Abstract
The objective of this study was to investigate the effects of glucose-based peritoneal dialysis (PD) fluids and icodextrin-based PD fluids on the expression of Toll-like receptor 2 (TLR2)/TLR4 and subsequent ligand-induced mitogen-activated protein kinase (MAPK) and NF-κB signaling and tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) mRNA expression in human peritoneal mesothelial cells (HPMCs). A human peritoneal mesothelial cell line (HMrSV5) was stimulated with glucose-based and icodextrin-based peritoneal dialysis fluids. Cell viability was assessed using MTT [3-(4,5-dimethylthiazolyl)-2,5-diphenyl-2H-tetrazolium bromide]. TLR2/TLR4 expression was determined by real-time PCR, Western blotting, and an immunofluorescence assay. In addition, cells were pretreated with different PD solutions and then incubated with Pam3CSK4 or lipopolysaccharide (LPS), and the degrees of MAPK and NF-κB activation were reflected by detecting the phosphorylation levels of extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), p38, and p65, using a Western blot method. TNF-α and IL-1β mRNA expression was measured by real-time PCR. Glucose-based peritoneal dialysis fluids suppressed the expression of TLR2 and TLR4 proteins in HPMCs. Challenge of cells with either Pam3CSK4 or LPS resulted in impaired TNF-α and IL-1β production. Moreover, reduced TLR2 and TLR4 levels in glucose-based peritoneal dialysis solution-treated mesothelial cells were accompanied by reduced p42/44 (ERK1/2), JNK, p38 MAPK, and NF-κB p65 phosphorylation upon TLR ligand engagement. No significant changes in MAPK and NF-κB signaling and TNF-α and IL-1β mRNA expression were observed in icodextrin-based PD solution-treated mesothelial cells. Glucose-based PD solution, but not icodextrin-based PD solution, downregulates expression of TLR2/TLR4 by human peritoneal mesothelial cells and triggers hyporesponsiveness to pathogen-associated molecular patterns.
Continuous ambulatory peritoneal dialysis (PD) has been used as a treatment for chronic renal failure for over 3 decades (10). Bacterial peritonitis is a major complication of PD and a leading cause of technique failure (6). The composition of most PD fluids is clearly nonphysiologic because of low pH, hyperosmolality, and high glucose and lactate content. It has therefore been suggested that continuous exposure of peritoneal cells, including leukocytes, mesothelial cells, and peritoneal macrophages, to conventional, glucose-containing PD solutions may result in an impairment of the local peritoneal host defense mechanisms (9, 14).
Toll-like receptors (TLRs) play important roles in the initial recognition of bacterial components in the host defense system, and 10 TLR genes have been reported so far. Among them, TLR4 principally recognizes lipopolysaccharide (LPS) derived from Gram-negative bacteria and mediates LPS signal transduction (11, 31). TLR2 can recognize lipoprotein, peptidoglycan, and lipoteichoic acids (LTA) derived from Gram-positive bacteria (15, 21-23, 29) and mediates the activation of downstream signaling molecules.
When TLRs sense the presence of intruders, they engage a common intracellular downstream signaling pathway of all members of the TLR/intereukin-1 receptor (IL-1R) superfamily that culminates in the activation of the mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK), as well as the nuclear factor κB (NF-κB) transcription factor. Binding of this transcription factor to specific DNA binding sites culminates in transcriptional activation of proinflammatory genes, such as those for tumor necrosis factor alpha (TNF-α), IL-1β, IL-6, and numerous other effectors of the innate immune response (1, 11, 31).
Peritoneal mesothelial cells (PMCs) have been shown to constitutively express TLR1, -2, -3, -4, -5, and -6. TLR4 is directly involved in LPS-induced peritoneal inflammation, in an NF-κB-dependent manner (8). Conventional, glucose-containing PD solutions can inhibit the local peritoneal host defense (14). However, whether glucose-containing PD solutions can influence the expression of TLRs and ligand-induced functional consequences is unknown. Our previous study demonstrated that angiotensin II (Ang II) upregulates the expression of TLR4 in rat peritoneal mesothelial cells (RPMCs), resulting in enhanced NF-κB signaling and induction of CD40, TNF-α, and IL-6 expression (27). In this study, we investigated the effects of glucose-based PD solutions and icodextrin-based PD solutions on the expression of TLR2 and TLR4 in human peritoneal mesothelial cells (HPMCs) and analyzed the functional consequences.
MATERIALS AND METHODS
Antibodies and reagents.
