Abstract
Pseudorabies virus (PRV) is a neurotropic alphaherpesvirus that produces fatal encephalitis in newborn pigs, respiratory disorders in fattening pigs, and reproductive failure in sows. Following primary infection of the respiratory tract, PRV can develop into a systemic infection with dispersion of the virus via the lymphatic system that involves mononuclear cells in tracheobronchial lymph nodes (TBLNs). The objectives of the present study were to evaluate the pathogenesis and to determine the early immune cytokine profiles in TBLNs following experimental infection with a feral swine PRV isolate at 1, 3, 6, and 14 days postinfection (dpi). Forty healthy pigs were purchased from a PRV-negative herd. Twenty pigs received the Florida strain isolate (FS268) of feral swine PRV intranasally, and 20 uninfected controls received a sham inoculum. Compared to the levels in the controls, the levels of alpha interferon (IFN-α), interleukin-1β (IL-1β), IL-12, and IFN-γ were increased in TBLN homogenates from PRV-infected pigs at 1 dpi, whereas the IL-18 levels were decreased from 3 to 6 dpi. The protein levels of IL-4 and IL-10 did not differ between the controls and the PRV-infected pigs at any time point. Flow cytometric analysis of TBLN homogenates of PRV-infected pigs and the controls revealed increases in the percentages of B cells at 6 dpi, CD4+ cells at 14 dpi, and CD25 expression in TBLN homogenates (in the total mononuclear fraction and on B cells) in the PRV-infected pigs. Collectively, these findings demonstrate that a feral PRV in commercial swine can modulate the host's early immune response to allow the virus to establish an infection.
Pseudorabies virus (PRV) is a neurotropic alphaherpesvirus that produces fatal encephalitis in newborn pigs, respiratory disorders in fattening pigs, and reproductive failure in sows. There are three classes of swine in the United States, as defined in the USDA Veterinary Services Pseudorabies Virus Program Standards: feral swine that are wild and free roaming, transitional swine that are considered owned by someone and that may enter the food chain, and commercial swine that are raised in an environment in which there is no contact with feral swine. Studies of feral swine populations in the United States have demonstrated that PRV is indigenous (7), and it is believed to spread primarily through a venereal route rather than the respiratory route typical in domestic herds (34, 36). In contrast to the trigeminal ganglion latency of PRV in domestic swine, feral swine typically show latent infection of the sacral ganglia (35). Viral strain differences may explain the difference in tropism, as a comparison study of virus strains originating from either domestic or feral pigs found the feral strains to be markedly attenuated (18). Since the eradication of PRV from the U.S. commercial swine herd, there have been rare case reports of transitional swine becoming infected with feral swine PRV isolates (1a, 1b). The continued expansion of the feral swine population in North America presents an emerging threat to the PRV-free status of the U.S. commercial swine herd.
In general, the recognition and the subsequent elimination of herpesvirus-infected cells involve three major immune effector mechanisms: antibody-dependent lysis, major histocompatibility complex (MHC) class I-restricted cytolytic T lymphocyte (CTL)-mediated lysis, and natural killer (NK) cell-mediated lysis. PRV virions contain several envelope glycoproteins important for interactions between the virion and the host cell and constitute major targets for the immune response of the infected host (reviewed in references 27 and 28). Glycoprotein D (gD) is required for entry of the virus into host cells and is probably the most potent inducer of neutralizing antibodies (25). gC is the major attachment protein of PRV and a target of neutralizing antibodies (2), CTLs (31), and effector/memory CD4+ CD8+ T cells (21, 48). gB is required for entry and cell-to-cell spread and is highly conserved among herpesviruses. It represents a major constituent of the viral envelope and has been shown to induce complement-dependent and -independent neutralizing antibodies and helper T cells (21). gE is important for the neurovirulence of PRV and plays a role in transneuronal spread, although it is nonessential for viral replication in cell culture (29).
After acute infection, pigs develop specific antibody and T-cell responses against PRV (7, 8, 21, 46). Besides the induction of type I interferons (IFNs), gamma interferon (IFN-γ) production by peripheral blood mononuclear cells (PBMCs) is an indicator of antigen-specific cell-mediated immunity in swine with PRV and other viral infections (26, 40, 46, 47). IFNs not only play an important role as antiviral agents but also are interconnected regulators in the innate and adaptive immune responses (4). Administration of potent IFN-γ-eliciting cytokines, such as interleukin-12 (IL-12) and IL-18, with PRV antigens promotes the antigen-specific induction of IFN-γ and IL-12 yet suppresses PRV-specific antibody production and increases the susceptibility of pigs to subsequent challenge with virulent PRV (44).
