Abstract
A multiplexed human papillomavirus (HPV) immunoassay has been developed for the detection of human IgG antibodies to HPV type 6, 11, 16, 18, 31, 33, 45, 52, and 58 virus-like particle (VLP) types in serum following natural infection or immunization with VLP-based vaccines. The VLP antigens were covalently conjugated to carboxyl Luminex microspheres (MS) using a carbodiimide chemistry. Antibody (Ab) titers were determined in a direct binding format, in which an IgG1- to -4-specific, phycoerythrin (PE)-labeled monoclonal antibody (MAb) (HP6043) binds to human serum IgG antibodies. Pooled serum samples from rhesus macaques immunized with a 9-valent VLP-based vaccine served as the reference standard. The overall specificity of the assay was >99%, and the linearity (parallelism) of the assay was <7% per 10-fold dilution. Total assay precision was <19% across 3 different VLP-microsphere lots, 2 secondary antibody lots, and 2 different operators over a period of 3 weeks. Three different methods were used to evaluate serostatus cutoffs (SCO): (i) a clinical sensitivity/specificity analysis based on “likely negative” and “likely positive” samples from nonvaccinees, (ii) stringent upper tolerance limits on samples from “likely negatives,” and (iii) stringent upper tolerance limits from the same “likely negative” sample set after VLP adsorption. Depending on the method to set the serostatus cutoff, the percentage of seropositive samples at the month 48 time point following vaccination with the HPV 6/11/16/18 quadrivalent vaccine ranged from 70% to 100%. This assay has proven useful for measuring the levels of serum antibody to the nine HPV VLPs following natural infection or administration of VLP-based vaccines.
Several different types of human papillomavirus (HPV) antibody (Ab) assays have been developed to monitor the immune status of individuals from epidemiology studies and vaccine clinical trials (22). In addition, there is interest in monitoring antibody levels at the population level to understand the impact of introducing HPV vaccines into the general vaccination schedule. These assays include pseudo-neutralization assays (21), competitive (epitope-specific) immunoassays (9, 20), and direct binding virus-like particle (VLP)-IgG assays (8). Neutralization assays that measure total immunoglobulin (IgM, IgA, and IgG) are often considered the “gold standard” for measuring the functional antibody response following vaccination or natural infection (27). However, since it is extremely difficult to grow HPV in vitro, researchers have developed pseudo-neutralization assays for VLP L1 and L2 and other surrogate assays to measure the immune response. In vitro pseudo-neutralization assays involve measuring the inhibition of HPV pseudovirion binding and infection of cultured cells and usually employ a reporter gene to score infection (2, 21). These pseudo-neutralization assays detect antibodies likely to be relevant to protection and cross-protection (23). However, they are complex and labor intensive, have a high coefficient of variation, and are not amenable to high-throughput testing.
As a surrogate for neutralization assays, competitive immunoassays utilizing neutralizing monoclonal antibodies (MAbs) that bind to conformational epitopes on L1 can be used to measure the type-specific antibody responses to neutralizing epitopes on the VLPs (8, 9, 20). These assays are sensitive and type specific and do not measure antibodies to denatured L1 protein. However, only a subset of the total anti-VLP antibodies are measured, as binding to only one neutralizing epitope is monitored. Therefore, the results in a competitive assay may underrepresent the total protective antibody levels.
In addition to pseudo-neutralization and competitive immunoassays, direct binding IgG assays can be used to measure antibody levels to the VLPs. These assays are sensitive, reproducible, simple to perform, and amenable to high-throughput testing. To further understand the immune response following HPV infection and vaccination with VLP-based vaccines, we have developed a direct binding IgG assay specific for the HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58 VLP types. This assay has been shown to be sensitive (with a ≥4-fold dynamic range), precise, robust, linear (parallel), and rugged and appears fit for its intended purpose of measuring antibodies following natural infection or vaccination with VLP-based vaccines.
MATERIALS AND METHODS
VLPs.
The VLPs used in the serology assay are the same final-manufacturing-product (FMP) VLPs that are used in Gardasil and in an experimental 9-valent HPV vaccine. Recombinant HPV L1 major capsid VLPs were independently produced intracellularly in a Saccharomyces cerevisiae expression system. The yeast cells were harvested and lysed, and the self-assembled L1 protein VLPs were purified chromatographically to >95% purity as previously described (3, 6, 16).
HPV VLP conjugation to Luminex MS.
Yeast-derived VLPs were coupled to a set of nine distinct fluorescent Luminex microspheres (MS) through a carbodiimide coupling procedure that has been described previously (9, 20). Improvements in the conjugation procedure from the original method were to conjugate all the VLPs at 100 μg per 2.0 × 108 microspheres and to store the VLP-microspheres in a bovine serum albumin (BSA)-free, blocking reagent (StabilGuard - Surmodics, Eden Prairie, MN). VLP-MS were enumerated on a Coulter counter (Beckman-Coulter, Miami, FL) and stored at a concentration of 1.8 × 106 VLP-microspheres/ml in storage buffer (20 mM histidine, 1% BSA, pH 6.2) in monoplex, at 4°C.
Reference and control sera.
A serum pool generated from six Rhesus macaques immunized with HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58 VLPs was used as a standard reference serum in the assay. The reference serum titer to HPV 16 was calibrated to the National Institute for Biologics Standards and Controls (NIBSC) 05/134 reference reagent. For the purposes of development of this assay, titers to HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58 are reported in milli-Merck units per milliliter (mMU/ml).
The high, medium, low, and negative controls used for this assay were comprised of HPV-negative normal human serum, as determined in the HPV 6, 11, 16, and 18 competitive Luminex immunoassay (cLIA), or samples comprised of high-, medium-, or low-titer serum from VLP-immunized rhesus macaques added to HPV-negative human serum.
Serum samples.
Human serum samples were purchased from commercial biobrokers or were retained samples from subjects enrolled in Merck clinical trials. Panels were specifically chosen to assess (i) assay precision and ruggedness (panel A [n = 52] and panel Rug [n = 4]), (ii) assay serostatus cutoff values (panel Neg [n = 64] and panels H6 through H58 [n = 288]), (iii) assay dilutability/linearity (panel Dil [n = 24]), (iv) analytical specificity (panel Spec [n = 24]); and (v) antibody persistence in HPV quadrivalent vaccinees (panel Vac [n = 96]) (Table 1). For assessing serostatus cutoffs, samples were chosen on the basis of historical titers obtained in a cLIA for HPV 6, 11, 16, and 18 or based on the individual's reported history of sex partners or HPV-related disease history. Panels A, Dil, and Spec were comprised of samples from individuals vaccinated with both the quadrivalent HPV (type 6/11/16/18) vaccine (Gardasil) and an experimental 5-valent vaccine against HPV 31, 33, 45, 52, and 58. The Vac panel was comprised of samples from the latest available postvaccination time point. Ninety-five of the 96 subjects whose serum samples comprised panel Vac received the full vaccination series. One sample came from a subject who received only one dose of the quadrivalent vaccine. Samples were stored at −70°C until use. All samples had undergone 2 freeze-thaw cycles and were heat inactivated at 56°C for 30 min. Serum samples were collected in accordance with institutional review board guidelines and informed consent.