Rabbit anti-human TLR2 polyclonal antibody, rabbit anti-human TLR4 monoclonal antibody (MAb), rabbit anti-human polyclonal antibodies against p38 MAPK, phospho-p38 MAPKThr180/Tyr182, JNK, phospho-JNKThr183/Tyr185, p44/42 MAPK, NF-κB p65, and phospho-NF-κB p65Ser536, and mouse anti-human polyclonal antibodies against phospho-p44/42 MAPKThr202/Tyr204 were purchased from Cell Signaling Technology Inc. (Danvers, MA). Phycoerythrin (PE)-labeled anti-human TLR4 MAb (HTA-125; mouse IgG2a), fluorescein isothiocyanate (FITC)-labeled anti-human TLR2 MAb (TL2.1; mouse IgG2a), and isotype-matched immunoglobulins (IgGs) of irrelevant specificities (FITC- or PE-labeled mouse IgG2a) were purchased from eBioscience (San Diego, CA). Ultra-pure LPS (upLPS) from Escherichia coli (O111:B4) and Pam3CSK4 were obtained from Invivogen (San Diego, CA). The PD solutions tested included 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and Extraneal (7.5% icodextrin), all from Baxter Healthcare Corporation (Deerfield, IL). Dulbecco's modified Eagle's medium (DMEM) and fetal calf serum (FCS) were purchased from Gibco-BRL (Grand Island, NY) (with endotoxin levels of ≤50 EU/ml [routinely ≤10 EU/ml]). A reverse transcription (RT) kit and Trizol reagent were purchased from Invitrogen (Carlsbad, CA). SYBR Ex Taq premix was obtained from Takara (Japan).
Cell culture.
The simian virus 40 (SV40)-immortalized human peritoneal mesothelial cell line HMrSV5 has been described previously (18, 19). Cells were grown in type I collagen-coated dishes in DMEM containing 10% FCS. All experiments on immortalized mesothelial cells were performed between passages 5 and 10.
Assessment of cell viability.
Cells were seeded into 96-well plates at a density of 104 cells/cm2 and cultured in DMEM supplemented with 10% FCS. Near-confluent cells were incubated with serum-free medium for 24 h to arrest and synchronize cell growth. Afterward, the medium was changed to different PD solutions diluted 2-fold with DMEM (low glucose [1 g/liter]). At selected time points (24, 48, and 72 h), MTT [3-(4,5-dimethylthiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] was added at a final concentration of 0.5 mg/ml, and the cells were incubated for a further 4 h in a humidified environment. Dimethyl sulfoxide (DMSO) was added, and 96-well plates were gently shaken for 10 min at room temperature, and the effects of different PD solutions on cell viability were determined by spectrophotometry at 490 nm.
Effects of different PD solutions on TLR2/TLR4 mRNA and protein expression.
Cells were detached with 0.25% trypsin-0.02% EDTA-Na2 and were seeded into 60-mm-diameter tissue culture plates (Corning Co., Corning, NY). Subconfluent cells were washed twice with D-Hanks' balanced salt solution (D-HBSS) and incubated with FCS-free DMEM for 24 h. The medium was then changed to 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, or Extraneal diluted 2-fold with DMEM (low glucose [1 g/liter]). At 6, 12, 24, 36, and 48 h, cells were lysed and TLR2/TLR4 mRNA expression was measured by real-time PCR. At 24, 48, and 72 h, cells were lysed and TLR2/TLR4 protein expression was measured by Western blotting. At 48 h, cells were fixed and TLR2/TLR4 protein expression was measured by immunofluorescence microscopy.
Effects of different PD solutions on TLR2/TLR4 ligand-induced MAPK and NF-κB activation.
Cells were incubated with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, or Extraneal diluted 2-fold with DMEM for 48 h and then with Pam3CSK4 (250 ng/ml) or LPS (1 μg/ml) for 1 h. The activation of MAPK and NF-κB was reflected by detecting the phosphorylation levels of p38 MAPK, p44/42 MAPK, JNK, and NF-κB p65, using Western blotting.
Effects of different PD solutions on TLR2/TLR4 ligand-induced TNF-α and IL-1β mRNA expression.
Cells were incubated with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, or Extraneal diluted 2-fold with DMEM for 48 h and then with Pam3CSK4 (250 ng/ml) or LPS (1 μg/ml) for 2 h. The expression of TNF-α and IL-1β mRNAs was measured by real-time PCR.
Western blotting.
Treated/untreated subconfluent cells were lysed in lysis buffer (Cell Signaling Technology Inc., Danvers, MA). Protein concentrations were measured using the Bradford method, and 20 μg of protein was analyzed by 10% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions and electrotransferred to nitrocellulose membranes. Nonspecific protein binding was blocked by incubating the membranes with blocking solution (Tris-buffered saline-Tween 20 [TBST] and 5% nonfat dried milk) for 60 min at room temperature. Polyclonal antibodies specific for TLR2 (1:1,000), TLR4 (1:1,000), p38 MAPK (1:1,000), phospho-p38 MAPKThr180/Tyr182 (1:1,000), JNK (1:1,000), phospho-JNKThr183/Tyr185 (1:1,000), p44/42 MAPK (1:1,000), phospho-p44/42 MAPKThr202/Tyr204 (1:1,000), NF-κB p65 (1:1,000), or phospho-NF-κB p65Ser536 (1:1,000) were applied to the membrane and incubated overnight at 4°C. After rinsing of the blots with 1× TBST, 1:2,000-diluted peroxidase-conjugated anti-rabbit and anti-mouse IgG antibodies were added for 60 min at room temperature. The detection of specific signals was performed by using an enhanced chemiluminescence system (Cell Signaling Technology Inc., Danvers, MA).