In vitro studies have demonstrated that porcine IFN-α, IFN-β, and IFN-γ act synergistically to inhibit the replication of PRV (43). PRV avoids innate defenses via the active suppression of type I IFNs and downstream IFN-β-stimulated genes (6). Numerous studies have been performed to evaluate the protective efficacy of PRV vaccines (27, 28), and the association of vaccine efficacy with balanced IFN-γ and IL-4 memory responses has been described (3, 12, 17). Using the murine model, Bianchi et al. reported that both IFN-γ- and CD4+-producing T cells play important roles in conferring protection against a lethal PRV infection (3). Studies evaluating the kinetics of cytokine production by leukocytes in the lung-draining lymph nodes of PRV-infected pigs, however, have not yet been reported, especially for feral PRV isolates. Accordingly, the goals of the present study were 2-fold: first, to evaluate the pathogenic effects of a feral swine PRV isolate and, second, to characterize the production of cytokines by cells of the tracheobronchial lymph node (TBLN) that drains infected lungs, one of the target organs in PRV-infected pigs.
MATERIALS AND METHODS
Virus, animals, and experimental design.
Forty conventionally raised 4- to 5-week-old pigs, free of clinical disease, were purchased and tested negative for porcine circovirus 2, swine influenza virus, porcine respiratory and reproductive virus, and PRV. The pigs were allotted to two equal treatment groups, and each group was housed in an isolation room for about 1 week prior to the beginning of the experiment. On 0 days postinoculation (dpi), the pigs received an intranasal challenge with 3 ml of a sham inoculum (n = 20), prepared from swine testicle (ST) cell culture, or an inoculum (n = 20) of the Florida strain isolate (FS268) of feral swine PRV (34) at 1 × 106 50% cell culture infectious doses (CCID50s) per pig. Five pigs from each group were euthanized and necropsied on 1, 3, 6, and 14 dpi.
Clinical signs, gross pathology, and pulmonary histopathology.
Pigs were monitored daily for clinical signs, including rectal temperature and a clinical respiratory score, as described previously (19). Blood samples were collected on 0, 1, 3, 6, and 14 dpi. The rectal temperatures of pigs intended for necropsy on 14 dpi were recorded daily. Pig weights were recorded on 0 dpi and at necropsy. During the postmortem examination, gross lung lesions were evaluated by visual inspection and each lung lobe was scored to reflect the approximate volume or percentage of the lung tissue affected (19). Bronchoalveolar lavage fluid (BALF) and TBLNs were collected. The BALF was cultured for the presence of bacterial pathogens. Two milliliters of BALF was frozen at −80°C. A section of the TBLN was homogenized with a pellet pestle for flow cytometry analysis. Another section of TBLN was homogenized in tissue lysis buffer for cytokine analysis (16).
Antibody detection.
For the PRV group, sera collected at 0 and 14 dpi were tested for antibody to the gB antigen of PRV by using the commercial HerdChek PRV gB blocking enzyme-linked immunosorbent assay (ELISA; IDEXX, Westbrook, ME), according to the manufacturer's directions. Test values were reported as the sample optical density value as a ratio of the positive control provided in the kit (S/N ratio). The cutoff S/N values for the PRV gB assay were as follows: negative, greater than 0.70; positive, less than or equal to 0.60; and suspect, greater than 0.60 and less than or equal to 0.70.
Virus detection.
On 0, 1, 3, 6, and 14 dpi, all serum and BALF samples were tested for virus. Real-time PCR assays for the specific detection of the gB and gE genes of PRV were performed by the use of TaqMan chemistry, as described previously (24). The analytical sensitivity of the assays was approximately 0.1 PFU per reaction (24).
Virus isolation for the detection of PRV in the samples collected was performed with an ST cell line, as described previously (32). Briefly, each well of 24-well plates with 80 to 90% confluent monolayers of ST cells was inoculated with 100 μl of diluted medium in which the nasal swab specimen, BALF, or tissue homogenate had been placed. The inoculated cells were incubated at 37°C in a 5% CO2 atmosphere for 72 h and monitored daily for the development of cytopathic effects.
Cytokine assays.
After removal, approximately 1 g of TBLN was homogenized in 750 μl of lysis buffer containing 0.5% Triton X-100, 150 mM NaCl, 15 mM Tris, 1 mM CaCl, and 1 mM MgCl, pH 7.40, with a tissue homogenizer (Biospec Products, Bartlesville, OK). The homogenates were incubated on ice for 30 min and then centrifuged at 2,500 rpm for 10 min (16). The supernatants were collected, passed through a 0.45-μm-pore-size filter (Gelman Sciences, Ann Arbor, MI), and then stored at −20°C prior to the assessment of cytokine levels.
The total protein concentration in the supernatants was measured by use of a commercially available bicinchoninic acid protein kit (Sigma-Aldrich, St. Louis, MO), following the manufacturer's directions, on a NanoDrop-1000 spectrophotometer (Thermo Scientific, Wilmington, DE).
Tumor necrosis factor alpha (TNF-α), IL-1β, IL-4, IL-6, IL-8, IL-10, and IL-12 protein levels were measured by use of the respective porcine DuoSet ELISA development kits (R&D Systems Inc., Minneapolis, MN), according to the manufacturer's protocol. IL-18 and IFN-γ protein levels were measured by use of the respective porcine ELISA kits (Biosource International, Inc., Camarillo, CA).