TABLE 1.
Assay prevalidation/qualification serum panels
| Panel | No. of samples | Criteriaa | Purpose |
|---|---|---|---|
| A | 52 | ≥9 highs, ≥9 meds, ≥9 lows, and ≥9 negatives for each of the 9 HPV types | To assess precision and ruggedness of the HPV-9 IgG |
| Rug | 4 | Samples Rug1-4: 4 samples from individuals immunized with 9 VLPs | To assess precision and ruggedness of the HPV-9 IgG |
| Neg | 64 | 9- to 12-year-old and 16- to 26-year-old individuals who reported ≤1 lifetime sex partner and were in RMITT-2 for P013/015 | Primary sample set for setting the serostatus cutoff |
| H06 | 32 | HPV 6 PCR+ and sero+ samples* | To assess serostatus cutoff |
| H11 | 32 | HPV 11 PCR+ and sero+ samples* | To assess serostatus cutoff |
| H16 | 32 | HPV 16 PCR+ and sero+ samples* | To assess serostatus cutoff |
| H18 | 32 | HPV 18 PCR+ and sero+ samples* | To assess serostatus cutoff |
| H31 | 32 | HPV 31 sero+ samples* | To assess serostatus cutoff |
| H33 | 32 | HPV 33 PCR+ samples (day 1 sample that became a CIN 1 or worse MITT-3 case)* | To assess serostatus cutoff |
| H45 | 32 | HPV 45 sero+/PCR+ samples (14 sero+ and 18 PCR+ individuals that became a CIN 1 or worse MITT-3 cases)* | To assess serostatus cutoff |
| H52 | 32 | HPV 52 sero+ samples* | To assess serostatus cutoff |
| H58 | 32 | HPV 58 sero+ samples (29 sero+ and 3 PCR+ that became CIN 1 or worse MITT-3 cases)* | To assess serostatus cutoff |
| Vac | 96 | Samples from mo 48 vaccinees who received the quadrivalent vaccine | To assess the percentage of samples above the serostatus cutoff |
| Dil | 24 | Serum samples from humans vaccinated (mo 3) with the quadrivalent vaccine and an exptl 5-valent vaccine with a wide range of antibody titers as determined in a 9-plex competitive immunoassay | To assess the effect of dilution on antibody titers and to demonstrate the assay's ability to measure high-titer samples |
| Spec | 24 | Approximately 8 samples historically <LLOQ and ∼16 samples with a wide range of titers from low to high as determined in a 9-plex competitive immunoassay. Samples were from humans vaccinated with the quadrivalent vaccine and an exptl 5-valent vaccine | To assess the analytical specificity of the assay |
*, samples for serostatus cutoff evaluation are from baseline PCR-positive and/or historically seropositive (sero+) individuals in an HPV 4 cLIA (9) or an HPV 8 cLIA; CIN 1, cervical intraepithelial neoplasia 1; MITT, modified intent to treat; RMITT-2, restricted modified intent to treat (subjects who were seronegative to HPV 6, 11, 16, and 18 and PCR negative to HPV 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 prior to the first vaccination and who had a normal Pap test result prior to the first vaccination); MITT-3, all subjects who received at least one vaccination, regardless of their baseline serostatus or PCR status.
Multiplexed HPV 9 IgG immunoassay.
The IgG assay for HPV 6, 11, 16, 18, 31, 45, 52, and 58 was performed on a prewet, 96-well-microtiter filter plate (Millipore, Billerica, MA). A 12-point standard curve with the reference serum starting at a 1:100 dilution and subsequent 3-fold dilutions, 4 controls, and 16 samples tested at the 1:50 and 1:500 dilutions were added to the plate in duplicate. VLP-microspheres for types 6, 11, 16, 18, 31, 33, 45, 52, and 58 were added to each well at 5,000 VLP-microspheres per HPV type. The plates were covered with foil and incubated for 15 to 60 min. The filter plates were washed twice with PBS-1% Triton X-100 and resuspended in 7.5 μg/ml of a phycoerythrin (PE)-tagged mouse monoclonal antibody (clone HP6043; Biotrend, Destin, FL) that binds equally to human IgG1 to -4 (13). The plates were covered with foil and incubated for an additional 15 to 60 min. Following the second incubation period, the plates were washed twice and then analyzed on a BioPlex200 (Bio-Rad, Hercules, CA). A design-of-experiment (DOE) analysis of primary and secondary incubation times of 15 to 60 min showed that there was less than a 7% difference in antibody titers. The correlation of median fluorescent intensity (MFI) units to mMU/ml of VLP-specific IgG was made by serially diluting the reference serum and interpolating the MFI data through a 4-parameter curve-fitting algorithm.
Analytical specificity.
Specificity testing was performed by removing the HPV-type-specific antibody responses in human serum through preincubation of serum samples with HPV VLPs. Recombinant IsdB was also tested, as a nonspecific antigen derived from a Saccharomyces cerevisiae yeast expression similar to that employed in the production of VLPs. Serum samples were incubated with 2.5 μg of a single VLP-type or IsdB antigen, 22.5 μg of multiple VLP types (2.5 μg per HPV type), or PBS for 1 h, prior to performing the protocol as described above.
Statistical analysis.
For standard curve modeling and sample inclusion criteria, the following criteria were assessed. The goodness of fit of the standard curve was determined based on the root mean square error of the 12 points in the curve (1). The 50% effective dose (EC50) and slope were determined using a standard 4-PL curve model based on the method of O'Connell (19). For sample acceptance criteria, the variability or extravariability (EXV) between duplicate sample results was determined. EXV assessment of duplicate MFI readings is used as a quality control measure for an individual sample preparation to ensure that the duplicate readings are comparable. The 3σ upper EXV limit for duplicate MFI readings was established using the 10th root-transformed MFI readings. The upper 99/99 tolerance limits were used to determine the serostatus cutoffs for methods 2 and 3 using “likely negative” samples that were or were not adsorbed with VLPs (17). An upper 99/99 tolerance limit ensures with 99% confidence that at least 99% of the negative sample of the “likely negative” population will have a lower Ab titer than the serostatus cutoff.
Prevalidation/qualification parameters. (i) Precision.