Immunofluorescence microscopy.
Cells were seeded into six-well slide chambers, incubated with fresh FCS-free DMEM for 24 h, and stimulated with the different PD solutions mentioned above for 48 h. Cells were washed twice with cold phosphate-buffered saline (PBS), fixed in fresh 100% methanol for 15 min at −20°C, and rinsed with PBS. Nonspecific binding was blocked with 5% bovine serum albumin (BSA) in PBS for 30 min at room temperature, followed by incubation with FITC-labeled anti-human TLR2 (1:50) monoclonal antibody and PE-labeled anti-human TLR4 (1:50) monoclonal antibody in 5% BSA in PBS at 4°C overnight. Cells were rinsed with PBS, mounting medium for fluorescence was added, and slides were sealed with coverslips and examined under an LSM 510 confocal immunofluorescence microscope (Carl Zeiss, Inc., Jena, Germany). The images were obtained with an LSM image browser.
Real-time PCR.
Cells were washed with HBSS and lysed in 1 ml Trizol reagent, and total RNA was prepared according to the manufacturer's instructions. The purity and quantity of the extract were determined by UV absorption and gel electrophoresis. A total of 2 μg RNA was reverse transcribed to cDNA, using an Invitrogen first-strand synthesis kit.
Quantitative RT-PCR of target cDNA was conducted for TLR2, TLR4, TNF-α, and IL-1β and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression. The following primers were used (3′-5′): CCC ATT CTC CCT CCG TAG (forward) and ACC TTC GAC CAC CGT TAT (reverse) for TLR2, TGA GCA GTC GTG CTG GTA TC (forward) and TTT TCT GCC AGT GCC TCT TT (reverse) for TLR4, CCA ACA GTG AGA GGG GTC AT (forward) and GCA GCT CTA GGG GGA GAA GT (reverse) for TNF-α, GAC TGA CAG GAC CGA CTA (forward) and GAA TGT GGG AGC GAA TGA C (reverse) for IL-1β, and TAT GGT GGT TTA GGC AAC (forward) and AGC CTT CTC CAT GGT GGT G (reverse) for GAPDH. Experiments were performed in 96-well plates in triplicate, using SYBR Ex Taq premix (Takara, Japan). Real-time PCR amplification was performed on an ABI Prism 7000 sequence detection system. Two-step PCR conditions were 95°C for 30 s and then 40 cycles at 95°C for 5 s and 55°C for 31 s, according to the manufacturer's instructions.
Statistical analysis.
Data are expressed as means ± standard deviations (SD) for three determinations. Statistical differences among groups were assessed by one-way analysis of variance (ANOVA), followed by the Bonferroni (post hoc) test for normally distributed continuous variables and by the Mann-Whitney test for continuous variables without a normal distribution. A P value of <0.05 was considered statistically significant. The statistical calculations were performed by means of the statistical package SPSS for Windows 13.0.
RESULTS
Effects of different PD solutions on cell viability.
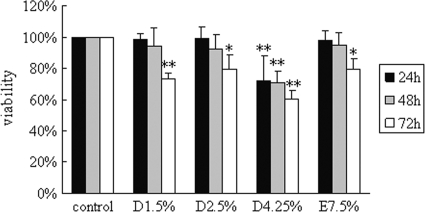
As shown in Fig. 1, the viability of cells incubated with medium alone was set to 100%, and that of cells after treatment for 24 h with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and Extraneal was 98.4% ± 3.9%, 99.4% ± 7.3%, 72.0% ± 16.2%, and 98.1% ± 6.2%, respectively. At the 48-h time point, the viability of cells was 94.4% ± 11.4%, 92.7% ± 9.2%, 71.2% ± 7.0%, and 94.8% ± 8.1%, respectively. Compared to the control, significant decreases in cell viability in PD solutions were observed at 72 h, with levels of 73.4% ± 3.9%, 79.3% ± 9.4%, 60.5% ± 5.4%, and 79.5% ± 7.1%, respectively (P < 0.05).
FIG. 1.
Effects of glucose-based PD solutions and icodextrin-based PD solutions on cell viability in human peritoneal mesothelial cells. Cells were treated with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and 7.5% icodextrin (Extraneal) for 24, 48, and 72 h. MTT assay was used to evaluate cell viability. Cells incubated with medium alone (DMEM with low glucose [1 g/liter]) were used as a control, and their cell viability was set to 100%. Data are expressed as means ± SD for three individual experiments. D1.5%, D2.5%, D4.25%, and E7.5% represent 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and 7.5% icodextrin, respectively. *, P < 0.05; **, P < 0.01 versus control.
Glucose-based peritoneal dialysis solutions decrease TLR2/TLR4 mRNA and protein expression.
