that of the Enferplex TB assay at a comparable specificity level of 97% (73.3% [22/30] versus 78% [25/30]), although this difference was not statistically significant. We have recently identified another IGRA antigen that complemented ESAT-6 and CFP-10 as a DIVA reagent by increasing the sensitivity of the test to levels comparable to those achieved with PPD (19), and it will be of interest to compare these improved IGRA DIVA reagents with the Enferplex TB serum assay in future studies.
TABLE 2.
Evaluation of the DIVA potential of the Enferplex TB assaya
| Test performed | % of animals testing positive (95% CIb) | No. of animals testing positive/total no. | Pc |
|---|---|---|---|
| Enferplex TBd | 0 (0, 9) | 0/39 | NA |
| SICCT | |||
| Standard interpretation | 66.6 (49.8, 80.9) | 26/39 | <0.0001 |
| Severe interpretation | 87.2 (72.5, 95.7) | 34/39 | <0.0001 |
| Bovigam IGRAe | |||
| PPD-B and -A | 38.5 (23.4, 55.4) | 15/39 | <0.0001 |
| ESAT-6 and CFP-10 | 7.7 (1.6, 20.8) | 3/39 | 0.2403 |
Calves were neonatally vaccinated with BCG, and serum samples were collected 6 months postvaccination and 2 to 5 weeks after the SICCT was performed.
CI, confidence interval.
For comparison of the SICCT or the Bovigam IGRA to the Enferplex TB assay by Fisher's exact test. To account for multiple-comparison errors, a P value smaller than 0.0125 was used to define statistical significance. NA, not applicable.
Animals tested negative with all 5 of the interpretation criteria.
For the Bovigam IGRA, the cutoff was a difference of >0.1 OD450 unit between the PPD-B and PPD-A results or between the results with the cocktail of ESAT-6- and CFP-10-derived synthetic peptides and the results with the no-antigen control.
However, in considering the specificities of the Bovigam test, it should be noted that these BCG vaccinees were close to the minimum eligibility cutoff age of 6 months for standard IGRA application. Thus, at least some of these responses were likely due to nonspecific (natural killer [NK]) cell activity, described previously (14), resulting in false-positive responses for these young animals. In Great Britain, cattle younger than 6 months are excluded from Bovigam testing in order to minimize the risk of such nonspecific IFN-γ production. It appears that the multiplex assay is generally less affected by this nonspecific reactivity and could therefore be used for this age group. The higher specificity of the Enferplex TB assay across all interpretation criteria for the BCG vaccinee cohort than for the TB-free field animals (Table 1) is likely due to the fact that the former animals are younger and were kept from birth under laboratory conditions that prevented exposure to environmental bacteria that could affect test specificity.
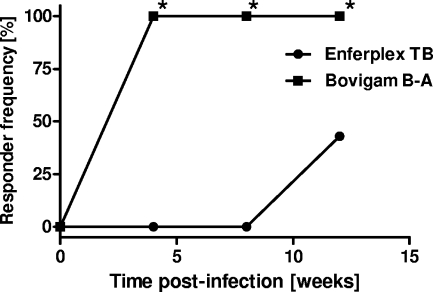
Interestingly, when we compared the response kinetics of the IGRA and the serum responses measured by the multiplex assay using sera from experimentally infected animals, we found that all 21 animals tested IGRA positive (using an OD difference [bovine minus avian PPD] of >0.1 as a cutoff) 4 weeks postinfection, while serum responses took longer to develop, with only about 43% (9/21) of infected calves giving positive responses at 12 weeks postinfection (Fig. 2; specificity setting, level 4, as shown in Table 1). While these results are in agreement with other observations that suggest a spectral disease progression of bTB in cattle (17), with cellular immune responses developing before humoral responses, the present finding is in contrast to that of an earlier study indicating that experimentally infected cattle could be detected as early as 5 weeks postinfection by this assay (27). However, different challenge inocula were used in the two experiments, as well as different challenge strains. Therefore, the two data sets are not directly comparable.
FIG. 2.
Kinetics of Bovigam IGRA and Enferplex TB multiplex assay responses in experimentally infected cattle. The analysis is based on 21 animals experimentally infected with 2,000 CFU M. bovis AF2122/97 via the intratracheal route. The Bovigam assay interpretation is based on the differences in OD450 values obtained after stimulation with bovine versus avian PPD (cutoff, >0.1 OD450 unit). Enferplex TB interpretation level 4, as listed in Table 1, was used. Squares, Bovigam IGRA; circles, Enferplex TB assay. *, P < 0.0001 by Fisher's exact test.