Variability estimates for intra-, inter-, and total precision were obtained using samples from the ruggedness panel and the control samples. Intra-assay precision was estimated using the variability across the four plates (P) within each run (R) (σ̂P(R)2) and the sample (S) by plate interaction (σ̂P(R) × S2). To determine interassay variability, estimates of run-to-run variability (σ̂R2) and sample-by-run variability (σ̂S × R2) were combined. Variability estimates were obtained on the natural log-transformed titers using the mixed procedure in SAS (SAS Institute, Cary, NC). An estimate of the total assay precision (percent RSD) was calculated as 100%(e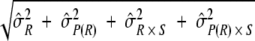 − 1). A statistically meaningful fold rise in test sample titer was calculated as e3 ×
− 1). A statistically meaningful fold rise in test sample titer was calculated as e3 × 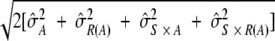 .
.
(ii) Standard curve modeling.
The reference standard dilution series was modeled using the four-parameter logistic function (19). The formula [f(xi;θ̂)](9/5) was used as the weight in the iterative nonlinear fit, indicating that the variance in the MFI signal is proportional to the mean level of the MFI signal raised to the nine-fifth power. Test sample concentrations were interpolated from the fitted standard curves.
(iii) Limit of detection and limits of quantitation.
For each of the 45 standard curves generated, the limit of detection (LOD) and limits of quantitation (LOQs) were determined using the methods described by O'Connell et al. (19).
(iv) Linearity/dilutability.
For the linearity (dilutability) experiments, the overall dilution effect per 10-fold dilution was calculated as follows: percent bias per 10-fold dilution = 100% × 10b − 1, where b represents the slope from the linear regression fit of the log10-transformed dilution-corrected antibody values against the log10-transformed dilution factor. Slopes for each sample were pooled and estimated using the mixed procedure in SAS.
(v) Specificity.
The percent specificity of the assay to either the VLPs or an irrelevant, yeast-derived antigen, Staphylococcus aureus IsdB, was estimated by the following: 1 − (VLPtreated − LLOQ/VLPmock − LLOQ) × 100 or [1 − (IsdBtreated − LLOQ/IsdBmock − LLOQ)] × 100, respectively, where VLPmock and IsdBmock denote the antibody concentration from serum mock adsorbed with PBS and LLOQ is the lower LOQ. VLPtreated and IsdBtreated denote the antibody concentration from samples preadsorbed with either VLPs or IsdB antigens, respectively.
RESULTS
Reference standard.
Prior to the start of assay development, a multivalent reference serum was created by pooling serum samples from six rhesus macaques that had been immunized at day 0, week 8, and week 24 with 2 μg each of VLPs 6, 11, 16, 18, 31, 33, 45, 52, and 58 adsorbed with amorphous aluminum hydroxyphosphate sulfate adjuvant (AAHS). The serum samples were collected at week 28 and pooled. The reference serum was calibrated to an existing reference serum for HPV 6, 11, 16 and 18, and the new types 31, 33, 45, 52, and 58 were cross-standardized to type 11 in 15 runs based on the method of Concepcion and Frasch (5). The potencies of the reference serum for HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58 were determined to be 3,817, 2,889, 23,061, 5,271, 3,942, 2,672, 1,489, 1,274, and 2,263 mMU/ml, respectively. For HPV type 16, the World Health Organization (WHO) has developed a reference reagent (NIBSC 05/134) from the serum samples of 3 individuals that have nonvaccine-induced titers to HPV 16 (11). The lyophilized standard has an assigned concentration of 10 IU per ml when reconstituted in 0.5 ml of distilled water (dH2O) (11). The rhesus reference serum was calibrated to the NIBSC 05/134 reference reagent for HPV 16 in 15 runs and determined to have a potency of 1,861 IU/ml.
Ruggedness.
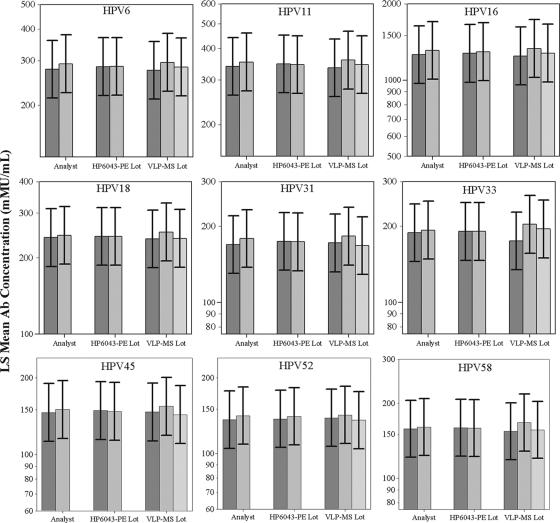
The objective of this assay development project was to develop and evaluate a multiplexed HPV 9 IgG assay that could be used to support sero-epidemiology studies and vaccine clinical trials that will span multiple years. Therefore, we chose to evaluate the assay for ruggedness, analytical sensitivity, analytical specificity, precision, and linearity (parallelism). Separately, we also evaluated setting serostatus cutoff levels using distinct panels of samples from individuals at low or high risk for HPV infection and from individuals who had been vaccinated with VLP-based vaccines. Since multiple reagent lots and operator changes are likely to occur during the course of a clinical trial, we first evaluated the ruggedness of the assay to operators, VLP-microsphere lots, and secondary detection antibody lots. A 1.3-fold ruggedness design goal was set based on past experience, as a 30% difference between factors would suggest that we had developed a robust VLP-microsphere manufacturing process and a robust assay that could be transferred to a clinical testing environment. Two operators tested a panel of 56 samples from panels A and Rug (Table 1) using 2 secondary detection antibody lots and 3 VLP-microsphere lots (2 × 2 × 3 = 12) in a full factorial design over a 3-week period. The panel of 56 samples was chosen to span the range of negative, low-, medium-, and high-titer samples that would be collected in a vaccine clinical trial. The design goal of less than a 1.3-fold difference between reagent lots was met, with minimal differences observed between VLP-microsphere lots, HP6043 detection antibody lots, and different operators (Fig. 1). Overall, the differences in antibody titers were less than 2.5%, 5.0%, and 15.0% between HP6043 lots, operators, and VLP-microsphere lots, respectively.
FIG. 1.
Ruggedness of the HPV 9 IgG serology assay to VLP-microsphere lots, operators, and secondary detection antibody lots. A panel of 56 samples was tested over a period of 3 weeks by 2 analysts using 3 VLP-microsphere lots and 2 different mouse anti-human IgG lots (HP6043). The least-squares (LS) mean antibody (Ab) concentrations (mMU/ml) of the 3 conditions were analyzed for the ruggedness of the assay. Error bars represent the ±1.3-fold design goal. All conditions were within a ±1.3-fold difference, indicating that the assay should be acceptably rugged for use in clinical testing.
Sensitivity and dynamic range.
