Abstract
Reports from our clinical laboratory database show that 75% of children <2 years old tested for celiac serology who were found positive for deamidated gliadin peptide (DGP) antibodies had negative results for tissue transglutaminase IgA. DGP levels were shown to decline and disappear without a gluten-free diet. This observation questions DGP's specificity for diagnosis of celiac disease.
Celiac serology tests are the necessary tool for correctly referring individuals for duodenal biopsies when diagnosing celiac disease (4). Testing for tissue transglutaminase IgA (tTG) antibodies (Abs) is currently the test of choice used for this purpose (5, 17). Since there are reports indicating lack of tTG sensitivity in infants (3, 7), we decided that all serum samples from infants <2 years old referred to our clinical laboratory for celiac disease testing should be additionally tested for deamidated gliadin peptide (DGP) (IgG and IgA) Abs (6, 13). Gliadin Abs exhibit weak performance (4), and endomysial IgA (EMA) testing adds limited diagnostic value to the tissue transglutaminase (tTG) assay (12, 19); therefore, these assays were not included in our laboratory practice. Total IgA was measured to identify IgA deficiency (5). In many cases, inconsistency between serology results, namely, DGP positive/tTG negative, was observed. We have retrospectively analyzed this observation by contacting the referring physicians and learning the clinical outcome for these infants.
Over a period of 17 months (July 2007 to December 2008), we tested serum samples from 5,036 infants (<2 years old) for celiac disease-specific Abs using two commercial enzyme-linked immunosorbent assay (ELISA) kits, one for DGP Abs (Quanta Lite DGP IgG+IgA screen [Inova Diagnostics, San Diego, CA]: intraassay coefficient of variation [CV], 0.5 to 4.7%; interassay CV, 2.4 to 5.8%; standard deviation, 0.1 to 3.8) and one for tTG Abs (Celikey tTG-IgA; Phadia, Freiburg, Germany). We compiled reports from the laboratory's database, listing all infants with contradicting positive and negative results.
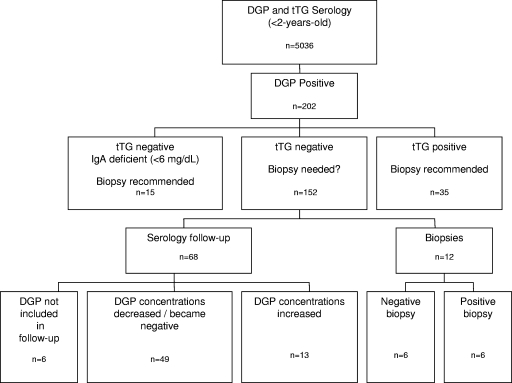
The DGP results were positive in 202 infants; of these, only 35 (17%) were positive for tTG (Fig. 1). Positive results for both Ab entities are highly suggestive of celiac disease and indicate the desirability of performing duodenal biopsies. Since these cases were not of interest in our study, no further follow-up was conducted. IgA deficiency (IgA at <6 mg/dl, determined with a BNII nephelometer; Siemens) was determined in 15 (7.4%) infants. These infants were referred for biopsies in order to make a final diagnosis. In the remaining 152 (75%) infants, tTG was negative (<3 U/ml) (Fig. 1). We contacted the referring physicians in order to discover the clinical outcome in 80 of these cases with contradictory serology results. Twelve infants were referred for duodenal biopsies, mainly due to their physician's approach, claiming that when clinical features are present, one positive serology measurement is an adequate indication for performing biopsies. Celiac disease was diagnosed in six cases (50%), as indicated by specific histological characteristics of villous atrophy according to the modified Marsh criteria (11). In the remaining six cases, the biopsy findings were negative for celiac disease. The clinical findings and DGP concentrations of these 12 infants were similar to those of all other infants. Furthermore, in the 12 infants who underwent biopsies, the DGP concentrations were not a prognostic factor for indicating biopsy outcome. The DGP concentrations measured in the biopsy-positive infants did not differ significantly from those measured in the biopsy-negative infants (ranges of 23 to 61 U and 25 to 95 U, respectively).
FIG. 1.
Scheme of results obtained for DGP-positive infants over a period of 17 months.