Our study is limited to some degree in that all our samples were collected from cattle that underwent tuberculin skin testing prior to sampling. The sensitivity estimates reported in Table 1 for the Enferplex TB assay are, therefore, potentially biased (overestimated), since skin test positivity was used as the initial selection criterion for infected cattle. Several studies have shown that the skin test boosts serum antibody responses and consequently increases sensitivity (11-13, 26). In contrast, IFN-γ production is little affected by skin testing when the SICCT is used (7, 9), although IFN-γ responses have been shown to be boosted following the caudal fold tuberculin test (6, 30). Therefore, further studies are needed to assess whether prior skin testing will affect sensitivity. However, if the Enferplex TB assay is used as an ancillary test to the skin test, like the Bovigam IGRA, this consideration will be less relevant. In this scenario, further studies have to be performed with diseased animals that escape detection by skin testing, since such animals would be the primary target population for any ancillary test. The Bovigam IGRA is used predominantly in this way, and our data from a recent study demonstrated that the sensitivities of the Bovigam IGRA (PPD-B minus PPD-A results) are identical for animals with confirmed bTB in SICCT-positive and SICCT-negative populations (89.72% and 91.36%, respectively, at 96.5% specificity [21a]). The use of both tests together in parallel, therefore, significantly increases the proportion of diseased animals that are detected, and one would expect a similar outcome by using the Enferplex TB assay in combination with skin tests and/or IGRAs. However, further comparative test validations are required in order to fully appreciate whether the multiplex serology assay can be used alone as a DIVA test or whether it must be used in combination with the tests probing cell-mediated immunity (the skin test and the Bovigam IGRA) in order to maximize sensitivity and specificity.
In conclusion, the present study has reaffirmed the high sensitivity (77%) of the Enferplex TB assay, even at maximal specificity levels. Furthermore, our study also demonstrated that the Enferplex TB assay would have considerable logistical advantages over blood tests using cellular immunity as a readout and could therefore contribute to the cost-effective application of a cattle vaccination policy. Furthermore, it could potentially be used in combination with other antemortem tests, the SICCT and the Bovigam IGRA, as part of a diagnostic program aimed at detecting animals at different disease stages.
Acknowledgments
These studies were funded in part by the Department for Environment, Food and Rural Affairs (Defra), United Kingdom.
We thank the testing team from Enfer Scientific that ran the Enferplex TB assay. We also express our sincere appreciation to the Animal Services Unit at VLA.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Amanfu, W. 2006. The situation of tuberculosis and tuberculosis control in animals of economic interest. Tuberculosis (Edinb.) 86:330-335. [DOI] [PubMed] [Google Scholar]
- 2.Aranaz, A., L. De Juan, J. Bezos, J. Alvarez, B. Romero, F. Lozano, J. L. Paramio, J. Lopez-Sanchez, A. Mateos, and L. Dominguez. 2006. Assessment of diagnostic tools for eradication of bovine tuberculosis in cattle co-infected with Mycobacterium bovis and M. avium subsp. paratuberculosis. Vet. Res. 37:593-606. [DOI] [PubMed] [Google Scholar]
- 3.Bezos, J., L. de Juan, B. Romero, J. Alvarez, F. Mazzucchelli, A. Mateos, L. Dominguez, and A. Aranaz. 2010. Experimental infection with Mycobacterium caprae in goats and evaluation of immunological status in tuberculosis and paratuberculosis co-infected animals. Vet. Immunol. Immunopathol. 133:269-275. [DOI] [PubMed] [Google Scholar]
- 4.Buddle, B. M., G. W. de Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 5.Buddle, B. M., N. A. Parlane, D. L. Keen, F. E. Aldwell, J. M. Pollock, K. Lightbody, and P. Andersen. 1999. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 6:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coad, M., D. Clifford, S. G. Rhodes, R. G. Hewinson, H. M. Vordermeier, and A. O. Whelan. 2010. Repeat tuberculin skin testing leads to desensitisation in naturally infected tuberculous cattle which is associated with elevated interleukin-10 and decreased interleukin-1 beta responses. Vet. Res. 41:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coad, M., R. G. Hewinson, D. Clifford, H. M. Vordermeier, and A. O. Whelan. 2007. Influence of skin testing and blood storage on interferon-gamma production in cattle affected naturally with Mycobacterium bovis. Vet. Rec. 160:660-662. [DOI] [PubMed] [Google Scholar]
- 8.Cockle, P. J., S. V. Gordon, R. G. Hewinson, and H. M. Vordermeier. 2006. Field evaluation of a novel differential diagnostic reagent for detection of Mycobacterium bovis in cattle. Clin. Vaccine Immunol. 13:1119-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gormley, E., M. B. Doyle, K. McGill, E. Costello, M. Good, and J. D. Collins. 2004. The effect of the tuberculin test and the consequences of a delay in blood culture on the sensitivity of a gamma-interferon assay for the detection of Mycobacterium bovis infection in cattle. Vet. Immunol. Immunopathol. 102:413-420. [DOI] [PubMed] [Google Scholar]