The 45 standard curves performed within the qualification were used to determine the overall LOD and LOQs for the 9 HPV types. The calculated 95th percentile of all LODs from the 45 standard curves for the 9 HPV types ranged from 0.8 mMU/ml for type 45 to 4.6 mMU/ml for type 16 (Table 2). To approximate the sensitivity of the assay in ng/ml, we also tested the HPV 16.V5 antibody (25) in the assay. Recognizing that a mouse-specific, rather than a human-specific, secondary antibody might cause slight differences in determinations of the sensitivity of the assay, the overall sensitivity of the assay was approximated to be 50 ng/ml (data not shown). The lower and upper LOQs (ULOQs) for each plate were determined by evaluating the variability over the range of MFIs corresponding to the standard curve concentrations. Additionally, the percent relative error and its associated 95% confidence limits at each calibration concentration were determined by the method of Findlay et al. (12). The dilution-corrected LLOQs ranged from 1.5 mMU/ml for types 11, 45, and 52 and the ULOQs ranged from 2,100 mMU/ml for type 52 to 20,850 mMU/ml for type 16 (Table 2). In all cases, the range of quantitation was determined to be greater than 1,000-fold for all HPV types (Table 2).
TABLE 2.
Assay qualification summary
| Assay characteristic | HPV typea |
Design goal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 11 | 16 | 18 | 31 | 33 | 45 | 52 | 58 | ||
| RMSE upper limits (MFI) | ||||||||||
| 97.7% limit | ≤0.30 | ≤0.30 | ≤0.30 | ≤0.30 | ≤0.30 | ≤0.30 | ≤0.30 | ≤0.30 | ≤0.30 | NA |
| 99.9% limit | <0.37 | <0.37 | <0.37 | <0.37 | <0.37 | <0.37 | <0.37 | <0.37 | <0.37 | NA |
| EC50 limits (mMU/ml) | ||||||||||
| 2σ | 4.9, 18.6 | 3.3, 14.0 | 13, 54.3 | 7.6, 28.4 | 3.7, 14.2 | 4.1, 18.4 | 2.0, 7.8 | 1.8, 7.5 | 3.4, 12.5 | NA |
| 3σ | 3.5, 26 | 2.3, 20.1 | 9.1, 77.7 | 5.5, 39.4 | 2.7, 19.9 | 2.8, 26.7 | 1.4, 11.0 | 1.2, 10.7 | 2.4, 17.3 | NA |
| Slope limits (MFI/mMU/ml) | ||||||||||
| 2σ | 0.90, 0.99 | 0.90, 0.98 | 0.89, 0.97 | 0.91, 1.00 | 0.90, 0.98 | 0.90, 1.00 | 0.91, 1.00 | 0.87, 0.96 | 0.91, 0.98 | NA |
| 3σ | 0.88, 1.01 | 0.88, 1.00 | 0.87, 0.99 | 0.89, 1.02 | 0.88, 1.00 | 0.88, 1.03 | 0.89, 1.02 | 0.86, 0.98 | 0.89, 1.00 | NA |
| Control 1 limits (mMU/ml) | ||||||||||
| 2σ | 243, 425 | 185, 323 | 1422, 2487 | 335, 587 | 254, 445 | 171, 300 | 98, 171 | 90, 157 | 150, 262 | NA |
| 3σ | 211, 488 | 161, 372 | 1237, 2860 | 292, 675 | 221, 511 | 149, 345 | 85, 197 | 78, 180 | 130, 301 | NA |
| Control 2 limits (mMU/ml) | ||||||||||
| 2σ | 27, 46 | 19, 34 | 156, 273 | 34, 60 | 27, 47 | 18, 31 | 12, 21 | 11, 20 | 15, 27 | NA |
| 3σ | 23, 53 | 17, 39 | 136, 314 | 30, 69 | 24, 54 | 16, 36 | 10, 24 | 10, 23 | 13, 31 | NA |
| Control 3 limits (mMU/ml) | ||||||||||
| 2σ | 13, 23 | 10, 17 | 71, 125 | 16, 28 | 13, 22 | ≤14.3 | 5, 9 | ≤7.0 | 7, 13 | NA |
| 3σ | 12, 27 | 8, 19 | 62, 143 | 14, 32 | 11, 25 | ≤16.4 | 4, 10 | ≤8.1 | 6, 14 | NA |
| Control 4 limits (mMU/ml) | ||||||||||
| 2σ | ≤4.0 | ≤2.6 | ≤11.8 | ≤5.0 | ≤2.6 | ≤3.5 | ≤2.6 | ≤2.6 | ≤3.6 | NA |
| 3σ | ≤4.6 | ≤3.0 | ≤13.6 | ≤5.0 | ≤3.0 | ≤3.5 | ≤3.0 | ≤3.0 | ≤4.1 | NA |
| Limit of detection (dilution-corrected; mMU/ml) | 1.2 | 1.0 | 4.6 | 2.6 | 1.1 | 3.2 | 0.8 | 1.2 | 1.6 | ≤LLOQ |
| Limits of quantitation (dilution-corrected; mMU/ml) | 3.0, 6,350 | 1.5, 4,800 | 8.0, 20,850 | 5.0, 8,800 | 2.0, 6,550 | 3.5, 4,450 | 1.5, 2,500 | 1.5, 2,100 | 2.0, 3,750 | LLOQ < cutoff, >10-fold range |
| Precision (% RSD) | ||||||||||
| Intra | 3.5 | 4.7 | 3.7 | 4.2 | 2.3 | 4.5 | 2.8 | 2.8 | 3.6 | NA |
| Inter | 11.3 | 10.5 | 10.1 | 10.2 | 17.0 | 14.9 | 11.2 | 9.7 | 12.8 | NA |
| Total | 11.9 | 11.6 | 10.8 | 11.1 | 17.2 | 15.7 | 11.6 | 10.2 | 13.4 | ≤25 |
| Human sample dilution effect % bias per 10-fold dilution (%) | ||||||||||
| 1:50 to 1:500 | 23.7 | 35.6 | 18.9 | 16.1 | 14.5 | 26.3 | 12.4 | 18.8 | 17.0 | <100 |
| 1:500 to 1:5,000 | 14.0 | 16.3 | 5.5 | −5.3 | 1.2 | 5.5 | −3.0 | 6.1 | −2.4 | <100 |
| 1:5,000 to 1:50,000 | −12.0 | −7.4 | −14.1 | −6.3 | −18.7 | −9.6 | −17.7 | −9.4 | −15.3 | <100 |
| Specificity for samples with HPV L1 VLP titers >10 mMU/ml (%) | ||||||||||
| Type-specific VLP specificity | 99.8 | 99.8 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 99.8 | ≥85 |
| Nonspecificity IsdB antigen | −1.0 | −0.7 | −0.2 | −1.1 | −2.1 | −2.5 | −1.3 | −4.2 | −1.6 | ≤15 |
EXV limit of 10 or 0.09 for untransformed and 10th root-transformed MFIs.
Precision.