Serological follow-up was performed for 68 infants who had not undergone biopsies, over a period of between 2 weeks and 1 year from the initial testing date. The tTG concentrations remained negative in all 68 individuals. Surprisingly, the DGP concentrations declined in 49 (72%) individuals, of whom 44 (65%) became negative without maintaining a gluten-free diet (GFD). Since the follow-up intervals were not consistent among all infants, we investigated the time it took for DGP levels to become negative, namely, the first measurement under the cutoff value of 20 U (n = 44). DGP turned negative within 1 month in 8 (18%) cases, within 4 months in 17 (39%) cases, within 8 months in 11 (25%) cases, and within 1 year in 8 (18%) cases. We selected the patients with strong positive (>40 U) and moderate positive (30 to 40 U) initial DGP levels and looked into the time it took for Ab levels to decline to low positive (20 to 30 U) and negative (<20 U) levels. Table 1 demonstrates the changes in DGP levels in 20 patients with initial strong positive (n = 10) and moderate positive (n = 10) DGP concentrations and the subsequent measurements during the 12-month follow-up period. Sixteen patients turned negative, while DGP in patients 7, 11, and 13 declined to low positive levels. DGP in patient 8 declined from strong positive to moderate positive levels. The additional low positive DGP samples (n = 29) (not shown in Table 1) exhibited similar rates of Ab decrease. Initially high DGP concentrations (Table 1) and the age of tested infants (data not shown) were not associated with the rate of Ab decline.
TABLE 1.
Decline of DGP concentrations without a gluten-free diet during the 12-month follow-up perioda
| Patient | Initial DGP measurement (U) | Follow-up DGP measurement (U) at age (mo): |
|||
|---|---|---|---|---|---|
| 0.5-1 | 2-4 | 5-8 | 9-12 | ||
| 1 | 96 | Negative | |||
| 2 | 74 | 26 | Negative | ||
| 3 | 73 | Negative | |||
| 4 | 72 | 39 | Negative | ||
| 5 | 63 | 58 | Negative | ||
| 6 | 55 | Negative | |||
| 7 | 48 | 28 | |||
| 8 | 47 | 39 | |||
| 9 | 44 | 25 | Negative | ||
| 10 | 41 | Negative | |||
| 11 | 39 | 27; 28 | |||
| 12 | 37 | Negative | |||
| 13 | 37 | 26 | |||
| 14 | 37 | 28 | Negative | ||
| 15 | 37 | Negative | |||
| 16 | 35 | Negative | |||
| 17 | 33 | 32 | Negative | ||
| 18 | 33 | 24 | Negative | ||
| 19 | 33 | 23; negative | |||
| 20 | 31 | Negative | |||
The table shows results for the 20 infants whose initial DGP concentrations were strongly positive (>40 U) or moderately positive (30 to 40 U). The DGP levels became low positive (20 to 29 U) or negative (<20 U) during the 12-month follow-up period, without maintenance of a gluten-free diet. All infants were tTG negative. Assay cutoff is 20 U.
Among the 68 samples in the follow-up group, DGP concentrations increased in 13 (19%) cases, and in six (9%) cases, DGP was not measured during the follow-up visit (Fig. 1).
The high percentage of DGP-positive/tTG-negative results raised questions as to the population studied. Further inquiries into the medical records of these infants indicated that the presenting clinical symptoms did not differ significantly from those of biopsy-proven celiac children (10). In the majority of cases (64%), the clinical symptoms persisted throughout the follow-up period. The most frequent symptom was failure to thrive (27%), followed by diarrhea (15%), respiratory infections (13%), other gastrointestinal manifestations (9%), viral and bacterial infections (9%), unexplained fever (4%), anemia (3%), feeding problems (3%), and elevated liver enzymes (1%). Nutritional deficits, such as those shown by folic acid, vitamin B12, hemoglobin, and iron levels, were not measured consistently throughout the follow-up period.