IFN-α protein was measured by a porcine IFN-α-specific ELISA with monoclonal antibody (MAb) F17 and MAb K9 (R&D Systems Inc.), as described previously (10). MAb K9 was conjugated with horseradish peroxidase (HRP) by use of a peroxidase labeling kit (Roche Molecular Biochemical, Indianapolis, IN). Immulon 2 flat-bottomed 96-well plates (Fisher Scientific, Houston, TX) were coated overnight at 4°C with MAb F17 at a concentration of 0.3 μg/well in coating buffer (100 mM carbonate buffer, pH 9.6; Sigma Inc., St. Louis, MO). After the wells were blocked with 1% nonfat dried milk and 0.05% Tween 20 in phosphate-buffered saline (PBS) for 1 h at 37°C, the plates were washed five times with 0.05% Tween 20 in PBS. Samples (50 μl) containing 50 μl of 1% nonfat dried milk and 0.05% Tween 20 in PBS were added to each well, and the plates were incubated for 2 h at 37°C. Following five washes, 100 μl of peroxidase-conjugated MAb K9 was added to each well. After 1 h of incubation at 37°C and five washes, 100 μl of tetramethylbenzidine substrate solution (KPL Inc., Gaithersburg, MD) was added to each well. After 30 min, the reaction was stopped with tetramethylbenzidine stop solution (KPL Inc.) and the optical density at 450 nm was measured with an ELISA plate reader. Quantified recombinant porcine IFN-α (rIFN-α; R&D Systems Inc.) was used as a standard, and the IFN-α concentrations were calculated on the basis of the concentrations on a standard curve. One unit per milliliter of rIFN-α was equivalent to 26 pg/ml.
Flow cytometry analysis.
Cells from the TBLNs were phenotyped by flow cytometry, as described previously (38). Commercially available antibodies to the following markers were used: CD3 (2B3C), CD25 (PGBL25A), MHC class II (TH16B), B cell (BB6-11C9), CD4 (PT90A), CD8 (76-2-11), and γ/δ T-cell (PGBL22A) (VMRD Inc., Pullman, WA). Secondary antibodies (VMRD) targeted to murine primary antibodies included IgG1-fluorescein isothiocyanate (FITC), IgG2a-FITC, IgG2b-phycoerythrin (PE), IgG1-PE, IgG2a-PE, and IgM-PE. Data were acquired with CellQuest Pro software (BD Biosciences, San Jose, CA) on an LSR II flow cytometer (Becton Dickinson) and were analyzed with FlowJo software (TreeStar, Ashland, OR).
Statistical analysis.
Differences in virus titers, body temperatures, and respiratory scores between the study groups were evaluated by analysis of variance (ANOVA). Two-way ANOVA and posttests were used for comparison of the cytokine titers. Spearman rank correlation tests were used to compare the individual virus titers, cytokine titers, body temperatures, and respiratory scores with the other parameters.
RESULTS
Clinical evaluation and gross pathology.
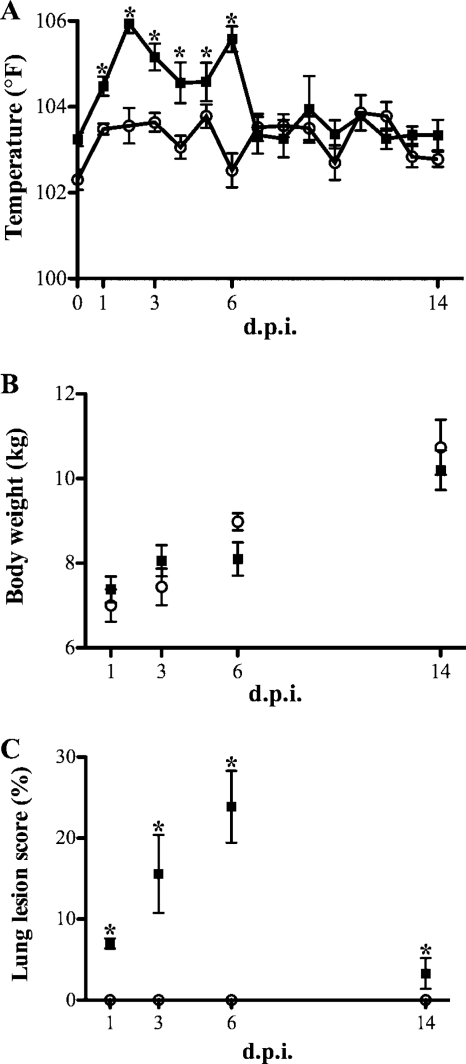
PRV-inoculated pigs developed a mild clinical disease typical of PRV infection, as reflected by fever (Fig. 1A), anorexia, sneezing, increased respiration rates, and dyspnea that began at 2 to 3 dpi and that resolved by 14 dpi. Control animals did not display clinical signs or gross lung lesions consistent with PRV infection. Pigs inoculated with PRV had an increased (P < 0.05) rectal temperature from 1 to 6 dpi (Fig. 1A). No significant differences (P > 0.05) in daily weight gain (Fig. 1B) were found between pigs inoculated with PRV (average, 0.6 kg/day) and control pigs (average, 0.6 kg/day). Gross lung lesions in PRV-inoculated pigs mainly consisted of hilar multifocal areas of purple to red areas of consolidation. The lesions were most pronounced at 3 and 6 dpi (Fig. 1C). Bacteria typically associated with porcine respiratory disease were not isolated from the BALF of any of the pigs. Pig serum was negative for PRV gB antibody at 0 dpi (mean S/N ratio, 1.22 ± 0.03) and positive at 14 dpi (mean S/N ratio, 0.26 ± 0.2).