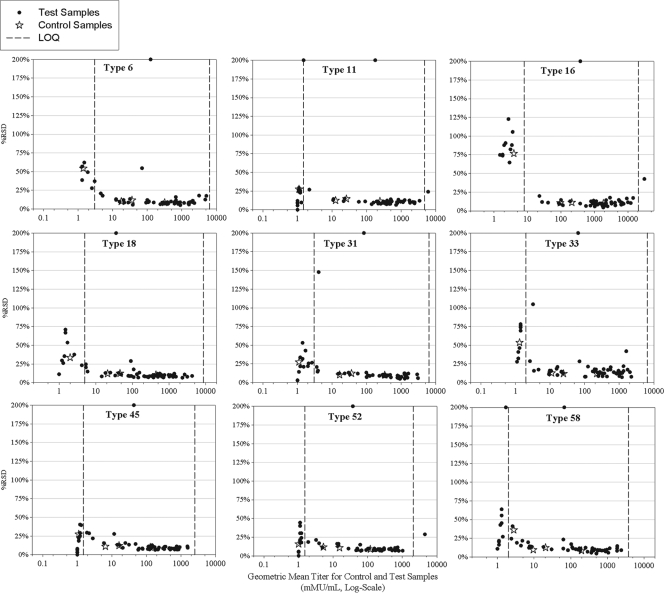
The intra-, inter-, and total precision of the assay was determined using the 56 samples tested in the ruggedness portion of the assay qualification (Table 1). The intra-assay precision was a <10% relative standard deviation (RSD) and interassay precision was a <18% RSD across all 9 HPV types. Total assay precision for a mix of serum samples of low, medium, and high HPV titer was determined to be a <18% RSD for all 9 HPV types, including the effect of multiple operators and reagent lots (Table 2 and Fig. 2). Based on these values, a ≥2-fold rise in antibody titer was determined to be statistically meaningful.
FIG. 2.
Precision of the HPV-9 IgG assay. Assay precision was determined by testing 56 samples over a 3-week period by two analysts using three VLP-microsphere lots and two HP6043 secondary antibody lots. The intra-assay precision was <10% relative standard deviation (RSD). The total assay precision for a mix of low, medium, and high HPV titer serum samples was determined to be <18% RSD for all nine HPV types across the dynamic range of the assay. Vertical dashed lines represent the limits of quantitation.
Dilutability (linearity).
Dilutability, also referred to as linearity or parallelism, is an attribute of a biological assay that demonstrates that a sample can be serially diluted and yield equivalent dilution-corrected values throughout the series. Dilutability of the HPV 9 IgG assay was evaluated by testing 24 samples at 1:50, 1:500, and 1:5,000 dilutions from individuals that had been vaccinated with the quadrivalent vaccine and an experimental 5-valent vaccine for HPV 31, 33, 45, 52, and 58. Overall, the assay exhibited a minimal dilution effect of <1.40-fold between the 1:50 and 1:500 dilutions and a dilution effect of 1.17-fold between the 1:500 and 1:5,000 dilutions (Table 2). All dilution effect estimates were within the design goal of a <2-fold difference per 10-fold dilution.
Analytical specificity.
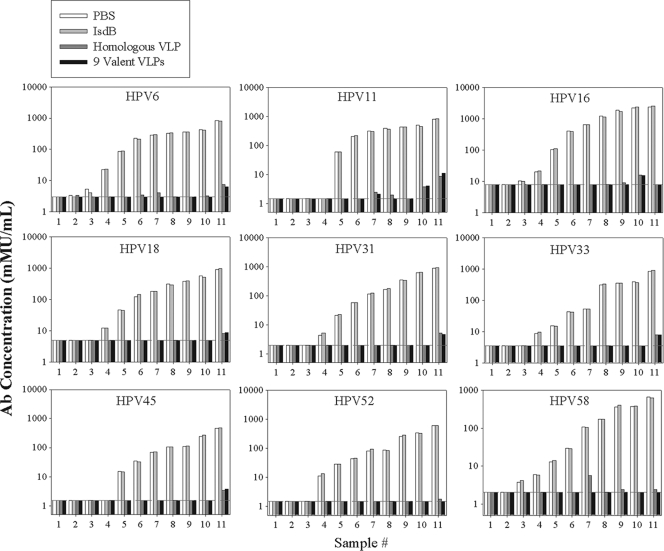
Analytical specificity is the ability of an assay to report only the analyte that it claims to measure and not other substances in the sample (10). The analytical specificity was tested by preadsorbing serum from individuals vaccinated with both the quadrivalent vaccine and a 5-valent vaccine for VLPs 31, 33, 45, 52, and 58 or HPV-negative human serum with HPV VLPs or with a nonspecific, yeast-derived Staphylococcus aureus antigen (IsdB). Serum that was historically HPV positive and had an antibody concentration of >10 mMU/ml for one or more HPV types showed a >99% decrease in antibody concentration when preadsorbed with one or more VLP types corresponding to the type for which that sample was positive (Fig. 3). Following preadsorption, antibody levels in HPV-positive samples were comparable to the background signal measured in HPV-negative samples. The nonspecific IsdB yeast antigen had no significant effect on measured antibody concentration. The analytical specificity for the HPV 9 IgG assay met the design goal of ≥85% for serum preadsorbed with type-specific VLPs and ≤15% for the nonspecific IsdB antigen.
FIG. 3.
Analytical specificity and nonspecificity of the HPV 9 IgG assay. A panel of 11 samples with undetectable or low antibodies (samples 1 to 3), medium antibody levels (4 to 7), and high antibody levels (8 to 11) were tested in the HPV 9 IgG assay following mock adsorption with PBS, adsorption with 2.5 μg of a nonspecific, yeast-derived antigen, IsdB, adsorption with 2.5 μg of the homologous VLP, or adsorption with 22.5 μg of a cocktail of 9 VLPs. The dashed horizontal line in each figure represents the lower limit of quantitation. Results show that the assay is >99% specific for VLP-specific antibodies for samples with antibody titer values of >10 mMU/ml.
Serostatus cutoffs.
Several different sample panels were created to determine the serostatus cutoffs (Table 1). The samples were chosen from nonvaccinees that were unlikely to have been exposed to HPV, nonvaccinees with a high likelihood of exposure to HPVs or documented HPV disease, and females who had been vaccinated with the quadrivalent vaccine. The likely negative serum samples (panel Neg) were either from individuals 9 to 12 years old or from females 16 to 26 years of age who reported 0 or 1 lifetime sex partner and, therefore, were likely to be at low risk of HPV infection. These samples have historically tested negative in the cLIA for HPV 6, 11, 16, and 18 or an experimental 8-plex cLIA for HPV 6, 11, 16, 18, 31, 45, 52, and 58 and were PCR negative for type 33. Sample panels designated H6, H11, H16, H18, H31, H45, H52, and H58 were determined to be both seropositive in the 4-plex or 8-plex HPV cLIA and HPV DNA positive by a multiplex PCR assay (15) for at least the HPV type for their respective category. Serum samples for panel H33 came from individuals who were PCR positive for type 33. Since HPV infection does not produce a robust immune response and only 60% of individuals seroconvert following infection (4), we tested the serostatus cutoff panels at the 1:50 and 1:500 dilutions.