We found these observations quite challenging. As a clinical laboratory, we are confronted daily with physicians questioning how to respond to DGP-positive/tTG-negative results. According to our experience, a single measurement of positive DGP may lead to the performance of unnecessary, invasive, and costly biopsies. Our recommendations are to continue serological follow-up and HLA testing when possible. According to the accepted understanding of celiac serology, it does not seem reasonable that true celiac patients demonstrate a decrease in Ab levels without a GFD (16). However, one should bear in mind that of the 80 infants for whom we have information, six (7.5%) were actually diagnosed with celiac disease during their first year of follow-up. DGP was the only serological marker which picked up the biopsy-positive infants and, as such, cannot be dismissed from our laboratory menu. Furthermore, DGP remained positive with intensifying concentrations in 13 infants. A longer period of follow-up would better estimate whether additional celiac infants would be revealed.
The sensitivities and specificities of the DGP assay vary significantly (1, 8, 13, 14). In our current observations, DGP exhibited a transient appearance, thus suggesting low specificity for celiac disease, as opposed to previous reports (18). Perhaps the observed decreased specificity is due to the low-risk population attending our outpatient clinic. Due to the heterogeneous nature of celiac disease and the increasing awareness in the medical community, a wide range of clinical conditions may prompt physicians to check for celiac serology (4, 15). The presenting symptoms of the DGP-positive/tTG-negative infants could indicate celiac disease but are common in many other disease states as well. Furthermore, a recent Swedish study described 3,736 individuals with positive celiac disease serology with normal mucosa (9). In order to further assess DGP's specificity, we tested 38 serum samples from children <2 years old with no clinical indications suggesting celiac disease. All 38 samples were negative for DGP. These findings emphasize the need for continuous serological follow-up of the DGP-positive/tTG-negative infants, especially when clinical findings are suggestive of celiac disease.
In contrast to the 152 cases of DGP-positive/tTG-negative infants, only one infant had DGP-negative/tTG-positive results. This infant had been diagnosed with celiac disease in the past and was on a GFD. The DGP concentrations declined and became negative more rapidly than the tTG concentrations. This difference in Ab kinetics has been described earlier (2, 8).
To conclude, positive DGP in the absence of tTG occurred frequently in infants <2 years old. In the majority of these cases, DGP declined without a GFD. Larger prospective studies are needed for calculating the sensitivities and specificities of DGP assays in comparison to biopsy findings. Our retrospective observation emphasizes that positive DGP results should not call for immediate performance of biopsies but, rather, warrant serological follow-up. Additionally, clinical checkups, monitoring of growth and weight gain charts, records of family history of celiac disease, and possibly, HLA testing, could all assist the physician when deciding whether to consider or reject biopsies in these infants.
Acknowledgments
We are thankful to Pearl Lilos for drafting the manuscript.
Footnotes
Published ahead of print on 31 March 2010.
REFERENCES
- 1.Agardh, D. 2007. Antibodies against synthetic deamidated gliadin peptides and tissue transglutaminase for the identification of childhood celiac disease. Clin. Gastroenterol. Hepatol. 5:1276-1281. [DOI] [PubMed] [Google Scholar]
- 2.Basso, D., G. Guariso, P. Fogar, A. Meneghel, C. F. Zambon, F. Navaglia, E. Greco, S. Schiavon, M. Rugge, and M. Plebani. 2009. Antibodies against synthetic deamidated gliadin peptides for celiac disease diagnosis and follow-up in children. Clin. Chem. 55:150-157. [DOI] [PubMed] [Google Scholar]