FIG. 1.
Clinical evaluation and gross pathology. (A) Rectal temperature for control (○) and PRV-inoculated (▪) pigs measured each day out to 14 dpi; (B) body weight measured prior to necropsy for control (○) and PRV-inoculated (▪) pigs; (C) macroscopic lesion score for lungs at necropsy for control (○) and PRV-inoculated (▪) pigs. Data are expressed as means ± standard errors (n = 5). *, P < 0.05.
Quantitative PCR for virus nucleic acid and virus isolation.
Table 1 summarizes the results of the virus detection assays. After intranasal infection, PRV was detected in the tonsil, lungs, BALF, and nasal swab of the inoculated pigs from 1 to 6 dpi; however, PRV was not detected in the brain, spleen, or sacral ganglia.
TABLE 1.
Distribution of PRV in tissues of intranasally inoculated pigsa
| Tissue sampled | No. of pigs positive for PRV by the indicated assay/total no. of pigs tested on the following dpi: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 |
3 |
6 |
14 |
|||||
| RT-PCR | VI | RT-PCR | VI | RT-PCR | VI | RT-PCR | VI | |
| Brain | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Spleen | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Sacral ganglion | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Tonsil | 3/5 | 4/5 | 4/5 | 5/5 | 3/5 | 2/5 | 0/5 | 0/5 |
| Lung | 5/5 | 5/5 | 4/5 | 4/5 | 4/5 | 1/5 | 1/5 | 0/5 |
| BALF | 5/5 | 5/5 | 5/5 | 4/5 | 4/5 | 0/5 | 2/5 | 0/5 |
| Nasal swab | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 0/5 | 0/5 |
No PRV was detected in the tissue from control animals (data not shown). RT-PCR, reverse transcription-PCR; VI, virus isolation assay.
Cytokine assays.
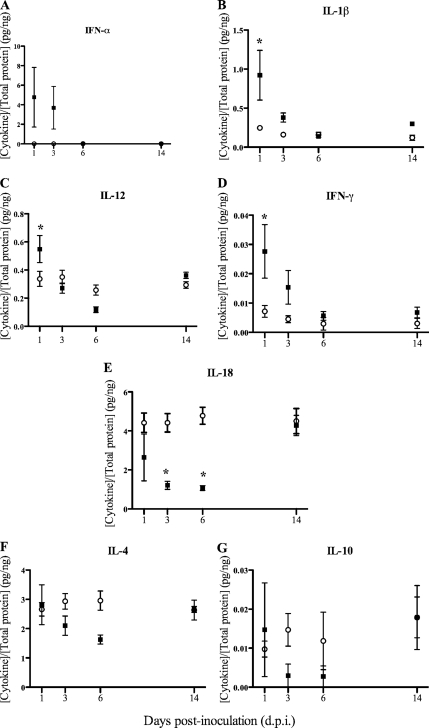
Cytokine protein level measurements were normalized to the total amount of protein per gram of TBLN. The ex vivo protein levels of the proinflammatory cytokines IFN-α and IL-1β were increased (P = 0.06 and P < 0.05, respectively) in the TBLN homogenates of PRV-inoculated pigs compared with those in the control pigs at 1 dpi (Fig. 2A and B), whereas the IL-6, IL-8, and TNF-α levels did not differ (P > 0.05) between the treatment groups at any time point. As with IFN-α and IL-1β, at 1 dpi the levels of the Th1 cytokines IL-12 and IFN-γ were increased (P < 0.05) in the PRV-inoculated pigs compared with the levels in the control pigs (Fig. 2C and D). In contrast, at 3 and 6 dpi the IL-18 level in the PRV-inoculated pigs was decreased (P < 0.05) compared with that in the control pigs (Fig. 2E). At 6 dpi, the IL-12 level in the PRV-inoculated pigs was also decreased (P < 0.05) compared with the level in the control pigs (Fig. 2C). Protein levels of the Th2/regulatory T cell cytokines IL-4 and IL-10 did not differ between the treatment groups at any time point (Fig. 2F and G).
FIG. 2.
Cytokine concentration in homogenates of TBLNs from pigs inoculated with PRV (▪) or controls (○) normalized to the total protein concentration per gram of TBLN. Due to differences in the concentrations of the cytokines, the graphs use different scales. Means and standard errors of the means (n = 5) are shown. *, significantly different value than that for the control (P < 0.05).
Flow cytometry.