The serostatus cutoffs were evaluated using three different methods. The first method set the serostatus cutoffs by a clinical sensitivity/specificity algorithm similar to a receiver operator control plot analysis (18). This method sought to maximize the number of “likely negative” samples testing negative and the number of “likely positive” nonvaccinee samples testing positive and is similar to what was performed historically to set the serostatus cutoffs for HPV types 6, 11, 16, and 18 in a competitive immunoassay (9). The second method established the serostatus cutoffs by determining the 99/99 upper tolerance limit of a panel of “likely negative” samples (Fig. 4). Lastly, the third method established the serostatus cutoffs by determining the 99/99 upper tolerance limit of the same sample panel of “likely negative” samples after the samples were preadsorbed with all nine HPV VLP types (Fig. 4). The third method is similar to what has been reported to set serostatus cutoffs for other HPV serology assays (14) but not the 4-plex cLIA.
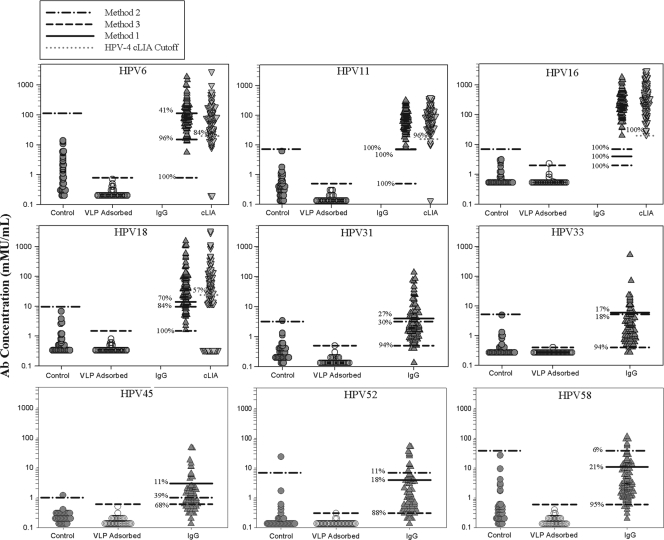
FIG. 4.
Antibody level and serostatus of control “likely negative” serum samples and month 48 serum samples from quadrivalent vaccinees. The control and VLP-adsorbed Ab titers are from 32 samples from individuals with 0 or 1 lifetime sex partner. The IgG and cLIA Ab titers are from month 48 samples from 96 females that received the quadrivalent vaccine. The samples were tested at a 1:50 dilution, and data are reported in mMU/ml. The three horizontal lines in the IgG column of data represent the serostatus cutoff values determined by the three different methods. Method 1, maximized number of negative and positive samples in the “likely negative” and “likely positive” groups, respectively; method 2, the 99/99 upper tolerance limit on the likely negative population; method 3, the 99/99 upper tolerance limit on the VLP-adsorbed likely negative samples. The horizontal line in the cLIA data column represents the historical cLIA serostatus cutoff values of 20, 16, 20, and 24 mMU/ml for types 6, 11, 16, and 18, respectively. The percentages represent the percentage of seropositive samples for each type using the different serostatus cutoffs.
The three different methods were used to set provisional serostatus cutoffs for the 9 HPV types (Table 3). The serostatus cutoffs ranged from 0.3 mMU/ml for HPV 52 using method 3 at the 1:50 dilution, which produced the lowest serostatus cutoff values, to 114 mMU/ml for HPV 6 using method 2 at the 1:50 dilution, which produced the highest serostatus cutoff values. The high serostatus cutoff value for type 6 was due to the higher-than-expected number of samples from the “likely negative” group producing an antibody value. The results for method 1 were in general between those of methods 2 and 3 and ranged from 3 to 15 mMU/ml for the nine HPV types. Testing the samples at the two dilutions gave similar serostatus cutoff values for the 9 HPV types by the use of methods 1 and 2. Setting the serostatus cutoffs at the 1:50 dilution using the VLP-adsorption method gave approximately 5-fold lower serostatus cutoffs due to the increased sensitivity of the assay at the 1:50 dilution (Table 3).
TABLE 3.
Provisional serostatus cutoffs
| Method | Dilution | Cutoff (mMU/ml)for HPV type: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 11 | 16 | 18 | 31 | 33 | 45 | 52 | 58 | ||
| 1 | 1:50 | 15.0 | 7.0 | 4.0 | 7.0 | 4.0 | 6.0 | 3.0 | 4.0 | 11.0 |
| 1:500 | 18.0 | 7.0 | 10.0 | 8.0 | 5.0 | 6.0 | 4.0 | 8.0 | 11.0 | |
| 2 | 1:50 | 114.0 | 7.2 | 7.0 | 9.7 | 3.2 | 5.2 | 1.0 | 7.0 | 39.0 |
| 1:500 | 38.0 | 6.3 | 11.0 | 9.2 | 4.9 | 5.1 | 2.5 | 13.0 | 24.0 | |
| 3 | 1:50 | 0.8 | 0.5 | 2.0 | 1.5 | 0.5 | 0.4 | 0.6 | 0.3 | 0.6 |
| 1:500 | 3.4 | 2.2 | 7.8 | 4.7 | 2.6 | 2.9 | 2.3 | 1.8 | 2.9 | |
Based on the serostatus cutoffs set using the panel of “likely negative” and “likely positive” samples from nonvaccinees, we then applied the three different serostatus cutoff values to a panel of 96 randomly selected month 48 samples from 16- to 26-year-old females who had been vaccinated with the quadrivalent 6/11/16/18 vaccine (Fig. 4). The samples were selected because they represented the latest available postvaccination time point for evaluating serostatus in late postvaccination samples. A total of 95 of the 96 samples were from subjects who had received all 3 doses of the quadrivalent vaccine (day 1, month 2, and month 6), whereas 1 sample came from a subject who received only 1 dose of the vaccine. A number of subjects had an HPV 6, 11, 16, or 18 PCR-positive result from a cervical sample collected on the day of the first vaccination or at month 7. These included seven samples for type 6, one for type 11, nine for type 16, and five for type 18. The samples were tested at a 1:50 dilution, and the percentage of seropositive samples was determined using each method (Fig. 4). For comparison, historical data for these samples for HPV 6, 11, 16, and 18 from the competitive Luminex immunoassay and the respective serostatus cutoffs of 20, 16, 20, and 24 mMU/ml for types 6, 11, 16, and 18, respectively (9) are also presented in Fig. 4. The results show that some samples from the “likely negative” panel exhibited some measurable antibody that could be adsorbed with all nine VLPs. The data also show that using serostatus cutoffs based on the “likely negative” samples that had been preadsorbed with the VLPs resulted in 100% seropositivity for month 48 vaccinee samples for types 6, 11, 16, and 18. Importantly, antibody titers from the 50 samples with the lowest titers could be adsorbed out by preincubation with VLPs (data not shown). There were also considerable levels of cross-reactive antibodies elicited by the quadrivalent vaccine that could be measured to related types 31, 33, 45, 52, and 58 (Fig. 4). In summary, using the HPV VLP-specific, total IgG assay and method 3, serostatus cutoff showed that immunization with the quadrivalent vaccine elicited high levels of VLP type-specific IgG that could be measured in 100% of individuals 4 years postvaccination.