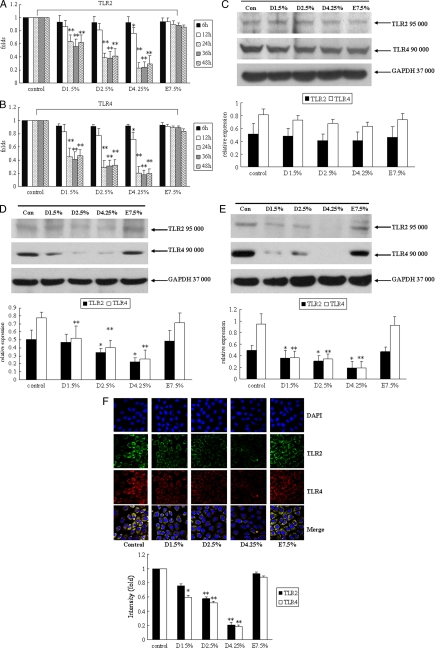
To assess the effects of different PD solutions on TLR2 and TLR4 mRNA expression, human peritoneal mesothelial cells were exposed to 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and Extraneal diluted 2-fold with DMEM for 6, 12, 24, 36, and 48 h. As shown in Fig. 2A and B, at 6 and 12 h, there were no significant differences between the Dianeal groups and the control group (except at 12 h for 4.25% Dianeal). However, treatment with 1.5% Dianeal, 2.5% Dianeal, and 4.25% Dianeal for 24, 36, and 48 h significantly decreased TLR2 and TLR4 mRNA expression compared to the control levels. Icodextrin-based PD solutions did not influence the TLR2 and TLR4 expression levels.
FIG. 2.
Glucose-based peritoneal dialysis solutions decrease TLR2 and TLR4 expression in human peritoneal mesothelial cells. Cells were treated with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and 7.5% icodextrin (Extraneal) for 6, 12, 24, 36, and 48 h for mRNA expression and for 24, 48, and 72 h for protein expression. Real-time PCR and immunoblot analyses were used to determine the TLR2 and TLR4 mRNA and protein expression levels. Incubation of cells with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and 7.5% icodextrin for 6 and 12 h did not influence TLR2 (A) and TLR4 (B) mRNA expression. However, at 24, 36, and 48 h, TLR2 and TLR4 mRNA expression in the glucose-based PD solution treatment groups was significantly downregulated compared to that in the control group (P < 0.01). (C) In addition, incubation of cells with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and 7.5% icodextrin for 24 h did not influence TLR2 and TLR4 protein expression. However, at 48 (D) and 72 (E) h, TLR2 and TLR4 protein expression in the glucose-based PD solution treatment groups was significantly downregulated compared to that in the control group (P < 0.05). Icodextrin-based PD solutions did not influence TLR2 and TLR4 expression. (F) Cells were analyzed at 48 h by immunofluorescence with anti-TLR2 and anti-TLR4 antibodies, and the intensity values of the cells were measured with LSM 510 confocal software. The positions of the nuclei are indicated by DAPI (4′,6-diamidino-2-phenylindole) fluorescence. Data are expressed as means ± SD for three individual experiments. D1.5%, D2.5%, D4.25%, and E7.5% represent 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and 7.5% icodextrin, respectively. *, P < 0.05; **, P < 0.01 versus control.
To assess the influence of different PD solutions on TLR2 and TLR4 protein expression, human peritoneal mesothelial cells were exposed to 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and Extraneal diluted 2-fold with DMEM for 24, 48, and 72 h. The protein expression of TLR2 and TLR4 was determined by immunoblot analysis. As shown in Fig. 2C, treatment with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and Extraneal for 24 h did not influence TLR2 and TLR4 protein expression. After incubation of cells with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and Extraneal for 48 h, TLR2 expression decreased by 5.5% ± 2.8%, 31.4% ± 7.5%, 54.9% ± 1.9%, and 4.4% ± 4.5%, respectively, and TLR4 expression decreased by 32.9% ± 17.6%, 47.7% ± 13.5%, 66.4% ± 13.5%, and 8.9% ± 8.6%, respectively, compared to the control levels (Fig. 2D). In addition, at the 72-h treatment time point, TLR2 expression decreased by 29.4% ± 14.7%, 38.9% ± 9.9%, 63.5% ± 16.5%, and 5.3% ± 3.3%, respectively, and TLR4 expression decreased by 59.5% ± 16.8%, 63.1% ± 9.5%, 79.2% ± 14.0%, and 1.2% ± 11.9%, respectively, compared to the control levels (Fig. 2E).
We further performed immunofluorescence microscopy to assess TLR2 and TLR4 expression in response to different PD solutions. As shown in Fig. 2F, at 48 h, TLR2 protein (green signal) and TLR4 protein (red signal) expression in the glucose-based PD solution treatment groups was significantly downregulated compared to that in the control group. Icodextrin-based PD solutions did not influence TLR2 and TLR4 expression. This was consistent with the results of immunoblot analysis.
TLR ligand-induced MAPK and NF-κB signaling is reduced in glucose-based PD solution-treated mesothelial cells.