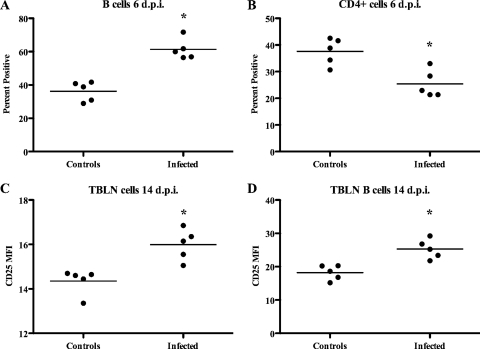
Flow cytometric analysis of TBLN homogenates revealed increases (P < 0.05) in B-cell percentages (Fig. 3A) and a concomitant decrease in CD4+ cells at 6 dpi in PRV-infected pigs compared with the results for the control pigs (Fig. 3B). By 14 dpi, the percentages of CD4+ cells were increased (P < 0.05) in PRV-infected pigs compared with the percentages in the control pigs (33.4% ± 1.2% and 26.0% ± 2.5%, respectively). The mean fluorescence intensity of CD25 was increased (P < 0.05) both in total TBLN homogenates (Fig. 3C) and on B cells in TBLN homogenates (Fig. 3D) at 14 dpi in PRV-infected pigs compared with the mean fluorescence intensity for the control pigs. Differences (P > 0.05) between treatment groups in the numbers of B cells and CD4+ cells were not detected at 1 and 3 dpi. Differences (P > 0.05) between treatment groups in the numbers of CD4+ CD8+, γδ T-cell receptor-positive, CD8+, CD3+, or MHC class II-positive cells were not detected.
FIG. 3.
Flow cytometric analysis of homogenates of TBLNs from pigs inoculated with PRV or controls. (A) B-cell percentages; (B) CD4+-cell percentages; (C) mean fluorescence intensity (MFI) of CD25 for total TBLN homogenates; (D) mean fluorescence intensity of B cells in TBLN homogenates. *, significant difference (P < 0.05) from the values for the controls.
DISCUSSION
Feral swine PRV isolate FS268 was pathogenic in this study, inducing fever, anorexia, listlessness, and dyspnea following experimental infection of young pigs. The presence of clinical signs was related to the detection of PRV in tissues associated with the respiratory tract. Likewise, the lack of central nervous system (CNS) clinical signs may be related to the absence of detectable virus in CNS tissues. The results of this study indicate that while isolate FS268 may induce moderate to severe pneumonia, it may have a limited effect on the CNS, supporting the assumptions of others that feral swine PRV isolates may have a phenotype different from that of isolates from commercial swine. Few studies evaluating the kinetics of cytokine production in PRV-infected pigs have been reported (5, 7, 14, 21, 33, 42, 48). Previous studies used PBMCs in vitro or from pigs infected with attenuated (gE- or thymidine kinase-deleted) laboratory or wild-type PRV strains.
During the 14-day course of this study, the infected pigs began to recover clinically, and this was reflected in a variety of cellular and cytokine changes in the TBLNs. Overall, an early increase in proinflammatory cytokine levels was elicited by PRV in TBLNs, leading to an increase in the Th1 (IL-12 and IFN-γ) responses concurrently with increased percentages of B cells at 6 dpi and unchanged Th2 (IL-4 and IL-10) responses. It has previously been observed in PRV (domestic strain)-primed pigs that upon PRV reexposure of PBMCs in vitro, there is a significantly enhanced transcription of Th1-type cytokines (IL-2 and IFN-γ) but not of Th2-type cytokines (IL-4 and IL-10), but in naïve pigs there was no increased transcription of cytokines (14). The activation of B cells, as evidenced by the upregulation of CD25 (IL-2 receptor α chain marker), and their proliferation and expansion (likely with the support of Th cells) were observed at 14 dpi in our study. A significant decrease in the IL-18 response early following PRV infection could be a virulence mechanism and could be related to the persistence and latency of PRV, given that the IL-18-interacting Th1 cytokine (IL-12 and IFN-γ) responses were not sustained. IL-18 expression can be activated by Toll-like receptor signaling (1) or can be induced by IFN-γ via interferon consensus sequence-binding protein and activator protein 1 pathways (20), by inflammasome activation (39), or by autocrine signaling through the NF-κB pathway (15). In the presence of IL-12, IL-18 induces strong Th1-type immune responses (9, 23, 30, 41), primarily through the induction of IFN-γ expression by T cells and natural killer cells (11, 45). Impairment of IL-18 expression, which is involved in the induction of antiviral cytokines, could represent a strategy that the virus uses to evade the immune response of the host.
A B-cell response is induced after PRV inoculation but alone is not a protective response (7). The proliferation of T cells after restimulation with PRV suggests that T cells are involved in protective immunity (21). These cells may produce IFN-γ and TNF-α (13). IFN-γ can be involved in the prevention of PRV replication either by a direct local antiviral effect or, indirectly, by the induction of proinflammatory cytokines, or by a combination of both mechanisms (17, 37). IFN-γ and TNF-α can also activate macrophages and natural killer cells, which are involved in the clearance of PRV (17). It has previously been demonstrated that CD8+ T cells lysed PRV-infected cells and that CD2+ CD4− and CD8− or CD8dull+ T lymphocytes (i.e., NK cells) lysed PRV-infected target cells (22, 48). More cytolytic cells may be found at specific sites in tissues involved in the infection process, such as mucosal tissues, tonsils, and draining lymph nodes (7). Lymphocytes that proliferate after pigs are inoculated with PRV probably migrate to the site of infection. This possibility is supported by the observation of a very localized influx of various T-lymphocyte subsets to the site of PRV infection, which was restricted to specific areas in the draining lymphoid tissues (5).