DISCUSSION
In this report we describe the development and evaluation of a multiplex IgG serology assay specific for HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58 L1 VLPs for the measurement of VLP-specific IgG serum antibodies to the nine HPV types. To date, the assay has proven useful for measuring VLP-specific IgG antibodies in serum samples from individuals who were naturally exposed to HPV or vaccinated with VLP-based vaccines. An improvement from the first-generation VLP-microsphere-based Luminex assays was to standardize the coating concentration to 100 μg of VLP per 2.0 × 108 microspheres across the nine HPV types and to replace a BSA-based blocking buffer with a non-BSA-based blocking buffer, StabilGuard. The use of StabilGuard improved both the lot-to-lot consistency of the VLP-microspheres and decreased nonspecific IgG binding to the VLP-microspheres, as it has been shown that there can be both BSA-specific and microsphere-specific IgG in serum (26).
We observed an approximately 5-fold improvement in the analytical sensitivity of the HPV 9 IgG assay for types 6, 11, 16, and 18 compared to our historical competitive assay. Presumably this is due to the assay measuring IgG antibodies binding anywhere on the entire VLP rather than restricted to a single neutralizing epitope. The overall analytical specificity of the assay was good, with more than a 99% reduction in the detection of VLP type-specific antibodies upon preadsorption of the sera with type-specific VLPs (Fig. 3 and Table 2). There was little or no cross-reactivity (<5%) to another yeast-derived antigen, IsdB (Fig. 3 and Table 2). The good specificity of the assay also allowed us to examine using VLP-specific, antibody-depleted sera as the negative control for setting a serostatus cutoff.
Another important observation from this study was the level of cross-reactive antibodies induced to types 31, 33, 45, 52, and 58 following vaccination with the quadrivalent vaccine (Fig. 4). Although antibody levels were approximately 10-fold lower than the quadrivalent vaccine types, the cross-reactive antibodies were measurable in 94%, 94%, 68%, 88%, and 95% of the subjects for types 31, 33, 45, 52, and 58, respectively. The multiplexed VLP-IgG assay format may prove useful in helping to understand the role of cross-reactive antibodies in both natural history and vaccine clinical studies.
Currently, there are no international correlates of protection or serostatus cutoffs for HPV serology. The U.S. Food and Drug Administration, European Union Committee for Medicinal Products for Human Use, and the World Health Organization HPV Laboratory Network do not have recommended guidelines on a method to establish a serostatus cutoff. Thus, it is important for each laboratory to describe the method and rationale used to set serostatus cutoffs. In this study, we evaluated 3 different methods to set the serostatus cutoffs. Method 1 used a modified receiver operator control (18) approach to maximize the number of “likely negative” samples testing negative and the number of “likely positive” samples testing positive in the assay. Method 2 used 99/99 upper tolerance limits (17) based on the “likely negative” samples to set the serostatus cutoffs, and method 3 used the same 99/99 upper tolerance limits based on the same panel of “likely negatives” that had been immunodepleted with the 9 VLPs. The 99/99 upper-tolerance limit is a more stringent approach than using 3 standard deviations above background, as there is 99% confidence that at least 99% of the likely negative samples test negative. Interestingly, there were a number of samples from the “likely negative” group that showed low levels of antibodies in the assay that could be immunodepleted with the VLPs. This was most pronounced for type 6, which had approximately 30% of the samples with measurable antibody levels. There are at least three possible explanations for this observation. One, these are true positives, as our “likely negative” panel included individuals who had reported 1 sex partner; two, HPV 6 is more easily transmitted via hand-to-genital contact without intercourse; and three, the total VLP-IgG assay measures cross-reactive antibodies induced by other related HPVs. Future studies are planned to test a larger panel of samples from adolescents and from individuals who reported never having had intercourse to better understand sero-reactivity in the total IgG format. Depending on the scientific question, it may be practical to use different cutoff values for epidemiology studies and inclusion criteria for enrolling subjects in vaccine efficacy clinical trials versus monitoring the long-term presence of antibodies following HPV vaccination.
The results presented herein show that 100% of women were seropositive (using method 3) for HPV 6, 11, 16, and 18 at 48 months following vaccination with the quadrivalent vaccine (Fig. 4). This included one subject in the randomly selected set that had received only one of the three recommended vaccinations. A number of the randomly chosen samples came from subjects that were HPV PCR positive and HPV seronegative at day 0 or month 7. In general, these subjects had higher month 48 antibody titers for the relevant type than those that were HPV PCR negative at day 0 or month 7. Presumably, this is due to the quadrivalent vaccine boosting preexisting immunity from an HPV exposure and is consistent with a previous report showing that vaccinating an HPV-seropositive individual can result in an anamnestic immune response (24). Results using the type-specific total IgG assay supplement those reported previously using the competitive immunoassay in which up to 40% of month 48 samples may test seronegative using a serostatus cutoff approach similar to method 1. The competitive immunoassay is likely to underestimate the total antibody response to the VLPs, as it measures antibodies to a single, type-specific, neutralizing epitope. Although an immune correlate of protection has not been established, it is known that very low levels of HPV antibody are able to neutralize HPV 16. The 50% inhibitory concentration ranged from 1.9 picomolar to 5.4 nanomolar for 3 monoclonal antibodies to HPV 16, including the H16.V5 antibody (7) used in this assay.
In summary, this multiplex direct binding VLP-IgG assay has been shown to be sensitive, specific, rugged to analysts, VLP-microsphere lots, and secondary antibody lots, linear (parallel), and precise. The assay appears fit for its intended purpose of measuring HPV antibodies following natural infection or vaccination with VLP-based vaccines. Future work will include development of 9-valent human reference sera and a formal validation with a larger panel of samples from individuals with a history of 0 sex partners, samples from individuals who received a 9-valent vaccine containing each of the HPV types studied here, and samples from clinically confirmed cervical cancer subjects. In addition, we plan to perform a formal comparison between the competitive immunoassay format and the IgG assay format to determine concordance between the two methods and to determine the level of analytical sensitivity in a single epitope versus a whole VLP assay.
Acknowledgments
We thank Scott Vuocolo, Paul Liberator, and Steven Lobel for critical review of the manuscript.
Footnotes
Published ahead of print on 17 March 2010.
REFERENCES
- 1.Brady, J. F. 2006. Mathematical aspects of immunoassays, p. 249-270. In J. M. Van Emon (ed.), Immunoassay and other bioanalytical techniques. CRC Press, Boca Raton, FL.