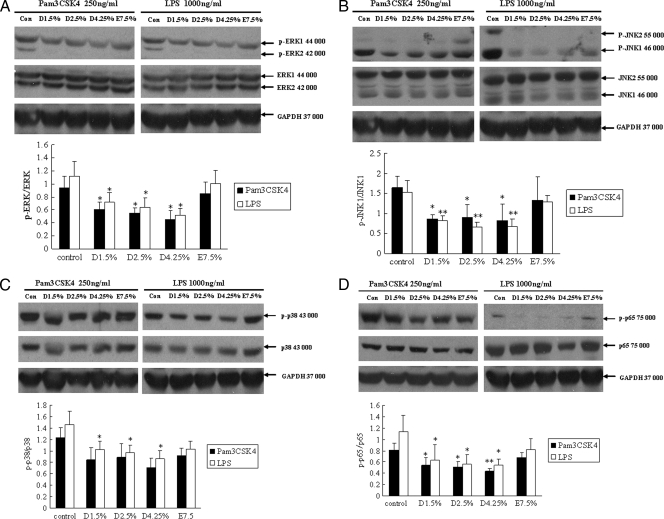
Since TLR2 and TLR4 were downregulated in mesothelial cells treated with glucose-based PD solutions, we were interested in investigating TLR downstream signaling. Using phospho-specific antibodies, we analyzed p38 MAPK, JNK, and p44/42 MAPK signaling in human peritoneal mesothelial cells stimulated with ligands for TLR2 (Pam3CSK4) and TLR4 (LPS), respectively. As shown in Fig. 3A and B, mesothelial cells pretreated with glucose-based PD solutions that were stimulated with either Pam3CSK4 or LPS exhibited lower levels of phosphorylated p44/42 MAPK (ERK1/2) and JNK than did untreated cells. Although Pam3CSK4-induced phosphorylated p38 MAPK protein expression did not decrease significantly in cells pretreated with glucose-based PD solutions (compared to the control), LPS-induced phosphorylated p38 MAPK was impaired significantly (Fig. 3C). These results suggest that TLR downregulation by glucose-based PD solutions impacts subsequent MAPK phosphorylation. Cells preincubated with icodextrin-based PD solutions did not show an influence on TLR ligand-induced MAPK signaling.
FIG. 3.
TLR ligand-induced MAPK and NF-κB signaling is reduced in glucose-based PD solution-treated human peritoneal mesothelial cells. Cells were pretreated with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and 7.5% icodextrin (Extraneal) for 48 h and then treated with TLR2 (Pam3CSK4; 250 ng/ml) or TLR4 (LPS; 1 μg/ml) ligand for 60 min. Immunoblot analysis was used to determine phosphorylated p38 MAPK, JNK, and p44/42 MAPK protein levels. Cells pretreated with glucose-based PD solutions that were stimulated with either Pam3CSK4 or LPS exhibited lower levels of phosphorylated p44/42 MAPK (A), JNK (B), p38 (C), and NF-κB p65 (D) than did untreated cells. Cells preincubated with icodextrin-based PD solutions did not shown an influence on TLR ligand-induced MAPK and NF-κB signaling. Data are expressed as means ± SD for three individual experiments. D1.5%, D2.5%, D4.25%, and E7.5% represent 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and 7.5% icodextrin, respectively. *, P < 0.05; **, P < 0.01 versus control.
We further investigated NF-κB signaling in human peritoneal mesothelial cells stimulated with ligands for TLR2 and TLR4, using phospho-specific antibodies to the p65 subunit of NF-κB. As shown in Fig. 3D, mesothelial cells pretreated with glucose-based PD solutions that were stimulated with either Pam3CSK4 or LPS exhibited lower levels of phosphorylated NF-κB p65 than did untreated cells. No significant changes in phosphorylated NF-κB p65 protein level were observed in icodextrin-based PD solution-treated mesothelial cells.
Decreased cytokine production in glucose-based PD solution-treated mesothelial cells upon TLR ligand induction.
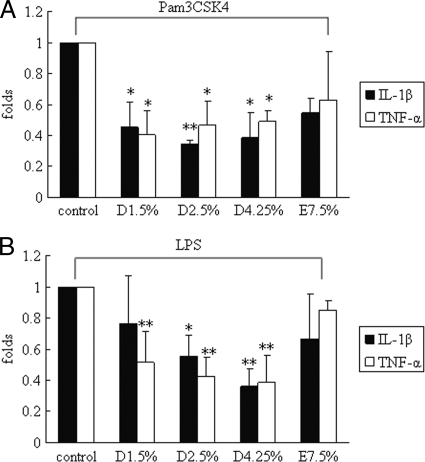
Cells were pretreated with glucose-based PD solutions for 48 h and, afterwards, were incubated with either 250 ng Pam3CSK4 or 1 μg LPS for 4 h. TNF-α and IL-1β mRNA expression was determined by real-time PCR. In accordance with TLR2 and TLR4 downregulation, TNF-α and IL-1β syntheses upon Pam3CSK4 and LPS challenge were decreased in glucose-based PD solution-treated mesothelial cells compared to those in the control (untreated cells) (P < 0.05). No significant changes in TNF-α and IL-1β mRNA expression were observed in icodextrin-based PD solution-treated mesothelial cells (Fig. 4A and B).