A change of lymphocyte subset frequency in TBLNs can be caused by an expansion or depletion of specific subsets after the inoculation of PRV. Our results showed that the proportion of various lymphocyte subsets in TBLNs changed after inoculation (Fig. 3C and D). This finding is in accordance with the observations of leukopenia after challenge with PRV and lower percentages of total T and B lymphocytes (33, 42) and is in contrast to the finding that the lymphocyte subset proportion did not change after inoculation of an avirulent strain of PRV that induced protective immunity (7).
In conclusion, the TBLNs from PRV-infected pigs presented an altered cytokine protein expression profile. It was evident that the key cytokines responsible for driving the immune responses (IFN-α, IL-1β, IL-12, IL-18, and IFN-γ) showed changes in their levels of protein expression in the TBLNs of diseased pigs. These changes suggest a lack of induction of the IL-4 and IL-10 responses and a decrease in the IL-18 response. To our knowledge, this is the first in vivo study of the cytokine response to a feral pseudorabies virus strain in the TBLNs of pigs. Moreover, the feral swine strain of PRV used in this study caused mild clinical disease during the acute phase that included fever and lung lesions, which are associated with an inflammatory cytokine response. These resolved by 14 dpi, and there was an increase in the B-cell percentages in association with the induction of immune cytokines in the TBLNs. The presence of PRV-specific antibodies in serum at 14 dpi was consistent with the observation of increased B-cell percentages in TBLN homogenates.
Acknowledgments
This work was supported by interagency agreement 0414701 between the U.S. Department of Agriculture's Agricultural Research Service and Animal and Plant Health Inspection Service.
We thank S. Pohl and D. Adolphson for technical assistance and S. Ohlendorf for secretarial assistance with the preparation of the manuscript.
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 1a.Animal and Plant Health Inspection Service. 2009. Animal health monitoring and surveillance. Status of reportable diseases in the United States. Animal and Plant Health Inspection Service, U.S. Department of Agriculture.
- 1b.Anonymous. 15 June 2007. Wisconsin responds to pseudorabies cases. JAVMA News. http://www.avma.org/onlnews/javma/jun07/070615r.asp.
- 2.Ben-Porat, T., J. M. DeMarchi, B. Lomniczi, and A. S. Kaplan. 1986. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology 154:325-334. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi, A. T., H. W. Moonen-Leusen, F. J. van Milligen, H. F. Savelkoul, R. J. Zwart, and T. G. Kimman. 1998. A mouse model to study immunity against pseudorabies virus infection: significance of CD4+ and CD8+ cells in protective immunity. Vaccine 16:1550-1558. [DOI] [PubMed] [Google Scholar]
- 4.Biron, C. A., and G. C. Sen. 2007. Innate response to viral infections, p. 249-278. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, S. E. Straus, M. A. Martin, and B. Roizman (ed.), Fields virology, vol. 1, 5th ed. Wolters Kluwer Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 5.Bouma, A., R. J. Zwart, M. G. De Bruin, M. C. De Jong, T. G. Kimman, and A. T. Bianchi. 1997. Immunohistological characterization of the local cellular response directed against pseudorabies virus in pigs. Vet. Microbiol. 58:145-154. [DOI] [PubMed] [Google Scholar]
- 6.Brukman, A., and L. W. Enquist. 2006. Suppression of the interferon-mediated innate immune response by pseudorabies virus. J. Virol. 80:6345-6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bruin, M. G., Y. E. De Visser, T. G. Kimman, and A. T. Bianchi. 1998. Time course of the porcine cellular and humoral immune responses in vivo against pseudorabies virus after inoculation and challenge: significance of in vitro antigenic restimulation. Vet. Immunol. Immunopathol. 65:75-87. [DOI] [PubMed] [Google Scholar]
- 8.de Bruin, T. G., E. M. van Rooij, Y. E. de Visser, J. J. Voermans, J. N. Samsom, T. G. Kimman, and A. T. Bianchi. 2000. Discrimination of different subsets of cytolytic cells in pseudorabies virus-immune and naive pigs. J. Gen. Virol. 81:1529-1537. [DOI] [PubMed] [Google Scholar]
- 9.Denton, A. E., P. C. Doherty, S. J. Turner, and N. L. La Gruta. 2007. IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. Eur. J. Immunol. 37:368-375. [DOI] [PubMed] [Google Scholar]