- 2.Buck, C. B., D. V. Pastrana, D. R. Lowy, and J. T. Schiller. 2005. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 119:445-462. [DOI] [PubMed] [Google Scholar]
- 3.Capen, R., M. L. Shank-Retzlaff, H. Sings, M. Esser, C. Sattler, M. Washabaugh, and R. Sitrin. 2007. Establishing potency specifications for antigen vaccines. BioProcess Int. 5:30-43. [Google Scholar]
- 4.Carter, J. J., L. A. Koutsky, J. P. Hughes, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 2000. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 181:1911-1919. [DOI] [PubMed] [Google Scholar]
- 5.Concepcion, N., and C. E. Frasch. 1998. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin. Diagn. Lab Immunol. 5:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook, J. C., J. G. Joyce, H. A. George, L. D. Schultz, W. M. Hurni, K. U. Jansen, R. W. Hepler, C. Ip, R. S. Lowe, P. M. Keller, and E. D. Lehman. 1999. Purification of virus-like particles of recombinant human papillomavirus type 11 major capsid protein L1 from Saccharomyces cerevisiae. Protein Expr. Purif. 17:477-484. [DOI] [PubMed] [Google Scholar]
- 7.Day, P. M., R. Gambhira, R. B. Roden, D. R. Lowy, and J. T. Schiller. 2008. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies. J. Virol. 82:4638-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dessy, F. J., S. L. Giannini, C. A. Bougelet, T. J. Kemp, M. P. David, S. M. Poncelet, L. A. Pinto, and M. A. Wettendorff. 2008. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum. Vaccin. 4:425-434. [DOI] [PubMed] [Google Scholar]
- 9.Dias, D., J. Van Doren, S. Schlottmann, S. Kelly, D. Puchalski, W. Ruiz, P. Boerckel, J. Kessler, J. M. Antonello, T. Green, M. Brown, J. Smith, N. Chirmule, E. Barr, K. U. Jansen, and M. T. Esser. 2005. Optimization and validation of a multiplexed Luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin. Diagn. Lab Immunol. 12:959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA. 2001. Guidance for industry: bioanalytical method validation. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Rockville, MD.
- 11.Ferguson, M., A. Heath, S. Johnes, S. Pagliusi, and J. Dillner. 2006. Results of the first WHO international collaborative study on the standardization of the detection of antibodies to human papillomaviruses. Int. J. Cancer 118:1508-1514. [DOI] [PubMed] [Google Scholar]
- 12.Findlay, J. W., W. C. Smith, J. W. Lee, G. D. Nordblom, I. Das, B. S. DeSilva, M. N. Khan, and R. R. Bowsher. 2000. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J. Pharm. Biomed. Anal. 21:1249-1273. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton, R. G. 1990. Engineered human antibodies as immunologic quality control reagents. Ann. Biol. Clin. (Paris) 48:473-477. [PubMed] [Google Scholar]
- 14.Harper, D. M., E. L. Franco, C. M. Wheeler, A. B. Moscicki, B. Romanowski, C. M. Roteli-Martins, D. Jenkins, A. Schuind, S. A. Costa Clemens, and G. Dubin. 2006. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 367:1247-1255. [DOI] [PubMed] [Google Scholar]
- 15.Iftner, T., L. Germ, R. Swoyer, S. K. Kjaer, J. G. Breugelmans, C. Munk, F. Stubenrauch, J. Antonello, J. T. Bryan, and F. J. Taddeo. 2009. Study comparing human papillomavirus (HPV) real-time multiplex PCR and hybrid capture II INNO-LiPA v2 HPV genotyping PCR assays J. Clin. Microbiol. 47:2106-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mach, H., D. B. Volkin, R. D. Troutman, B. Wang, Z. Luo, K. U. Jansen, and L. Shi. 2006. Disassembly and reassembly of yeast-derived recombinant human papillomavirus virus-like particles (HPV VLPs). J. Pharm. Sci. 95:2195-2206. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery, D. C. (ed.). 2001. Introduction to statistical quality control, 4th ed. John Wiley & Sons, New York, NY.
- 18.NCCLS. 2001. Assessment of the clinical accuracy of laboratory tests using receiver operating characteristic (ROC) plots. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 19.O'Connell, M., B. Belanger, and P. Haaland. 1992. The four parameter logistic model for calibration and assay development, p. 180-185. In American Statistical Association Proceedings of the Biopharmaceutical Section. American Statistical Association, Alexandria, VA.
- 20.Opalka, D., C. E. Lachman, S. A. MacMullen, K. U. Jansen, J. F. Smith, N. Chirmule, and M. T. Esser. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed Luminex assay. Clin. Diagn. Lab Immunol. 10:108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastrana, D. V., C. B. Buck, Y. Y. Pang, C. D. Thompson, P. E. Castle, P. C. FitzGerald, S. Kruger Kjaer, D. R. Lowy, and J. T. Schiller. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321:205-216. [DOI] [PubMed] [Google Scholar]
- 22.Schiller, J. T., and D. R. Lowy. 2009. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J. Infect. Dis. 200:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, J. F., M. Brownlow, M. Brown, R. Kowalski, M. T. Esser, W. Ruiz, E. Barr, D. R. Brown, and J. T. Bryan. 2007. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum. Vaccin. 3:109-115. [DOI] [PubMed] [Google Scholar]
- 24.Villa, L. L., K. A. Ault, A. R. Giuliano, R. L. Costa, C. A. Petta, R. P. Andrade, D. R. Brown, A. Ferenczy, D. M. Harper, L. A. Koutsky, R. J. Kurman, M. Lehtinen, C. Malm, S. E. Olsson, B. M. Ronnett, F. E. Skjeldestad, M. Steinwall, M. H. Stoler, C. M. Wheeler, F. J. Taddeo, J. Yu, L. Lupinacci, R. Railkar, R. Marchese, M. T. Esser, J. Bryan, K. U. Jansen, H. L. Sings, G. M. Tamms, A. J. Saah, and E. Barr. 2006. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 24:5571-5583. [DOI] [PubMed] [Google Scholar]
- 25.Wang, Z., N. Christensen, J. T. Schiller, and J. Dillner. 1997. A monoclonal antibody against intact human papillomavirus type 16 capsids blocks the serological reactivity of most human sera. J. Gen. Virol. 78(9):2209-2215. [DOI] [PubMed] [Google Scholar]
- 26.Waterboer, T., P. Sehr, and M. Pawlita. 2006. Suppression of non-specific binding in serological Luminex assays. J. Immunol. Methods 309:200-204. [DOI] [PubMed] [Google Scholar]
- 27.WHO. 2006. Guidelines to assure the quality, safety and efficacy of recombinant human papillomavirus virus-like particle vaccines. WHO/BS/06.2050. World Health Organization, Geneva, Switzerland.