- 10.Green, L. R., C. C. Jones, A. L. Sherwood, I. V. Garkavi, G. A. Cangelosi, T. C. Thacker, M. V. Palmer, W. R. Waters, and C. V. Rathe. 2009. Single-antigen serological testing for bovine tuberculosis. Clin. Vaccine Immunol. 16:1309-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lightbody, K. A., J. McNair, S. D. Neill, and J. M. Pollock. 2000. IgG isotype antibody responses to epitopes of the Mycobacterium bovis protein MPB70 in immunised and in tuberculin skin test-reactor cattle. Vet. Microbiol. 75:177-188. [DOI] [PubMed] [Google Scholar]
- 12.Lightbody, K. A., R. A. Skuce, S. D. Neill, and J. M. Pollock. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet. Rec. 142:295-300. [DOI] [PubMed] [Google Scholar]
- 13.Lyashchenko, K., A. O. Whelan, R. Greenwald, J. M. Pollock, P. Andersen, R. G. Hewinson, and H. M. Vordermeier. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen, I., P. Boysen, S. Kulberg, J. C. Hope, G. Jungersen, and A. K. Storset. 2005. Bovine NK cells can produce gamma interferon in response to the secreted mycobacterial proteins ESAT-6 and MPP14 but not in response to MPB70. Infect. Immun. 73:5628-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollock, J. M., and P. Andersen. 1997. Predominant recognition of the ESAT-6 protein in the first phase of interferon with Mycobacterium bovis in cattle. Infect. Immun. 65:2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollock, J. M., J. McNair, M. D. Welsh, R. M. Girvin, H. E. Kennedy, D. P. Mackie, and S. D. Neill. 2001. Immune responses in bovine tuberculosis. Tuberculosis (Edinb.) 81:103-107. [DOI] [PubMed] [Google Scholar]
- 17.Ritacco, V., B. Lopez, I. N. De Kantor, L. Barrera, F. Errico, and A. Nader. 1991. Reciprocal cellular and humoral immune responses in bovine tuberculosis. Res. Vet. Sci. 50:365-367. [DOI] [PubMed] [Google Scholar]
- 18.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1990. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust. Vet. J. 67:134-137. [DOI] [PubMed] [Google Scholar]
- 19.Sidders, B., C. Pirson, P. J. Hogarth, R. G. Hewinson, N. G. Stoker, H. M. Vordermeier, and K. Ewer. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect. Immun. 76:3932-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steele, J. H. 1995. Regional and country status report, p. 169-172. In C. O. Thoen (ed.), Mycobacterium bovis infection in animals and humans. Iowa State University Press, Ames, IA.
- 21.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Veterinary Laboratories Agency. 2009. Annex A.1. Analytical workstream report. Project SB4008. Veterinary Laboratories Agency, New Haw, Addlestone, Surrey, United Kingdom. http://www.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/tb/documents/gamma-annex1.pdf.
- 22.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vordermeier, H. M., P. C. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vordermeier, M., A. Aranaz, and J. M. Pollock. 2001. Immunodiagnosis of bovine tuberculosis. Summary of a satellite workshop of the Mycobacterium bovis 2000 conference, Cambridge, UK, 17 August 2000. Tuberculosis (Edinb.) 81:177-180. [DOI] [PubMed] [Google Scholar]
- 25a.Vordermeier, M., and K. Ewer. April 2006. Specificity trial of the BOVIGAM IFN-gamma test in GB cattle. Veterinary Laboratories Agency, New Haw, Addlestone, Surrey, United Kingdom. Defra surveillance project SB4021. http://www.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/tb/documents/gifn_specificityreport.pdf.
- 26.Waters, W. R., M. V. Palmer, T. C. Thacker, J. P. Bannantine, H. M. Vordermeier, R. G. Hewinson, R. Greenwald, J. Esfandiari, J. McNair, J. M. Pollock, P. Andersen, and K. P. Lyashchenko. 2006. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin. Vaccine Immunol. 13:648-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whelan, C., E. Shuralev, G. O'Keeffe, P. Hyland, H. F. Kwok, P. Snoddy, A. O'Brien, M. Connolly, P. Quinn, M. Groll, T. Watterson, S. Call, K. Kenny, A. Duignan, M. J. Hamilton, B. M. Buddle, J. A. Johnston, W. C. Davis, S. A. Olwill, and J. Clarke. 2008. Multiplex immunoassay for serological diagnosis of Mycobacterium bovis infection in cattle. Clin. Vaccine Immunol. 15:1834-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood, P. R., L. A. Corner, J. S. Rothel, C. Baldock, S. L. Jones, D. B. Cousins, B. S. McCormick, B. R. Francis, J. Creeper, and N. E. Tweddle. 1991. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust. Vet. J. 68:286-290. [DOI] [PubMed] [Google Scholar]
- 29.Wood, P. R., L. A. Corner, J. S. Rothel, J. L. Ripper, T. Fifis, B. S. McCormick, B. Francis, L. Melville, K. Small, K. de Witte, et al. 1992. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet. Microbiol. 31:71-79. [DOI] [PubMed] [Google Scholar]
- 30.Wood, P. R., and S. L. Jones. 2001. BOVIGAM: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis (Edinb.) 81:147-155. [DOI] [PubMed] [Google Scholar]
- 31.Wood, P. R., and J. S. Rothel. 1994. In vitro immunodiagnostic assays for bovine tuberculosis. Vet. Microbiol. 40:125-135. [DOI] [PubMed] [Google Scholar]