- 10.Diaz de Arce, H., K. Artursson, R. L'Haridon, A. Perers, C. La Bonnardiere, and G. V. Alm. 1992. A sensitive immunoassay for porcine interferon-alpha. Vet. Immunol. Immunopathol. 30:319-327. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello, C. A. 1999. Interleukin-18. Methods 19:121-132. [DOI] [PubMed] [Google Scholar]
- 12.Dory, D., T. Fischer, V. Beven, R. Cariolet, H. J. Rziha, and A. Jestin. 2006. Prime-boost immunization using DNA vaccine and recombinant orf virus protects pigs against pseudorabies virus (herpes suid 1). Vaccine 24:6256-6263. [DOI] [PubMed] [Google Scholar]
- 13.Feduchi, E., M. A. Alonso, and L. Carrasco. 1989. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J. Virol. 63:1354-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, T., M. Buttner, and H. J. Rziha. 2000. T helper 1-type cytokine transcription in peripheral blood mononuclear cells of pseudorabies virus (suid herpesvirus 1)-primed swine indicates efficient immunization. Immunology 101:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortin, C. F., T. Ear, and P. P. McDonald. 2009. Autocrine role of endogenous interleukin-18 on inflammatory cytokine generation by human neutrophils. FASEB J. 23:194-203. [DOI] [PubMed] [Google Scholar]
- 16.Greenberger, M. J., R. M. Strieter, S. L. Kunkel, J. M. Danforth, R. E. Goodman, and T. J. Standiford. 1995. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumoniae. J. Immunol. 155:722-729. [PubMed] [Google Scholar]
- 17.Grob, P., V. E. Schijns, M. F. van den Broek, S. P. Cox, M. Ackermann, and M. Suter. 1999. Role of the individual interferon systems and specific immunity in mice in controlling systemic dissemination of attenuated pseudorabies virus infection. J. Virol. 73:4748-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn, E. C., G. R. Page, P. S. Hahn, K. D. Gillis, C. Romero, J. A. Annelli, and E. P. Gibbs. 1997. Mechanisms of transmission of Aujeszky's disease virus originating from feral swine in the USA. Vet. Microbiol. 55:123-130. [DOI] [PubMed] [Google Scholar]
- 19.Halbur, P. G., P. S. Paul, M. L. Frey, J. Landgraf, K. Eernisse, X. J. Meng, M. A. Lum, J. J. Andrews, and J. A. Rathje. 1995. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 32:648-660. [DOI] [PubMed] [Google Scholar]
- 20.Kim, Y. M., J. Y. Im, S. H. Han, H. S. Kang, and I. Choi. 2000. IFN-gamma up-regulates IL-18 gene expression via IFN consensus sequence-binding protein and activator protein-1 elements in macrophages. J. Immunol. 165:3198-3205. [DOI] [PubMed] [Google Scholar]
- 21.Kimman, T. G., T. M. De Bruin, J. J. Voermans, B. P. Peeters, and A. T. Bianchi. 1995. Development and antigen specificity of the lymphoproliferation responses of pigs to pseudorabies virus: dichotomy between secondary B- and T-cell responses. Immunology 86:372-378. [PMC free article] [PubMed] [Google Scholar]
- 22.Kimman, T. G., T. G. DeBruin, J. J. Voermans, and A. T. Bianchi. 1996. Cell-mediated immunity to pseudorabies virus: cytolytic effector cells with characteristics of lymphokine-activated killer cells lyse virus-infected and glycoprotein gB- and gC-transfected L14 cells. J. Gen. Virol. 77(Pt 5):987-990. [DOI] [PubMed] [Google Scholar]
- 23.Liu, B., I. Mori, M. J. Hossain, L. Dong, K. Takeda, and Y. Kimura. 2004. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J. Gen. Virol. 85:423-428. [DOI] [PubMed] [Google Scholar]
- 24.Ma, W., K. M. Lager, J. A. Richt, W. C. Stoffregen, F. Zhou, and K. J. Yoon. 2008. Development of real-time polymerase chain reaction assays for rapid detection and differentiation of wild-type pseudorabies and gene-deleted vaccine viruses. J. Vet. Diagn. Invest. 20:440-447. [DOI] [PubMed] [Google Scholar]
- 25.Marchioli, C. C., R. J. Yancey, Jr., E. A. Petrovskis, J. G. Timmins, and L. E. Post. 1987. Evaluation of pseudorabies virus glycoprotein gp50 as a vaccine for Aujeszky's disease in mice and swine: expression by vaccinia virus and Chinese hamster ovary cells. J. Virol. 61:3977-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mateu de Antonio, E., R. J. Husmann, R. Hansen, J. K. Lunney, D. Strom, S. Martin, and F. A. Zuckermann. 1998. Quantitative detection of porcine interferon-gamma in response to mitogen, superantigen and recall viral antigen. Vet. Immunol. Immunopathol. 61:265-277. [DOI] [PubMed] [Google Scholar]
- 27.Mettenleiter, T. C. 1996. Immunobiology of pseudorabies (Aujeszky's disease). Vet. Immunol. Immunopathol. 54:221-229. [DOI] [PubMed] [Google Scholar]
- 28.Mettenleiter, T. C. 1994. Pseudorabies (Aujeszky's disease) virus: state of the art. August 1993. Acta Vet. Hung. 42:153-177. [PubMed] [Google Scholar]