FIG. 4.
Decreased cytokine production in glucose-based PD solution-treated human peritoneal mesothelial cells upon TLR ligand induction. Cells were pretreated with 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and 7.5% icodextrin (Extraneal) for 48 h and then treated with TLR2 (Pam3CSK4; 250 ng/ml) (A) or TLR4 (LPS; 1 μg/ml) (B) ligand for 2 h. Real-time PCR was used to determine TNF-α and IL-1β mRNA expression. TNF-α and IL-1β syntheses were decreased upon Pam3CSK4 and LPS challenge in glucose-based PD solution-treated mesothelial cells compared to those in the control (untreated cells) (P < 0.05). No significant changes in TNF-α and IL-1β mRNA expression were observed in icodextrin-based PD solution-treated mesothelial cells. Data are expressed as means ± SD for three individual experiments. D1.5%, D2.5%, D4.25%, and E7.5% represent 1.5% Dianeal, 2.5% Dianeal, 4.25% Dianeal, and 7.5% icodextrin, respectively. *, P < 0.05; **, P < 0.01 versus control.
DISCUSSION
The introduction of PD more than 3 decades ago provoked much interest in the biology of mesothelial cells. In the peritoneal cavity, HPMCs represent the largest population of resident cells, whose primary function is to provide a nonadhesive and protective layer against the invasion of foreign particles and injury to the peritoneum consequent to chemical or surgical insult (30). Our in vitro data demonstrate that conventional glucose-containing PD solutions downregulate TLR2 and TLR4 in HPMCs, accompanied by decreased p42/44 (ERK1/2), JNK, p38 MAPK, and NF-κB p65 phosphorylation and TNF-α and IL-1β expression upon Pam3CSK4/LPS engagement. These results may suggest an impaired inflammatory response to bacterial pathogen-associated molecular patterns (PAMPs) in HPMCs treated with glucose-based PD solutions, at least in part due to TLR downregulation. Since TLRs are key components in pathogen recognition and are crucial mediators in the early inflammatory response to foreign microorganisms, downregulation of TLR2 and TLR4 by glucose-containing PD solutions clearly represents an important and novel immunomodulating effect. Based on this findings, we assume that long-term application of conventional glucose-containing PD solutions may increase the risk of microbial invasion and peritoneal infection. However, further in vivo exploration is needed to prove this assumption.
There is accumulating evidence that TLR expression, especially TLR4 expression, is influenced by various endogenous and exogenous factors and that this modulation has important clinical implications. Methe et al. (13) demonstrated that statins downregulated TLR4 and decreased LPS-induced IL-1R-associated kinase (IRAK) phosphorylation and TNF-α, IL-6, IL-12, and B7-1 expression in human CD14+ monocytes. They concluded that interactions with innate immune mechanisms are a potential mechanism of statins to mediate anti-atherosclerotic effects by reducing TLR4 expression, which plays an important role in cardiovascular disease (5, 25, 28). Sadeghi and colleagues (20) reported that vitamin D3 downregulated TLR2/TLR4 expression and decreased LTA/LPS-induced phosphorylated p38 and p42/44 MAPK and TNF-α expression in human monocytes, and they indicated the immunomodulatory effect of 1,25(OH)2D3 from a novel perspective. In contrast, Wolf et al. (26) showed that Ang II upregulated TLR4 and enhanced LPS-induced NF-κB activation and monocyte chemotactic protein 1 (MCP-1) and RANTES (regulated upon activation, normal T-cell expressed, and secreted) expression. They provided a better understanding of how exogenous infections may trigger renal autoimmune processes, particularly in pathophysiologic situations with high renal Ang II concentrations, and the development of inflammation in many noninfectious renal diseases. Our previous research found that Ang II upregulates expression of TLR4 in RPMCs, resulting in enhanced NF-κB signaling and induction of CD40, TNF-α, and IL-6 expression. This may suggest that locally produced Ang II in the peritoneum may have an amplified role in LPS-induced peritoneal inflammation (27). Our current study gave similar results to these reports. However, Dasu et al. (3) demonstrated that high glucose levels induce TLR2/TLR4 expression in human monocytes in a time- and concentration-dependent manner. This difference may be explained as follows. First, due to the inhibition by conventional glucose-containing PD solutions of local peritoneal host defense, our study compared the effects of glucose-containing PD solutions and icodextrin-based PD solutions on TLR2/TLR4 expression. This study did not focus on the effect of glucose itself per se. Although 4.25% Dianeal (3.86% [wt/vol]), which contains the highest glucose concentration, had the most obvious effect in downregulating the expression of TLR2/TLR4 at 72 h, we could not make the conclusion that glucose-containing PD solutions decreased TLR2/TLR4 expression in a time- and concentration-dependent manner by statistical analysis. Second, different cell types may have different responses to the same stimulation. TLR2/TLR4 levels, especially TLR4 expression, could be upregulated or downregulated in different cell types in response to the same stimulation. Human monocytes are the first and key target after high-glucose stimulation or hyperglycemia. For example, NADPH oxidase-derived oxidants mediate high-glucose-induced NF-κB activation and result in enhanced IL-6 and IL-1 expression in monocytes (2, 4). However, peritoneal mesothelial cells, which play a key role in the control of fluid and solute transport, immune surveillance, and regulation of inflammatory processes and wound healing (30), are totally different from monocytes. Third, our current study did not investigate the mechanism of TLR2/TLR4 downregulation, such as whether inhibition of protein kinase C (PKC) by specific inhibitors or small interfering RNA or inhibition of NADPH oxidase could validate the TLR2/TLR4 upregulation induced by glucose (3). Future studies are needed to determine the mechanism of TLR2/TLR4 downregulation.