- 3.Bürgin-Wolff, A., H. Gaze, F. Hadziselimovic, H. Huber, M. J. Lentze, D. Nusslé, and C. Reymond-Berthet. 1991. Antigliadin and antiendomysium antibody determination for coeliac disease. Arch. Dis. Child. 66:941-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasano, A., M. Araya, S. Bhatnagar, D. Cameron, C. Catassi, M. Dirks, M. L. Mearin, L. Ortigosa, A. Phillips, and Celiac Disease Working Group, FISPGHAN. 2008. Federation of International Societies of Pediatric Gastroenterology, Hepatology and Nutrition consensus report on celiac disease. J. Ped. Gastroenterol. Nutr. 47:214-219. [DOI] [PubMed] [Google Scholar]
- 5.Hill, I. D., M. H. Dirks, G. S. Liptak, R. B. Colletti, A. Fasano, S. Guandalini, E. J. Hoffenberg, K. Horvath, J. A. Murray, M. Pivor, and E. G. Seidman. 2005. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 40:1-19. [DOI] [PubMed] [Google Scholar]
- 6.Kaukinen, K., P. Collin, K. Laurila, T. Kaartinen, J. Partanen, and M. Mäki. 2007. Resurrection of gliadin antibodies in celiac disease: deamidated gliadin peptide antibody test provides additional diagnostic benefit. Scand. J. Gastroenterol. 42:1428-1433. [DOI] [PubMed] [Google Scholar]
- 7.Lagerqvist, C., I. Dahlbom, T. Hansson, E. Jidell, P. Juto, P. Olcén, H. Stenlund, O. Hernell, and A. Ivarsson. 2008. Antigliadin immunoglobulin A best in finding celiac disease in children younger than 18 months of age. J. Pediatr. Gastroenterol. Nutr. 47:428-435. [DOI] [PubMed] [Google Scholar]
- 8.Liu, E., M. Li, L. Emery, I. Taki, K. Barriga, C. Tiberti, G. S. Eisenbarth, M. J. Rewers, and E. J. Hoffenberg. 2007. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J. Pediatr. Gastroenterol. Nutr. 45:293-300. [DOI] [PubMed] [Google Scholar]
- 9.Ludvigsson, J. F., L. Brandt, and S. M. Montgomery. 2009. Symptoms and signs in individuals with serology positive for celiac disease but normal mucosa. BMC Gastroenterol. 9:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lurz, E., U. Scheidegger, J. Spalinger, M. Schöni, and S. Schibli. 2009. Clinical presentation of celiac disease and the diagnostic accuracy of serologic markers in children. Eur. J. Pediatr. 168:839-845. [DOI] [PubMed] [Google Scholar]
- 11.Oberhuber, G., G. Granditsch, and H. Vogelsang. 1999. The histopathology of celiac disease: time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 11:1185-1194. [DOI] [PubMed] [Google Scholar]
- 12.Parizade, M., Y. Bujanover, B. Weiss, V. Nachmias, and B. Shainberg. 2009. Performance of serology assays for diagnosing celiac in a clinical setting. Clin. Vaccine. Immunol. 16:1576-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prince, H. E. 2006. Evaluation of the INOVA diagnostics enzyme-linked immunosorbent assay kits for measuring serum immunoglobulin G (IgG) and IgA to deamidated gliadin peptides. Clin. Vaccine Immunol. 13:150-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rashtak, S., M. W. Ettore, H. Homburger, and J. A. Murray. 2008. Comparative usefulness of deamidated gliadin antibodies in the diagnosis of celiac disease. Clin. Gastroenterol. Hepatol. 6:426-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravikumara, M., D. P. Tuthill, and H. R. Jenkins. 2006. The changing clinical presentation of coeliac disease. Arch. Dis. Child. 91:969-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rostom, A., J. A. Murray, and M. F. Kagnoff. 2006. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology 131:1981-2002. [DOI] [PubMed] [Google Scholar]
- 17.Setty, M., L. Hormaza, and S. Guandalini. 2008. Celiac disease: risk assessment, diagnosis, and monitoring. Mol. Diagn. Ther. 12:289-298. [DOI] [PubMed] [Google Scholar]
- 18.Tonutti, E., D. Visentini, A. Picierno, N. Bizzaro, D. Villalta, R. Tozzoli, G. Kodermaz, A. Carroccio, G. Iacono, S. Teresi, S. M. La Chiusa, and I. Brusca. 2009. Diagnostic efficacy of the ELISA test for the detection of deamidated anti-gliadin peptide antibodies in the diagnosis and monitoring of celiac disease. J. Clin. Lab. Anal. 23:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Meensel, B., M. Hiele, I. Hoffman, S. Vermeire, P. Rutgeerts, K. Geboes, and X. Bossuyt. 2004. Diagnostic accuracy of ten second-generation (human) tissue transglutaminase antibody assays in celiac disease. Clin. Chem. 50:2125-2135. [DOI] [PubMed] [Google Scholar]