- 29.Mettenleiter, T. C., N. Lukacs, and H. J. Rziha. 1985. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J. Virol. 56:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura, K., H. Okamura, K. Nagata, T. Komatsu, and T. Tamura. 1993. Purification of a factor which provides a costimulatory signal for gamma interferon production. Infect. Immun. 61:64-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ober, B. T., A. Summerfield, C. Mattlinger, K. H. Wiesmuller, G. Jung, E. Pfaff, A. Saalmuller, and H. J. Rziha. 1998. Vaccine-induced, pseudorabies virus-specific, extrathymic CD4+CD8+ memory T-helper cells in swine. J. Virol. 72:4866-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onyekaba, C., L. Bueon, P. King, J. Fahrmann, and S. M. Goyal. 1987. Susceptibility of various cell culture systems to pseudorabies virus. Comp. Immunol. Microbiol. Infect. Dis. 10:163-166. [DOI] [PubMed] [Google Scholar]
- 33.Page, G. R., F. I. Wang, and E. C. Hahn. 1992. Interaction of pseudorabies virus with porcine peripheral blood lymphocytes. J. Leukoc. Biol. 52:441-448. [DOI] [PubMed] [Google Scholar]
- 34.Romero, C. H., P. Meade, J. Santagata, K. Gillis, G. Lollis, E. C. Hahn, and E. P. Gibbs. 1997. Genital infection and transmission of pseudorabies virus in feral swine in Florida, USA. Vet. Microbiol. 55:131-139. [DOI] [PubMed] [Google Scholar]
- 35.Romero, C. H., P. N. Meade, B. L. Homer, J. E. Shultz, and G. Lollis. 2003. Potential sites of virus latency associated with indigenous pseudorabies viruses in feral swine. J. Wildl. Dis. 39:567-575. [DOI] [PubMed] [Google Scholar]
- 36.Romero, C. H., P. N. Meade, J. E. Shultz, H. Y. Chung, E. P. Gibbs, E. C. Hahn, and G. Lollis. 2001. Venereal transmission of pseudorabies viruses indigenous to feral swine. J. Wildl. Dis. 37:289-296. [DOI] [PubMed] [Google Scholar]
- 37.Schijns, V. E., R. Van der Neut, B. L. Haagmans, D. R. Bar, H. Schellekens, and M. C. Horzinek. 1991. Tumour necrosis factor-alpha, interferon-gamma and interferon-beta exert antiviral activity in nervous tissue cells. J. Gen. Virol. 72(Pt 4):809-815. [DOI] [PubMed] [Google Scholar]
- 38.Stabel, T. J., S. R. Bolin, B. A. Pesch, and T. E. Rahner. 2000. A simple and rapid flow cytometric method for detection of porcine cell surface markers. J. Immunol. Methods 245:147-152. [DOI] [PubMed] [Google Scholar]
- 39.Stasakova, J., B. Ferko, C. Kittel, S. Sereinig, J. Romanova, H. Katinger, and A. Egorov. 2005. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1beta and 18. J. Gen. Virol. 86:185-195. [DOI] [PubMed] [Google Scholar]
- 40.Suradhat, S., R. Thanawongnuwech, and Y. Poovorawan. 2003. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 84:453-459. [DOI] [PubMed] [Google Scholar]
- 41.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383-390. [DOI] [PubMed] [Google Scholar]
- 42.Williams, P. P. 1995. Study of immune function in inbred miniature pigs vaccinated and challenged with suid herpesvirus 1. Can. J. Vet. Res. 59:285-293. [PMC free article] [PubMed] [Google Scholar]
- 43.Yao, Q., P. Qian, Y. Cao, Y. He, Y. Si, Z. Xu, and H. Chen. 2007. Synergistic inhibition of pseudorabies virus replication by porcine alpha/beta interferon and gamma interferon in vitro. Eur. Cytokine Netw. 18:71-77. [DOI] [PubMed] [Google Scholar]
- 44.Yoon, H. A., A. G. Aleyas, J. A. George, S. O. Park, Y. W. Han, J. H. Lee, H. Y. Kang, S. H. Kang, J. G. Cho, and S. K. Eo. 2006. Modulation of immune responses induced by DNA vaccine expressing glycoprotein B of pseudorabies virus via coadministration of IFN-gamma-associated cytokines. J. Interferon Cytokine Res. 26:730-738. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimoto, T., H. Okamura, Y. I. Tagawa, Y. Iwakura, and K. Nakanishi. 1997. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proc. Natl. Acad. Sci. U. S. A. 94:3948-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuckermann, F. A. 2000. Aujeszky's disease virus: opportunities and challenges. Vet. Res. 31:121-131. [DOI] [PubMed] [Google Scholar]
- 47.Zuckermann, F. A., S. Martin, R. J. Husmann, and J. Brandt. 1999. Use of interleukin 12 to enhance the cellular immune response of swine to an inactivated herpesvirus vaccine. Adv. Vet. Med. 41:447-461. [DOI] [PubMed] [Google Scholar]
- 48.Zuckermann, F. A., L. Zsak, T. C. Mettenleiter, and T. Ben-Porat. 1990. Pseudorabies virus glycoprotein gIII is a major target antigen for murine and swine virus-specific cytotoxic T lymphocytes. J. Virol. 64:802-812. [DOI] [PMC free article] [PubMed] [Google Scholar]