Over the past few decades, icodextrin, a glucose polymer, has increasingly been used as an alternative osmotic agent to glucose. It has been shown that a 7.5% icodextrin-based PD solution can provide sustained positive ultrafiltration that is equivalent to the effect of a 3.86% glucose PD solution (16). In vitro studies indicated that compared to currently used glucose-based PD solutions, icodextrin-based PD solutions caused less suppression of phagocytic activity (24), did not cause damage to the intracellular junctions of HPMCs (7), and had a weaker effect on the production of transforming growth factor β1 (TGF-β1) (7), plasminogen activator inhibitor 1 (PAI-1), and tissue-type plasminogen activator (t-PA) (12). Furthermore, long-term clinical study provided evidence that the use of icodextrin solution did not result in deterioration of peritoneal defense determinants more than was seen with glucose, and icodextrin had a positive effect on some aspects of the peritoneal defense system, for example, causing an increase in absolute numbers and percentages of effluent peritoneal macrophages (17). Icodextrin appears to be more biocompatible than glucose-based solutions in that it has fewer detrimental effects on normal cell and membrane function, as demonstrated in the above-mentioned reports. Our study shows that icodextrin-based PD solutions exert fewer effects on TLR2 and TLR4 expression and subsequent MAPK and NF-κB signaling and TNF-α and IL-1β mRNA expression upon Pam3CSK4/LPS engagement than do glucose-based PD solutions. These results indicate, from a novel perspective, the better biocompatibility of icodextrin than glucose and are in agreement with previous findings. Ex vivo experiments will be conducted in our subsequent research.
Taken together, our results provide evidence that glucose-based PD solutions, but not icodextrin-based PD solutions, prime human peritoneal mesothelial cells to respond less effectively to bacterial PAMPs, most likely via modulation of TLRs.
Acknowledgments
We thank Qin Zhou and Ning Luo for their technical assistance.
This work was supported by the Guangdong Natural Science Foundation of China (grant 9151008901000051).
Footnotes
Published ahead of print on 3 March 2010.
REFERENCES
- 1.Barton, G. M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 300:1524-1525. [DOI] [PubMed] [Google Scholar]
- 2.Dasu, M. R., S. Devaraj, and I. Jialal. 2007. High glucose induces IL-1beta expression in human monocytes: mechanistic insights. Am. J. Physiol. Endocrinol. Metab. 293:E337-E346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasu, M. R., S. Devaraj, L. Zhao, D. H. Hwang, and I. Jialal. 2008. High glucose induces Toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57:3090-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaraj, S., S. K. Venugopal, U. Singh, and I. Jialal. 2005. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta}. Diabetes 54:85-91. [DOI] [PubMed] [Google Scholar]
- 5.Edfeldt, K., J. Swedenborg, G. K. Hansson, and Z. Q. Yan. 2002. Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105:1158-1161. [PubMed] [Google Scholar]
- 6.Gokal, R. 2002. Peritoneal dialysis in the 21st century: an analysis of current problems and future developments. J. Am. Soc. Nephrol. 13(Suppl. 1):S104-S116. [PubMed] [Google Scholar]
- 7.Ito, T., N. Yorioka, Y. Kyuden, Y. Asakimori, K. Kiribayashi, T. Ogawa, and N. Kohno. 2003. Effect of glucose polymer on the intercellular junctions of cultured human peritoneal mesothelial cells. Nephron Clin. Pract. 93:c97-c105. [DOI] [PubMed] [Google Scholar]
- 8.Kato, S., Y. Yuzawa, N. Tsuboi, S. Maruyama, Y. Morita, T. Matsuguchi, and S. Matsuo. 2004. Endotoxin-induced chemokine expression in murine peritoneal mesothelial cells: the role of Toll-like receptor 4. J. Am. Soc. Nephrol. 15:1289-1299. [PubMed] [Google Scholar]
- 9.Kazancioglu, R. 2009. Peritoneal defense mechanisms—the effects of new peritoneal dialysis solutions. Perit. Dial. Int. 29(Suppl. 2):S198-S201. [PubMed] [Google Scholar]
- 10.Krediet, R. T. 2007. 30 years of peritoneal dialysis development: the past and the future. Perit. Dial. Int. 27(Suppl. 2):S35-S41. [PubMed] [Google Scholar]
- 11.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Invest. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masahira, K., I. Takafumi, M. Takao, K. Nobuoki, and Y. Noriaki. 2007. Glucose-based PD solution, but not icodextrin-based PD solution, induces plasminogen activator inhibitor-1 and tissue-type plasminogen activator in human peritoneal mesothelial cells via ERK1/2. Ther. Apher. Dial. 11:94-100. [DOI] [PubMed] [Google Scholar]
- 13.Methe, H., J. O. Kim, S. Kofler, M. Nabauer, and M. Weis. 2005. Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arterioscler. Thromb. Vasc. Biol. 25:1439-1445. [DOI] [PubMed] [Google Scholar]
- 14.Mortier, S., N. H. Lameire, and A. S. De Vriese. 2004. The effects of peritoneal dialysis solutions on peritoneal host defense. Perit. Dial. Int. 24:123-138. [PubMed] [Google Scholar]
- 15.Opitz, B., N. W. Schroder, I. Spreitzer, K. S. Michelsen, C. J. Kirschning, W. Hallatschek, U. Zahringer, T. Hartung, U. B. Gobel, and R. R. Schumann. 2001. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappaB translocation. J. Biol. Chem. 276:22041-22047. [DOI] [PubMed] [Google Scholar]
- 16.Pecoits-Filho, R., S. Mujais, and B. Lindholm. 2002. Future of icodextrin as an osmotic agent in peritoneal dialysis. Kidney Int. 2002(Suppl.):S80-S87. [DOI] [PubMed] [Google Scholar]
- 17.Posthuma, N., P. ter Wee, A. J. Donker, H. A. Dekker, P. L. Oe, and H. A. Verbrugh. 1999. Peritoneal defense using icodextrin or glucose for daytime dwell in CCPD patients. Perit. Dial. Int. 19:334-342. [PubMed] [Google Scholar]
- 18.Rougier, J. P., S. Guia, J. Hagege, G. Nguyen, and P. M. Ronco. 1998. PAI-1 secretion and matrix deposition in human peritoneal mesothelial cell cultures: transcriptional regulation by TGF-beta 1. Kidney Int. 54:87-98. [DOI] [PubMed] [Google Scholar]
- 19.Rougier, J. P., P. Moullier, R. Piedagnel, and P. M. Ronco. 1997. Hyperosmolality suppresses but TGF beta 1 increases MMP9 in human peritoneal mesothelial cells. Kidney Int. 51:337-347. [DOI] [PubMed] [Google Scholar]
- 20.Sadeghi, K., B. Wessner, U. Laggner, M. Ploder, D. Tamandl, J. Friedl, U. Zugel, A. Steinmeyer, A. Pollak, E. Roth, G. Boltz-Nitulescu, and A. Spittler. 2006. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 36:361-370. [DOI] [PubMed] [Google Scholar]
- 21.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 22.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, et al. 1999. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 24.Thomas, S., U. Schenk, F. P. Fischer, T. Mettang, J. Passlick-Deetjen, and U. Kuhlmann. 1997. In vitro effects of glucose polymer-containing peritoneal dialysis fluids on phagocytic activity. Am. J. Kidney Dis. 29:246-253. [DOI] [PubMed] [Google Scholar]
- 25.Vink, A., A. H. Schoneveld, J. J. van der Meer, B. J. van Middelaar, J. P. Sluijter, M. B. Smeets, P. H. Quax, S. K. Lim, C. Borst, G. Pasterkamp, and D. P. de Kleijn. 2002. In vivo evidence for a role of Toll-like receptor 4 in the development of intimal lesions. Circulation 106:1985-1990. [DOI] [PubMed] [Google Scholar]
- 26.Wolf, G., J. Bohlender, T. Bondeva, T. Roger, F. Thaiss, and U. O. Wenzel. 2006. Angiotensin II upregulates Toll-like receptor 4 on mesangial cells. J. Am. Soc. Nephrol. 17:1585-1593. [DOI] [PubMed] [Google Scholar]
- 27.Wu, J., X. Yang, Y. F. Zhang, S. F. Zhou, R. Zhang, X. Q. Dong, J. J. Fan, M. Liu, and X. Q. Yu. 2009. Angiotensin II upregulates Toll-like receptor 4 and enhances lipopolysaccharide-induced CD40 expression in rat peritoneal mesothelial cells. Inflamm. Res. 58:473-482. [DOI] [PubMed] [Google Scholar]
- 28.Xu, X. H., P. K. Shah, E. Faure, O. Equils, L. Thomas, M. C. Fishbein, D. Luthringer, X. P. Xu, T. B. Rajavashisth, J. Yano, S. Kaul, and M. Arditi. 2001. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104:3103-3108. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. T. Golenbock. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 165:1-5. [PubMed] [Google Scholar]
- 30.Yung, S., and T. M. Chan. 2009. Intrinsic cells: mesothelial cells—central players in regulating inflammation and resolution. Perit. Dial. Int. 29(Suppl. 2):S21-S27. [PubMed] [Google Scholar]
- 31.Zhang, G., and S. Ghosh. 2001. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J. Clin. Invest. 107:13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]